Integration of Transcriptomics and Metabolomics Reveals the Antitumor Mechanism of Protopanaxadiol Triphenylphosphate Derivative in Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Results
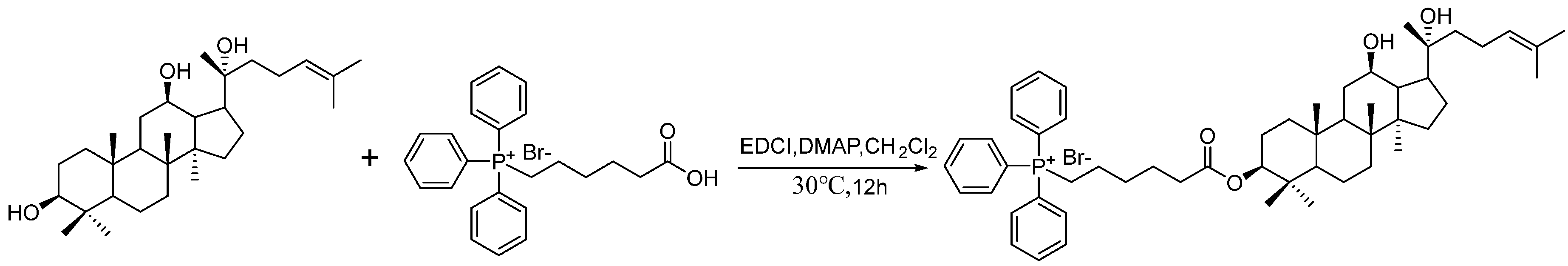
2.1. Synthesis of CTPPPPD
2.2. CTPPPPD Inhibiting A549 Cell Proliferation Activity
2.3. Analysis of Gene Expression Patterns and Anti-A549 Cell Activity of CTPPPPD
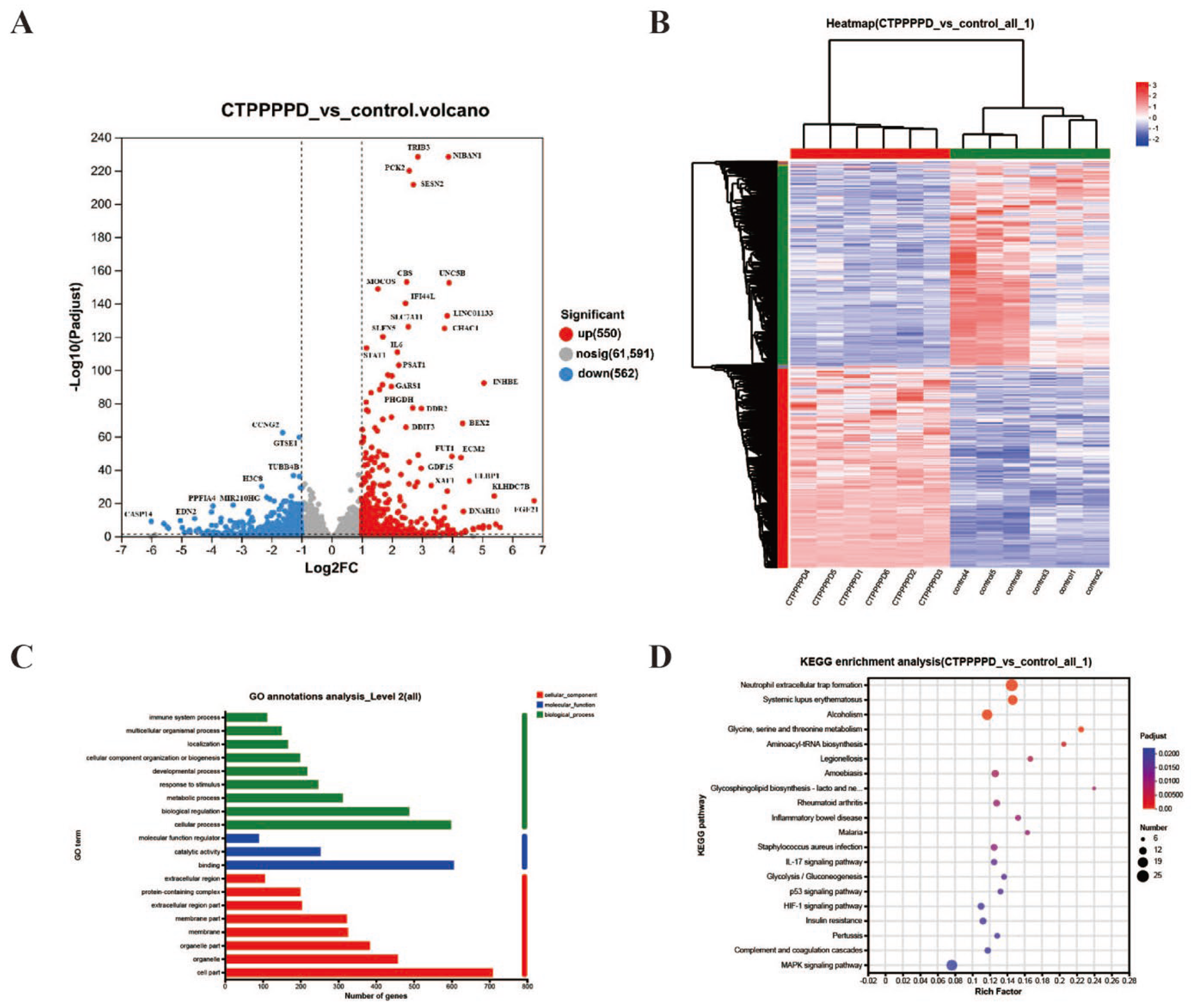
2.3.1. Quality Control of RNA-Seq Data
2.3.2. Differentially Expressed Genes (DEGs) in A549 Cells
2.4. Analysis of Accumulated Metabolites of Anti-A549 Cell Activity of CTPPPPD
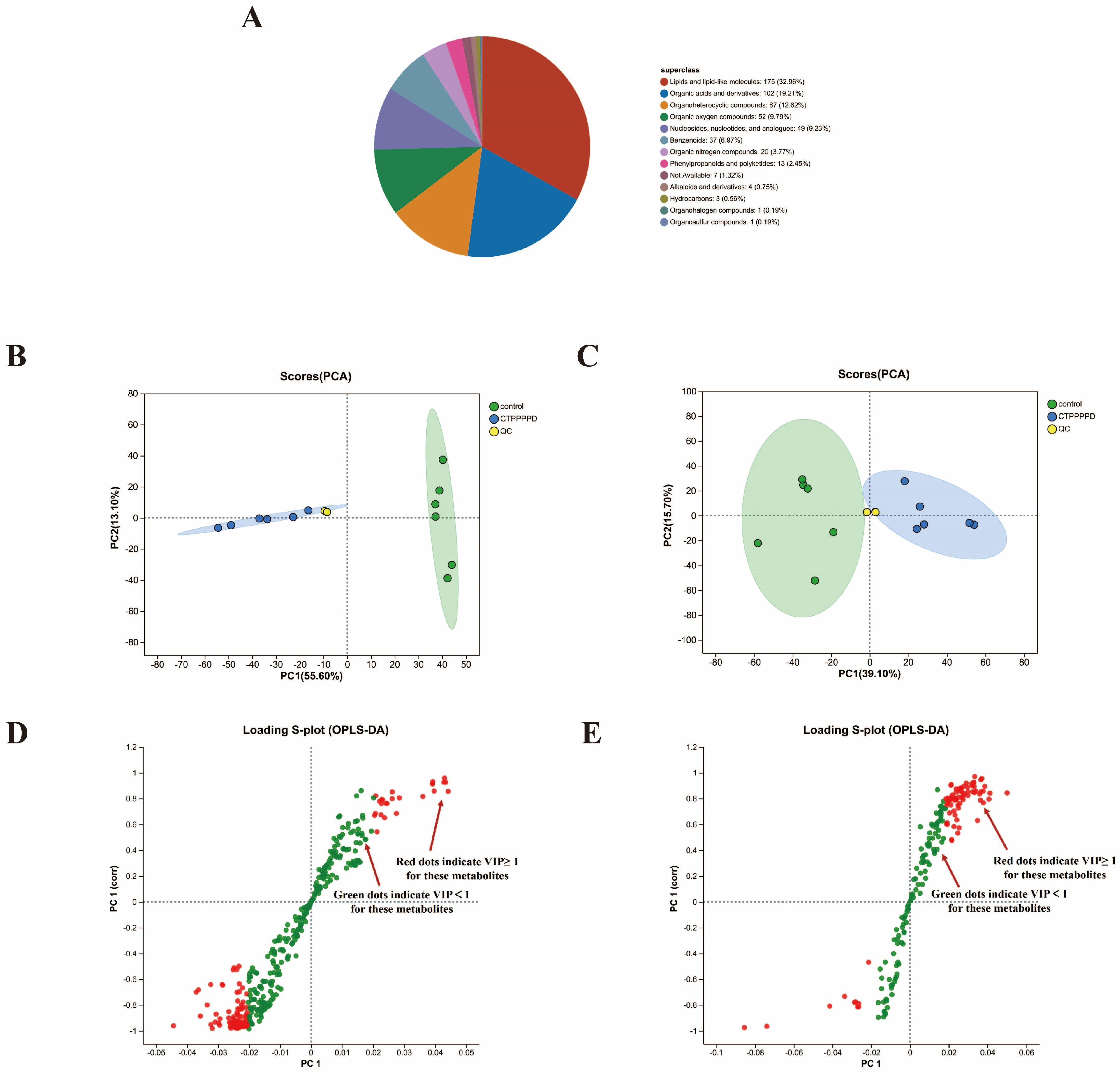
2.4.1. Identification and Characterization of Metabolites
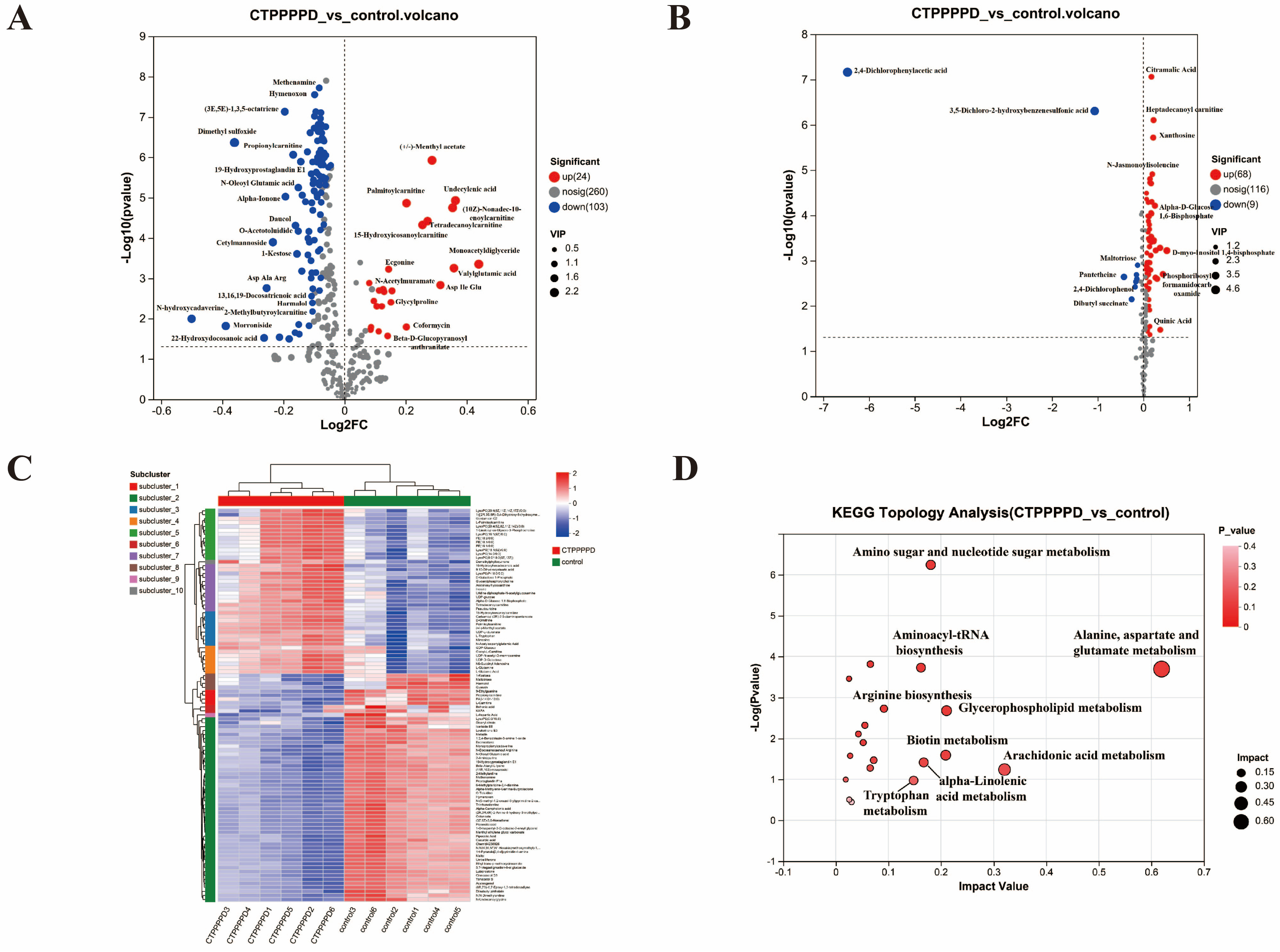
2.4.2. Differentially Accumulated Metabolites (DAMs) in A549 Cells
2.5. Integrated Analysis of CTPPPPD Effects on A549 Cells
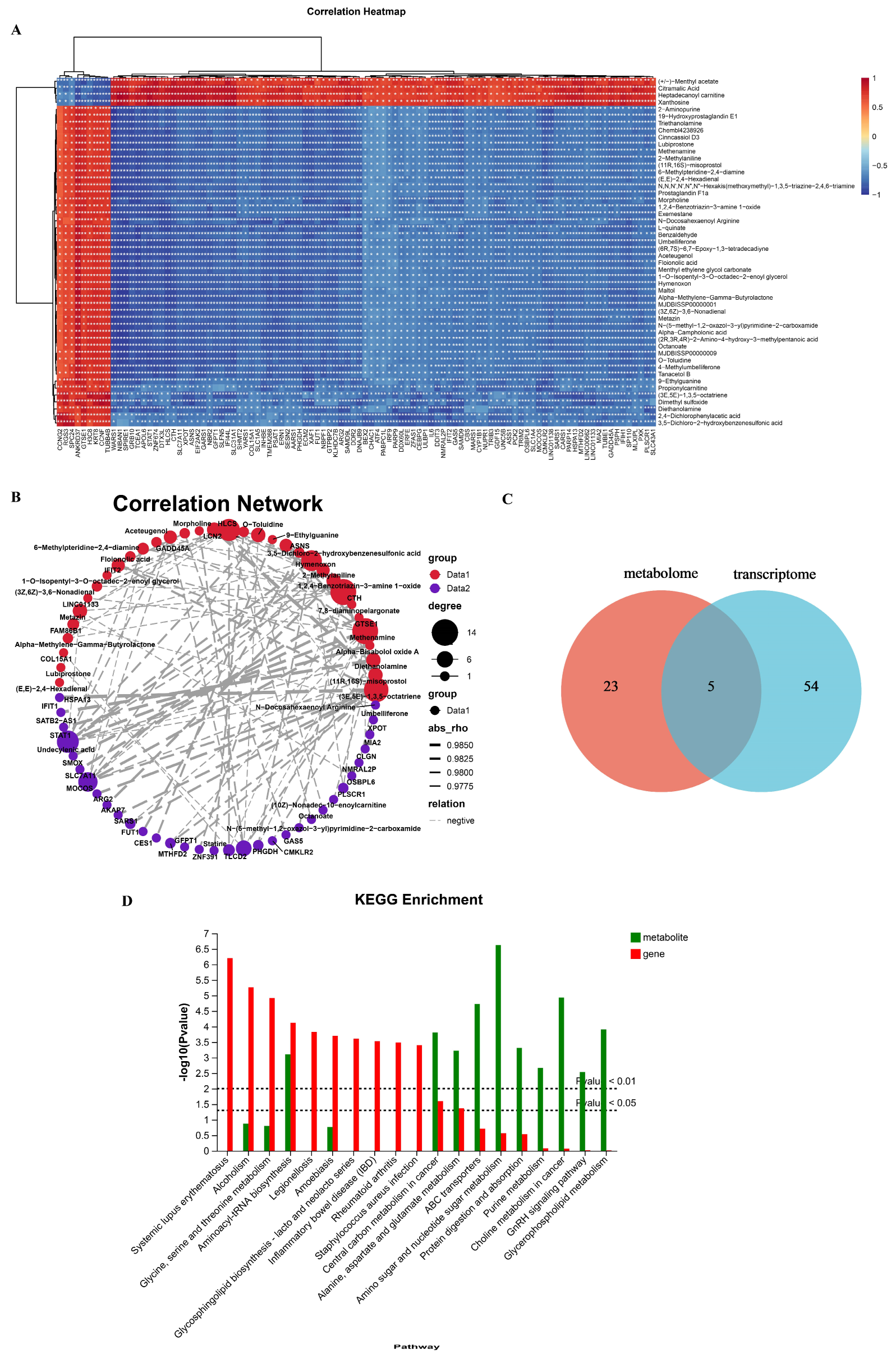
2.5.1. Analyzing Transcriptome and Metabolome Association in Metabolic Pathways
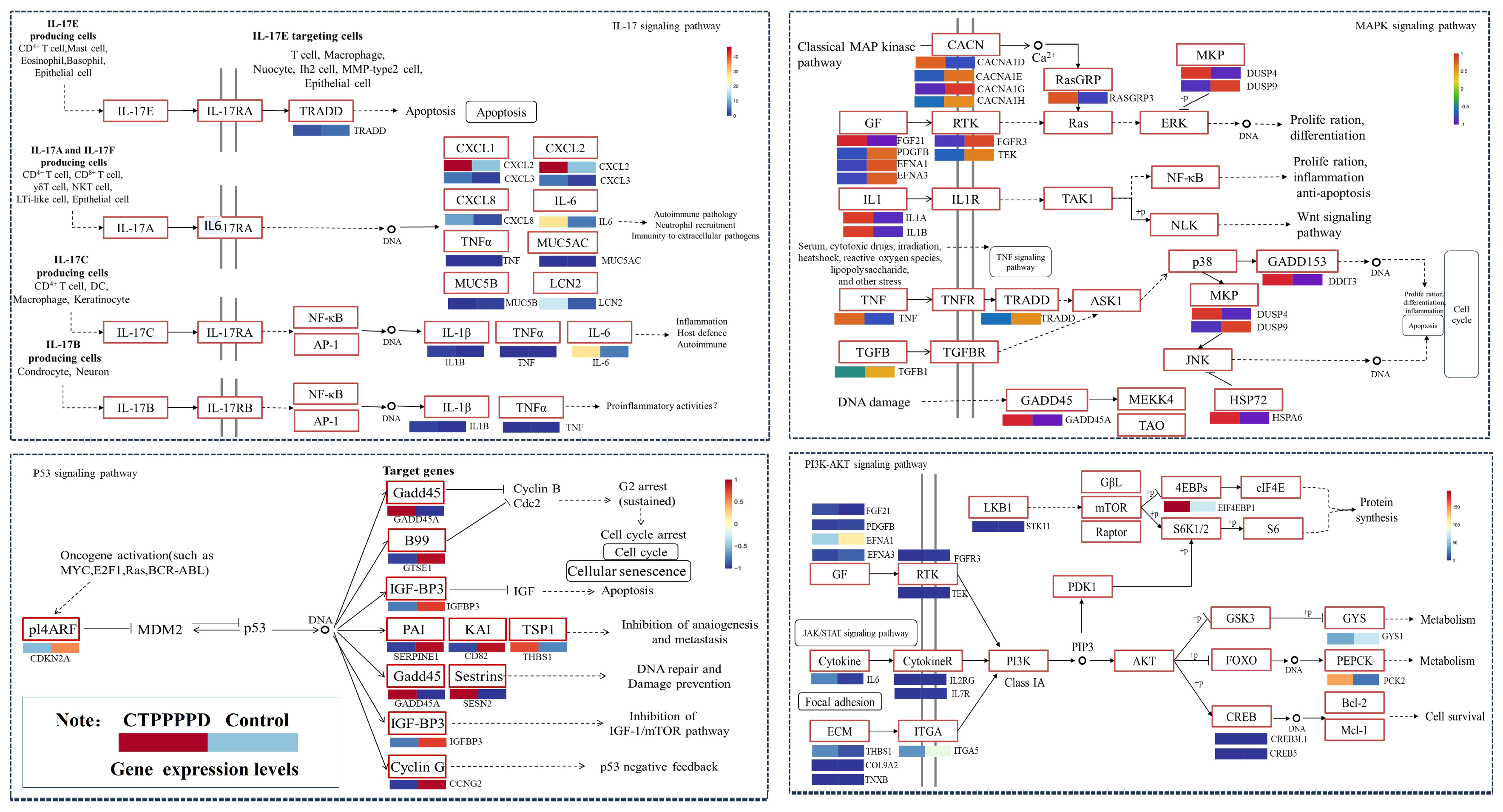
2.5.2. Effect of CTPPPPD on Important Relevant Pathways in NSCLC
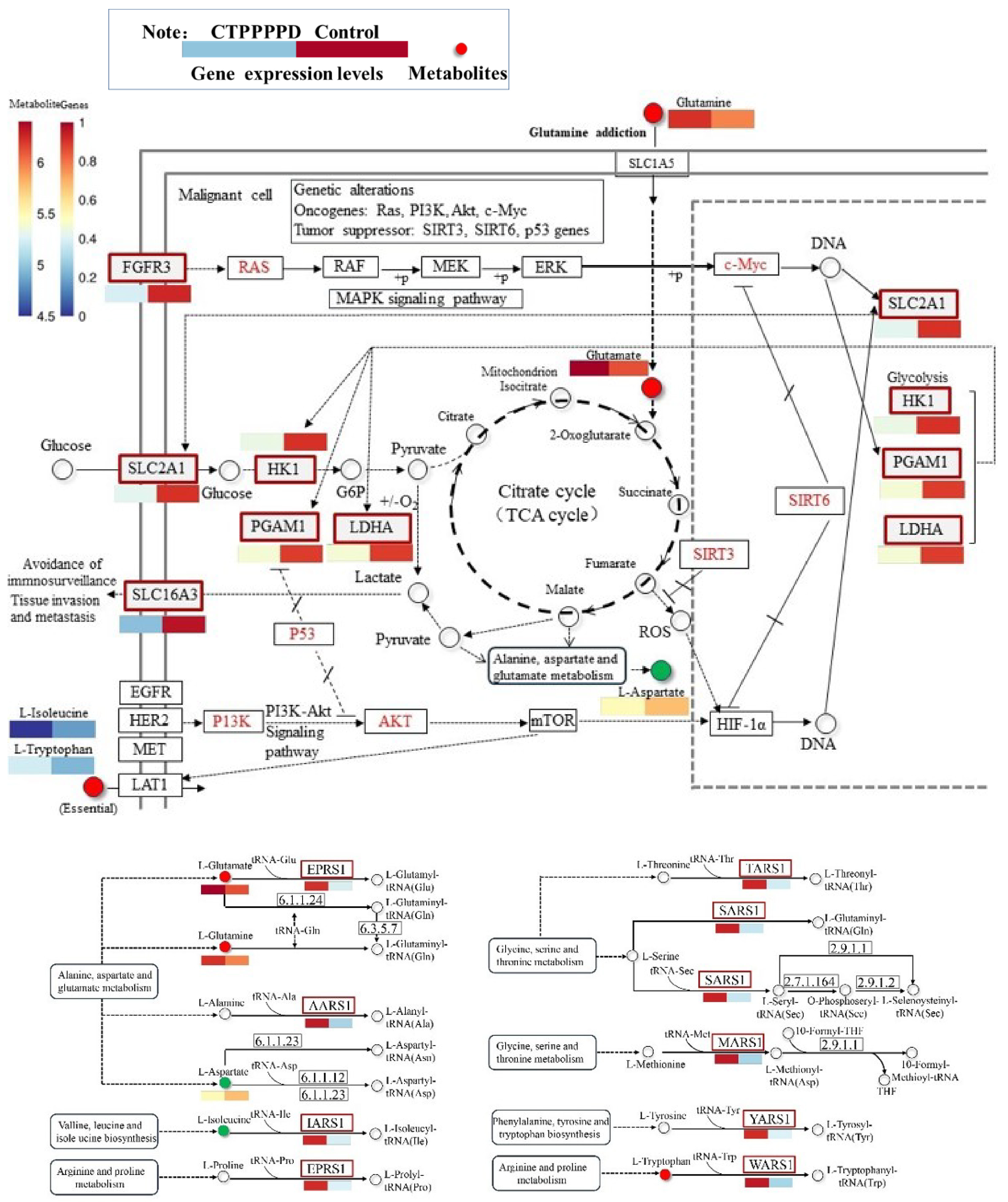
2.5.3. Effects of CTPPPPD on Pathways Related to NSCLC Metabolism
2.5.4. Quantitative Real-Time PCR (qRT-PCR)
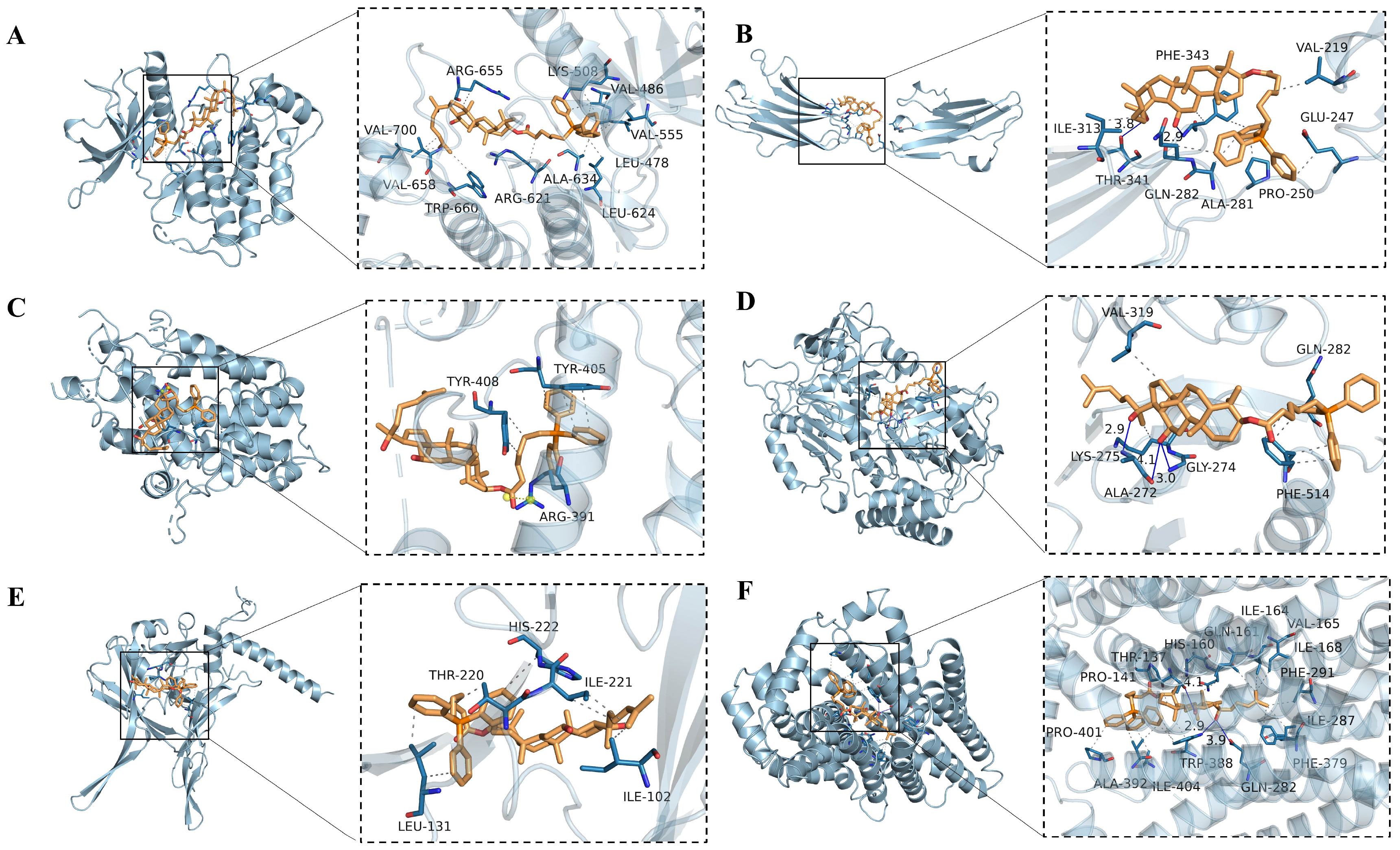
2.5.5. Molecular Docking of CTPPPPD
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of CTPPPPD
4.3. Cell Culture
4.4. Effect of CTPPPPD on A549 Cell Morphology
4.5. Apoptosis Analysis
4.6. Mitochondrial Membrane Potential (JC-1) Detection
4.7. Extraction of Total RNA and Transcriptome Analysis
4.8. Untargeted LC-MS Metabolomics Analysis
4.9. Real-Time Quantitative PCR (RT-qPCR)
