Hierarchical Nanostructures of Iron Phthalocyanine Nanowires Coated on Nickel Foam as Catalysts for the Oxygen Evolution Reaction
Abstract
1. Introduction
2. Result and Discussion
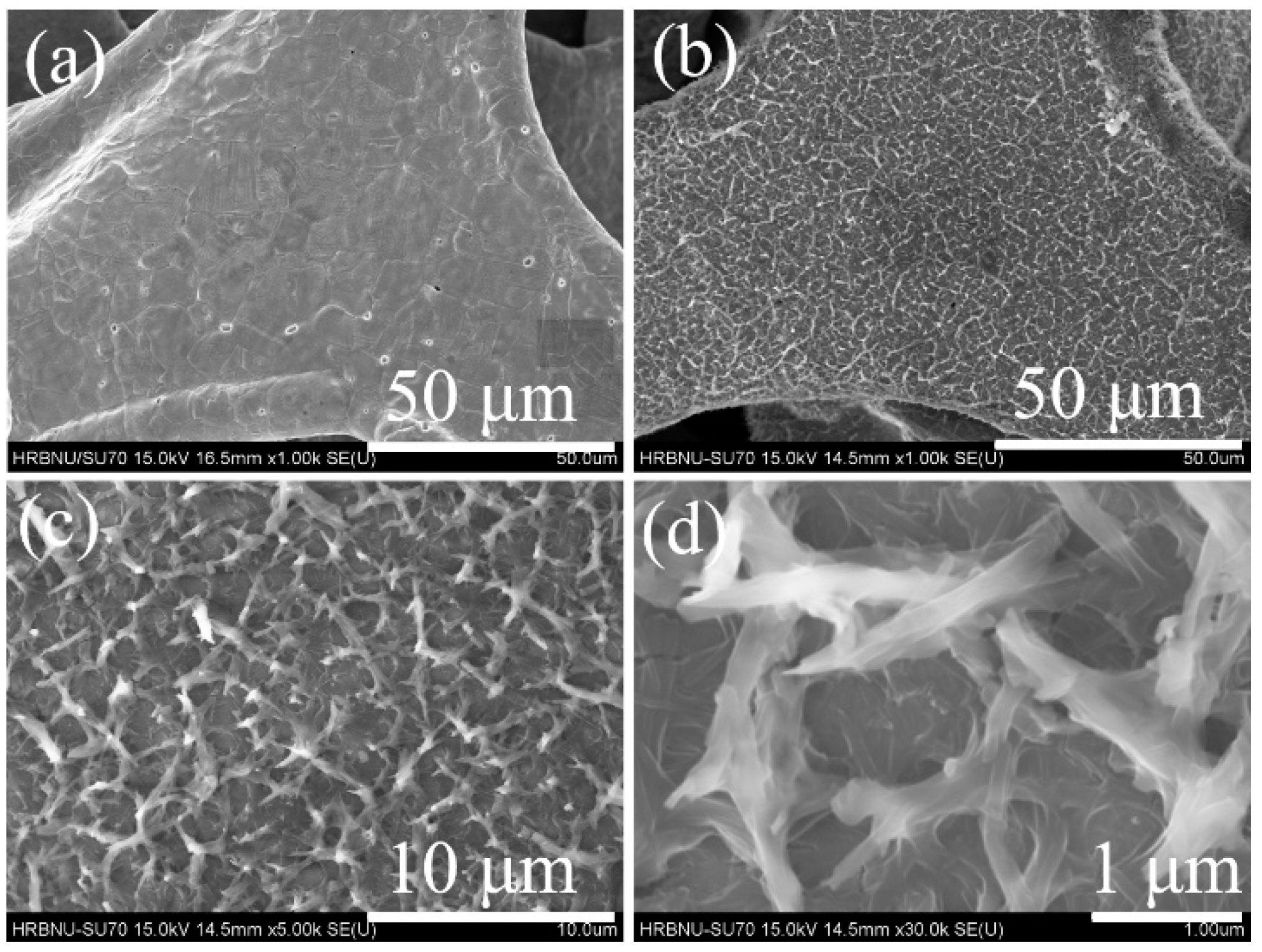
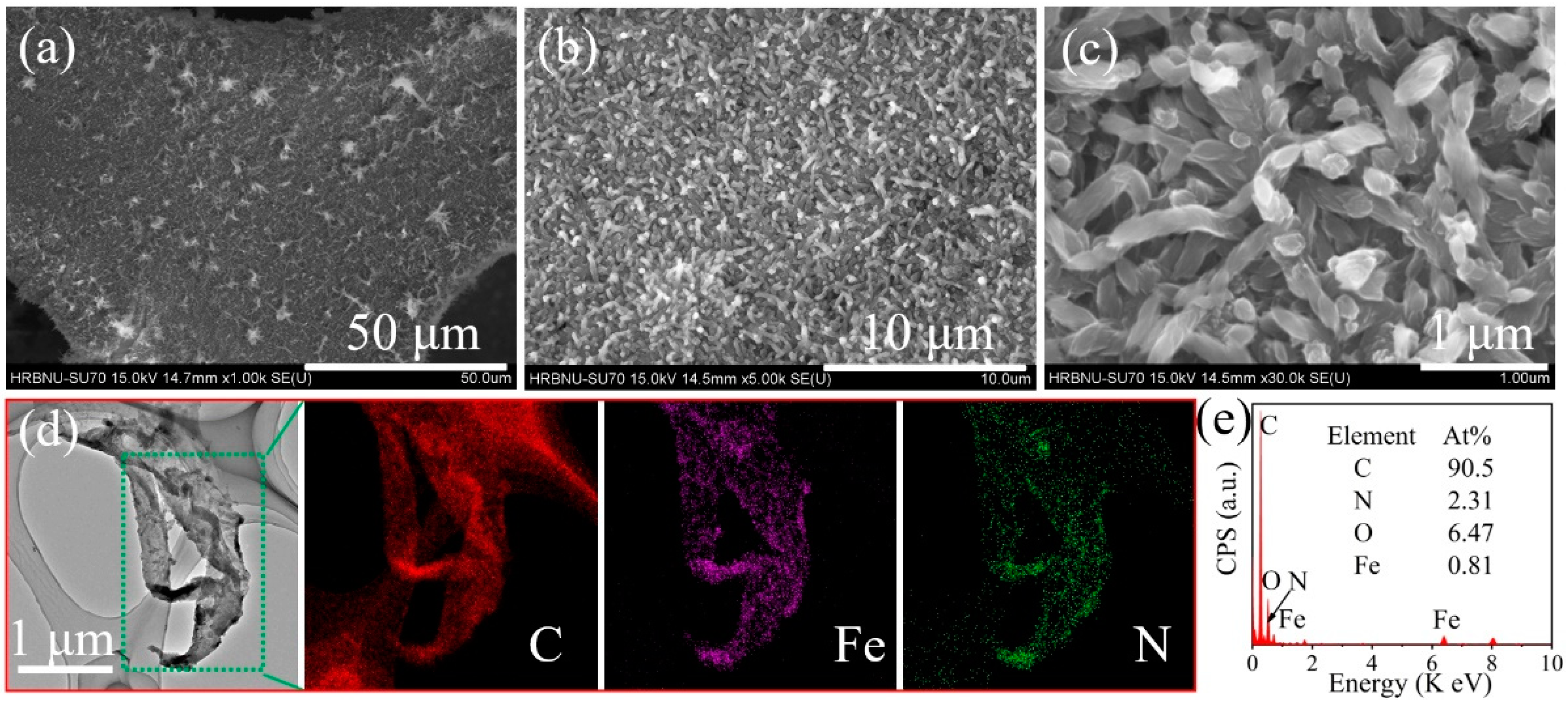
2.1. SEM and TEM Images, EDX Spectra
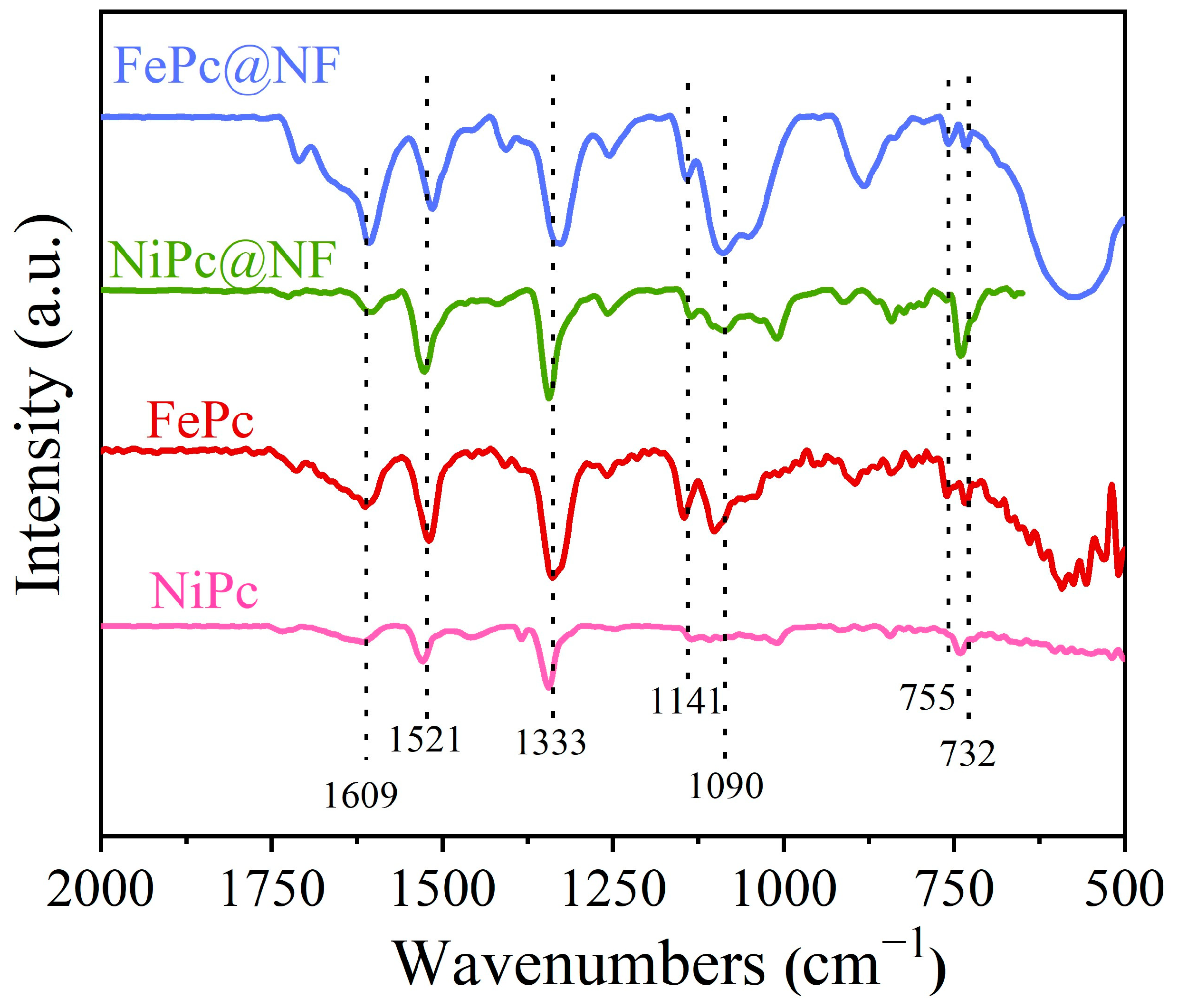
2.2. Fourier Transform Infrared (FT-IR) Spectra
2.3. X-ray Photoelectron Spectroscopy (XPS) Spectra
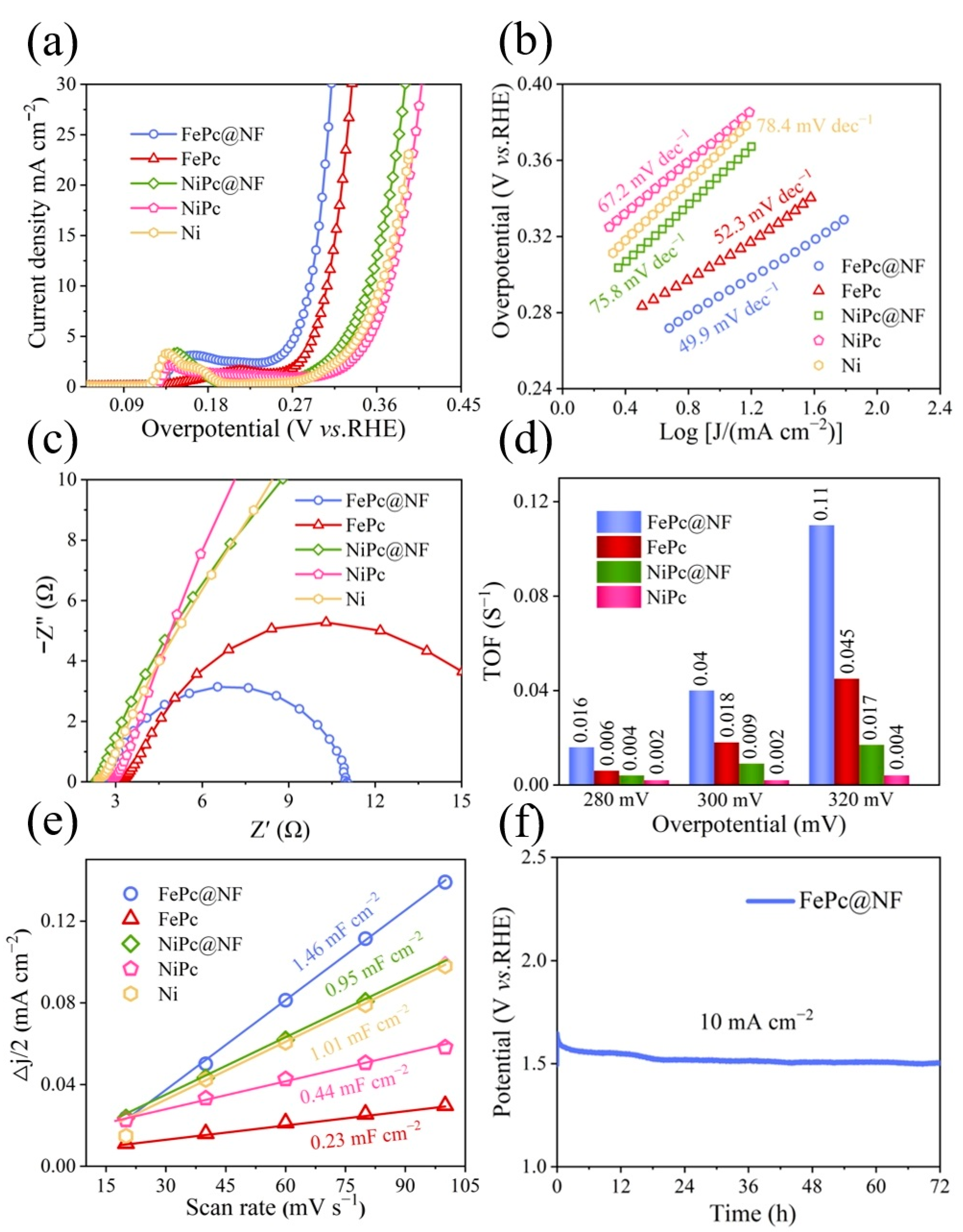
2.4. Oxygen Evolution Reaction (OER) Activity
3. Experimental Section
3.1. Materials
3.2. Synthesis of Catalysts
3.3. Material Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, D.; Gu, Y.; Yu, X.; Lin, Z.; Xue, H.; Feng, L. Nanostructured Ni2P-C as an Efficient Catalyst for Urea Electrooxidation. ChemElectroChem 2018, 5, 659–664. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Li, L.; Ye, X.; Chen, H.; Wei, Z. Mo2N–Ni/NF Heterostructure Boosts Electrocatalytic Hydrogen Evolution with Pt-Like Activity. Inorg. Chem. 2020, 59, 16514–16521. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Jones, C.W.; Linic, S.; Stamenkovic, V.R. Best Practices in Pursuit of Topics in Heterogeneous Electrocatalysis. ACS Catal. 2017, 7, 6392–6393. [Google Scholar] [CrossRef]
- Chandrakala, K.B.; Giddaerappa; Reddy, K.R.V.; Shivaprasad, K.H. Investigational undertaking descriptors for reduced graphene oxide-phthalocyanine composite based catalyst for electrochemical oxygen evolution reaction. J. Electroanal. Chem. 2022, 919, 116558. [Google Scholar] [CrossRef]
