2-Bromopyridines as Versatile Synthons for Heteroarylated 2-Pyridones via Ru(II)-Mediated Domino C–O/C–N/C–C Bond Formation Reactions
Abstract
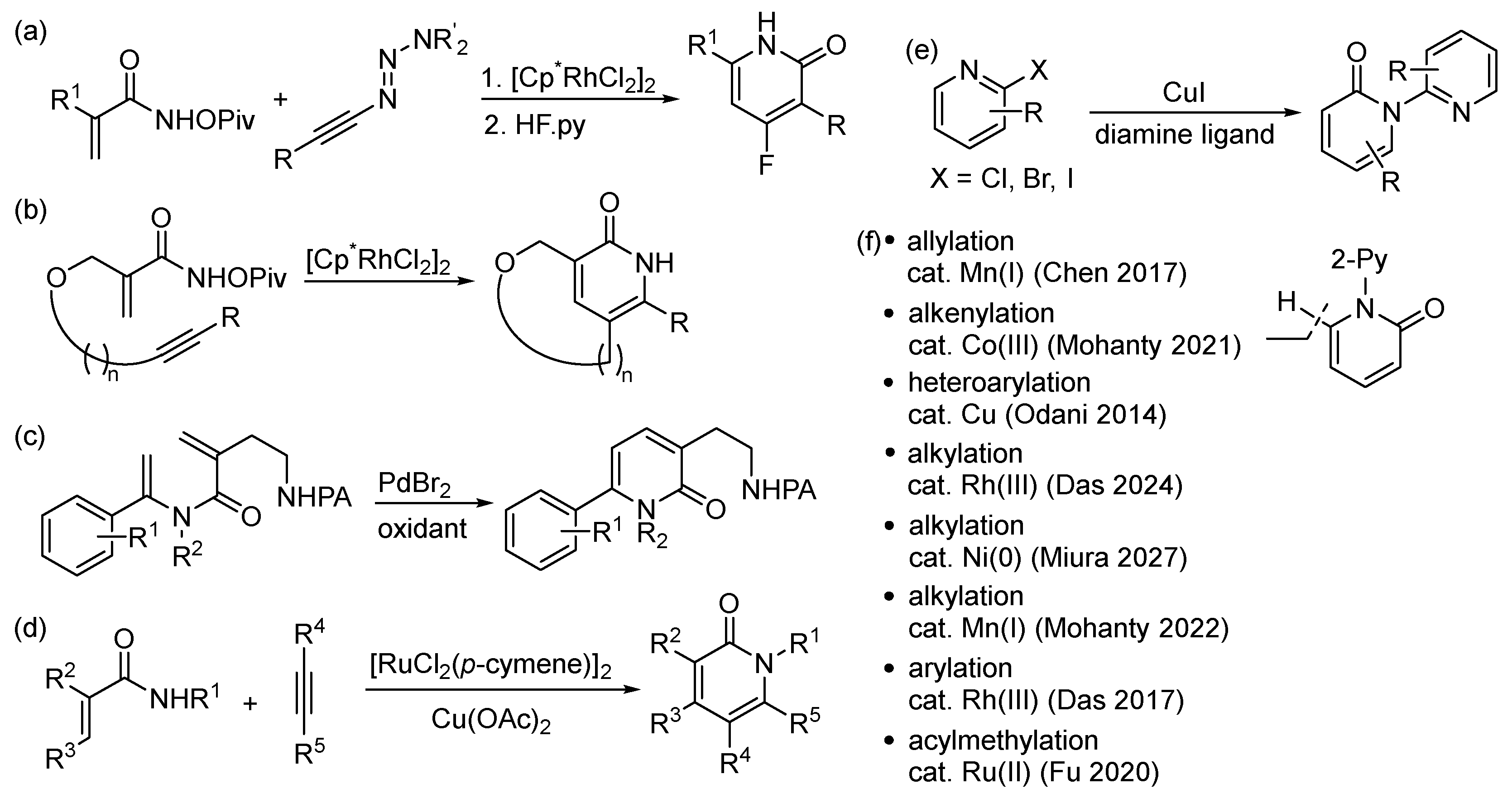
1. Introduction
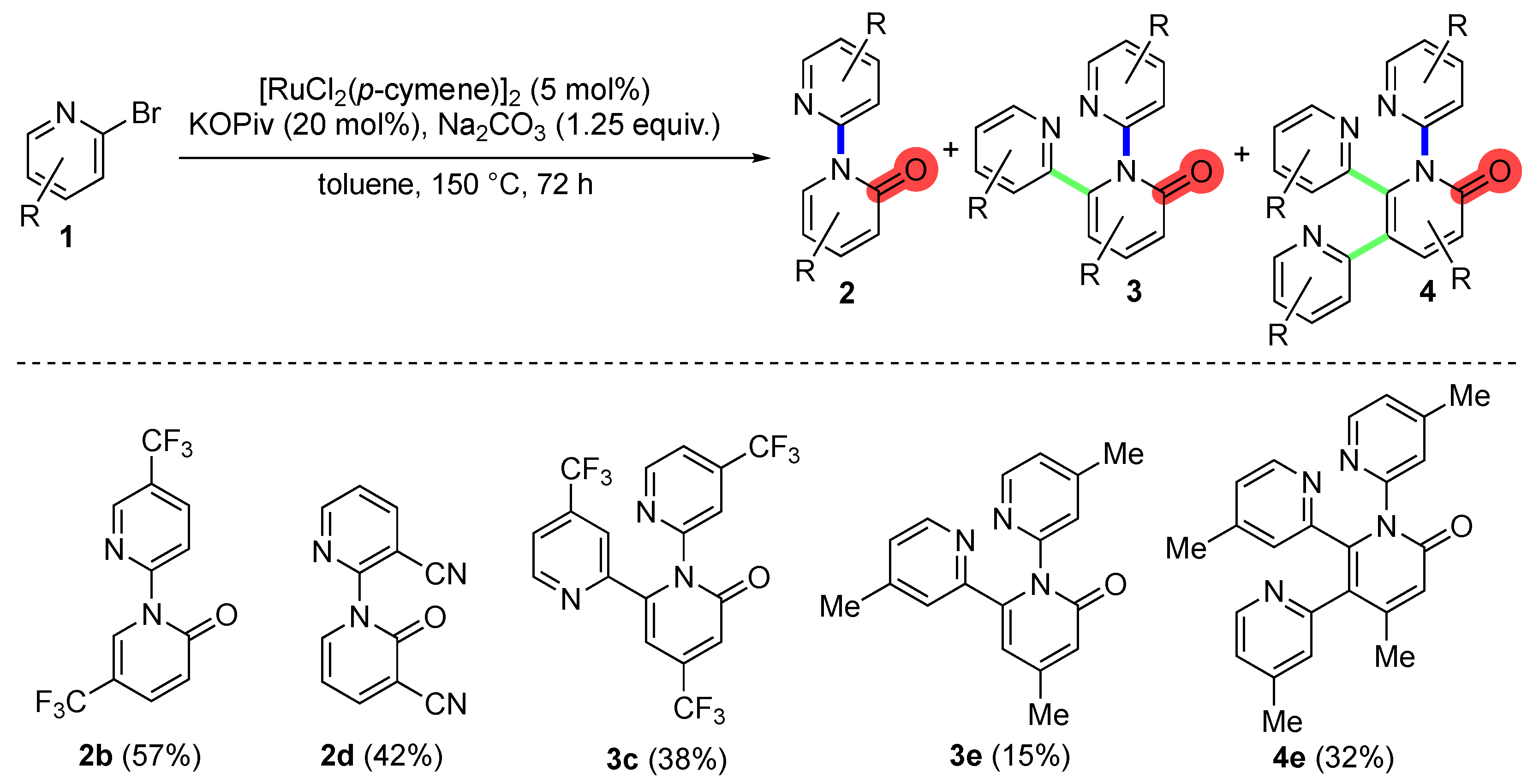
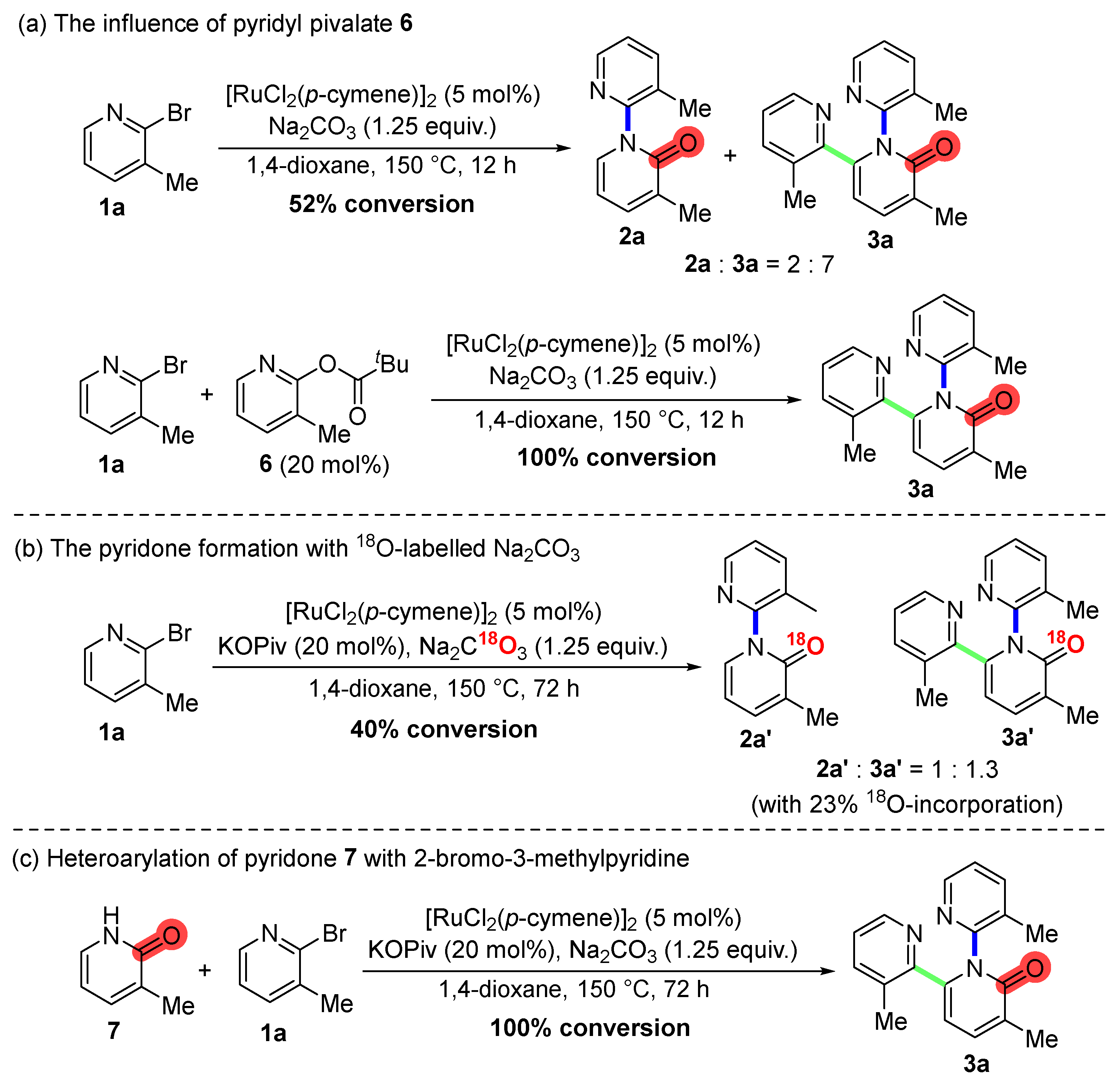
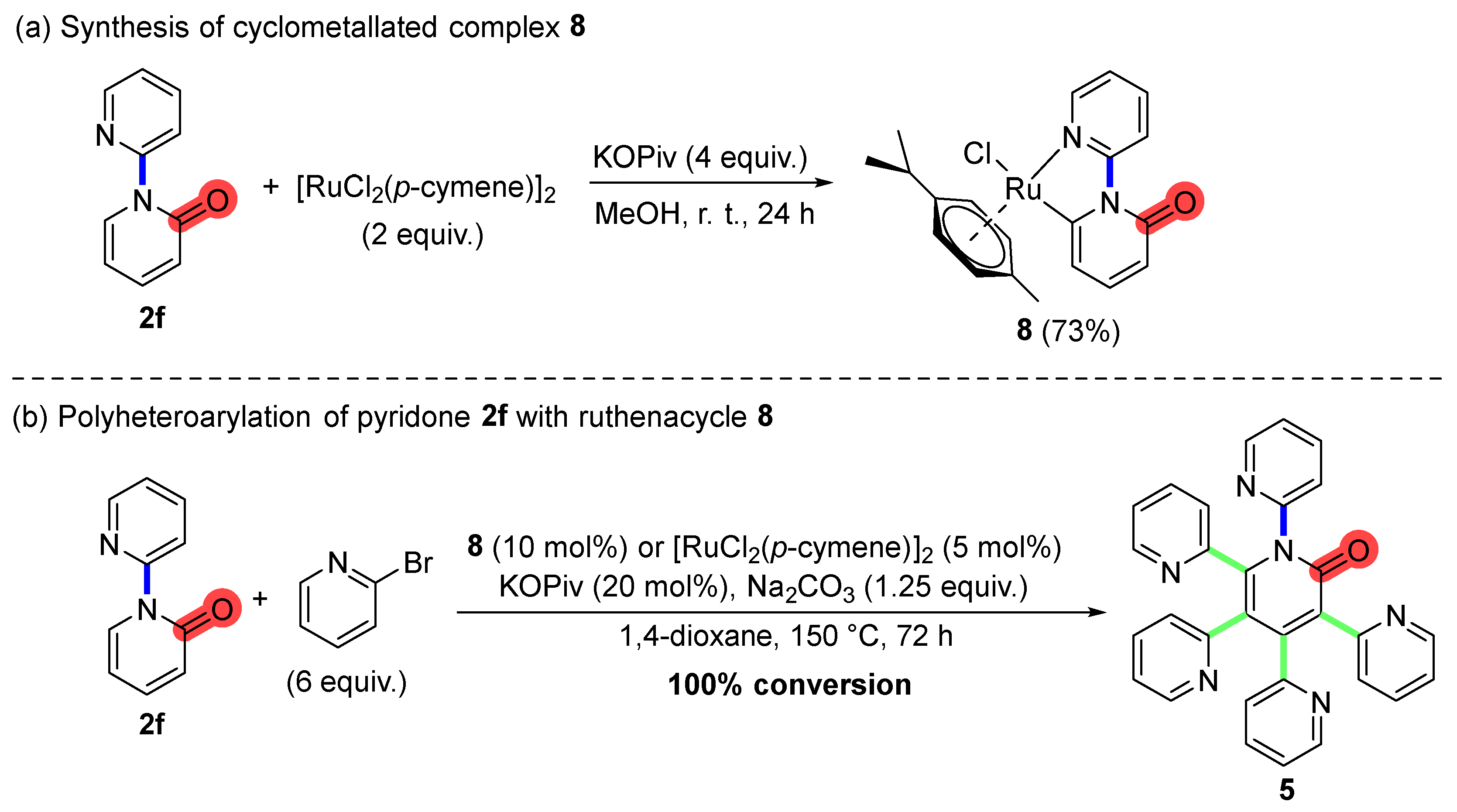
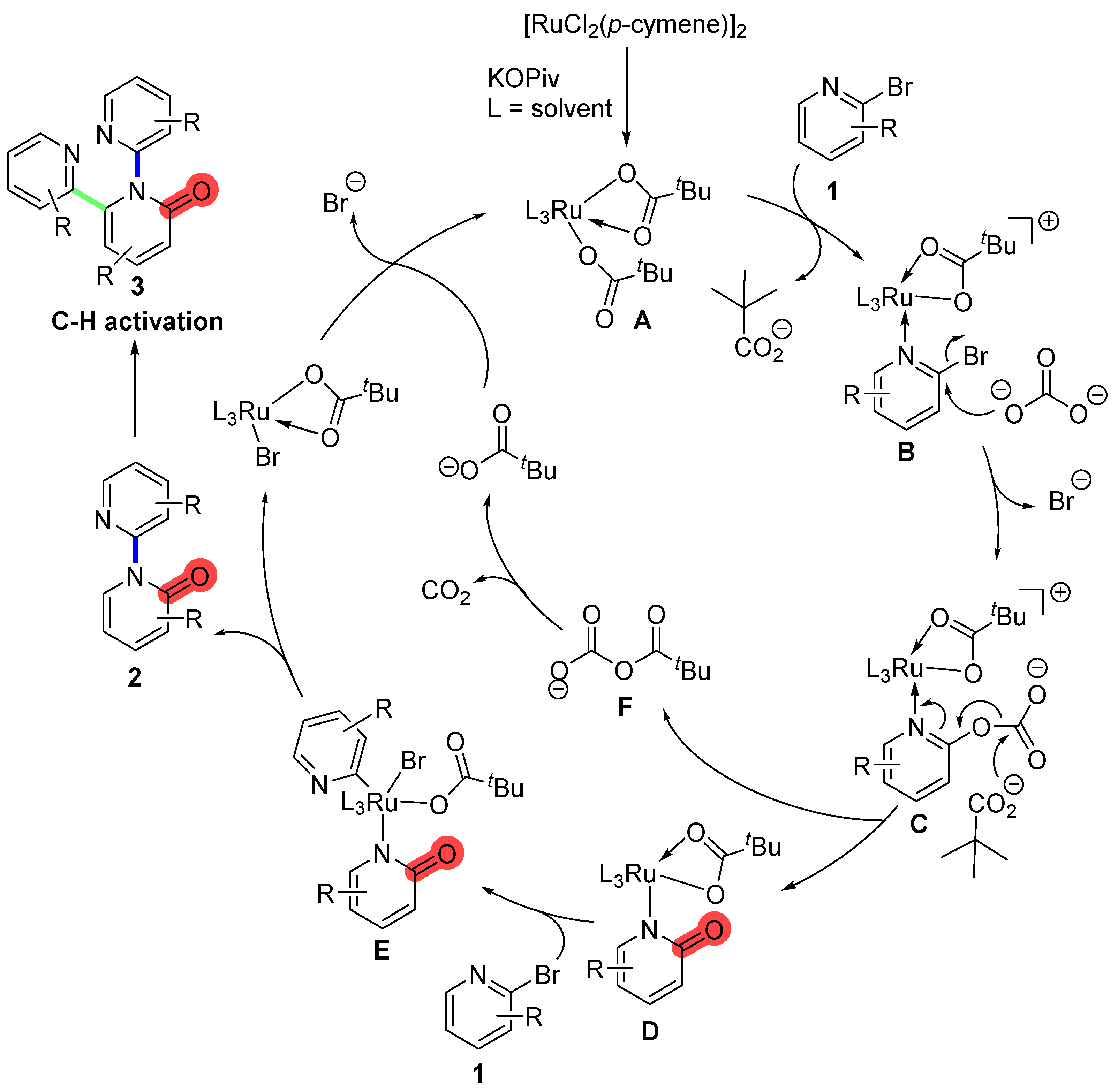
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Synthesis of Pyridones 2–5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geuns-Meyer, S.; Cee, V.C.; Deak, H.L.; Du, B.; Hodous, B.L.; Nguyen, H.N.; Olivieri, P.R.; Schenkel, L.B.; Vaida, K.R.; Andrews, P.; et al. Discovery of N-(4-(3-(2-Aminopyrimidin-4-yl)pyridin-2-yloxy)phenyl)-4-(4-methylthiophen-2-yl)phthalazin-1-amine (AMG 900), A Highly Selective, Orally Bioavailable Inhibitor of Aurora Kinases with Activity against Multidrug-Resistant Cancer Cell Lines. J. Med. Chem. 2015, 58, 5189–5207. [Google Scholar] [CrossRef] [PubMed]
- Linardopoulos, S.; Blagg, J. Aurora Kinase Inhibition: A New Light in the Sky? J. Med. Chem. 2015, 58, 5186–5188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.-C.; Li, M.; Wu, Q.; Liu, C.-L.; Chang, X.-H. Design, synthesis and insecticidal evaluation of aryloxy dihalopropene derivatives. Bioorg. Med. Chem. 2016, 24, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Veale, C.G.L. Unpacking the Pathogen Box—An Open Source Tool for Fighting Neglected Tropical Disease. ChemMedChem 2019, 14, 386–453. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Fan, Y.; Guo, H.; Ma, T.; Wen, J.; Liu, D.; Zhao, L. Synthesis and biological evaluation of quinoline derivatives as potential anti-prostate cancer agents and Pim-1 kinase inhibitors. Bioorg. Med. Chem. 2016, 24, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Hodges, A.S.; Lee, S.C.Y.; Hardcastle, K.I.; Saadein, M.R.; Eichler, J.F. A report describing the effect of di-2-pyridyl ligand architecture on the coordination properties with gold(III). Inorg. Chim. Acta 2011, 368, 252–256. [Google Scholar] [CrossRef]
