Influence of Solvent Relative Permittivity in Swab Spray Mass Spectrometry
Abstract
1. Introduction
1.1. Discovery of Electrospray
1.2. Principles of Electrospray
1.3. Electrospray Ionization in Ambient Mass Spectrometry
2. Results and Discussion
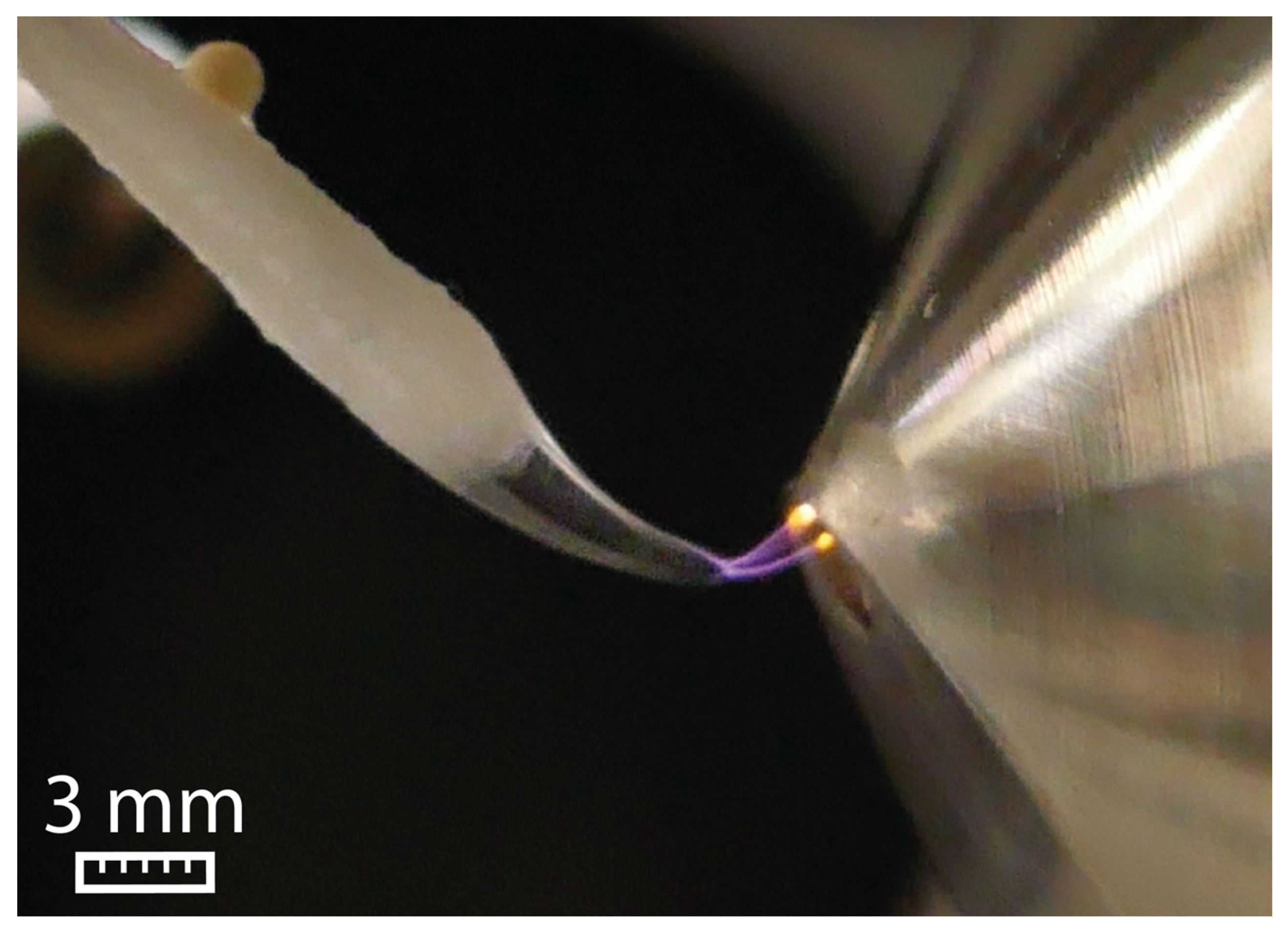
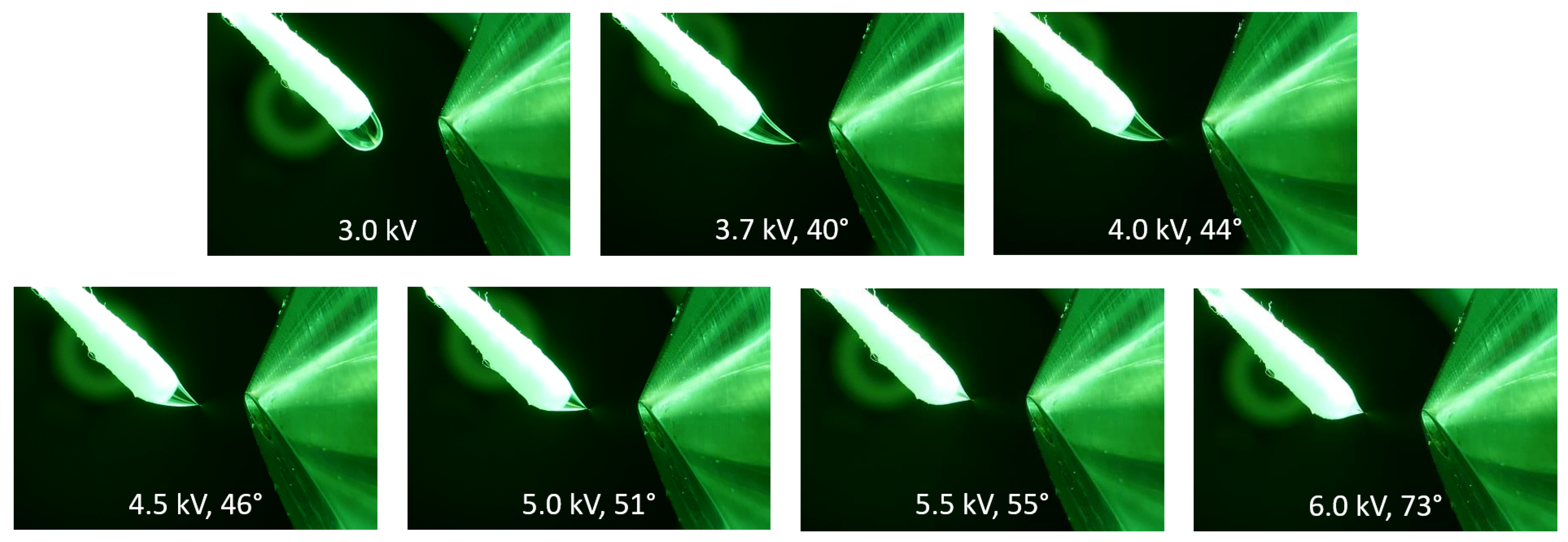
2.1. Impact of the Electric Potential on Taylor Cone Formation
2.2. The Different Taylor Cone Modes
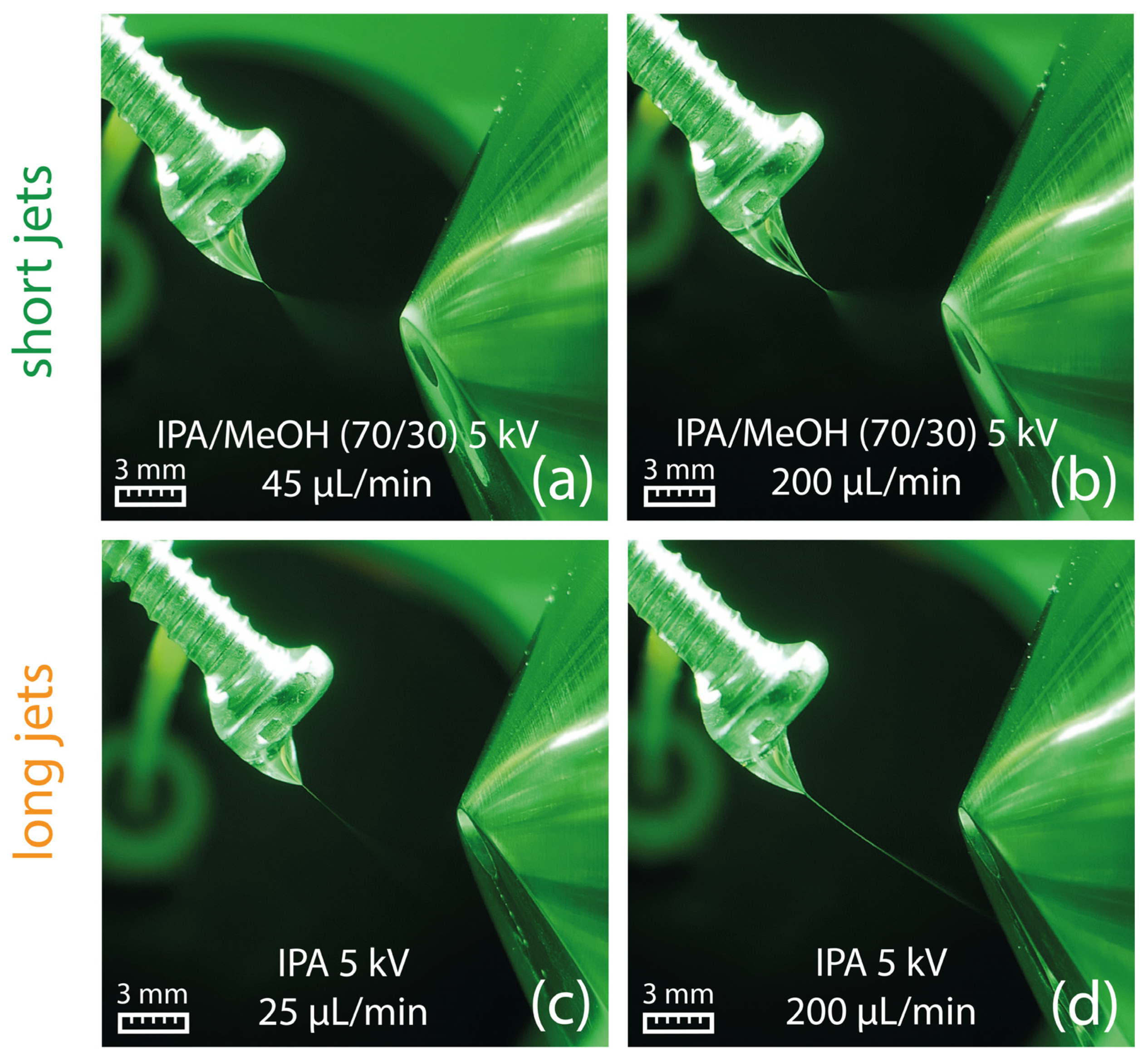
2.3. Taylor Cones with Short Jets
2.4. Taylor Cones with Long Jets
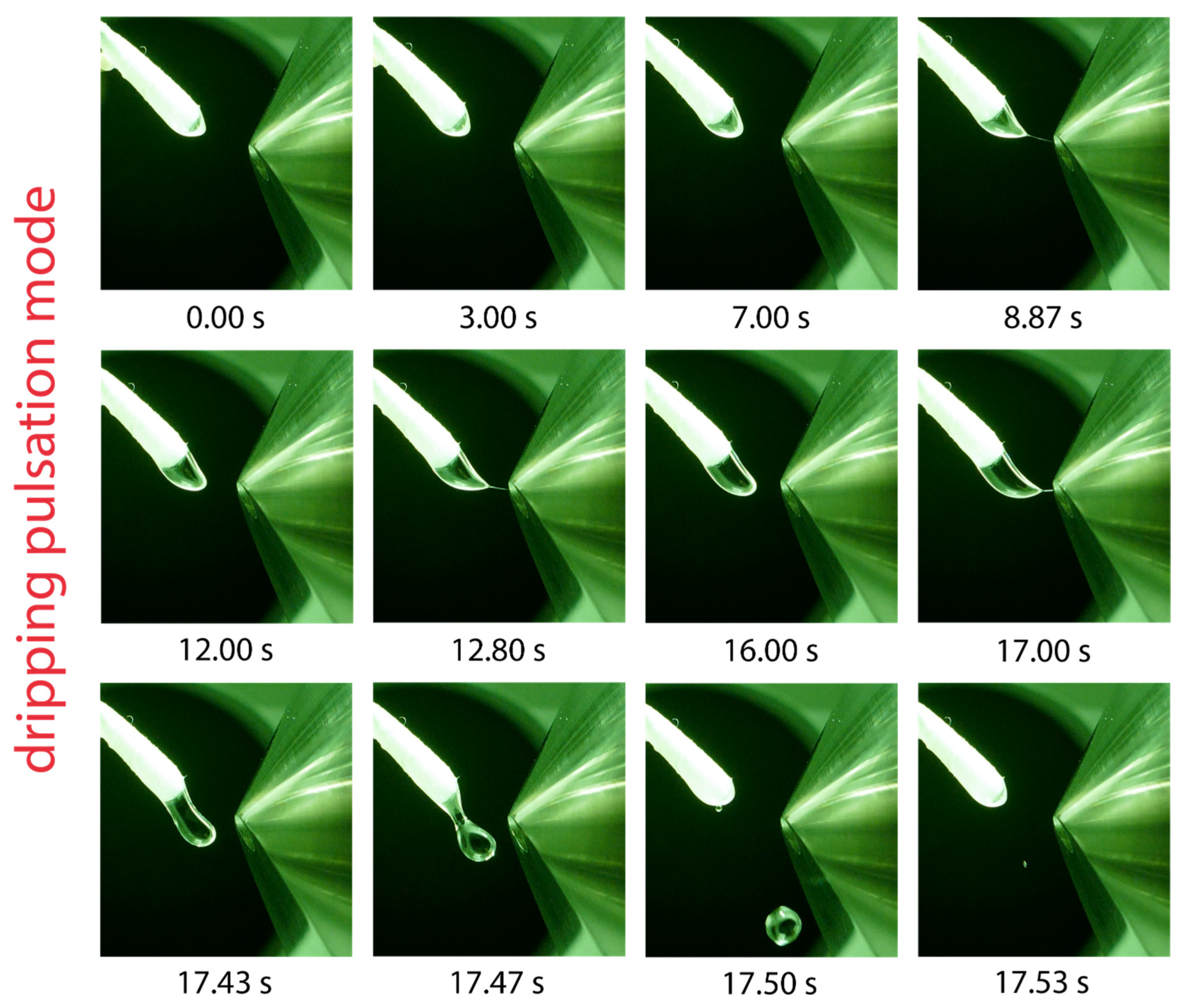
2.5. Dripping Pulsation Mode
2.6. Spindle Pulsation Mode
2.7. Taylor Cones with Long Jets by Non-Viscous Solvents
2.8. Jet Formation in Capillary-Based Electrospray Ion Sources
2.9. Jet Formation in Paper Spray
3. Material and Methods
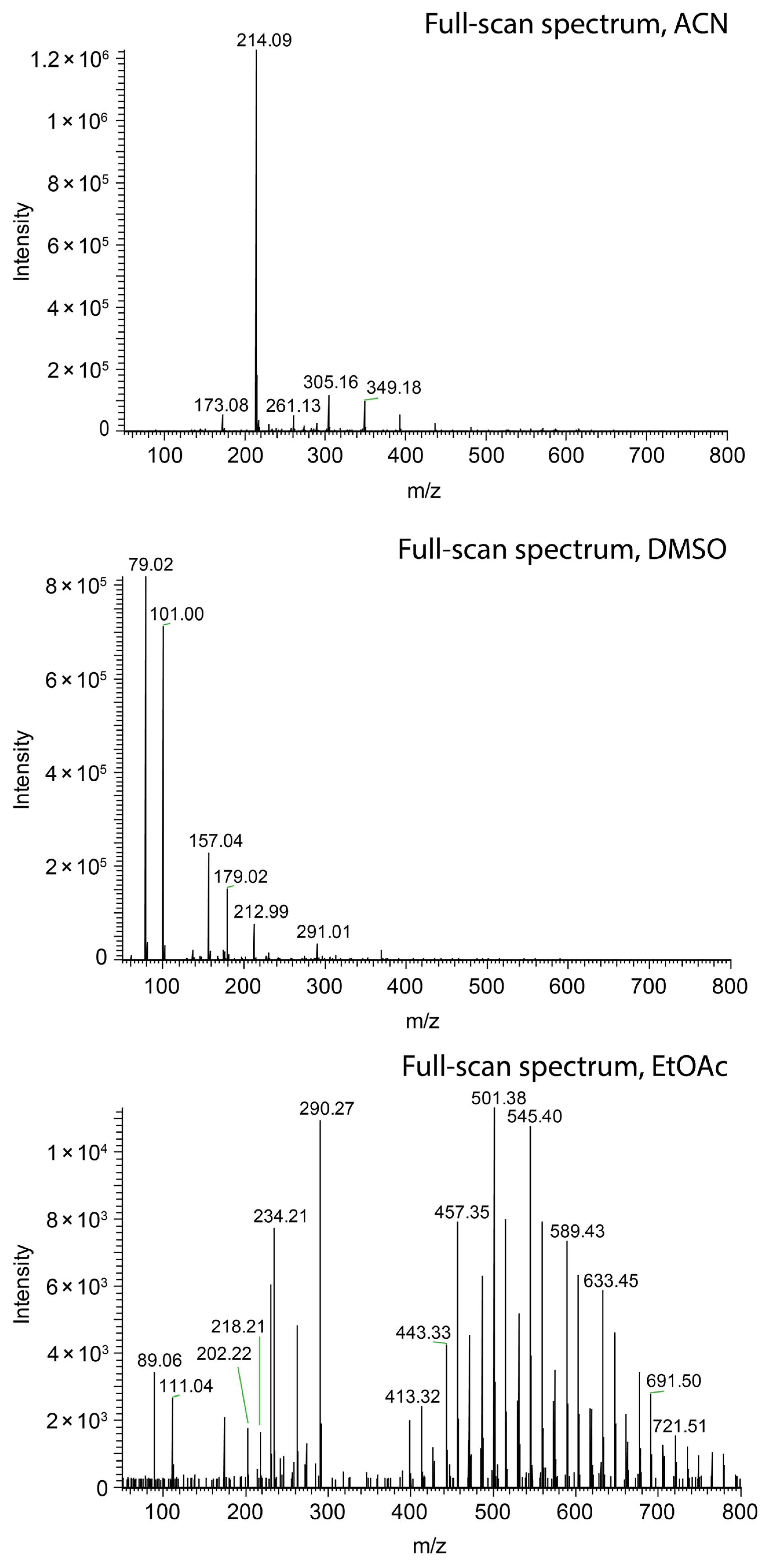
3.1. Instrumentation
3.2. Chemicals and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeleny, J. The Electrical Discharge from Liquid Points, and a Hydrostatic Method of Measuring the Electric Intensity at Their Surfaces. Phys. Rev. 1914, 3, 69–91. [Google Scholar] [CrossRef]
- Taylor, G.I. Disintegration of Water Drops in an Electric Field. Proc. R. Soc. Lond. A Math. Phys. Sci. 1964, 280, 383–397. [Google Scholar] [CrossRef]
- Melcher, J.R.; Taylor, G.I. Electrohydrodynamics: A Review of the Role of Interfacial Shear Stresses. Annu. Rev. Fluid. Mech. 1969, 1, 111–146. [Google Scholar] [CrossRef]
- Cloupeau, M.; Prunet-Foch, B. Electrohydrodynamic Spraying Functioning Modes: A Critical Review. J. Aerosol Sci. 1994, 25, 1021–1036. [Google Scholar] [CrossRef]
- de la Mora, J.F. The Fluid Dynamics of Taylor Cones. Annu. Rev. Fluid. Mech. 2006, 39, 217–243. [Google Scholar] [CrossRef]
