Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma
Abstract
1. Introduction
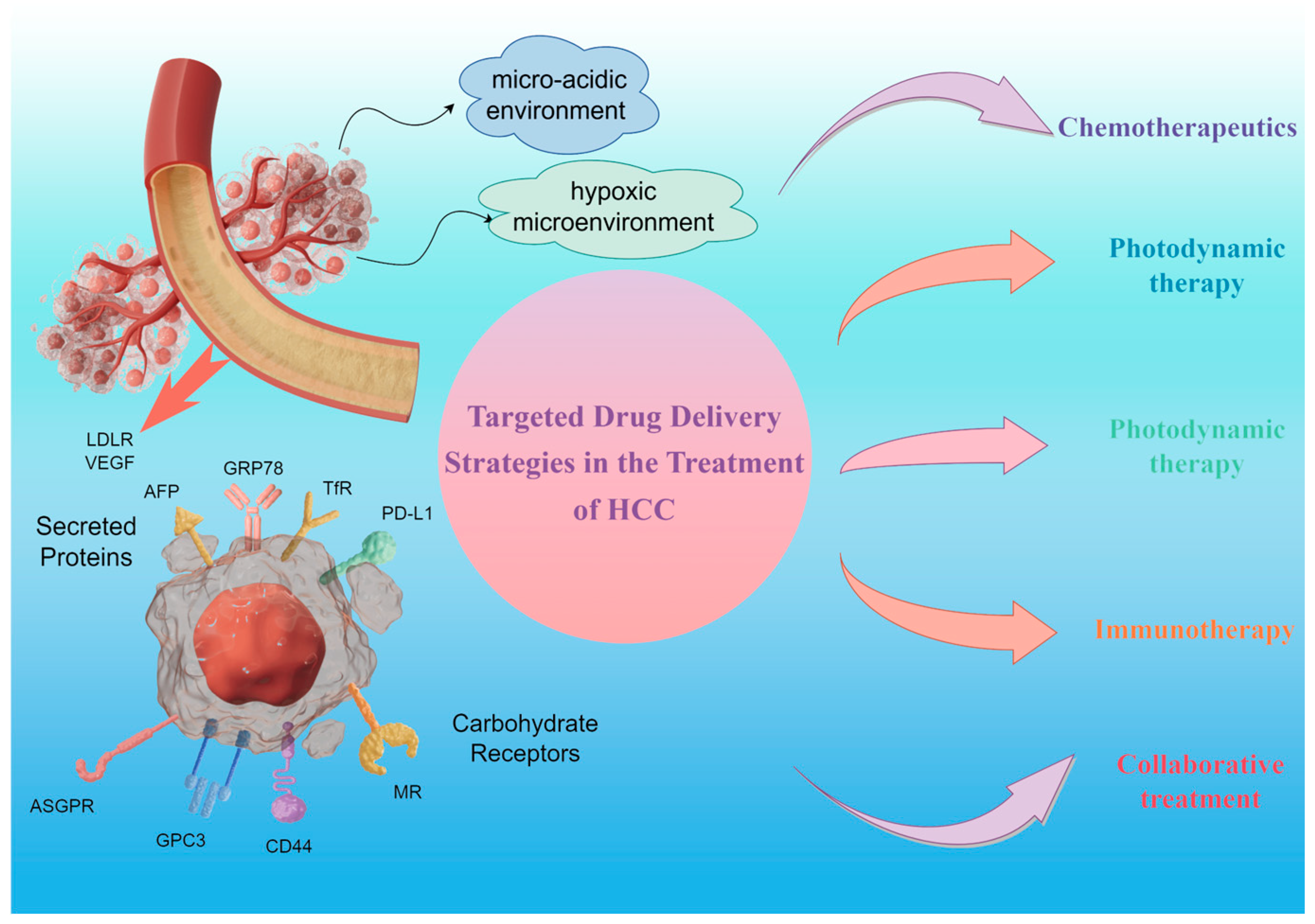
2. Targeting Strategies for HCC
2.1. Active Targeting
2.1.1. Targeting Tumors with Secreted Proteins
2.1.2. Targeting Tumors with Carbohydrate Receptors
2.1.3. Targeting Tumors with the Vasculature
2.2. Tumor Microenvironment Stimulus Response Targeting
2.2.1. pH-Responsive Drug Release
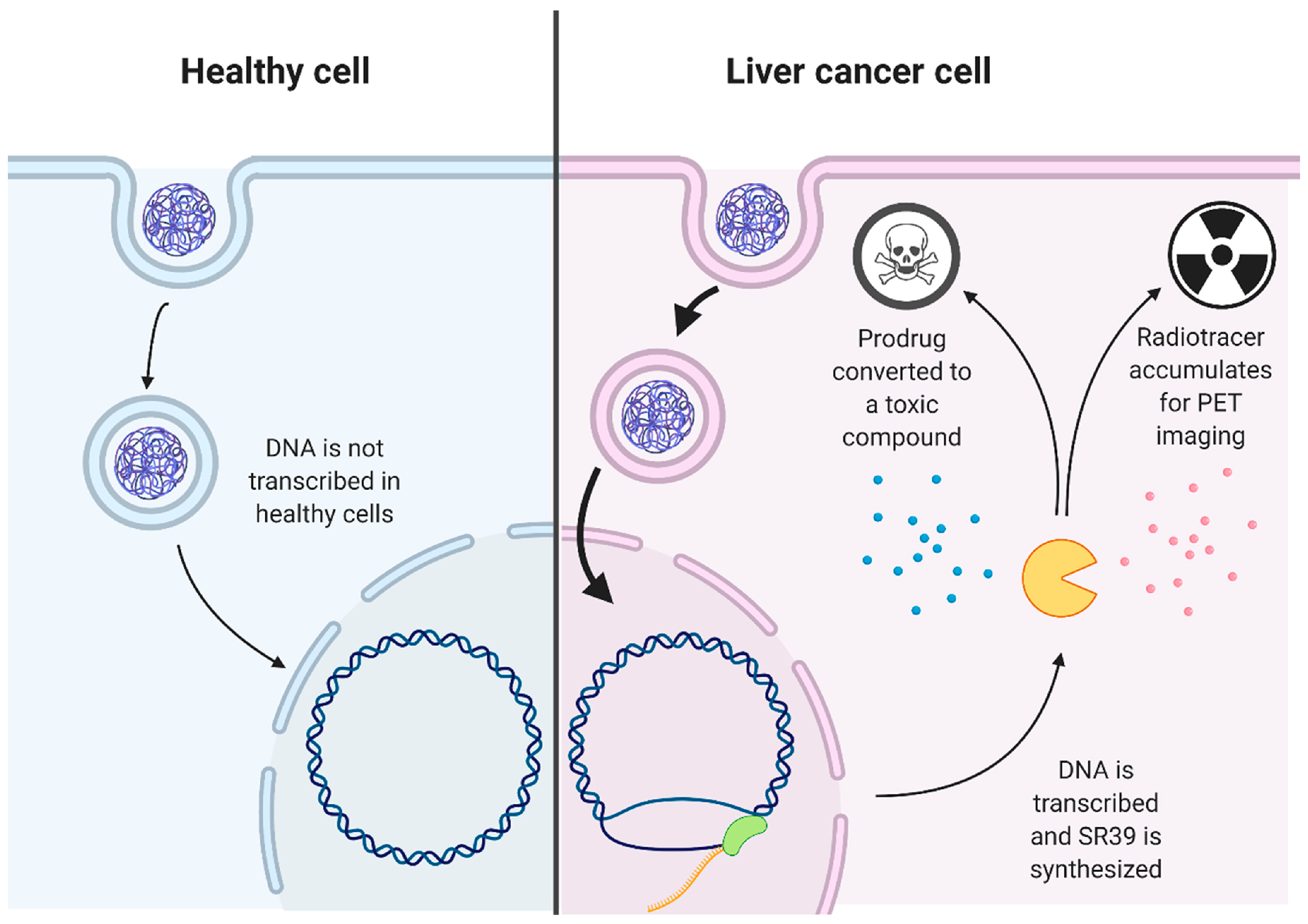
2.2.2. Environmentally Responsive Drug Release in Hypoxia
3. Targeted Therapies for HCC
3.1. Chemotherapeutics
3.2. Photodynamic Therapy
3.3. Photothermal Therapy
3.4. Immunotherapy
3.5. Collaborative Treatment
4. Conclusions and Outlook
- (1)
- Currently, the therapeutic effect of a single treatment modality for HCC is limited. How to innovatively develop a synergistic strategy between multiple therapeutic means, so that the therapeutic effects of various therapeutic mechanisms can be mutually enhanced and they can complement each other’s strengths, and together play a more significant tumor suppression, has become a direction of exploration for subsequent studies.
- (2)
- The biosafety of nanomedicines cannot be guaranteed due to the lack of a unified and standardized methodology for biosafety evaluation in current research [28]. For example, heavy metal components may potentially impair liver and kidney function, while the acidic and alkaline nature of nanoparticles may cause damage to the walls of blood vessels, in addition to the fact that numerous nanomaterials may trigger significant changes in hemodynamics, all of which may further contribute to the failure of vital organs of the body. Regulators lack a clear or even harmonized regulatory assessment pathway for complex medicines such as nanomedicines. In the EMA’s evaluation process, in addition to routine human bioequivalence or pharmacokinetic (PK) data, non-clinical evaluations need to be supplemented to add valuable and comparable data through a stepwise approach. In contrast, the FDA does not rely on non-clinical evaluations of such nanoparticle drugs because they believe that animal data may not accurately predict the human response [193]. Therefore, there is a need to explore and assess the biological safety of drugs, including, but not limited to, toxic responses, in vivo distribution, metabolic pathways, and long-term effects. By establishing a more comprehensive, scientific, and standardized biosafety evaluation system, safer and less toxic nanomedicines that are truly beneficial to the human body can be screened for.
