Molecular Dynamics Simulation of Lipid Nanoparticles Encapsulating mRNA
Abstract
1. Introduction
2. Results
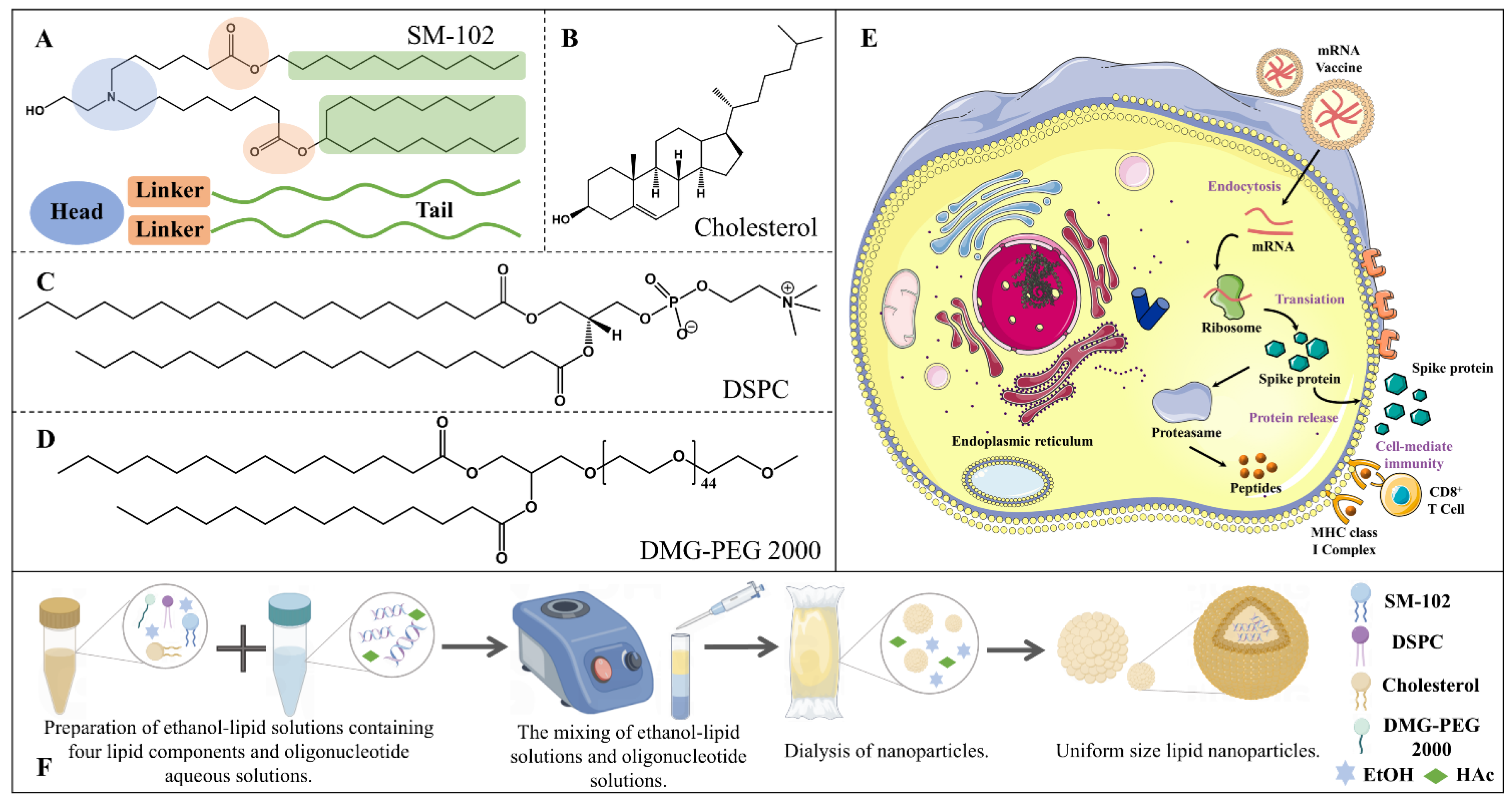
2.1. Construction of the Investigated Systems
2.2. Convergence of MD Trajectories
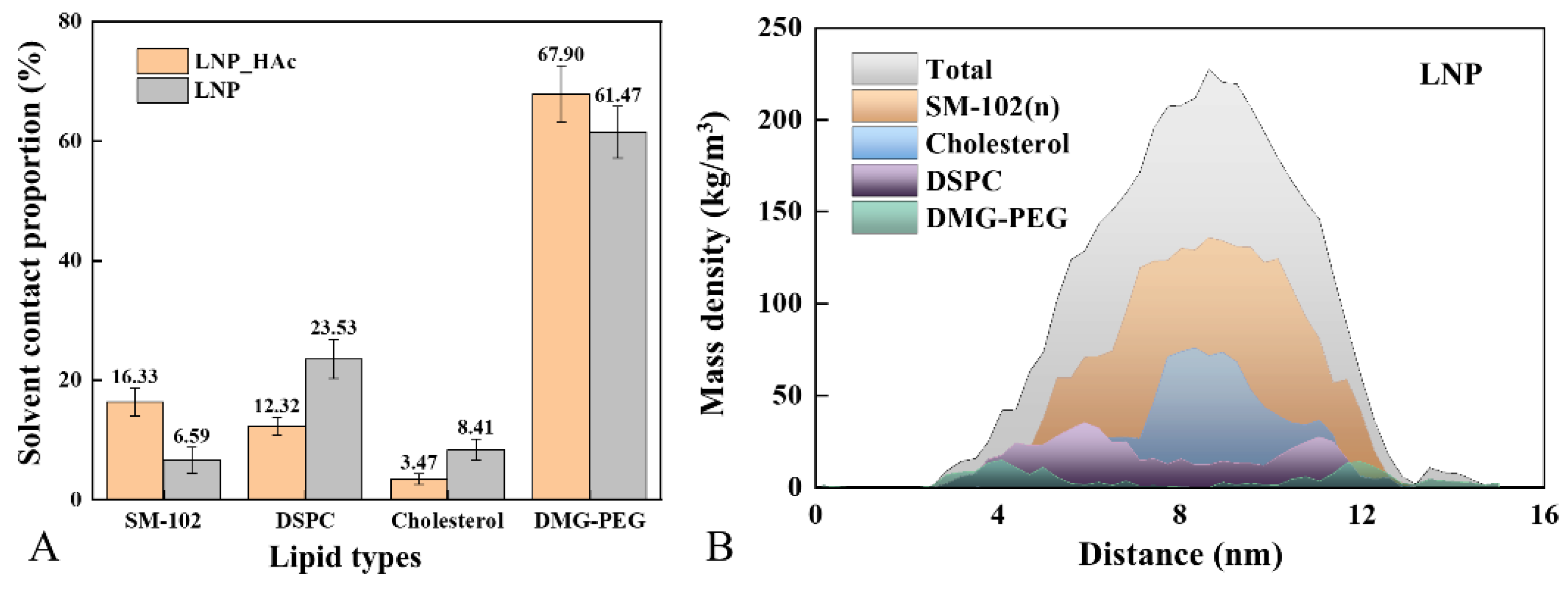
2.3. Macroscopic Characterization of System Dynamics
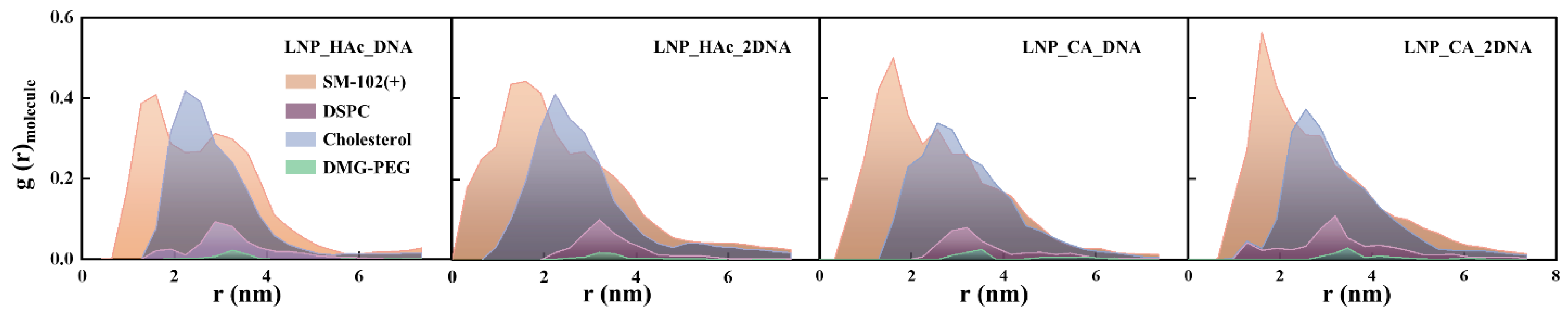
2.4. Impact of pH on Lipid Cluster Structure
2.5. The Effect of Introducing Different Acids on the Structure of Lipid Clusters
2.6. Radial Distribution of Solvent
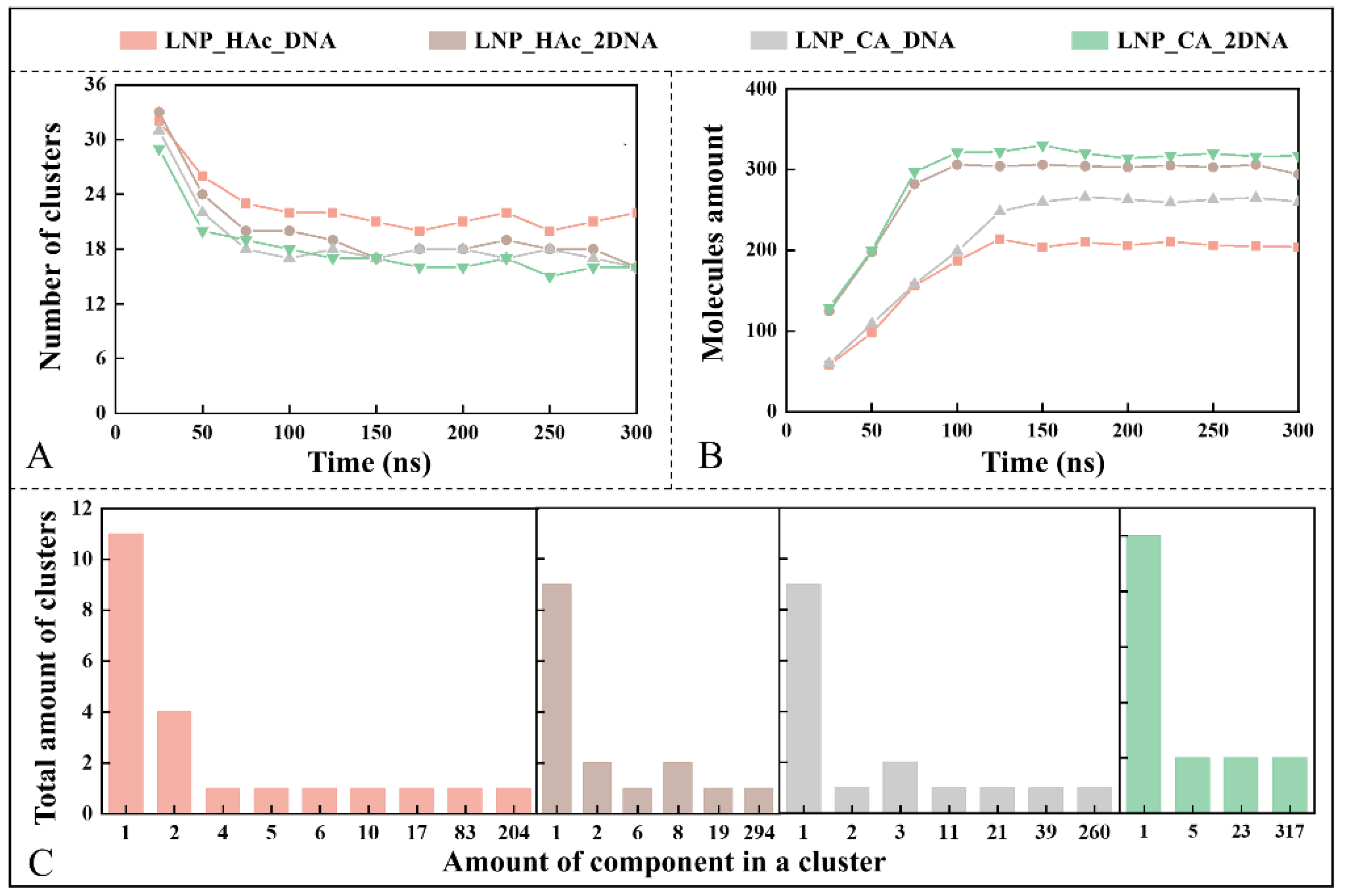
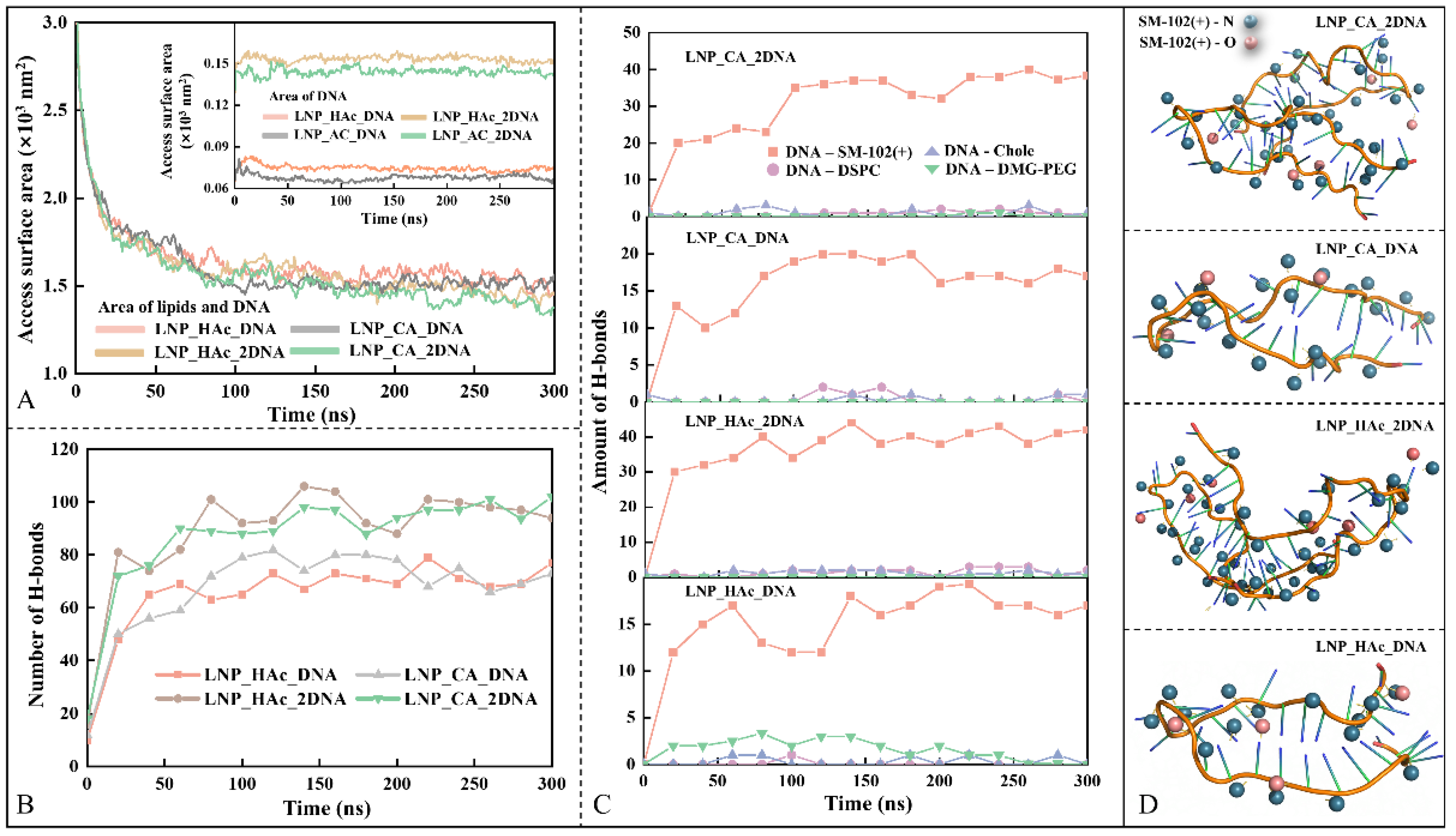
2.7. Dynamic Process of mRNA Encapsulation
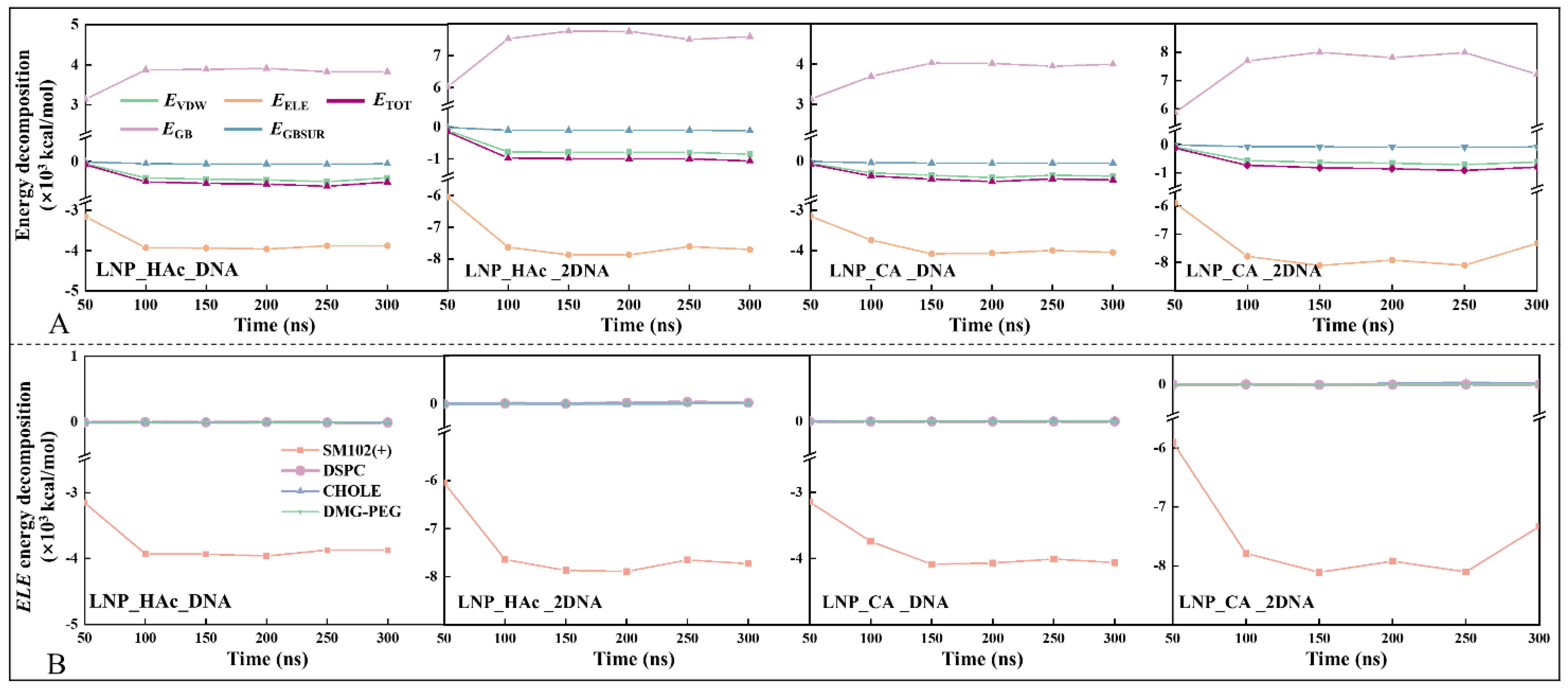
2.8. Driving Force of mRNA Encapsulation
2.9. Stability Factor of mRNA–LNPs
3. Discussion
4. Calculation Methods
4.1. System Preparation
4.2. Molecular Dynamics Simulation
4.3. Spatial Density Distribution
4.4. Radial Distribution Function
4.5. Binding Free Energy Calculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.J.; Kulkarni, J.A.; van der Meel, R.; Cullis, P.R.; Vader, P.; Schiffelers, R.M. State-of-the-art design and rapid-mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods 2018, 2, 1700375. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, F.; Persano, S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem. 2020, 8, 589959. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2021, 55, 2–12. [Google Scholar] [CrossRef]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic lipids for gene delivery: Many players, one goal. Chem. Phys. Lipids 2021, 235, 105032. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mayta, R.; Murdoch, T.J.; Warzecha, C.C.; Billingsley, M.M.; Shepherd, S.J.; Gong, N.; Wang, L.; Wilson, J.M.; Lee, D. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater. Sci. 2021, 9, 1449–1463. [Google Scholar] [CrossRef]
