Synthesis of Tumor Selective Indole and 8-Hydroxyquinoline Skeleton Containing Di-, or Triarylmethanes with Improved Cytotoxic Activity
Abstract
1. Introduction
2. Results and Discussions
2.1. Synthesis
2.2. Biological Evaluations
Cytotoxicity Assay
3. Materials and Methods
3.1. Biological Assays
3.1.1. Cell Lines and Their Maintenance
3.1.2. MTT Assay
3.2. Preparation Protocols for the Synthesis of the New Derivatives
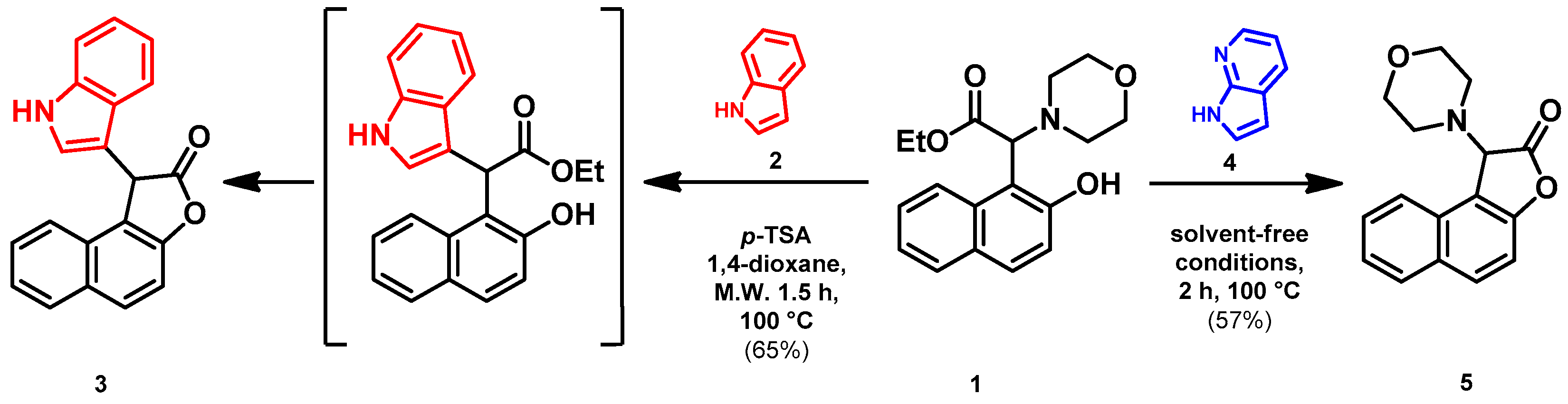
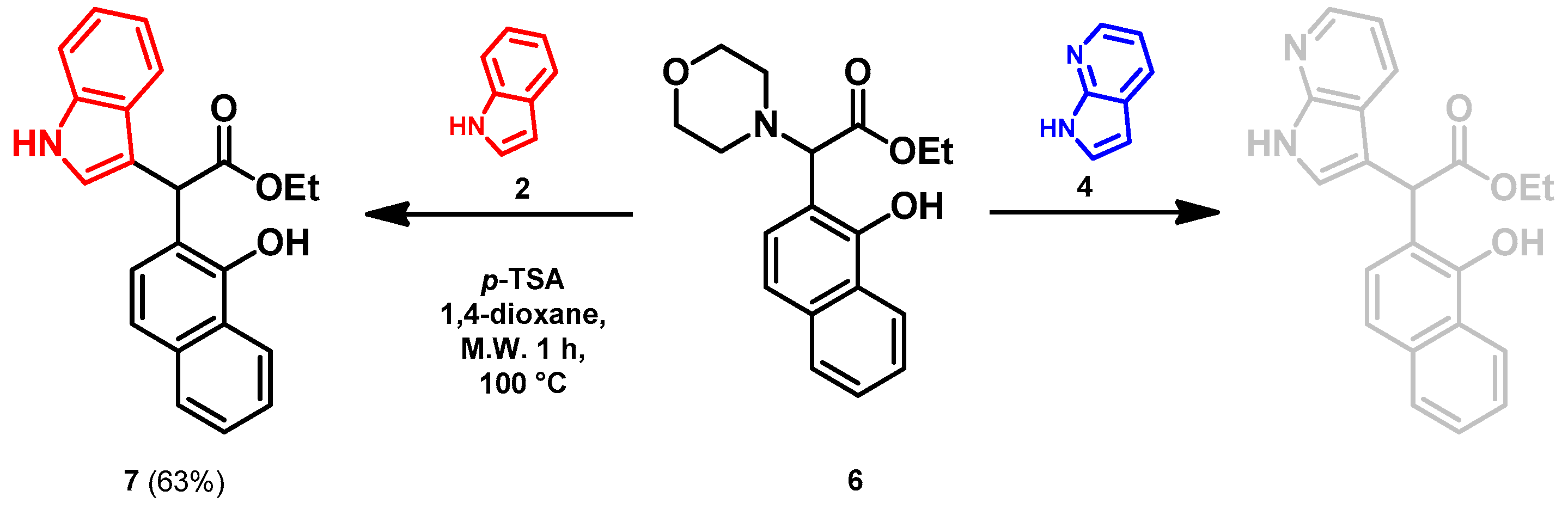
- Ethyl 2-(1-hydroxynaphthalen-2-yl)-2-(1H-indol-3-yl)acetate (7)
- In a 35 mL pressurized reaction vial, the mixture of ethyl 2-(1-hydroxynaphthalen-2-yl)-2-morpholinoacetate (6; 50.0 mg, 0.16 mmol) and indole (2; 17.5 mg, 0.16 mmol) in the presence of 10 mol% (2.8 mg, 0.016 mmol) p-TSA in 1,4-dioxane was heated at 100 °C for 1 h under MW irradiation. Following the removal of the solvent, the residue was purified by column chromatography (n-hexane:EtOAc, 4:1); Rf value = 0.32 (n-hexane:EtOAc, 4:1); 34.9 mg, (63%); oil; 1H-NMR (CDCl3): 1.35 (t, 3H, J = 7.15 Hz), 4.24–4.40 (m, 2H), 5.36 (s, 1H), 7.09 (t, 1H, J = 7.51 Hz), 7.19–7.21 (m, 1H), 7.34 (t, 2H, J = 8.86 Hz), 7.39 (d, 1H, J = 8.51 Hz), 7.44–7.52 (m, 3H), 7.74–7.80 (m, 1H), 8.08 (brs, 1H), 8.31–8.36 (m, 1H), 8.99 (s, 1H).13C NMR (CDCl3): 14.1; 29.7; 48.7; 111.1; 111.4; 115.9; 118.7; 119.9; 120.1; 122.5; 122.7; 123.6; 125.3; 126.3; 126.3; 126.5; 127.2; 128.9; 134.4; 136.5; 151.6; 176.0 (Figures S1 and S2). HRMS calculated for [M − H]− = 344.1292 m/z, [2M − H]− = 689.2657 m/z found 344.1287 m/z (Δmi = −1.3 ppm) and 689.2659 m/z (Δmi = 0.3 ppm). The ethoxy loss followed by ring-closing yielded proposed fragment ions with 298.0875 (Δmi = 0.3 ppm) m/z during in-source fragmentation (Figure S3). FTIR in Figure S4.