4.10. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Rodríguez, D.D.N.; Navarro-Martin, A.; Cigarral, C.; Chicas-Sett, R.; García, R.; Garcia, V.; Gonzalez, J.A.; Gonzalo, S.; Murcia-Mejía, M.; Robaina, R.; et al. GOECP/SEOR radiotheraphy guidelines for non-small-cell lung cancer. World J. Clin. Oncol. 2022, 13, 237–266. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Tan, K.L.; Lee, H.C.; Cheah, P.S.; Ling, K.H. Mitochondrial Dysfunction in Down Syndrome: From Pathology to Therapy. Neuroscience 2023, 511, 1–12. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Gao, R.; Qu, Y.; Huang, Y.; Zhang, N.; Hu, S.; Fan, F.; Zou, Y.; Hu, K.; et al. Intravenous Transplantation of an Ischemic-specific Peptide-TPP-mitochondrial Compound Alleviates Myocardial Ischemic Reperfusion Injury. ACS Nano 2023, 17, 896–909. [Google Scholar] [CrossRef]
- Thuy, L.T.; Lee, S.; Dongquoc, V.; Choi, J.S. Nanoemulsion Composed of α-Tocopherol Succinate and Dequalinium Shows Mitochondria-Targeting and Anticancer Effects. Antioxidants 2023, 12, 437. [Google Scholar] [CrossRef]
- Heise, N.; Becker, S.; Mueller, T.; Bache, M.; Csuk, R.; Güttler, A. Mitochondria-Targeting 1,5-Diazacyclooctane-Spacered Triterpene Rhodamine Conjugates Exhibit Cytotoxicity at Sub-Nanomolar Concentration against Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 10695. [Google Scholar] [CrossRef]
- Zou, Y.; Nishikawa, M.; Kang, H.G.; Cheng, G.; Wang, W.; Wang, Y.; Komatsu, N. Effect of Protein Corona on Mitochondrial Targeting Ability and Cytotoxicity of Triphenylphosphonium Conjugated with Polyglycerol-Functionalized Nanodiamond. Mol. Pharm. 2021, 18, 2823–2832. [Google Scholar] [CrossRef]
- Peng, X.; Tang, S.; Tang, D.; Zhou, D.; Li, Y.; Chen, Q.; Wan, F.; Lukas, H.; Han, H.; Zhang, X.; et al. Autonomous metal-organic framework nanorobots for active mitochondria-targeted cancer therapy. Sci. Adv. 2023, 9, eadh1736. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Ma, J.; Wang, Y.; Qu, X.; Qiu, J.; Hua, K. Mitochondria-Targeted Mesoporous Organic Silica Nanoplatforms for Overcoming Cisplatin Resistance by Disturbing Mitochondrial Redox Homeostasis. Front. Chem. 2022, 10, 875818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, C.Z.; Mohammadi, S.; Sawadogo, W.R.; Ma, Q.; Yuan, C.S. Pharmacological Effects of Ginseng: Multiple Constituents and Multiple Actions on Humans. Am. J. Chin. Med. 2023, 51, 1085–1104. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, X.; Yang, C.; Liang, Z.; Guan, D.; Yuan, T.; Qi, W.; Zhao, D.; Li, X.; Dong, H.; et al. Structural Characters and Pharmacological Activity of Protopanaxadiol-Type Saponins and Protopanaxatriol-Type Saponins from Ginseng. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 9096774. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.J.; Shui, Y.M.; Wan, J.B.; Gao, J.L. Anticancer Activities of Protopanaxadiol- and Protopanaxatriol-Type Ginsenosides and Their Metabolites. Evid. Based Complement. Altern. Med. 2016, 2016, 5738694. [Google Scholar] [CrossRef]
- Kang, S.; Sunwoo, K.; Jung, Y.; Hur, J.K.; Park, K.H.; Kim, J.S.; Kim, D. Membrane-Targeting Triphenylphosphonium Functionalized Ciprofloxacin for Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 758. [Google Scholar] [CrossRef]
- Qin, L.; Cheng, X.; Wang, S.; Gong, G.; Su, H.; Huang, H.; Chen, T.; Damdinjav, D.; Dorjsuren, B.; Li, Z.; et al. Discovery of Novel Aminobutanoic Acid-Based ASCT2 Inhibitors for the Treatment of Non-Small-Cell Lung Cancer. J. Med. Chem. 2024, 67, 988–1007. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Ren, B.; Feng, J.; Yang, N.; Guo, Y.; Chen, C.; Qin, Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int. Immunopharmacol. 2021, 98, 107841. [Google Scholar] [CrossRef]
- Jo, H.; Jang, D.; Park, S.K.; Lee, M.G.; Cha, B.; Park, C.; Shin, Y.S.; Park, H.; Baek, J.M.; Heo, H.; et al. Ginsenoside 20(S)-protopanaxadiol induces cell death in human endometrial cancer cells via apoptosis. J. Ginseng Res. 2021, 45, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Huang, L.; Chen, M.; Zeng, W.; Feng, Z.; Huang, S.; Liu, T. Integrated Analysis of the Transcriptome and Metabolome Reveals Genes Involved in Terpenoid and Flavonoid Biosynthesis in the Loblolly Pine (Pinus taeda L.). Front. Plant Sci. 2021, 12, 729161. [Google Scholar] [CrossRef]