- Kon, E.; Elia, U.; Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.S.; Schlegel, A.; Maxeiner, K.; Weber, B.; Barz, M.; Schroer, M.A.; Blanchet, C.E.; Svergun, D.I.; Ramishetti, S.; Peer, D. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 2020, 3, 10634–10645. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Strategy Update; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. MRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef] [PubMed]
- Tatematsu, M.; Funami, K.; Seya, T.; Matsumoto, M. Extracellular RNA sensing by pattern recognition receptors. J. Innate Immun. 2018, 10, 398–406. [Google Scholar] [CrossRef] [PubMed]
- De Beuckelaer, A.; Pollard, C.; Van Lint, S.; Roose, K.; Van Hoecke, L.; Naessens, T.; Udhayakumar, V.K.; Smet, M.; Sanders, N.; Lienenklaus, S. Type I interferons interfere with the capacity of mRNA lipoplex vaccines to elicit cytolytic T cell responses. Mol. Ther. 2016, 24, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.; Rejman, J.; De Haes, W.; Verrier, B.; Van Gulck, E.; Naessens, T.; De Smedt, S.; Bogaert, P.; Grooten, J.; Vanham, G. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Karikó, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J. MRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef]
- Gürbüz, E.; Keresteci, B.; Günneç, C.; Baysal, G. Encapsulation Applications and Production Techniques in the Food Industry. J. Nutr. Health Sci. 2020, 7, 106. [Google Scholar]