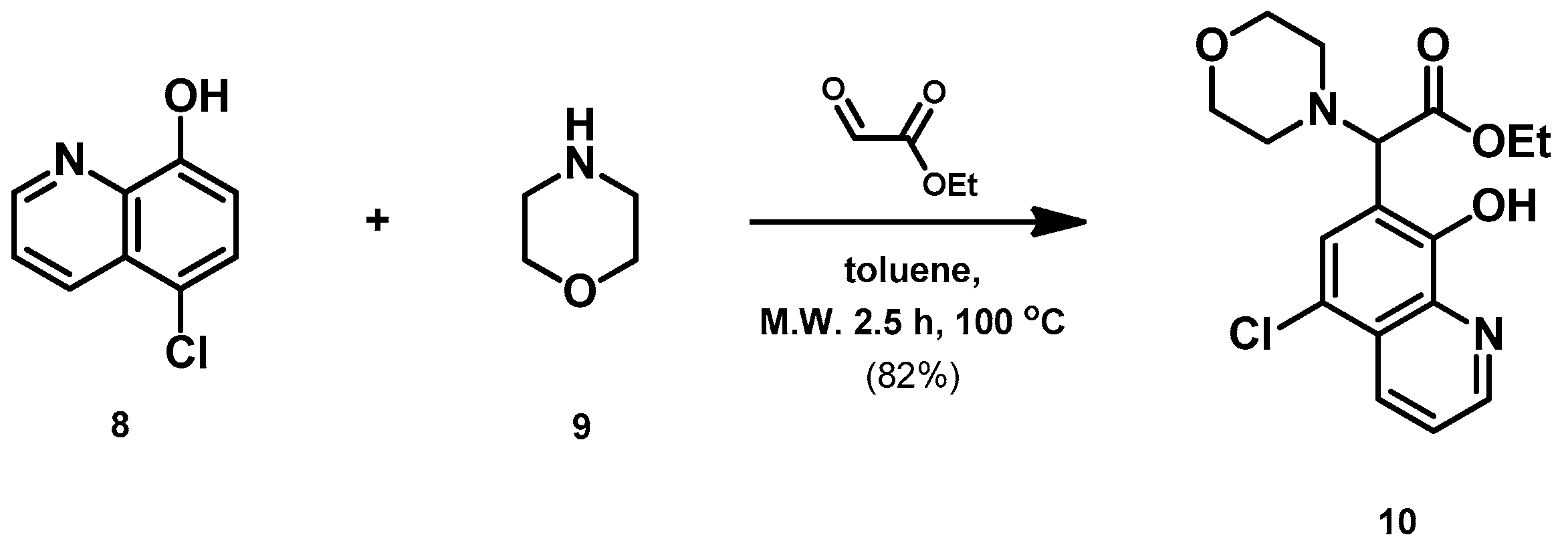
- Ethyl 2-(5-chloro-8-hydroxyquinolin-7-yl)-2-morpholinoacetate (10)
- A mixture of 5-chloro-8-hydroxyquinoline (8; 1.0 g, 5.56 mmol), ethyl glyoxylate (1.2 g, 5.93 mmol; 50% in toluene) and morpholine (9; 0.5 g, 5.54 mmol) in toluene (15 mL) was placed into a 35-mL pressurized reaction vial and heated at 100 °C for 2.5 h under MW irradiation. The solvent was removed in vacuo. The desired product was isolated by crystallization with EtOAc (10 mL), recrystallized from iPr2O (5 mL); Rf value = 0.43 (CH2Cl2: EtOAc, 4:1); 1.6 g, (82%); white crystals; m.p. 124–126 °C; 1H-NMR (CDCl3): 1.23 (t, 3H, J = 7.12 Hz), 2.55–2.68 (m, 4H), 3.74–3.81 (m, 4H), 4.12–4.26 (m, 2H), 4.60 (s, 1H), 7.54–7.59 (m, 1H), 7.68 (s, 1H), 8.50 (d, 1H, J = 8.51 Hz), 8.87 (d, 1H, J = 4.36 Hz). 13C NMR (CDCl3): 14.1; 51.3; 61.4; 66.8; 68.2; 116.5; 120.8; 122.8; 126.3; 127.1; 133.2; 139.0; 149.0; 150.8; 170.1 (Figures S5 and S6). HRMS calculated for [M + H]+ =351.1106 m/z, [2M + Na]+ =723.1959 m/z found 351.1091 m/z (Δmi = −4.3 ppm) and 723.1927 m/z (Δmi = −4.4 ppm). The loss of the morpholino group can explain the observed 264.0411 m/z (Δmi = −4.2 ppm) peak during in-source fragmentation (Figure S7). FTIR in Figure S8.
