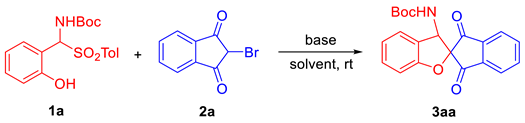
Abstract
An efficient cascade cyclization strategy was developed to synthesize aminobenzofuran spiroindanone and spirobarbituric acid derivatives utilizing 2-bromo-1,3-indandione, 5-bromo-1,3-dimethylbarbituric acid, and ortho-hydroxy α-aminosulfones as substrates. Under the optimized reaction conditions, the corresponding products were obtained with high efficiency, exceeding 95% and 85% yields for the respective derivatives. This protocol demonstrates exceptional substrate versatility and robust scalability up to the Gram scale, establishing a stable platform for the synthesis of 3-aminobenzofuran derivative. The successful synthesis paves the way for further biological evaluations with potential implications in scientific research.
1. Introduction
Benzofuran, a crucial fused heterocycle, exhibits diverse pharmacological properties, including antitumor, anti-inflammatory, antiviral, and anti-osteoporotic activities [1,2,3,4,5,6,7,8]. For instance, compound A can stimulate bone formation, accelerate bone turnover, augment the proportion of osteoblasts, and effectively prevent and treat glucocorticoid-induced osteoporosis by upregulating BMP-2 expression [9]. Compound B demonstrates exceptional anticancer activity against breast cancer cells [10,11].
The 3-aminobenzofuran scaffold represents a significant subclass within the diverse family of benzofuran-based compounds, occupying a prominent position at the forefront of medical treatment advancements and innovations [12,13,14]. Its unique structural features contribute to its promising potential in developing novel therapeutic agents [15,16,17] (Figure 1). Compound C exhibits potent antitumor properties by inducing cell cycle arrest and promoting apoptosis within cellular environments [18]. Compound D stands as a crucial constituent in therapeutic agents aimed at alleviating the symptoms of Alzheimer’s disease [19].
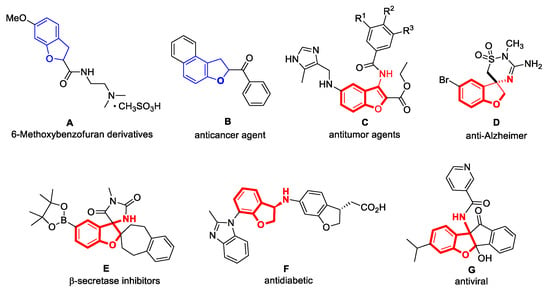
Figure 1.
Compounds with benzofuran and 3-aminobenzofuran as core scaffolds.
The 3-amino substitution enhances the biological activities of benzofuran derivatives, prompting research into innovative synthetic strategies to facilitate their clinical applications [20,21,22,23,24]. Recently, Helgren et al. introduced a microwave-facilitated, unprotected group synthesis of 3-amino-2,3-dihydrobenzofurans via chalcone precursors. This synthesis uses acid-catalyzed aldol condensation, reduction, and epoxidation reactions, facilitating C2, C3, and A-ring modifications [25]. Wang and colleagues have developed a method for the synthesis of enantiomerically enriched 2,3-dihydrobenzofurans bearing quaternary carbon stereocenters in moderate to excellent yields (31–98%) through the copper/BOX complex-catalyzed reaction of 2-imino-substituted phenols with aryl diazoacetates. This reaction employs readily accessible catalysts and mild reaction conditions, demonstrating broad functional group tolerance, single diastereoisomer formation, and excellent enantioselectivity (88–97% ee). The reaction proceeds via a distinctive mechanism in which the copper catalyst exerts two distinct functions. Initially, it reacts with the diazo compound to generate a metallacarbene. Subsequently, it acts as a Lewis acid to activate the imine following the formation of an oxonium ylide [26]. Panday and coworkers swiftly synthesized 3-amino-2-arylbenzofurans at room temperature via Cs2CO3-catalyzed route, advancing to Cu(II)-catalyzed N-arylation [27]. Abtahi and coworkers achieved one-pot 3-aminobenzofuran synthesis using CuI in a deep eutectic solvent [28]. The current synthetic methodologies for the construction of 3-aminobenzofuran frameworks, which encompass radical cyclization, coupling-cyclization, and Lewis acid/transition metal catalysis, are frequently impeded by complex purification procedures and rigorous reaction conditions. This hinders their practical applicability.
Meanwhile, indanone and barbituric acid are prevalent in natural products and bioactive pharmaceutical molecules [29,30,31,32,33]. The structures of 1,3-indanedione and 1,3-dimethylbarbituric acid, which serve as pivotal pharmacophore scaffolds, have garnered extensive research attention. The conventional utilization of 1,3-indandione [34] and 1,3-dimethylbarbituric acid [35,36] structures has been primarily focused on their benzylidene unsaturation moiety, offering a rich platform for chemical modifications and diverse functionalization. However, there is a scarcity of reports detailing their involvement in tandem cyclization reactions, highlighting an underexplored area with significant potential for further investigation and development [37,38,39,40]. The combined pharmacophore strategy allows us to harness the potential of 1,3-indandione and 1,3-dimethylbarbituric acid to engage in tandem reactions with ortho-hydroxy α-amino sulfones, thereby constructing a series of target products featuring an aminobenzofuranospiroindanone and spirobarbiturate core skeleton. While devising a synthetic route, several unsuccessful attempts were made to utilize halogen-substituted N-succinimides as the primary halogen source (Scheme 1, path a). We then strategically shifted our focus to bromine-substituted 1,3-indandione as the substrate (Scheme 1, path b), a pivotal decision that ultimately led to the synthesis of a product structure with profound implications for the development of novel therapeutic agents.
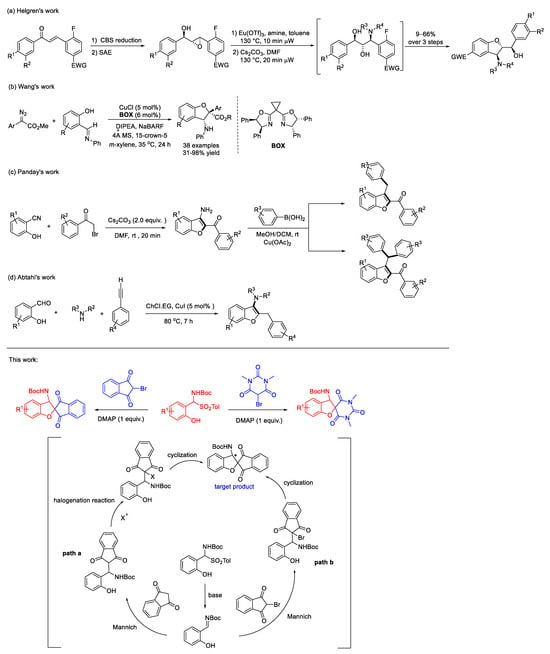
Scheme 1.
Synthesis of 3-aminobenzofuran derivatives and our contributions.