- Njogu, E.M.; Omondi, B.; Nyamori, V.O. Multimetallic silver(I)–pyridinyl complexes: Coordination of silver(I) and luminescence. J. Coord. Chem. 2015, 68, 3389–3431. [Google Scholar] [CrossRef]
- Glasson, C.R.K.; Lindoy, L.F.; Meehan, G.V. Recent developments in the d-block metallo-supramolecular chemistry of polypyridyls. Coord. Chem. Rev. 2008, 252, 940–963. [Google Scholar] [CrossRef]
- Drev, M.; Grošelj, U.; Kočar, D.; Perdih, F.; Svete, J.; Štefane, B.; Požgan, F. Self-assembly of multinuclear sandwich silver(I) complexes by cooperation of Hexakis (azaheteroaryl) benzene ligands, argentophilic interactions, and fluoride inclusion. Inorg. Chem. 2020, 59, 3993–4001. [Google Scholar] [CrossRef]
- Wang, C.-S.; Dixneuf, P.H.; Soulé, J.-F. Photoredox Catalysis for Building C–C Bonds from C(sp2)–H Bonds. Chem. Rev. 2018, 118, 7532–7585. [Google Scholar] [CrossRef]
- Torres, M.; Gil, S.; Parra, M. New Synthetic Methods to 2-Pyridone Rings. Curr. Org. Chem. 2005, 9, 1757–1779. [Google Scholar] [CrossRef]
- Lagoja, I.M. Pyrimidine as Constituent of Natural Biologically Active Compounds. Chem. Biodivers. 2005, 2, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Jessen, H.J.; Gademann, K. 4-Hydroxy-2-pyridone alkaloids: Structures and synthetic approaches. Nat. Prod. Rep. 2010, 27, 1168–1185. [Google Scholar] [CrossRef] [PubMed]