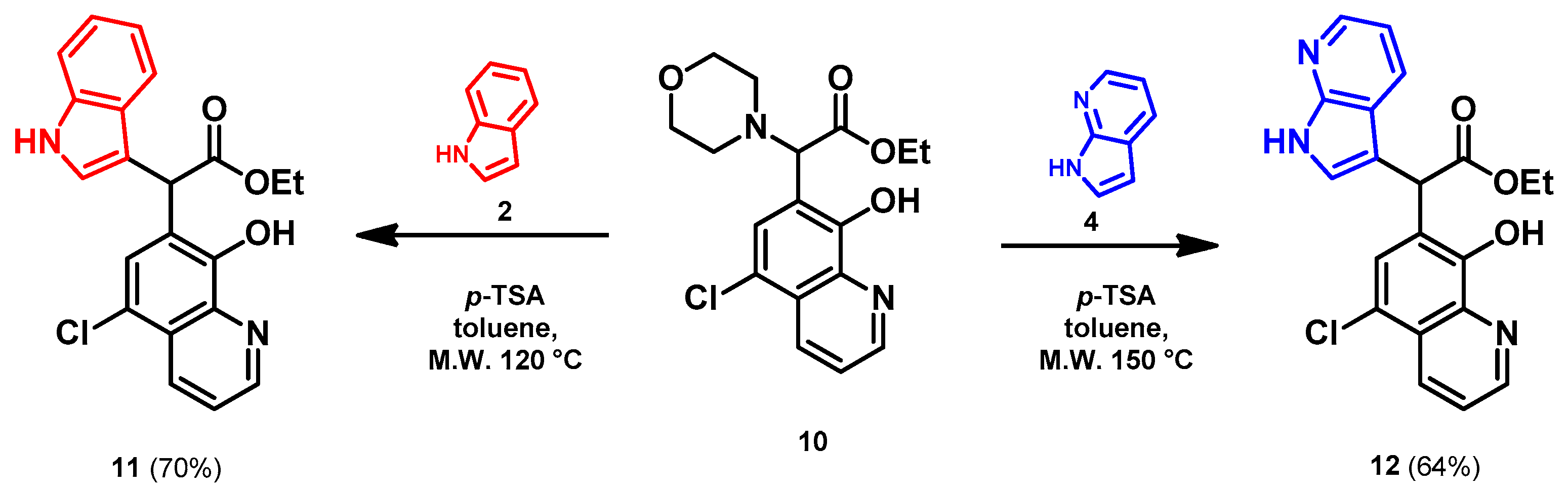
- Ethyl 2-(5-chloro-8-hydroxyquinolin-7-yl)-2-(1H-indol-3-yl)acetate (11)
- Bifunctional precursor 10 (50.0 mg, 0.14 mmol) and indole (2; 17.0 mg, 0.14 mmol) in the presence of 10 mol% (2.4 mg, 0.014 mmol) p-TSA were dissolved in toluene (10 mL) in a 35 mL pressurized reaction vial and heated at 120 °C for 4 h under MW irradiation. Following column chromatography purification (EtOAc:n-hexane, 1:1), the eluent was removed in vacuo; Rf value = 0.63 (EtOAc:n-hexane, 1:1); 37.7 mg, (70%); oil; 1H-NMR (CDCl3): 1.27 (t, 3H, J = 7.16 Hz), 4.21–4.29 (m, 2H), 5.92 (s, 1H), 7.07 (t, 1H, J = 7.46 Hz), 7.19 (t, 1H, J = 7.65 Hz), 7.36–7.40 (m, 2H), 7.50–7.53 (m, 1H), 7.58–7.61 (m, 2H), 8.15 (brs, 1H), 8.45 (d, 1H, J = 8.49 Hz), 8.82 (d, 1H, J = 4.33 Hz). 13C NMR (CDCl3): 14.2; 41.4; 61.3; 111.2; 112.9; 119.1; 119.9; 120.4; 121.0; 122.3; 122.5; 122.9; 125.5; 126.7; 128.0; 133.3; 136.2; 138.5; 148.2; 148.4; 172.5 (Figures S9 and S10). HRMS calculated for [M + H]+ = 381.1000 m/z, [2M + Na]+ = 783.1748 m/z found 381.0986 m/z (Δmi = −3.7 ppm) and 783.1714 m/z (Δmi = −4.3 ppm) (Figure S11). FTIR in Figure S12.
- Ethyl 2-(5-chloro-8-hydroxyquinolin-7-yl)-2-(7-azaindole-3-yl)acetate (12)
- In a 35 mL pressurized reaction vial a mixture of product 10 (50.0 mg, 0.14 mmol) and 7-azaindole (4; 17.0 mg, 0.14 mmol) in the presence of 10 mol% (2.4 mg, 0.014 mmol) p-TSA in toluene was heated at 150 °C for 3 h under MW irradiation. The solvent was removed under reduced pressure. The desired product was isolated by crystallisation with Et2O (10 mL); Rf value = 0.52 (n-hexane:EtOAc, 1:4); 34.8 mg, (64%); beige crystals; m.p. 228–230 °C; 1H-NMR (DMSO): 1.16 (t, 3H, J = 7.11 Hz), 4.13–4.20 (q, 2H, J = 7.11 Hz), 5.73 (s, 1H), 7.03 (dd, 1H, J1 = 4.64 Hz, J2 = 7.91 Hz), 7.40 (s, 1H), 7.45–7.48 (m, 1H), 7.70–7.75 (m, 1H), 7.84 (d, 1H, J = 7.83 Hz), 8.22 (d, 1H, J = 4.79 Hz), 8.46 (d, 1H, J = 8.54 Hz), 8.98 (d, 1H, J = 4.25 Hz), 11.69 (brs, 1H). 13C NMR (DMSO): 14.5; 42.4; 61.3; 110.3; 115.9; 118.7; 119.0; 122.7; 123.5; 125.0; 125.3, 127.3; 127.9; 133.0, 139.1; 143.5; 149.0; 149.7; 150.2, 172.1 (Figures S13 and S14). HRMS calculated for [M + H]+ m/z = 382.0953, found m/z = 382.0939 (Δmi = −3.7 ppm) (Figure S15). FTIR in Figure S16.