- Kumar, Y.; Kozlova, E.J.; Kikas, A.; Kaarik, M.; Aruvali, J.; Kisand, V.; Leis, J.; Tamm, A.; Tammeveski, K. Bimetal Phthalocyanine-Modified Carbon Nanotube-Based Bifunctional Catalysts for Zinc-Air Batteries. ChemElectroChem 2021, 8, 2662–2670. [Google Scholar] [CrossRef]
- Murthy, A.P.; Theerthagiri, J.; Madhavan, J. Insights on Tafel Constant in The Analysis of Hydrogen Evolution Reaction. J. Phys. Chem. C 2018, 122, 23943–23949. [Google Scholar] [CrossRef]
- Sun, H.M.; Xu, X.B.; Yan, Z.H.; Chen, X.; Cheng, F.Y.; Weiss, P.S.; Chen, J. Porous Multishelled Ni2P Hollow Microspheres as Active Electrocatalyst for Hydrogen and Oxygen Evolution. Chem. Mater. 2017, 29, 8539–8547. [Google Scholar] [CrossRef]
- Dai, L.M.; Xue, Y.H.; Qu, L.T.; Choi, H.J.; Beak, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Ziani, A.; Shinagawa, T.; Stegenburga, L.; Takanabe, K. Generation of Transparent Oxygen Evolution Electrode Consisting of Regularly Ordered Nanoparticles from Self-Assembly Cobalt Phthalocyanine as a Template. ACS Appl. Mater. Interfaces 2016, 8, 32376–32384. [Google Scholar] [CrossRef]
- Helsel, N.; Choudhury, P. Investigation of bifunctionality of FePc-functionalized graphene for enhanced ORR/OER activity. Mol. Catal. 2023, 545, 113213. [Google Scholar] [CrossRef]
