Polyphenolic and Immunometric Profiling of Wheat Varieties: Impact of Organic and Conventional Farming on Allergenic and Bioactive Compounds
Abstract
1. Introduction
2. Results
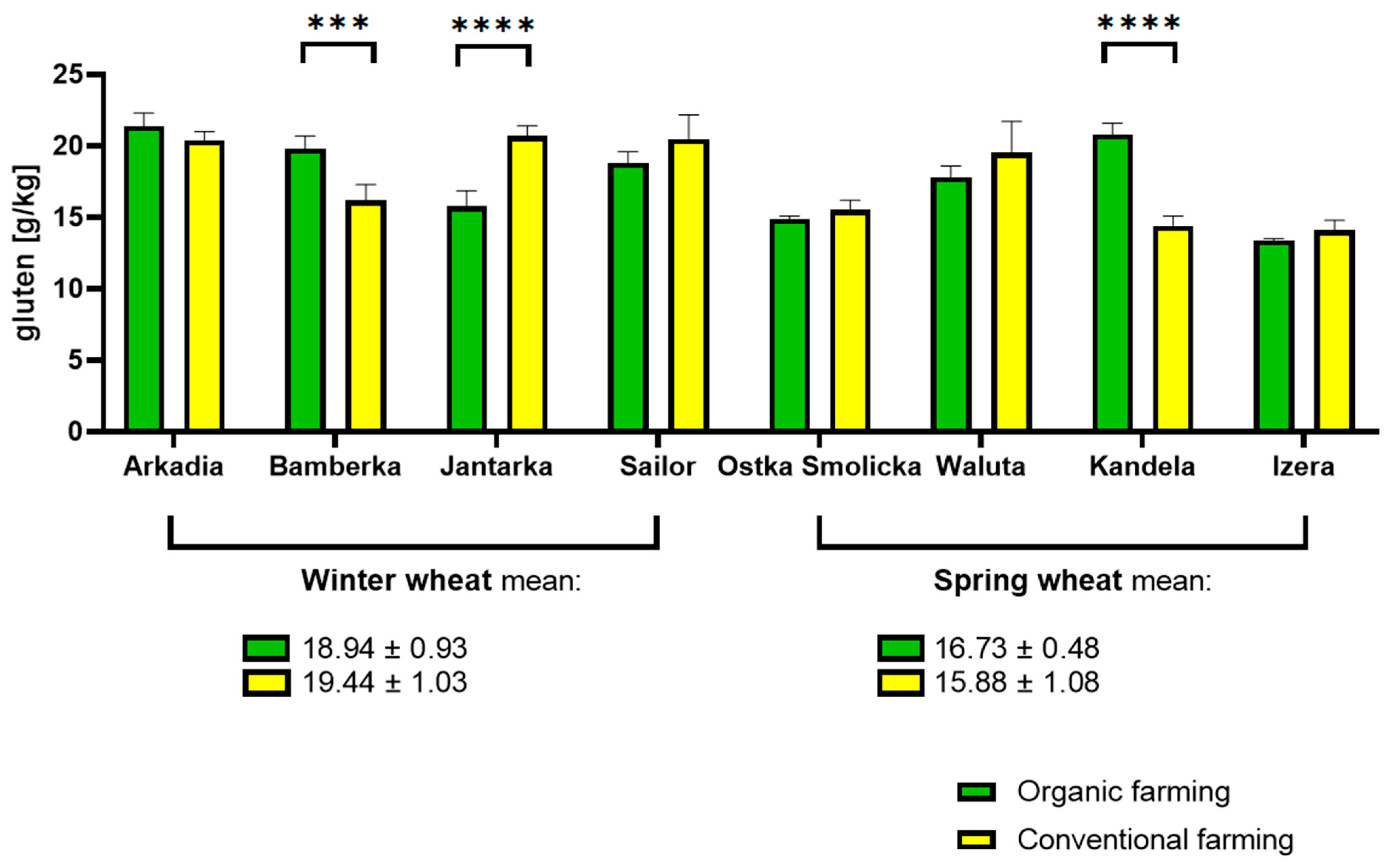
2.1. Gluten Quantification
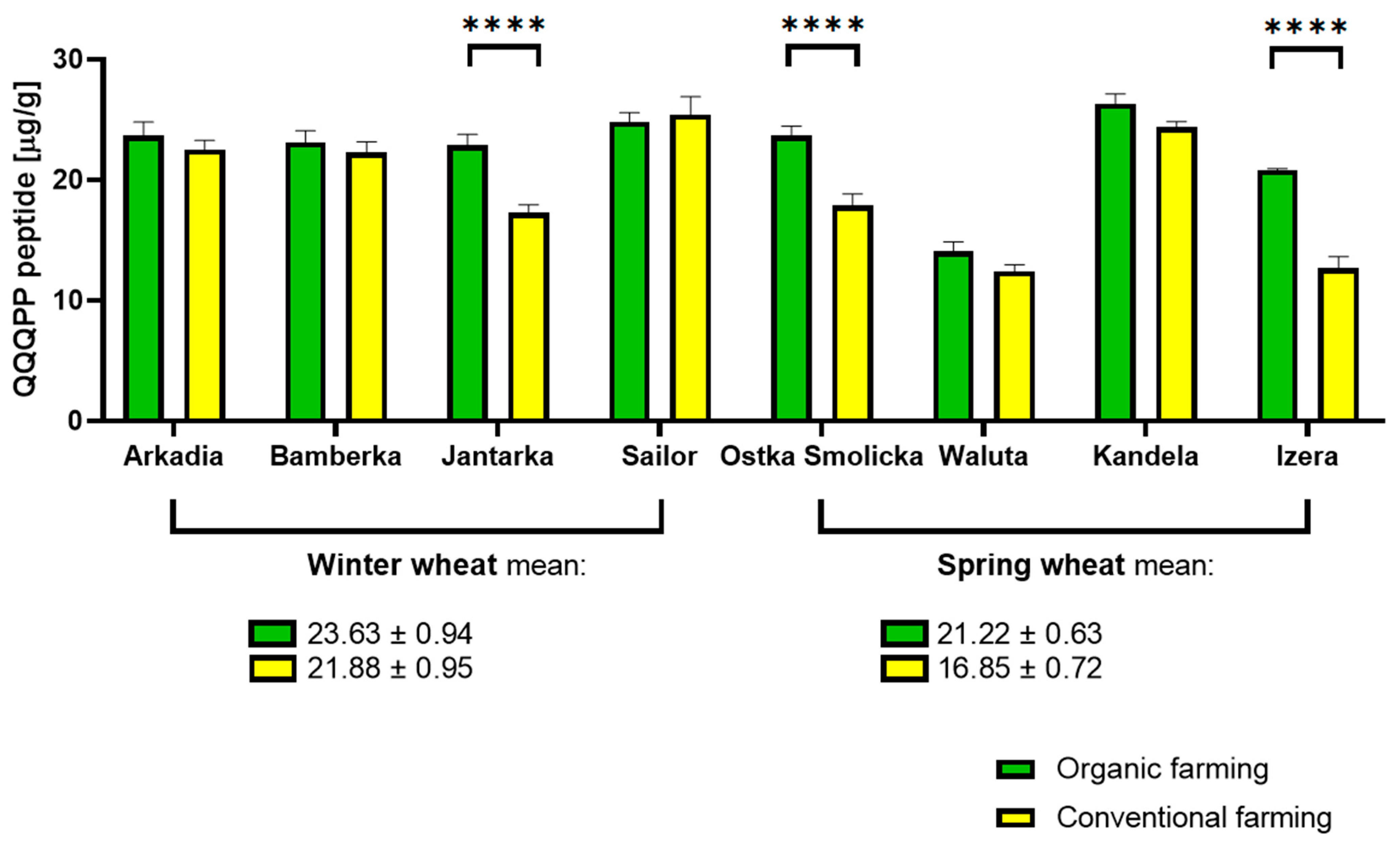
2.2. QQQPP Peptide Determination
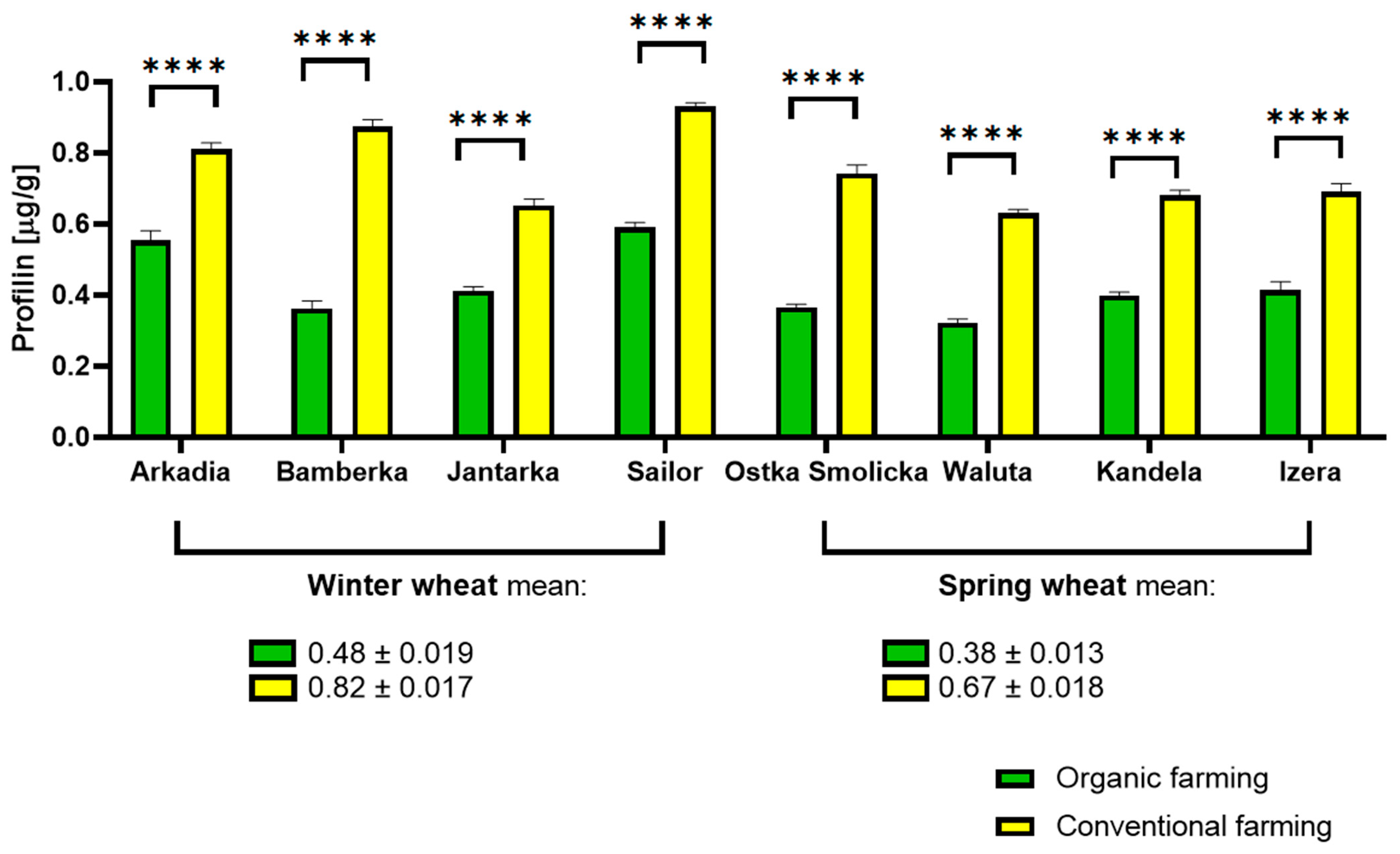
2.3. Profilin Determination
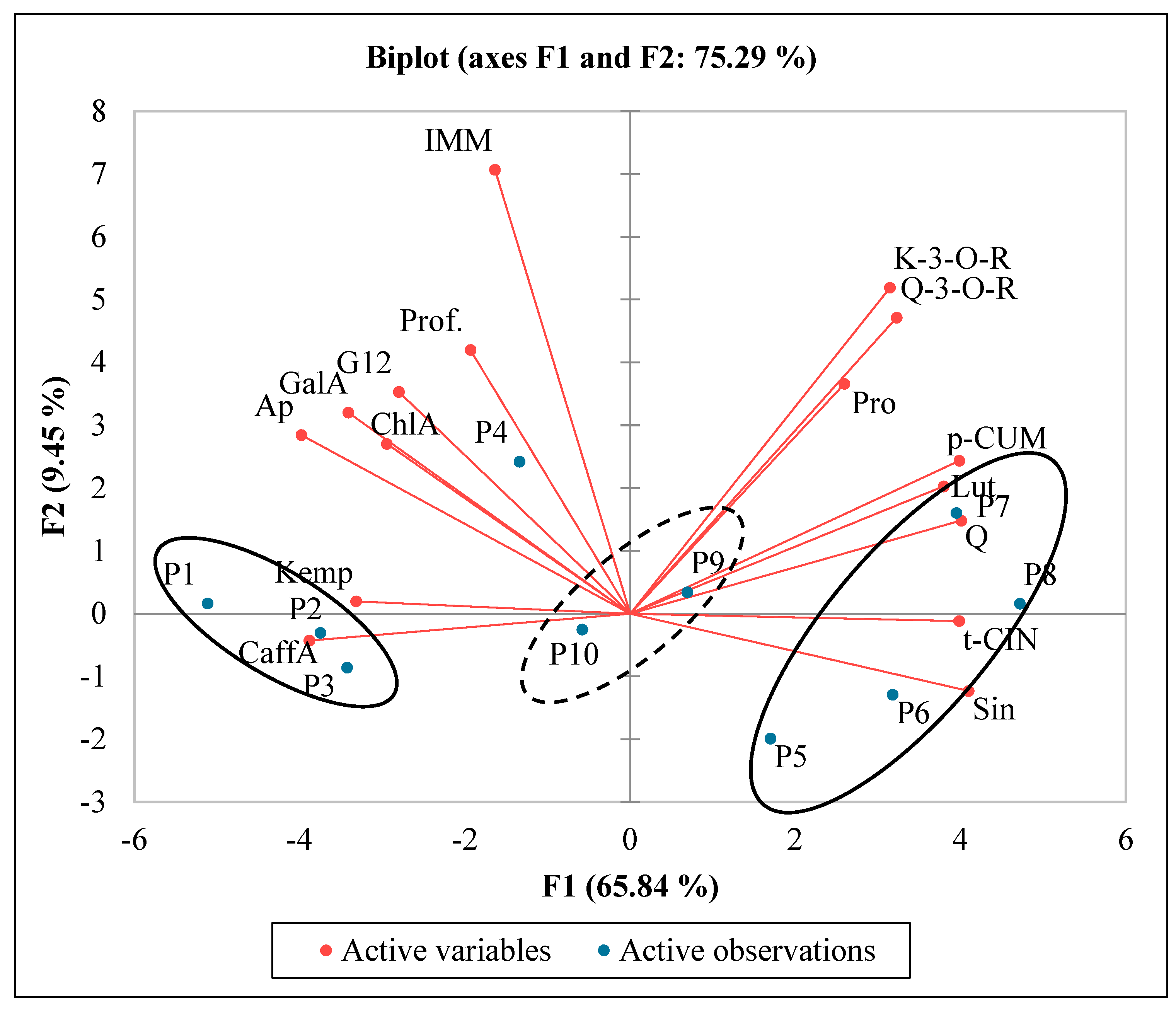
2.4. Analysis of Polyphenols
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Gluten Quantification
4.3. Enzyme-Linked Immunosorbent Assay (ELISA) for QQQPP Peptide Determination and Profilin Determination
- (a)
- QQQPP Peptide Determination
- (b) Profilin Determination
4.4. Analysis of Polyphenols
4.4.1. Sample Preparation
4.4.2. Separation by HPLC
4.4.3. Identification and Calculation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moysiadis, V.; Sarigiannidis, P.; Vitsas, V.; Khelifi, A. Smart Farming in Europe. Comput. Sci. Rev. 2021, 39, 100345. [Google Scholar] [CrossRef]
- Krasilnikov, P.; Taboada, M.A.; Amanullah. Fertilizer Use, Soil Health and Agricultural Sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Xie, E.; Zhao, Y.; Li, H.; Shi, X.; Lu, F.; Zhang, X.; Peng, Y. Spatio-temporal changes of cropland soil pH in a rapidly industrializing region in the Yangtze River Delta of China, 1980–2015. Agric. Ecosyst. Environ. 2019, 272, 95–104. [Google Scholar] [CrossRef]
- Dhankhar, N.; Kumar, J. Impact of increasing pesticides and fertilizers on human health: A review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Shao, L.; Qin, F.; Yang, J.; Gu, H.; Zhai, P.; Pan, X. Effects of organic fertilizers on yield, soil physico-chemical property, soil microbial community diversity and structure of Brassica rapa var. Chinensis. Front. Microbiol. 2023, 14, 1132853. [Google Scholar] [CrossRef]
- Yuan, D.; Hu, Y.; Jia, S.; Li, W.; Zamanian, K.; Han, J.; Huang, F.; Zhao, X. Microbial Properties Depending on Fertilization Regime in Agricultural Soils with Different Texture and Climate Conditions: A Meta-Analysis. Agronomy 2023, 13, 764. [Google Scholar] [CrossRef]
- Frattini, N.; Pulido Carrasquero, A.; Pronsato, L.; Milanesi, L.; Vasconsuelo, A. Effects of common fertilizers on the soil ecosystem. Bull. Natl. Res. Cent. 2023, 47, 78. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Shi, Z.; Yin, Y.; Fan, Y.; Zhang, Z.; Tian, X.; Yang, Y.; Pan, L. Excessive use of chemical fertilizers in catchment areas raises the seasonal pH in natural freshwater lakes of the subtropical monsoon climate region. Ecol. Indic. 2023, 154, 110477. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Riedo, J.; Wächter, D.; Gubler, A.; Wettstein, F.E.; Meuli, R.G.; Bucheli, T.D. Pesticide residues in agricultural soils in light of their on-farm application history. Environ. Pollut. 2023, 331, 121892. [Google Scholar] [CrossRef] [PubMed]
