Synergistic Antifungal Effect and In Vivo Toxicity of a Monoterpene Isoespintanol Obtained from Oxandra xylopioides Diels
Abstract
1. Introduction
2. Results
2.1. Obtaining and Identification of Isoespintanol
2.2. Isoespintanol Action in Combination with Commercial Antifungals
2.3. ISO In Vivo Toxicity Experiments
2.3.1. Animal Weight
2.3.2. Global Cell Count
2.3.3. Differential Cell Counting
2.3.4. GOT and GPT Dosage
2.3.5. Measurement of Cytokines IFN-γ and TNF
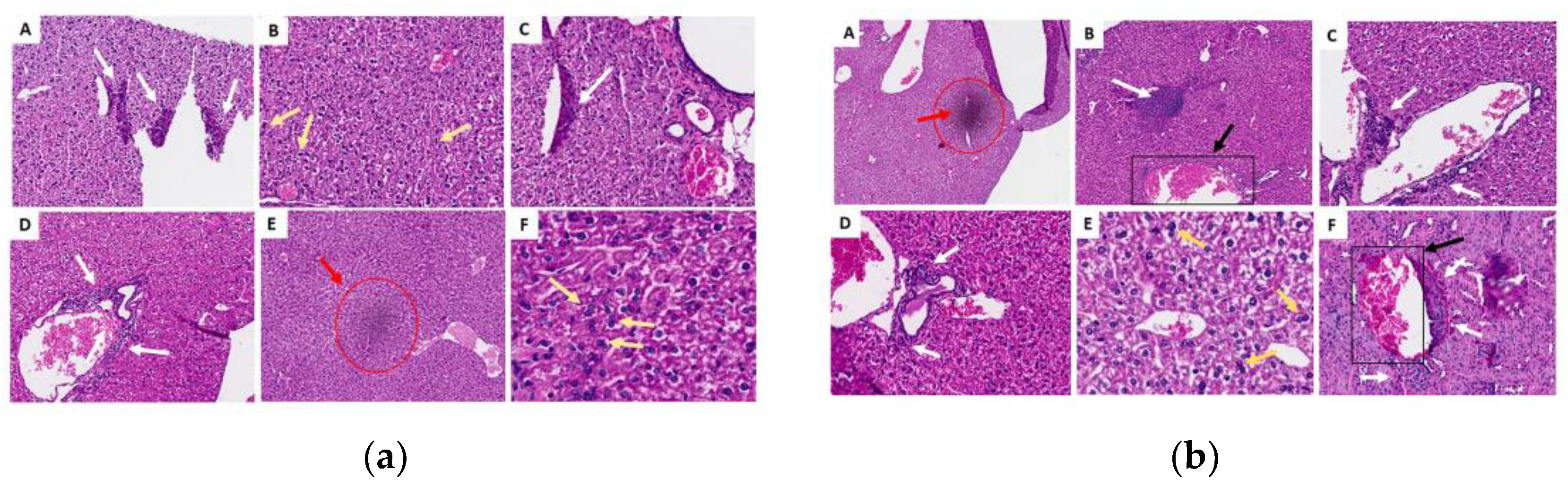
2.3.6. Histopathological Analysis of Organs (Lung, Liver and Kidney)
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Obtaining and Identification of Isoespintanol
4.3. Strains
4.4. Isoespintanol Action in Combination with Commercial Antifungals
4.5. Preparation of Isoespintanol for Toxicity Experiments
4.6. Animals
4.7. Exposure to Isoespintanol and Experimental Groups
4.8. Animal Weight Measurement
4.9. Euthanasia, Blood Cell Collection and Counting, PCL and BAL
4.10. Histological Evaluation of Lungs, Livers and Kidneys
4.11. GOT and GPT Dosage
4.12. Measurement of IFN-γ and TNF Cytokines in Plasma, BAL and PCL
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avato, P. Editorial to the Special Issue “Natural products and drug discovery”. Molecules 2020, 25, 1128. [Google Scholar] [CrossRef] [PubMed]
- Naman, C.B.; Benatrehina, P.A.; Kinghorn, A.D. Pharmaceuticals, plant drugs. In Breeding Genetics and Biotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 93–99. [Google Scholar] [CrossRef]
- Aylate, A.; Agize, M.; Ekero, D.; Kiros, A.; Ayledo, G.; Gendiche, K. In-vitro and in-vivo antibacterial activities of Croton macrostachyus methanol extract against E. coli and S. aureus. Adv. Anim. Vet. Sci. 2017, 5, 107–114. [Google Scholar]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Li, X.-J.; Zhang, H.-Y. Natural products and drug discovery can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Pappas, P.G. Antifungal combination therapy clinical potential. Drugs 2005, 65, 1461–1480. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Fu, Y.; Ibrahim, A.S.; Mortara, L.A.; Shafiq, M.C.; Edwards, J.E.; Criddle, R.S. In vitro determination of optimal antifungal combinations against Cryptococcus neoformans and Candida albicans. Antimicrob. Agents Chemother. 1995, 39, 2459–2465. [Google Scholar] [CrossRef][Green Version]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Amber, K.; Aijaz, A.; Immaculata, X.; Luqman, K.A.; Nikhat, M. Anticandidal effect of Ocimum sanctum essential oil and its synergy with fluconazole and ketoconazole. Phytomedicine 2010, 17, 921–925. [Google Scholar] [CrossRef]
- Giordani, R.; Regli, P.; Kaloustian, J.; Mikaïl, C.; Abou, L.; Portugal, H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother. Res. 2004, 18, 990–995. [Google Scholar] [CrossRef]
- de Castro, R.D.; de Souza, T.M.P.A.; Bezerra, L.M.D.; Ferreira, G.L.S.; de Brito Costa, E.M.M.; Cavalcanti, A.L. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement. Altern. Med. 2015, 15, 417. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Manzoor, N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. Sci. 2013, 48, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Rhee, M.S. Short-term antifungal treatments of caprylic acid with carvacrol or thymol induce synergistic 6-Log reduction of pathogenic Candida albicans by cell membrane disruption and efflux pump inhibition. Cell. Physiol. Biochem. 2019, 53, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the antifungal activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) essential oil and its synergistic interaction with azoles. Molecules 2019, 24, 3148. [Google Scholar] [CrossRef] [PubMed]