- Hamama, W.S.; Waly, M.; El-Hawary, I.; Zoorob, H.H. Developments in the Chemistry of 2-Pyridone. Synth. Commun. 2014, 44, 1730–1759. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Singh, S.; Wu, Y.; Wang, Z.; Hao, W.; Verma, P.; Qiao, J.X.; Sunoj, R.B.; Yu, J.-Q. Pd-Catalyzed γ-C(sp3)–H Fluorination of Free Amines. J. Am. Chem. Soc. 2020, 142, 9966–9974. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Wang, Z.; Wu, Y.; Wisniewski, S.R.; Qiao, J.X.; Ewing, W.R.; Eastgate, M.D.; Yu, J.-Q. Overcoming the Limitations of γ- and δ-C–H Arylation of Amines through Ligand Development. J. Am. Chem. Soc. 2018, 140, 17884–17894. [Google Scholar] [CrossRef]
- Xia, G.; Zhuang, Z.; Liu, L.-Y.; Schreiber, S.L.; Melillo, B.; Yu, J.-Q. Ligand-Enabled β-Methylene C(sp3)−H Arylation of Masked Aliphatic Alcohols. Angew. Chem. Int. Ed. 2020, 59, 7783–7787. [Google Scholar] [CrossRef]
- Pan, S.; Sarkar, S.; Ghosh, B.; Samanta, R. Transition metal catalysed direct construction of 2-pyridone scaffolds through C–H bond functionalizations. Org. Biomol. Chem. 2021, 19, 10516–10529. [Google Scholar] [CrossRef]
- Biswas, A.; Maity, S.; Pan, S.; Samanta, R. Transition Metal-Catalysed Direct C−H Bond Functionalizations of 2-Pyridone Beyond C3-Selectivity. Chem. Asian J. 2020, 14, 2092–2109. [Google Scholar] [CrossRef]
- Tan, J.-F.; Bormann, C.T.; Severin, K.; Cramer, N. Alkynyl Triazenes as Fluoroalkyne Surrogates: Regioselective Access to 4-Fluoro-2-pyridones by a Rh(III)-Catalyzed C–H Activation–Lossen Rearrangement–Wallach Reaction. ACS Catal. 2020, 10, 3790–3796. [Google Scholar] [CrossRef]
- Krieger, J.-P.; Lesuisse, D.; Ricci, G.; Perrin, M.-A.; Meyer, C.; Cossy, J. Rhodium(III)-Catalyzed C–H Activation/Heterocyclization as a Macrocyclization Strategy. Synthesis of Macrocyclic Pyridones. Org. Lett. 2017, 19, 2706–2709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Nian, Y.; Zhou, Y.; Jiang, H.; Liu, H. Palladium-catalyzed picolinamide-directed coupling of C(sp2)–H and C(sp2)–H: A straightforward approach to quinolinone and pyridone scaffolds. Chem. Commun. 2015, 51, 7509–7511. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Lygin, A.V.; Hofmann, N. Ruthenium-Catalyzed Oxidative Synthesis of 2-Pyridones through C–H/N–H Bond Functionalizations. Org. Lett. 2011, 13, 3278–3281. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Miura, M. A lesson for site-selective C–H functionalization on 2-pyridones: Radical, organometallic, directing group and steric controls. Chem. Sci. 2018, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.M.; McGlacken, G.P. Transition Metal Mediated C–H Activation of 2-Pyrones, 2-Pyridones, 2-Coumarins and 2-Quinolones. Eur. J. Org. Chem. 2018, 6068–6082. [Google Scholar] [CrossRef]
- Nakatani, A.; Hirano, K.; Satoh, T.; Miura, M. Manganese-mediated C3-selective direct alkylation and arylation of 2-pyridones with diethyl malonates and arylboronic acids. J. Org. Chem. 2014, 79, 1377–1385. [Google Scholar] [CrossRef]
- Modak, A.; Rana, S.; Maiti, D. Iron-catalyzed regioselective direct arylation at the C-3 position of N-alkyl-2-pyridone. J. Org. Chem. 2015, 80, 296–303. [Google Scholar] [CrossRef]
- Dutta, U.; Deb, A.; Lupton, D.W.; Maiti, D. The regioselective iodination of quinolines, quinolones, pyridones, pyridines and uracil. Chem. Commun. 2015, 51, 17744–17747. [Google Scholar] [CrossRef]
- Niwetmarin, W.; Saruengkhanphasit, R.; Eurtivong, C.; Ruchirawat, S. Visible-light-mediated decarboxylative alkylation of 2-pyridone derivatives via a C3-selective C–H functionalization. Org. Biomol. Chem. 2021, 19, 9231–9236. [Google Scholar] [CrossRef]
- Miura, W.; Hirano, K.; Miura, M. Iridium-catalyzed site-selective C–H borylation of 2-pyridones. Synthesis 2017, 49, 4745–4752. [Google Scholar]
- Chen, Y.Y.; Wang, F.; Jia, A.Q.; Li, X.W. Palladium-catalyzed selective oxidative olefination and arylation of 2-pyridones. Chem. Sci. 2012, 3, 3231–3236. [Google Scholar] [CrossRef]
