Determination of Antiepileptics in Biological Samples—A Review
Abstract
1. Introduction
- Simple partial seizures (carbamazepine, phenytoin, phenobarbital, primidone, valproate, gabapentin, and lamotrigine).
- Complex partial seizures (carbamazepine, phenobarbital, phenytoin, primidone, valproate, gabapentin, and lamotrigine).
- Partial with secondary generalized tonic–clonic seizures (carbamazepine, phenobarbital, phenytoin, primidone, valproate, gabapentin, and lamotrigine).
- Generalized absence seizures (clonazepam, ethosuximide, and valproate).
- Generalized myoclonic seizures (valproate).
- Tonic–clonic seizures (carbamazepine, phenobarbital, phenytoin, primidone, and valproate) [26].
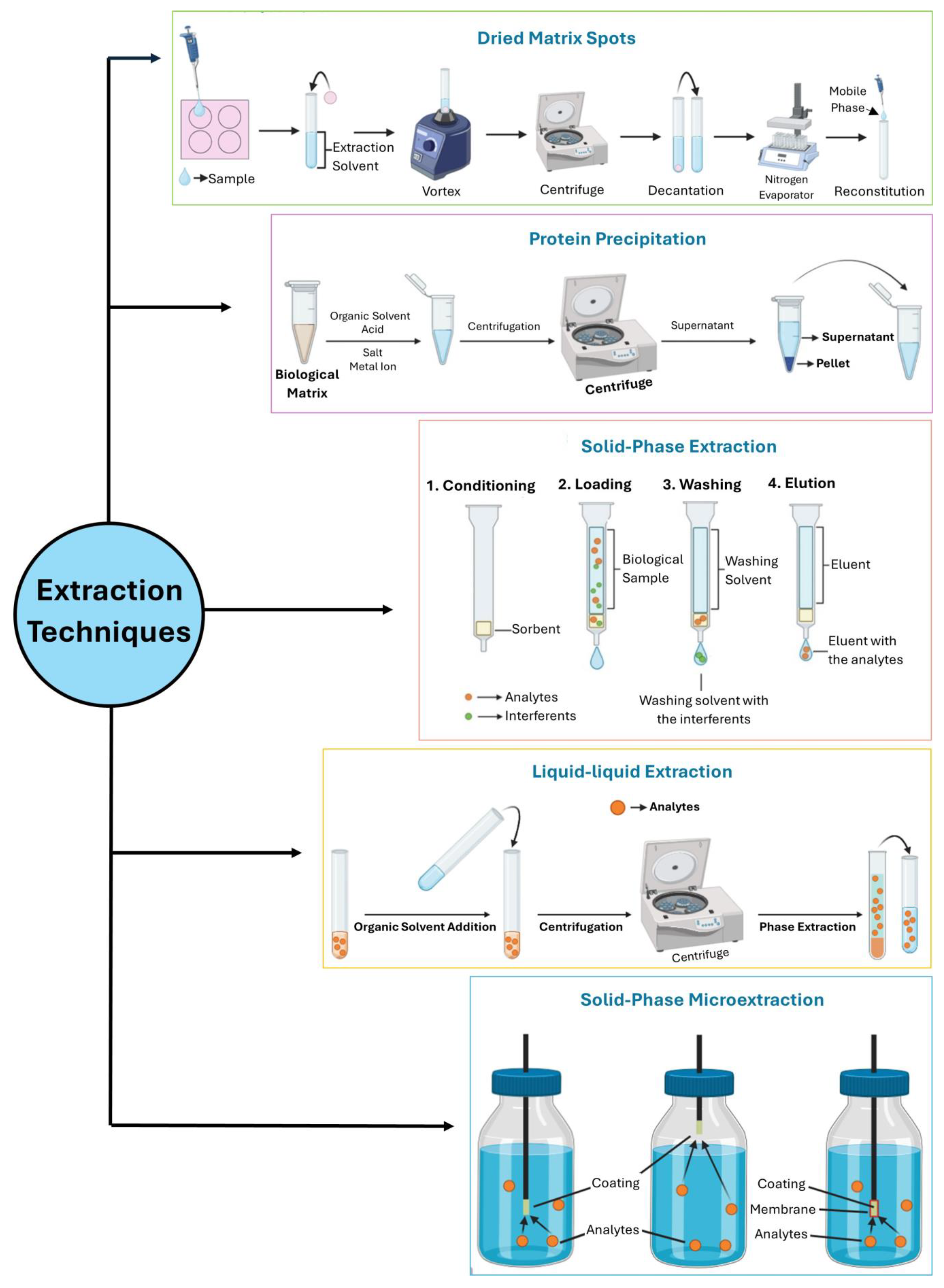
2. Strategies to Determine AEDs in Biological Specimens
2.1. Blood and Derivates
2.2. Urine
2.3. Oral Fluid
2.4. Hair
2.5. Exhaled Air
2.6. Breast Milk
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO | Epilepsy: A Public Health Imperative; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Hassan, S.A.; Helmy, A.H.; Youssef, N.F.; Weshahy, S.A.; El-zeany, B.A. Fluorescence imaging approaches for eco-friendly determination of perampanel in human plasma and application for therapeutic drug monitoring. Luminescence 2023, 38, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M. Clinical Characteristics, Treatment Outcome and Associated Factors of Epilepsy Among Children at Hospitals of North-West Ethiopia. Pediatr. Health Med. Ther. 2023, 14, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, B.S.; Silverman, A.; Eapen, B. Seizure Medications. In Anticonvulsants Toxicity; Springer, C., Nappe, T.M., Eds.; StatPearls Publishing: St. Petersburg, FL, USA, 2023; pp. 1–7. [Google Scholar]
- Landazuri, P.; Lecumberri, M.L.; Bertran, L.V.; Farrengurg, M.; Lhatoo, S. Seizure and Epilepsy Care The Pocket Epileptologist. Part of Cambridge Manuals in Neurology; Cambridge University Press: Cambidge, UK, 2023. [Google Scholar]
- Aaryashree; Choudhary, A.K.; Yoshimi, Y. Disposable Sensor Chips with Molecularly Imprinted Carbon Paste Electrodes for Monitoring Anti-Epileptic Drugs. Sensors 2023, 23, 3271. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABA(A) Receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef]
- Twyman, R.E.; Rogers, C.J.; Macdonald, R.L. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann. Neurol. 1989, 25, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Borden, L.A.; Murali Dhar, T.G.; Smith, K.E.; Weinshank, R.L.; Branchek, T.A.; Gluchowski, C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur. J. Pharmacol. 1994, 269, 219–224. [Google Scholar]
- Coulter, D.A.; Huguenard, J.R.; Prince, D.A. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann. Neurol. 1989, 25, 582–593. [Google Scholar] [CrossRef]
- Hendrich, J.; Van Minh, A.T.; Heblich, F.; Nieto-Rostro, M.; Watschinger, K.; Striessnig, J.; Wratten, J.; Davies, A.; Dolphin, A.C. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. USA 2008, 105, 3628–3633. [Google Scholar] [CrossRef]
- Trimethadione: Uses, Interactions, Mechanism of Action | DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00347 (accessed on 19 July 2024).