- Rojano, B.; Saez, J.; Schinella, G.; Quijano, J.; Vélez, E.; Gil, A.; Notario, R. Experimental and theoretical determination of the antioxidant properties of isoespintanol (2-Isopropyl-3,6-Dimethoxy-5-Methylphenol). J. Mol. Struct. 2008, 877, 1–6. [Google Scholar] [CrossRef]
- Rojano, B.; Pérez, E.; Figadère, B.; Martin, M.T.; Recio, M.C.; Giner, R.; Ríos, J.L.; Schinella, G.; Sáez, J. Constituents of Oxandra Cf. xylopioides with anti-inflammatory activity. J. Nat. Prod. 2007, 70, 835–838. [Google Scholar] [CrossRef]
- Gavilánez Buñay, T.C.; Colareda, G.A.; Ragone, M.I.; Bonilla, M.; Rojano, B.A.; Schinella, G.R.; Consolini, A.E. Intestinal, urinary and uterine antispasmodic effects of isoespintanol, metabolite from Oxandra xylopioides leaves. Phytomedicine 2018, 51, 20–28. [Google Scholar] [CrossRef]
- Rinaldi, G.J.; Rojano, B.; Schinella, G.; Mosca, S.M. Participation of NO in the vasodilatory action of isoespintanol. Vitae 2019, 26, 78–83. [Google Scholar] [CrossRef]
- González Arbeláez, L.; Ciocci Pardo, A.; Fantinelli, J.C.; Rojano, B.; Schinella, G.; Mosca, S.M. Isoespintanol, a monoterpene isolated from Oxandra Cf xylopioides, ameliorates the myocardial ischemia-reperfusion injury by AKT/PKCε/ENOS-Dependent pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 629–638. [Google Scholar] [CrossRef]
- Usuga, A.; Tejera, I.; Gómez, J.; Restrepo, O.; Rojano, B.; Restrepo, G. Cryoprotective effects of ergothioneine and isoespintanol on canine semen. Animals 2021, 11, 2757. [Google Scholar] [CrossRef]
- Di Sarli Gutiérrez, L.; Castro, M.C.; Farromeque Vásquez, S.; Villagarcía, H.G.; González Arbeláez, L.; Rojano, B.; Schinella, G.; Maiztegui, B.; Francini, F. Protective effect of monoterpene isoespintanol in a rat model of prediabetes induced by fructose. Pharmaceuticals 2024, 17, 47. [Google Scholar] [CrossRef]
- Contreras Martínez, O.I.; Angulo Ortíz, A.; Santafé Patiño, G. Antibacterial screening of isoespintanol, an aromatic monoterpene isolated from Oxandra xylopioides Diels. Molecules 2022, 27, 80004. [Google Scholar] [CrossRef] [PubMed]
- Contreras Martínez, O.I.; Angulo Ortíz, A.; Santafé Patiño, G. Antifungal potential of isoespintanol extracted from Oxandra xylopioides Diels (Annonaceae) against intrahospital isolations of Candida spp. Heliyon 2022, 8, 11110. [Google Scholar] [CrossRef]
- Contreras, O.; Angulo, A.; Santafé, G. Mechanism of antifungal action of monoterpene isoespintanol against clinical isolates of Candida tropicalis. Molecules 2022, 27, 5808. [Google Scholar] [CrossRef]
- Contreras Martínez, O.I.; Angulo Ortíz, A.; Santafé Patiño, G.; Peñata-Taborda, A.; Berrio Soto, R. Isoespintanol antifungal activity involves mitochondrial dysfunction, inhibition of biofilm formation, and damage to cell wall integrity in Candida tropicalis. Int. J. Mol. Sci. 2023, 24, 10187. [Google Scholar] [CrossRef]
- Contreras-Martínez, O.I.; Angulo-Ortíz, A.; Santafé-Patiño, G.; Aviña-Padilla, K.; Velasco-Pareja, M.C.; Yasnot, M.F. Transcriptional reprogramming of Candida tropicalis in response to isoespintanol treatment. J. Fungi 2023, 9, 1199. [Google Scholar] [CrossRef]