- Klauer, A.A.; van Hoof, A. Degradation of mRNAs that lack a stop codon: A decade of nonstop progress. Wiley Interdiscip. Rev. RNA 2012, 3, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Pogocki, D.; Schöneich, C. Chemical stability of nucleic acid–derived drugs. J. Pharm. Sci. 2000, 89, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Colotte, M.; Luis, A.; Tuffet, S.; Bonnet, J. An efficient method for long-term room temperature storage of RNA. Eur. J. Hum. Genet. 2014, 22, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. MRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Ziller, A.; Nogueira, S.S.; Hühn, E.; Funari, S.S.; Brezesinski, G.; Hartmann, H.; Sahin, U.; Haas, H.; Langguth, P. Incorporation of mRNA in lamellar lipid matrices for parenteral administration. Mol. Pharm. 2018, 15, 642–651. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; Van Der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef]
- Trollmann, M.F.; Böckmann, R.A. MRNA lipid nanoparticle phase transition. Biophys. J. 2022, 121, 3927–3939. [Google Scholar] [CrossRef]
- Li, M.; Jia, L.; Xie, Y.; Ma, W.; Yan, Z.; Liu, F.; Deng, J.; Zhu, A.; Siwei, X.; Su, W. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. NPJ Vaccines 2023, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Hussmann, G.P.; Peterson, N.C.; Santos, J.L.; Tuesca, A.D. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int. J. Pharm. 2022, 611, 121314. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Melamed, J.R.; Chaudhary, N.; Lamson, N.G.; Ball, R.L.; Yerneni, S.S.; Whitehead, K.A. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett. 2020, 20, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Kauffman, K.J.; Fenton, O.S.; Sadtler, K.; Patel, A.K.; Heartlein, M.W.; Derosa, F.; Anderson, D.G. Optimization of a degradable polymer–lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 2018, 18, 6449–6454. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.Y.; Leung, J.; Zhang, Y.; Strong, C.; Basha, G.; Momeni, A.; Chen, Y.; Jan, E.; Abdolahzadeh, A.; Wang, X. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv. Mater. 2023, 35, 2303370. [Google Scholar] [CrossRef]
- Duan, H.; Hu, K.; Zheng, D.; Cheng, Y.; Zhang, Z.; Wang, Y.; Liang, L.; Hu, J.; Luo, T. Recognition and release of uridine and hCNT3: From multivariate interactions to molecular design. Int. J. Biol. Macromol. 2022, 223, 1562–1577. [Google Scholar] [CrossRef]
- Zuo, K.; Liang, L.; Du, W.; Sun, X.; Liu, W.; Gou, X.; Wan, H.; Hu, J. 3D-QSAR, molecular docking and molecular dynamics simulation of Pseudomonas aeruginosa LpxC inhibitors. Int. J. Mol. Sci. 2017, 18, 761. [Google Scholar] [CrossRef]
- Rissanou, A.N.; Karatasos, K. Effects of the structure of lipid-based agents in their complexation with a single stranded mRNA fragment: A computational study. Soft Matter 2022, 18, 6229–6245. [Google Scholar] [CrossRef]
- Rissanou, A.N.; Ouranidis, A.; Karatasos, K. Complexation of single stranded RNA with an ionizable lipid: An all-atom molecular dynamics simulation study. Soft Matter 2020, 16, 6993–7005. [Google Scholar] [CrossRef]
- Gao, H.; Kan, S.; Ye, Z.; Feng, Y.; Jin, L.; Zhang, X.; Deng, J.; Chan, G.; Hu, Y.; Wang, Y. Development of in silico methodology for siRNA lipid nanoparticle formulations. Chem. Eng. J. 2022, 442, 136310. [Google Scholar] [CrossRef]