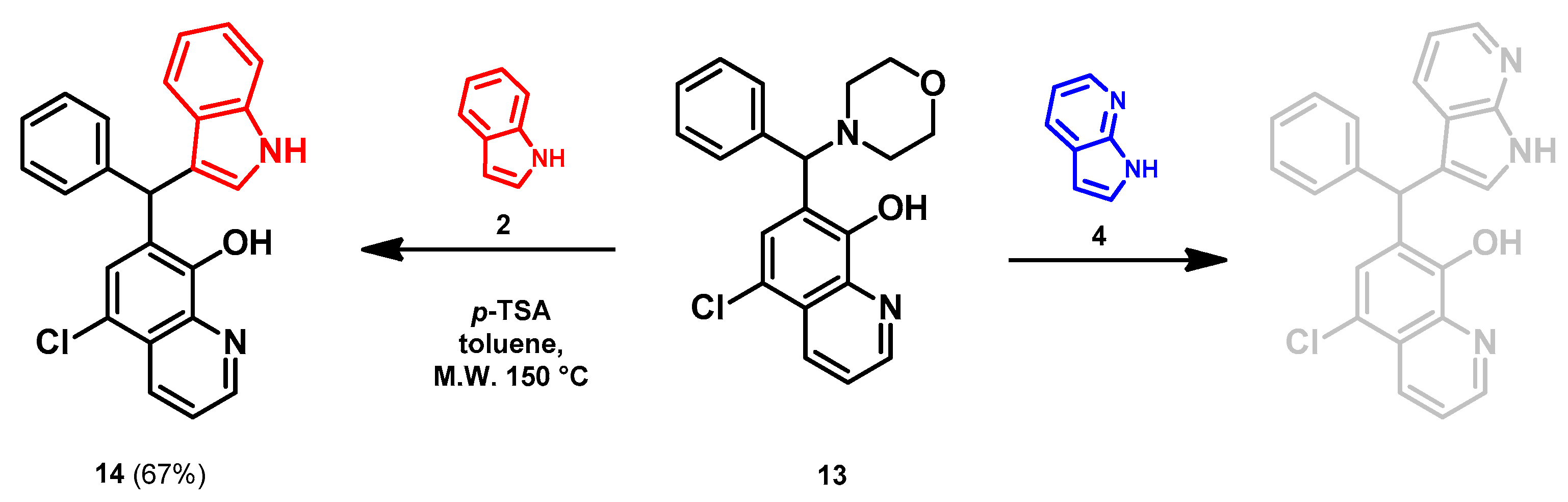
- 7-((1H-indol-3-yl)(phenyl)methyl)-5-chloroquinolin-8-ol (14)
- Glycine type precursor 13 (50.0 mg, 0.14 mmol) and indole (2; 16.5 mg, 0.14 mmol) in the presence of 10 mol% (2.4 mg, 0.014 mmol) p-TSA in toluene were heated in a 35 mL pressurized reaction vial at 150 °C for 4 h under MW irradiation. Following the removal of the solvent, the residue was purified by column chromatography (n-hexane:EtOAc, 3:1); Rf value = 0.44 (n-hexane:EtOAc, 3:1); 36.2 mg, (67%); oil; 1H-NMR (CDCl3): 6.34 (s, 1H), 6.70 (s, 1H), 6.99 (t, 1H, J = 7.50 Hz), 7.17 (t, 1H, J = 7.33 Hz), 7.21–7.25 (m, 1H), 7.27–7.34 (m, 5H), 7.35–7.40 (m, 2H), 7.48–7.53 (m, 1H), 7.99 (brs, 1H), 8.46 (d, 1H, J = 8.42 Hz), 8.80 (d, 1H, J = 4.13 Hz). 13C NMR (CDCl3): 41.3; 111.1; 118.6; 119.5; 119.8; 120.0; 122.0; 122.3; 123.9; 125.0; 125.9; 126.4; 127.0; 128.4; 128.5; 128.9; 133.3; 136.8; 138.7; 142.8; 148.1; 148.4 (Figures S17 and S18). HRMS calculated for [M + H]+ m/z = 385.1102, found m/z = 385.1088 (Δmi= −3.6 ppm). The in-source fragmentation resulted in 268.0514 m/z (−3.7 ppm) of fragment ion, and the exit of indole moiety might be responsible for its formation (Figure S19). FTIR in Figure S20.