- Bras, A.; Roy, A.; Heckel, D.G.; Anderson, P.; Karlsson Green, K. Pesticide resistance in arthropods: Ecology matters too. Ecol. Lett. 2022, 25, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Thia, J.A.; Maino, J.; Kelly, A.; Hoffmann, A.A.; Umina, P.A. Expanding risk predictions of pesticide resistance evolution in arthropod pests with a proxy for selection pressure. J. Pest Sci. 2023, 96, 1199–1212. [Google Scholar] [CrossRef]
- Scorza, F.A.; Beltramim, L.; Bombardi, L.M. Pesticide exposure and human health: Toxic legacy. Clinics 2023, 78, 100249. [Google Scholar] [CrossRef]
- Penuelas, J.; Coello, F.; Sardans, J. A better use of fertilizers is needed for global food security and environmental sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Wei, B.; Yang, Z. Government promotion, social networks and farmers’ adoption behavior of ecological farming technology. Chin. J. Eco-Agric. 2022, 30, 2036–2049. [Google Scholar] [CrossRef]
- Rega, C.; Thompson, B.; Niedermayr, A.; Desjeux, Y.; Kantelhardt, J.; D’Alberto, R.; Gouta, P.; Konstantidelli, V.; Schaller, L.; Latruffe, L.; et al. Uptake of Ecological Farming Practices by EU Farms: A Pan-European Typology. EuroChoices 2022, 21, 64–71. [Google Scholar] [CrossRef]
- Constantin, M.; Deaconu, M.E.; Petrescu, I.-E.; Istudor, M.; Tărăşilă, G.A. A review on the competitiveness and performance of ecological, organic and regenerative agricultural systems. Proc. Int. Conf. Bus. Excell. 2022, 16, 304–316. [Google Scholar] [CrossRef]
- Knapp, S.; van der Heijden, M.G.A. A global meta-analysis of yield stability in organic and conservation agriculture. Nat. Commun. 2018, 9, 3632. [Google Scholar] [CrossRef]
- Smith, O.M.; Cohen, A.L.; Rieser, C.J.; Davis, A.G.; Taylor, J.M.; Adesanya, A.W.; Jones, M.S.; Meier, A.R.; Reganold, J.P.; Orpet, R.J.; et al. Organic Farming Provides Reliable Environmental Benefits but Increases Variability in Crop Yields: A Global Meta-Analysis. Front. Sustain. Food Syst. 2019, 3, 82. [Google Scholar] [CrossRef]