- (3)
- Although short-term drug therapy may provide some relief from the symptoms of HCC, HCC remains a disease with a relatively poor prognosis. Particularly in advanced HCC cases and those who are multidrug-resistant, the development and implementation of treatment regimens face significant challenges [194]. The mechanism of multidrug resistance (MDR) in HCC is intricate and complex, and how to overcome this problem and improve the prognosis of hepatocellular carcinoma patients remains the future direction of nanomedicine.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A. International Trends in Liver Cancer Incidence Rates. Cancer Epidem. Biomar. 2011, 20, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Duran, S.R.; Jaquiss, R.D.B. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar]
- Medavaram, S.; Zhang, Y. Emerging therapies in advanced hepatocellular carcinoma. Exp. Hematol. Oncol. 2018, 7, 17. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.C.; Wang, Z.; Cong, W.; Zeng, M.; Zhou, W.P.; Bie, P.; Liu, L.X.; Wen, M.Q.; Kuang, M.; et al. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer 2023, 12, 405–444. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Highton, A.J.; Schuster, I.S.; Degli-Esposti, M.A.; Altfeld, M. The role of natural killer cells in liver inflammation. Semin. Immunopathol. 2021, 43, 519–533. [Google Scholar] [CrossRef]
- Bao, M.H.R.; Wong, C.C.L. Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells 2021, 10, 1715. [Google Scholar] [CrossRef]
- Pope, E.D.; Kimbrough, E.O.; Vemireddy, L.P.; Surapaneni, P.K.; Copland, J.A.; Mody, K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin. Ther. Tar. 2019, 23, 473–483. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Rong, D.W.; Zhang, B.; Zheng, W.B.; Wang, X.H.; Chen, Z.Y.; Tang, W.W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: Challenges and opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.L.; Liu, L.P.; Yang, S.L.; Ren, J.W.; Lai, P.B.S.; Chen, G.G. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2017, 1868, 564–570. [Google Scholar] [CrossRef]
- Raoul, J.L.; Kudo, M.; Finn, R.S.; Edeline, J.; Reig, M.; Galle, P.R. Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treat. Rev. 2018, 68, 16–24. [Google Scholar] [CrossRef]
- Galun, D.; Mijac, D.; Filipovic, A.; Bogdanovic, A.; Zivanovic, M.; Masulovic, D. Precision Medicine for Hepatocellular Carcinoma: Clinical Perspective. J. Pers. Med. 2022, 12, 149. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Lin, Y.; Zhang, J.Y.; Zhang, Y.M.; Li, Y.Q.; Liu, Z.; Li, Q.; Luo, M.; Liang, R.; Ye, J.Z. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J. Exp. Clin. Canc. Res. 2019, 38, 447. [Google Scholar] [CrossRef]
- Inarrairaegui, M.; Melero, I.; Sangro, B. Immunotherapy of Hepatocellular Carcinoma: Facts and Hopes. Clin. Cancer Res. 2018, 24, 1518–1524. [Google Scholar] [CrossRef]
- Zhu, X.D.; Sun, H.C. Emerging agents and regimens for hepatocellular carcinoma. J. Hematol. Oncol. 2019, 12, 110. [Google Scholar] [CrossRef]
- Chang, Y.S.; Su, C.W.; Chen, S.C.; Chen, Y.Y.; Liang, Y.J.; Wu, J.C. Upregulation of USP22 and ABCC1 during Sorafenib Treatment of Hepatocellular Carcinoma Contribute to Development of Resistance. Cells 2022, 11, 634. [Google Scholar] [CrossRef]
- Howell, J.; Pinato, D.J.; Ramaswami, R.; Bettinger, D.; Arizumi, T.; Ferrari, C.; Yen, C.; Gibbin, A.; Burlone, M.E.; Guaschino, G. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: A multi-center, prospective study. Aliment. Pharm. Ther. 2017, 45, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.L.; Zhang, L.M.; Zhu, A.X.; Bernards, R.; Qin, W.X.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastro. Hepat. 2023, 20, 203–222. [Google Scholar] [CrossRef]
- Campbell, R.B. Tumor physiology and delivery of nanopharmaceuticals. Anticancer Agents Med. Chem. 2006, 6, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Verslype, C.; Rosmorduc, O.; Rougier, P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, 41–48. [Google Scholar] [CrossRef]
- Netea-Maier, R.T.; Smit, J.W.A.; Netea, M.G. Metabolic changes in tumor cells and tumor-associated macrophages: A mutual relationship. Cancer Lett. 2018, 413, 102–109. [Google Scholar] [CrossRef]
- Kieliszek, M.; Blazejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [Google Scholar] [CrossRef]
- Cormode, D.P.; Skajaa, G.O.; Delshad, A.; Parker, N.; Jarzyna, P.A.; Calcagno, C.; Galper, M.W.; Skajaa, T.; Briley-Saebo, K.C.; Bell, H.M.; et al. A Versatile and Tunable Coating Strategy Allows Control of Nanocrystal Delivery to Cell Types in the Liver. Bioconjugate Chem. 2011, 22, 353–361. [Google Scholar] [CrossRef]
- Xu, M.J.; Yang, L.; Lin, Y.J.; Lu, Y.; Bi, X.Y.; Jiang, T.T.; Deng, W.; Zhang, L.; Yi, W.; Xie, Y.; et al. Emerging nanobiotechnology for precise theranostics of hepatocellular carcinoma. J. Nanobiotechnol. 2022, 20, 427. [Google Scholar] [CrossRef]
- Zhu, J.D.; Zhou, Z.C.; Yang, C.H.; Kong, D.L.; Wan, Y.; Wang, Z. Folate-conjugated amphiphilic star-shaped block copolymers as targeted nanocarriers. J. Biomed. Mater. Res. A 2011, 97, 498–508. [Google Scholar] [CrossRef]
- Ding, L.; Wang, X.; Wang, T.; Yu, B.; Han, M.H.; Guo, Y.F. Effect of Lipophilic Chains on the Antitumor Effect of a Dendritic Nano Drug Delivery System. Molecules 2023, 28, 69. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Islam, R.; Maeda, H.; Fang, J. Factors affecting the dynamics and heterogeneity of the EPR effect: Pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opin. Drug Del. 2022, 19, 199–212. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukigawa, K.; Fang, J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy—Problems, Solutions, and Prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef]