- Dole, M.; Mack, L.L.; Hines, R.L.; Mobley, R.C.; Ferguson, L.D.; Alice, M.B. Molecular Beams of Macroions. J. Chem. Phys. 1968, 49, 2240–2249. [Google Scholar] [CrossRef]
- Yamashita, M.; Fenn, J.B. Electrospray Ion Source. Another Variation on the Free-Jet Theme. J. Phys. Chem. 1984, 88, 4451–4459. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray Ionization for Mass Spectrometry of Large Biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef]
- Verenchikov, A.N.; Krasnov, N.V.; Shkurov, V.A. Electrospray Ionization Developed by Lidija Gall’s Group. Int. J. Mass Spectrom. 2023, 490, 117067. [Google Scholar] [CrossRef]
- Wilm, M. Principles of Electrospray Ionization. Mol. Cell. Proteom. 2011, 10, M111.009407. [Google Scholar] [CrossRef]
- Subbotin, A.V.; Semenov, A.N. Electrohydrodynamics of Stationary Cone-Jet Streaming. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150290. [Google Scholar] [CrossRef]
- Zong, D.; Hu, H.; Duan, Y.; Sun, Y. Viscosity of Water under Electric Field: Anisotropy Induced by Redistribution of Hydrogen Bonds. J. Phys. Chem. B 2016, 120, 4818–4827. [Google Scholar] [CrossRef] [PubMed]
- Bateni, A.; Amirfazli, A.; Neumann, A.W. Effects of an Electric Field on the Surface Tension of Conducting Drops. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 25–38. [Google Scholar] [CrossRef]
- Ismail, A.S.; Yao, J.; Xia, H.H.; Stark, J.P.W. Breakup Length of Electrified Liquid Jets: Scaling Laws and Applications. Phys. Rev. Appl. 2018, 10, 064010. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, Z.; Wan, X.; Fang, H.; Yu, D.-G.; Chen, X.; Liu, P. The Relationships between Process Parameters and Polymeric Nanofibers Fabricated Using a Modified Coaxial Electrospinning. Nanomaterials 2019, 9, 843. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Jiang, Y.; Yang, S. Experimental Study of Electro-Spraying Modes of Deionized Water in Atmospheric Environment. Exp. Comput. Multiph. Flow 2021, 3, 38–46. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, J.-H.; Ogata, A. Time-Resolved High-Speed Camera Observation of Electrospray. J. Aerosol Sci. 2011, 42, 249–263. [Google Scholar] [CrossRef]
- Nemes, P.; Marginean, I.; Vertes, A. Spraying Mode Effect on Droplet Formation and Ion Chemistry in Electrosprays. Anal. Chem. 2007, 79, 3105–3116. [Google Scholar] [CrossRef]
- Jaworek, A.; Krupa, A. Generation and Characteristics of the Precession Mode of Ehd Spraying. J. Aerosol Sci. 1996, 27, 75–82. [Google Scholar] [CrossRef]
- Pongrác, B.; Kim, H.-H.; Negishi, N.; Machala, Z. Influence of Water Conductivity on Particular Electrospray Modes with Dc Corona Discharge—Optical Visualization Approach. Eur. Phys. J. D 2014, 68, 224. [Google Scholar] [CrossRef]
- Castillo-Orozco, E.; Kar, A.; Kumar, R. Electrospray Mode Transition of Microdroplets with Semiconductor Nanoparticle Suspension. Sci. Rep. 2017, 7, 5144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, S.J.; Hong, J.G. Spray Mode and Monodisperse Droplet Properties of an Electrospray. ACS Omega 2022, 7, 28667–28674. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A. The Electrospray: Fundamentals and Applications. In Experimental Heat Transfer, Fluid. Mechanics and Thermodynamics 1993; Kelleher, M.D., Sreenivasan, K.R., Shah, R.K., Joshi, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 270–282. ISBN 978-0-444-81619-1. [Google Scholar]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the Mechanism of Electrospray Ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef]