- Cakmakci, S.; Cakmakci, R. Quality and Nutritional Parameters of Food in Agri-Food Production Systems. Foods 2023, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Rej, A.; Aziz, I.; Sanders, D.S. Coeliac disease and noncoeliac wheat or gluten sensitivity. J. Intern. Med. 2020, 288, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Spector Cohen, I.; Day, A.; Shaoul, R. Should the Glu Be Ten or Twenty? An Update on the Ongoing Debate on Gluten Safety Limits for Patients with Celiac Disease. Gastrointest. Disord. 2020, 2, 202–211. [Google Scholar] [CrossRef]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef]
- García-Ramírez, B.; Mares-Mejía, I.; Rodríguez-Hernández, A.; Cano-Sánchez, P.; Torres-Larios, A.; Ortega, E.; Rodríguez-Romero, A. A native IgE in complex with profilin provides insights into allergen recognition and cross-reactivity. Commun. Biol. 2022, 5, 748. [Google Scholar] [CrossRef]
- Alvarado, M.I.; Jimeno, L.; De La Torre, F.; Boissy, P.; Rivas, B.; Lázaro, M.J.; Barber, D. Profilin as a severe food allergen in allergic patients overexposed to grass pollen. Allergy 2014, 69, 1610–1616. [Google Scholar] [CrossRef]
- Yigezu, Y.A.; Mugera, A.; El-Shater, T.; Aw-Hassan, A.; Piggin, C.; Haddad, A.; Khalil, Y.; Loss, S. Enhancing adoption of agricultural technologies requiring high initial investment among smallholders. Technol. Forecast. Soc. Change 2018, 134, 199–206. [Google Scholar] [CrossRef]
- Colivicchi, I. From Industrial Agriculture Towards Ecological Farming-Necessity or Opportunity; Economics of Sustainability; Scientific Publications; University of Economics in Katowice: Katowice, Poland, 2022; pp. 399–420. [Google Scholar]
- Popa, M.E.; Mitelut, A.C.; Popa, E.E.; Stan, A.; Popa, V.I. Organic foods contribution to nutritional quality and value. Trends Food Sci. Technol. 2019, 84, 15–18. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 704–714. [Google Scholar] [CrossRef]
- Bhagavathula, A.S.; Vidyasagar, K.; Khubchandani, J. Organic Food Consumption and Risk of Obesity: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Gosling, C.J.; Goncalves, A.; Ehrminger, M.; Valliant, R. Association of organic food consumption with obesity in a nationally representative sample. Br. J. Nutr. 2021, 125, 703–711. [Google Scholar] [CrossRef]
- Rahman, A.; Baharlouei, P.; Koh, E.H.Y.; Pirvu, D.G.; Rehmani, R.; Arcos, M.; Puri, S. A Comprehensive Analysis of Organic Food: Evaluating Nutritional Value and Impact on Human Health. Foods 2024, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef]
- Krejčířová, L.; Capouchová, I.; Petr, J.; Bicanová, E.; Faměra, O. The effect of organic and conventional growing systems on quality and storage protein composition of winter wheat. Plant Soil Environ. 2007, 53, 499–505. [Google Scholar] [CrossRef]