- Rehan, F.; Zhang, M.J.; Fang, J.; Greish, K. Therapeutic Applications of Nanomedicine: Recent Developments and Future Perspectives. Molecules 2024, 29, 2073. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, Y.; Yang, W.Z.; Niu, P.; Li, X.; Chen, Y.D.; Li, Z.Y.; Liu, Y.; An, Y.L.; Liu, Y.; et al. Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy. Nano Today 2020, 35, 100970. [Google Scholar] [CrossRef]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.I.; Lee, Y.J.; Kim, K.M.; Nahm, S.S.; Park, Y.S.; Cheon, G.J.; Lim, S.M.; Kang, J.H. Non-invasive monitoring of hepatocellular carcinoma in transgenic mouse with bioluminescent imaging. Cancer Lett. 2011, 310, 53–60. [Google Scholar] [CrossRef]
- Vaughan, H.J.; Zamboni, C.; Hassan, L.F.; Radant, N.P.; Jacob, D.; Mease, R.C.; Minn, I.; Tzeng, S.Y.; Gabrielson, K.L.; Bhardwaj, P.; et al. Polymeric nanoparticles for dual-targeted theranostic gene delivery to hepatocellular carcinoma. Sci. Adv. 2022, 8, eabo6406. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Yang, X.; Tian, J. Targeted polypyrrole nanoparticles for the identification and treatment of hepatocellular carcinoma. Nanoscale 2018, 10, 9594–9601. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Ni, M.; Gill, P.; Lee, A.S. Cell Surface Relocalization of the Endoplasmic Reticulum Chaperone and Unfolded Protein Response Regulator GRP78/BiP. J. Biol. Chem. 2010, 285, 15065–15075. [Google Scholar] [CrossRef]
- Lo, A.; Lin, C.T.; Wu, H.C. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol. Cancer Ther. 2008, 7, 579–589. [Google Scholar] [CrossRef]
- Mohamed, N.K.; Hamad, M.A.; Hafez, M.Z.E.; Wooley, K.L.; Elsabahy, M. Nanomedicine in management of hepatocellular carcinoma: Challenges and opportunities. Int. J. Cancer 2017, 140, 1475–1484. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, R.; Zhang, J.; Hou, Y.; Chen, X.; Zhou, M.; Tian, X.; Hao, C.; Fan, K.; Yan, X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics 2019, 9, 2167–2182. [Google Scholar] [CrossRef]
- Fan, K.L.; Cao, C.Q.; Pan, Y.X.; Lu, D.; Yang, D.L.; Feng, J.; Song, L.; Liang, M.M.; Yan, X.Y. Magnetoferritin nanoparticles for targeting and visualizing tumor tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef]
- Muthu, M.S.; Kutty, R.V.; Luo, Z.T.; Xie, J.P.; Feng, S.S. Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials 2015, 39, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Jiang, M.Y.; Jiang, D.; Feng, X.Y.; Yao, J.H.; Song, Q.X.; Chen, H.Z.; Gao, X.L.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharmaceut. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Makwana, H.; Mastrotto, F.; Magnusson, J.P.; Sleep, D.; Hay, J.; Allen, S.; Alexander, C. Engineered Polymer-Transferrin Conjugates as Self-Assembling Targeted Drug Delivery Systems. Biomacromolecules 2017, 18, 1532–1543. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Moos, T. Revisiting nanoparticle technology for blood-brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J. Control. Release 2016, 222, 32–46. [Google Scholar] [CrossRef]
- Sakurai, K.; Sohda, T.; Ueda, S.; Tanaka, T.; Hirano, G.; Yokoyama, K.; Morihara, D.; Aanan, A.; Takeyama, Y.; Irie, M.; et al. Immunohistochemical Demonstration of Transferrin Receptor 1 and 2 in Human Hepatocellular Carcinoma Tissue. Hepato-Gastroenterology 2014, 61, 426–430. [Google Scholar]
- Tortorella, S.; Karagiannis, T.C. Transferrin Receptor-Mediated Endocytosis: A Useful Target for Cancer Therapy. J. Membr. Biol. 2014, 247, 291–307. [Google Scholar] [CrossRef]
- Karagiannis, T.C.; Lobachevsky, P.N.; Leung, B.K.Y.; White, J.M.; Martin, R.F. Receptor-mediated DNA-targeted photoimmunotherapy. Cancer Res. 2006, 66, 10548–10552. [Google Scholar] [CrossRef]
- Szwed, M.; Matusiak, A.; Laroche-Clary, A.; Robert, J.; Marszalek, I.; Jozwiak, Z. Transferrin as a drug carrier: Cytotoxicity, cellular uptake and transport kinetics of doxorubicin transferrin conjugate in the human leukemia cells. Toxicol. Vitr. 2014, 28, 187–197. [Google Scholar] [CrossRef]
- Clark, A.J.; Davis, M.E. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc. Natl. Acad. Sci. USA 2015, 112, 12486–12491. [Google Scholar] [CrossRef]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Han, T.L.V.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Zhang, H.J.; Hou, L.; Jiao, X.J.; Ji, Y.D.; Zhu, X.L.; Zhang, Z.Z. Transferrin-mediated fullerenes nanoparticles as Fe2+-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials 2015, 37, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gu, X.; Cheng, L.; Meng, F.H.; Storm, G.; Zhong, Z. Low-toxicity transferrin-guided polymersomal doxorubicin for potent chemotherapy of orthotopic hepatocellular carcinoma in vivo. Acta Biomater. 2019, 92, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Chen, F.M.; Liu, R.; Luo, J.Q.; Huang, Y.C.; Shu, N.; Zheng, S.J.; Shao, D.; Leong, K.W.; Du, J.Z. Nanoparticle-enabled concurrent modulation of phagocytosis and repolarization of macrophages for enhanced cancer immunotherapy. Nano Today 2022, 47, 101651. [Google Scholar] [CrossRef]
- Wei, Z.H.; Zhang, X.Q.; Zhang, Z.L.; Yong, T.Y.; Zhan, G.T.; Lv, W.L.; Ding, Z.Q.; Sun, K.L.; Yang, X.L.; Gan, L. Engineered Iron-Based nanoplatform amplifies repolarization of M2-Like Tumor-Associated Macrophages for enhanced cancer immunotherapy. Chem. Eng. J. 2022, 433, 133847. [Google Scholar] [CrossRef]
- Wei, S.M.; Shao, X.X.; Liu, Y.; Xiong, B.Y.; Cui, P.F.; Liu, Z.L.; Li, Q.S. Genome editing of PD-L1 mediated by nucleobase-modified polyamidoamine for cancer immunotherapy. J. Mater. Chem. B 2022, 10, 1291–1300. [Google Scholar] [CrossRef]