- Reiss, W.G.; Oles, K.S. Acetazolamide in the treatment of seizures. Ann. Pharmacother. 1996, 30, 514–519. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Hanada, T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol. Scand. 2013, 127, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Pisani, A.; De Murtas, M.; Mercuri, N.B.; Marciani, M.G.; Calabresi, P. Action of GP 47779, the active metabolite of oxcarbazepine, on the corticostriatal system. II. Modulation of high-voltage-activated calcium currents. Epilepsia 1995, 36, 997–1002. [Google Scholar] [CrossRef]
- Sofia, R.D. Mechanism of action of the anticonvulsant felbamate: Opposing effects on N-methyl-D-aspartate and gamma-aminobutyric acidA receptors. Ann. Neurol. 1994, 36, 677–678. [Google Scholar] [CrossRef] [PubMed]
- Temperini, C.; Innocenti, A.; Scozzafava, A.; Parkkila, S.; Supuran, C.T. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: The antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J. Med. Chem. 2010, 53, 850–854. [Google Scholar] [CrossRef]
- Nakamura, M.; Cho, J.-H.; Shin, H.; Jang, I.-S. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur. J. Pharmacol. 2019, 855, 175–182. [Google Scholar] [CrossRef]
- Sharma, R.; Song, W.S.; Nakamura, M.; Neupane, C.; Shin, H.; Melnick, S.M.; Glenn, K.J.; Jang, I.-S.; Kim, M.-H.; Park, J.B. Effects of Cenobamate on GABA-A Receptor Modulation (P1.5-033). Neurology 2019, 92, 15. [Google Scholar] [CrossRef]
- Löscher, W. Valproate: A reappraisal of its pharmacodynamic properties and mechanisms of action. Prog. Neurobiol. 1999, 58, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Ticku, M.K.; Davis, W.C. Effect of valproic acid on [3H]diazepam and [3H]dihydropicrotoxinin binding sites at the benzodiazepine-GABA receptor-ionophore complex. Brain Res. 1981, 223, 218–222. [Google Scholar] [CrossRef]
- Zhang, X.; Velumian, A.A.; Jones, O.T.; Carlen, P.L. Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia 2000, 41, 52–60. [Google Scholar] [CrossRef]
- Simeone, T.A.; Wilcox, K.S.; White, H.S. Subunit selectivity of topiramate modulation of heteromeric GABAA receptors. Neuropharmacology 2006, 50, 845–857. [Google Scholar] [CrossRef]
- Gibbs, J.W., 3rd; Sombati, S.; DeLorenzo, R.J.; Coulter, D.A. Cellular actions of topiramate: Blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia 2000, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Potnis, V.V.; Albhar, K.G.; Nanaware, P.A.; Pote, V.S. A Review on Epilepsy and its Management. J. Drug Deliv. Ther. 2020, 10, 273–279. [Google Scholar] [CrossRef]
- Johannessen Landmark, C.; Johannessen, S.I.; Patsalos, P.N. Therapeutic drug monitoring of antiepileptic drugs: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2020, 16, 227–238. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.D.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Perucca, E. Clinically important drug interactions in epilepsy: Interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003, 2, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Opuni, K.F.M.; Boadu, J.A.; Amponsah, S.K.; Okai, C.A. High performance liquid chromatography: A versatile tool for assaying antiepileptic drugs in biological matrices. J. Chromatogr. B 2021, 1179, 122750. [Google Scholar] [CrossRef]
- Feriduni, B.; Farajzadeh, M.A.; Jouyban, A. Determination of two antiepileptic drugs in urine by homogenous liquid-liquid extraction performed in a narrow tube combined with dispersive liquid-liquid microextraction followed by gas chromatography-flame ionization detection. Iran. J. Pharm. Res. 2019, 18, 620–630. [Google Scholar]
- Erarpat, S.; Bodur, S.; Ayyıldız, M.F.; Günkara, Ö.T.; Erulaş, F.; Chormey, D.S.; Turak, F.; Budak, T.B.; Bakırdere, S. Accurate simple determination of oxcarbazepine in human plasma urine samples using switchable-hydrophilicity solvent in, G.C.–.M.S. Biomed. Chromatogr. 2020, 34, e4915. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, W.; Wang, C.; Liu, Z.; Chen, Z. Direct coupling in-tube solid-phase microextraction with mass spectrometry using polymer coated open-tubular column for rapid analysis of antiepileptic drugs in biofluids. Anal. Chim. Acta 2023, 1240 (Suppl. S2022), 340775. [Google Scholar] [CrossRef]
- Möller, I.; Held, K.; Klimpel, D.; Nadulski, T.; Dufaux, B. Development and validation of an LC–MS/MS method for relevant drugs in epilepsy patients using dried blood spots. Biomed. Chromatogr. 2021, 35, e5130. [Google Scholar] [CrossRef]
- Carvalho, J.; Rosado, T.; Barroso, M.; Gallardo, E. Determination of antiepileptic drugs using dried saliva spots. J. Anal. Toxicol. 2019, 43, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Barroso, M.; Queiroz, J.A. LC-MS: A powerful tool in workplace drug testing. Drug Test. Anal. 2009, 1, 109–115. [Google Scholar] [CrossRef] [PubMed]
- de Campos, E.G.; da Costa, B.R.B.; dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Santos Junior, W.J.R.; De Martinis, B.S. Alternative matrices in forensic toxicology: A critical review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Peters, F.T.; Wissenbach, D.K.; Busardo, F.P.; Marchei, E.; Pichini, S. Method Development in Forensic Toxicology. Curr. Pharm. Des. 2018, 23, 5455–5467. [Google Scholar] [CrossRef] [PubMed]
- Mouskeftara, T.; Alexandridou, A.; Krokos, A.; Gika, H.; Mastrogianni, O.; Orfanidis, A.; Raikos, N. A Simple Method for the Determination of Lacosamide in Blood by, G.C.-M.S. J. Forensic Sci. 2020, 65, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shang, X.; Yao, S.; Wang, F. A novel and nonderivatization method for the determination of valproic acid in human serum by two-dimensional liquid chromatography. Biomed. Chromatogr. 2020, 34, e4695. [Google Scholar] [CrossRef] [PubMed]
- Velghe, S.; Delahaye, L.; Ogwang, R.; Hotterbeekx, A.; Colebunders, R.; Mandro, M.; Idro, R.; Stove, C.P. Dried Blood Microsampling-Based Therapeutic Drug Monitoring of Antiepileptic Drugs in Children With Nodding Syndrome and Epilepsy in Uganda and the Democratic Republic of the Congo. Ther. Drug Monit. 2020, 42, 481–490. [Google Scholar] [CrossRef]