- Rozbicki, J.; Ceglińska, A.; Gozdowski, D.; Jakubczak, M.; Cacak-Pietrzak, G.; Mądry, W.; Golba, J.; Piechociński, M.; Sobczyński, G.; Studnicki, M.; et al. Influence of the cultivar, environment and management on the grain yield and bread-making quality in winter wheat. J. Cereal Sci. 2015, 61, 126–132. [Google Scholar] [CrossRef]
- Sobolewska, M.; Stankowski, S. The influence of farming systems on the technological quality of grain and flour cultivars of winter wheat. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2017, 332, 73–82. [Google Scholar] [CrossRef]
- Zhou, E.; Xue, X.; Xu, H.; Zhao, L.; Wu, L.; Li, Q. Effects of covalent conjugation with quercetin and its glycosides on the structure and allergenicity of Bra c p from bee pollen. Food Chem. 2023, 406, 135075. [Google Scholar] [CrossRef]
- Davey, R.J.; Moens, P.D. Profilin: Many facets of a small protein. Biophys. Rev. 2020, 12, 827–849. [Google Scholar] [CrossRef]
- Suanno, C.; Aloisi, I.; Parrotta, L.; Fernández-González, D.; Del Duca, S. Allergenic risk assessment of urban parks: Towards a standard index. Environ. Res. 2021, 200, 111436. [Google Scholar] [CrossRef]
- Jeon, Y.H. Pollen-food allergy syndrome in children. Clin. Exp. Pediatr. 2020, 63, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Akinfenwa, O.; Huang, H.J.; Linhart, B.; Focke-Tejkl, M.; Vrtala, S.; Poroshina, A.; Nikonova, A.; Khaitov, M.; Campion, N.J.; Eckl-Dorna, J.; et al. Preventive Administration of Non-Allergenic Bet v 1 Peptides Reduces Allergic Sensitization to Major Birch Pollen Allergen, Bet v 1. Front. Immunol. 2021, 12, 744544. [Google Scholar] [CrossRef]
- Olivieri, M.; Spiteri, G.; Brandi, J.; Cecconi, D.; Fusi, M.; Zanoni, G.; Rizzi, C. Glucose/Ribitol Dehydrogenase and 16.9 kDa Class I Heat Shock Protein 1 as Novel Wheat Allergens in Baker’s Respiratory Allergy. Molecules 2022, 27, 1212. [Google Scholar] [CrossRef]
- Costantino, A.; Aversano, G.M.; Lasagni, G.; Smania, V.; Doneda, L.; Vecchi, M.; Roncoroni, L.; Pastorello, E.A.; Elli, L. Diagnostic management of patients reporting symptoms after wheat ingestion. Front. Nutr. 2022, 9, 1007007. [Google Scholar] [CrossRef]
- Zhang, Y.; Che, H.; Li, C.; Jin, T. Food Allergens of Plant Origin. Foods 2023, 12, 2232. [Google Scholar] [CrossRef] [PubMed]
- Słowianek, M.; Skorupa, M.; Hallmann, E.; Rembiałkowska, E.; Leszczyńska, J. Allergenic Potential of Tomatoes Cultivated in Organic and Conventional Systems. Plant Foods Hum. Nutr. 2016, 71, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, E.; Rozpara, E.; Slowianek, M.; Leszczynska, J. The effect of organic and conventional farm management on the allergenic potency and bioactive compounds status of apricots (Prunus armeniaca L.). Food Chem. 2019, 279, 171–178. [Google Scholar] [CrossRef]