- Yang, Z.K.; Wu, H.T.; Lin, Q.; Wang, X.; Kang, S.J. Lymphopenic condition enhanced the antitumor immunity of PD-1-knockout T cells mediated by CRISPR/Cas9 system in malignant melanoma. Immunol. Lett. 2022, 250, 15–22. [Google Scholar] [CrossRef]
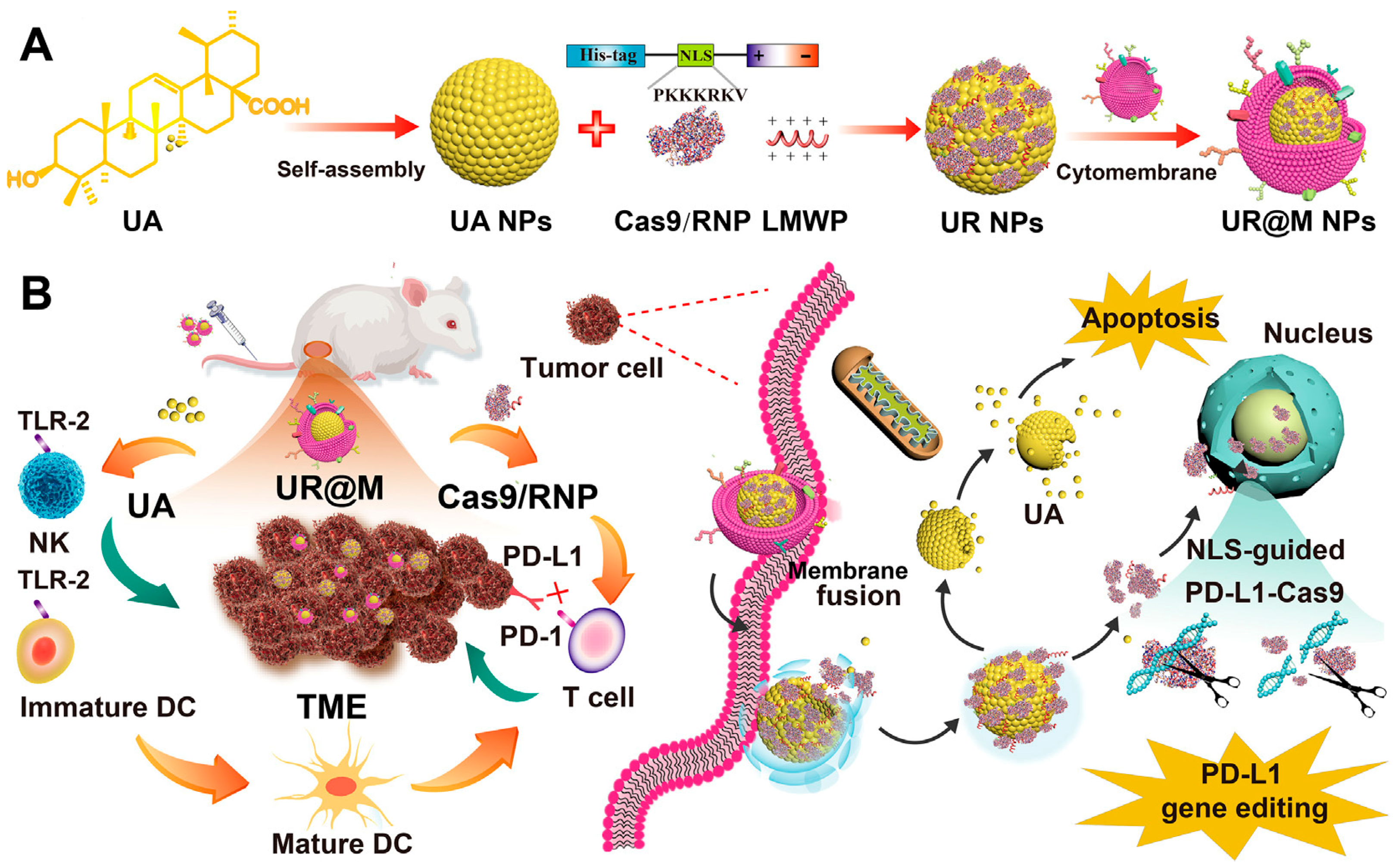
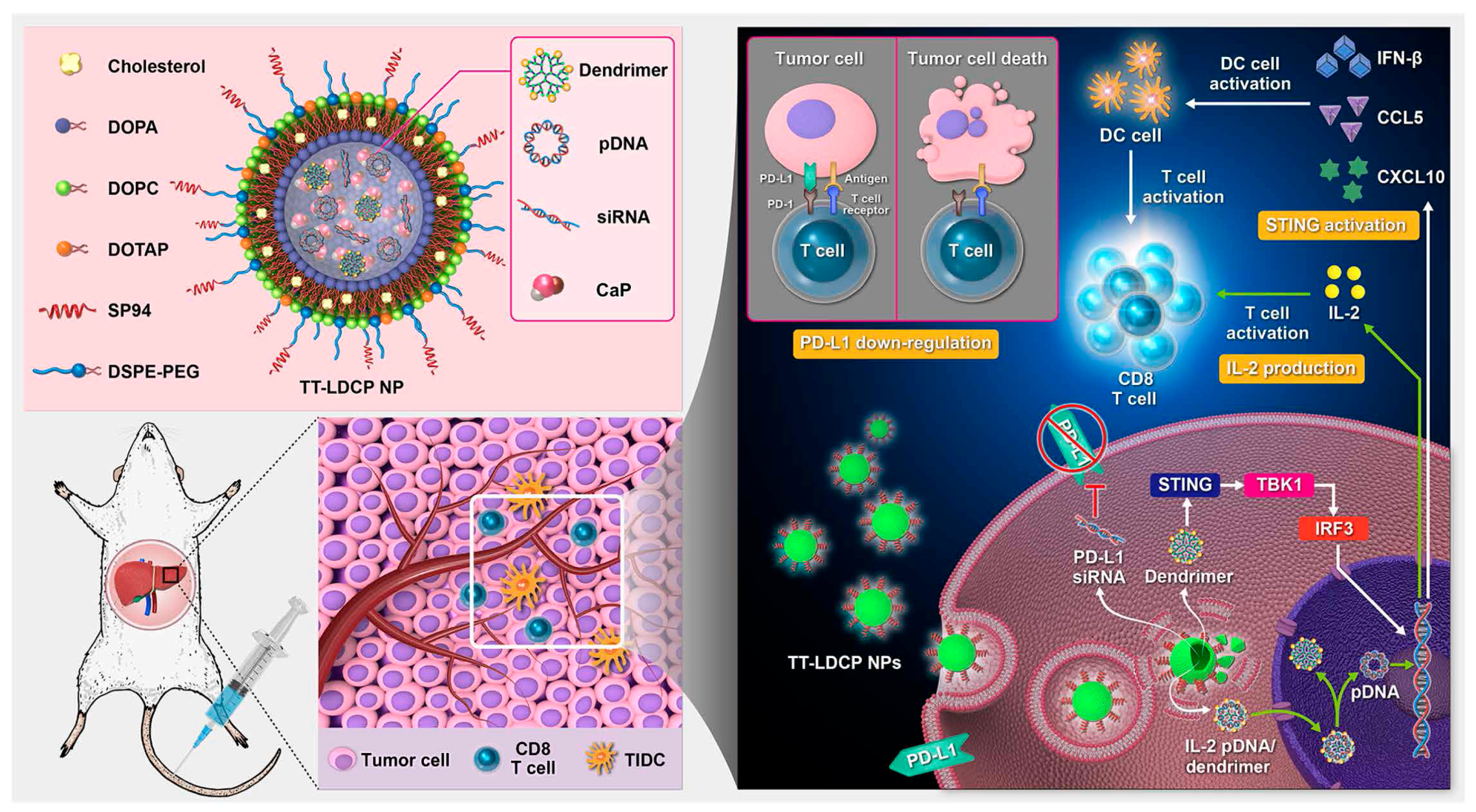
- Zhang, B.C.; Lai, C.M.; Luo, B.Y.; Shao, J.W. Triterpenoids-templated self-assembly nanosystem for biomimetic delivery of CRISPR/Cas9 based on the synergy of TLR-2 and ICB to enhance HCC immunotherapy. Acta Pharm. Sin. B 2024, 14, 3205–3217. [Google Scholar] [CrossRef]
- Fan, L.L.; Zhang, B.C.; Xu, A.C.; Shen, Z.C.; Guo, Y.; Zhao, R.R.; Yao, H.L.; Shao, J.W. Carrier-Free, Pure Nanodrug Formed by the Self-Assembly of an Anticancer Drug for Cancer Immune Therapy. Mol. Pharmaceut. 2018, 15, 2466–2478. [Google Scholar] [CrossRef]
- Zhang, B.C.; Jiang, J.L.; Wu, P.Y.; Zou, J.J.; Le, J.Q.; Lin, J.F.; Li, C.; Luo, B.Y.; Zhang, Y.J.; Huang, R.; et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm. Sin. B 2021, 11, 246–257. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.L. Application of glycosylation in targeted drug delivery. Eur. J. Med. Chem. 2019, 182, 111612. [Google Scholar] [CrossRef]
- Huang, G.L.; Lv, M.J.; Hu, J.C.; Huang, K.L.; Xu, H. Glycosylation and Activities of Natural Products. Mini-Rev. Med. Chem. 2016, 16, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Ma, Y.; Sun, X.L. Recent Developments in Carbohydrate-Decorated Targeted Drug/Gene Delivery. Med. Res. Rev. 2010, 30, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Kano, A.; Obara, K.; Saito, M.; Chen, X.; Park, T.G.; Akaike, T.; Maruyama, A. Nuclear localization and antisense effect of PNA internalized by ASGP-R-mediated endocytosis with protein/DNA conjugates. J. Control. Release 2011, 155, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, A.M.; Chiellini, F. A 3D model for the human hepatic asialoglycoprotein receptor (ASGP-R). J. Biomol. Struct. Dyn. 2000, 18, 435–451. [Google Scholar] [CrossRef]
- Cavallaro, G.; Farra, R.; Craparo, E.F.; Sardo, C.; Porsio, B.; Giammona, G.; Perrone, F.; Grassi, M.; Pozzato, G.; Grassi, G.; et al. Galactosylated polyaspartamide copolymers for siRNA targeted delivery to hepatocellular carcinoma cells. Int. J. Pharmaceut. 2017, 525, 397–406. [Google Scholar] [CrossRef]
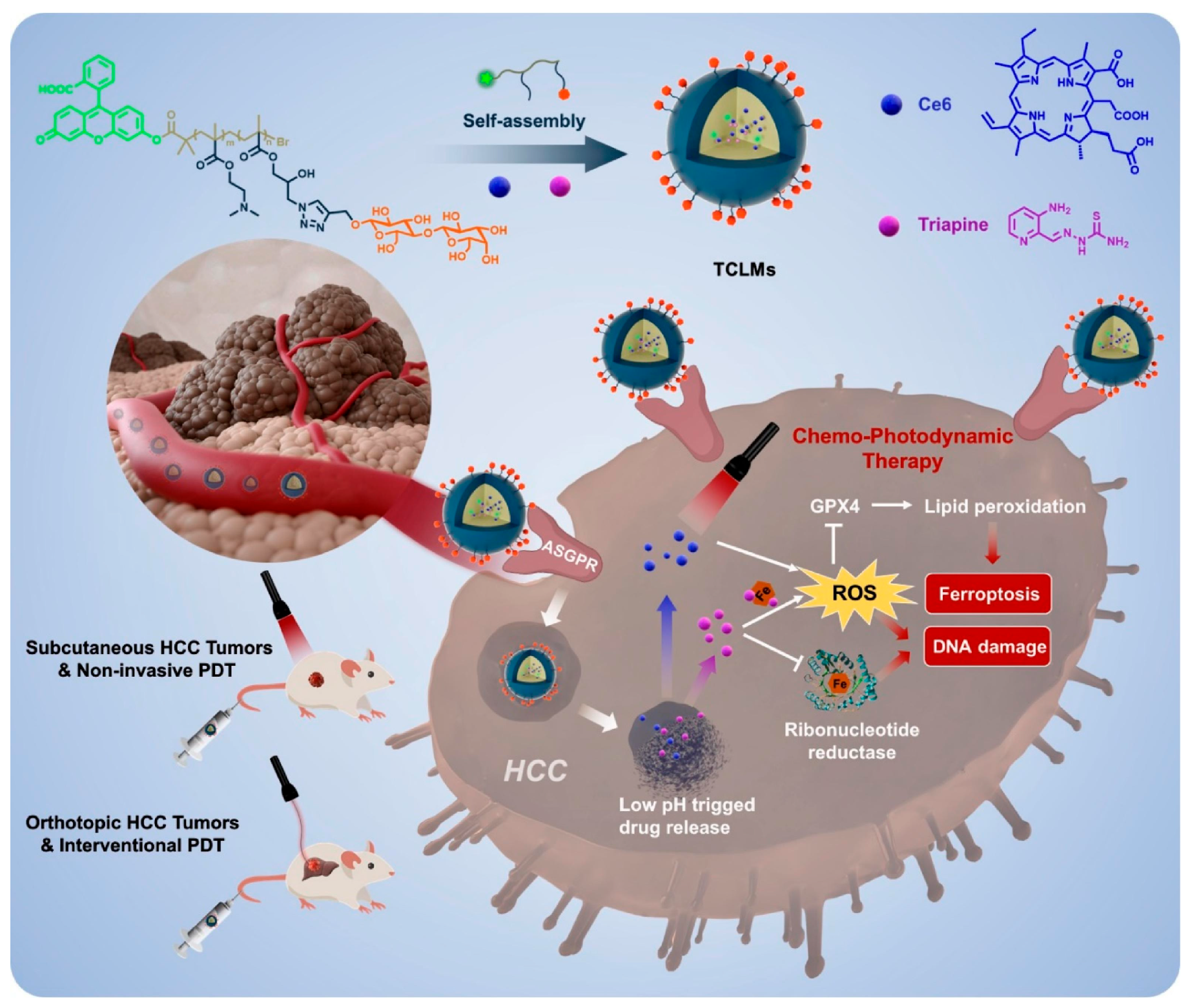
- Zhang, P.F.; Liu, C.; Wu, W.R.; Mao, Y.; Qin, Y.F.; Hu, J.; Hu, J.; Fu, J.J.; Hua, D.; Yin, J. Triapine/Ce6-loaded and lactose-decorated nanomicelles provide an effective chemo-photodynamic therapy for hepatocellular carcinoma through a reactive oxygen species-boosting and ferroptosis-inducing mechanism. Chem. Eng. J. 2021, 425, 131543. [Google Scholar] [CrossRef]
- Heffeter, P.; Pape, V.F.S.; Enyedy, E.A.; Keppler, B.K.; Szakacs, G.; Kowol, C.R. Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development. Antioxid. Redox. Sign. 2019, 30, 1062–1082. [Google Scholar] [CrossRef]
- Mrozek-Wilczkiewicz, A.; Malarz, K.; Rams-Baron, M.; Serda, M.; Bauer, D.; Montforts, F.P.; Ratuszna, A.; Burley, T.; Polanski, J.; Musiol, R. Iron Chelators and Exogenic Photosensitizers. Synergy through Oxidative Stress Gene Expression. J. Cancer 2017, 8, 1979–1987. [Google Scholar] [CrossRef]
- Popovic-Bijelic, A.; Kowol, C.R.; Lind, M.E.S.; Luo, J.H.; Himo, F.; Enyedy, E.A.; Arion, V.B.; Graslund, A. Ribonucleotide reductase inhibition by metal complexes of Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone): A combined experimental and theoretical study. J. Inorg. Biochem. 2011, 105, 1422–1431. [Google Scholar] [CrossRef]
- Zheng, D.W.; Lei, Q.; Zhu, J.Y.; Fan, J.X.; Li, C.X.; Li, C.; Xu, Z.S.; Cheng, S.X.; Zhang, X.Z. Switching Apoptosis to Ferroptosis: Metal-Organic Network for High-Efficiency Anticancer Therapy. Nano Lett. 2017, 17, 284–291. [Google Scholar] [CrossRef]
- Meng, X.; Deng, J.; Liu, F.; Guo, T.; Liu, M.Y.; Dai, P.P.; Fan, A.P.; Wang, Z.; Zhao, Y.J. Triggered All-Active Metal Organic Framework: Ferroptosis Machinery Contributes to the Apoptotic Photodynamic Antitumor Therapy. Nano Lett. 2019, 19, 7866–7876. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ma, Y.Y.; Yuan, Q.L.; Hu, H.X.; Hu, X.K.; Qian, Z.Y.; Rolle, J.K.; Gu, Y.Q.; Li, S.W. Enhanced Ferroptosis by Oxygen-Boosted Phototherapy Based on a 2-in-1 Nanoplatform of Ferrous Hemoglobin for Tumor Synergistic Therapy. ACS Nano 2020, 14, 3414–3425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.B.; Shang, W.T.; Yu, X.L.; Tian, J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med. Res. Rev. 2018, 38, 741–767. [Google Scholar] [CrossRef]
- Shi, D.H.; Shi, Y.P.; Kaseb, A.O.; Qi, X.X.; Zhang, Y.; Chi, J.C.; Lu, Q.; Gao, H.P.; Jiang, H.; Wang, H.M.; et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. 2020, 26, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Qiu, Y.J.; Shi, Y.Y.; Wen, W.; He, X.P.; Dong, L.W.; Tan, Y.X.; Long, Y.T.; Tian, H.; Wang, H.Y. Glypican-3-targeted precision diagnosis of hepatocellular carcinoma on clinical sections with a supramolecular 2D imaging probe. Theranostics 2018, 8, 3268–3274. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Wu, T.; Zhu, N.; Huang, Y.; Yang, X.Y.; Yuan, L.; Wu, Y.J.; Liang, X.F.; Jiang, X.Q. The clinical significance of CTC enrichment by GPC3-IML and its genetic analysis in hepatocellular carcinoma. J. Nanobiotechnol. 2021, 19, 74. [Google Scholar]
- Deng, H.; Shang, W.T.; Wang, K.; Guo, K.X.; Liu, Y.; Tian, J.; Fang, C.H. Targeted-detection and sequential-treatment of small hepatocellular carcinoma in the complex liver environment by GPC-3-targeted nanoparticles. J. Nanobiotechnol. 2022, 20, 156. [Google Scholar] [CrossRef]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Hu, Y.R.; Lin, J.; Huang, P. Glucose Oxidase-Instructed Multimodal Synergistic Cancer Therapy. Adv. Mater. 2019, 31, 1808325. [Google Scholar] [CrossRef]