- Gu, X.; Yu, S.; Peng, Q.; Ma, M.; Hu, Y.; Zhou, B. Determination of unbound valproic acid in plasma using centrifugal ultrafiltration and gas chromatography:Application in TDM. Anal. Biochem. 2020, 588, 113475. [Google Scholar] [CrossRef]
- Bharwad, K.D.; Shah, P.A.; Shrivastav, P.S.; Sharma, V.S. Selective quantification of lacosamide in human plasma using UPLC–MS/MS: Application to pharmacokinetic study in healthy subjects with different doses. Biomed. Chromatogr. 2020, 34, e4928. [Google Scholar] [CrossRef]
- Önal, C.; Kul, A.; Ozdemir, M.; Sagirli, O. Determination of levetiracetam in human plasma by online heart-cutting liquid chromatography: Application to therapeutic drug monitoring. J. Sep. Sci. 2020, 43, 3590–3596. [Google Scholar] [CrossRef]
- Seyfinejad, B.; Khoubnasabjafari, M.; Ziaei, S.E.; Ozkan, S.A.; Jouyban, A. Electromembrane extraction as a new approach for determination of free concentration of phenytoin in plasma using capillary electrophoresis. DARU J. Pharm. Sci. 2020, 28, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Madej, K.; Paprotny, Ł.; Wianowska, D.; Kasprzyk, J.; Herman, M.; Piekoszewski, W. A fully validated HPLC–UV method for determination of sulthiame in human serum/plasma samples. Biomed. Chromatogr. 2021, 35, e5002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yu, L.H.; Wang, T.T.; Feng, J.; Ma, L.; Yu, J.; Sun, L.; Li, H.; Sun, Y. Development and validation of an innovative UPLC method to quantify lacosamide, oxcarbazepine, and lamotrigine in the serum of children with epilepsy in China. Biomed. Chromatogr. 2021, 35, e5022. [Google Scholar] [CrossRef] [PubMed]
- Sabença, R.; Bicker, J.; Silva, R.; Carona, A.; Silva, A.; Santana, I.; Sales, F.; Falcão, A.; Fortuna, A. Development and application of an HPLC-DAD technique for human plasma concentration monitoring of perampanel and lamotrigine in drug-resistant epileptic patients. J. Chromatogr. B 2021, 1162, 122491. [Google Scholar] [CrossRef]
- Hess, C.; Zeidler, S.; Roehrich, J. Forensic application and evaluation of a commercially available pregabalin immunoassay test in serum on an Olympus AU480. Drug Test. Anal. 2021, 13, 1216–1218. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Cao, H.; Lin, H.; Ren, W.; Huang, J.; Zhang, J. Therapeutic drug monitoring of valproic acid using a dried plasma spot sampling device. J. Mass Spectrom. 2021, 56, e4603. [Google Scholar] [CrossRef]
- Kim, D.Y.; Moon, J.; Shin, Y.W.; Lee, S.T.; Jung, K.H.; Park, K.I.L.; Jung, K.Y.; Kim, M.; Lee, S.H.; Yu, K.S.; et al. Usefulness of saliva for perampanel therapeutic drug monitoring. Epilepsia 2020, 61, 1120–1128. [Google Scholar] [CrossRef]
- Franco, V.; Gatti, G.; Mazzucchelli, I.; Marchiselli, R.; Fattore, C.; Rota, P.; Galimberti, C.A.; Capovilla, G.; Beccaria, F.; De Giorgis, V.; et al. Relationship between saliva and plasma rufinamide concentrations in patients with epilepsy. Epilepsia 2020, 61, e79–e84. [Google Scholar] [CrossRef]
- Hassib, S.T.; Hashem, H.M.A.; Mahrouse, M.A.; Mostafa, E.A. Development and Bio-Analytical Validation of Chromatographic Determination Method of Rufinamide in Presence of its Metabolite in Human Plasma. J. Chromatogr. Sci. 2021, 59, 458–464. [Google Scholar] [CrossRef]
- Schaefer, V.D.; de Lima Feltraco Lizot, L.; Hahn, R.Z.; Schneider, A.; Antunes, M.V.; Linden, R. Simple determination of valproic acid serum concentrations using BioSPME followed by gas chromatography-mass spectrometric analysis. J. Chromatogr. B 2021, 1167, 122574. [Google Scholar] [CrossRef]
- Phillips, S.J.; Oliveto, A.; Mancino, M.J.; Hendrickson, H.P. Development and validation of a rapid liquid chromatography/tandem mass spectrometry method to quantitate gabapentin and buprenorphine in human serum. Rapid Commun. Mass Spectrom. 2021, 35, e9104. [Google Scholar] [CrossRef] [PubMed]
- Seyfinejad, B.; Ozkan, S.A.; Jouyban, A. Ultrasound-assisted electromembrane extraction of clonazepam from plasma and determination using capillary electrophoresis. J. Chromatogr. B 2021, 1181, 122928. [Google Scholar] [CrossRef]
- Kruizinga, M.D.; Zuiker, R.G.J.A.; Bergmann, K.R.; Egas, A.C.; Cohen, A.F.; Santen, G.W.E.; van Esdonk, M.J. Population pharmacokinetics of clonazepam in saliva and plasma: Steps towards noninvasive pharmacokinetic studies in vulnerable populations. Br. J. Clin. Pharmacol. 2022, 88, 2236–2245. [Google Scholar] [CrossRef]
- Schoonjans, A.S.; Roosens, L.; Dewals, W.; Paelinck, B.P.; Ceulemans, B. Therapeutic drug monitoring of fenfluramine in clinical practice: Pharmacokinetic variability and impact of concomitant antiseizure medications. Epilepsia 2022, 63, 686–696. [Google Scholar] [CrossRef]
- Ma, D.; Ji, Z.; Cao, H.; Huang, J.; Zeng, L.; Yin, L. LC–MS 3 Strategy for Quantification of Carbamazepine in Human Plasma and Its Application in Therapeutic Drug Monitoring. Molecules 2022, 27, 1224. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, S.; Kim, E.; Hwang, I.; Cho, J.Y.; Chung, J.Y.; Jang, I.; Oh, J. The pharmacokinetic, safety, and tolerability profiles of eslicarbazepine acetate are comparable between Korean and White subjects. Clin. Transl. Sci. 2022, 15, 2116–2126. [Google Scholar] [CrossRef]
- Rmandić, M.; Stajić, A.; Jančić, J.; Samardžić, J.; Jović, N.; Malenović, A. Quantification of Zonisamide in Dried Blood Spots and Dried Plasma Spots by UPLC–MS/MS: Application to Clinical Practice. Molecules 2022, 27, 4899. [Google Scholar] [CrossRef] [PubMed]
- Pascali, J.P.; Giorgetti, A.; Barone, R.; Pelletti, G.; Fais, P. Valproic acid determination by liquid chromatography coupled to mass spectrometry (LC–MS/MS) in whole blood for forensic purposes. Drug Test. Anal. 2023, 15, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, H.J.; Zhang, H.L.; Feng, J.; Yu, J.; Wang, T.T.; Sun, Y.; Yu, L.H. Impact of ABCC2 1249G>A and-24C>T Polymorphisms on Lacosamide Efficacy and Plasma Concentrations in Uygur Pediatric Patients with Epilepsy in China. Ther. Drug Monit. 2023, 45, 117–125. [Google Scholar] [CrossRef]
- Namera, A.; Uekusa, K.; Saito, T.; Yoshimoto, K.; Ishiuchi, N.; Murata, K.; Nagao, M. A method for determining valproic acid in human whole blood and urine via gas chromatography-mass spectrometry and small-scale inter-laboratory trial. Leg. Med. 2022, 59, 102133. [Google Scholar] [CrossRef]