- Gigant, N.; Bäckvall, J.-E. Synthesis of conjugated dienes via a biomimetic aerobic oxidative coupling of two Cvinyl–H bonds. Chem. Eur. J. 2013, 19, 10799–10803. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, A.J.; Lloyd-Jones, G.C. Room-temperature gold-catalysed arylation of heteroarenes: Complementarity to palladium catalysis. Chem. Eur. J. 2016, 22, 12641–12645. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, H.; Wang, H. Manganese(I)-catalyzed direct C–H allylation of arenes with allenes. J. Org. Chem. 2017, 82, 11173–11181. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Han, X.; Li, C.; Liu, L.; Cong, Z.; Liu, H. Cobalt(III)-catalyzed site-selective C–H amidation of pyridones and isoquinolones. RSC Adv. 2018, 8, 32659–32663. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Wang, J.; Li, C.; Zhu, W.; Jiang, H.; Liu, H. Cu(II)-catalyzed C6-selective C–H thiolation of 2-pyridones using air as the oxidant. RSC Adv. 2016, 6, 57441–57445. [Google Scholar] [CrossRef]
- Mohanty, S.R.; Prusty, N.; Gupta, L.; Biswal, P.; Ravikumar, P.C. Cobalt(III)-Catalyzed C-6 Alkenylation of 2-Pyridones by Using Terminal Alkyne with High Regioselectivity. J. Org. Chem. 2021, 86, 9444–9454. [Google Scholar] [CrossRef]
- Odani, R.; Hirano, K.; Satoh, T.; Miura, M. Copper-mediated C6-selective dehydrogenative heteroarylation of 2-pyridones with 1,3-azoles. Angew. Chem. Int. Ed. 2014, 53, 10784–10788. [Google Scholar] [CrossRef]
- Das, D.; Biswas, A.; Karmakar, U.; Chand, S.; Samanta, R. C6-Selective Direct Alkylation of Pyridones with Diazo Compounds under Rh(III)-Catalyzed Mild Conditions. J. Org. Chem. 2016, 3, 842–848. [Google Scholar] [CrossRef]
- Miura, W.; Hirano, K.; Miura, M. Nickel-Catalyzed Directed C6-Selective C–H Alkylation of 2-Pyridones with Dienes and Activated Alkenes. J. Org. Chem. 2017, 82, 5337–5344. [Google Scholar] [CrossRef]