- Thinius, M.; Polaczek, C.; Langner, M.; Bräkling, S.; Haack, A.; Kersten, H.; Benter, T. Charge Retention/Charge Depletion in ESI-MS: Experimental Evidence. J. Am. Soc. Mass Spectrom. 2020, 31, 773–784. [Google Scholar] [CrossRef]
- Valeja, S.G.; Tipton, J.D.; Emmett, M.R.; Marshall, A.G. New Reagents for Enhanced Liquid Chromatographic Separation and Charging of Intact Protein Ions for Electrospray Ionization Mass Spectrometry. Anal. Chem. 2010, 82, 7515–7519. [Google Scholar] [CrossRef][Green Version]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Manicke, N.E.; Lin, J.-M.; Cooks, R.G.; Ouyang, Z. Development, Characterization, and Application of Paper Spray Ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Pirro, V.; Jarmusch, A.K.; Vincenti, M.; Cooks, R.G. Direct Drug Analysis from Oral Fluid Using Medical Swab Touch Spray Mass Spectrometry. Anal. Chim. Acta 2015, 861, 47–54. [Google Scholar] [CrossRef]
- Yang, B.; Wang, F.; Yang, X.; Zou, W.; Wang, J.; Zou, Y.; Liu, F.; Liu, H.; Huang, O. Medical Swab Touch Spray-Mass Spectrometry for Newborn Screening of Nicotine and Cotinine in Meconium. J. Mass Spectrom. 2016, 51, 1237–1242. [Google Scholar] [CrossRef]
- Pirro, V.; Llor, R.S.; Jarmusch, A.K.; Alfaro, C.M.; Cohen-Gadol, A.A.; Hattab, E.M.; Cooks, R.G. Analysis of Human Gliomas by Swab Touch Spray-Mass Spectrometry: Applications to Intraoperative Assessment of Surgical Margins and Presence of Oncometabolites. Analyst 2017, 142, 4058–4066. [Google Scholar] [CrossRef]