- Aninowski, M.; Leszczyńska, J. The determination of potentially allergenicity of selected herbs. Biotechnol. Food Sci. 2019, 83, 3–11. [Google Scholar]
- Hallmann, E.; Ponder, A.; Aninowski, M.; Narangerel, T.; Leszczyńska, J. The Interaction between Antioxidants Content and Allergenic Potency of Different Raspberry Cultivars. Antioxidants 2020, 9, 256. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological Potential of Kaempferol, a Flavonoid in the Management of Pathogenesis via Modulation of Inflammation and Other Biological Activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef]
- Tehami, W.; Nani, A.; Khan, N.A.; Hichami, A. New Insights Into the Anticancer Effects of p-Coumaric Acid: Focus on Colorectal Cancer. Dose Response 2023, 21, 15593258221150704. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Zhang, D.; Xiao, C.L.; Zhou, Y.; Li, X.; Wang, L.; He, Z.; Reilly, J.; Xiao, Z.Y.; Shu, X. P-Coumaric Acid Reverses Depression-Like Behavior and Memory Deficit Via Inhibiting AGE-RAGE-Mediated Neuroinflammation. Cells 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, H.; Tan, Y.; Yu, X.-D.; Xiao, C.; Li, Y.; Reilly, J.; He, Z.; Shu, X. Protection of p-Coumaric acid against chronic stress-induced neurobehavioral deficits in mice via activating the PKA-CREB-BDNF pathway. Physiol. Behav. 2024, 273, 114415. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Dai, C.; Fang, Y.; Wu, J. Development of poly(p-coumaric acid) as a self-anticancer nanocarrier for efficient and biosafe cancer therapy. Biomater. Sci. 2022, 10, 2263–2274. [Google Scholar] [CrossRef]
- Hadidi, M.; Linan-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Holghoomi, R.; Kiani, M.H.; Rahdar, A.; Hashemi, S.M.; Romanholo Ferreira, L.F.; Fathi-karkan, S. Nanoparticle-delivered gallic acid: A new frontier in cancer therapy. J. Drug Deliv. Sci. Technol. 2024, 101, 106129. [Google Scholar] [CrossRef]
- Behera, P.K.; Devi, S.; Mittal, N. Therapeutic potential of gallic acid in obesity: Considerable shift! Obes. Med. 2023, 37, 100473. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef]
- Kowalska, I.; Soluch, A.; Mołdoch, J.; Jończyk, K. The Effect of Farming Systems and Cultivars on the Qualitative and Quantitative Composition of Bioactive Compounds in Winter Wheat (Triticum aestivum L.). Molecules 2025, 30, 902. [Google Scholar] [CrossRef]
- Bartos, A.; Majak, I.; Leszczynska, J. Detection of Bet v 1 Homologous Proteins and Plant Profilins by Indirect ELISA. Methods Mol. Biol. 2024, 2717, 305–310. [Google Scholar] [CrossRef] [PubMed]
