Melting points (uncorrected) were measured using a Mel-Temp melting point apparatus. The Microanalysis Unit of the University of Otago, New Zealand, performed microanalyses. Infrared spectra were recorded as Nujol mulls on a Perkin-Elmer 298 (Beaconsfield, UK) or a Perkin-Elmer 580B spectrometer. Ultraviolet–visible spectra were recorded in methanol (unless otherwise stated) on a Hitachi UV-3200 spectrometer (Hitachinaka, Japan). 1H and 13C NMR spectra were obtained for the designated solvents on a Bruker AC300F (300 MHz) spectrometer (Bruker Pty Ltd., Preston, NSW, Australia). 1H NMR data were recorded as follows: chemical shift measured in parts per million (ppm) downfield from TMS (δ), multiplicity, observed coupling constant (J) in Hertz (Hz), proton count. Multiplicities are reported as singlet (s), broad singlet (bs), doublet (d), triplet (t), quartet (q), quintet (quin), and multiplet (m). 13C NMR chemical shifts are reported in ppm downfield from TMS and identifiable carbons are given. The EI and ES mass spectra were recorded on an AEI MS 12 mass spectrometer (Washington, D.C., USA) at 70 eV ionizing potential and 8000 V accelerating voltage with an ion source temperature of 210 °C. Kieselgel 60H (Merck, Rahway, NJ, USA, Art 7736) was employed for flash chromatography and thin layer chromatography (TLC) was performed on DC Aluminium Foil Kieselgel F254 (Merck, Art 5554). Solvents and reagents were purified by literature methods. Petroleum ether refers to the hydrocarbon fraction of boiling range 60–80 °C. Compounds were detected by short and long ultraviolet light and with iodine vapor.
MCF-7, H-460, A-431, and DU-145 cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, while HT-29 was maintained in RPMI media (Gibco, Life Technologies) supplemented with 10% fetal bovine serum. All cell lines were cultured at 37 °C, under 5% CO2 in a humidified atmosphere.
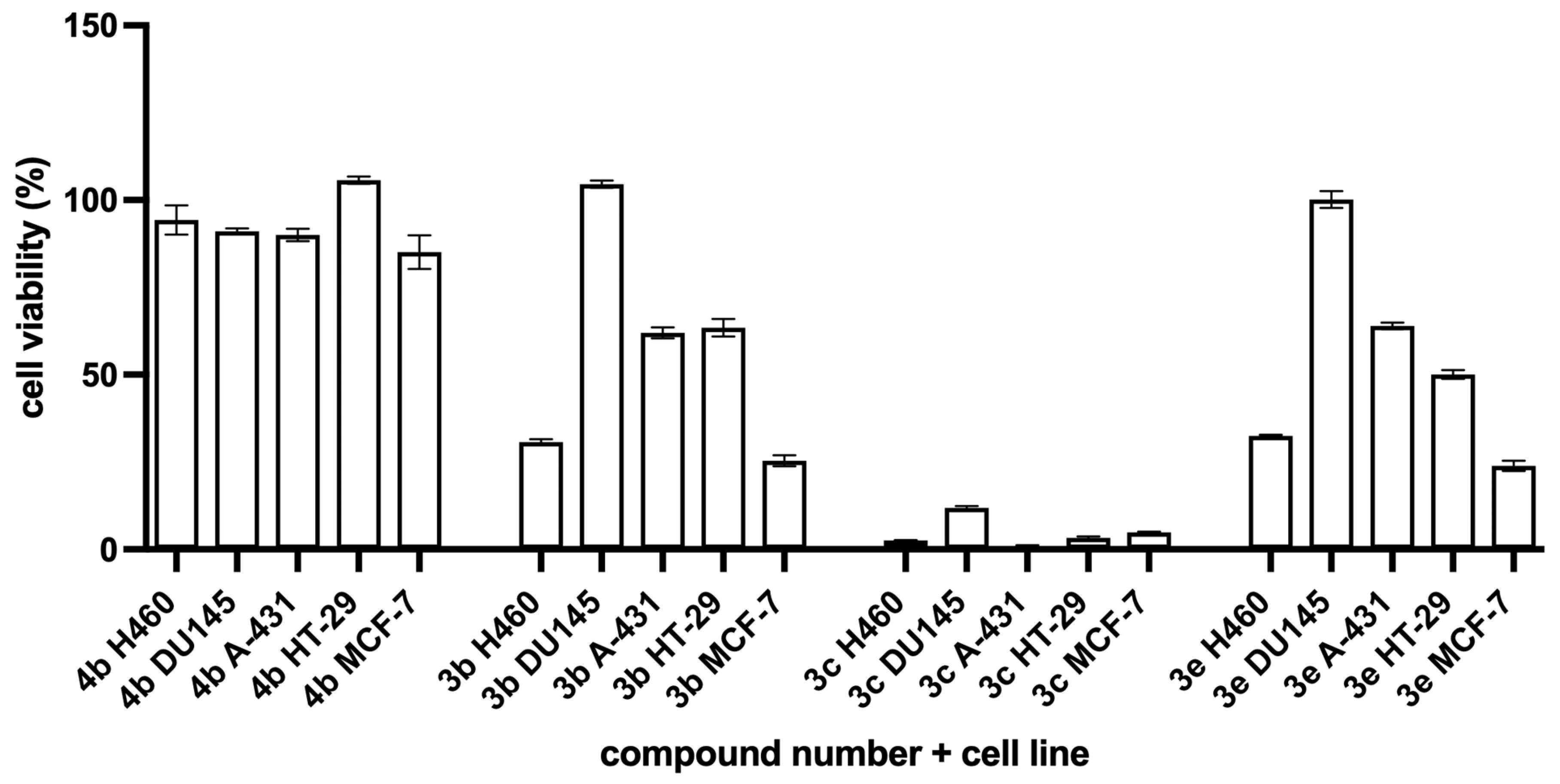
3.2. Experimental Data
3-(4-Bromophenyl)-5,7-dimethoxy-4-phenyl-1,2,3,4-tetrahydroquinolin-4-ol (5a)
White solid (0.9 g, 77%). M.p. 164–166 °C; UV (MeOH): λmax 201 (ε 49,266 cm−1M−1), 221 (68532) nm; IR (KBr): νmax 3367, 3007, 2944, 1605, 1589, 1501, 1487, 1201, 1149, 1132, 1075, 1061, 994, 819, 751, 721, 699 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.12 (dd, J = 3.0, 9.0 Hz, 1H, H2a), 3.24 (dd, J = 3.0, 9.0 Hz, 1H, H2b), 3.35 (s, 3H, OMe), 3.43–3.49 (m, 1H, H3), 3.79 (s, 4H, OMe, OH), 4.14 (bs, 1H, NH), 5.87 (s, 2H, ArH6, ArH8), 6.90 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 7.11–7.20 (m, 5H, Ph), 7.30 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′); 13C NMR (75.6 MHz, CDCl3): δ 43.7 (CH2NH), 53.4 (CHAr′), 55.1 (OMe), 55.2 (OMe), 74.0 (C-OH), 90.2 (ArC6), 91.6 (ArC8), 108.5 (ArC), 120.3 (ArC4′), 125.5 (ArC2″, ArC6″), 126.0 (ArC4″), 127.2 (ArC3″, ArC5″), 130.3 (ArC2′, ArC6′), 131.4 (ArC3′, ArC5′), 146.9 (ArC1″), 150.0 (ArC1′, ArC), 159.8 (ArC7), 160.7 (ArC5); HRMS (ESI) m/z Calcd. for C23H23BrNO3 (M + H)+ 440.0861. Found 440.0848; Anal. Calcd. for C23H22BrNO3: C, 62.74; H, 5.04; N, 3.18. Found: C, 62.97; H, 5.14; N, 3.14.
5,7-Dimethoxy-3,4-bis(4-methoxyphenyl)-1,2,3,4-tetrahydroquinolin-4-ol (5b)
White solid (1.4 g, 72.3%). M.p. 172–174 °C; UV (MeOH): λmax 203 (ε 58,256 cm−1M−1), 222 (58631) nm; IR (KBr): νmax 3388, 3000, 2938, 2838, 1603, 1585, 1508, 1201, 1180, 1165, 1149, 1132, 1067, 1025, 824, 751, 728, 701 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.85 (dd, J = 3.0, 12.0 Hz, 1H, H2a), 2.91 (dt, J = 3.0, 12.0 Hz, 1H, H2b), 3.16 (s, 3H, OMe), 3.40–3.46 (m, 1H, H3), 3.64 (s, 3H, OMe), 3.65 (s, 3H, OMe), 3.66 (s, 3H, OMe), 4.60 (s, 1H, OH), 5.69 (d, J = 3.0 Hz, 1H, ArH6), 5.85 (d, J = 3.0 Hz, 1H, ArH8), 6.08 (bs, 1H, NH), 6.62 (m, 4H, ArH2′, ArH3′, ArH5′, ArH6′), 6.78 (d, J = 9.0 Hz, 2H, ArH2″, ArH6″), 6.87 (d, J = 9.0 Hz, 2H, ArH2″, ArH6″); 13C NMR (75.6 MHz, CDCl3): δ 43.1 (CH2NH), 53.6 (CHAr′), 55.0 (OMe), 55.1 (2 × OMe), 55.3 (OMe), 73.1 (C-OH), 89.0 (ArC6), 91.5 (ArC8), 108.9 (ArC), 112.3 (ArC3′, ArC5′), 112.6 (ArC3″, ArC5″), 126.6 (ArC2′, ArC6′), 130.8 (ArC2″, ArC6″), 132.4 (ArC1″), 143.0 (ArC1′), 147.9 (ArC), 157.0 (ArC4′), 157.7 (ArC4″), 160.2 (ArC7), 160.2 (ArC5); HRMS (ESI) m/z Calcd. for C25H28NO5 (M + H)+: 422.1967. Found: 422.1953.