- Costa, C.; van Es, E.M.; Sears, P.; Bunch, J.; Palitsin, V.; Cooper, H.; Bailey, M.J. Exploring a Route to a Selective and Sensitive Portable System for Explosive Detection—Swab Spray Ionisation Coupled to of High-Field Assisted Waveform Ion Mobility Spectrometry (FAIMS). Forensic Sci. Int. 2019, 1, 214–220. [Google Scholar] [CrossRef]
- Bain, R.M.; Fedick, P.W.; Dilger, J.M.; Cooks, R.G. Analysis of Residual Explosives by Swab Touch Spray Ionization Mass Spectrometry. Propellants Explos. Pyrotech. 2018, 43, 1139–1144. [Google Scholar] [CrossRef]
- Jarmusch, A.K.; Pirro, V.; Kerian, K.S.; Cooks, R.G. Detection of Strep Throat Causing Bacterium Directly from Medical Swabs by Touch Spray-Mass Spectrometry. Analyst 2014, 139, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Fedick, P.W.; Bain, R.M. Swab Touch Spray Mass Spectrometry for Rapid Analysis of Organic Gunshot Residue from Human Hand and Various Surfaces Using Commercial and Fieldable Mass Spectrometry Systems. Forensic Chem. 2017, 5, 53–57. [Google Scholar] [CrossRef]
- Morato, N.M.; Pirro, V.; Fedick, P.W.; Cooks, R.G. Quantitative Swab Touch Spray Mass Spectrometry for Oral Fluid Drug Testing. Anal. Chem. 2019, 91, 7450–7457. [Google Scholar] [CrossRef] [PubMed]
- Jarmusch, A.K.; Pirro, V.; Logsdon, D.L.; Cooks, R.G. Direct Ion Generation from Swabs. Talanta 2018, 184, 356–363. [Google Scholar] [CrossRef]
- Beneito-Cambra, M.; Gilbert-López, B.; Moreno-González, D.; Bouza, M.; Franzke, J.; García-Reyes, J.F.; Molina-Díaz, A. Ambient (Desorption/Ionization) Mass Spectrometry Methods for Pesticide Testing in Food: A Review. Anal. Methods 2020, 12, 4831–4852. [Google Scholar] [CrossRef]
- Muggli, T.M.; Schürch, S. Analysis of Pesticide Residues on Fruit Using Swab Spray Ionization Mass Spectrometry. Molecules 2023, 28, 6611. [Google Scholar] [CrossRef]