2. Results and Discussion
2.1. Optimization of Reaction Conditions
To ascertain the optimal synthetic conditions for the benzofuran-spiroindenone derivative, we employed ortho-hydroxy α-aminosulfone 1a and 2-bromo-1,3-indandione 2a as the starting materials and stirred them in dichloromethane solvent at ambient temperature. This approach facilitated the identification of the most favorable experimental parameters. A preliminary investigation was conducted to assess the impact of various bases on the reaction efficiency (Table 1, entries 1–9). When using NaHCO₃ as the base, the desired product, namely 3aa, was obtained in 42% yield after 20 h reaction (Table 1, entry 1). Subsequently, Na2CO3, K2CO3, and Cs2CO3 were subjected to screening. The results demonstrated that the effects of Na2CO3 and K2CO3 were comparable (Table 1, entries 2 and 3). However, when Cs2CO3 was utilized, there was a considerable reduction in the yield (41%) (Table 1, entry 4). A selection of organic bases (Table 1, entries 5–9) was subjected to preliminary screening. When Et3N was used, there was no discernible improvement in the reaction yield, which remained at approximately 45% (Table 1, entry 5). Additionally, the other three organic bases, DABCO, DBU, and TMG, were also subjected to preliminary screening. When using these bases in the template reaction, there was no discernible alteration in the actual reaction outcome, with the yield fluctuating around 30% (Table 1, entries 6–8). It is noteworthy that when the organic base DMAP was subjected to evaluation (Table 1, entry 9), the reaction outcome exhibited a notable improvement, resulting in a yield increase of 70%.

Table 1.
Optimization of reaction conditions a.
Following the identification of the optimal base, a screening of solvents for the reaction was conducted, with the solvents listed in Table 1 (entries 10–15). When dichloroethane (DCE) was employed as the solvent (Table 1, entry 10), the yield was further augmented to 85%. In contrast, when the aromatic hydrocarbon solvent toluene was used, the yield of the reaction was not significantly enhanced (Table 1, 11). The large polar solvents, CH₃CN and EtOH, were also tested (Table 1, 12, and 14), but no improvement was observed in the reaction. Apart from exploring protic solvents, we also evaluated aprotic solvents, including THF (Table 1, entry 13) and MTBE (Table 1, entry 15). Nevertheless, the outcomes did not meet our initial expectations of significant enhancement. Through method optimization, it was established that the most favorable reaction conditions entailed utilizing DMAP as the base and DCE as the solvent, leading to a high yield of up to 85% for the desired product.
2.2. Substrate Scope
Once the optimal reaction conditions had been determined, a comprehensive investigation was conducted into the substrate scope of this reaction (Scheme 2). The examination of the 5-position substituents on ortho-hydroxy α-aminosulfone revealed that substitution with chlorine or fluorine at this position facilitated the synthesis of the target products 3ca and 3da with good to excellent yields. Unexpectedly, the substitution of the 5-position with bromine led to the synthesis of the desired product 3ba, albeit with a modest yield of merely 50%. It is noteworthy that the strategic substitution of the 5-position in ortho-hydroxy α-aminosulfone with electron-rich groups, particularly methoxy and methyl groups, resulted in the successful synthesis of target compounds 3ea and 3fa, which exhibited good yields. Moreover, a comprehensive examination was undertaken to ascertain the impact of substituents at the 4-position on the physicochemical properties of these α-aminosulfones. The substitution of the 4-position with halogen atoms, such as chlorine or bromine, resulted in the successful acquisition of target products 3ga and 3ha with satisfactory yields. Subsequently, the substitution pattern at the 3-position of ortho-hydroxy α-aminosulfone was investigated. When the 3-position was substituted with the electron-donating methoxy group, the reaction failed to yield the desired target product. However, when the 3-position was substituted with halogen bromine or fluorine, the target products 3ia and 3ja were obtained with excellent yields of 90% and 95%, respectively. This highlights the superior reactivity of halogen-substituted ortho-hydroxy α-aminosulfones in comparison to those bearing electron-donating groups. Lastly, the reactivity of the 6-chloro-substituted ortho-hydroxy α-aminosulfone was evaluated, revealing a smooth reaction course that led to the formation of product 3ka with a good yield of 81%. These findings underscore the intricate interplay between substitution patterns and reactivity patterns in the synthesis of functionalized ortho-hydroxy α-aminosulfones.
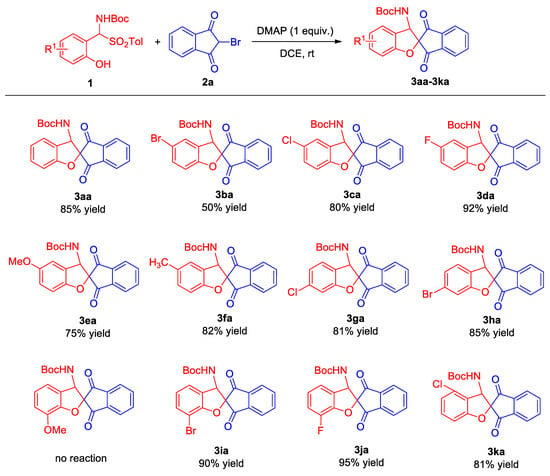
Scheme 2.
Substrate expansion for the tandem cyclization reaction of ortho-hydroxy α-aminosulfone and 2-bromo-1,3-indandione. The reaction was conducted under the following conditions: ortho-hydroxy α-aminosulfone 1 (0.15 mmol), 2-bromo-1,3-indandione 2 (0.1 mmol), and DMAP (1 equiv.) in DCE (1.0 mL) was stirred for 20 h at room temperature, followed by silica gel column chromatography for the separation of the products.
Building upon the successful outcomes of the preceding experiment, the tandem cyclization reaction between ortho-hydroxy α-aminosulfone and bromo-substituted 1,3-dimethylbarbituric acid was subjected to an extensive optimization process. This refinement effort revealed that the reaction, when conducted with DMAP serving as the base and DCE as the solvent, was capable of producing the target product 5aa in 80% yield.
Prompted by this finding, an investigation into the versatility of the reaction toward a diverse array of substituted ortho-hydroxy α-aminosulfone substrates was undertaken (Scheme 3). When the substituent is located at the 5-position of the aromatic ring, whether it is an electron-donating group or a halogen substituent, the target products (5ba–5ea) can be obtained with a relatively high yield (80–85%). For the 3-position-substituted ortho-hydroxy α-aminosulfones, their performance did not show significant improvement. Specifically, the 3-fluoro-substituted ortho-hydroxy α-aminosulfone only produced the target product 5fa in 70% yield, while the 3-position electron-donating methoxy-substituted ortho-hydroxy α-aminosulfone failed to yield the target product. It is worthy of note that the 3-methoxy-substituted ortho-hydroxy α-aminosulfone also exhibited no observable reaction with 2-bromo-1,3-indandione during the substrate extension of the tandem cyclization reaction between ortho-hydroxy α-aminosulfone and 2-bromo-1,3-indandione. The 4-chloro- and 4-bromo-substituted ortho-hydroxy α-aminosulfones exhibited good performances, and 5ga and 5ha were obtained in 72% and 79% yields, respectively. Overall, in the substrate extension of these two parts, ortho-hydroxy α-aminosulfones demonstrated better performance in the tandem cyclization reaction with 2-bromo-1,3-indandione.
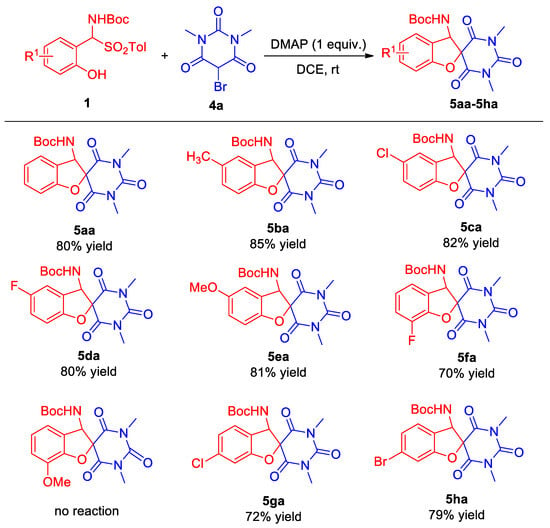
Scheme 3.
Substrate scope for benzofuranobarbituric acid. ortho-hydroxy α-aminosulfone 1 (0.15 mmol) and 5-bromo-1,3-dimethylbarbituric acid 4 (0.1 mmol) in DCE (1.0 mL) were stirred for 10 h at room temperature. The product yield was isolated.