White solid (0.55 g, 66%). M.p. 192–194 °C; UV (MeOH): λmax 207 (ε 61,432 cm−1M−1), 255 (53,581), 343 (3880) nm; IR (KBr): νmax 2961, 1609, 1578, 1366, 1204, 1150, 1082, 1040, 829, 771, 753, 723, 708, 692 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.38 (s, 3H, OMe), 3.96 (s, 3H, OMe), 6.46 (d, J = 3.0 Hz, 1H, ArH6), 6.92 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 7.00–7.04 (m, 2H, Ph), 7.13 (d, J = 3.0 Hz, 1H, ArH8), 7.17–7.19 (m, 3H, Ph), 7.25 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 8.75 (s, 1H, H2); 13C NMR (75.6 MHz, CDCl3): δ 55.3 (OMe), 55.5 (OMe), 99.8 (ArC6), 100.4 (ArC8), 114.5 (ArC), 120.8 (ArC3), 126.3 (ArC3′, ArC5′), 126.6 (ArC4′, ArC1′), 129.0 (ArC4″), 130.7 (ArC2′, ArC6′), 131.8 (ArC2″, ArC6″), 137.4 (ArC3″, ArC5″), 139.9 (ArC4), 144.9 (ArC1″), 150.7 (ArC), 151.2 (ArC2), 157.6 (ArC7), 160.9 (ArC5); HRMS (ESI) m/z Calcd. for C23H19BrNO2 (M + H)+ 420.0599. Found: 420.0584; Anal. Calcd. for C23H18BrNO2: C, 65.73; H, 4.32; N, 3.33. Found: C, 65.79; H, 4.34; N, 3.27.
White solid (0.7 g, 54%). M.p. 180–182 °C; UV (MeOH): λmax 202 (ε 380,769 cm−1M−1), 206 (380,769), 212 (384,615), 256 (280,769), 341 (65,384) nm; IR (KBr): νmax 2935, 2837, 1610, 1578, 1555, 1510, 1450, 1408, 1365, 1330, 1286, 1203, 1147, 1117, 1042, 1027, 826, 801, 787, 722, 656 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.43 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.79 (s, 3H, OMe), 3.96 (s, 3H, OMe), 6.46 (d, J = 3.0 Hz, 1H, ArH6), 6.71–6.75 (m, 4H, ArH3′, ArH5′, ArH3″, ArH5″), 6.93–6.98 (m, 4H, ArH2′, ArH6′, ArH2″, ArH6″), 7.12 (d, J = 3.0 Hz, 1H, ArH8), 8.75 (s, 1H, H2); 13C NMR (75.6 MHz, CDCl3): δ 55.1 (OMe), 55.2 (OMe), 55.5 (OMe), 55.6 (OMe), 99.8 (ArC6, ArC8), 100.5 (ArC), 112.1 (ArC3′, ArC5′), 112.2 (ArC3″, ArC5″), 113.2 (ArC1′), 115.0 (ArC), 130.3 (ArC1″), 130.4 (ArC2′), 131.0 (ArC6′), 131.4 (ArC2″), 132.5 (ArC6″), 132.8 (ArC4), 150.5 (ArC), 152.0 (ArC2), 157.8 (ArC7), 157.9 (ArC5), 158.1 (ArC4′), 160.5 (ArC4″); HRMS (ESI) m/z Calcd. for C25H24NO4 (M + H)+: 402.1705. Found: 402.1692.
White solid (0.08 g, 46%). M.p. 216–218 °C; UV (MeOH): λmax 209 (ε 70,446 cm−1M−1), 257 (73,791), 347 (4089) nm; IR (KBr): νmax 2961, 2837, 1614, 1510, 1366, 1240, 1203, 1149, 1116, 1041, 1026, 837, 829, 767, 700 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.38 (s, 3H, OMe), 3.74 (s, 3H, OMe), 3.96 (s, 3H, OMe), 6.45 (d, J = 3.0 Hz, 1H, ArH6), 6.70 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 6.97 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 7.02–7.06 (m, 2H, Ph), 7.13 (d, J = 3.0 Hz, 1H, ArH8), 7.17–7.19 (m, 3H, Ph), 8.76 (s, 1H, ArH2); 13C NMR (75.6 MHz, CDCl3): δ 55.0 (OMe), 55.3 (OMe), 55.4 (OMe), 99.7 (ArC6, ArC8), 100.4 (ArC), 113.0 (ArC5′, ArC3′), 114.6 (ArC1′), 126.0 (ArC3), 126.5 (ArC2″, ArC6″), 129.0 (ArC3″, ArC5″), 130.7 (ArC4″), 131.3 (ArC2′, ArC6′), 132.0 (ArC4), 140.4 (ArC1″), 144.6 (ArC), 150.3 (ArC2), 157.6 (ArC4′), 158.1 (ArC7), 160.5 (ArC5); HRMS (ESI) m/z Calcd. for C24H22NO3Na (M + Na)+: 393.1979. Found: 393.1968; Anal. Calcd. for C24H21NO3: C, 77.61; H, 5.70; N, 3.77. Found: C, 77.38; H, 5.81; N, 3.73.
White solid (0.06 g, 38%). M.p. 172–174 °C; UV (MeOH): λmax 205 (ε 48,702 cm−1M−1), 257 (34,961) nm; IR (KBr): νmax 2923, 2853, 1615, 1511, 1449, 1204, 1148, 1125, 1105, 1030, 824, 591 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.30 (s, 9H, 3 × Me), 3.36 (s, 3H, OMe), 3.74 (s, 3H, OMe), 3.96 (s, 3H, OMe), 6.45 (d, J = 3.0 Hz, 1H, ArH6), 6.70 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 6.93–6.99 (m, 4H, ArH2″, ArH3″, ArH5″, ArH6″), 7.14 (d, J = 3.0 Hz, 1H, ArH8), 7.20 (d, J = 8.4 Hz, 2H, ArH2′, ArH6′), 8.77 (s, 1H, ArH2); 13C NMR (75.6 MHz, CDCl3): δ 30.1 (MeC), 31.5 (MeC), 32.0 (MeC), 34.5 (CMe), 55.2 (2 × OMe), 55.6 (OMe), 100.0 (ArC6, ArC8), 100.4 (ArC), 113.2 (ArC3′, ArC5′), 117.8 (ArC1′), 122.9 (CHAr′), 123.5 (ArC2″, ArC6″), 128.9 (ArC3″, ArC5″), 131.0 (ArC2′, ArC6′), 131.5 (ArC1′), 132.3 (ArC4), 137.5 (ArC), 149.1 (ArC2), 151.9 (ArC4″), 157.9 (ArC5), 158.2 (ArC7), 160.7 (ArC4′); HRMS (ESI) m/z Calcd. for C28H30NO3 (M + H)+: 428.2226. Found: 428.2213.
White solid (0.08 g, 46.5%). M.p. 186–188 °C; UV (MeOH): λmax 211 (ε 70,077 cm−1M−1), 256 (48,353), 340 (11,212) nm; IR (KBr): νmax 2954, 1614, 1511, 1449, 1239, 1203, 1150, 1040, 1030, 840, 832, 811, 719, 582 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.24 (s, 3H, MeAr″), 3.32 (s, 3H, OMe), 3.67 (s, 3H, OMe), 3.88 (s, 3H, OMe), 6.37 (d, J = 2.4 Hz, 1H, ArH6), 6.63 (d, J = 8.7 Hz, 2H, ArH3′, ArH5′), 6.84 (d, J = 8.1 Hz, 2H, ArH3″, ArH5″), 6.88–6.92 (m, 4H, ArH2′, ArH6′, ArH2″, ArH6″), 7.05 (d, J = 2.1 Hz, 1H, ArH8), 8.67 (s, 1H, ArH2); 13C NMR (75.6 MHz, CDCl3): δ 21.2 (MeAr″), 55.1 (OMe), 55.2 (OMe), 55.5 (OMe), 99.8 (ArC6, ArC8), 100.5 (ArC), 113.1 (ArC3′, ArC5′), 113.2 (ArC1′, ArC3), 127.2 (ArC2″, ArC6″), 127.3 (ArC3″, ArC5″), 129.0 (ArC2′, ArC6′), 131.4 (ArC1″, ArC4″), 135.4 (ArC4), 144.9 (ArC), 150.5 (ArC2), 157.8 (ArC5), 158.1 (ArC7), 160.5 (ArC4′); HRMS (ESI) m/z Calcd. for C25H24NO3 (M + H)+: 386.1756. Found: 386.1744.
White solid (1.6 g, 88.5%). M.p. 144–146 °C; UV (MeOH): λmax 219 (ε 64,000 cm−1M−1), 247 (16,666), 336 (7333) nm; IR (KBr): νmax 3532, 2982, 1706, 1576, 1488, 1455, 1384, 1304, 1237, 1213, 1066, 1042, 1010, 823, 759, 723, 704 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.19 (t, J = 6.0 Hz, 3H, CH3CH2), 3.02 (dd, J = 3.0, 12.0 Hz, 1H, H2a), 3.19 (s, 3H, OMe), 3.82 (t, J = 12.0 Hz, 1H, H3), 3.74 (s, 3H, OMe), 4.11–4.18 (m, 3H, CH2CH3, H2b), 6.28 (d, J = 3.0 Hz, 1H, ArH6), 6.63 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 6.89–6.92 (m, 2H, Ph), 7.00 (d, 3.0 Hz, 1H, ArH8), 7.07–7.14 (m, 3H, Ph), 7.27 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′); 13C NMR (75.6 MHz, CDCl3): δ 14.4 (Me), 46.0 (CH2N), 54.5 (CHAr′), 55.3 (OMe), 55.5 (OMe), 62.1 (OCH2), 74.5 (C-OH), 97.0 (ArC6, ArC8), 100.1 (ArC), 116.7 (ArC4′), 120.7 (ArC4″), 125.1 (ArC2″, ArC6″), 126.2 (ArC5″), 127.2 (ArC3″), 130.3 (ArC2′, ArC6′), 131.5 (ArC3′, ArC5′), 136.2 (ArC), 140.0 (ArC1′), 148.7 (ArC1″), 154.1 (COO), 158.5 (ArC7), 159.5 (ArC5); HRMS (ESI) m/z Calcd. for C26H26BrNO5Na (M + Na)+: 534.0892. Found: 534.0881; Anal. Calcd. for C26H26BrNO5: C, 60.95; H, 5.11; N, 2.73. Found: C, 60.94; H, 5.18; N, 2.69.