- Li, J.W.; Liu, P.; Mao, J.X.; Yan, J.Y.; Song, W.B. Two-dimensional conductive metal-organic frameworks with dual metal sites toward the electrochemical oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 1623–1629. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Arico, A.S.; Monforte, G.; Baglio, V. EDTA-derived Co-N-C and Fe-N-C Electro-Catalysts for The Oxygen Reduction Reaction in Acid Environment. Renew. Energy 2018, 120, 342–349. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline. Iron Cobalt Sci. 2011, 332, 443–447. [Google Scholar]
- Arul, A.; Pak, H.; Moon, K.U.; Christy, M.; Oh, M.Y.; Nahm, K.S. Metallomacrocyclic carbon complex: A study of bifunctional electrocatalytic activity for oxygen reduction and oxygen evolution reactions and their lithium-oxygen battery applications. Appl. Catal. B—Environ. 2018, 220, 488–496. [Google Scholar] [CrossRef]
- Isvoranu, C.; Wang, B.; Ataman, E.; Schulte, K.; Knudsen, J.; Andersen, J.N.; Bocquet, M.L.; Schnadt, J. Ammonia adsorption on iron phthalocyanine on Au (111): Influence on adsorbate-substrate coupling and molecular spin. J. Chem. Phys. 2011, 134, 114710. [Google Scholar] [CrossRef]
- Matsuda, S.; Mori, S.; Hashimoto, K.; Nakanishi, S. Transition Metal Complexes with Macrocyclic Ligands Serve as Efficient Electrocatalysts for Aprotic Oxygen Evolution on Li2O2. J. Phys. Chem. C 2014, 118, 28435–28439. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Poldsepp, E.; Kozlova, J.; Rahn, M.; Treshchalov, A.; Kikas, A.; Kisand, V.; Aruvali, J.; Tamm, A.; Douglin, J.C.; et al. Bifunctional Oxygen Electrocatalysis on Mixed Metal Phthalocyanine-Modified Carbon Nanotubes Prepared via Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 41507–41516. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Mirahmadi, E. Electrocatalytic activity of iron and nickel phthalocyanines supported on multi-walled carbon nanotubes towards oxygen evolution reaction. Electrochim. Acta 2013, 105, 92–98. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, G.; Liu, W.; Sun, X. Electrocatalysis and activity descriptors with metal phthalocyanines for energy conversion reactions. J. Electroanal. Chem. 2022, 922, 116799. [Google Scholar] [CrossRef]
- Shen, M.X.; Zheng, L.R.; He, W.H.; Ruan, C.P.; Jiang, C.H.; Ai, K.L.; Lu, L.H. High-Performance Oxygen Reduction Electrocatalysts Derived from Uniform Cobalt-Adenine Assemblies. Nano Energy 2015, 17, 120–130. [Google Scholar] [CrossRef]
- Yamada, Y.; Kura, J.; Toyoda, Y.; Tanaka, K. High catalytic methane oxidation activity of monocationic μ-nitrido-bridged iron phthalocyanine dimer with sixteen methyl groups. Dalton Trans. 2021, 50, 6718–6724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Zhang, B.S.; Liu, X.; Wang, D.W.; Su, D.S. Unravelling The Structure of Electrocatalytically Active Fe-N Complexes in Carbon for The Oxygen Reduction Reaction. Angew. Chem.-Int. Ed. 2014, 53, 10673–10677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Liu, C.Y.; Bian, L.Z.; Qi, J.; Yang, L.L.; Wei, P.Y.; Fu, P.; Han, S.W.; Han, W.; Hu, Z.X.; et al. In-situ construction of Ni–Fe alloy nanoparticles on perovskite surface for CO2 direct electrolysis. Int. J. Hydrogen Energy 2024, 80, 418–426. [Google Scholar] [CrossRef]