| Polyphenol | Cultivation System | p-Value | |
|---|---|---|---|
| Organic Wheat | Conventional Wheat | ||
| gallic | 21.21 ± 2.46 a | 20.58 ± 2.42 b | <0.0001 |
| chlorogenic | 2.05 ± 0.2 a | 1.90 ± 0.23 b | <0.0001 |
| caffeic | 3.82 ± 0.61 a | 3.61 ± 0.61 b | <0.0001 |
| p-coumaric | 29.45 ± 4.09 a | 27.22 ± 3.89 b | <0.0001 |
| sinapic | 2.46 ± 0.64 a | 2.19 ± 0.65 b | <0.0001 |
| t-cinaminic | 2.62 ± 0.35 a | 2.55 ± 0.40 b | 0.0048 |
| quercetin-3-O-rutinoside | 3.23 ± 0.45 a | 2.95 ± 0.45 b | <0.0001 |
| kaempferol-3-O-glucoside | 3.01 ± 0.58 a | 2.70 ± 0.56 b | <0.0001 |
| luteolin | 0.63 ± 0.09 a | 0.56 ± 0.08 b | <0.0001 |
| quercetin | 1.15 ± 0.17 a | 1.04 ± 0.18 b | 0.0003 |
| apigenin | 0.46 ± 0.10 b | 0.47 ± 0.08 a | <0.0001 |
| kaempferol | 36.96 ± 2.67 b | 38.16 ± 3.14 a | <0.0001 |
| Polyphenol | Organic Winter Wheat | Conventional Winter Wheat | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Arkadia | Bamberka | Jantarka | Sailor | Arkadia | Bamberka | Jantarka | Sailor | ||
| gallic | 25.68 ± 0.17 a | 21.93 ± 0.24 c | 23.44 ± 0.17 ab | 22.17 ± 0.20 b | 25.00 ± 0.19 a | 21.67 ± 0.20 c | 22.90 ± 0.02 b | 20.81 ± 0.13 c | 0.003 |
| chlorogenic | 2.01 ± 0.04 b | 2.16 ± 0.03 b | 2.46 ± 0.04 a | 2.21 ± 0.05 b | 1.92 ± 0.01 c | 1.88 ± 0.01 c | 2.45 ± 0.03 a | 1.96 ± 0.04 c | 0.0001 |
| caffeic | 4.72 ± 0.03 a | 4.63 ± 0.05 a | 4.25 ± 0.01 a | 3.76 ± 0.03 a | 4.57 ± 0.01 a | 4.38 ± 0.04 a | 4.05 ± 0.12 a | 3.58 ± 0.02 a | n.s. |
| p-coumaric | 23.34 ± 0.29 b | 24.34 ± 0.026 b | 26.41 ± 0.14 ab | 32.02 ± 1.89 a | 21.53 ± 0.55 c | 21.58 ± 0.96 c | 25.80 ± 0.54 b | 26.92 ± 0.54 ab | 0.011 |
| sinapic | 1.57 ± 0.03 a | 1.67 ± 0.04 a | 2.17 ± 0.04 a | 2.02 ± 0.01 a | 1.35 ± 0.01 a | 1.40 ± 0.01 a | 1.87 ± 0.02 a | 1.69 ± 0.02 a | n.s. |
| t-cinaminic | 2.05 ± 0.02 c | 2.15 ± 0.03 c | 2.60 ± 0.01 a | 2.50 ± 0.02 b | 1.85 ± 0.03 c | 2.11 ± 0.02 c | 2.95 ± 0.01 a | 2.26 ± 0.06 b | <0.0001 |
| quercetin-3-O-rutinoside | 2.81 ± 0.04 ab | 2.97 ± 0.01 a | 2.78 ± 0.04 b | 3.22 ± 0.04 a | 2.40 ± 0.04 c | 2.79 ± 0.04 b | 2.66 ± 0.06 c | 3.04 ± 0.01 a | 0.0001 |
| kaempferol-3-O-glucoside | 2.27 ± 0.08 b | 2.33 ± 0.04 b | 2.59 ± 0.03 b | 3.83 ± 0.11 a | 1.84 ± 0.05 c | 2.12 ± 0.02 c | 2.22 ± 0.03 b | 3.33 ± 0.03 a | 0.015 |
| luteolin | 0.55 ± 0.01 a | 0.54 ± 0.02 a | 0.56 ± 0.01 a | 0.58 ± 0.01 a | 0.48 ± 0.01 b | 0.46 ± 0.01 b | 0.49 ± 0.01 b | 0.55 ± 0.01 a | 0.048 |
| quercetin | 0.91 ± 0.02 a | 0.98 ± 0.02 a | 1.04 ± 0.01 a | 1.08 ± 0.01 a | 0.78 ± 0.01 b | 0.87 ± 0.02 b | 0.97 ± 0.01 a | 1.03 ± 0.02 a | 0.034 |