White solid (1.6 g, 84.5%). M.p. 108–110 °C; UV (MeOH): λmax 220 (ε 56,085 cm−1M−1), 276 (3365), 337 (5608) nm; IR (KBr): νmax 3464, 2934, 1677, 1610, 1582, 1509, 1172, 1144, 1062, 1027, 956, 891, 826, 765 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.24 (t, J = 6.0 Hz, 3H, CH3CH2), 3.06 (dd, J = 3.0, 12.0 Hz, 1H, H2a), 3.36 (s, 3H, OMe), 3.77 (s, 6H, 2 × OMe), 3.83 (dd, J = 3.0, 9.0 Hz, 1H, H2b), 3.84 (s, 3H, OMe), 4.19 (q, J = 6.0 Hz, 2H, CH2CH3), 4.28 (dd, J = 3.0, 12.0 Hz, 1H, H3), 6.23 (d, J = 3.0 Hz, 1H, ArH6), 6.69 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 6.73–6.82 (m, 4H, ArH2″, ArH3″, ArH5″, ArH6″), 6.90 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 7.16 (d, J = 3.0 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.4 (Me), 46.5 (CH2N), 54.3 (OMe), 55.0 (OMe), 55.3 (2 × OMe), 55.6 (CHAr′), 61.9 (OCH2), 74.5 (C-OH), 97.0 (ArC6, ArC8), 100.0 (ArC), 112.4 (ArC3′, ArC5′), 112.7 (ArC3″, ArC5″), 117.0 (ArC), 126.3 (ArC2′, ArC6′), 130.7 (ArC2″, ArC6″), 140.1 (ArC1″), 141.4 (ArC1′), 154.2 (COO), 157.7 (ArC4″), 158.2 (ArC4′), 158.6 (ArC7), 159.3 (ArC5); HRMS (ESI) m/z Calcd. for C28H31NO7Na (M + Na)+: 516.1998. Found: 516.1981.
White solid (1.5 g, 85%). M.p. 140–142 °C; UV (MeOH): λmax 219 (ε 41,395 cm−1M−1), 337 (3255) nm; IR (KBr): νmax 3561, 2971, 1699, 1607, 1577, 1385, 1302, 1237, 1178, 1042, 1008, 829, 765, 752, 736, 703 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.26 (t, J = 6.0 Hz, 3H, CH3CH2), 3.08 (dd, J = 3.0, 9.0 Hz, 1H, H2a), 3.32 (s, 3H, OMe), 3.77 (s, 3H, OMe), 3.83 (dd, J = 3.0 Hz, 9.0 Hz, 1H, H2b), 3.84 (s, 3H, OMe), 4.21 (q, J = 6.0 Hz, 2H, CH2CH3), 4.33 (dd, J = 3.0 Hz, 12.0 Hz, 1H, H3), 6.23 (d, J = 3.0 Hz, 1H, ArH6), 6.72–6.79 (m, 4H, ArH3′, ArH5′, ArH3″, ArH5″), 6.97–7.00 (m, 2H, ArH4″, ArH8), 7.13–7.18 (m, 4H, ArH2′, ArH6′, ArH2″, ArH6″); 13C NMR (75.6 MHz, CDCl3): δ 14.4 (Me), 54.2 (CH2N), 54.9 (CHAr′), 55.0 (OMe), 55.3 (OMe), 55.5 (OMe), 61.9 (OCH2), 74.7 (C-OH), 96.5 (ArC6), 97.0 (ArC8), 100.1 (ArC), 112.6 (ArC3′, ArC5′), 125.2 (ArC3″), 125.5 (ArC5″), 125.9 (ArC4″), 127.1 (ArC2′, ArC6′), 129.2 (ArC2″), 129.6 (ArC6″), 140.1 (ArC), 140.6 (ArC1′), 149.1 (ArC1″), 154.2 (COO), 157.4 (ArC4′), 157.7 (ArC7), 158.3 (ArC5); HRMS (ESI) m/z Calcd. for C27H29NO6Na (M + Na)+: 486.1893. Found: 486.1881; Anal. Calcd. for C27H29NO6: C, 69.96; H, 6.31; N, 3.02. Found: C, 69.75; H, 6.37; N, 3.01.
White solid (1.6 g, 80%). M.p. 118–120 °C; UV (MeOH): λmax 202 (ε 102,479 cm−1M−1), 222 (113,813) nm; IR (KBr): νmax 3551, 2957, 1703, 1584, 1515, 1237, 1140, 1060, 1037, 827, 756, 736 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.23 (t, J = 6.0 Hz, 3H, CH3CH2), 1.27 (s, 9H, 3 × Me), 3.13 (dd, J = 2.1, 10.2 Hz, 1H, H2a), 3.35 (s, 3H, OMe), 3.79 (s, 3H, OMe), 3.86 (s, 3H, OMe), 4.10–4.19 (m, 3H, CH2CH3, H2b), 4.24 (dd, J = 2.7, 12.9 Hz,1H, H3), 6.26 (d, J = 3.0 Hz, 1H, ArH6), 6.76 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 6.84 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 6.94 (d, J = 8.4 Hz, 2H, ArH2″, ArH6″), 7.17–7.20 (m, 3H, ArH8, ArH3″, ArH5″); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 31.3 (MeC), 31.4 (MeC), 34.3 (MeC), 46.7 (CMe), 54.0 (CH2N), 55.1 (OMe), 55.4 (OMe), 55.7 (OMe), 60.4 (CHAr′), 62.9 (OCH2), 74.8 (C-OH), 97.2 (ArC6, ArC8), 100.3 (ArC), 112.8 (ArC3′, ArC5′), 124.0 (ArC3″, ArC5″), 125.0 (ArC2′, ArC6′), 129.7 (ArC), 130.7 (ArC2″, ArC6″), 140.2 (ArC1′), 146.2 (ArC1″), 148.9 (ArC4″), 154.4 (COO), 158.3 (ArC4′), 158.8 (ArC7), 159.4 (ArC5); HRMS (ESI) m/z Calcd. for C31H37NO6Na (M + Na)+: 542.2519. Found: 542.2496.
White solid (1.5 g, 82%). M.p. 108–110 °C; UV (MeOH): λmax 220 (ε 47,281 cm−1M−1), 336 (5673) nm; IR (KBr): νmax 3470, 2935, 2838, 1684, 1611, 1584, 1511, 1374, 1313, 1179, 1143, 1057, 1029, 956, 829, 817, 764 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.16 (t, J = 6.0 Hz, 3H, CH3CH2), 2.22 (s, 3H, MeAr″), 2.99 (dd, J = 1.8, 10.8 Hz, 1H, H2a), 3.27 (s, 3H, OMe), 3.69 (s, 3H, OMe), 3.71 (s, 1H, OH), 3.76 (s, 3H, OMe), 3.77 (dd, J = 2.1, 10.5 Hz, 1H, H2b), 4.11 (q, J = 6.0 Hz, 2H, CH2CH3), 4.19 (dd, J = 2.7, 12.6 Hz, 1H, H3), 6.15 (d, J = 3.0 Hz, 1H, ArH6), 6.65–6.75 (m, 4H, ArH3′, ArH5′, ArH3″, ArH5″), 6.79 (d, J = 8.1 Hz, 2H, ArH2′, ArH6′), 6.88 (d, J = 8.1 Hz, 2H, ArH2″, ArH6″), 7.08 (d, J = 3.0 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 21.0 (MeAr″), 46.6 (CH2N), 55.3 (OMe), 55.4 (OMe), 55.6 (OMe), 55.7 (CHAr′), 62.0 (OCH2), 74.8 (C-OH), 97.1 (ArC6), 97.2 (ArC8), 100.2 (ArC), 112.8 (ArC3′, ArC5′), 125.3 (ArC2′, ArC6′), 128.0 (ArC2″, ArC6″), 129.4 (ArC3″, ArC5″), 130.7 (ArC4″), 135.4 (ArC), 140.2 (ArC1′), 144.8 (ArC1″), 154.4 (COO), 158.7 (ArC4′), 159.1 (ArC7), 159.4 (ArC5); HRMS (ESI) m/z Calcd. for C28H31NO6Na (M + Na)+: 500.2049. Found: 500.2035.
White solid (1.55 g, 85%). M.p. 142–144 °C; UV (MeOH): λmax 219 (ε 42,936 cm−1M−1), 243 (12,881), 337 (4293) nm; IR (KBr): νmax 3232, 1614, 1511, 1449, 1239, 1203, 1150, 1040, 1030, 840, 832, 811, 719, 582 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.29 (t, J = 6.0 Hz, 3H, CH3CH2), 3.17 (dd, J = 2.1, 10.8 Hz, 1H, H2a), 3.35 (s, 3H, OMe), 3.85 (d, J = 1.3 Hz, 1H, H2b), 3.88 (s, 3H, OMe), 3.93 (s, 1H, OH), 4.25 (q, J = 7.2 Hz, 2H, CH2CH3), 4.40 (dd, J = 2.7, 12.6 Hz, 1H, H3), 6.27 (d, J = 3.0 Hz, 1H, ArH6), 6.88–6.91 (m, 2H, ArH′), 7.02–7.05 (m, 2H, ArH″), 7.18–7.24 (m, 7H, ArH′, ArH″, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 46.4 (CH2N), 55.1 (OMe), 55.4 (OMe), 55.6 (CHAr′), 62.1 (OCH2), 74.9 (C-OH), 97.1 (ArC6, ArC8), 100.2 (ArC), 117.2 (ArC4′), 125.3 (ArC4″), 126.1 (ArC6′), 126.7 (ArC2′), 127.1 (ArC6″), 127.2 (ArC2″), 127.3 (ArC5′), 127.7 (ArC3′), 129.3 (ArC5″), 129.9 (ArC3″), 137.3 (ArC), 140.3 (ArC1′), 149.2 (ArC1″), 154.3 (COO), 158.6 (ArC7), 159.5 (ArC5); HRMS (ESI) m/z Calcd. for C26H28NO5 (M + H)+: 434.1967. Found: 434.1949; Anal. Calcd. for C26H27NO5: C, 72.04; H, 6.28; N, 3.23. Found: C, 72.20; H, 6.34; N, 3.19.