2.3. Scaled-Up Synthesis
To demonstrate the practicality and stability of the DMAP-catalyzed tandem cyclization reaction involving ortho-hydroxy α-aminosulfone, an endeavor has also been made to conduct the reaction on a Gram-scale basis. As depicted in Scheme 4, the initial reaction scale was enlarged by approximately 55-fold. For the Gram-scale synthesis of ortho-hydroxy α-aminosulfone 1a with 2-bromo-1,3-indandione 2a, the target product 3aa was ultimately achieved with the same high yield (85%) as in previous smaller-scale reactions. Nevertheless, in the Gram-scale reaction involving ortho-hydroxy α-aminosulfone 1a and 5-bromo-1,3-dimethylbarbituric acid 4a, the yield decreased slightly but remained moderate (73%), enabling the successful isolation of the target product 5aa. This underscores the considerable potential of this methodology for the large-scale production of such compounds via tandem cyclization reactions.
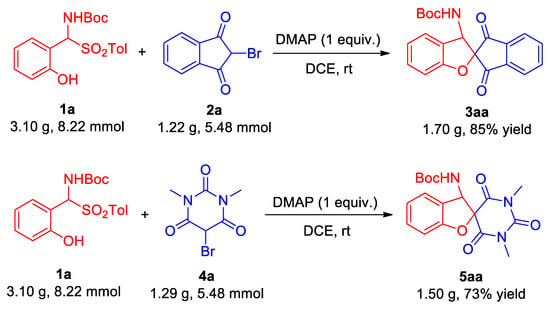
Scheme 4.
Gram-scale synthesis of 3aa and 5aa.
2.4. Further Evaluation of Substrate Scope of Dicarbonyl α-Bromo-Substituted Compounds
Based on the structural features of 2-bromo-1,3-indandione and 5-bromo-1,3-dimethylbarbituric acid, several analogous dicarbonyl α-bromo-substituted compounds have been synthesized and applied to the tandem reaction with ortho-hydroxy α-aminosulfone 1a (Scheme 5). Initially, linear chain-derived dicarbonyl α-bromo-substituted compounds, namely, α-bromo-substituted 1,3-diphenylpropane-1,3-dione and diethyl malonate, were used as reactants. The 2-bromo-1,3-diphenylpropane-1,3-dione was found to be unreactive with 1a. In contrast, diethyl 2-bromomalonate exhibited reactivity, although the yield was deemed inadequate, with only a minimal amount of product observed via TLC. Moreover, a six-membered ring-derived dicarbonyl α-bromo-substituted compound, 2-bromocyclohexane-1,3-dione, was used as a reactant. However, the reaction between 2-bromocyclohexane-1,3-dione and 1a exhibited a low efficiency.
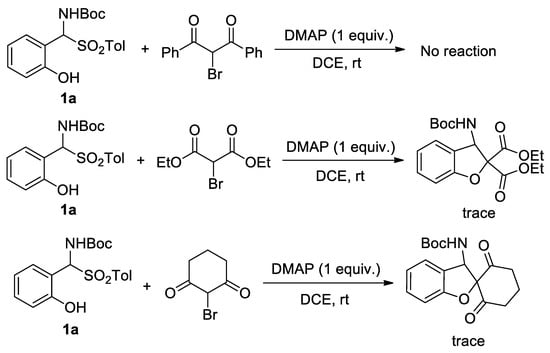
Scheme 5.
Further valuation of substrate scope.
2.5. Asymmetric Catalytic Reaction Trials
Drawing upon our previous investigations into asymmetric catalysis [41,42,43], we have embarked on an extended exploration of this particular reaction, aiming for a more profound understanding. Consequently, catalysts C1–C4 with varying structural types were selected for application in this asymmetric catalytic reaction (Scheme 6). Firstly, the reactions of cinchona alkaloid-derived squaramide catalysts (C1 and C2) catalyzed addition/cyclization of ortho-hydroxy-α-aminosulfone 1a with 2-bromo-1,3-indandione 2a showed relatively good catalytic performance, with catalyst C2 exhibiting the best performance. The catalytic product 3aa was obtained in 85% yield with 17% ee. When cinchona alkaloid and 1,2-diaminocyclohexane-derived thiourea catalysts (C3 and C4) were employed, the catalytic effect of the reactions was found to be even worse. Furthermore, catalyst C1 was used to catalyze the asymmetric tandem cyclization reaction of ortho-hydroxy α-aminosulfone 1a and 5-bromo-1,3-dimethylbarbituric acid 4a, yet the enantiomeric excess of the product was only 22% ee. Through analysis of the results, it can be seen that the multifunctional hydrogen bond-derived thiourea and squaramide catalysts exhibit generally poor asymmetric catalytic performance for this type of reaction.
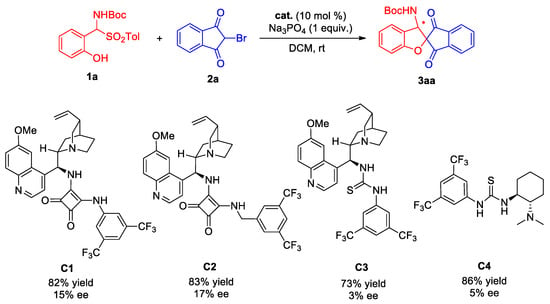
Scheme 6.
Preliminary evaluation for asymmetric catalytic reaction. * The chiral carbon, absolute configuration not determined.
3. Materials and Methods
3.1. General Information
In the context of this study, commercially sourced reagents were utilized without undergoing any additional purification procedures. Commonly used solvents, including petroleum ether (boiling range 60–90 °C), ethyl acetate, dichloromethane, xylene, and methanol, as well as other analytical grade reagents, were produced by the Beijing Chemical Plant (Beijing, China). Column chromatography silica gel (200–300 mesh) and thin-layer chromatography silica gel plates were manufactured by Yantai Xinnuo New Materials Technology Co., Ltd. (Yantai, China) and Yantai Dexin Biotechnology Co., Ltd. (Yantai, China), respectively. The melting points were determined using an XT-4 melting point apparatus, and these values were reported without any correction. For nuclear magnetic resonance (NMR) analysis, 1H NMR spectra were acquired on a Bruker Ascend 400 MHz spectrometer, with chemical shifts expressed in δ (ppm) units, utilizing tetramethylsilane (TMS) as the internal standard for calibration. 13C NMR spectra were recorded at 100 MHz on the same 400 MHz spectrometer, with chemical shifts also reported in ppm, referenced to TMS, and further calibrated against the solvent peak (CDCl3, δC = 77.00). For high-resolution mass spectrometry, electron spray ionization (ESI) mass spectra were obtained using an Agilent 6520 Accurate-Mass Q-TOF MS system (Santa Clara, CA, USA), which was equipped with an ESI source and ensured precise and accurate mass measurements.
3.2. Experimental Materials for Tandem Reactions
The products 1a–1k were prepared according to the literature reported by Liu and coworkers [43,44].
2-Bromo-1H-indene-1,3(2H)-dione (2a) was synthesized according to the literature [45,46].
A mixture of 1,3-indandione (219 mg, 1.5 mmol), KBr (835 mg, 7.5 mmol), 1 M HCl (7.5 mL), and 30% H2O2 (3.4 mL) in toluene (7.5 mL) was stirred at a room temperature. Reaction progress was monitored by TLC. Upon completion, the reaction was quenched with saturated Na2SO3 (10 mL) and neutralized with saturated NaHCO3 (10 mL). The organic layer was separated, extracted with EtOAc (2 × 20 mL), washed with water and brine, dried over MgSO4, and concentrated under vacuum to give product 2a (300 mg, 90% yield).