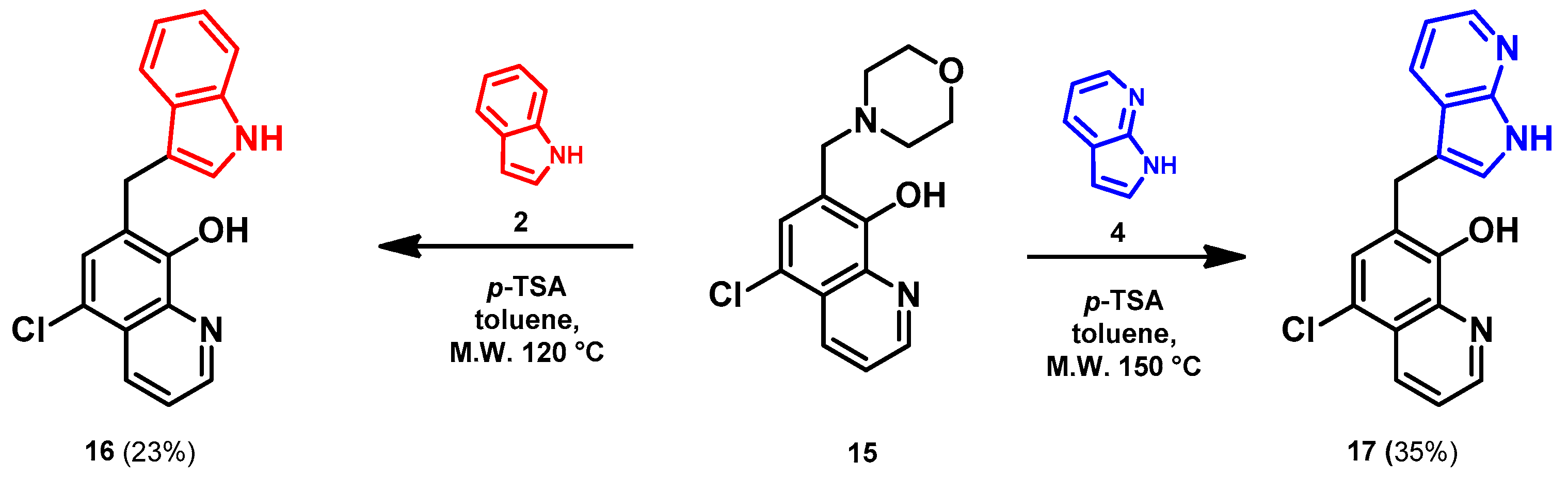
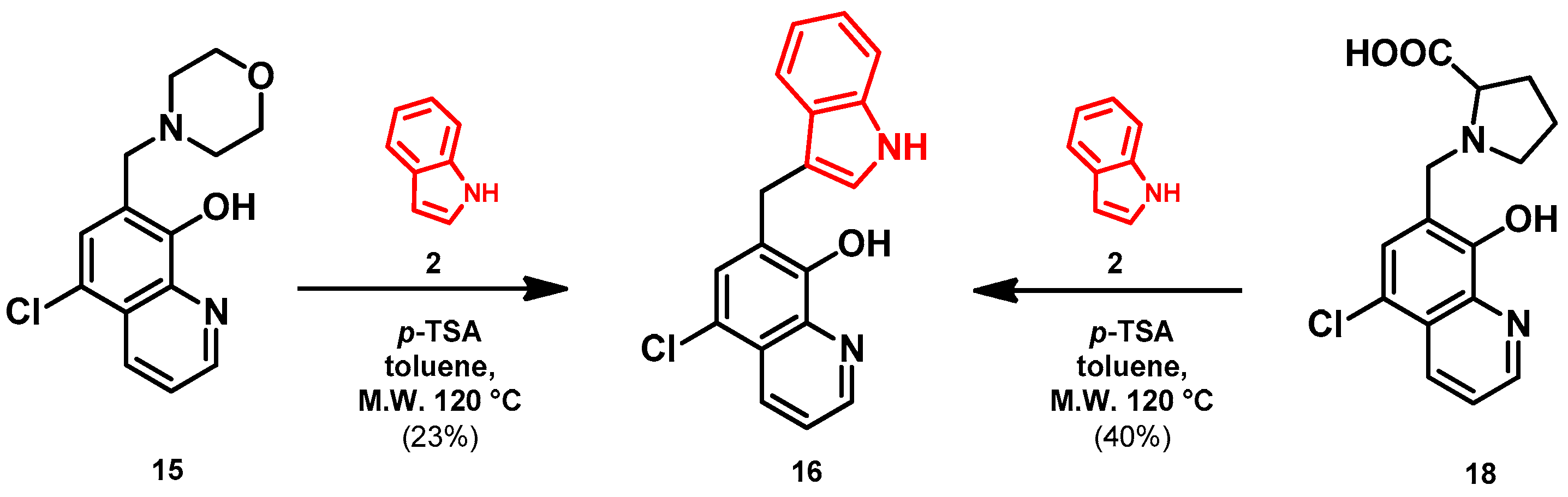
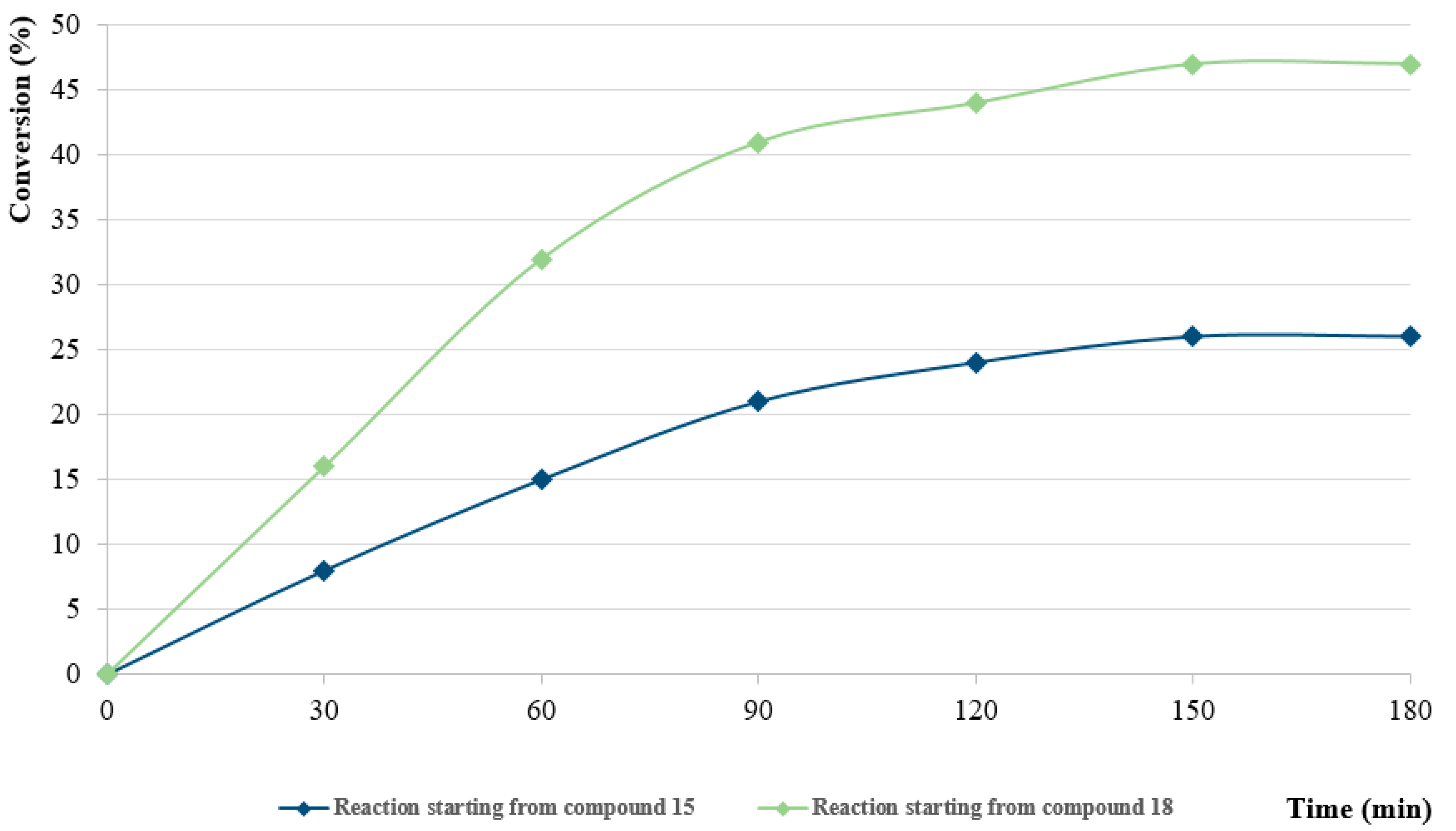
- General procedure for the synthesis of 7-((1H-indol-3-yl)methyl)-5-chloroquinolin-8-ol (16) from precursor 15 and 18
- Indole (2; 20.0 mg, 0.17 mmol) and Mannich base 15 or 18 (0.17 mmol) in the presence of 10 mol% (2.9 mg, 0.017 mmol) p-TSA in toluene were heated at 120 °C for 3 h under MW irradiation. Following column chromatography purification (EtOAc:n-hexane, 1:2 in the case of reaction starting from precursor 15; EtOAc:n-hexane, 1:3 in the case of reaction starting from precursor 18), the eluent was removed in vacuo; Rf value = 0.68 (EtOAc:n-hexane, 1:2); 12.1 mg, (23%) in the case of reaction starting from precursor 15; Rf value = 0.51 EtOAc:n-hexane, 1:3); 21.0 mg (40%) in the case of reaction starting from precursor 18; oil; 1H-NMR (CDCl3): 4.32 (s, 2H), 7.06–7.11 (m, 2H), 7.18 (t, 1H, J = 7.44 Hz), 7.36 (d, 1H, J = 8.21 Hz), 7.43 (s, 1H), 7.46–7.51 (m, 1H), 7.65 (d, 1H, J = 7.88 Hz), 7.99 (brs, 1H), 8.45 (d, 1H, J = 8.39 Hz), 8.81 (d, 1H, J = 4.28 Hz). 13C NMR (CDCl3): 24.8; 111.1; 114.5; 119.2; 119.5; 120.0; 121.7; 122.2; 122.5; 123.4; 124.8; 127.4; 129.3; 133.3; 136.4; 138.6; 148.1; 148.3 (Figures S21 and S22). HRMS calculated for [M + H]+ 309.0789, found m/z = 309.0779 (Δmi = −3.2 ppm) (Figure S23). FTIR in Figure S24.