White solid (1.5 g, 78%). M.p. 170–172 °C; UV (MeOH): λmax 219 (ε 66,666 cm−1M−1) nm; IR (KBr): νmax 3585, 2956, 2939, 2905, 1697, 1581, 1453, 1397, 1320, 1280, 1225, 1146, 1080, 1057, 1030, 953, 845, 813, 762, 700 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.27 (t, J = 6.0 Hz, 3H, CH3CH2), 2.34 (s, 3H, MeAr″), 3.17 (dd, J = 2.7, 10.2 Hz, 1H, H2a), 3.29 (s, 1H, OH), 3.39 (s, 3H, OMe), 3.88 (s, 3H, OMe), 3.90 (dd, J = 2.1, 10.5 Hz, 1H, H2b), 4.22 (q, J = 7.2 Hz, 2H, CH2CH3), 4.35 (dd, J = 2.7, 12.9 Hz, 1H, H3), 6.28 (d, J = 3.0 Hz, 1H, ArH6), 6.89–7.02 (m, 6H, ArH′, ArH″), 7.21–7.24 (m, 4H, ArH′, ArH″, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 21.2 (MeAr″), 46.5 (CH2N), 55.1 (OMe), 55.4 (OMe), 55.7 (CHAr′), 62.1 (OCH2), 74.8 (C-OH), 97.1 (ArC6, ArC8), 100.2 (ArC), 126.6 (ArC4′), 127.3 (ArC2′, ArC6′), 127.7 (ArC2″, ArC6″), 127.8 (ArC5′), 128.5 (ArC3′), 129.1 (ArC5″), 129.9 (ArC3″), 135.5 (ArC4″), 137.5 (ArC), 140.2 (ArC1″), 146.2 (ArC1′), 154.4 (COO), 158.7 (ArC7), 159.4 (ArC5); HRMS (ESI) m/z Calcd. for C27H29NO5Na (M + Na)+: 470.1943. Found: 470.1927; Anal. Calcd. for C27H29NO5: C, 72.46; H, 6.53; N, 3.13. Found: C, 72.66; H, 6.61; N, 3.09.
White solid (0.8 g, 55%). M.p. 188–190 °C; UV (MeOH): λmax 215 (ε 55,234 cm−1M−1), 247 (19,910), 329 (14,129) nm; IR (KBr): νmax 2977, 1699, 1597, 1568, 1402, 1325, 1204, 1152, 1045, 1008, 831, 818, 766, 755, 701 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.29 (t, J = 6.0 Hz, 3H, CH3CH2), 3.23 (s, 3H, OMe), 3.84 (s, 3H, OMe), 4.24 (q, J = 6.0 Hz, 2H, CH2CH3), 4.54 (s, 2H, CH2N), 6.19 (d, J = 3.0 Hz, 1H, ArH6), 6.80 (d, J = 6.0 Hz, 2H, ArH2′, ArH6′), 6.95 (d, J = 3.0 Hz, 1H, ArH8), 6.97–6.99 (m, 2H, Ph), 7.12–7.14 (m, 3H, Ph), 7.24 (d, J = 6.0 Hz, 2H, ArH3′, ArH5′); 13C NMR (75.6 MHz, CDCl3): δ 14.4 (Me), 48.5 (CH2N), 55.3 (OMe), 55.4 (OMe), 62.1 (OCH2), 96.5 (ArC6, ArC8), 100.3 (ArC), 113.8 (Ar′C4), 120.1 (ArC3), 125.9 (ArC2′, ArC6′), 127.2 (ArC3″, ArC5″), 129.0 (ArC2″, ArC6″), 130.1 (ArC4″), 130.8 (ArC3′, ArC5′), 133.3 (ArC4), 138.6 (ArC1′), 139.8 (ArC), 140.0 (ArC1″), 153.4 (COO), 157.6 (ArC7), 159.9 (ArC5); HRMS (ESI) m/z Calcd. for C26H24BrNO4Na (M + Na)+: 516.0786. Found: 516.0766; Anal. Calcd. for C26H24BrNO4: C, 63.17; H, 4.89; N, 2.83. Found: C, 63.37; H, 4.98; N, 2.80.
White solid (0.75 g, 52%). M.p. 148–150 °C; UV (MeOH): λmax 251 (ε 16,507 cm−1M−1), 325 (11,279) nm; IR (KBr): νmax 2966, 2935, 2841, 1704, 1607, 1569, 1508, 1446, 1324, 1244, 1219, 1150, 1050, 831, 819, 758 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.28 (t, J = 6.0 Hz, 3H, CH3CH2), 3.28 (s, 3H, OMe), 3.74 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.83 (s, 3H, OMe), 4.23 (q, J = 6.0 Hz, 2H, CH2CH3), 4.53 (s, 2H, CH2N), 6.20 (d, J = 3.0 Hz, 1H, ArH6), 6.68 (d, J = 9.0 Hz, 4H, ArH3′, ArH5′, ArH3″, ArH5″), 6.88 (d, J = 9.0 Hz, 4H, ArH2′, ArH6′, ArH2″, ArH6″), 6.98 (d, J = 3.0 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.4 (Me), 48.9 (CH2N), 55.0 (2 × OMe), 55.3 (OMe), 55.4 (OMe), 61.9 (OCH2), 96.6 (ArC6, ArC8), 100.2 (ArC), 112.5 (ArC3′, ArC5′), 113.0 (ArC3″, ArC5″), 114.3 (CHAr′), 129.6 (ArC2″, ArC6″), 130.2 (ArC2″, ArC6″), 131.3 (ArC4), 132.3 (ArC1′), 132.9 (ArC1″), 139.7 (ArC), 153.5 (COO), 157.5 (ArC4′, ArC4″), 157.6 (ArC7), 159.4 (ArC5); HRMS (ESI) m/z Calcd. for C28H30NO6 (M + H)+: 476.2073. Found: 476.2059.
White solid (0.8 g, 54%). M.p. 148–150 °C; UV (MeOH): λmax 215 (ε 57,573 cm−1M−1), 247 (20,562), 327 (14,393) nm; IR (KBr): νmax 2964, 2901, 2840, 1707, 1601, 1573, 1365, 1307, 1216, 1172, 1144, 1041, 1028, 825, 748, 703 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.28 (t, J = 6.0 Hz, 3H, CH3CH2), 3.22 (s, 3H, OMe), 3.73 (s, 3H, OMe), 3.84 (s, 3H, OMe), 4.24 (q, J = 6.0 Hz, 2H, CH2CH3), 4.55 (s, 2H, CH2N), 6.19 (d, J = 3.0 Hz, 1H, ArH6), 6.65 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 6.85 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 6.99–7.01 (m, 3H, Ph, ArH8), 7.10–7.13 (m, 3H, Ph); 13C NMR (75.6 MHz, CDCl3): δ 14.4 (Me), 55.0 (2 × OMe), 55.3 (OMe), 61.9 (CH2N), 65.8 (OCH2), 96.5 (ArC6, ArC8), 100.2 (ArC), 113.0 (ArC5′), 114.2 (ArC3′), 125.5 (ArC3), 127.1 (ArC4″, ArC4), 129.2 (ArC3″, ArC5″), 129.6 (ArC2″, ArC6″), 131.7 (ArC2′, ArC6′), 132.1 (ArC1′), 139.6 (ArC), 140.6 (ArC1″), 153.5 (COO), 157.4 (ArC4′), 157.7 (ArC7), 159.5 (ArC5); HRMS (ESI) m/z Calcd. for C27H27NO5Na (M + Na)+: 468.1787. Found: 468.1773; Anal. Calcd. for C27H27NO5: C, 72.79; H, 6.11; N, 3.14. Found: C, 72.84; H, 6.18; N, 3.19.
White solid (0.8 g, 57%). M.p. 118–120 °C; UV (MeOH): λmax 212 (ε 67,271 cm−1M−1), 245 (22,589), 325 (15,303) nm; IR (KBr): νmax 2936, 1693, 1606, 1513, 1451, 1329, 1248, 1202, 1168, 1138, 1032, 858, 831, 811, 760, 656 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.27 (s, 9H, 3 × Me), 1.28 (t, J = 7.2 Hz, 3H, CH3CH2), 3.19 (s, 3H, OMe), 3.74 (s, 3H, OMe), 3.83 (s, 3H, OMe), 4.24 (q, J = 7.2 Hz, 2H, CH2CH3), 4.54 (s, 2H, CH2N), 6.18 (d, J = 3.0 Hz, 1H, ArH6), 6.65 (d, J = 8.7 Hz, 2H, ArH3′, ArH5′), 6.87 (d, J = 6.3 Hz, 2H, ArH3″, ArH5″), 6.90 (d, J = 5.7 Hz, 2H, ArH2″, ArH6″), 6.99 (d, J = 3.0 Hz, 1H, ArH8), 7.13 (d, J = 8.3 Hz, 2H, ArH2′, ArH6′); 13C NMR (75.6 MHz, CDCl3): δ 14.6 (Me), 31.4 (3 × MeC), 34.4 (CMe), 49.0 (CH2N), 55.1 (OMe), 55.4 (OMe), 55.5 (OMe), 62.1 (OCH2), 96.9 (ArC6, ArC8), 100.4 (ArC), 113.0 (ArC3′, ArC5′), 114.7 (CHAr′), 124.0 (ArC3″, ArC5″), 128.9 (ArC4), 129.8 (ArC2′, ArC6′), 131.8 (ArC1′), 132.5 (ArC2″, ArC6″), 137.7 (ArC1″), 139.8 (ArC), 148.5 (ArC4″), 153.7 (COO), 157.7 (ArC4′) 157.8 (ArC7), 159.5 (ArC5); HRMS (ESI) m/z Calcd. for C31H36NO5 (M + H)+: 502.2593. Found: 502.2579.