- Morgos, D.T.; Stefani, C.; Miricescu, D.; Greabu, M.; Stanciu, S.; Nica, S.; Stanescu-Spinu, I.I.; Balan, D.G.; Balcangiu-Stroescu, A.E.; Coculescu, E.C.; et al. Targeting PI3K/AKT/mTOR and MAPK Signaling Pathways in Gastric Cancer. Int. J. Mol. Sci. 2024, 25, 1848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Meng, N.N.; Liu, C.; Li, K.L.; Wang, M.X.; Lv, Z.B.; Chen, S.Y.; Guo, X.; Wang, X.K.; Wang, Q.; et al. PDB-1 from Potentilla discolor Bunge induces apoptosis and autophagy by downregulating the PI3K/Akt/mTOR signaling pathway in A549 cells. Biomed. Pharmacother. 2020, 129, 110378. [Google Scholar] [CrossRef]
- Smolle, E.; Leko, P.; Stacher-Priehse, E.; Brcic, L.; El-Heliebi, A.; Hofmann, L.; Quehenberger, F.; Hrzenjak, A.; Popper, H.H.; Olschewski, H.; et al. Distribution and prognostic significance of gluconeogenesis and glycolysis in lung cancer. Mol. Oncol. 2020, 14, 2853–2867. [Google Scholar] [CrossRef]
- Dingemans, A.M.; van den Boogaart, V.; Vosse, B.A.; van Suylen, R.J.; Griffioen, A.W.; Thijssen, V.L. Integrin expression profiling identifies integrin alpha5 and beta1 as prognostic factors in early stage non-small cell lung cancer. Mol. Cancer 2010, 9, 152. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, C.; Li, R. Integrin and gene network analysis reveals that ITGA5 and ITGB1 are prognostic in non-small-cell lung cancer. OncoTargets Ther. 2016, 9, 2317–2327. [Google Scholar] [CrossRef]
- Weng, T.Y.; Wang, C.Y.; Hung, Y.H.; Chen, W.C.; Chen, Y.L.; Lai, M.D. Differential Expression Pattern of THBS1 and THBS2 in Lung Cancer: Clinical Outcome and a Systematic-Analysis of Microarray Databases. PLoS ONE 2016, 11, e0161007. [Google Scholar] [CrossRef]