- 7-((7-azaindole-3-yl)methyl)-5-chloroquinolin-8-ol (17)
- A mixture of precursor 15 (50.0 mg, 0.18 mmol) and 7-azaindole (4; 21.0 mg, 0.18 mmol) in the presence of 10 mol% (3.1 mg, 0.018 mmol) p-TSA in toluene (15 mL) was placed into a 35-mL pressurized reaction vial and heated at 150 °C for 3 h under MW irradiation. Following column chromatography purification (EtOAc:n-hexane, 47:3), the eluent was removed in vacuo; oil; Rf value = 0.79 (EtOAc:n-hexane, 47:3); 19.5 mg, (35%); 1H-NMR (CDCl3): 5.68 (s, 2H), 6.48 (d, 1H, J = 3.33 Hz), 7.07–7.12 (m, 1H), 7.38 (d, 1H, J = 3.37 Hz), 7.45 (s, 1H), 7.49–7.54 (m, 1H), 7.93 (d, 1H, J = 7.71 Hz), 8.38 (d, 1H, J = 4.34 Hz), 8.45 (d, 1H, J = 8.20 Hz), 8.85 (s, 1H). 13C NMR (CDCl3): 42.5; 100.2; 115.9; 120.1; 120.7; 120.7; 122.5; 126.0; 128.1; 128.3; 129.2; 133.2; 139.2; 142.8; 147.4; 148.8; 149.4 (Figures S25 and S26). HRMS calculated for [M + H]+ m/z = 310.0742, found m/z = 310.0729 (Δmi = −4.2 ppm) (Figure S27). FTIR in Figure S28.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tramontini, M.; Angiolini, L. Further advances in the chemistry of mannich bases. Tetrahedron 1990, 46, 1791–1837. [Google Scholar] [CrossRef]
- Paqett, L.A. (Ed.) Encyclopedia of Reagents for Organic Synthesis; Wiley: UK, 2001; Volume 4, p. 21982. [Google Scholar]
- Kos, J.; Ku, C.F.; Kapustikova, I.; Oravec, M.; Zhang, H.; Jampilek, J. 8-Hydroxyquinoline-2-carboxanilides as antiviral agents against avian influenza virus. ChemistrySelect 2019, 4, 4582–4587. [Google Scholar] [CrossRef]
- Krawczyk, M.; Pastuch-Gawolek, G.; Mrozek-Wilczkiewicz, A.; Kuczak, M.; Skonieczna, M.; Musiol, R. Synthesis of 8-hydroxyquinoline glycoconjugates and preliminary assay of their B1,4-GalT inhibitory and anti-cancer properties. Bioorg. Chem. 2019, 84, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Pape, V.F.S.; Palkó, R.; Tóth, S.; Szabó, M.J.; Sessler, J.; Dormán, G.; Enyedy, É.A.; Soós, T.; Szatmári, I.; Szakács, G. Structure–activity relationships of 8-hydroxyquinoline-derived Mannich bases with tertiary amines targeting multi-drug-resistant cancer. J. Med. Chem. 2022, 65, 7729–7745. [Google Scholar] [CrossRef]
- Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological poten-tial. MedChemComm 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Baez-Gonzalez, A.S.; Carrazco-Carrillo, J.A.; Figueroa-Gonzalez, G.; Quintas-Granados, L.I.; Padilla-Benavides, T.; Reyes-Hernandez, O.D. Functional effect of indole-3 carbinol in the viability and invasive properties of cultured cancer cells. Biochem. Biophys. Rep. 2023, 35, 101492. [Google Scholar] [CrossRef] [PubMed]
- Amare, D.E.; Bovee, T.F.H.; Mulder, P.P.J.; Hamers, A.; Hoogenboom, R.L.A.P. Acid condensation products of in-dole-3-carbinol and their in-vitro (anti)estrogenic, (anti)androgenic and aryl hydrocarbon receptor activities. Arab. J. Chem. 2020, 13, 7199–7211. [Google Scholar] [CrossRef]
- Puri, S.; Stefan, K.; Khan, S.L.; Pahnke, J.; Stefan, S.M.; Juvale, K. Indole derivatives as new structural class of potent and antiproliferative inhibitors of Monocarboxylate Transporter 1 (MCT1; SLC16A1). J. Med. Chem. 2023, 66, 657–676. [Google Scholar] [CrossRef]
- El-Boghdady, N.A.; El-Hakk, S.A.; Abd-Elmawla, M.A. The lncRNAs UCA1 and CRNDE target miR-145/TLR4/NF-қB/TNF-α axis in acetic acid-induced ulcerative colitis model: The beneficial role of 3,3-diindolylmethane. Int. Immunopharmacol. 2023, 121, 110541. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Köse, M.; Sylvester, K.; Weighardt, H.; Thimm, D.; Borges, G.; Förster, I.; Kügelgen, I.; Müller, C.E. Diindol-ylmethane derivatives: Potent agonists of the immunostimulatory Orphan G Protein-Coupled Receptor GPR84. J. Med. Chem. 2017, 60, 3636–3655. [Google Scholar] [CrossRef]
- Deb, M.L.; Pegu, C.D.; Deka, B.; Dutta, P.; Kotmale, A.S.; Baruah, P.K. Brønsted-Acid-Mediated Divergent Reactions of Betti Bases with Indoles: An Approach to Chromeno[2,3-b]indoles through Intramolecular Dehydrogenative C2-Alkoxylation of Indole. Eur. J. Org. Chem. 2016, 2016, 3441–3448. [Google Scholar] [CrossRef]
- Pegu, C.D.; Nasrin, S.B.; Deb, M.L.; Das, D.J.; Saikia, K.K.; Baruah, P.K. CAN-Catalyzed microwave promoted reaction of indole with betti bases under solvent-free condition and evaluation of antibacterial activity of the products. Synth. Commun. 2017, 21, 2007–2014. [Google Scholar] [CrossRef]
- Lőrinczi, B.; Simon, P.; Szatmári, I. Synthesis of indole-coupled KYNA derivatives via C–N bond cleavage of Mannich bases. Int. J. Mol. Sci. 2022, 23, 7152. [Google Scholar] [CrossRef]