- Shamraeva, M.A.; Bormotov, D.S.; Shamarina, E.V.; Bocharov, K.V.; Peregudova, O.V.; Pekov, S.I.; Nikolaev, E.N.; Popov, I.A. Spherical Sampler Probes Enhance the Robustness of Ambient Ionization Mass Spectrometry for Rapid Drugs Screening. Molecules 2022, 27, 945. [Google Scholar] [CrossRef]
- Budhwani, K.I.; Pekmezi, G.M.; Selim, M.M. Measuring Surface and Interfacial Tension In Situ in Microdripping Mode for Electrohydrodynamic Applications. Micromachines 2020, 11, 687. [Google Scholar] [CrossRef]
- Maheshwari, S.; Chetwani, N.; Chang, H.-C. Alternating Current Electrospraying. Ind. Eng. Chem. Res. 2009, 48, 9358–9368. [Google Scholar] [CrossRef]
- Rumble, J. (Ed.) CRC Handbook of Chemistry and Physics, 104th ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429170195. [Google Scholar]
- Neumaier, L.; Schilling, J.; Bardow, A.; Gross, J. Dielectric Constant of Mixed Solvents Based on Perturbation Theory. Fluid Phase Equilibria 2022, 555, 113346. [Google Scholar] [CrossRef]
- Chen, W.-S.; Hsieh, M.-Y. Dielectric Constant Calculation Based on Mixture Equations of Binary Composites at Microwave Frequency. Ceram. Int. 2017, 43, S343–S350. [Google Scholar] [CrossRef]
- Tang, K.; Smith, R.D. Physical/Chemical Separations in the Break-up of Highly Charged Droplets from Electrosprays. J. Am. Soc. Mass Spectrom. 2001, 12, 343–347. [Google Scholar] [CrossRef]
- Gao, J.; Austin, D.E. Mechanistic Investigation of Charge Separation in Electrospray Ionization Using Microparticles to Record Droplet Charge State. J. Am. Soc. Mass Spectrom. 2020, 31, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.P.A.; Brunner, D.J.; Camelot, D.M.A.; Marijnissen, J.C.M.; Scarlett, B. Jet break-up in electrohydrodynamic atomization in the cone-jet mode. J. Aerosol Sci. 2000, 31, 65–95. [Google Scholar] [CrossRef]