- Kuznetsova, I.; Lebedeva, O.; Kultin, D.; Mashkin, M.; Kalmykov, K.; Kustov, L. Enhancing efficiency of nitrate reduction to ammonia by Fe and Co nanoparticle-based bimetallic electrocatalyst. Int. J. Mol. Sci. 2024, 25, 7089. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.F.; Su, X.; Wang, X.; Ye, L.T.; Xie, K. In situ exsolved CoFe alloys over perovskite toward enhanced ammonia synthesis. New J. Chem. 2024, 48, 10060–10066. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.M.; Guan, D.Q.; Lin, Z.Z.; Hu, Z.W.; Ni, M.; Huang, H.T.; Bhatelia, T.; Jiang, S.P.; Shao, Z.P. New nanocomposites derived from cation nonstoichiometric Bax(Co, Fe, Zr, Y)O3-δ as efficient electrocatalysts for water oxidation in alkaline solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhong, Y.J.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z.P. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Xu, X.M.; Shao, Z.P.; Jiang, S.P. High-entropy materials for water electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Yuceel, C.; Sahin, Z.; Isci, U. Substituent effect on iron phthalocyanines as cyclohexene oxidation catalysts. J. Porphyr. Phthalocyanines 2022, 26, 452–457. [Google Scholar] [CrossRef]
- Jia, H.X.; Yao, Y.C.; Zhao, J.T.; Gao, Y.Y.; Luo, Z.L.; Du, P.W. A novel two-dimensional nickel phthalocyanine-based metal-organic framework for highly efficient water oxidation catalysis. J. Mater. Chem. A 2018, 6, 1188–1195. [Google Scholar] [CrossRef]