- Guo, C.L.; Wang, L.J.; Zhao, Y.; Liu, H.; Li, X.Q.; Jiang, B.; Luo, J.; Guo, S.J.; Wu, N.; Shi, D.Y. A Novel Bromophenol Derivative BOS-102 Induces Cell Cycle Arrest and Apoptosis in Human A549 Lung Cancer Cells via ROS-Mediated PI3K/Akt and the MAPK Signaling Pathway. Mar. Drugs 2018, 16, 43. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, H.; Jiang, T.; Xiao, W.; Zhao, S.; Yu, X.; Han, F. FGFR3 silencing by siRNA inhibits invasion of A549 cells. Oncol. Lett. 2016, 12, 4319–4326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, M.J.; Wang, X.; Chen, Y.; Li, G.J.; Zhao, G.Q.; Xiang, B.Q.; Wei, X.Q.; Lei, Y.J.; Huang, Y.C. The influences of TGF-β1 upon the human adenocarcinoma cell of lung A549 and cellular immunity. Ann. Transl. Med. 2020, 8, 1076. [Google Scholar] [CrossRef]
- Tan, W.; Liao, Y.; Qiu, Y.; Liu, H.; Tan, D.; Wu, T.; Tang, M.; Zhang, S.; Wang, H. miRNA 146a promotes chemotherapy resistance in lung cancer cells by targeting DNA damage inducible transcript 3 (CHOP). Cancer Lett. 2018, 428, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Vaddavalli, P.L.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in cancer and aging. Trends Genet. 2022, 38, 598–612. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Zhou, Z.S.; Wang, G.Z.; Tu, J.Y.; Cheng, H.B.; Ma, S.Z.; Ke, C.; Wang, Y.; Jian, Q.P.; Shu, Y.H.; et al. MiR-122-5p regulates the mevalonate pathway by targeting p53 in non-small cell lung cancer. Cell Death Dis. 2023, 14, 234. [Google Scholar] [CrossRef]
- Chi, F.; Wang, Z.; Li, Y.; Chang, N. Knockdown of GINS2 inhibits proliferation and promotes apoptosis through the p53/GADD45A pathway in non-small-cell lung cancer. Biosci. Rep. 2020, 40, BSR20193949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Meng, J.; Jiang, H.; Feng, X.; Wei, D.; Meng, W. GTSE1 Facilitates the Malignant Phenotype of Lung Cancer Cells via Activating AKT/mTOR Signaling. Anal. Cell. Pathol. 2021, 2021, 5589532. [Google Scholar] [CrossRef]
- Wang, K.; Mei, Z.; Zheng, M.; Liu, X.; Li, D.; Wang, H. FTO-mediated autophagy inhibition promotes non-small cell lung cancer progression by reducing the stability of SESN2 mRNA. Heliyon 2024, 10, e27571. [Google Scholar] [CrossRef]