5-bromo-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (4a) was prepared following the same procedure as 2a.
The 1,3-indanedione utilized in the synthesis reaction was procured from Innochem (Beijing) Technology Co., Ltd. (Beijing, China). The potassium bromide and hydrogen peroxide were procured from Beijing MREDA Technology Co., Ltd. (Beijing, China) and Xilong Scientific Co., Ltd. (Shantou, China), respectively. The cinchona alkaloid-derived squaramide catalysts and cyclohexanediamine-derived thiourea catalyst utilized in the primary test were prepared in accordance with the literature reports [47,48,49,50].
3.3. Procedure for the Synthesis of Aminobenzofuran Spiroindanone 3
- ortho-Hydroxy α-aminosulfone 1 (0.15 mmol), 2-bromo-1,3-indandione 2a (0.1 mmol), and 4-dimethylaminopyridine (DMAP) (1.0 equiv.) were combined in dry DCE (1.0 mL) in a 5 mL glass reaction vessel. The reaction system was stirred at room temperature for 20 h. After the completion of the reaction, which was monitored by thin-layer chromatography, the crude product was separated by means of a silica gel rapid column chromatography (ethyl acetate/petroleum ether 1:5) to obtain 3.
- tert-Butyl (1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3aa). Compound 3aa (31.0 mg, 85% yield) was isolated as a yellow solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 148–150 °C. 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 6.8 Hz, 1H, ArH), 7.98–7.88 (m, 3H, ArH), 7.32–7.26 (m, 2H, ArH), 7.02 (t, J = 7.6 Hz, 1H, ArH), 6.93 (d, J = 8.4 Hz, 1H, ArH), 5.70 (d, J = 8.8 Hz, 1H, NH), 5.11 (d, J = 8.4 Hz, 1H, CH), 1.22 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ195.5, 194.1, 159.9, 154.9, 141.5, 141.4, 136.5, 136.3, 130.8, 124.8, 124.3, 123.7, 123.4, 122.3, 110.6, 88.2, 80.5, 59.7, 27.9 ppm. HRMS (ESI): m/z calcd. for C21H19NNaO5 [M + Na]+ 388.1156, found 388.1151.
- tert-Butyl (5-bromo-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)-carbamate (3ba). Compound 3ba (22.2 mg, 50% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 140–142 °C. 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 6.8 Hz, 1H, ArH), 7.99–7.90 (m, 3H, ArH), 7.43–7.39 (m, 2H, ArH), 6.86 (d, J = 8.4 Hz, 1H, ArH), 5.69 (d, J = 8.8 Hz, 1H, NH), 5.07 (d, J = 8.8 Hz, 1H, CH), 1.22 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 195.0, 193.6, 159.1, 154.7, 141.4, 136.8, 136.5, 133.7, 127.8, 126.1, 124.5, 123.5, 114.2, 112.3, 88.6, 80.9, 59.2, 27.8 ppm. HRMS (ESI): m/z calcd. for C21H1879BrNNaO5 [M + Na]+ 466.0261, found 466.0271; calcd. for C21H1881BrNNaO5 [M + Na]+ 468.0241, found 468.0252.
- tert-Butyl (5-chloro-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)-carbamate (3ca). Compound 3ca (31.9 mg, 80% yield) was isolated as a yellow solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 217–219 °C. 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 6.8 Hz, 1H, ArH), 7.99–7.88 (m, 3H, ArH), 7.28–7.25 (m, 2H, ArH), 6.90 (d, J = 8.4 Hz, 1H, ArH), 5.68 (d, J = 8.8 Hz, 1H, NH), 5.08 (d, J = 8.4 Hz, 1H, CH), 1.22 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 195.0, 193.6, 158.6, 154.7, 141.4, 136.7, 136.5, 130.8, 127.2, 125.6, 124.9, 124.5, 123.5, 111.7, 88.6, 80.8, 59.3, 27.9 ppm. HRMS (ESI): m/z calcd. for C21H18ClNNaO5 [M + Na]+ 422.0766, found 422.0764.
- tert-Butyl (5-fluoro-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3da). Compound 3da (35.3 mg, 92% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 213–215 °C. 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 6.8 Hz, 1H, ArH), 7.99–7.90 (m, 3H, ArH), 7.03–6.98 (m, 2H, ArH), 6.89 (dd, J1 = 3.8 Hz, J2 = 8.6 Hz, 1H, ArH), 5.69 (d, J = 8.8 Hz, 1H, NH), 5.09 (d, J = 8.4 Hz, 1H, CH), 1.22 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 195.2, 193.9, 158.4 (d, 1JC–F = 238.9 Hz), 155.8, 154.7, 141.4, 136.7, 136.5, 125.0 (d, 3JC–F = 8.2 Hz), 124.4, 123.5, 117.4 (d, 2JC–F = 24.4 Hz), 111.8 (d, 2JC–F = 25.2 Hz), 111.2 (d, 3JC–F = 8.3 Hz), 88.7, 80.8, 59.5, 27.9 ppm. HRMS (ESI): m/z calcd. for C21H18FNNaO5 [M + Na]+ 406.1062, found 406.1043.
- tert-Butyl (5-methoxy-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3ea). Compound 3ea (29.6 mg, 75% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 224–226 °C. 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 6.8 Hz, 1H, ArH), 7.98–7.86 (m, 3H, ArH), 6.89–6.81 (m, 3H, ArH), 5.67 (d, J = 8.8 Hz, 1H, NH), 5.10 (d, J = 8.8 Hz, 1H, CH), 3.78 (s, 3H, OMe), 1.23 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ155.5, 154.8, 153.9, 141.5, 141.4, 136.5, 136.3, 124.4, 124.3, 123.4, 116.7, 110.9, 109.9, 80.6, 59.9, 56.1, 28.2, 27.9 ppm. HRMS (ESI): m/z calcd. for C22H21NNaO6 [M + Na]+ 418.1261, found 418.1252.
- tert-Butyl (5-methyl-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3fa). Compound 3fa (31.1 mg, 82% yield) was isolated as a yellow solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 181–183 °C. 1H NMR (400 MHz, CDCl3): δ 8.08 (d, J = 6.8 Hz, 1H, ArH), 7.97–7.86 (m, 3H, ArH), 7.11–7.07 (m, 2H, ArH), 6.85 (d, J = 8.0 Hz, 1H, ArH), 5.65 (d, J = 8.8 Hz, 1H, NH), 5.06 (d, J = 8.4 Hz, 1H, CH), 2.31 (s, 3H, CH3), 1.22 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 195.6, 194.3, 158.0, 154.8, 141.5, 141.4, 136.5, 136.3, 131.8, 131.3, 125.1, 124.3, 123.5, 123.4, 110.2, 88.4, 80.5, 59.8, 27.9, 20.7 ppm. HRMS (ESI): m/z calcd. for C22H21NNaO5 [M + Na]+ 402.1312, found 402.1310.
- tert-Butyl (6-chloro-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3ga). Compound 3ga (32.3 mg, 81% yield) was isolated as a yellow solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 225–227 °C. 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 6.8 Hz, 1H, ArH), 7.98–7.88 (m, 3H, ArH), 7.18 (d, J = 7.6 Hz, 1H, ArH), 7.02–6.98 (m, 2H, ArH), 5.65 (d, J = 8.8 Hz, 1H, NH), 5.05 (d, J = 8.8 Hz, 1H, CH), 1.22 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 194.9, 193.5, 160.7, 154.8, 141.4, 136.7, 136.5, 136.4, 125.4, 124.5, 123.5, 122.7, 122.6, 111.5, 88.7, 80.8, 59.0, 27.9 ppm. HRMS (ESI): m/z calcd. for C21H18ClNNaO5 [M + Na]+ 422.0766, found 422.0765.