- Shimizu, T.; Wakamatsu, K.; Yamada, Y.; Toyoda, Y.; Akine, S.; Yoza, K.; Yoshikawa, H. Application of μ-Nitrido- and μ-Carbido-Bridged Iron Phthalocyanine Dimers as Cathode-Active Materials for Rechargeable Batteries. ACS Appl. Mater. Interfaces 2021, 13, 40612–40617. [Google Scholar] [CrossRef] [PubMed]
- Mihara, N.; Yamada, Y.; Takaya, H.; Kitagawa, Y.; Aoyama, S.; Igawa, K.; Tomooka, K.; Tanaka, K. Oxygen Reduction to Water by a Cofacial Dimer of Iron (III)-Porphyrin and Iron (III)- Phthalocyanine Linked through a Highly Flexible Fourfold Rotaxane. Chem.-Eur. J. 2017, 23, 7508–7514. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Miwa, Y.; Toyoda, Y.; Phung, Q.M.; Oyama, K.I.; Tanaka, K. Evaluation of CH4 oxidation activity of high-valent iron-oxo species of a μ-nitrido-bridged heterodimer of iron porphycene and iron phthalocyanine. Catal. Sci. Technol. 2023, 13, 1725–1734. [Google Scholar] [CrossRef]
- Isvoranu, C.; Knudsen, J.; Ataman, E.; Schulte, K.; Wang, B.; Bocquet, M.L.; Andersen, J.N.; Schnadt, J. Adsorption of ammonia on multilayer iron phthalocyanine. J. Chem. Phys. 2011, 134, 114711. [Google Scholar] [CrossRef]
- Yamada, Y.; Sugiura, T.; Morita, K.; Ariga-Miwa, H.; Tanaka, K. Improved synthesis of monocationic μ-nitrido-bridged iron phthalocyanine dimer with no peripheral substituents. Inorg. Chim. Acta 2019, 489, 160–163. [Google Scholar] [CrossRef]
- Bata, P.; Notheisz, F.; Kluson, P.; Zsigmond, A. Iron phthalocyanine as new efficient catalyst for catalytic transfer hydrogenation of simple aldehydes and ketones. Appl. Organomet. Chem. 2015, 29, 45–49. [Google Scholar] [CrossRef]