White solid (0.8 g, 60.5%). M.p. 124–126 °C; UV (MeOH): λmax 214 (ε 41,875 cm−1M−1), 248 (17,296), 326 (11,834) nm; IR (KBr): νmax 2957, 2837, 1702, 1599, 1507, 1443, 1241, 1148, 1109, 1051, 1021, 824, 806, 775, 762, 720 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.30 (t, J = 6.0 Hz, 3H, CH3CH2), 2.30 (s, 3H, MeAr″), 3.26 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.85 (s, 3H, OMe), 4.26 (q, J = 6.0 Hz, 2H, CH2CH3), 4.55 (s, 2H, CH2N), 6.21 (d, J = 3.0 Hz, 1H, ArH6), 6.69 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 6.87–6.92 (m, 4H, ArH2″, ArH3″, ArH5″, ArH6″), 6.95 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 7.01 (d, J = 3.0 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 21.2 (MeAr″), 49.0 (CH2N), 55.1 (OMe), 55.4 (OMe), 55.5 (OMe), 62.0 (OCH2), 96.8 (ArC6, ArC8), 100.4 (ArC), 113.1 (ArC3′, ArC5′), 114.6 (CHAr′), 127.9 (ArC3″, ArC5″), 129.1 (ArC4′), 129.7 (ArC2′, ArC6′), 131.8 (ArC1′), 132.5 (ArC2″, ArC6″), 135.0 (ArC1″), 137.5 (ArC), 139.8 (ArC4″), 153.6 (COO), 157.7 (ArC4′), 157.8 (ArC7), 159.5 (ArC5); HRMS (ESI) m/z Calcd. for C28H29NO5Na (M + Na)+: 482.1943. Found: 482.1930; Anal. Calcd. for C28H29NO5: C, 73.18; H, 6.36; N, 3.05. Found: C, 73.29; H, 6.47; N, 3.01.
White solid (0.8 g, 58%). M.p. 112–114 °C; UV (MeOH): λmax 216 (ε 65,759 cm−1M−1), 248 (27,210), 324 (15,873) nm; IR (KBr): νmax 2973, 2840, 1698, 1595, 1567, 1443, 1396, 1323, 1259, 1146, 1133, 1049, 1031, 1014, 885, 848, 832, 765, 699 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.32 (t, J = 6.0 Hz, 3H, CH3CH2), 3.26 (s, 3H, OMe), 3.88 (s, 3H, OMe), 4.28 (q, J = 7.2 Hz, 2H, CH2CH3), 4.61 (s, 2H, CH2N), 6.23 (d, J = 3.0 Hz, 1H, ArH6), 6.96–7.04 (m, 5H, ArH′, Ar″H), 7.11–7.19 (m, 6H, ArH′, ArH″, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 48.9 (CH2N), 55.4 (2 × OMe), 62.1 (OCH2), 96.7 (ArC6, ArC8), 100.4 (ArC), 114.2 (CHAr′), 125.7 (ArC6′), 126.3 (ArC2′), 127.1 (ArC4′, ArC4″), 127.7 (ArC2″, ArC6″), 128.5 (ArC3′, ArC5′), 129.3 (ArC3″, ArC5″), 132.7 (ArC4), 139.8 (ArC), 139.9 (ArC1′), 140.5 (ArC1″), 153.6 (COO), 157.7 (ArC7), 159.8 (ArC5); HRMS (ESI) m/z Calcd. for C26H25NO4Na (M + Na)+: 438.1681. Found: 438.1667; Anal. Calcd. for C26H25NO4: C, 75.16; H, 6.06; N, 3.37. Found: C, 75.19; H, 6.16; N, 3.33.
White solid (0.7 g, 53%). M.p. 118–120 °C; UV (MeOH): λmax 203 (ε 100,357 cm−1M−1), 252 (54,057), 317 (30,190) nm; IR (KBr): νmax 2938, 1708, 1599, 1574, 1443, 1397, 1320, 1265, 1203, 1151, 1136, 1047, 1034, 968, 939, 899, 808, 762, 699 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.28 (t, J = 6.0 Hz, 3H, CH3CH2), 2.28 (s, 3H, MeAr″), 3.23 (s, 3H, OMe), 3.85 (s, 3H, OMe), 4.24 (q, J = 7.2 Hz, 2H, CH2CH3), 4.55 (s, 2H, CH2N), 6.20 (d, J = 3.0 Hz, 1H, ArH6), 6.82–7.00 (m, 7H, ArH′, ArH″), 7.08–7.11 (m, 3H, ArH′, ArH″, ArH8′); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 21.2 (MeAr″), 48.9 (CH2N), 55.4 (OMe), 55.5 (OMe), 62.1 (OCH2), 96.8 (ArC6, ArC8), 114.4 (ArC), 126.2 (CHAr′), 127.7 (ArC6′), 127.8 (ArC2′), 127.9 (ArC4′), 128.0 (ArC5′), 128.3 (ArC3′), 128.5 (ArC5″), 129.1 (ArC3″), 132.5 (ArC4), 135.1 (ArC2″, ArC6″), 137.3 (ArC, ArC4″), 140.0 (ArC1″), 140.2 (ArC1′), 153.6 (COO), 157.8 (ArC7), 159.7 (ArC5); HRMS (ESI) m/z Calcd. for C27H27NO4Na (M + Na)+: 452.1838. Found: 452.1828; Anal. Calcd. for C27H27NO4: C, 75.50; H, 6.34; N, 3.26. Found: C, 75.56; H, 6.51; N, 3.14.
White solid (0.6 g, 92%). M.p. 122–124 °C; UV (MeOH): λmax 216 (ε 454,959 cm−1M−1), 284 (31,847), 336 (50,045) nm; IR (KBr): νmax 2971, 2905, 2841, 1703, 1607, 1584, 1511, 1375, 1239, 1169, 1154, 1039, 1023, 936, 889, 811, 763 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.33 (t, J = 9.0 Hz, 3H, CH3CH2), 3.31 (dt, J = 3.0, 12.0 Hz, 1H, H3), 3.60 (s, 3H, OMe), 3.61–3.70 (m, 1H, H2a), 3.72 (s, 3H, OMe), 3.79 (s, 3H, OMe), 3.85 (s, 3H, OMe), 4.06 (ddd, J = 3.0, 3.0, 12.0 Hz, 1H, H2b), 4.29 (q, J = 9.0 Hz, 2H, CH2CH3), 4.42 (dd, J = 3.0, 6.0 Hz, 1H, H4), 6.22 (d, J = 3.0 Hz, 1H, ArH6), 6.48 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 6.59 (d, J = 9.0 Hz, 2H, ArH3″, ArH5″), 6.70–6.77 (m, 4H, ArH2′, ArH6′, ArH2″, ArH6″), 7.44 (d, J = 3.0 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 42.8 (ArC4), 42.9 (ArC3), 44.8 (CH2N), 55.0 (OMe), 55.1 (OMe), 55.3 (OMe), 55.6 (OMe), 61.9 (OCH2), 94.4 (ArC6, ArC8), 99.2 (ArC), 112.5 (ArC3′, ArC5′), 113.2 (ArC3″, ArC5″), 129.2 (ArC2′, ArC6′), 130.4 (ArC2″, ArC6″), 131.6 (ArC), 132.2 (ArC1″), 138.8 (ArC1′), 154.9 (COO), 156.9 (ArC5), 157.8 (ArC5), 158.2 (ArC4′), 158.7 (ArC4″); HRMS (ESI) m/z Calcd. for C28H31NO6Na (M + Na)+: 500.2049. Found: 500.2034; Anal. Calcd. for C28H31NO6: C, 70.42; H, 6.54; N, 2.93. Found: C, 70.58; H, 6.72; N, 2.88.
White solid (0.6 g, 95%). M.p. 160–162 °C; UV (MeOH): λmax 216 (ε 54,328 cm−1M−1), 283 (2388), 336 (5970) nm; IR (KBr): νmax 3001, 2977, 2950, 2837, 1710, 1590, 1516, 1251, 1239, 1060, 1043, 830, 758, 701 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.33 (t, J = 6.0 Hz, 3H, CH3CH2), 3.36 (dt, J = 3.0, 12.0 Hz, 1H, H3), 3.58 (s, 3H, OMe), 3.67 (t, J = 12.0 Hz, 1H, H2a), 3.78 (s, 3H, OMe), 3.85 (s, 3H, OMe), 4.09 (ddd, J = 3.0, 6.0, 12.0 Hz, 1H, H2b), 4.29 (q, J = 6.0 Hz, 2H, CH2CH3), 4.45 (d, J = 3.0, 1H, H4), 6.21 (d, J = 3.0 Hz, 1H, ArH6), 6.56–6.59 (m, 2H, Ph), 6.68–6.75 (m, 4H, ArH2′, ArH3′, ArH5′, ArH6′), 7.01–7.10 (m, 3H, Ph), 7.45 (d, J = 3.0 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.5 (Me), 42.9 (ArC4), 43.7 (ArC3), 44.8 (CH2N), 55.1 (OMe), 55.3 (OMe), 55.6 (OMe), 61.9 (OCH2), 94.4 (ArC6, ArC8), 99.2 (ArC), 113.2 (ArC3′, ArC5′), 125.9 (ArC4″), 127.1 (ArC2′, Ar′C6), 129.1 (ArC2″, ArC6″), 129.5 (ArC3″, ArC5″), 132.1 (ArC), 138.9 (ArC1″), 139.7 (ArC1′), 154.9 (COO), 157.0 (ArC5), 158.3 (ArC5), 158.8 (ArC4′); HRMS (ESI) m/z Calcd. for C27H29NO5Na (M + Na)+: 470.1943. Found: 470.1927; Anal. Calcd. for C27H29NO5: C, 72.46; H, 6.53; N, 3.13. Found: C, 72.65; H, 6.45; N, 3.19.
White solid (0.65 g, 91.5%). M.p. 116–118 °C; UV (MeOH): λmax 203 (ε 71,732 cm−1M−1), 220 (74,830) nm; IR (KBr): νmax 3154, 2959, 1712, 1590, 1512, 1373, 1267, 1240, 1060, 1042, 838, 812, 762 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.28 (s, 9H, 3 × Me), 1.38 (t, J = 6.0 Hz, 3H, CH3CH2), 3.36 (dt, J = 6.0, 12.0 Hz, 1H, H3), 3.64 (s, 3H, OMe), 3.72 (t, J = 15.0 Hz, 1H, H2a), 3.82 (s, 3H, OMe), 3.89 (s, 3H, OMe), 4.08 (ddd, J = 3.0, 6.0, 12.0 Hz, 1H, H2b), 4.34 (q, J = 6.0 Hz, 2H, CH2CH3), 4.48 (d, J = 6.0, 1H, H4), 6.26 (d, J = 3.0 Hz, 1H, ArH6), 6.54 (d, J = 9.0 Hz, 2H, ArH3′, ArH5′), 6.71–6.78 (m, 4H, ArH2′, ArH6′, ArH2″, ArH6″), 7.09 (d, J = 9H, 2H, ArH3″, ArH5″), 7.47 (d, J = 3.0 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.6 (Me), 31.4 (3 × MeC), 34.3 (CMe), 43.2 (ArC4, ArC3), 45.2 (CH2N), 55.2 (OMe), 55.4 (OMe), 55.7 (OMe), 62.0 (OCH2), 94.5 (ArC6, ArC8), 99.5 (ArC), 113.2 (ArC3′, ArC5′), 124.1 (ArC3″, ArC5″), 129.2 (ArC2′, ArC6′), 129.3 (ArC2″, ArC6″), 132.3 (ArC), 136.3 (ArC1″), 138.9 (ArC1′), 148.8 (ArC4″), 155.1 (COO), 157.0 (ArC5), 158.4 (ArC7), 158.8 (ArC4′); HRMS (ESI) m/z Calcd. for C31H37NO5Na (M + Na)+: 526.2569. Found: 526.2555; Anal. Calcd. for C31H37NO5: C, 73.93; H, 7.41; N, 2.78. Found: C, 73.65; H, 7.45; N, 2.74.