- Du, L.; Zhang, Z.; Xu, Q.; Chen, N. Central metabolic pathway modification to improve L-tryptophan production in Escherichia coli. Bioengineered 2019, 10, 59–70. [Google Scholar] [CrossRef]
- Montal, E.D.; Dewi, R.; Bhalla, K.; Ou, L.; Hwang, B.J.; Ropell, A.E.; Gordon, C.; Liu, W.J.; DeBerardinis, R.J.; Sudderth, J.; et al. PEPCK Coordinates the Regulation of Central Carbon Metabolism to Promote Cancer Cell Growth. Mol. Cell 2015, 60, 571–583. [Google Scholar] [CrossRef]
- Lv, J.; Zhou, Z.; Wang, J.; Yu, H.; Lu, H.; Yuan, B.; Han, J.; Zhou, R.; Zhang, X.; Yang, X.; et al. Prognostic value of lactate dehydrogenase expression in different cancers: A meta-analysis. Am. J. Med. Sci. 2019, 358, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chong, Y.; Chen, M.; Dai, W.; Zhou, X.; Ji, Y.; Qiu, G.; Du, X. Targeting lactate dehydrogenase a improves radiotherapy efficacy in non-small cell lung cancer: From bedside to bench. J. Transl. Med. 2021, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, H.; Kong, T.; Chen, S.; Li, P.; Chen, L.; Cheng, J.; Cui, G.; Zhang, G. PGAM1, regulated by miR-3614-5p, functions as an oncogene by activating transforming growth factor-β (TGF-β) signaling in the progression of non-small cell lung carcinoma. Cell Death Dis. 2020, 11, 710. [Google Scholar] [CrossRef]
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer—From tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chai, B.; Wang, X.; Wu, Z.; Gu, Z.; Liu, X.; Zhao, Y.; Chen, T.; Ma, Z.; Sun, Q. miRNA-199a-5p/SLC2A1 axis regulates glucose metabolism in non-small cell lung cancer. J. Cancer 2022, 13, 2352–2361. [Google Scholar] [CrossRef]
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef]
- Tao, Q.; Li, X.; Zhu, T.; Ge, X.; Gong, S.; Guo, J.; Ma, R. Lactate Transporter SLC16A3 (MCT4) as an Onco-Immunological Biomarker Associating Tumor Microenvironment and Immune Responses in Lung Cancer. Int. J. Gen. Med. 2022, 15, 4465–4474. [Google Scholar] [CrossRef]