| apigenin | 0.62 ± 0.005 a | 0.57 ± 0.012 ab | 0.50 ± 0.003 b | 0.52 ± 0.004 b | 0.59 ± 0.004 a | 0.55 ± 0.001 b | 0.52 ± 0.006 b | 0.55 ± 0.003 b | <0.0001 |
| kaempferol | 38.01 ± 0.09 b | 39.89 ± 0.4 b | 41.05 ± 0.11 a | 38.40 ± 0.88 b | 38.15 ± 0.44 b | 43.35 ± 0.26 a | 42.79 ± 0.23 a | 38.35 ± 0.45 b | 0.0001 |
| Polyphenol | Organic Winter Wheat | Conventional Winter Wheat | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ostka Smolicka | Waluta | Kandela | Izera | Ostka Smolicka | Waluta | Kandela | Izera | ||
| gallic | 18.42 ± 0.20 a | 18.00 ± 0.21 a | 19.30 ± 0.15 a | 20.78 ± 0.16 a | 17.87 ± 0.16 a | 17.30 ± 0.28 a | 19.25 ± 0.17 a | 19.82 ± 0.33 a | n.s. |
| chlorogenic | 1.93 ± 0.03 a | 1.86 ± 0.03 b | 1.82 ± 0.02 b | 1.94 ± 0.05 a | 1.66 ± 0.05 c | 1.79 ± 0.01 b | 1.70 ± 0.06 c | 1.79 ± 0.07 b | 0.013 |
| caffeic | 3.19 ± 0.03 b | 3.67 ± 0.07 a | 3.19 ± 0.01 b | 3.10 ± 0.03 b | 3.03 ± 0.08 b | 3.36 ± 0.02 b | 3.05 ± 0.01 b | 2.85 ± 0.01 c | 0.047 |
| p-coumaric | 29.37 ± 0.32 c | 32.14 ± 0.19 a | 33.04 ± 0.12 a | 34.95 ± 0.36 a | 27.84 ± 0.15 c | 31.10 ± 0.36 b | 31.40 ± 0.21 b | 31.61 ± 0.59 b | 0.0007 |
| sinapic | 2.88 ± 0.04 b | 2.98 ± 0.04 b | 3.17 ± 0.03 a | 3.24 ± 0.03 a | 2.71 ± 0.02 c | 2.59 ± 0.01 c | 2.80 ± 0.02 b | 3.11 ± 0.02 a | <0.0001 |
| t-cinaminic | 2.78 ± 0.08 c | 2.80 ± 0.01 b | 3.13 ± 0.05 a | 2.96 ± 0.01 a | 2.81 ± 0.02 b | 2.63 ± 0.03 c | 2.96 ± 0.01 a | 2.82 ± 0.02 b | 0.003 |
| quercetin-3-O-rutinoside | 2.76 ± 0.01 b | 3.58 ± 0.06 a | 3.73 ± 0.05 a | 3.96 ± 0.02 a | 2.38 ± 0.03 b | 3.15 ± 0.04 a | 3.54 ± 0.12 a | 3.65 ± 0.03 a | 0.035 |
| kaempferol-3-O-glucoside | 2.86 ± 0.05 b | 3.01 ± 0.01 b | 3.35 ± 0.05 ab | 3.85 ± 0.01 a | 2.68 ± 0.04 c | 2.87 ± 0.05 b | 2.96 ± 0.04 c | 3.55 ± 0.10 a | 0.011 |
| luteolin | 0.62 ± 0.02 a | 0.66 ± 0.01 a | 0.73 ± 0.06 a | 0.81 ± 0.02 a | 0.52 ± 0.02 a | 0.62 ± 0.01 a | 0.63 ± 0.01 a | 0.71 ± 0.02 a | n.s. |
| quercetin | 1.17 ± 0.02 b | 1.24 ± 0.02 b | 1.36 ± 0.02 a | 1.42 ± 0.02 a | 1.03 ± 0.02 c | 1.12 ± 0.02 c | 1.17 ± 0.01 c | 1.38 ± 0.03 a | 0.001 |
| apigenin | 0.37 ± 0.01 c | 0.36 ± 0.01 c | 0.39 ± 0.01 b | 0.38 ± 0.01 b | 0.41 ± 0.01 a | 0.38 ± 0.01 b | 0.40 ± 0.01 a | 0.39 ± 0.01 ab | 0.027 |
| kaempferol | 34.86 ± 0.23 a | 33.30 ± 0.26 a | 34.01 ± 0.30 a | 36.14 ± 0.22 a | 36.12 ± 0.25 a | 34.27 ± 0.37 a | 35.20 ± 0.17 a | 37.02 ± 0.71 a | n.s. |
| Crop Production System | ||
|---|---|---|
| Specification | Organic | Conventional |
| Crop rotation |
|
|
| Organic fertilization | compost (30 t∙ha−1) to potato; catch crop | rape straw winter wheat straw |