- tert-Butyl (6-bromo-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3ha). Compound 3ha (37.7 mg, 85% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 164–166 °C. 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 6.8 Hz, 1H, ArH), 7.98–7.88 (m, 3H, ArH), 7.17–7.12 (m, 3H, ArH), 5.63 (d, J = 8.8 Hz, 1H, NH), 5.07 (d, J = 8.4 Hz, 1H, CH), 1.22 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 194.9, 193.5, 160.7, 154.8, 141.4, 136.7, 136.5, 125.8, 125.5, 124.4, 124.1, 123.5, 123.1, 114.4, 88.6, 80.8, 59.1, 27.9 ppm. HRMS (ESI): m/z calcd. for C21H1879BrNNaO5 [M + Na]+ 466.0261, found 466.0271; calcd. for C21H1881BrNNaO5 [M + Na]+ 468.0241, found 468.0252.
- tert-Butyl (7-bromo-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3ia). Compound 3ia (39.9 mg, 90% yield) was isolated as a yellow solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 172–174 °C. 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 6.8 Hz, ArH), 7.99–7.88 (m, 3H, ArH), 7.46 (d, J = 8.0 Hz, 1H, ArH), 7.21 (d, J = 7.6 Hz, 1H, ArH), 6.92 (t, J = 7.6 Hz, 1H, ArH), 5.77 (d, J = 8.8 Hz, 1H, NH), 5.12 (d, J = 8.8 Hz, 1H, CH), 1.23 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 194.8, 193.3, 157.2, 154.8, 141.5 136.7, 136.5, 133.9, 125.2, 124.5, 123.8, 123.7, 123.5, 103.4, 87.6, 80.8, 60.0, 27.9 ppm. HRMS (ESI): m/z calcd. for C21H1879BrNNaO5 [M + Na]+ 466.0261, found 466.0271, C21H1881BrNNaO5 [M + Na]+ 468.0241, found 468.0264.
- tert-Butyl (7-fluoro-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3ja). Compound 3ja (36.4 mg, 95% yield) was isolated as a yellow solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 175–177 °C. 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 6.4 Hz, 1H, ArH), 7.99–7.90 (m, 3H, ArH), 7.12–7.05 (m, 2H, ArH), 7.00–6.95 (m, 1H, ArH), 5.74 (d, J = 8.8 Hz, 1H, NH), 5.12 (d, J = 8.8 Hz, 1H, CH), 1.23 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 194.7, 193.3, 154.8, 147.2 (d, 1J C–F = 247.0 Hz), 146.7 (2JC–F = 11.6 Hz), 141.44, 141.36, 136.7, 136.5, 127.2, 124.5, 123.5, 123.1 (d, 3J C–F = 5.5 Hz), 120.0 (d, 3JC–F = 3.3 Hz), 117.9 (d, 2JC–F = 16.5 Hz), 88.5, 80.8, 59.6, 27.8 ppm. HRMS (ESI): m/z calcd. for C21H18FNNaO5 [M + Na]+ 406.1062, found 406.1057.
- tert-Butyl (4-chloro-1′,3′-dioxo-1′,3′-dihydro-3H-spiro[benzofuran-2,2′-inden]-3-yl)carbamate (3ka). Compound 3ka (32.3 mg, 81% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 178–180 °C. 1H NMR (400 MHz, CDCl3): δ 8.10–8.08 (m, 1H, ArH), 7.99–7.89 (m, 3H, ArH), 7.26 (t, J = 8.2 Hz, 1H, ArH), 6.99 (d, J = 8.0 Hz, 1H, ArH), 6.88 (d, J = 8.4 Hz, 1H, ArH), 5.73 (d, J = 8.8 Hz, 1H, NH), 5.07 (d, J = 8.4 Hz, 1H, CH), 1.27 (s, 9H, CH3) ppm. 13C NMR (176 MHz, CDCl3): δ 194.7, 193.1, 161.1, 154.7, 141.8, 140.8, 136.7, 136.6, 132.0, 131.3, 124.5, 123.5, 122.9, 121.6, 109.2, 88.2, 80.6, 58.9, 27.9 ppm. HRMS (ESI): m/z calcd. for C21H18ClNNaO5 [M + Na]+ 422.0766, found 422.0748.
3.4. Procedure for the Synthesis of Aminobenzofuran Spirobarbituric 5
A mixture comprising ortho-hydroxy α-aminosulfone 1 (0.15 mmol), 5-bromo-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione 4a (0.1 mmol), and 4-dimethlaminopyridine (DMAP) (1.0 equiv.) was assembled in anhydrous dichloroethane (DCE, 1.0 mL) within a 10 mL glass reaction vessel. This reaction ensemble was agitated at ambient temperature for 10 h. Upon completion of the reaction, monitored via thin-layer chromatography (TLC), the crude product mixture underwent purification via silica gel flash column chromatography, employing a solvent system of ethyl acetate and petroleum ether in a ratio of 1:5, affording the desired product 5.
- tert-Butyl (1′,3′-dimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro[benzofuran-2,5′-pyrimidin]-3-yl)carbamate (5aa). Compound 5aa (30.0 mg, 80% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 198–200 °C. 1H NMR (400 MHz, CDCl3): δ 7.33 (t, J = 7.6 Hz, 1H, ArH), 7.22 (d, J = 7.6 Hz, 1H, ArH), 7.03–7.00 (m, 2H, ArH), 5.75 (d, J = 8.4 Hz, 1H, NH), 5.14 (d, J = 8.4 Hz, 1H, CH), 3.40 (s, 3H, CH3), 3.30 (s, 3H, CH3), 1.43 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 167.4, 165.2, 159.9, 155.0, 150.3, 131.2, 124.7, 122.5, 121.9, 110.8, 87.6, 81.3, 63.9, 29.2, 28.8, 28.1 ppm. HRMS (ESI): m/z calcd. for C18H21N3NaO6 [M + Na]+ 398.1322, found398.1307.
- tert-Butyl (1′,3′,5-trimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro[benzo-furan-2,5′-pyrimidin]-3-yl)carbamate (5ba). Compound 5ba (31.1 mg, 85% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 203–205 °C. 1H NMR (400 MHz, CDCl3): δ 7.11 (d, J = 8.4 Hz, 1H, ArH), 7.02 (s, 1H, ArH), 6.90 (d, J = 8.4 Hz, 1H, ArH), 5.70 (d, J = 8.8 Hz, 1H, NH), 5.12 (d, J = 8.4 Hz, 1H, CH), 3.38 (s, 3H, CH3), 3.28 (s, 3H, CH3), 2.30 (s, 3H, CH3), 1.42 (s, 9H, CH3) ppm. 13C NMR (176 MHz, CDCl3): δ 167.5, 165.3, 157.9, 154.9, 150.3, 132.0, 131.7, 124.9, 121.7, 110.3, 87.8, 81.3, 63.9, 29.2, 28.7, 28.1, 20.7 ppm. HRMS (ESI): m/z calcd. for C19H23N3NaO6 [M + Na]+ 412.1479, found 412.1471.
- tert-Butyl (5-chloro-1′,3′-dimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro-[benzofuran-2,5′-pyrimidin]-3-yl)carbamate (5ca). Compound 5ca (33.5 mg, 82% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 218–220 °C. 1H NMR (400 MHz, CDCl3): δ 7.29 (dd, J1 = 8.8 Hz, J2 = 2.0 Hz, 1H, ArH), 7.21 (s, 1H, ArH), 6.95 (d, J = 8.4 Hz, 1H, ArH), 5.73 (d, J = 8.8 Hz, 1H, NH), 5.12 (d, J = 8.4 Hz, 1H, CH), 3.39 (s, 3H, CH3), 3.29 (s, 3H, CH3), 1.42 (s, 9H, CH3) ppm. 13C NMR (176 MHz, CDCl3): δ 167.0, 164.8, 158.5, 154.8, 150.1, 131.2, 127.4, 124.8, 123.8, 111.8, 87.9, 81.6, 63.4, 29.3, 28.8, 28.1 ppm. HRMS (ESI): m/z calcd. for C18H20ClN3NaO6 [M + Na]+ 432.0933, found 432.0924.