- Jia, X.B.; Zhang, Q.; Xu, L.; Yao, W.J.; Wei, L. Lotus leaf flavonoids induce apoptosis of human lung cancer A549 cells through the ROS/p38 MAPK pathway. Biol. Res. 2021, 54, 7. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Rasoulian, F.; Eskandani, M.; Akbari Nakhjavani, S.; Eskandani, M. Acriflavine-loaded solid lipid nanoparticles: Preparation, physicochemical characterization, and anti-proliferative properties. Pharm. Dev. Technol. 2021, 26, 934–942. [Google Scholar] [CrossRef]
- Bian, X.; Chen, L.; Bian, X.; Li, L.; Liu, D.; Liu, S.; Xu, L.; Huo, X.; Yang, X. Protective effect of Tibetan medicine Qiwei Tiexie pills on liver injury induced by acetaminophen overdose: An integrated strategy of network pharmacology, metabolomics and transcriptomics. Phytomedicine 2024, 123, 155221. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, Y.; Shi, Y.; Feng, C.; Xu, L.; Chen, Z.; Sun, X.; Zhao, Y.; Sun, X. Multi-omics reveals that 5-O-methylvisammioside prevention acute liver injury in mice by regulating the TNF/MAPK/NF-κB/arachidonic acid pathway. Phytomedicine 2024, 128, 155550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xie, C.; Wang, K.; Takahashi, S.; Krausz, K.W.; Lu, D.; Wang, Q.; Luo, Y.; Gong, X.; Mu, X.; et al. Comprehensive analysis of transcriptomics and metabolomics to understand triptolide-induced liver injury in mice. Toxicol. Lett. 2020, 333, 290–302. [Google Scholar] [CrossRef] [PubMed]



| Pathway | Metabolites | Gene Symbol |
|---|---|---|
| Central carbon metabolism in cancer | L-Glutamine; L-Tryptophan; L-Glutamic Acid; L-Isoleucine; L-Aspartic Acid | SLC16A3; FGFR3; LDHA; HK1; PGAM1; SLC2A1 |
| Aminoacyl-tRNA biosynthesis | L-Glutamine; L-Tryptophan; L-Glutamic Acid; L-Isoleucine; L-Aspartic Acid | YARS1; MARS1; AARS1; SARS1; EPRS1; IARS1; WARS1; TARS1 |