White solid (0.65 g, 95.5%). M.p. 140–142 °C; UV (MeOH): λmax 217 (ε 61,337 cm−1M−1), 283 (2067), 336 (7581) nm; IR (KBr): νmax 2978, 1704, 1586, 1513, 1376, 1241, 1169, 1058, 1042, 1024, 937, 889, 809, 761 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.36 (t, J = 7.2 Hz, 3H, CH3CH2), 2.26 (s, 3H, MeAr″), 3.35 (dt, J = 4.2, 13.5 Hz, 1H, H3), 3.62 (s, 3H, OMe), 3.69 (t, J = 13.5 Hz, 1H, H2a), 3.81 (s, 3H, OMe), 3.87 (s, 3H, OMe), 4.08 (ddd, J = 1.5, 4.50, 12.3 Hz, 1H, H2b), 4.31 (q, J = 7.2 Hz, 2H, CH2CH3), 4.46 (d, J = 3.6 Hz, 1H, H4), 6.23 (d, J = 2.4 Hz, 1H, ArH6), 6.47 (d, J = 8.1 Hz, 2H, ArH3′, ArH5′), 6.76 (s, 4H, ArH2″, ArH3″, ArH5″, ArH6″), 6.86 (d, J = 7.8 Hz, 2H, ArH2′, ArH6′), 7.47 (d, J = 2.1 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.6 (Me), 21.0 (MeAr″), 43.0 (ArC4), 43.3 (ArC3), 45.0 (CH2N), 55.2 (OMe), 55.4 (OMe), 55.7 (OMe), 62.0 (OCH2), 94.5 (ArC6, ArC8), 99.3 (ArC), 113.3 (ArC3′, ArC5′), 128.0 (ArC2′, ArC6′), 129.3 (ArC2″, ArC6″), 129.5 (ArC3″, ArC5″), 132.2 (ArC), 135.5 (ArC4″), 136.4 (ArC1′), 138.9 (ArC1″), 155.0 (COO), 157.0 (ArC5), 158.4 (ArC7), 158.8 (ArC4′); HRMS (ESI) m/z Calcd. for C28H31NO5Na (M + Na)+: 484.2100. Found: 484.2087; Anal. Calcd. for C28H31NO5: C, 72.86; H, 6.77; N, 3.03. Found: C, 72.89; H, 6.93; N, 2.98.
White solid (0.65 g, 93%). M.p. 90–92 °C; UV (MeOH): λmax 215 (ε 49,140 cm−1M−1), 247 (20,147), 337 (10,319) nm; IR (KBr): νmax 3001, 2939, 2839, 1687, 1579, 1489, 1454, 1368, 1307, 1214, 1202, 1173, 1063, 1039, 1022, 949, 882, 836, 823, 758, 656 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.37 (t, J = 7.2 Hz, 3H, CH3CH2), 3.46 (dt, J = 4.2, 13.2 Hz, 1H, H3), 3.61 (s, 3H, OMe), 3.77 (t, J = 12.9 Hz, 1H, H2a), 3.89 (s, 3H, OMe), 4.20 (ddd, J = 1.5, 4.2, 12.6 Hz, 1H, H2b), 4.33 (q, J = 7.2 Hz, 2H, CH2CH3), 4.55 (d, J = 4.8 Hz, 1H, H4), 6.26 (d, J = 2.4 Hz, 1H, ArH6), 6.57–6.61 (m, 2H, ArH′), 6.82–6.85 (m, 2H, ArH″), 7.03–7.14 (m, 3H, ArH′), 7.21–7.25 (m, 3H, ArH″), 7.50 (d, J = 2.4 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.6 (Me), 43.7 (ArC4), 43.9 (ArC3), 44.5 (CH2N), 55.4 (OMe), 55.7 (OMe), 62.0 (OCH2), 94.6 (ArC6), 99.3 (ArC8), 113.5 (ArC), 126.0 (ArC4′), 126.8 (ArC4″), 127.2 (ArC2″, ArC6″), 127.9 (ArC3′, ArC5′), 128.2 (ArC2′, ArC6′), 129.6 (ArC3″, ArC5″), 139.1 (ArC), 139.9 (ArC1″), 140.1 (ArC1′), 155.0 (COO), 157.2 (ArC5), 159.0 (ArC7); HRMS (ESI) m/z Calcd. for C26H27NO4Na (M + Na)+: 440.1838. Found: 440.1822; Anal. Calcd. for C26H27NO4: C, 74.80; H, 6.52; N, 3.35. Found: C, 74.96; H, 6.66; N, 3.32.
White solid (0.6 g, 95%). M.p. 122–124 °C; UV (MeOH): λmax 216 (ε 134,352 cm−1M−1) nm; IR (KBr): νmax 2939, 1723, 1690, 1589, 1455, 1373, 1325, 1287, 1243, 1170, 1140, 1057, 1044, 940, 885, 821, 808, 761, 698 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.33 (t, J = 6.9 Hz, 3H, CH3CH2), 2.23 (s, 3H, MeAr″), 3.38 (dt, J = 4.2, 13.2 Hz, 1H, H3), 3.59 (s, 3H, OMe), 3.72 (t, J = 13.2 Hz, 1H, H2a), 3.85 (s, 3H, OMe), 4.12 (ddd, J = 1.5, 4.2, 12.3 Hz, 1H, H2b), 4.29 (q, J = 6.9 Hz, 2H, CH2CH3), 4.48 (d, J = 4.2 Hz, 1H, H4), 6.21 (d, J = 2.4 Hz, 1H, ArH6), 6.43 (d, J = 8.1 Hz, 2H, ArH3″, ArH5″), 6.81–6.84 (m, 4H, Ph), 7.18–7.21 (m, 3H, ArH2″, ArH6″, Ph), 7.45 (d, J = 2.4 Hz, 1H, ArH8); 13C NMR (75.6 MHz, CDCl3): δ 14.6 (Me), 21.0 (MeAr″), 43.3 (ArC4), 43.7 (ArC3), 44.7 (CH2N), 55.4 (OMe), 55.7 (OMe), 62.0 (OCH2), 94.5 (ArC6), 99.3 (ArC8), 113.9 (ArC), 126.7 (ArC4′), 127.9 (ArC2′, ArC6′), 128.0 (Ar″C2, Ar″C6), 128.3 (ArC3′, ArC5′), 129.4 (ArC3″, ArC5″), 135.5 (ArC4″), 136.4 (ArC), 139.0 (ArC1″), 140.2 (ArC1′), 155.0 (COO), 157.1 (ArC5), 158.9 (ArC7); HRMS (ESI) m/z Calcd. for C27H29NO4Na (M + Na)+: 454.1994. Found: 454.1980; Anal. Calcd. for C27H29NO4: C, 75.15; H, 6.77; N, 3.25. Found: C, 75.40; H, 6.87; N, 3.22.