- Salzmann, L.; Wild, J.; Singh, N.; Schierscher, T.; Liesch, F.; Bauland, F.; Geistanger, A.; Risch, L.; Geletneky, C.; Seger, C.; et al. An isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS)-based candidate reference measurement procedure (RMP) for the quantification of gabapentin in human serum and plasma. Clin. Chem. Lab. Med. 2023, 61, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, Y.; Li, W.; He, C.; Di, X.; Duan, Y.; Chen, L.; Wang, Z. Quantitation of levetiracetam concentrations in plasma and saliva samples by ultra-performance liquid chromatography–tandem mass spectrometry: Application to therapeutic drug monitoring for pregnant women with epilepsy. Biomed. Chromatogr. 2023, 38, e5777. [Google Scholar] [CrossRef] [PubMed]
- Nuchtavorn, N.; Dvořák, M.; Kubáň, P. Paper-based molecularly imprinted-interpenetrating polymer network for on-spot collection and microextraction of dried blood spots for capillary electrophoresis determination of carbamazepine. Anal. Bioanal. Chem. 2020, 412, 2721–2730. [Google Scholar] [CrossRef]
- Mendoza Aguilera, M.; Bellés Medall, M.D.; Álvarez Martín, T.; Pascual Marmaneu, Ó.; Liñana Granell, C.; Ferrando Piqueres, R. Therapeutic drug monitoring of levetiracetam in daily clinical practice: High-performance liquid chromatography versus immunoassay. Eur. J. Hosp. Pharm. Sci. Pract. 2020, 27, e2–e6. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, A.; Klimpel, D.; Bien, C.G.; Brandt, C.; May, T.W. Influence of dose and antiepileptic comedication on brivaracetam serum concentrations in patients with epilepsy. Epilepsia 2020, 61, e43–e48. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, L.; Liu, L.; Kuang, H.; Xu, C. Development of a monoclonal antibody-based immunochromatographic assay for the detection of carbamazepine and carbamazepine-10, 11-epoxide. J. Chromatogr. B 2020, 1141, 122036. [Google Scholar] [CrossRef]
- Li, X.-Y.; Hu, C.; Zhu, X.-H.; Wang, Y.; Shu, S.-Q.; Luo, Z. Pharmacokinetics and safety of Padsevonil in healthy Chinese subjects and comparison of two sampling methods for Padsevonil quantification. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4698–4707. [Google Scholar]
- Mavri, A.; Ilc, S. The efficacy of direct oral anticoagulants in patients on concomitant treatment with levetiracetam. Sci. Rep. 2023, 13, 9257. [Google Scholar] [CrossRef]
- Birnbaum, A.K.; Meador, K.J.; Karanam, A.; Brown, C.; May, R.C.; Gerard, E.E.; Gedzelman, E.R.; Penovich, P.E.; Kalayjian, L.A.; Cavitt, J.; et al. Antiepileptic Drug Exposure in Infants of Breastfeeding Mothers With Epilepsy. JAMA Neurol. 2020, 77, 441–450. [Google Scholar] [CrossRef]
- Itabashi, S.; Bito, R.; Nishina, M.; Fukumoto, M.; Soda, M.; Doi, M.; Usui, S.; Kitaichi, K. Determination of lamotrigine in human plasma using liquid chromatography-tandem mass spectrometry. Neuropsychopharmacol. Rep. 2019, 39, 48–55. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, H.; Fan, Y.; Zhang, J.; Cao, G.; Yu, J.; Chen, Y.; Guo, B. Determination of DP-VPA and its active metabolite, VPA, in human plasma, urine, and feces by UPLC-MS/MS: A clinical pharmacokinetics and excretion study. Drug Test. Anal. 2019, 11, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Zhao, K.; Sun, S.; Lu, Y.; Li, X. A new derivatization method for the determination of valproic acid in serum using tetramethylammonium hydroxide as a catalyst. Biomed. Chromatogr. 2019, 33, e4440. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xia, T.; Yun, Y.; Li, M.; Zhang, F.; Gao, S.; Chen, W. UHPLC-MS/MS method for simultaneous determination of carbamazepine and its seven major metabolites in serum of epileptic patients. J. Chromatogr. B 2019, 1108, 17–24. [Google Scholar] [CrossRef]
- Guo, M.; Shao, L.; Du, Y.; Qian, Z.; Huang, T.; Tang, D. Microporous polymer based on the new compound “bi-(4-vinyl phenylquinoline) amide” for enrichment and quantitative determination of lamotrigine in rat and human serum. Anal. Bioanal. Chem. 2019, 411, 3353–3360. [Google Scholar] [CrossRef]
- Velghe, S.; Deprez, S.; Stove, C.P. Fully automated therapeutic drug monitoring of anti-epileptic drugs making use of dried blood spots. J. Chromatogr. A 2019, 1601, 95–103. [Google Scholar] [CrossRef]
- Toki, T.; Iwasaki, T.; Ishii, M. Topiramate Blood Levels During Polytherapy for Epilepsy in Children. Am. J. Ther. 2019, 26, e18–e24. [Google Scholar] [CrossRef]
- Liu, T.; Kotha, R.R.; Jones, J.W.; Polli, J.E.; Kane, M.A. Fast liquid chromatography-tandem mass spectrometry method for simultaneous determination of eight antiepileptic drugs and an active metabolite in human plasma using polarity switching and timed selected reaction monitoring. J. Pharm. Biomed. Anal. 2019, 176, 112816. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-Z.; Shao, L.; Chen, X.; Li, H.-J.; Wang, L.; Pan, Y.-J.; Tang, D.-Q. Assay of dried blood spot from finger prick for sodium valproate via ink auxiliary headspace gas chromatography mass spectrometry. J. Chromatogr. A 2019, 1601, 335–339. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhou, Y.; Cui, Y.-M.; Yang, T.; Zhao, X.; Wu, Y. Population pharmacokinetics and dose simulation of oxcarbazepine in Chinese paediatric patients with epilepsy. J. Clin. Pharm. Ther. 2019, 44, 300–311. [Google Scholar] [CrossRef]
- Høj, L.J.; Mollerup, C.B.; Rasmussen, B.S.; Johansen, S.S.; Linnet, K.; Dalsgaard, P.W. Identification of phenobarbital and other barbiturates in forensic drug screening using positive electrospray ionization liquid chromatography-high resolution mass spectrometry. Drug Test. Anal. 2019, 11, 1258–1263. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lv, J.; Ma, L.; Peng, L.; Wang, J.; Nie, X.; Xia, L.; Zan, X. The influence of concomitant antiepileptic drugs on lamotrigine serum concentrations in Northwest Chinese Han population with epilepsy. PLoS ONE 2019, 14, e0210600. [Google Scholar] [CrossRef] [PubMed]