| Proximal tubule bicarbonate reclamation | L-Glutamine; L-Glutamic Acid | AQP1; ATP1A3; PCK2 |
| Arginine biosynthesis | L-Aspartic Acid; L-Glutamine; L-Glutamic Acid | NAGS; ASS1; ARG2 |
| Alanine, aspartate and glutamate metabolism | N-Acetylaspartylglutamic Acid; L-Glutamine; L-Glutamic Acid; L-Aspartic Acid | ASNS; GFPT1; ASS1; RIMKLA |
| Gene | PDB ID | Compound | Binding Affinity (kcal/mol) |
|---|---|---|---|
| FGFR3 | 4K33 | CTPPPPD | −7.9 |
| FGFR3 | 3GRW | CTPPPPD | −7.1 |
| SESN2 | 5CUF | CTPPPPD | −7.3 |
| PCK2 | 5I67 | CTPPPPD | −9.0 |
| TGF-β1 | 5VQP | CTPPPPD | −6.9 |
| SLC2A1 | 6THA | CTPPPPD | −9.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Bian, X.; Ma, X.; Ren, T.; Li, Y.; Huang, L.; Tang, Z.; Gao, L.; Chang, S.; Sun, X. Integration of Transcriptomics and Metabolomics Reveals the Antitumor Mechanism of Protopanaxadiol Triphenylphosphate Derivative in Non-Small-Cell Lung Cancer. Molecules 2024, 29, 4275. https://doi.org/10.3390/molecules29174275
Han L, Bian X, Ma X, Ren T, Li Y, Huang L, Tang Z, Gao L, Chang S, Sun X. Integration of Transcriptomics and Metabolomics Reveals the Antitumor Mechanism of Protopanaxadiol Triphenylphosphate Derivative in Non-Small-Cell Lung Cancer. Molecules. 2024; 29(17):4275. https://doi.org/10.3390/molecules29174275
Chicago/Turabian StyleHan, Liu, Xingbo Bian, Xiangyu Ma, Ting Ren, Yawei Li, Lijing Huang, Zebo Tang, Liancong Gao, Sheng Chang, and Xin Sun. 2024. "Integration of Transcriptomics and Metabolomics Reveals the Antitumor Mechanism of Protopanaxadiol Triphenylphosphate Derivative in Non-Small-Cell Lung Cancer" Molecules 29, no. 17: 4275. https://doi.org/10.3390/molecules29174275
APA StyleHan, L., Bian, X., Ma, X., Ren, T., Li, Y., Huang, L., Tang, Z., Gao, L., Chang, S., & Sun, X. (2024). Integration of Transcriptomics and Metabolomics Reveals the Antitumor Mechanism of Protopanaxadiol Triphenylphosphate Derivative in Non-Small-Cell Lung Cancer. Molecules, 29(17), 4275. https://doi.org/10.3390/molecules29174275