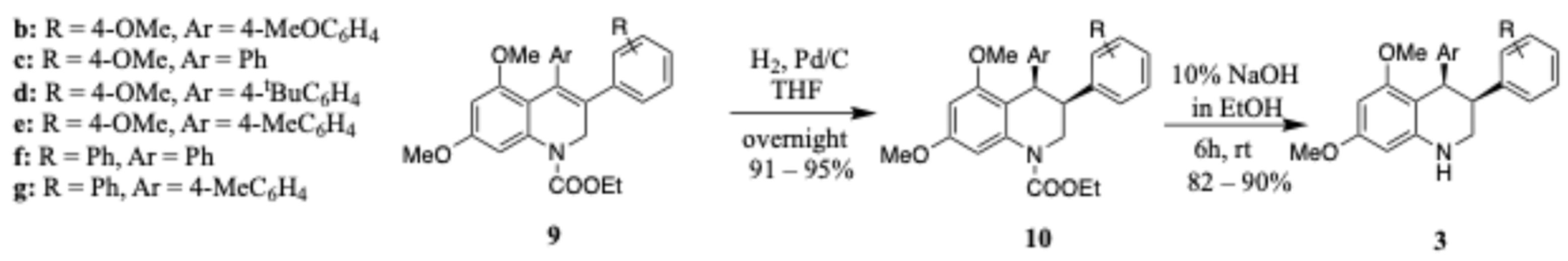
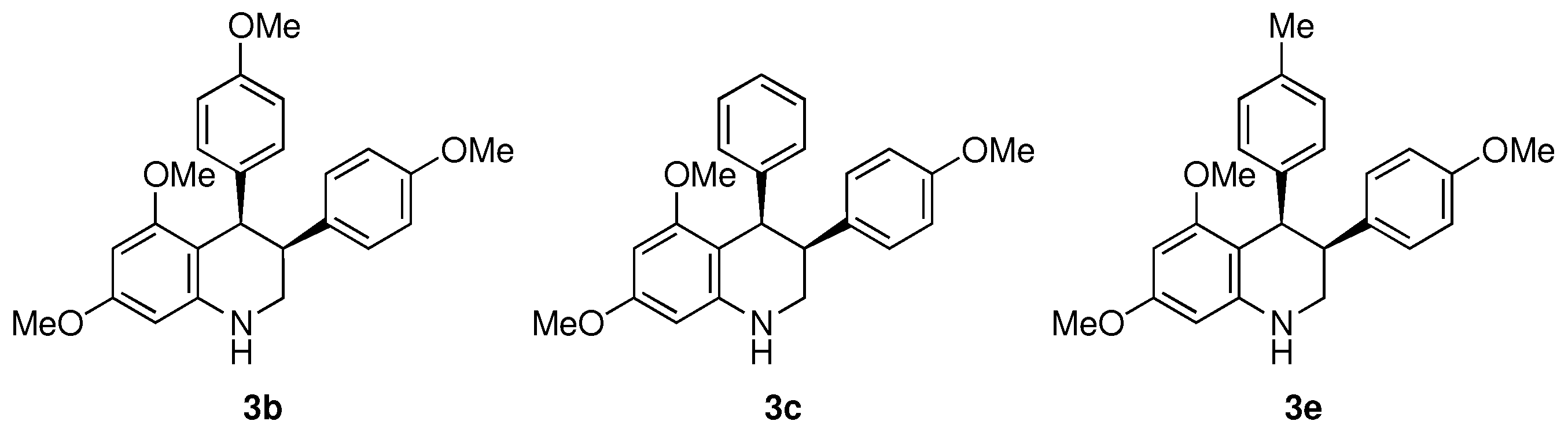
cis-5,7-Dimethoxy-3,4-bis(4-methoxyphenyl)-1,2,3,4-tetrahydroquinoline (3b)
White solid (0.35 g, 82%). M.p. 102–104 °C; UV (MeOH): λmax 214 (ε 62,383 cm−1M−1), 246 (23,705), 337 (13,100) nm; IR (KBr): νmax 2935, 1703, 1608, 1583, 1511, 1234, 1204, 1168, 1143, 1032, 953, 826, 763 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.21 (ddd, J = 3.0, 6.0, 9.0 Hz, 1H, H2b), 3.31 (dt, J = 6.0, 12.0 Hz, 1H, H3), 3.53–3.60 (m, 1H, H2a), 3.57 (s, 3H, OMe), 3.72 (s, 3H, OMe), 3.77 (s, 3H, OMe), 3.78 (s, 3H, OMe), 4.13 (bs, 1H, NH), 4.31 (dd, J = 3.0, 6.0 Hz, 1H, H4), 5.82 (d, J = 3.0 Hz, 1H, ArH6), 5.84 (d, J = 3.0 Hz, 1H, ArH8), 6.54–6.58 (m, 4H, ArH3′, ArH5′, ArH3″, ArH5″), 6.63 (d, J = 9.0 Hz, 2H, ArH2″, ArH6″), 6.70 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′); 13C NMR (75.6 MHz, CDCl3): δ 40.9 (ArC4), 42.0 (ArC3), 42.4 (CH2N), 54.9 (OMe), 55.0 (OMe), 55.1 (OMe), 55.4 (OMe), 87.9 (ArC6), 90.7 (ArC8), 105.5 (ArC), 112.1 (ArC3′, ArC5′), 113.0 (ArC3″, ArC5″), 129.0 (ArC2′, ArC6′), 130.6 (ArC2″, ArC6″), 133.4 (ArC1″), 134.3 (ArC1′), 145.0 (ArC), 157.4 (ArC5), 157.9 (ArC7), 158.3 (ArC4′), 159.9 (ArC1″); HRMS (ESI) m/z Calcd. for C25H28NO4 (M + H)+: 406.2018. Found: 406.2006; Anal. Calcd. for C25H27NO4.0.25 H2O: C, 73.24; H, 6.76; N, 3.42. Found: C, 73.48; H, 6.82; N, 3.38.
cis-5,7-Dimethoxy-3-(4-methoxyphenyl)-4-phenyl-1,2,3,4-tetrahydroquinoline (3c)
White solid (0.4 g, 87%). M.p. 166–168 °C; UV (MeOH): λmax 203 (ε 51,440 cm−1M−1), 216 (51,440), 337 (5144) nm; IR (KBr): νmax 3425, 3006, 2964, 2906, 2838, 2877, 1603, 1503, 1402, 1207, 1111, 1134, 1090, 1054, 810, 796, 728 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.21 (ddd, J = 3.0, 3.0, 9.0 Hz, 1H, H2b), 3.35 (dt, J = 3.0, 12.0 Hz, 1H, H3), 3.56 (s, 3H, OMe), 3.59–3.64 (m, 1H, H2a), 3.76 (s, 3H, OMe), 3.78 (s, 3H, OMe), 4.14 (bs, 1H, NH), 4.35 (d, J = 3.0, 1H, H4), 5.82 (d, J = 3.0 Hz, 1H, ArH6), 5.85 (d, J = 3.0 Hz, 1H, ArH8), 6.60–6.71 (m, 6H, ArH3′, ArH5′, ArH2″, ArH4″, ArH5″, ArH6″), 7.01–7.06 (m, 3H, ArH2′, ArH6′, ArH3″); 13C NMR (75.6 MHz, CDCl3): δ 40.9 (ArC4), 41.9 (ArC3), 43.3 (CH2N), 55.0 (OMe), 55.1 (OMe), 55.3 (OMe), 87.9 (ArC6), 90.6 (ArC8), 105.2 (ArC), 113.0 (ArC3′, ArC5′), 125.5 (ArC4″), 126.7 (ArC2′, ArC6′), 128.9 (ArC2″, ArC6″), 129.8 (ArC3″, ArC5″), 133.3 (ArC), 142.1 (ArC1″), 145.1 (ArC1′), 157.9 (ArC5), 158.4 (ArC7), 159.9 (ArC4′); HRMS (ESI) m/z Calcd. for C24H26NO3 (M + H)+: 376.1913. Found: 376.1902; Anal. Calcd. for C24H25NO3: C, 76.77; H, 6.71; N, 3.73. Found: C, 77.00; H, 6.98; N, 3.70.
cis-4-(4-(tert-Butyl)phenyl)-5,7-dimethoxy-3-(4-methoxyphenyl)-1,2,3,4-tetrahydroquinoline (3d)
White solid (0.4 g, 90.5%). M.p. 118–120 °C; UV (MeOH): λmax 203 (ε 85,611 cm−1M−1), 216 (80,755) nm; IR (KBr): νmax 3427, 2960, 1609, 1509, 1222, 1211, 1133, 1109, 1050, 1023, 831, 805, 704 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.23 (s, 9H, 3 × Me), 3.24 (ddd, J = 3.0, 6.0, 15.0 Hz, 1H, H2b), 3.35 (dt, J = 3.0, 15.0 Hz, 1H, H3), 3.56–3.63 (m, 1H, H2a), 3.57 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.77 (s, 3H, OMe), 3.84 (bs, 1H, NH), 4.32 (d, J = 3.0, 1H, H4), 5.89 (d, J = 3.0 Hz, 1H, ArH6), 5.97 (d, J = 3.0 Hz, 1H, ArH8), 6.53–6.57 (m, 4H, ArH3′, ArH5′, ArH2″, ArH4″), 6.65 (d, J = 9.0 Hz, 2H, ArH2′, ArH6′), 7.03 (d, J = 9.0 Hz, 2H, ArH3″, ArH5″); 13C NMR (75.6 MHz, CDCl3): δ 31.4 (3 × Me), 34.2 (CMe), 41.2 (ArC4, ArC3), 42.7 (CH2N), 55.2 (2 × OMe), 55.5 (OMe), 89.5 (ArC6), 91.8 (ArC8), 106.9 (ArC), 113.0 (ArC3′, ArC5′), 123.7 (Ar″C3, Ar″C5), 129.1 (ArC2′, ArC6′), 129.3 (ArC2″, ArC6″), 133.3 (ArC), 138.6 (ArC1″, ArC1′), 148.4 (ArC4″), 158.1 (ArC5), 158.5 (ArC7), 160.0 (Ar′C4); HRMS (ESI) m/z Calcd. for C25H24NO4 (M + H)+: 402.1705. Found: 402.1692.
cis-5,7-Dimethoxy-3-(4-methoxyphenyl)-4-(p-tolyl)-1,2,3,4-tetrahydroquinoline (3e)
White solid (0.4 g, 87%). M.p. 170–172 °C; UV (MeOH): λmax 203 (ε 54,535 cm−1M−1), 212 (55,648), 246 (21,702), 336 (11,686) nm; IR (KBr): νmax 3418, 3002, 2918, 2837, 1613, 1511, 1223, 1110, 1099, 1049, 1030, 822, 767 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.16 (s, 3H, MeAr″), 3.13 (ddd, J = 1.5, 3.6, 10.8 Hz, 1H, H2a), 3.25 (dt, J = 3.9, 12.3 Hz, 1H, H3), 3.41–3.54 (m, 1H, H2b), 3.49 (s, 3H, OMe), 3.69 (s, 3H, OMe), 3.70 (s, 3H, OMe), 4.05 (bs, 1H, NH), 4.26 (d, J = 3.6, 1H, H4), 5.74 (d, J = 2.4 Hz, 1H, ArH6), 5.77 (d, J = 2.1 Hz, 1H, ArH8), 6.46 (d, J = 8.1 Hz, 2H, ArH3′, ArH5′), 6.55 (d, J = 8.7 Hz, 2H, ArH2″, ArH6″), 6.63 (d, J = 9.0 Hz, 2H, ArH3″, ArH5″), 6.77 (d, J = 8.4 Hz, 2H, ArH2′, ArH6′); 13C NMR (75.6 MHz, CDCl3): δ 21.0 (MeAr″), 41.1 (ArC4), 42.0 (ArC3), 42.9 (CH2N), 55.1 (OMe), 55.2 (OMe), 55.5 (OMe), 88.0 (ArC6), 90.8 (ArC8), 105.6 (ArC), 113.1 (ArC3′, ArC5′), 127.6 (ArC2′, ArC6′), 129.1 (ArC2″, ArC6″), 129.8 (ArC3″, ArC5″), 133.6 (ArC), 134.9 (ArC4″), 139.0 (ArC1″), 145.1 (ArC1′), 158.0 (ArC5), 158.5 (ArC7), 160.0 (ArC4′); HRMS (ESI) m/z Calcd. for C25H28NO3 (M + H)+: 390.2069. Found: 390.2054; Anal. Calcd. for C25H27NO3: C, 77.09; H, 6.99; N, 3.60. Found: C, 76.97; H, 7.09; N, 3.54.