- Alvani-Alamdari, S.; Jouyban, A.; Khoubnasabjafari, M.; Nokhodchi, A.; Rahimpour, E. Efficiency comparison of nylon-6-based solid-phase and stir bar sorptive extractors for carbamazepine extraction. Bioanalysis 2019, 11, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.I.; Carl, P.; Schneider, R.J.; Segundo, M.A. Automated lab-on-valve sequential injection ELISA for determination of carbamazepine. Anal. Chim. Acta 2019, 1076, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Aicua-Rapun, I.; André, P.; Rossetti, A.O.; Decosterd, L.A.; Buclin, T.; Novy, J. Intravenous brivaracetam in status epilepticus: Correlation between loading dose, plasma levels and clinical response. Epilepsy Res. 2019, 149, 88–91. [Google Scholar] [CrossRef]
- Dao, K.; Thoueille, P.; Decosterd, L.A.; Mercier, T.; Guidi, M.; Bardinet, C.; Lebon, S.; Choong, E.; Castang, A.; Guittet, C.; et al. Sultiame pharmacokinetic profile in plasma and erythrocytes after single oral doses: A pilot study in healthy volunteers. Pharmacol. Res. Perspect. 2020, 8, e00558. [Google Scholar] [CrossRef]
- Favié, L.M.A.; Groenendaal, F.; van den Broek, M.P.H.; Rademaker, C.M.A.; de Haan, T.R.; van Straaten, H.L.M.; Dijk, P.H.; van Heijst, A.; Simons, S.H.P.; Dijkman, K.P.; et al. Phenobarbital, Midazolam Pharmacokinetics, Effectiveness, and Drug-Drug Interaction in Asphyxiated Neonates Undergoing Therapeutic Hypothermia. Neonatology 2019, 116, 154–162. [Google Scholar] [CrossRef]
- Qu, L.; Pan, L.; Wang, L.; Liu, C.; Tian, Y.; Hao, Z. Development of an online solid-phase extraction-liquid chromatography-mass spectrometric analysis of oxcarbazepine and its active metabolite licarbazepine from plasma with a direct injection step. J. Chromatogr. B 2019, 1125, 121710. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.; Tanaka, R.; Suzuki, Y.; Sato, Y.; Itoh, H. Comparison of performance characteristics between high-performance liquid chromatography and latex agglutination turbidimetric immunoassay for therapeutic drug monitoring of zonisamide. J. Clin. Lab. Anal. 2019, 33, e22940. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, X.; Tang, S.-F.; Wang, H.; Chen, Y.; Zhang, N. Preparation of a β-cyclodextrin grafted magnetic biochar for efficient extraction of four antiepileptic drugs in plasma samples. J. Chromatogr. A 2024, 1724, 464893. [Google Scholar] [CrossRef]
- Fallik, N.; Trakhtenbroit, I.; Fahoum, F.; Goldstein, L. Therapeutic drug monitoring in pregnancy: Levetiracetam. Epilepsia 2024, 65, 1285–1293. [Google Scholar] [CrossRef]
- Zhao, T.; Li, H.-J.; Zhang, H.-L.; Feng, J.-R.; Yu, J.; Sun, K.-F.; Feng, J.; Sun, Y.; Yu, L.-H. Effects of CYP3A4 genetic polymorphisms on the pharmacokinetics and efficacy of perampanel in Chinese pediatric patients with epilepsy. Seizure 2024, 120, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, A.; Herting, A.; Klimpel, D.; Bien, C.G.; Polster, T. Ethosuximide lowers lamotrigine serum concentrations: Evidence for a clinically relevant interaction. Epilepsia 2024, 65, e73–e78. [Google Scholar] [CrossRef] [PubMed]
- Pigliasco, F.; Cafaro, A.; Barco, S.; Stella, M.; Mattioli, F.; Riva, A.; Mancardi, M.M.; Lattanzi, S.; Bandettini, R.; Striano, P.; et al. Innovative LC-MS/MS method for therapeutic drug monitoring of fenfluramine and cannabidiol in the plasma of pediatric patients with epilepsy. J. Pharm. Biomed. Anal. 2024, 245, 116174. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Lin, B.; Zhang, H.; Sun, Y.; Wu, Y.; Ye, W.; Miao, J. Effect of Age, Comedications, and CYP3A4/5 Polymorphisms on Perampanel Exposure in Chinese Pediatric Patients with Epilepsy. J. Clin. Pharmacol. 2024, 64, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Schierscher, T.; Salzmann, L.; Singh, N.; Fischer, V.; Kobel, A.; Bauland, F.; Geistanger, A.; Risch, L.; Geletneky, C.; Seger, C.; et al. An isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS)-based candidate reference measurement procedure (RMP) for the quantification of primidone in human serum and plasma. Clin. Chem. Lab. Med. 2024, 62, 1327–1338. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Chen, Q.; Wang, Z.; Duan, Y.; Chen, L. Monitoring levetiracetam concentration in saliva during pregnancy is stable and feasible. CNS Neurosci. Ther. 2024, 30, e14827. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Younis, H.M. A novel spectrofluorimetric method using optical sensor Eu(3+)-ACAC as a highly selective photo probe to determine Pregabalin in biological samples and pharmaceutical form. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 322, 124811. [Google Scholar] [CrossRef]
- Abady, M.M.; Jeong, J.-S.; Kwon, H.-J. Simultaneous quantification of 11 antiepileptic drugs using limited isotope-labeled internal standards in LC-MS/MS: An accuracy assessment. J. Chromatogr. B 2024, 1240, 124143. [Google Scholar] [CrossRef]
- Protti, M.; Mandrioli, R.; Mercolini, L. Tutorial: Volumetric absorptive microsampling (VAMS). Anal. Chim. Acta 2019, 1046, 32–47. [Google Scholar] [CrossRef]
- Zailani, N.N.B.; Ho, P.C.L. Dried Blood Spots—A Platform for Therapeutic Drug Monitoring (TDM) and Drug/Disease Response Monitoring (DRM). Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 467–494. [Google Scholar] [CrossRef]
- Dodeja, P.; Giannoutsos, S.; Caritis, S.; Venkataramanan, R. Applications of Volumetric Absorptive Microsampling Technique: A Systematic Critical Review. Ther. Drug Monit. 2023, 45, 431–462. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, A.; Conti, M.; Pigliasco, F.; Barco, S.; Bandettini, R.; Cangemi, G. Biological Fluid Microsampling for Therapeutic Drug Monitoring: A Narrative Review. Biomedicines 2023, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Bridgewater, B.; Strickland, E.C.; McIntire, G. A rapid LC–MS-MS method for the quantitation of antiepileptic drugs in urine. J. Anal. Toxicol. 2020, 44, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Mariyappan, V.; Sundaresan, R.; Chen, S.M.; Ramachandran, R. Ultrasensitive electrochemical sensor for the detection of carbamazepine based on gadolinium vanadate nanostructure decorated functionalized carbon nanofiber nanocomposite. Chemosphere 2022, 307, 135803. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.A.; Ali, M.F.B.; Rageh, A.H.; Mostafa, A.M. Highly sensitive UHPLC-DAD method for simultaneous determination of two synergistically acting antiepileptic drugs; levetiracetam and lacosamide: Application to pharmaceutical tablets and human urine. Biomed. Chromatogr. 2019, 33, e4554. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; Toffoli, A.L.D.; Alves, J.d.S.; Lanças, F.M. Multidimensional Liquid Chromatography Employing a Graphene Oxide Capillary Column as the First Dimension: Determination of Antidepressant and Antiepileptic Drugs in Urine. Molecules 2020, 25, 1092. [Google Scholar] [CrossRef] [PubMed]