| Mineral fertilization NPK (kg∙ha−1) | According to the results of soil analysis and crop needs, natural P + K fertilizers (42 + 60) in 2013 | Spring wheat: N (110) + P (35) + K (70) Winter wheat: N (140) + P (60) + K (80) |
| Herbicides | - | 2 treatments (see Table 2) |
| Fungicides | - | 2 treatments (see Table 2) |
| Insecticides | - | 2 treatments (see Table 2) |
| Crop Production System | Plant Protection Products | ||
|---|---|---|---|
| Herbicides | Fungicides | Insecticides | |
| Organic | - | - | - |
| Conventional | Mustang Forte 195SE 0.8 L ha−1 | Tilt Turbo 575EC 1.0 L ha−1 | Decis Mega 0.125 L ha−1 |
| Axial 100EC 0.4 L ha−1 + Agritox Turbo 750SL 1.0 L ha−1 | Menara 410EC 0.4 L ha−1 + Amistar 250 SC 0.6 L ha−1 | Furry 100EW 0.1 L ha−1 | |
| Mustang Forte 195 SE: aminopyralid—10 g·L−1, florasulam—5 g·L−1, 2,4D—180 g·L−1; Axial 100EC: pinoxaden—100 g·L−1; Agritox Turbo 750SL: MCPA—660 g·L−1, dicamb—90 g·L−1 | Tilt Turbo 575EC: propiconazole—125 g·L−1, fenpropidine—450 g·L−1; Menara 410EC: propiconazole 250 g·L−1, Amistar 250SC: azoxystrobin 250 g·L−1 | Decis Mega: deltamethrin—50 g·L−1; Furry 100EW: zeta-cypermethrin —100 g·L−1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartos, A.; Malik, A.; Feledyn-Szewczyk, B.; Jończyk, K.; Kazimierczak, R.; Hallmann, E.; Leszczyńska, J. Polyphenolic and Immunometric Profiling of Wheat Varieties: Impact of Organic and Conventional Farming on Allergenic and Bioactive Compounds. Molecules 2025, 30, 1313. https://doi.org/10.3390/molecules30061313
Bartos A, Malik A, Feledyn-Szewczyk B, Jończyk K, Kazimierczak R, Hallmann E, Leszczyńska J. Polyphenolic and Immunometric Profiling of Wheat Varieties: Impact of Organic and Conventional Farming on Allergenic and Bioactive Compounds. Molecules. 2025; 30(6):1313. https://doi.org/10.3390/molecules30061313
Chicago/Turabian StyleBartos, Adrian, Alicja Malik, Beata Feledyn-Szewczyk, Krzysztof Jończyk, Renata Kazimierczak, Ewelina Hallmann, and Joanna Leszczyńska. 2025. "Polyphenolic and Immunometric Profiling of Wheat Varieties: Impact of Organic and Conventional Farming on Allergenic and Bioactive Compounds" Molecules 30, no. 6: 1313. https://doi.org/10.3390/molecules30061313
APA StyleBartos, A., Malik, A., Feledyn-Szewczyk, B., Jończyk, K., Kazimierczak, R., Hallmann, E., & Leszczyńska, J. (2025). Polyphenolic and Immunometric Profiling of Wheat Varieties: Impact of Organic and Conventional Farming on Allergenic and Bioactive Compounds. Molecules, 30(6), 1313. https://doi.org/10.3390/molecules30061313