cis-5,7-Dimethoxy-3,4-diphenyl-1,2,3,4-tetrahydroquinoline (3f)
White solid (0.4 g, 86.5%). M.p. 180–182 °C; UV (MeOH): λmax 214 (ε 43,196 cm−1M−1), 337 (6479) nm; IR (KBr): νmax 3424, 2873, 1598, 1507, 1451, 1401, 1367, 1219, 1203, 1132, 1095, 1078, 1046, 936, 796, 764, 662 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.27 (ddd, J = 1.5, 3.6, 11.1 Hz, 1H, H2a), 3.41 (dt, J = 3.9, 12.6 Hz, 1H, H3), 3.56 (s, 3H, OMe), 3.66 (t, J = 12.3 Hz, 1H, H2b), 3.78 (s, 3H, OMe), 4.05 (bs, 1H, NH), 4.41 (dd, J = 1.2, 4.8 Hz, 1H, H4), 5.83 (d, J = 2.4 Hz, 1H, ArH6), 5.87 (d, J = 2.1 Hz, 1H, ArH8), 6.62–6.65 (m, 2H, ArH′), 6.70–6.74 (m, 2H, ArH″), 6.99–7.07 (m, 3H, ArH′), 7.12–7.16 (m, 3H, ArH″); 13C NMR (75.6 MHz, CDCl3): δ 40.7 (ArC4), 42.8 (ArC3), 43.4 (CH2N), 55.1 (OMe), 55.4 (OMe), 88.2 (ArC6), 90.9 (ArC8), 105.4 (ArC), 125.7 (ArC4″), 126.3 (ArC4′), 126.8 (ArC2′, ArC6′), 127.7 (ArC2″, ArC6″), 128.1 (ArC3′, ArC5′), 129.8 (ArC3″, ArC5″), 141.3 (ArC), 142.2 (ArC1″), 145.0 (ArC1′), 158.5 (ArC5), 160.1 (ArC7); HRMS (ESI) m/z Calcd. for C23H24NO2 (M + H)+: 346.1807. Found: 346.1794; Anal. Calcd. for C23H23NO2: C, 79.97; H, 6.71; N, 4.05. Found: C, 80.00; H, 6.82; N, 4.02.
cis-5,7-Dimethoxy-3-phenyl-4-(p-tolyl)-1,2,3,4-tetrahydroquinoline (3g)
White solid (0.4 g, 91%). M.p. 184–186 °C; UV (MeOH): λmax 216 (ε 110,601 cm−1M−1) nm; IR (KBr): νmax 3395, 2850, 1617, 1589, 1514, 1470, 1351, 1315, 1222, 1170, 1130, 1096, 1046, 945, 755, 695 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.23 (s, 3H, MeAr″), 3.26 (ddd, J = 1.8, 3.6, 11.1 Hz, 1H, H2a), 3.38 (dt, J = 3.9, 12.3 Hz, 1H, H3), 3.57 (s, 3H, OMe), 3.65 (t, J = 11.1 Hz, 1H, H2b), 3.78 (s, 3H, OMe), 4.39 (dd, J = 1.2, 4.2 Hz, 1H, H4), 5.83 (d, J = 2.1 Hz, 1H, ArH6), 5.86 (d, J = 2.1 Hz, 1H, ArH8), 6.51 (d, J = 8.1 Hz, 2H, ArH3″, ArH5″), 6.73–6.76 (m, 2H, Ph), 6.83 (d, J = 7.8 Hz, 2H, ArH2″, ArH6″), 7.15–7.17 (m, 3H, Ph); 13C NMR (75.6 MHz, CDCl3): δ 21.0 (MeAr″), 40.7 (ArC4), 42.8 (ArC3), 42.9 (CH2N), 55.1 (OMe), 55.5 (OMe), 88.2 (ArC6), 90.9 (ArC8), 105.6 (ArC), 126.3 (ArC4′), 127.6 (ArC2′, ArC6′), 127.7 (ArC2″, ArC6″), 128.2 (ArC3′, ArC5′), 129.7 (ArC3″, ArC5″), 135.0 (ArC4″), 139.0 (ArC), 142.5 (ArC1″), 145.0 (ArC1′), 158.5 (ArC5), 160.0 (ArC7); HRMS (ESI) m/z Calcd. for C24H26NO2 (M + H)+: 360.1964. Found: 360.1952; Anal. Calcd. for C24H25NO2: C, 80.19; H, 7.01; N, 3.90. Found: C, 80.00; H, 7.13; N, 3.85.
Yellow solid (0.06 g, 30%). M.p. 232–234 °C; UV (MeOH): λmax 203 (ε 63,067 cm−1M−1), 216 (65,388) nm; IR (KBr): νmax 3470, 3383, 2959, 2925, 2853, 1619, 1512, 1461, 1376, 1218, 1197, 1179, 1125, 1106, 1076, 1054, 825, 798, 726 cm−1; 1H NMR (300 MHz, Acetone-d6): δ 1.31 (s, 9H, 3 × Me), 3.22–3.36 (m, 2H, H2a, H3), 3.68–3.82 (m, 1H, H2b), 3.72 (s, 3H, OMe), 4.20 (dd, J = 0.9, 4.5 Hz, 1H, H4), 5.33 (bs, 1H, NH), 5.80 (d, J = 2.4 Hz, 1H, ArH6), 5.91 (d, J = 2.1 Hz, 1H, ArH8), 6.62–6.73 (m, 6H, ArH2′, ArH3′, ArH5′, ArH6′, ArH2″, ArH6″), 7.14 (d, J = 8.4 Hz, 2H, ArH3″, ArH5″), 7.71 (s, 1H, OH), 8.16 (s, 1H, OH); 13C NMR (75.6 MHz, CDCl3): δ 30.9 (3 × MeC), 33.9 (CMe), 40.9 (ArC4), 43.1 (ArC3), 43.2 (CH2N), 54.1 (OMe), 90.2 (ArC6, ArC8), 104.1 (ArC), 114.6 (ArC3′, ArC5′), 123.4 (ArC3″, ArC5″), 128.9 (ArC2″, ArC6″), 129.6 (ArC2′, ArC6′), 139.8 (ArC1″), 139.8 (ArC1′), 147.9 (ArC), 148.0 (ArC4″), 155.5 (ArC4′), 158.2 (ArC5), 160.1 (ArC7); HRMS (ESI) m/z Calcd. for C26H30NO3 (M + H)+: 404.2226. Found: 404.2209.
5-Hydroxy-7-methoxy-3,4-diphenyl-1,2,3,4-tetrahydroquinoline (13f)
Yellow solid (0.07 g, 38.5%). M.p. 198–200 °C; UV (MeOH): λmax 216 (ε 53,867 cm−1M−1), 246 (14,304), 337 (7152) nm; IR (KBr): νmax 3675, 3377, 2971, 2901, 1625, 1597, 1490, 1450, 1410, 1375, 1231, 1212, 1153, 1118, 1080, 1050, 798, 764, 662 cm−1; 1H NMR (300 MHz, Acetone-d6): δ 3.24–3.31 (m, 1H, H2a), 3.38 (dt, J = 3.9, 12.3 Hz, 1H, H3), 3.62–3.80 (m, 1H, H2b), 3.69 (s, 3H, OMe), 4.45 (dd, J = 1.2, 4.8 Hz, 1H, H4), 5.39 (bs, 1H, NH), 5.76 (d, J = 2.4 Hz, 1H, ArH6), 5.89 (d, J = 2.1 Hz, 1H ArH8), 6.67–6.70 (m, 2H, ArH′), 6.79–6.82 (m, 2H, ArH″), 6.99–7.06 (m, 3H, ArH′), 7.14–7.18 (m, 3H, ArH″), 7.78 (s, 1H, OH); 13C NMR (75.6 MHz, Acetone-d6): δ 40.3 (ArC4), 42.7 (ArC3), 43.7 (CH2N), 54.1 (OMe), 90.1 (ArC6), 90.2 (ArC8), 103.6 (ArC), 125.4 (ArC4″), 126.1 (ArC4′), 126.6 (ArC2′, ArC6′), 127.6 (ArC2″, ArC6″), 128.0 (ArC3′, ArC5′), 130.0 (ArC3″, ArC5″), 141.9 (ArC), 142.7 (ArC1″), 146.3 (ArC1′), 155.6 (ArC5), 160.0 (ArC7); HRMS (ESI) m/z Calcd. for C22H22NO2 (M + H)+: 332.1951. Found: 332.1636.
Yellow solid (0.06 g, 32%). M.p. 128–130 °C; UV (MeOH): λmax 203 (ε 25,755 cm−1M−1) nm; IR (KBr): νmax 2922, 2852, 1610, 1510, 1451, 1366, 1330, 1286, 1238, 1202, 1111, 1028, 826, 802, 722, 697 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.22 (s, 3H, MeAr″), 3.24 (ddd, J = 1.2, 3.3, 10.8 Hz, 1H, H2a), 3.43 (dt, J = 4.8, 12.3 Hz, 1H, H3), 3.63 (t, J = 12.3 Hz, 1H, H2b), 3.73 (s, 3H, OMe), 4.25 (d, J = 4.8 Hz, 1H, H4), 5.78 (d, J = 2.1 Hz, 1H, ArH6), 5.85 (d, J = 2.1 Hz, 1H, ArH8), 6.55 (d, J = 8.1 Hz, 2H, ArH3″, ArH5″), 6.71–6.74 (m, 2H, Ph), 6.84 (d, J = 7.80 Hz, 2H, ArH2″, ArH6″), 7.12–7.15 (m, 3H, Ph); 13C NMR (75.6 MHz, CDCl3): δ 21.0 (MeAr″), 40.6 (ArC4), 43.3 (ArC3), 43.5 (CH2N), 54.1 (OMe), 91.6 (ArC6), 91.9 (ArC8), 104.2 (ArC), 126.4 (ArC4′), 127.8 (ArC2′, ArC6′), 128.1 (ArC2″, ArC6″), 128.2 (ArC3′, ArC5′), 129.8 (ArC3″, ArC5″), 136.0 (ArC4″), 137.7 (ArC), 140.9 (ArC1″), 145.6 (Ar′C1), 154.7 (ArC5), 160.1 (ArC7); HRMS (ESI) m/z Calcd. for C23H24NO2 (M + H)+: 346.1807. Found: 346.1791.