- Eugenia Gallardo, T.R.; Barroso, M. The Potential of Oral Fluid in 1Drug Monitoring: An Update. Bioanalysis 2023, 15, 657–660. [Google Scholar] [CrossRef]
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef]
- Chiș, I.-A.; Andrei, V.; Muntean, A.; Moldovan, M.; Mesaroș, A.; Dudescu, M.C.; Ilea, A. Salivary Biomarkers of Anti-Epileptic Drugs: A Narrative Review. Diagnostics 2023, 13, 1962. [Google Scholar] [CrossRef]
- Tommasini, M.; Lucotti, A.; Stefani, L.; Trusso, S.; Ossi, P.M. SERS Detection of the Anti-Epileptic Drug Perampanel in Human Saliva. Molecules 2023, 28, 4309. [Google Scholar] [CrossRef]
- Carona, A.; Bicker, J.; Silva, R.; Silva, A.; Santana, I.; Sales, F.; Falcão, A.; Fortuna, A. HPLC method for the determination of antiepileptic drugs in human saliva and its application in therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2021, 197, 113961. [Google Scholar] [CrossRef] [PubMed]
- Dziurkowska, E.; Wesolowski, M. Simultaneous Quantification of Antipsychotic and Antiepileptic Drugs and Their Metabolites in Human Saliva Using UHPLC-DAD. Molecules 2019, 24, 2953. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Barroso, M.; Vieira, D.N.; Gallardo, E. Trends in microextraction approaches for handling human hair extracts—A review. Anal. Chim. Acta 2021, 1185, 338792. [Google Scholar] [CrossRef]
- Barroso, M.; Gallardo, E.; Vieira, D.N.; López-Rivadulla, M.; Queiroz, J.A. Hair: A complementary source of bioanalytical information in forensic toxicology. Bioanalysis 2011, 3, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Yum, H.; Jang, M.; Rhee, J.; Lee, S.; Han, S.B. Simultaneous determination of barbiturates, phenytoin and topiramate in hair by LC-MS/MS and application to real samples. J. Pharmacol. Toxicol. Methods 2020, 106, 106931. [Google Scholar] [CrossRef]
- Pleil, J.D. Breath biomarkers in toxicology. Arch. Toxicol. 2016, 90, 2669–2682. [Google Scholar] [CrossRef]
- Awchi, M.; Singh, K.D.; Dill, P.E.; Frey, U.; Datta, A.N.; Sinues, P. Prediction of systemic free and total valproic acid by off-line analysis of exhaled breath in epileptic children and adolescents. J. Breath Res. 2023, 17, 046013. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Xu, C.; Huang, Z.; Hu, B. Detection of abused drugs in human exhaled breath using mass spectrometry: A review. Rapid Commun. Mass Spectrom. 2023, 37, e9503. [Google Scholar] [CrossRef]
- Seyfinejad, B.; Meshkini, A.; Habibolahi, P.; Ozkan, S.A.; Jouyban, A. Determination of phenytoin in exhaled breath condensate using electromembrane extraction followed by capillary electrophoresis. Electrophoresis 2020, 41, 666–677. [Google Scholar] [CrossRef]
- Hale, T.W.; Krutsch, K. Hale’s Medications & Mothers’ Milk 2021: A Manual of Lactational Pharmacology, 19th ed.; Springer Publishing Company: New York, NY, USA, 2021. [Google Scholar]
- Dinavitser, N.; Kohn, E.; Berlin, M.; Brandriss, N.; Bar-Chaim, A.; Keidar, R.; Livne, A.; Stepensky, D.; Berkovitch, M.; Sheinberg, R. Levetiracetam in lactation: How much is excreted into human breast milk? Br. J. Clin. Pharmacol. 2022, 88, 199–205. [Google Scholar] [CrossRef]
| Mechanism of Action | Drugs | Reference |
|---|---|---|
| Modulation of voltage-gated sodium channels |
| [4,7] |
| GABA receptors modulation |
| [4,7,8,9,10] |
| Modulation of calcium channels |
| [7,11,12,13] |
| Carbonic anhydrase modulation |
| [7,14] |
| Modulation of glutamate receptors and others |
| [7,15] |
| Unknown mechanism of action |
| [4,7] |
| Several mechanisms of action |
| [4,7,16,17,18,19,20,21,22,23,24,25] |
| Compounds | Matrix | Volume | Extraction Method | Detection Method | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Lacosamide | Blood | 500 µL | LLE (methanol, NaOH 0.01 M, and ethyl acetate) | GC-MS | 0.1 µg/mL | 0.5 µg/mL | 78.6–121.9 | [39] |
| Valproic Acid | Plasma | 300 μL | PP (85:15 (v/v) solution of perchloric acid–ethylene glycol and 50% ammonium acetate solution) | 2D-LC-UV | 1.00 μg/mL | n.s. | 95.2–98.0 | [40] |
| (a) Valproic Acid (b) CBZ (c) CBZ-E (d) Phenobarbital | Blood | n.s. | DBS | LC-MS/MS | n.s. | (a) 25 µg/mL (b) 2 µg/mL (c) n.s. (d) 1 µg/mL | (a) 58.7 ± 8.33 (b) 62.6 ± 9.36 (c) 61.0 ± 9.99 (d) 61.2 ± 9.79 | [41] |
| (a) Valproic Acid (b) CBZ (c) CBZ-E (d) Phenobarbital | Blood | n.s. | VAMS (acetonitrile/water (80/20 v/v)) | LC-MS/MS | n.s. | (a) 25 µg/mL (b) 2 µg/mL (c) n.s. (d) 1 µg/mL | (a) 85.2 ± 6.1 (b) 86.4 ± 5.9 (c) 91.4 ± 4.6 (d) 93.7 ± 4.6 | [41] |
| Valproic Acid | Plasma | 200 μL | CF-UF/PP (dichloromethane) | GC-FID | n.s. | 0.56 µg/mL | 101.45 ± 2.08 to 102.58 ± 3.38 | [42] |
| Oxcarbazepine | Plasma | 1.0 g | VA–SHS–LPME (N,N-dimethylbenzylamine, distilled water (1:1, v/v), dry ice, and sodium hydroxide) | GC-MS | 6.2 μg/kg | 21 μg/kg | 97–100 | [32] |
| Lacosamide | Plasma | n.s. | PP (methanol:water (50:50, v/v), acetonitrile:methanol, 50:50 (v/v), and 0.1% formic acid in water (80:20, v/v)) | UHPLC-MS/MS | n.s. | n.s. | 97.2–99.7 | [43] |
| Levetiracetam | Plasma | 300 μL | PP (methanol) | HCLC-UV-PDA | n.s. | 6 µg/mL | n.s. | [44] |
| Phenytoin | Plasma | n.s. | EME | CE-DAD | 0.005 μg/mL | 0.03 μg/mL | n.s. | [45] |
| Sulthiame | Serum/Plasma | 50 μL | PP (acetonitrile) | RP-HPLC–UV | 0.19 μL/mL | 0.58 μL/mL | ≈100 | [46] |
| (a) Lacosamide (b) Oxcarbazepine (c) Lamotrigine | Serum | 90 μL | PP (protein precipitator) | UHPLC-DAD | (a) 0.25 μg/mL (b) 0.50 μg/mL (c) 0.25 μg/mL | (a) 0.5 µg/mL (b) 2.5 µg/mL (c) 0.5 µg/mL | 96.6–106.2 | [47] |
| (a) Perampanel (b) Lamotrigine | Plasma | 200 μL | LLE (ethyl acetate) | HPLC-DAD | n.s. | (a) 0.03 µg/mL (b) 0.25 µg/mL | (a) 90.0–114.6 (b) 93.3–112.9 | [48] |