- Cannito, S.; Bincoletto, V.; Turato, C.; Pontisso, P.; Scupoli, M.T.; Ailuno, G.; Andreana, I.; Stella, B.; Arpicco, S.; Bocca, C. Hyaluronated and PEGylated Liposomes as a Potential Drug-Delivery Strategy to Specifically Target Liver Cancer and Inflammatory Cells. Molecules 2022, 27, 1062. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, Y. Prognostic value of CD44 expression in patients with hepatocellular carcinoma: Meta-analysis. Cancer Cell Int. 2016, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Zhang, H.Z.; Liu, Y.W.; He, Y.Q.; Wang, W.J.; Du, Y.; Yang, C.X.; Gao, F. CD44 clustering is involved in monocyte differentiation. Acta Bioch. Bioph. Sin. 2014, 46, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Spadea, A.; De La Rosa, J.M.R.; Tirella, A.; Ashford, M.B.; Williams, K.J.; Stratford, I.J.; Tirelli, N.; Mehibel, M. Evaluating the Efficiency of Hyaluronic Acid for Tumor Targeting via CD44. Mol. Pharmaceut. 2019, 16, 2481–2493. [Google Scholar] [CrossRef]
- Dhar, D.; Antonucci, L.; Nakagawa, H.; Kim, J.Y.; Glitzner, E.; Caruso, S.; Shalapour, S.; Yang, L.; Valasek, M.A. Liver Cancer Initiation Requires p53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell 2018, 33, 1061. [Google Scholar] [CrossRef]
- Yusupov, M.; Privat-Maldonado, A.; Cordeiro, R.M.; Verswyvel, H.; Shaw, P.; Razzokov, J.; Smits, E.; Bogaerts, A. Oxidative damage to hyaluronan-CD44 interactions as an underlying mechanism of action of oxidative stress-inducing cancer therapy. Redox. Biol. 2021, 43, 101968. [Google Scholar] [CrossRef]
- Zhang, F.; Jia, Y.; Zheng, X.; Shao, D.; Zhao, Y.W.; Wang, Z.; Dawulieti, J.; Liu, W.L.; Sun, M.; Sun, W.; et al. Janus nanocarrier-based co-delivery of doxorubicin and berberine weakens chemotherapy-exacerbated hepatocellular carcinoma recurrence. Acta Biomater. 2019, 100, 352–364. [Google Scholar] [CrossRef]
- Shao, D.; Li, M.Q.; Wang, Z.; Zheng, X.; Lao, Y.; Chang, Z.M.; Zhang, F.; Lu, M.M.; Yue, J.; Hu, H.; et al. Bioinspired Diselenide-Bridged Mesoporous Silica Nanoparticles for Dual-Responsive Protein Delivery. Adv. Mater. 2018, 30, 1801198. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.S.; Chang, Z.M.; Li, L.; Zhang, Y.; Lu, M.M.; Zheng, X.; Li, M.Q.; Shao, D.; Li, J.; et al. Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinoma. Chem. Biol. Drug Des. 2017, 89, 464–469. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Shao, D.; Ji, W.Y.; Li, J.; Chen, L.; Song, J. The nanotoxicity investigation of optical nanoparticles to cultured cells in vitro. Toxicol. Rep. 2014, 1, 137–144. [Google Scholar] [CrossRef]
- Zhong, L.; Xu, L.; Liu, Y.Y.; Li, Q.S.; Zhao, D.Y.; Li, Z.B.; Zhang, H.C.; Zhang, H.; Kan, Q.; Wang, Y.J.; et al. Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy. Acta Bioch. Bioph. Sin. B 2019, 9, 397–409. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, J.W.; Choi, W.S.; Won, J.E.; Kim, G.H.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Kang, T.H.; Jung, I.D.; et al. CD44-Targeting PLGA Nanoparticles Incorporating Paclitaxel and FAK siRNA Overcome Chemoresistance in Epithelial Ovarian Cancer. Cancer Res. 2018, 78, 6247–6256. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.Q.; Xu, C.R.; Zhao, X.M.; Lin, C.S.; Yang, X.; Xin, X.F.; Zhang, L.; Qn, C.; Han, X.P.; Yang, L.; et al. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
- Irjala, H.; Alanen, K.; Grénman, R.; Heikkilä, P.; Joensuu, H.; Jalkanen, S. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)-1 direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res. 2003, 63, 4671–4676. [Google Scholar] [PubMed]
- Xiong, M.H.; Lei, Q.; You, X.Y.; Gao, T.T.; Song, X.J.; Xia, Y.; Ye, T.H.; Zhang, L.D.; Wang, N.Y.; Yu, L.T. Mannosylated liposomes improve therapeutic effects of paclitaxel in colon cancer models. J. Microencapsul. 2017, 34, 513–521. [Google Scholar] [CrossRef]
- Pasieka, T.J.; Maresova, L.; Shiraki, K.; Grose, C. Regulation of varicella-zoster virus-induced cell-to-cell fusion by the endocytosis-competent glycoproteins gH and gE. J. Virol. 2004, 78, 2884–2896. [Google Scholar] [CrossRef]
- Xiao, Z.C.; Li, T.; Zheng, X.Y.; Lin, L.T.; Wang, X.B.; Li, B.; Huang, J.J.; Wang, Y.; Shuai, X.T.; Zhu, K.S. Nanodrug enhances post-ablation immunotherapy of hepatocellular carcinoma via promoting dendritic cell maturation and antigen presentation. Bioact. Mater. 2023, 21, 57–68. [Google Scholar] [CrossRef]
- Hagiwara, S.; Nishida, N.; Kudo, M. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastro. Hepat. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug. Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Verteporfin. Drugs Aging 2000, 16, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.H.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
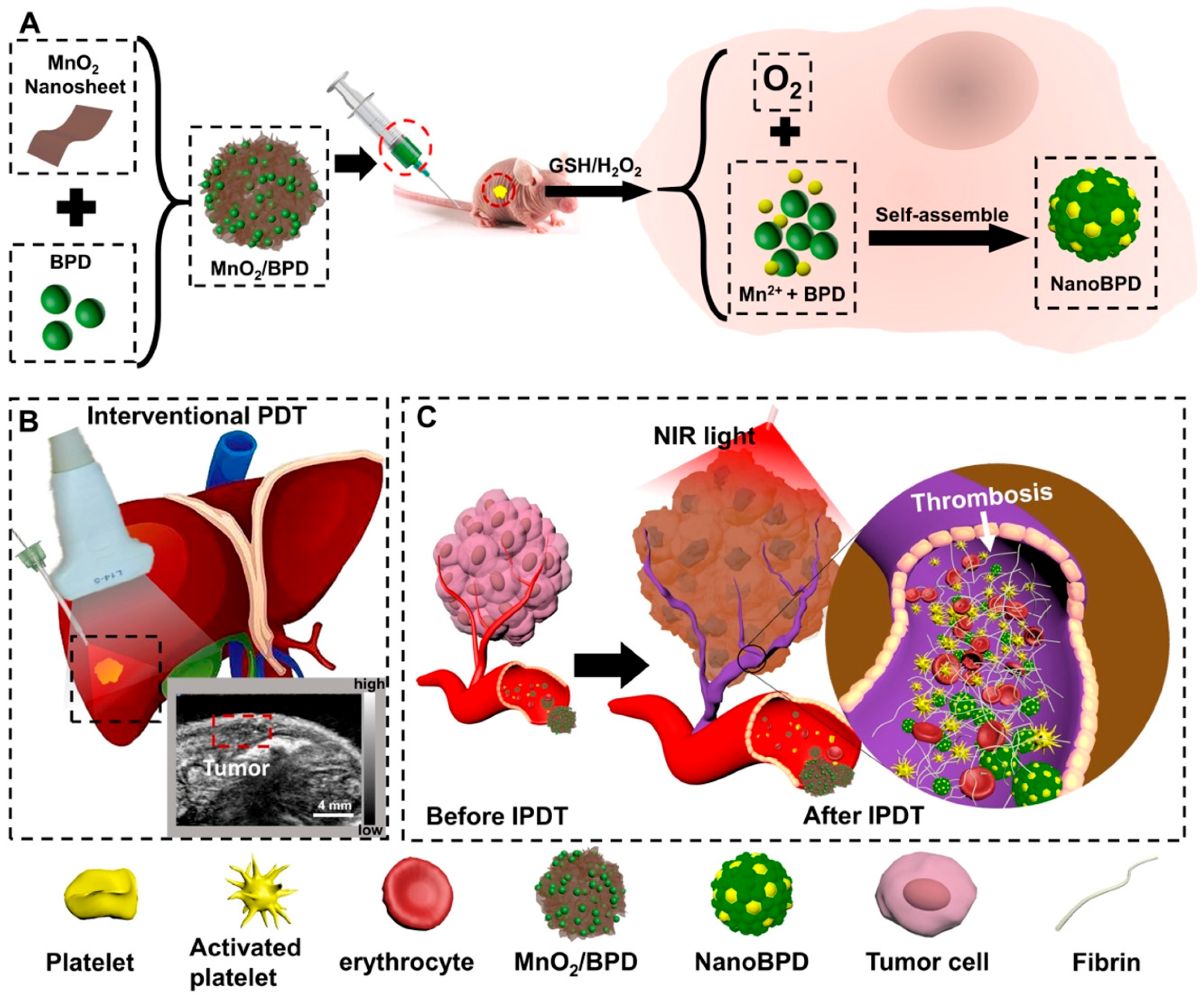
- Wang, Y.Q.; Shang, W.T.; Zhong, H.S.; Luo, T.; Niu, M.; Xu, K.; Tian, J. Tumor Vessel Targeted Self-Assemble Nanoparticles for Amplification and Prediction of the Embolization Effect in Hepatocellular Carcinoma. ACS Nano 2020, 14, 14907–14918. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer. Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Wang, H.; Shen, X.; Zhu, Z.S.; Cai, S.X. Enhancing HepG2 cell apoptosis with a combined nanoparticle delivery of miR-128-3p agomir and Oroxin B: A novel drug delivery approach based on PI3K-AKT and VEGF pathway crosstalk. Asian J. Pharm. Sci. 2024, 100909. [Google Scholar] [CrossRef]