- tert-Butyl (5-fluoro-1′,3′-dimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro-[benzofuran-2,5′-pyrimidin]-3-yl)carbamate (5da). Compound 5da (31.4 mg, 80% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 205–207 °C. 1H NMR (400 MHz, CDCl3): δ 7.02 (td, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H, ArH), 6.96–6.93 (m, 2H, ArH), 5.74 (d, J = 8.8 Hz, 1H, NH), 5.19 (d, J = 8.4 Hz, 1H, CH), 3.39 (s, 3H, CH3), 3.29 (s, 3H, CH3), 1.42 (s, 9H, CH3) ppm. 13C NMR (176 MHz, CDCl3): δ 167.2, 165.0, 158.4 (1JC–F = 240.8 Hz), 155.7, 154.9, 150.1, 123.2 (3JC–F = 8.8 Hz), 117.8 (2JC–F = 24.6 Hz), 111.7 (2JC-F = 25.7 Hz), 111.3 (3JC–F = 8.1 Hz), 88.0, 81.6, 63.6, 29.2, 28.8, 28.1 ppm. HRMS (ESI): m/z calcd. for C18H20FN3NaO6 [M + Na]+ 416.1228, found 416.1209.
- tert-Butyl (5-methoxy-1′,3′-dimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro-[benzofuran-2,5′-pyrimidin]-3-yl)carbamate (5ea). Compound 5ea (32.8 mg, 81% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 228–230 °C. 1H NMR (400 MHz, CDCl3): δ 6.93 (d, J = 8.8 Hz, 1H, ArH), 6.86 (dd, J1 = 2.6 Hz, J2 = 9.0 Hz, 1H, ArH), 6.74 (d, J = 2.0 Hz, 1H, ArH), 5.72 (d, J = 8.8 Hz, 1H, NH), 5.13 (d, J = 8.4 Hz, 1H, CH), 3.76 (s, 3H, OCH3), 3.39 (s, 3H, CH3), 3.29 (s, 3H, CH3), 1.43 (s, 9H, CH3) ppm. 13C NMR (176 MHz, CDCl3): δ 167.5, 165.3, 155.5, 154.9, 153.8, 150.3, 122.5, 117.0, 111.0, 109.8, 87.9, 81.4, 64.0, 56.1, 29.2, 28.7, 28.1 ppm. HRMS (ESI): m/z calcd. for C19H23N3NaO7 [M + Na]+ 428.1428, found 428.1417.
- tert-Butyl (7-fluoro-1′,3′-dimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro-[benzofuran-2,5′-pyrimidin]-3-yl)carbamate (5fa). Compound 5fa (27.5 mg, 70% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 201–203 °C. 1H NMR (400 MHz, CDCl3): δ 7.13–7.09 (m, 1H, ArH), 7.01–6.94 (m, 2H, ArH), 5.79 (d, J = 8.4 Hz, 1H, NH), 5.17 (d, J = 8.4 Hz, 1H, CH), 3.40 (s, 3H, CH3), 3.30 (s, 3H, CH3), 1.42 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 166.8, 164.5, 155.0, 150.1, 147.1 (1JC–F = 247.6 Hz), 146.7 (2JC–F = 11.5 Hz), 125.5 (4JC–F = 2.5 Hz), 123.3 (3JC–F = 5.3 Hz), 119.8 (3JC–F = 3.4 Hz), 118.2 (2JC–F = 16.4 Hz), 87.9, 81.6, 63.9, 29.2, 28.8, 28.1 ppm. HRMS (ESI): m/z calcd. for C18H20FN3NaO6 [M + Na]+ 416.1228, found 416.1224.
- tert-Butyl (6-chloro-1′,3′-dimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro-[benzofuran-2,5′-pyrimidin]-3-yl)carbamate (5ga). Compound 5ga (29.4 mg, 72% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 202–204 °C. 1H NMR (400 MHz, CDCl3): δ 7.14 (d, J = 8.0 Hz, 1H, ArH), 7.03–6.99 (m, 2H, ArH), 5.70 (d, J = 8.8 Hz, 1H, NH), 5.10 (d, J = 8.4 Hz, 1H, CH), 3.39 (s, 3H, CH3), 3.29 (s, 3H, CH3), 1.42 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 166.9, 164.8, 160.5, 154.9, 150.1, 136.8, 125.3, 122.8, 120.7, 111.6, 88.0, 81.6, 63.2, 29.2, 28.8, 28.1 ppm. HRMS (ESI): m/z calcd. for C18H20ClN3NaO6 [M + Na]+ 432.0933, found 432.0928.
- tert-Butyl (6-bromo-1′,3′-dimethyl-2′,4′,6′-trioxo-1′,3′,4′,6′-tetrahydro-2′H,3H-spiro-[benzofuran-2,5′-pyrimidin]-3-yl)carbamate (5ha). Compound 5ha (35.8 mg, 79% yield) was isolated as a white solid through a standard purification process involving column chromatography on silica gel (200–300 mesh), utilizing a mixture of petroleum ether and ethyl acetate (5:1) as the eluent. m.p. 198–200 °C. 1H NMR (400 MHz, CDCl3): δ 7.18–7.14 (m, 2H, ArH), 7.08 (d, J = 8.0 Hz, 1H, ArH), 5.68 (d, J = 8.8 Hz, 1H, NH), 5.20 (d, J = 8.8 Hz, 1H, CH), 3.38 (s, 3H, CH3), 3.27 (s, 3H, CH3), 1.42 (s, 9H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 166.9, 164.7, 160.5, 154.9, 150.1, 125.7, 125.6, 124.4, 121.3, 114.4, 87.9, 81.5, 63.3, 29.2, 28.7, 28.1 ppm. HRMS (ESI): m/z calcd. for C18H2179BrN3O6 [M + H]+ 454.0608, found 454.0305, calcd. for C18H2181BrN3O6 [M + H]+ 456.0588, found 456.0287.
3.5. Procedure for the Scaled-Up Synthesis of Compound 3aa and 5aa
A 100 mL round-bottomed flask was charged with ortho-hydroxy α-aminosulfone 1a (3.10 g, 8.22 mmol), 2-bromo-1H-indene-1,3(2H)-dione 2a (1.22 g, 5.48 mmol), and 4-dimethylaminopyridine (DMAP) (668.6 mg, 5.48 mmol). The mixture was dissolved in 55 mL of DCE and stirred at room temperature while monitoring the reaction progress using TLC. After completion of the reaction, the resulting mixture was separated and purified via silica gel column chromatography using an eluent composed of ethyl acetate/petroleum ether in a ratio of 1:5. Finally, a product yield of 85% (1.70 g) for compound 3aa was achieved.
The Gram-scale preparation method of 5aa is analogous to the aforementioned 3aa.
3.6. Asymmetric Catalyzed Cyclization
The ortho-hydroxy α-aminosulfone 1a (56.6 mg, 0.15 mmol), 2-bromo-1,3-indandione 2a (22.3 mg, 0.10 mmol), trisodium phosphate (38 mg, equimolar amount), and catalyst C2 (6.5 mg, 0.5 mol%) were combined in a 5 mL glass vial. The mixture was dissolved in dichloromethane (1.0 mL) containing catalyst C2 at a concentration of 10 mol%. The reaction system was then stirred at room temperature until complete conversion of substrate 2a was confirmed by thin-layer chromatography analysis. Subsequently, the solvent was evaporated under vacuum rotation conditions, and the resulting product was purified using rapid column chromatography on silica gel with an eluent consisting of ethyl acetate/petroleum ether ratios ranging from 1:8 to 1:5, yielding catalytic product 3aa (30.3 mg, 83% yield, 17% ee).