| Pregabalin | Serum | (a) n.s. (b) 100 μL | (a) n.s. (b) n.s. | (a) IT (b) LC-MS/MS | n.s. | n.s. | n.s. | [49] |
| Valproic Acid | Plasma | (a) 40 μL (b) 30 μL | (a) DPS/PP (water–ACN, 50:50, v/v)) (b) PP (acetonitrile) | (a) LC–MS/MS (b) LC–MS/MS | n.s. | 10 µg/mL | (a) 82.6–86.0 (b) 98.4–99.8 | [50] |
| Perampanel | Plasma | (a) 50 µL (b) 1000 µL | n.s. | LC-MS/MS | n.s. | 0.5 ng/mL | n.s. | [51] |
| Rufinamide | Plasma | 250 µL | LLE (methanol, ammonium hydroxide solution pH 9.25, and dichloromethane) | HPLC-UV | 0.05 μg/mL | 0.25 μg/mL | 94.1 ± 4.7 | [52] |
| (a) Brivaracetam (b) Carbamazepine (c) Carbamazepine-epoxide (g) Gabapentin (h) Lacosamide (i) Lamotrigine (j) Lamotrigine-13C,15N4 (k) Levetiracetam (m) 10-OH-Carbazepine (n) Perampanel (o) Phenytoin (p) Pregabalin (q) Primidone (u) Rufinamide (w) Stiripentol (x) Sultiame (y) Topiramate (b’) Zonisamide | Blood | 20 μL | DBS | LC-MS/MS | n.s. | n.s. | n.s. | [34] |
| Rufinamide | Plasma | 50 μL | PP (methanol) | RP-HPLC-UV | n.s. | 0.5 µg/mL | n.s. | [53] |
| Valproic Acid | Serum | 50 µL | BioSPME (HCl 0.1 M, methanol) | GC-MS | n.s. | 10 µg/mL | n.s. | [54] |
| Gabapentin | Serum | 20 μL | PP (methanol, 95:5(v/v) 10 mM ammonium formate:methanol, and 0.1% formic acid) | LC-MS/MS | n.s. | 0.1 µg/mL | n.s. | [55] |
| Clonazepam | Plasma | n.s. | UA-EME | CE-DAD | 3.0 ng/mL | 0.01 µg/mL | 58 | [56] |
| Clonazepam | Plasma | 50 μL | n.s. | LC-MS | n.s. | 2 µg/mL | n.s. | [57] |
| (a) Fenfluramine (b) Norfenfluramine | Plasma | n.s. | PP | LC-MS/MS | n.s. | n.s. | n.s. | [58] |
| Carbamazepine | Plasma | 5 µL | PP (methanol) | LC-MS3 | 0.5 µg/mL | 0.5 µg/mL | 110.5 ± 7.0 | [59] |
| (a) Eslicarbazepine acetate (b) Eslicarbazepine (c) Oxcarbazepine (d) (R)-licarbazepine | Plasma | (a) and (c) 50 μL (b) and (d) 200 μL | PP (50% acetonitrile for eslicarbazepine acetate and oxcarbazepine; 100% acetonitrile for eslicarbazepine and (R)-licarbazepine) | LC-MS/MS | n.s. | n.s. | n.s. | [60] |
| Zonisamide | (a) Blood (b) Plasma | (a) 50 μL (b) 30 μL | (a) DBS (b) DPS | UHPLC–MS/MS | n.s. | (a) 0.125 µg/mL (b) 0.250 µg/mL | n.s. | [61] |
| Valproic Acid | Blood | 200 µL | PP (acetonitrile) | LC-MS/MS | 2 μg/mL | 5 μg/mL | n.s. | [62] |
| Lacosamide | Plasma | 100 µL | ODS | UHPLC-DAD | n.s. | 0.25 µg/mL | 96.6–106.2 | [63] |
| Valproic Acid | Blood | 100 µL | LLE (MTBE, TMSDM, and methanol) | GC-MS | 1 µg/mL | n.s. | 86.7–91.6 | [64] |
| Gabapentin | (a) Serum (b) Plasma | n.s. | PP (75% methanol in Milli-Q water (v/v)) | ID-LC-MS/MS | n.s. | n.s. | 99–108 | [65] |
| Topiramate | (a) Serum (b) Plasma | n.s. | PP (75% methanol in Milli-Q water (v/v)) | ID-LC-MS/MS | 0.0239 μg/mL | n.s. | 95–102 | [65] |
| (a) Carbamazepine (b) Oxcarbazepine | Plasma | n.s. | IT-SPME | MS | (a) 0.0002 µg/mL (b) 0.00025 µg/mL | (a) 0.00008 µg/mL (b) 0.0001 µg/mL | (a) 102.4–117.7 (b) 90.7–104.8 | [33] |
| Levetiracetam | Plasma | 40 μL | PP (protein precipitation solution) | UPLC-MS/MS | n.s. | 0.1 µg/mL | 97.4–101.1 | [66] |
| Carbamazepine | Blood | 15 μL | MI-IPN (DBS) | CE | n.s. | 4 µg/mL | 89.7–94.7 | [67] |
| Levetiracetam | Serum | n.s. | n.s. | (a) HPLC-UV (b) IT | n.s. | n.s. | n.s. | [68] |
| Brivaracetam | Serum | n.s. | n.s. | LC-MS/MS | 0.02 µg/mL | 0.1 µg/mL | n.s. | [69] |
| (a) Carbamazepine (b) Carbamazepine-epoxide | Plasma | 2 mL | PP (dichloromethane) | ICA | (a) 0.25 ng/mL (b) 1 ng/mL | n.s. | (a) 89.0–95.2 (b) 89.1–94.6 | [70] |
| Padsenovil | (a) Blood (b) Plasma | n.s. | (a) VAMS (b) n.s. | n.s. | n.s. | n.s. | n.s. | [71] |
| Levetiracetam | Plasma | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [72] |
| (a) Lamotrigine (b) Levetiracetam (c) Carbamazepine (d) Carbamazepine-epoxide (e) Topiramate (f) Valproic acid (g) Zonisamide (h) Oxcarbazepine | Plasma | n.s. | (a) n.s. (b) DBS | LC-MS | n.s. | (a) 0.1 µg/mL (b) 1.8 µg/mL (c) 0.7 µg/mL (d) 0.1 µg/mL (e) 1.6 µg/mL (f) 13.1 µg/mL (g) 1.0 µg/mL (h) 0.1 µg/mL | n.s. | [73] |
| Lamotrigine | Plasma | 50 μL | SPE | LC-MS/MS | n.s. | 0.2 µg/mL | 93.8–98.6 | [74] |
| Valproic Acid | Plasma | n.s. | SPE | UHPLC-MS/MS | n.s. | 0.05 µg/mL | 81.4–110 | [75] |
| Valproic Acid | Serum | 50 μL | PP (sulfuric acid, ether, and tetramethylammonium hydroxide) | HPLC-UV | 0.4 µg/mL | 1.0 µg/mL | 91.6–97.4 | [76] |
| (a) Carbamazepine (b) Carbamazepine-epoxide | Serum | 100 μL | PP (acetonitrile) | UHPLC-MS/MS | n.s. | (a) 0.05 µg/mL (b) 0.01 µg/mL | 74.7– 93.48 | [77] |
| Lamotrigine | Serum | 200 μL | PP (methanol/acetonitrile 1:1, v/v) and SPME | HPLC-DAD | n.s. | 0.625 µg/mL | 75.4–82.5 | [78] |
| (a) Carbamazepine (b) Valproic acid (c) Phenobarbital (d) Phenytoin (e) Carbamazepine-epoxide | Blood | 25 μL | DBS | LC-MS/MS | n.s. | (a) 2 µg/mL (b) 25 µg/mL (c) 1 µg/mL (d) 4 µg/mL (e) 0.25 µg/mL | (a) 53.24–71.96 (b) 50.37–67.03 (c) 51.41–70.99 (d) 50.75–68.25 (e) 51.21–70.79 | [79] |
| Topiramate | Blood | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [80] |
| (a) Levetiracetam (b) Lamotrigine (c) Zonisamide (d) Topiramate (e) Carbamazepine (f) Phenytoin (g) Valproic Acid (h) Oxcarbazepine (i) 10,11-dihydro- 10-hydroxycarbamazepine | Plasma | 50 μL | PP (acetonitrile) | LC-MS/MS | n.s. | (a) 0.005 µg/mL (b) 0.005 µg/mL (c) 0.01 µg/mL (d) 0.01 µg/mL (e) 0.005 µg/mL (f) 0.010 µg/mL (g) 0.05 µg/mL (h) 0.005 µg/mL (i) 0.005 µg/mL | (a) 93.7–102.9 (b) 95.6–103.8 (c) 93.7–105.7 (d) 100.1–109.3 (e) 98–104.4 (f) 98.6–104 (g) 68.9–73.9 (h) 98–104.6 (i) 93.7–103.6 | [81] |
| Valproic Acid | Blood | n.s. | DBS | GC-MS | n.s. | n.s. | n.s. | [82] |
| Oxcarbazepine | Plasma | 200 μL | PP (methanol) | HPLC–n.s. | n.s. | 2 µg/mL | n.s. | [83] |
| Phenobarbital | Blood | 0.1 g | PP (acetonitrile) | LC-HRMS | 0.25 mg/kg | 0.5 mg/kg | n.s. | [84] |
| Lamotrigine | Serum | n.s. | PP (methanol) | HPLC–n.s. | n.s. | n.s. | n.s. | [85] |
| Carbamazepine | Serum | n.s. | (a) MIP-SBSE (b) MIP-MSPE | HPLC-UV | n.s. | n.s. | n.s. | [86] |
| Carbamazepine | Serum | n.s. | n.s. | µ-BIS-LOV ELISA | n.s. | 1.0 µg/L | 93–110 | [87] |
| Brivaracetam | Plasma | n.s. | n.s. | UHPLC-MS/MS | n.s. | n.s. | n.s. | [88] |