- He, X.; Jing, Z.; Cheng, G. MicroRNAs: New regulators of Toll-like receptor signalling pathways. Biomed. Res. Int. 2014, 2014, 945169. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends. Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Kara, G.; Calin, G.A.; Ozpolat, B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv. Drug. Deliver. Rev. 2022, 182, 114113–114135. [Google Scholar] [CrossRef]
- Lin, Z.Y.; He, H.B.; Wang, M.; Liang, J.Y. MicroRNA-130a controls bone marrow mesenchymal stem cell differentiation towards the osteoblastic and adipogenic fate. Cell Proliferat. 2019, 52, e12688. [Google Scholar] [CrossRef]
- Li, N.N.; Men, W.X.; Zheng, Y.B.; Wang, H.C.; Meng, X.S. Oroxin B Induces Apoptosis by Down-Regulating MicroRNA-221 Resulting in the Inactivation of the PTEN/PI3K/AKT Pathway in Liver Cancer. Molecules 2019, 24, 4384–4398. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Xu, I.M.; Chiu, D.K.; Leibold, J.; Tse, A.P.; Bao, M.H.; Yuen, V.W.; Chan, C.Y.; Lai, R.K.; Chin, D.W.; et al. Induction of Oxidative Stress Through Inhibition of Thioredoxin Reductase 1 Is an Effective Therapeutic Approach for Hepatocellular Carcinoma. Hepatology 2019, 69, 1768–1786. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Zuo, M.X.; Li, W.; Chen, Q.F.; Wu, P.H. Infiltrative Hepatocellular Carcinoma: Transcatheter Arterial Chemoembolization Versus Hepatic Arterial Infusion Chemotherapy. Front. Oncol. 2021, 11, 747496. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.K.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Liu, X.; Huangfu, Y.N.; Wang, J.R.; Kong, P.X.; Tian, W.J.; Liu, P.; Fang, C.; Li, S.Y.; Nie, Y.; Feng, Z.J. Supramolecular Polymer-Nanomedicine Hydrogel Loaded with Tumor Associated Macrophage-Reprogramming polyTLR7/8a Nanoregulator for Enhanced Anti-Angiogenesis Therapy of Orthotopic Hepatocellular Carcinoma. Adv. Sci. 2023, 10, 2300637. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Adachi, Y.; Matsuki, M.; Watanabe, H.; Takase, K.; Kodama, K.; Matsui, J.; Funahashi, Y.; Nomoto, K. Antitumor and Antiangiogenic Activities of Lenvatinib in Mouse Xenograft Models of Vascular Endothelial Growth Factor-Induced Hypervascular Human Hepatocellular Carcinoma. Cancer Investig. 2019, 37, 185–198. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug. Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends. Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhu, R.T.; Zhou, M.Y.; Liu, J.J.; Dong, K.; Zhao, S.F.; Cao, J.H.; Wang, W.J.; Sun, C.G.; Wu, S.T. Homologous cancer cell membrane-camouflaged nanoparticles target drug delivery and enhance the chemotherapy efficacy of hepatocellular carcinoma. Cancer. Lett. 2023, 558, 216106. [Google Scholar] [CrossRef]
- Guo, R.; Long, Y.; Lu, Z.; Deng, M.; He, P.; Li, M.; He, Q. Enhanced stability and efficacy of GEM-TOS prodrug by co-assembly with antimetastatic shell LMWH-TOS. Acta Pharm Sin B. 2020, 10, 1977–1988. [Google Scholar] [CrossRef]
- Sin, S.Q.; Mohan, C.D.; Goh, R.M.W.J.; You, M.L.; Nayak, S.C.; Chen, L.; Sethi, G.; Rangappa, K.S.; Wang, L.Z. Hypoxia signaling in hepatocellular carcinoma: Challenges and therapeutic opportunities. Cancer Metast. Rev. 2023, 42, 741–764. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, Z.J.; Jia, F.; Xu, Q.; Shu, Z.L.; Deng, J.L.; Li, A.M.; Yu, M.; Yu, Z.Q. CXCR4-guided liposomes regulating hypoxic and immunosuppressive microenvironment for sorafenib-resistant tumor treatment. Bioact. Mater. 2022, 17, 147–161. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, W.; Zhou, Y.J.; Huang, S.J.; Yu, J.Z.; Yang, M.J.; Zhang, Z.H.; Ma, J.Q.; Luo, J.J.; Rao, S.X. Hypoxia-activated cascade nanovaccine for synergistic chemoembolization-immune therapy of hepatocellular carcinoma. Biomaterials 2024, 306, 122480. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xie, Y.; Li, J.; Peng, Z.H.; Sheinin, Y.; Zhou, J.P.; Oupicky, D. Tumor-Penetrating Nanoparticles for Enhanced Anticancer Activity of Combined Photodynamic and Hypoxia-Activated Therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef]
- Xin, F.L.; Wu, M.; Cai, Z.X.; Zhang, X.L.; Wei, Z.W.; Liu, X.L.; Liu, J.F. Tumor Microenvironment Triggered Cascade-Activation Nanoplatform for Synergistic and Precise Treatment of Hepatocellular Carcinoma. Adv. Healthc. Mater. 2021, 10, 2002036. [Google Scholar] [CrossRef] [PubMed]
- Abi-Jaoudeh, N.; Dayyani, F.; Chen, P.J.; Fernando, D.; Fidelman, N.; Javan, H.; Liang, P.C.; Hwang, J.I.; Imagawa, D.K. Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.J.; Li, L.M.; Chen, H.; Tang, M.H.; Cheng, W.; Lin, T.Y.; Li, L.Y.; Li, B.; Xue, X.D. Drug-drug conjugates self-assembled nanomedicines triggered photo- / immuno- therapy for synergistic cancer treatments. J. Control. Release 2023, 363, 361–375. [Google Scholar] [CrossRef]
- Phuengkham, H.; Song, C.; Lim, Y.T. A Designer Scaffold with Immune Nanoconverters for Reverting Immunosuppression and Enhancing Immune Checkpoint Blockade Therapy. Adv. Mater. 2019, 31, 1903242. [Google Scholar] [CrossRef]
- Liu, X.J.; Sun, Y.X.; Xu, S.S.; Gao, X.N.; Kong, F.P.; Xu, K.H.; Tang, B. Homotypic Cell Membrane-Cloaked Biomimetic Nanocarrier for the Targeted Chemotherapy of Hepatocellular Carcinoma. Theranostics 2019, 9, 5828–5838. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef]
- Sultan, A.S.; Xie, J.W.; LeBaron, M.J.; Ealley, E.L.; Nevalainen, M.T.; Rui, H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 2005, 24, 746–760. [Google Scholar] [CrossRef]
- Tian, H.; Luo, Z.Y.; Liu, L.L.; Zheng, M.B.; Chen, Z.; Ma, A.Q.; Liang, R.J.; Han, Z.Q.; Lu, C.Y.; Cai, L.T. Cancer Cell Membrane-Biomimetic Oxygen Nanocarrier for Breaking Hypoxia-Induced Chemoresistance. Adv. Funct. Mater. 2017, 27, 1703197. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.L.; Cai, B.; Xu, J.H.; Li, A.; Zhang, W.F.; Sun, Z.J.; Guo, S.S.; Liu, W.; Wang, T.H. Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv. Mater. 2016, 28, 3460. [Google Scholar] [CrossRef]