- Jürgens, D.C.; Deßloch, L.; Porras-Gonzalez, D.; Winkeljann, J.; Zielinski, S.; Munschauer, M.; Hörner, A.L.; Burgstaller, G.; Winkeljann, B.; Merkel, O.M. Lab-scale siRNA and mRNA LNP manufacturing by various microfluidic mixing techniques—An evaluation of particle properties and efficiency. OpenNano 2023, 12, 100161. [Google Scholar] [CrossRef]
- Paloncýová, M.; Čechová, P.; Šrejber, M.; Kührová, P.; Otyepka, M. Role of ionizable lipids in SARS-CoV-2 vaccines as revealed by molecular dynamics simulations: From membrane structure to interaction with mRNA fragments. J. Phys. Chem. Lett. 2021, 12, 11199–11205. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Homeyer, N.; Gohlke, H. Free energy calculations by the molecular mechanics Poisson− Boltzmann surface area method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef]
- Wu, Z.; Duan, H.; Cheng, Y.; Guo, D.; Peng, L.; Hu, Y.; Hu, J.; Luo, T. A novel ligand swing-mediated active site coordination change of human apurinic/apyrimidinic endonuclease 1: A potential cytotoxic mechanism of nickel ion in the base excision repair. Chem. Phys. 2022, 555, 111456. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Xiao, Y. 3dDNA: A computational method of building DNA 3D structures. Molecules 2022, 27, 5936. [Google Scholar] [CrossRef]
- Denning, E.J.; Priyakumar, U.D.; Nilsson, L.; Mackerell, A.D., Jr. Impact of 2′-hydroxyl sampling on the conformational properties of RNA: Update of the CHARMM all-atom additive force field for RNA. J. Comput. Chem. 2011, 32, 1929–1943. [Google Scholar] [CrossRef]