| Sultiame | (a) Blood (b) Plasma | 100 μL | PP (methanol) | HPLC-MS/MS | n.s. | n.s. | (a) n.s. (b) 1.61–16.73 | [89] |
| Phenobarbital | Plasma | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [90] |
| (a) Oxcarbazepine (b) Licarbazepine | Plasma | n.s. | SPE | LC-HRMS | 0.008 µg/mL | 92.34–104.27 | [91] | |
| Zonisamide | Serum | n.s. | n.s. | (a) LTIA (b) HPLC-UV | n.s. | (a) 3 µg/mL (b) 0.5 µg/mL | n.s. | [92] |
| (a) Lamotrigine (b) Carbamazepine (c) Oxcarbazepine (d) Cabamazepine-Epoxide | Plasma | 1 mL | PP (acetonitrile) and MSPE | HPLC-UV | (a) 0.01 µg/mL (b) 0.009 µg/mL (c) 0.007 µg/mL (d) 0.009 µg/mL | (a) 0.031 µg/mL (b) 0.027 µg/mL (c) 0.02 µg/mL (d) 0.028 µg/mL | (a) 86.8–101.8 (b) 82.5–99.2 (c) 80.6–98.9 (d) 78.7–98.5 | [93] |
| Levetiracetam | Serum/Plasma | n.s. | n.s. | EI | n.s. | n.s. | n.s. | [94] |
| Perampanel | Plasma | n.s. | n.s. | LC-MS/MS | n.s. | n.s. | n.s. | [95] |
| Lamotrigine | Serum | n.s. | n.s. | TI | n.s. | n.s. | n.s. | [96] |
| (a) Fenfluramine (b) Norfenfluramine | Plasma | 100 μL | PP (acetonitrile) | LC-MS/MS | n.s. | (a) 1.6 ng/mL (b) 0.82 ng/mL | n.s. | [97] |
| Perampanel | Plasma | n.s. | n.s. | HPLC-UV | n.s. | n.s. | n.s. | [98] |
| Primidone | Serum/Plasma | 50 μL | PP (75% methanol v/v) | ID-LC-MS/MS | 0.0620 µg/mL | n.s. | 97–103 | [99] |
| Carbamazepine | Serum/Plasma | 50 μL | PP (75% methanol v/v) | ID-LC-MS/MS | 0.115 µg/mL | n.s. | 101–105 | [99] |
| Phenobarbital | Serum/Plasma | n.s. | PP (75% methanol v/v) | ID-LC-MS/MS | 0.697 µg/mL | n.s. | 100–107 | [99] |
| Zonisamide | Serum/Plasma | 50 μL | PP(75% methanol v/v) | ID-LC-MS/MS | 0.317 µg/mL | 1.50 µg/mL | 98–101 | [99] |
| Levetiracetam | Plasma | 40 μL | PP (acetonitrile) | UHPLC-MS/MS | n.s. | 0.1 µg/mL | n.s. | [100] |
| Carbamazepine-epoxide | Serum/Plasma | 50 μL | PP (75% methanol v/v) | ID-LC-MS/MS | 0.0111 µg/mL | n.s. | 94–105 | [99] |
| Pregabalin | Serum | 2 mL | PP (methanol) | SM | 2.81 × 10−8 mol/L | 8.5 × 10−8 mol/L | 99.02–104.78 | [101] |
| (a) Vigabatrin (b) Levetiracetam (c) Pregabalin (d) Gabapentin (e) Lamotrigine (f) Lacosamide (g) Zonisamide (h) Rufinamide (i) Topiramate (j) Oxcarbazepine (k) Carbazepine | Serum | 150 μL | PP (acetonitrile) | LC-MS/MS | n.s. | n.s. | >93 | [102] |
| Compounds | Volume | Extraction Method | Detection Method | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Oxcarbazepine | 0.9 g | VA–SHS–LPME (N,N-dimethylbenzylamine, distilled water (1:1, v/v), and sodium hydroxide 1 M) | GC-MS | 6.2 μg/kg | 21 μg/kg | 97–100 | [32] |
| (a) Carbamazepine (b) Carbamazepine-10,11-epoxide (c) Eslicarbazepine (d) Lamotrigine (e) Levetiracetam (f) Oxcarbazepine (g) Phenytoin (h) 4-hydroxyphenytoin (i) Topiramate | 25 µL | SPE (80:18:2 DCM/IPA/NH4OH) | LC-MS/MS | (a) 0.05 µg/mL (b) 0.05 µg/mL (c) 0.5 µg/mL (d) 500 ng/mL (e) 0.5 µg/mL (f) 0.5 µg/mL (g) 0.5 µg/mL (h) 0.5 µg/mL (i) 0.5 µg/mL | (a) 0.05 µg/mL (b) 0.05 µg/mL (c) 0.5 µg/mL (d) 500 ng/mL (e) 0.5 µg/mL (f) 0.5 µg/mL (g) 0.5 µg/mL (h) 0.5 µg/mL (i) 0.5 µg/mL | (a) 106.2 (b) 24.7 (c) 102.0 (d) 102.9 (e) 14.9 (f) 92.6 (g) 105.6 (h) 100.8 (i) 92.8 | [107] |
| (a) Carbamazepine (b) Oxcarbazepine | n.s. | IT-SPME | MS | (a) 0.00008 µg/mL (b) 0.0001 µg/mL | (a) 0.0003 µg/mL (b) 0.0003 µg/mL | (a) 98.7–108.6 (b) 90.2–107.2 | [33] |
| Valproic Acid | 100 µL | LLE (MTBE, TMSDM, and methanol) | GC-MS | 1 µg/mL | n.s. | 86.2–98.0 | [64] |
| Carbamazepine | n.s. | n.s. | Electrochemical Sensor | 0.0018 μM | 0.006 μM | 99.0–100.7 | [108] |
| (a) Levetiracetam (b) Lacosamide | 1 mL | Filtration | UHPLC-DAD | (a) 0.026 µg/mL (b) 0.023 µg/mL | (a) 0.096 µg/mL (b) 0.093 µg/mL | 98.69–101.87 | [109] |
| Carbamazepine | n.s. | CEC | MHPLC-MS/MS | n.s. | n.s. | n.s. | [110] |
| Valproic Acid | n.s. | SPE | UHPLC-MS/MS | n.s. | 0.2 µg/mL | 74.1–112.3 | [75] |
| Sultiame | n.s. | PP (methanol) | LC-MS/MS | n.s. | n.s. | 1.61–16.73 | [89] |
| Pregabalin | 1 mL | n.s. | SM | 2.81 × 10−8 mol/L | 8.5 × 10−8 mol/L | 99.08–104.96 | [101] |
| Compounds | Volume | Extraction Method | Detection Method | LOD | LOQ | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Clonazepam | 20 μL | n.s. | LC-MS | n.s. | n.s. | n.s. | [57] |
| Perampanel | 10 μL | PSP (chloroform) | SERS | n.s. | n.s. | n.s. | [114] |
| (a) Carbamazepine (b) Carbamazepine-10,11-epoxide (c) S-licarbazepine (d) Lacosamide (e) Levetiracetam | 100 μL | PP (dichloromethane) | HPLC-DAD | n.s. | n.s. | (a) 80.1–95.4 (b) 82.5–95.1 (c) 80.0–94.2 (d) 79.8 –93.9 (e) 78.0–94.4 | [115] |
| Perampanel | (a) 50 µL (b) 1 mL | PP (acetonitrile) | LC-MS/MS | n.s. | n.s. | n.s. | [51] |
| Levetiracetam | 40 μL | PP (protein precipitation solution) | UHPLC-MS/MS | n.s. | 0.001 μg/mL | 108.8–113.5 | [66] |
| Rufinamide | 250 μL | LLE (methanol, ammonium hydroxide solution pH 9.25, and dichloromethan(e)) | HPLC-UV | 0.05 μg/mL | 0.25 μg/mL | 87.2 ± 3.9 | [52] |
| (a) Phenobarbital (b) Phenytoin (c) Carbamazepine (d) Carbamazepine-epoxide | 50 μL | LLE (acidified methanol pH 5.5) | HPLC-DAD | 0.05 μg/mL | 0.1 μg/mL | (a) 43–57.1 (b) 48.1–64.4 (c) 38.7–49.1 (d) 38.8–55 | [35] |
| (a) Carbamazepine (b) Carbamazepine-epoxide | n.s. | SPE | UHPLC-DAD | n.s. | n.s. | (a) 46.82–49.18 (b) 41.4–41.72 | [116] |
| Levetiracetam | 40 μL | PP (acetonitrile) | UHPLC-MS/MS | n.s. | 0.1 µg/mL | n.s. | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinho, J.; Simão, A.Y.; Barroso, M.; Gallardo, E.; Rosado, T. Determination of Antiepileptics in Biological Samples—A Review. Molecules 2024, 29, 4679. https://doi.org/10.3390/molecules29194679
Martinho J, Simão AY, Barroso M, Gallardo E, Rosado T. Determination of Antiepileptics in Biological Samples—A Review. Molecules. 2024; 29(19):4679. https://doi.org/10.3390/molecules29194679
Chicago/Turabian StyleMartinho, João, Ana Y. Simão, Mário Barroso, Eugenia Gallardo, and Tiago Rosado. 2024. "Determination of Antiepileptics in Biological Samples—A Review" Molecules 29, no. 19: 4679. https://doi.org/10.3390/molecules29194679
APA StyleMartinho, J., Simão, A. Y., Barroso, M., Gallardo, E., & Rosado, T. (2024). Determination of Antiepileptics in Biological Samples—A Review. Molecules, 29(19), 4679. https://doi.org/10.3390/molecules29194679