4. Conclusions
In summary, a comprehensive series of benzofuran-fused indanone and barbiturate derivatives were synthesized with remarkable success through tandem cyclization reactions, featuring 2-bromo-1,3-indenedione, 5-bromo-1,3-dimethylbarbituric acid, and ortho-hydroxy α-aminosulfones as pivotal reactants. By meticulously optimizing the reaction conditions with DMAP as the base catalyst, DCE as the solvent, and gentle stirring at room temperature, we achieved exceptional yields of up to 95% and 85% for benzofuran-fused indanone and barbiturate derivatives, respectively. The extension of the substrate scope and the successful execution of Gram-scale syntheses underscored the robustness and versatility of this methodology, significantly expanding the benzofuran derivative library and facilitating further biological evaluations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29163725/s1, Copies of 1H and 13C NMR spectra of new compounds.
Author Contributions
R.-R.Z. wrote the preliminary manuscript; X.-Q.H. performed the experiments and acquired and analyzed the original data; D.-M.D. designed the research plan, supervised the experiments, modified all figures and schemes, analyzed and checked all the data, and revised this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article and Supplementary Materials.
Acknowledgments
We thank the Analysis and Testing Center of the Beijing Institute of Technology for the measurement of NMR and mass spectrometry.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Khanam, H.; Uzzaman, S. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ojika, M.; Suzuki, S.; Murakami, M.; Sakagamia, Y. Iantherans A and B, unique dimeric polybrominated benzofurans as Na,K-ATPase inhibitors from a marine sponge, Ianthella sp. Bioorg. Med. Chem. 2001, 9, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Mitsui, C.; Illies, L.; Sato, Y.; Nakamura, E. Synthesis and properties of 2,3,6,7-tetraarylbenzo [1,2-b:4,5-b‘] difurans as hole-transporting material. J. Am. Chem. Soc. 2007, 129, 11902–11903. [Google Scholar] [CrossRef]
- Patel, P.; Shakya, R.; Vishakha, V.A.; Kurmi, B.D.; Verma, S.K.; Gupta, G.D.; Rajak, H. Furan and benzofuran derivatives as privileged scaffolds as anticancer agents: SAR and docking studies (2010 to till date). J. Mol. Struct. 2024, 1299, 137098–137118. [Google Scholar] [CrossRef]
- Soni, J.N.; Soman, S.S. Synthesis and antimicrobial evaluation of amide derivatives of benzodifuran-2-carboxylic acid. Eur. J. Med. Chem. 2014, 75, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Meng, Q.Y.; Daniliuc, C.G.; Studer, A. Aroyl fluorides as bifunctional reagents for dearomatizing fluoroaroylation of benzofurans. J. Am. Chem. Soc. 2022, 144, 7072–7079. [Google Scholar] [CrossRef]
- Abbas, A.A.; Dawood, K.M. Anticancer therapeutic potential of benzofuran scaffolds. RSC Adv. 2023, 13, 11096–11120. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Hamidia, H.; Hajiabbas, P.; Amiria, T. Total synthesis of natural products containing benzofuran rings. RSC Adv. 2017, 7, 24470–24521. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Sun, L.Q.; Han, X.Y.; Wang, Y.J.; Xie, Z.S.; Xue, S.T.; Li, Z.R. Efficacy, mechanism, and structure–activity relationship of 6-methoxy benzofuran derivatives as a useful tool for senile osteoporosis. J. Med. Chem. 2023, 66, 1742–1760. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Lv, Z.S.; Feng, L.S.; Wang, Y.L.; Zhang, F.; Bai, L.Y.; Deng, J.L. Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur. J. Med. Chem. 2019, 162, 266–276. [Google Scholar] [CrossRef]
- Chen, Z.; Pitchakuntla, M.; Jia, Y.X. Synthetic approaches to natural products containing 2,3-dihydrobenzofuran skeleton. Nat. Prod. Rep. 2019, 36, 666. [Google Scholar] [CrossRef]
- Smith, D.T.; Vitaku, E.; Njardarson, J.T. Dearomatization approach to 2-trifluoromethylated benzofuran and dihydrobenzofuran products. Org. Lett. 2017, 19, 3508–3511. [Google Scholar] [CrossRef]
- Xiao, B.X.; Du, W.; Chen, Y.C. Asymmetric dearomatizative Diels–Alder reaction for the construction of hydrodibenzo[b,d]furan frameworks with tetrasubstituted stereogenic centers. Adv. Synth. Catal. 2017, 359, 1018–1027. [Google Scholar] [CrossRef]
- Danel, J.M.; Fleige, M.; Schluens, D.; Wollenburg, M.; Daniliuc, C.G.; Neugebauer, J.; Glorius, F. NHC-catalyzed enantioselective dearomatizing hydroacylation of benzofurans and benzothiophenes for the synthesis of spirocycles. ACS Catal. 2016, 6, 5735–5739. [Google Scholar]
- Dwarakanath, D.; Gaonkar, S.L. Advances in synthetic strategies and medicinal importance of benzofurans: A review. Asian J. Org. Chem. 2022, 11, e202200282. [Google Scholar] [CrossRef]
- Goyal, D.; Kaur, A.; Goyal, B. Benzofuran and indole: Promising scaffolds for drug development in alzheimer’s disease. Chem. Med. Chem. 2018, 13, 1275–1299. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Zou, Y.; Zhou, M.; Li, J.; Wu, C.; Tan, R.; Liao, Y.; Li, W.; Zheng, J. Metabolic activation of 3-aminodibenzofuran mediated by P450 enzymes and sulfotransferases. Toxicol. Lett. 2022, 360, 44–52. [Google Scholar] [CrossRef]
- Lauria, A.; Gentile, C.; Mingoia, F.; Piccionello, A.P.; Bartolotta, R.; Delisi, R.; Buscemi, S.; Martorana, A. Design synthesis, and biological evaluation of a new class of benzo[b]furan derivatives as antiproliferative agents, with in silico predicted antitubulin activity. Chem. Biol. Drug Des. 2018, 91, 39–49. [Google Scholar] [CrossRef]
- Cabrera-Pardo, J.R.; Fuentealba, J.; Gavilán, J.; Cajas, D.; Becerra, J.; Napiórkowska, M. Exploring the multi–target neuroprotective chemical space of benzofuran scaffolds: A new strategy in drug development for alzheimer’s disease. Front. Pharmacol. 2020, 10, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Chen, W.J.; Chen, F.Y.; Zhang, H.Y.; Xu, H.Y.; Zhou, Z.; Yi, W. Synthesis of 2-aminobenzofurans via base-mediated [3+2] annulation of N-phenoxy amides with gem-difluoroalkenes. Org. Chem. Front. 2021, 8, 4452–4458. [Google Scholar] [CrossRef]
- Li, L.S.; Li, C.Y.; Zhang, S.T.; Wang, X.R.; Fu, P.; Wang, Y. Catalytic asymmetric synthesis of 3,4′-piperidinoyl spirooxindoles via [3+3] annulation of 3-aminobenzofurans and isatin-derived enals. J. Org. Chem. 2024, 89, 5170–5180. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.X.; Zhang, Y.J.; Guo, Y.S.; Jin, Q.H.; Zhu, H.Y.; Xiu, H.S.; Liu, Z.H.; Wang, Y. The [4+1] cyclization reaction of 2-hydroxylimides and trimethylsulfoxonium iodide for the synthesis of 3-amino-2,3-dihydrobenzofurans. New J. Chem. 2022, 46, 18124–18127. [Google Scholar] [CrossRef]