- Vennin, C.; Cattaneo, C.M.; Bosch, L.; Vegna, S.; Ma, X.; Damstra, H.G.J.; Martinovic, M.; Tsouri, E.; Ilic, M.; Azarang, L.; et al. Taxanes trigger cancer cell killing in vivo by inducing non-canonical T cell cytotoxicity. Cancer Cell 2023, 41, 1170–1185.e12. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Mechanism of candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef]
- Černáková, L.; Light, C.; Salehi, B.; Rogel-Castillo, C.; Victoriano, M.; Martorell, M.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Novel therapies for biofilm-based Candida spp. infections. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1214, pp. 93–123. [Google Scholar] [CrossRef]
- Salci, T.P.; Negri, M.; Abadio, A.K.R.; Svidzinski, T.I.E.; Kioshima, É.S. Targeting Candida spp. to develop antifungal agents. Drug Discov. Today 2018, 23, 802–814. [Google Scholar] [CrossRef]
- Donkor, M.N.; Donkor, A.M.; Mosobil, R. Combination therapy: Synergism among three plant extracts against selected pathogens. BMC Res. Notes 2023, 16, 83. [Google Scholar] [CrossRef]
- Zapata-Zapata, C.; Rojas-López, M.; García, L.T.; Quintero, W.; Terrón, M.C.; Luque, D.; Mesa-Arango, A.C. Lippia origanoides essential oil or thymol in combination with fluconazole produces damage to cells and reverses the azole-resistant phenotype of a Candida tropicalis strain. J. Fungi 2023, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; MacDougall, C.; Ostrosky-Zeichner, L.; Perfect, J.R.; Rex, J.H. Combination antifungal therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. [Google Scholar] [CrossRef]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef]
- Lupetti, A.; Danesi, R.; Campa, M.; Del Tacca, M.; Steven, K. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 2002, 8, 76–81. [Google Scholar] [CrossRef]
- Guimarães, M.; Donadi, E. Immune cellular response to HPV: Current concepts. Braz. J. Infect. Dis. 2004, 8, 1–9. Available online: https://europepmc.org/article/med/15137933 (accessed on 4 June 2024).
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Hosseini Farash, B.R.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In vitro and in vivo effectiveness of carvacrol, thymol and linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A. Toxicity of selected monoterpenes and essential oils rich in these compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef] [PubMed]
- Donadu, M.G.; Peralta-ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; et al. Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant Candida strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef]
- Wadhwa, K.; Kaur, H.; Kapoor, N.; Brogi, S. Identification of Sesamin from Sesamum indicum as a potent antifungal agent using an integrated in silico and biological screening platform. Molecules 2023, 28, 4658. [Google Scholar] [CrossRef]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A Novel interpretation of the fractional inhibitory concentration index: The case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef] [PubMed]









| C. tropicalis | MIC90 Single | MIC90 in Combination | FIC Indices | Effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FLZ | AFB | CASP | ISO | ISO-FLZ | ISO-AFB | ISO-CASP | ISO-FLZ | ISO-AFB | ISO-CASP | ISO-FLZ | ISO-AFB | ISO-CASP | |