- Morad, M.R.; Rajabi, A.; Razavi, M.; Sereshkeh, S.R.P. A Very Stable High Throughput Taylor Cone-Jet in Electrohydrodynamics. Sci. Rep. 2016, 6, 38509. [Google Scholar] [CrossRef]
- Collins, R.T.; Harris, M.T.; Basaran, O.A. Breakup of Electrified Jets. J. Fluid Mech. 2007, 588, 75–129. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Kim, B.; Kim, S. Optimization and Application of Paper-Based Spray Ionization Mass Spectrometry for Analysis of Natural Organic Matter. Anal. Chem. 2018, 90, 12027–12034. [Google Scholar] [CrossRef]
- McBride, E.M.; Mach, P.M.; Dhummakupt, E.S.; Dowling, S.; Carmany, D.O.; Demond, P.S.; Rizzo, G.; Manicke, N.E.; Glaros, T. Paper Spray Ionization: Applications and Perspectives. TrAC Trends Anal. Chem. 2019, 118, 722–730. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Song, W.-Y.; Kim, T.-Y. Characterization of Spray Modes and Factors Affecting the Ionization Efficiency of Paper Spray Ionization. Front. Chem. 2022, 10, 864184. [Google Scholar] [CrossRef] [PubMed]


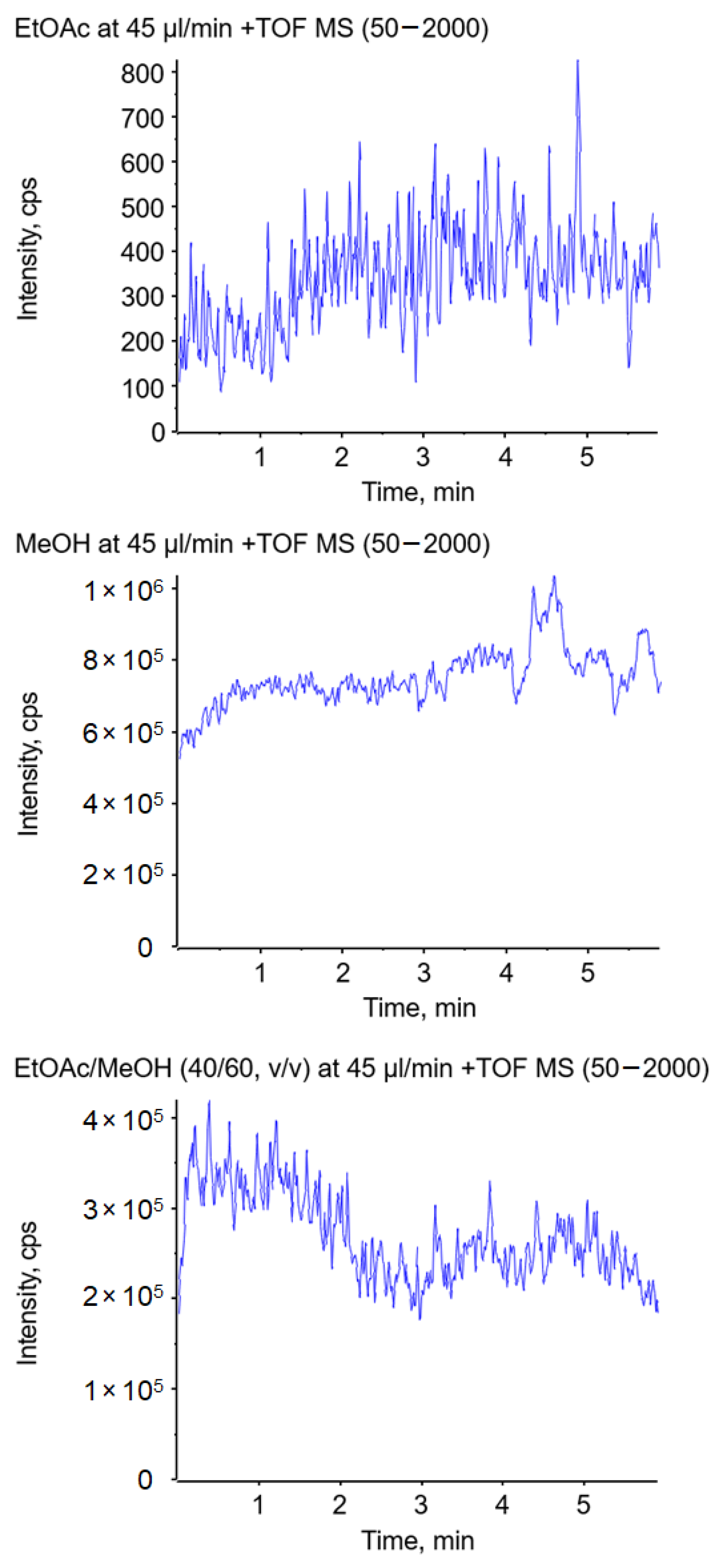

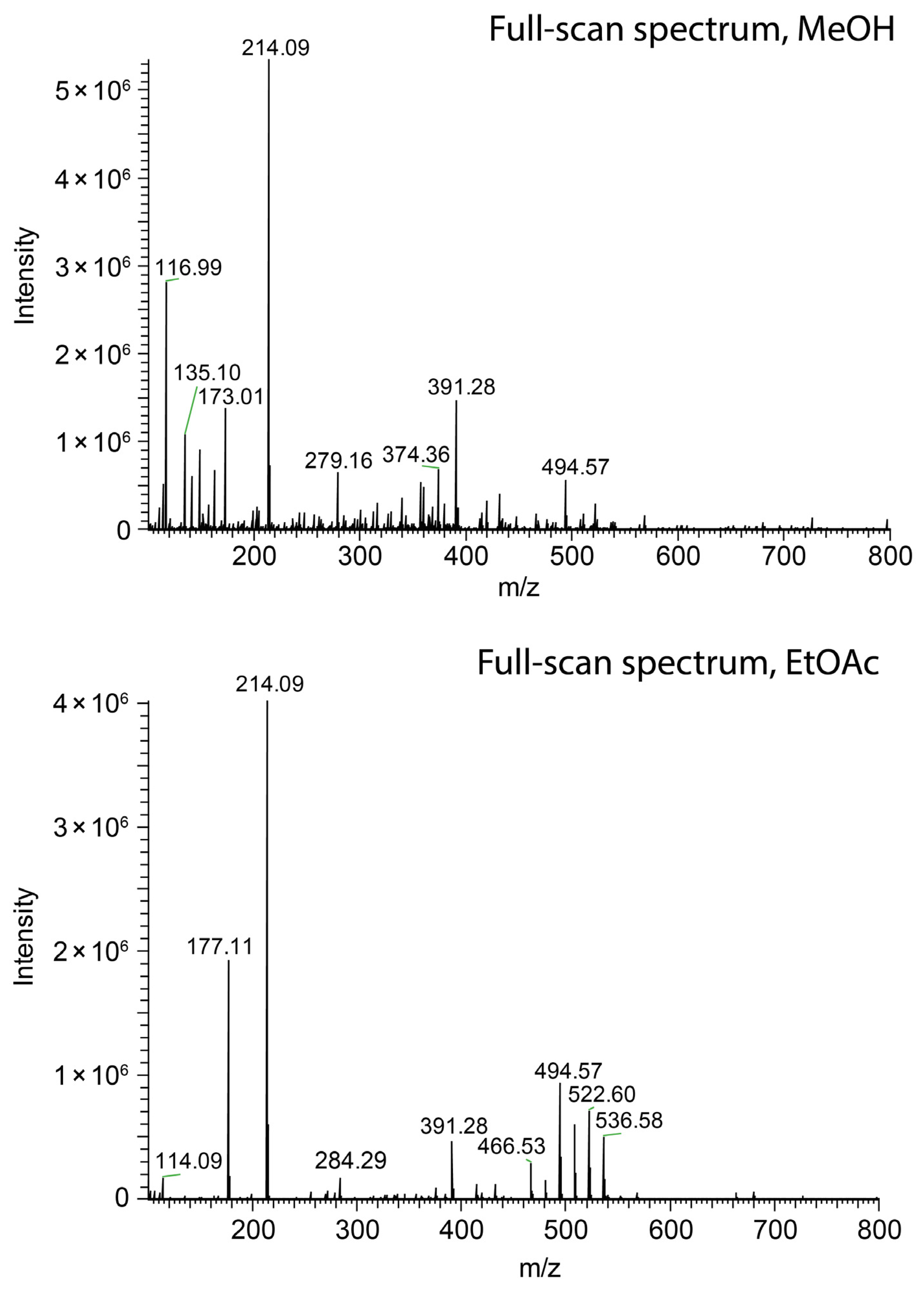
| Spray Mode | Solvent | MeOH Content v/v in % for Short Jet | Relative Permittivity εr (20° C) | Viscosity η (25 °C) mPa•s | Surface Tension γ (25 °C) mN/m |
|---|---|---|---|---|---|
| dripping pulsation | hexane | immiscible | 1.887 | 0.300 | 17.88 |
| 1,4-dioxane | ≥60 | 2.219 | 1.177 | 32.75 | |
| toluene | ≥50 | 2.379 (23 °C) | 0.560 | 27.91 | |
| dibutyl ether | ≥60 | 3.083 | 0.637 | N/A | |
| Taylor cone with long jet | diethyl ether | ≥30 | 4.267 | 0.224 | 16.50 |
| anisole | ≥50 | 4.302 (21 °C) | 1.056 | 35.10 | |
| trichloromethane | ≥20 | 4.807 | 0.537 | 26.65 | |