- Xu, Y.; Vanommeslaeghe, K.; Aleksandrov, A.; Mackerell, A.D., Jr.; Nilsson, L. Additive CHARMM force field for naturally occurring modified ribonucleotides. J. Comput. Chem. 2016, 37, 896–912. [Google Scholar] [CrossRef]
- Mu, Z.; Tan, Y.; Zhang, B.; Liu, J.; Shi, Y. Ab initio predictions for 3D structure and stability of single-and double-stranded DNAs in ion solutions. PLoS Comput. Biol. 2022, 18, e1010501. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, C.; Sun, X.; Yang, J.; Li, X.; Yang, G.; Jin, J.; Shi, X. Circular RNA circMYLK4 shifts energy metabolism from glycolysis to OXPHOS by binding to the calcium channel auxiliary subunit CACNA2D2. J. Biol. Chem. 2024, 300, 107426. [Google Scholar] [CrossRef] [PubMed]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; Mackerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Klauda, J.B. Update of the CHARMM36 united atom chain model for hydrocarbons and phospholipids. J. Phys. Chem. B 2020, 124, 6797–6812. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef] [PubMed]
- Berensen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.; Fraaije, J.G. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Ponti, A. Simulation of magnetic resonance static powder lineshapes: A quantitative assessment of spherical codes. J. Magn. Reson. 1999, 138, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Hogues, H.; Sulea, T.; Purisima, E.O. Exhaustive docking and solvated interaction energy scoring: Lessons learned from the SAMPL4 challenge. J. Comput. Aided Mol. Des. 2014, 28, 417–427. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Duan, H.; Luo, Q.; Liu, W.; Liang, L.; Wan, H.; Chang, S.; Hu, J.; Shi, H. Inhibition Mechanism of Indoleamine 2, 3-Dioxygenase 1 (IDO1) by Amidoxime Derivatives and its Revelation in Drug Design: Comparative Molecular Dynamics Simulations. Front. Mol. Biosci. 2020, 6, 164. [Google Scholar] [CrossRef]



| Components | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systems | SM-102(+) | SM-102(n.) | DSPC | Cholesterol | DMG-PEG | HAc | Ac− | CA | CA− | EtOH | DNA | N/P |
| LNP | 0 | 144 | 30 | 111 | 4 | 0 | 0 | 0 | 0 | 31,408 | 0 | 0 |
| LNP_HAc | 144 | 0 | 30 | 111 | 4 | 33 | 2 | 0 | 0 | 31,408 | 0 | 0 |
| LNP_HAc_DNA | 174 | 0 | 35 | 134 | 5 | 33 | 2 | 0 | 0 | 31,408 | 1 | 6:1 |
| LNP_HAc_2DNA | 174 | 0 | 35 | 134 | 5 | 33 | 2 | 0 | 0 | 31,408 | 2 | 3:1 |
| LNP_CA_DNA | 174 | 0 | 35 | 134 | 5 | 0 | 0 | 12 | 22 | 31,408 | 1 | 6:1 |
| LNP_CA_2DNA | 174 | 0 | 35 | 134 | 5 | 0 | 0 | 12 | 22 | 31,408 | 2 | 3:1 |
| Systems | Components | Rg (nm) | Volume (nm3) | SASA (nm2) | Density (g/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SM-102(+) | SM-102(n.) | DSPC | Cholesterol | DMG-PEG | DNA | |||||
| LNP | 0 | 144 | 30 | 111 | 4 | 0 | 3.42 | 238.08 | 609.91 | 717.50 |
| LNP_HAc | 144 | 0 | 30 | 111 | 4 | 0 | 5.10 | 290.99 | 874.40 | 795.04 |
| LNP_HAc_DNA | 174 | 0 | 35 | 134 | 5 | 1 | 3.88 | 311.04 | 817.75 | 703.20 |
| LNP_HAc_2DNA | 174 | 0 | 35 | 134 | 5 | 2 | 4.34 | 446.93 | 1112.78 | 715.76 |
| LNP_CA_DNA | 174 | 0 | 35 | 134 | 5 | 1 | 4.25 | 402.03 | 1091.92 | 687.03 |
| LNP_CA_2DNA | 174 | 0 | 35 | 134 | 5 | 2 | 4.72 | 479.61 | 1285.55 | 700.02 |
| Aspects | Literature | Comparison and Differences |
|---|---|---|