- Sun, H.P.; Su, J.H.; Meng, Q.S.; Yin, Q.; Chen, L.L.; Gu, W.W.; Zhang, Z.W.; Yu, H.J.; Zhang, P.C.; Wang, S.L. Cancer Cell Membrane-Coated Gold Nanocages with Hyperthermia-Triggered Drug Release and Homotypic Target Inhibit Growth and Metastasis of Breast Cancer. Adv. Funct. Mater. 2017, 27, 1604300. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zheng, D.W.; Zhang, M.K.; Yu, W.Y.; Qiu, W.X.; Hu, J.J.; Feng, J.; Zhang, X.Z. Preferential Cancer Cell Self-Recognition and Tumor Self-Targeting by Coating Nanoparticles with Homotypic Cancer Cell Membranes. Nano Lett. 2016, 16, 5895–5901. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Yang, C.X.; Yan, X.P. Biomimetic Persistent Luminescent Nanoplatform for Autofluorescence-Free Metastasis Tracking and Chemophotodynamic Therapy. Anal. Chem. 2018, 90, 4188–4195. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.; Khort, P.; Fedotov, O.; Chumachenko, V.; Virych, P.; Warren, H.S.; Booth, B.W.; Bliznyuk, V.; Kutsevol, N. Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer. Molecules 2024, 29, 2224. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.L.; Carter, S.L.; Wileyto, E.P.; Miller, J.; Yuan, M.; Yu, G.Q.; Durham, A.C.; Busch, T.M. Tumor vascular microenvironment determines responsiveness to photodynamic therapy. Cancer. Res. 2012, 72, 2079–2088. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Lan, X.Y.; Lin, H.M.; Xi, Q.Y.; Wang, M.C.; Quan, X.L.; Yao, G.Y.; Yu, Z.Q.; Wang, Y.X.; Yu, M. Tumor microenvironment-regulating nanomedicine design to fight multi-drug resistant tumors. Wires Nanomed. Nanobi. 2023, 15, e1842. [Google Scholar] [CrossRef]
- Wan, Y.L.; Fu, L.H.; Li, C.Y.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, 2103978. [Google Scholar] [CrossRef]
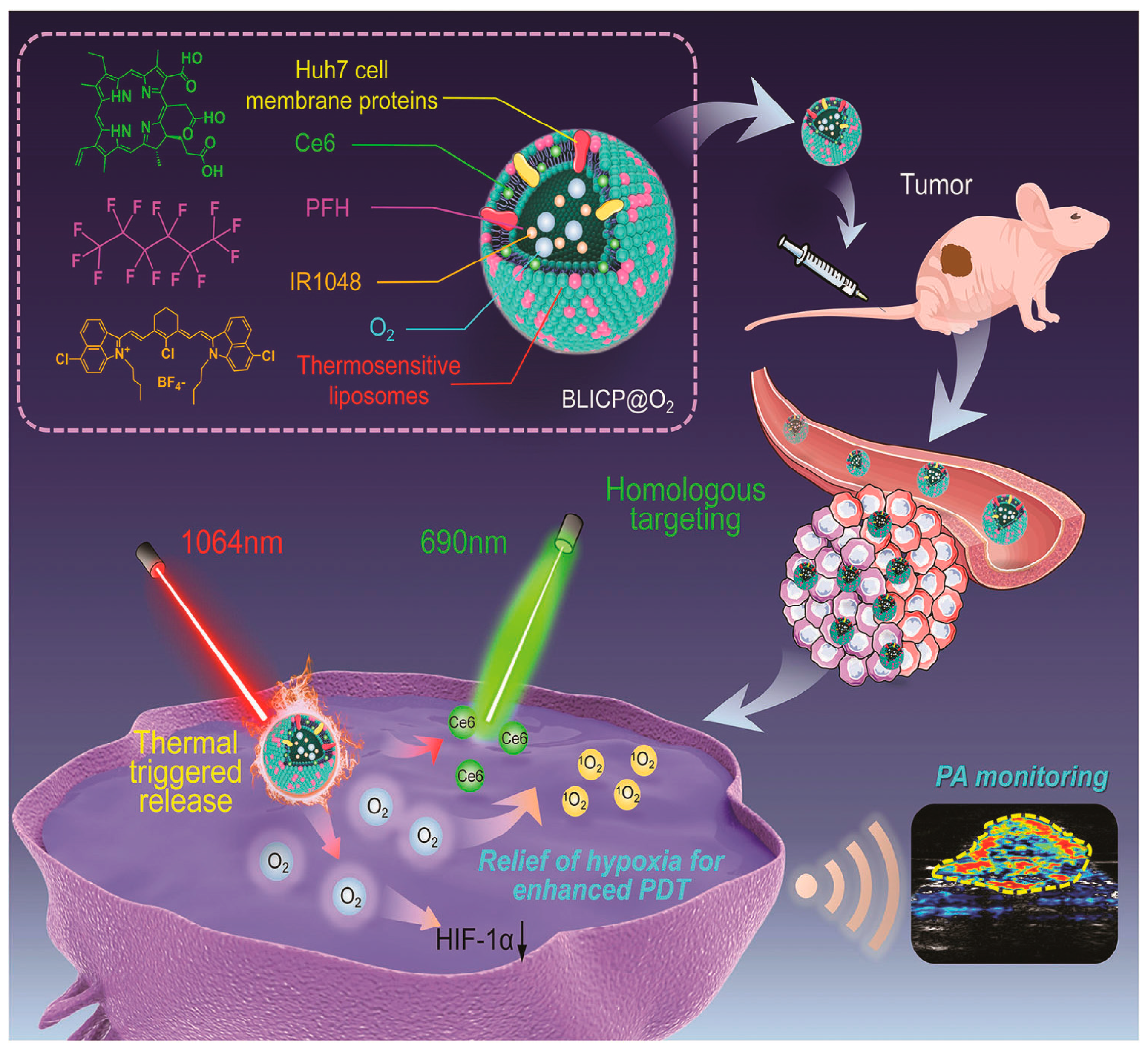
- Zeng, S.L.; Chen, J.Q.; Gao, R.K.; Chen, R.; Xue, Q.; Ren, Y.G.; Liu, L.J.; Tang, C.Y.; Hu, H.Y.; Zeng, N. NIR-II Photoacoustic Imaging-Guided Oxygen Delivery and Controlled Release Improves Photodynamic Therapy for Hepatocellular Carcinoma. Adv. Mater. 2024, 36, 2308780. [Google Scholar] [CrossRef]
- Lu, G.H.; Wang, X.J.; Li, F.; Wang, S.; Zhao, J.W.; Wang, J.Y.; Liu, J.; Lyu, C.L.; Ye, P.; Tan, H. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 2022, 13, 4214. [Google Scholar] [CrossRef]
- Xu, H.W.; Zhang, Y.; Zhang, H.T.; Zhang, Y.R.; Xu, Q.Q.; Lu, J.Y.; Feng, S.P.; Luo, X.Y.; Wang, S.L.; Zhao, Q.F. Smart polydopamine-based nanoplatforms for biomedical applications: State-of-art and further perspectives. Coordin. Chem. Rev. 2023, 488, 215153. [Google Scholar] [CrossRef]
- Wang, L.P.; Wang, W.B.; Wang, Y.F.; Tao, W.L.; Hou, T.X.; Cai, D.F.; Liu, L.K.; Liu, C.; Jiang, K.; Lin, J.Y. The Graphene Quantum Dots Gated Nanoplatform for Photothermal-Enhanced Synergetic Tumor Therapy. Molecules 2024, 29, 615. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhao, S.J.; Li, B.L.; Wang, B.H.; Lan, M.H.; Song, X.Z. Low temperature photothermal therapy: Advances and perspectives. Coordin. Chem. Rev. 2022, 454, 214330. [Google Scholar] [CrossRef]
- Yi, X.; Duan, Q.Y.; Wu, F.G. Low-Temperature Photothermal Therapy: Strategies and Applications. Research 2021, 2021, 9816594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.J.; Li, T.Y.; Cheng, H.; Zhang, F.; Yang, X.Y.; Wang, S.H.; Zhou, J.P.; Ding, Y. Nanomedicine potentiates mild photothermal therapy for tumor ablation. Asian. J. Pharm. Sci. 2021, 16, 738–761. [Google Scholar] [CrossRef]
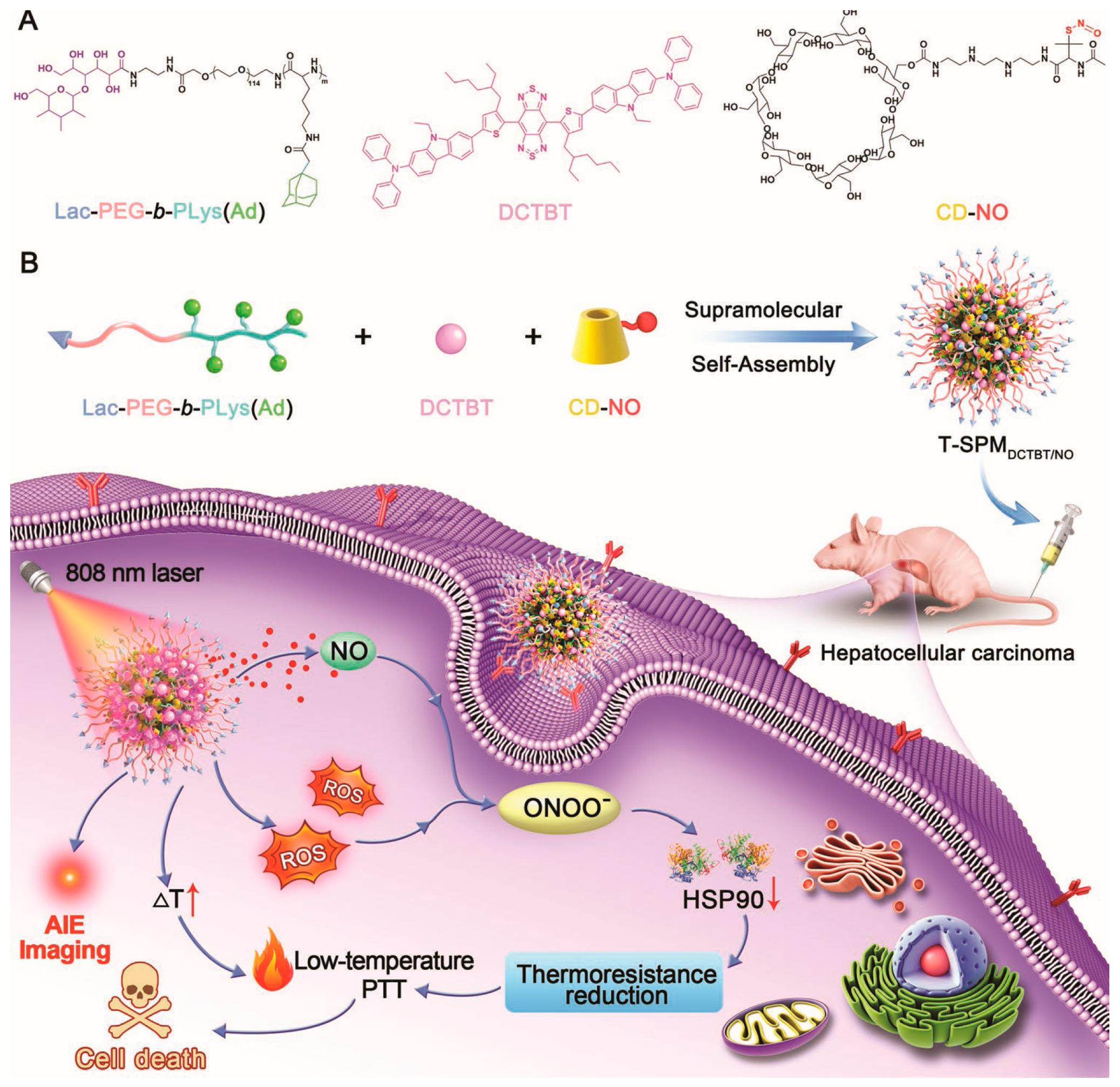
- Hu, H.T.; Li, D.; Dai, W.B.; Jin, Q.; Wang, D.; Ji, J.; Tang, B.Z.; Tang, Z. A NIR-II AIEgen-based supramolecular nanodot for peroxynitrite-potentiated mild-temperature photothermal therapy of hepatocellular carcinoma. Adv. Funct. Mater. 2023, 33, 2213134. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.W.; Hou, C.X.; Shang, K.; Yang, K.; Tian, Z.M.; Pei, Z.C.; Qu, Y.Q.; Pei, Y.X. Dual-responsive dithio-polydopamine coated porous CeO(2) nanorods for targeted and synergistic drug delivery. Int. J. Nanomed. 2018, 13, 2161–2173. [Google Scholar] [CrossRef]
- Schmidt, K.; Prakash, T.P.; Donner, A.J.; Kinberger, G.A.; Gaus, H.J.; Low, A.; Ostergaard, M.E.; Bell, M.; Swayze, E.E.; Seth, P.P. Characterizing the effect of GalNAc and phosphorothioate backbone on binding of antisense oligonucleotides to the asialoglycoprotein receptor. Nucleic. Acids. Res. 2017, 45, 2294–2306. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Shi, M.H.; Zhang, J.L.; Wang, Y.; Peng, C.; Hu, H.Y.; Qiao, M.X.; Zhao, X.L. Tumor-specific nitric oxide generator to amplify peroxynitrite based on highly penetrable nanoparticles for metastasis inhibition and enhanced cancer therapy. Biomaterials 2022, 283, 121448. [Google Scholar] [CrossRef]
- Ruf, B.; Heinrich, B.; Greten, T.F. Immunobiology and immunotherapy of HCC: Spotlight on innate and innate-like immune cells. Cell. Mol. Immunol. 2021, 18, 112–127. [Google Scholar] [CrossRef]
- Li, F.W.; Wen, Z.F.; Wu, C.X.; Yang, Z.B.; Wang, Z.R.; Diao, W.J.; Chen, D.H.; Xu, Z.R.; Lu, Y.L.; Liu, W.K. Simultaneous Activation of Immunogenic Cell Death and cGAS-STING Pathway by Liver- and Mitochondria-Targeted Gold(I) Complexes for Chemoimmunotherapy of Hepatocellular Carcinoma. J. Med. Chem. 2024, 67, 1982–2003. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Hsu, F.F.; Qiu, J.T.T.; Chern, G.J.; Lee, Y.A.; Chang, C.C.; Huang, Y.T.; Sung, Y.C.; Chiang, C.C.; Huang, R.L. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Tsai, C.C.; Lee, J.M.; Fang, C.H.; Chang, K.S.; Wong, K.K.; Lin, C.T.; Qiu, J.T. The efficacy of a novel vaccine approach using tumor cells that ectopically express a codon-optimized murine GM-CSF in a murine tumor model. Vaccine 2016, 34, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; López-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, 459. [Google Scholar] [CrossRef]