| 107 | 75.4 | 2.0 | 0.5 | 362.1 | 45.5–5.8 | 195.3–0.8 | 410.5–0.4 | 0.2 | 0.9 | 2.0 | Sng | Sng | S.I. |
| 98 | 8.0 | 2.1 | 0.5 | 304.1 | 44.8–5.7 | 439.4–1.8 | 339.2–0.3 | 0.9 | 2.3 | 1.8 | Sng | Ant | S.I. |
| 92 | 66.8 | 1.7 | 0.7 | 222.1 | 50.5–6.5 | 165.4–0.7 | 404.1–0.4 | 0.3 | 1.1 | 2.4 | Sng | S.I. | Ant |
| 84 | 358.3 | 2.4 | 0.5 | 416.0 | 394.1–50.5 | 483.3–1.9 | 511.9–0.5 | 1.1 | 2.0 | 2.4 | S.I. | S.I. | Ant |
| 81 | 529.3 | 1.6 | 0.5 | 299.8 | 141.7–18.1 | 237.6–1.0 | 341.9–0.3 | 0.5 | 1.4 | 1.8 | Sng | S.I. | S.I. |
| 74 | 428.5 | 2.5 | 0.5 | 360.1 | 24.0–3.1 | 121.4–0.5 | 175.3–0.2 | 0.1 | 0.5 | 0.9 | Sng | Sng | Sng |
| 03 | 410.0 | 2.0 | 0.6 | 332.7 | 44.1–5.6 | 347.5–1.4 | 316.6–0.3 | 0.1 | 1.7 | 1.5 | Sng | S.I. | S.I. |
| Days/Groups | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| 0 | 20.88 | 20.48 | 22.36 | 20.5 | 20.8 | 21.36 |
| 14 | 22.12 | 22.09 | 22.42 | 21.34 | 22.12 | 21.33 |
| Days/Groups | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| 0 | 21.39 | 20.6 | 21.16 | 19.94 | 21.45 | 22.85 |
| 14 | 21.99 | 21.66 | 22.79 | 21.05 | 23.18 | 23.66 |
| 28 | 22.72 | 22.45 | 23.58 | 21.52 | 24.31 | 24.08 |
| 42 | 22.51 | 22.91 | 23.51 | 21.81 | 24.66 | 23.77 |
| 56 | 23.21 | 23.19 | 24.70 | 21.99 | 25.10 | 24.37 |
| 70 | 24.13 | 23.47 | 24.87 | 21.79 | 25.35 | 24.83 |
| 84 | 24.09 | 21.76 | 25.17 | 22.67 | 25.71 | 25.11 |
| Experimental Group | Route of Administration | Exposure | Volume/Animal |
|---|---|---|---|
| G1: CTRL + water | Oral | Water | 100 µL |
| G2: CTRL + oil | Oral | Oil | 100 µL |
| G3:25 mg/mL | Oral | ISO 25 mg/mL | 100 µL |
| G4: 50 mg/mL | Oral | ISO 50 mg/mL | 100 µL |
| G5: 100 mg/mL | Oral | ISO 100 mg/mL | 100 µL |
| G6: 200 mg/mL | Oral | ISO 200 mg/mL | 100 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Martínez, O.I.; Angulo-Ortíz, A.; Santafé Patiño, G.; Sierra Martinez, J.; Berrio Soto, R.; de Almeida Rodolpho, J.M.; de Godoy, K.F.; de Freitas Aníbal, F.; de Lima Fragelli, B.D. Synergistic Antifungal Effect and In Vivo Toxicity of a Monoterpene Isoespintanol Obtained from Oxandra xylopioides Diels. Molecules 2024, 29, 4417. https://doi.org/10.3390/molecules29184417
Contreras-Martínez OI, Angulo-Ortíz A, Santafé Patiño G, Sierra Martinez J, Berrio Soto R, de Almeida Rodolpho JM, de Godoy KF, de Freitas Aníbal F, de Lima Fragelli BD. Synergistic Antifungal Effect and In Vivo Toxicity of a Monoterpene Isoespintanol Obtained from Oxandra xylopioides Diels. Molecules. 2024; 29(18):4417. https://doi.org/10.3390/molecules29184417
Chicago/Turabian StyleContreras-Martínez, Orfa Inés, Alberto Angulo-Ortíz, Gilmar Santafé Patiño, Jesus Sierra Martinez, Ricardo Berrio Soto, Joice Margareth de Almeida Rodolpho, Krissia Franco de Godoy, Fernanda de Freitas Aníbal, and Bruna Dias de Lima Fragelli. 2024. "Synergistic Antifungal Effect and In Vivo Toxicity of a Monoterpene Isoespintanol Obtained from Oxandra xylopioides Diels" Molecules 29, no. 18: 4417. https://doi.org/10.3390/molecules29184417
APA StyleContreras-Martínez, O. I., Angulo-Ortíz, A., Santafé Patiño, G., Sierra Martinez, J., Berrio Soto, R., de Almeida Rodolpho, J. M., de Godoy, K. F., de Freitas Aníbal, F., & de Lima Fragelli, B. D. (2024). Synergistic Antifungal Effect and In Vivo Toxicity of a Monoterpene Isoespintanol Obtained from Oxandra xylopioides Diels. Molecules, 29(18), 4417. https://doi.org/10.3390/molecules29184417