- Mohanty, S.R.; Prusty, N.; Banjare, S.K.; Nanda, T.; Ravikumar, P.C. Overcoming the Challenges toward Selective C(6)–H Functionalization of 2-Pyridone with Maleimide through Mn(I)-Catalyst: Easy Access to All-Carbon Quaternary Center. Org. Lett. 2022, 24, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Poddar, P.; Maity, S.; Samanta, R. Rhodium(III)-catalyzed C6-selective arylation of 2-pyridones and related heterocycles using quinone diazides: Syntheses of heteroarylated phenols. J. Org. Chem. 2017, 82, 3612–3621. [Google Scholar] [CrossRef] [PubMed]
- Miura, W.; Hirano, K.; Miura, M. Rhodium-catalyzed C6-selective C–H borylation of 2-pyridones. Org. Lett. 2016, 18, 3742–3745. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Wang, J.; Jiang, H.; Liu, H. Rhodium(III)-catalyzed site-selective C–H alkylation and arylation of pyridones using organoboron reagents. Org. Lett. 2016, 18, 5376–5379. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Z.; Zhang, Q.; Li, Z.; Liu, H.; Bi, X.; Wang, J. Ru(II)-catalyzed C6-selective C–H acylmethylation of pyridones using sulfoxonium ylides as carbene precursors. RSC Adv. 2020, 10, 6351–6355. [Google Scholar] [CrossRef]
- Drev, M.; Grošelj, U.; Ledinek, B.; Perdih, F.; Svete, J.; Štefane, B.; Požgan, F. Ruthenium(II)-Catalyzed Microwave-Promoted Multiple C–H Activation in Synthesis of Hexa(heteroaryl)benzenes in Water. Org. Lett. 2018, 17, 5268–5273. [Google Scholar] [CrossRef]
- Wang, P.; Liang, C.; Leung, M. An improved Ullmann–Ukita–Buchwald–Li conditions for CuI-catalyzed coupling reaction of 2-pyridones with aryl halides. Tetrahedron 2005, 61, 2931–2939. [Google Scholar] [CrossRef]