- Guo, Z.; Mu, J.; Chen, B. 3D Flower-Like Ironphthalocyanine Hierarchical Microstructures: Solvothermal-Fabrication and High Visible Light Photocatalytic Properties. Ceram. Int. 2015, 41, 4916–4922. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, B.; Mu, J. Iron Phthalocyanine/TiO2 Nanofiber Heterostructures with Enhanced Visible Photocatalytic Activity Assisted with H2O2. J. Hazard. Mater. 2012, 219, 156–163. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Yu, P.; Zhang, M. Hierarchical Nanostructures of Iron Phthalocyanine Nanowires Coated on Nickel Foam as Catalysts for the Oxygen Evolution Reaction. Molecules 2024, 29, 4272. https://doi.org/10.3390/molecules29174272
Meng X, Yu P, Zhang M. Hierarchical Nanostructures of Iron Phthalocyanine Nanowires Coated on Nickel Foam as Catalysts for the Oxygen Evolution Reaction. Molecules. 2024; 29(17):4272. https://doi.org/10.3390/molecules29174272
Chicago/Turabian StyleMeng, Xianying, Peng Yu, and Mingyi Zhang. 2024. "Hierarchical Nanostructures of Iron Phthalocyanine Nanowires Coated on Nickel Foam as Catalysts for the Oxygen Evolution Reaction" Molecules 29, no. 17: 4272. https://doi.org/10.3390/molecules29174272
APA StyleMeng, X., Yu, P., & Zhang, M. (2024). Hierarchical Nanostructures of Iron Phthalocyanine Nanowires Coated on Nickel Foam as Catalysts for the Oxygen Evolution Reaction. Molecules, 29(17), 4272. https://doi.org/10.3390/molecules29174272