- Huang, Y.H.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef]
- Liu, C.H.; Chern, G.J.; Hsu, F.F.; Huang, K.W.; Sung, Y.C.; Huang, H.C.; Qiu, J.T.; Wang, S.K.; Lin, C.C.; Wu, C.H. A multifunctional nanocarrier for efficient TRAIL-based gene therapy against hepatocellular carcinoma with desmoplasia in mice. Hepatology 2018, 67, 899–913. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Yan, J.Y.; Liu, C.Y.; Wu, Z.W.; Chien, C.T.; Chiu, W.C.; Lin, S.Y. Designed nucleus penetrating thymine-capped dendrimers: A potential vehicle for intramuscular gene transfection. J. Mater. Chem. B 2015, 3, 9060–9066. [Google Scholar] [CrossRef]
- Ou, D.L.; Liao, Z.X.; Kempson, I.M.; Li, L.; Yang, P.C.; Tseng, S.J. Nano-modified viruses prime the tumor microenvironment and promote the photodynamic virotherapy in liver cancer. J. Biomed. Sci. 2024, 31, 1. [Google Scholar] [CrossRef]
- Li, C.W.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, D.A.; Shutova, M.V.; Johnston, N.R.; Smith, O.P.; Fedorin, V.V.; Kukushkin, Y.S.; van der Loo, J.C.M.; Johnstone, E.C. From the analyst’s couch the clinical landscape for AAV gene therapies. Nat. Rev. Drug. Discov. 2021, 20, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.Y.; Liu, T.Q.; Tang, J.; Yang, Y.N.; Song, H.; Tuong, Z.K.; Fu, J.Y.; Yu, C.Z. Mechanism of Iron Oxide-Induced Macrophage Activation: The Impact of Composition and the Underlying Signaling Pathway. J. Am. Chem. Soc. 2019, 141, 6122–6126. [Google Scholar] [CrossRef]
- Chen, Y.B.; Song, Y.C.; Du, W.; Gong, L.L.; Chang, H.C.; Zou, Z.Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guérin, M.; Biton, J.; Ouakrim, H. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, E4041–E4050. [Google Scholar] [CrossRef]
- Tseng, S.J.; Huang, K.Y.; Kempson, I.M.; Kao, S.H.; Liu, M.C.; Yang, S.C.; Liao, Z.X.; Yang, P.C. Remote Control of Light-Triggered Virotherapy. Acs Nano 2016, 10, 10339–10346. [Google Scholar] [CrossRef]
- Tseng, S.J.; Kempson, I.M.; Huang, K.Y.; Li, H.J.; Fa, Y.C.; Ho, Y.C.; Liao, Z.X.; Yang, P.C. Targeting Tumor Microenvironment by Bioreduction-Activated Nanoparticles for Light-Triggered Virotherapy. Acs Nano 2018, 12, 9894–9902. [Google Scholar] [CrossRef]
- Tseng, S.J.; Liao, Z.X.; Kao, S.H.; Zeng, Y.F.; Huang, K.Y.; Li, H.J.; Yang, C.L.; Deng, Y.F.; Huang, C.F.; Yang, S.C. Highly specific in vivo gene delivery for p53-mediated apoptosis and genetic photodynamic therapies of tumour. Nat. Commun. 2015, 6, 6456. [Google Scholar] [CrossRef]
- Tseng, S.J.; Kempson, I.M.; Liao, Z.X.; Ho, Y.C.; Yang, P.C. An acid degradable, lactate oxidizing nanoparticle formulation for non-small cell lung cancer virotherapy. Nano Today 2022, 46, 101582. [Google Scholar] [CrossRef]
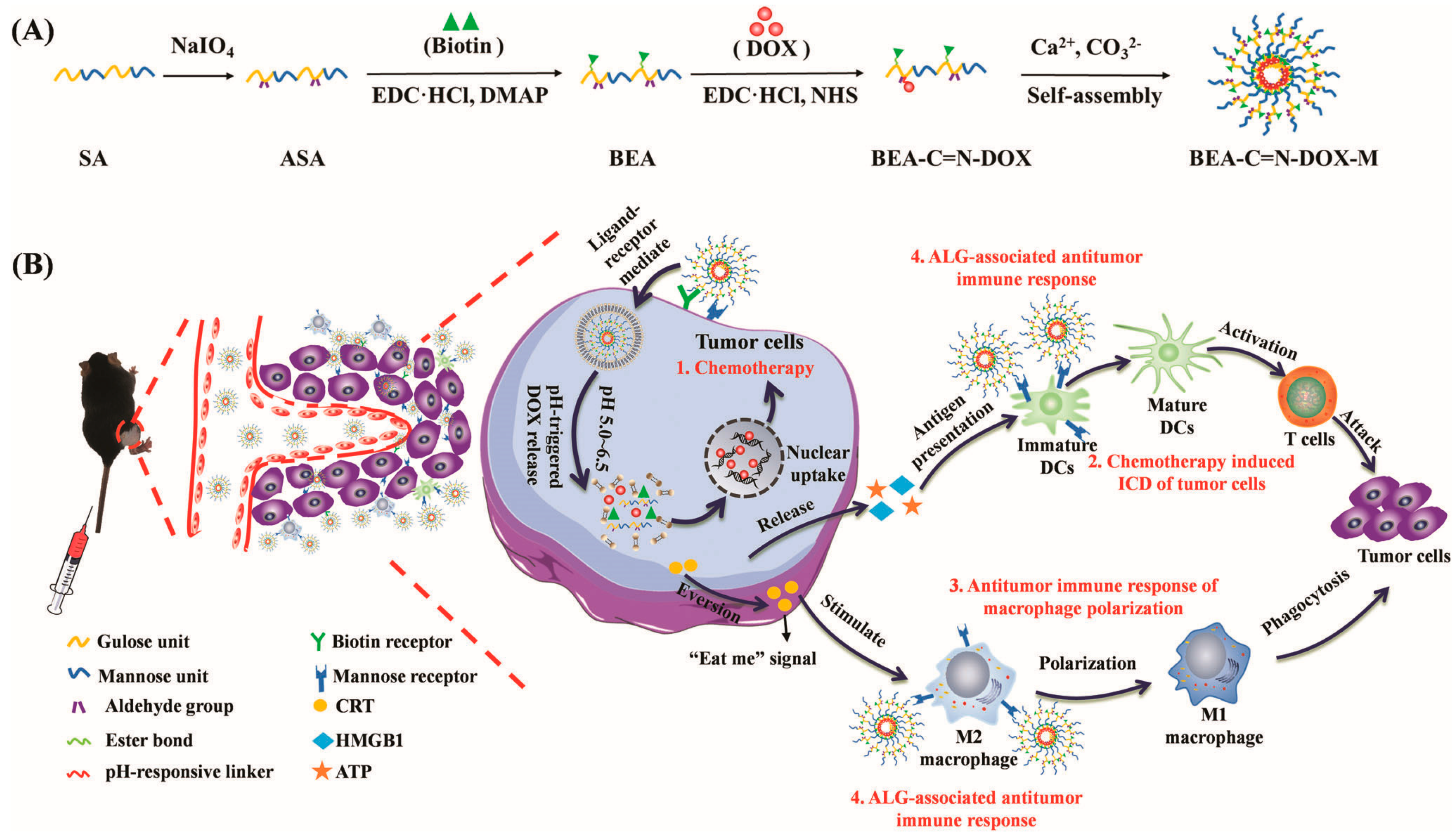
- Huang, C.; Xie, T.; Liu, Y.F.; Yan, S.; OuYang, F.J.; Zhang, H.T.; Lei, L.; He, D.X.; Wei, H.; Yu, C.Y. A Sodium Alginate-Based Multifunctional Nanoplatform for Synergistic Chemo-Immunotherapy of Hepatocellular Carcinoma. Adv. Mater. 2023, 35, 2301352. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: A focused review. Carbohyd. Polym. 2022, 277, 118881. [Google Scholar] [CrossRef] [PubMed]
- Spadari, C.C.; Lopes, L.B.; Ishida, K. Potential Use of Alginate-Based Carriers as Antifungal Delivery System. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Mühlebach, S. Regulatory challenges of nanomedicines and their follow-on versions: A generic or similar approach? Adv. Drug Deliv. Rev. 2018, 131, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.H.; Ye, Q.F.; Miao, X.Y.; Liu, X.; Huang, S.Q.; Xiong, L.; Wen, Y.; Zhang, Z.J. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics 2021, 11, 5464–5490. [Google Scholar] [CrossRef] [PubMed]
| Agent | Target | Clinical Stage |
|---|---|---|
| Sorafenib | VEGFR1–3, PDGFR, RAF, KIT | FDA-approved (2007) |
| Regorafenib | VEGFR1–3, PDGFR, RAF, FGFR1–2 | FDA-approved (2017) |
| Lenvatinib | VEGFR1–3, PDGFR, FGFR1–4, RET | FDA-approved (2018) |
| Pembrolizumab | PD1 | FDA-approved (2018) |
| Cabozantinib | VEGFR1–3, MET, RET | FDA-approved (2019) |
| Ramucirumab | VEGFR2 | FDA-approved (2019) |
| Atezolizumab + bevacizumab | PDL1 + VEGFA | FDA-approved (2020) |
| Donafenib | VEGFR1–3, PDGFR, RAF | Phase III (2020) |
| Apatinib | VEGFR2, KIT, RET, SRC | Phase III (2021) |
| Sintilimab + IBI305 | PD1 + VEGFA | Phase III (2021) |
| Atezolizumab + cabozantinib | PDL1 + multiple targets | Phase III (2021) |
| Durvalumab + tremelimumab | PDL1 + CTLA4 | Phase III (2022) |
| Durvalumab | PDL1 | Phase III (2022) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, Y.; Li, Z.; Wan, D.; Pan, J. Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma. Molecules 2024, 29, 4405. https://doi.org/10.3390/molecules29184405
Liu Y, Wu Y, Li Z, Wan D, Pan J. Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma. Molecules. 2024; 29(18):4405. https://doi.org/10.3390/molecules29184405
Chicago/Turabian StyleLiu, Yonghui, Yanan Wu, Zijian Li, Dong Wan, and Jie Pan. 2024. "Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma" Molecules 29, no. 18: 4405. https://doi.org/10.3390/molecules29184405
APA StyleLiu, Y., Wu, Y., Li, Z., Wan, D., & Pan, J. (2024). Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma. Molecules, 29(18), 4405. https://doi.org/10.3390/molecules29184405