- Li, B.; Roisnel, T.; Darcel, C.; Dixneuf, P.H. Cyclometallation of arylimines and nitrogen-containing heterocycles via room-temperature C–H bond activation with arene ruthenium(II) acetate complexes. Dalton Trans. 2012, 41, 10934–10937. [Google Scholar] [CrossRef]
- Li, B.; Darcel, C.; Roisnel, T.; Dixneuf, P.H. Cycloruthenation of aryl imines and N-heteroaryl benzenes via C–H bond activation with Ru(II) and acetate partners. J. Organomet. Chem. 2015, 793, 200–209. [Google Scholar] [CrossRef]
- Liao, C.; Li, J.; Chen, X.; Lu, J.; Liu, Q.; Chen, L.; Huang, Y.; Li, Y. Selective synthesis of pyridyl pyridones and oxydipyridines by transition-metal-free hydroxylation and arylation of 2-fluoropyridine derivatives. Org. Biomol. Chem. 2020, 18, 1185–1193. [Google Scholar] [CrossRef]
- Lamazzi, L.; Dreau, A.; Bufferne, B.; Flouzat, C.; Carlier, P.; ter Halle, R.; Besson, T. Microwave-induced by-products in the synthesis of 2-(4-methyl-2-phenylpiperazinyl)pyridine-3-carbonitrile. Tetrahedron Lett. 2009, 50, 4502–4505. [Google Scholar] [CrossRef]
- Taylor, E.C.; Harrison, K.A.; Rampal, J.B. Unusual “Hydrolysis” of 2 Nitrosopyridines: Formation of l-(2-Pyridyl)-2(lH)-pyridones. J. Org. Chem. 1986, 51, 102–105. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Q.; Liu, Y.; Wang, Q. 4-(N,N-Dimethylamino)pyridine Hydrochloride as a Recyclable Catalyst for Acylation of Inert Alcohols: Substrate Scope and Reaction Mechanism. Org. Lett. 2014, 16, 236–239. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, version 1.171.36.28; Rigaku Oxford Diffraction: Yarnton, UK, 2013.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
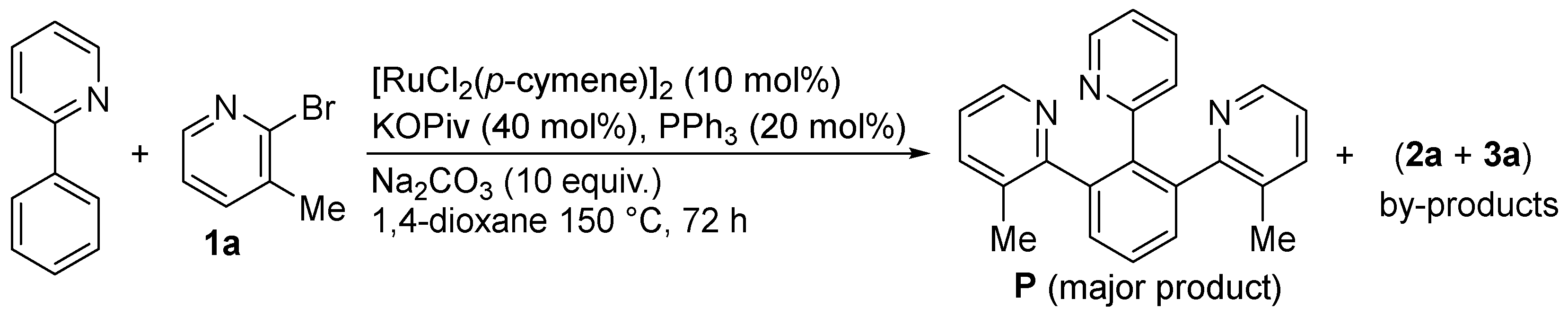
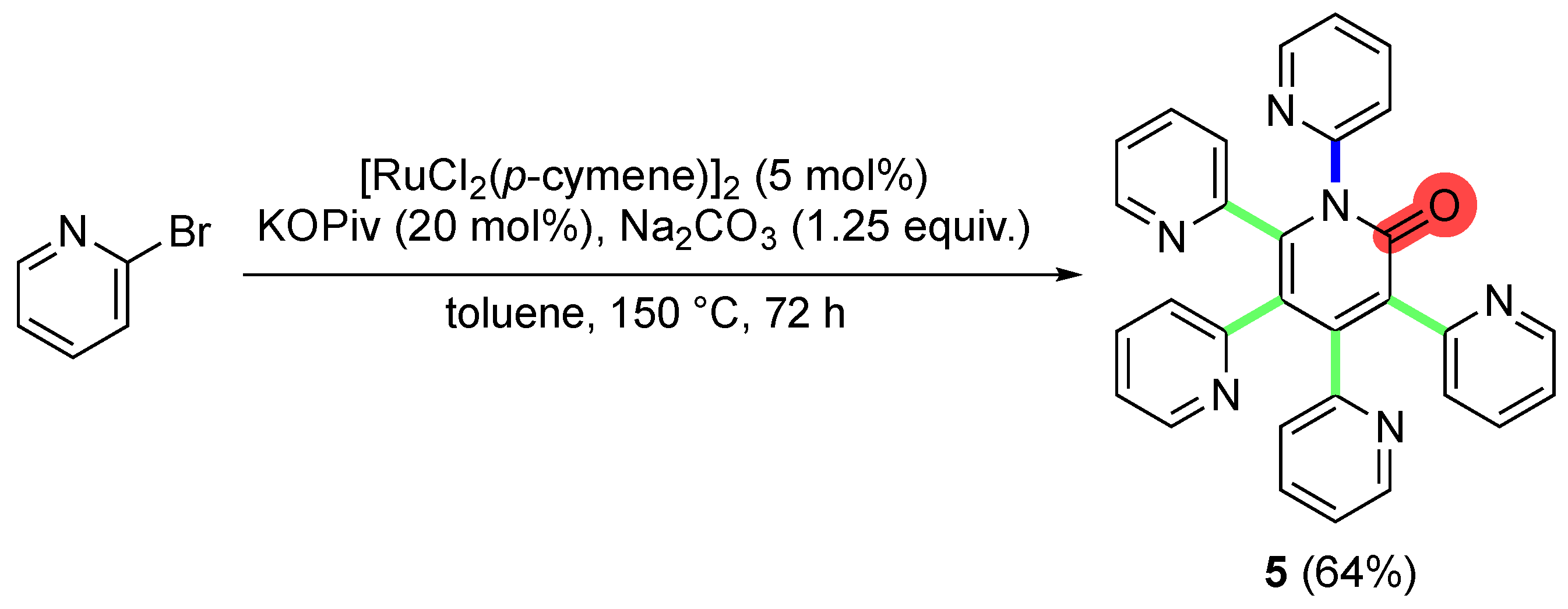
 | ||||
|---|---|---|---|---|
| Entry | [Ru] (mol%) | Additive (mol%) | Solvent | 1a/2a/3a 2 |
| 1 | (1.25) | KOPiv (20)/PPh3 (2.5)/Na2CO3 (125) | 1,4-dioxane | 25/40/35 |
| 2 | (1) | KOPiv (4) | 1,4-dioxane | 95/5/0 |