| propyl acetate | ≥30 | 5.622 | 0.544 | 23.80 | |
| ethyl acetate | ≥30 | 6.081 | 0.423 | 23.39 | |
| methyl acetate | ≥30 | 7.072 (15 °C) | 0.364 | 24.73 | |
| 1-chlorobutane | ≥40 | 7.276 | 0.422 | 23.18 | |
| tetrahydrofuran | ≥30 | 7.522 (22 °C) | 0.456 | 26.40 (20 °C) | |
| dichloromethane | ≥20 | 8.936 (25 °C) | 0.413 | 27.20 | |
| 3-pentanone | ≥30 | 17.002 | 0.444 | 24.74 | |
| 2-propanol | ≥30 | 20.182 | 2.040 | 20.92 | |
| acetone | ≥20 | 21.012 | 0.306 | 22.71 | |
| 1-nitropropane | ≥30 | 24.702 (15 °C) | 0.798 | N/A | |
| Taylor cone with short jet | ethanol | 25.320 | 1.074 | 21.91 | |
| methanol | 33.020 | 0.544 | 22.17 | ||
| acetonitrile | 36.642 | 0.369 | 28.66 | ||
| nitromethane | 37.272 | 0.630 | 36.53 | ||
| N,N-dimethylformamide | 38.250 | 0.794 | 35.52 | ||
| dimethyl sulfoxide | 47.242 | 1.987 | 42.92 | ||
| water | ≥90 | 80.120 | 0.890 | 72.06 | |
| glycerol | 46.532 | 934 | 62.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muggli, T.M.; Schürch, S. Influence of Solvent Relative Permittivity in Swab Spray Mass Spectrometry. Molecules 2024, 29, 4274. https://doi.org/10.3390/molecules29174274
Muggli TM, Schürch S. Influence of Solvent Relative Permittivity in Swab Spray Mass Spectrometry. Molecules. 2024; 29(17):4274. https://doi.org/10.3390/molecules29174274
Chicago/Turabian StyleMuggli, Thomas Michael, and Stefan Schürch. 2024. "Influence of Solvent Relative Permittivity in Swab Spray Mass Spectrometry" Molecules 29, no. 17: 4274. https://doi.org/10.3390/molecules29174274
APA StyleMuggli, T. M., & Schürch, S. (2024). Influence of Solvent Relative Permittivity in Swab Spray Mass Spectrometry. Molecules, 29(17), 4274. https://doi.org/10.3390/molecules29174274