- Ma, X.L.; Wang, Z.Q.; Liu, Z.R.; Li, Z. One-Pot Three-component synthesis of 2-methyl-3-aminobenzofurans using calcium carbide as a concise solid alkyne source. Chin. J. Chem. 2021, 39, 2990–2994. [Google Scholar] [CrossRef]
- Yang, W.L.; Sun, Z.T.; Zhang, J.; Li, Z.; Deng, W.P. Enantioselective synthesis of 3-amino-hydrobenzofuran-2,5-diones via Cu(i)-catalyzed intramolecular conjugate addition of imino esters. Org. Chem. Front. 2019, 6, 579–583. [Google Scholar] [CrossRef]
- Helgren, T.R.; Xu, L.L.; Sotelo, D.; Mehta, Y.R.; Korkmaz, M.A.; Pavlinov, I.; Aldrich, L.N. Microwave-assisted.; asymmetric synthesis of 3-amino-2,3-dihydrobenzofuran flavonoid derivatives from chalcones. Chem. Eur. J. 2018, 24, 4509–4514. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.S.; Li, R.D.; Wang, X.C. Copper-catalyzed asymmetric annulation reactions of carbenes with 2-iminyl- or 2-acyl-substituted phenols: Convenient access to enantioenriched 2,3-dihydrobenzofurans. Angew. Chem. Int. Ed. 2019, 58, 13885–13889. [Google Scholar] [CrossRef]
- Panday, A.K.; Ali, D.; Choudhury, L.H. Cs2CO3-mediated rapid room-temperature synthesis of 3-amino-2-aroyl benzofurans and their copper-catalyzed N-arylation reactions. ACS Omega 2020, 5, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, B.; Tavakol, H. CuI-catalyzed, one-pot synthesis of 3-aminobenzofurans in deep eutectic solvents. Appl. Organomet. Chem. 2021, 35, e6433. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Patil, S.A. Recent developments in biological activities of indanones. Eur. J. Med. Chem. 2017, 138, 182–198. [Google Scholar] [CrossRef]
- Turek, M.; Szczesna, D.; Koprowski, M.; Bałczewski, P. Synthesis of 1-indanones with a broad range of biological activity. Beilstein J. Org. Chem. 2017, 13, 451–494. [Google Scholar] [CrossRef]
- Yu, B.; Yu, D.Q.; Liu, H.M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Segovia, C.; Lebrêne, A.; Levacher, V.; Oudeyer, S.; Brière, J.F. Enantioselective catalytic transformations of barbituric acid derivatives. Catalysts 2019, 9, 131. [Google Scholar] [CrossRef]
- Bagherinejad, A.; Alizadeh, A. A review of the synthetic strategies toward spirobarbiturate-fused 3- to 7-membered rings. Org. Biomol. Chem. 2022, 20, 7188–7215. [Google Scholar] [CrossRef] [PubMed]
- Pigot, C.; Brunel, D.; Dumur, F. Indane-1,3-dione: From synthetic strategies to applications. Molecules. 2022, 27, 5976–6112. [Google Scholar] [CrossRef]
- Osman, E.E.A.; Hanafy, N.S.; George, R.F. Design and synthesis of some barbituric and 1,3-dimethylbarbituric acid derivatives: A non-classical scaffold for potential PARP1 inhibitors. Bioorg. Chem. 2020, 104, 104198. [Google Scholar]
- Sonoda, K.; Ujike, S.; Katayama, A.; Suzuki, N.; Kawaguchi, S.; Tsujita, T. Improving lipophilicity of 5-(1-acetyl-5-phenylpyrazolidin-3-ylidene)-1,3-dimethylbarbituric acid increases its efficacy to activate hypoxia-inducible factors. Bioorg. Med. Chem. 2022, 73, 117039. [Google Scholar] [CrossRef]
- Liu, M.M.; Yang, X.C.; Hua, Y.Z.; Chang, J.B.; Wang, M.C. Dinuclear zinc-catalyzed asymmetric tandem reaction of α-Hydroxy-1-indanone: Access to spiro [1-indanone-5,2′-γ-butyrolactones]. Org. Lett. 2019, 21, 7089–7093. [Google Scholar] [CrossRef]
- Schade, A.; Tchernook, I.; Bauer, M.; Oehlke, A.; Breugst, M.; Friedrich, J.; Spange, S. Kinetics of electrophilic alkylations of barbiturate and thiobarbiturate anions. J. Org. Chem. 2017, 82, 8476–8488. [Google Scholar] [CrossRef]
- Yan, X.B.; Shao, P.; Song, X.X.; Zhang, C.F.; Lu, C.; Liu, S.T.; Li, Y.L. Chemoselective syntheses of spirodihydrofuryl and spirocyclopropyl barbiturates via cascade reactions of barbiturate-based olefins and acetylacetone. Org. Biomol. Chem. 2019, 17, 2684–2690. [Google Scholar] [CrossRef]
- Alizadeh, A.; Bagherinejad, A.; Kayanian, J.; Vianello, R. An expedient metal-free cascade route to chromonyl diene scaffolds: Thermodynamic vs. kinetic control. RSC Adv. 2022, 12, 34946–34950. [Google Scholar] [CrossRef]
- Zhao, B.L.; Du, D.M. Organocatalytic cascade Michael/Michael reaction for the asymmetric synthesis of spirooxindoles containing five contiguous stereocenters. Chem. Commum. 2016, 52, 6162–6165. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hou, X.Q.; Li, B.Y.; Du, D.M. Organocatalytic remote asymmetric inverse-electron-demand Oxa-Diels-Alder reaction of allyl ketones with isatin-derived unsaturated keto esters. Adv. Synth. Catal. 2020, 362, 5728. [Google Scholar] [CrossRef]
- Hou, X.Q.; Lin, Y.; Du, D.M. Organocatalytic domino annulation of in situ generated tert-butyl 2-hydroxybenzylidenecarbamates with 2-isothiocyanato-1-indanones for synthesis of bridged and fused ring heterocycles. Org. Chem. Front. 2021, 8, 4183–4187. [Google Scholar] [CrossRef]
- Ren, Q.; Siau, W.Y.; Du, Z.Y.; Zhang, K.; Wang, J. Expeditious assembly of a 2-Amino-4H-chromene skeleton by using an enantioselective mannich intramolecular ring cyclization–tautomerization cascade sequence. Chem. Eur. J. 2011, 17, 7781–7785. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, S.; Khavasi, H.R.; Bazgir, A. Highly efficient construction of bisspirooxindoles containing vicinal spirocenters through an organocatalytic modified Feist–Bénary reaction. Chem. Eur. J. 2013, 19, 12553–12559. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Tajbakhsh, M.; Mohadjerani, M.; Lasemi, Z. Ethylenebis(N-methylimidazolium) ditribromide (EBMIDTB): An efficient reagent for the monobromination of 1,3-diketones and β-ketoesters. Monatsh. Chem. 2009, 140, 57–60. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Highly enantioselective Michael addition of nitroalkanes to chalcones using chiral squaramides as hydrogen bonding organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Du, D.M. Chiral squaramide-catalyzed highly enantioselective Michael addition of 2-hydroxy-1,4-naphthoquinones to nitroalkenes. Adv. Synth. Catal. 2011, 353, 1241–1246. [Google Scholar] [CrossRef]
- Anderson, J.C.; Koovits, P.J. An enantioselective tandem reduction/nitro-Mannich reaction of nitroalkenes using a simple thiourea organocatalyst. Chem. Sci. 2013, 4, 2897–2901. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).