| 3 | (1) | Na2CO3 (125) | 1,4-dioxane | 85/7/8 |
| 4 | (1) | KOPiv (4)/Na2CO3 (125) | 1,4-dioxane | 0/80/20 |
| 5 | (5) | KOPiv (20)/Na2CO3 (125) | 1,4-dioxane | 0/20 (12%) 3/80 (62%) 3 |
| 6 | (5) | KOPiv (20)/Na2CO3 (125) | NMP | 15/20/65 |
| 7 | (5) | KOPiv (20)/Na2CO3 (125) | 2-MeTHF | 0/50/50 |
| 8 | (5) | KOPiv (20)/Na2CO3 (125) | DEC | 0/25/75 |
| 9 | (5) | KOPiv (20)/Na2CO3 (125) | toluene | 0/5/95 (83) 3 |
| 10 | (5) | KOPiv (20)/Na2CO3 (125) | H2O | 100/0/0 |
| 11 | (1) | KOPiv (4)/Na2CO3 (125) | 1,4-dioxane/H2O (4:1) | 75/20/5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drev, M.; Brodnik, H.; Grošelj, U.; Perdih, F.; Svete, J.; Štefane, B.; Požgan, F. 2-Bromopyridines as Versatile Synthons for Heteroarylated 2-Pyridones via Ru(II)-Mediated Domino C–O/C–N/C–C Bond Formation Reactions. Molecules 2024, 29, 4418. https://doi.org/10.3390/molecules29184418
Drev M, Brodnik H, Grošelj U, Perdih F, Svete J, Štefane B, Požgan F. 2-Bromopyridines as Versatile Synthons for Heteroarylated 2-Pyridones via Ru(II)-Mediated Domino C–O/C–N/C–C Bond Formation Reactions. Molecules. 2024; 29(18):4418. https://doi.org/10.3390/molecules29184418
Chicago/Turabian StyleDrev, Miha, Helena Brodnik, Uroš Grošelj, Franc Perdih, Jurij Svete, Bogdan Štefane, and Franc Požgan. 2024. "2-Bromopyridines as Versatile Synthons for Heteroarylated 2-Pyridones via Ru(II)-Mediated Domino C–O/C–N/C–C Bond Formation Reactions" Molecules 29, no. 18: 4418. https://doi.org/10.3390/molecules29184418
APA StyleDrev, M., Brodnik, H., Grošelj, U., Perdih, F., Svete, J., Štefane, B., & Požgan, F. (2024). 2-Bromopyridines as Versatile Synthons for Heteroarylated 2-Pyridones via Ru(II)-Mediated Domino C–O/C–N/C–C Bond Formation Reactions. Molecules, 29(18), 4418. https://doi.org/10.3390/molecules29184418