| Structure of mRNA–LNPs | Schoenmaker, L., et al., 2021 [26]. | The presence of internal solvent cavities |
| N/P | Gao, H., et al., 2022 [42]. Jürgens, D.C., et al., 2023 [43]. | The effect of N/P ratio on cluster fusibility |
| Types of acidic environments | Cheng, M.H.Y., et al., 2023 [37]. | It was consistently found that citric acid is more conducive than acetic acid to the formation of larger lipid nanoparticles |
| Mechanism of mRNA encapsulation | Trollmann, M.F., et al., 2022 [31]. | It was consistently found that the key to the mRNA encapsulation process lies in the redistribution of ionizable lipids in response to pH changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Cheng, D.; Luo, W.; Hu, D.; Yang, T.; Hu, K.; Liang, L.; Liu, W.; Hu, J. Molecular Dynamics Simulation of Lipid Nanoparticles Encapsulating mRNA. Molecules 2024, 29, 4409. https://doi.org/10.3390/molecules29184409
Zhang Z, Cheng D, Luo W, Hu D, Yang T, Hu K, Liang L, Liu W, Hu J. Molecular Dynamics Simulation of Lipid Nanoparticles Encapsulating mRNA. Molecules. 2024; 29(18):4409. https://doi.org/10.3390/molecules29184409
Chicago/Turabian StyleZhang, Zhigang, Dazhi Cheng, Wenqin Luo, Donling Hu, Tiantian Yang, Kaixuan Hu, Li Liang, Wei Liu, and Jianping Hu. 2024. "Molecular Dynamics Simulation of Lipid Nanoparticles Encapsulating mRNA" Molecules 29, no. 18: 4409. https://doi.org/10.3390/molecules29184409
APA StyleZhang, Z., Cheng, D., Luo, W., Hu, D., Yang, T., Hu, K., Liang, L., Liu, W., & Hu, J. (2024). Molecular Dynamics Simulation of Lipid Nanoparticles Encapsulating mRNA. Molecules, 29(18), 4409. https://doi.org/10.3390/molecules29184409