- Hegedűs, D.; Szemerédi, N.; Gábor, M.; Sas, J.; Belasri, K.; Szatmári, I.; Spengler, G. Cyclic amines coupled to indole deriva-tives with improved efflux pump inhibiting activity in bacteria and cancer cells. Anticancer Res. 2024, 44, 1149–1160. [Google Scholar] [CrossRef]
- Hegedűs, D.; Szemerédi, N.; Spengler, G.; Szatmári, I. Application of partially aromatic ortho-quionone-methides for the synthesis of novel naphthoxazines with improved antibacterial activity. Eur. J. Med. Chem. 2022, 237, 114391. [Google Scholar] [CrossRef] [PubMed]
- Csütörtöki, R.; Szatmári, I.; Mándi, A.; Kurtán, T.; Fülöp, F. Synthesis of hydroxynaphthyl-substituted-amino acid derivatives via a modified Mannich reaction. Synlett 2011, 13, 1940–1946. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Singh, A. Synthesis of unsymmetrical α,α-diarylacetates. J. Org. Chem. 2016, 81, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, X.; Fang, H.; Hou, X. Design, Synthesis and preliminary bioactivity evaluations of 8-hydroxyquinoline de-rivatives as Matrix Metalloproteinase (MMP) inhibitors. Eur. J. Med. Chem. 2019, 181, 111563. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.O.; Shim, J.S.; Chong, C.R.; Bhat, S. Preparation of Quinoline Derivatives for Use as Human Methionine Aminopeptidase, sirT1 and Angiogenesis Inhibitors. Patent WO2010042163, 15 April 2010. [Google Scholar]
- Mészáros, J.P.; Poljarević, J.M.; Szatmári, I.; Csuvik, O.; Fülöp, F.; Szoboszlai, N.; Spengler, G.; Enyedy, É.A. An 8-hydroxyquinoline-proline hybrid with multidrug resistance reversal activity and the solution chemistry of its half-sandwich organometallic Ru and Rh complexes. Dalton Trans. 2020, 49, 7977–7992. [Google Scholar] [CrossRef]
- Sancha, S.A.R.; Szemerédi, N.; Spengler, G.; Ferreira, M.-J.U. Lycorine carbamate derivatives for reversing P-glycoprotein-mediated multidrug resistance in human Colon adenocarcinoma cells. Int. J. Mol. Sci. 2023, 24, 2061. [Google Scholar] [CrossRef]
- Pluchino, K.M.; Hall, M.D.; Goldsborough, A.S.; Callaghan, R.; Gottesman, M.M. Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resist. Updates 2012, 15, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Pivarcsik, T.; Dömötör, O.; Mészáros, J.P.; May, N.V.; Spengler, G.; Csuvik, O.; Szatmári, I.; Enyedy, É.A. 8-Hydroxyquinoline Amino Acid Hybrids and Their Half-Sandwich Rh and Ru Complexes: Synthesis, Anticancer Activities, Solution Chemistry and Interaction with Biomolecules. Int. J. Mol. Sci. 2021, 22, 11281. [Google Scholar] [CrossRef] [PubMed]
- Csuvik, O.; Szemerédi, N.; Spengler, G.; Szatmári, I. Synthesis of 4-Hydroxyquinolines as Potential Cytotoxic Agents. Int. J. Mol. Sci. 2022, 23, 9688. [Google Scholar] [CrossRef] [PubMed]
| Colo205 (µM) | Colo320 (µM) | MRC-5 (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV ± SD | AV ± SD | AV ± SD | ||||||||||
| 3 | 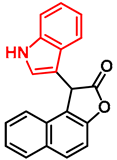 | - | - | - | >100 ± - | - | - | - | >100 ± - | - | - | >100 ± - |
| 7 | 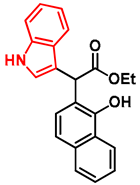 | - | - | - | >100 ± - | 53.82 | 54.92 | 52.18 | 53.64 ± 1.38 | - | - | >100 ± - |
| 10 | 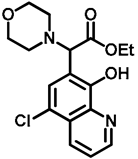 | 2.07 | 2.06 | 2.02 | 2.05 ± 0.02 | 1.565 | 1.813 | 1.79 | 1.72 ± 0.14 | - | - | >100 ± - |
| 11 |  | 5.51 | 4.71 | 4.42 | 4.88 ± 0.56 | 4.636 | 4.832 | 4.957 | 4.81 ± 0.16 | 54.46 | 55.08 | 54.77 ± 0.44 |
| 12 |  | 8.05 | 7.42 | 8.84 | 8.11 ± 0.71 | 5.157 | 4.923 | 5.109 | 5.06 ± 0.12 | 46.9 | 45.56 | 46.23 ± 0.95 |
| 14 | 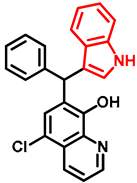 | 2.49 | 2.49 | 2.63 | 2.53 ± 0.08 | 2.552 | 2.84 | 2.781 | 2.72 ± 0.15 | 30.08 | 29.28 | 29.68 ± 0.57 |
| 16 |  | 13.56 | 13.37 | 12.26 | 13.06 ± 0.70 | 4.967 | 4.74 | 4.91 | 4.87 ± 0.12 | - | - | >100 ± - |
| 17 |  | 36.49 | 39.42 | 40.40 | 38.77 ± 2.03 | 22.98 | 21.89 | 22.54 | 22.47 ± 0.55 | - | - | >100 ± - |
| DOX | 0.84 | 0.89 | 0.81 | 0.85 ± 0.04 | 3.117 | 3.12 | 2.961 | 3.07 ± 0.09 | - | - | >8.62 ± - | |
| DMSO | - | - | - | >2% ± - | - | - | - | >2% ± - | - | - | >2% ± - | |
| MRC-5/Colo205 | MRC-5/Colo320 | Colo320/Colo205 | ||
|---|---|---|---|---|
| SI | RR | |||
| 3 | 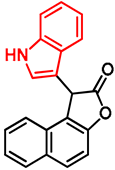 | - | - | NA |
| 7 |  | - | NA | NA |
| 10 | 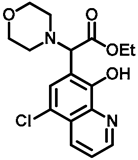 | >6 | >6 | 0.83 |
| 11 | 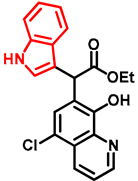 | 11.2 | 11.4 | 0.98 |
| 12 |  | 5.7 | 9.1 | 0.62 |
| 14 | 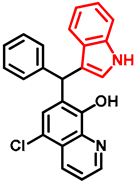 | 11.7 | 11 | 1.07 |
| 16 | 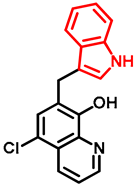 | >6 | >6 | 0.37 |
| 17 | 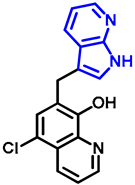 | NA | NA | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegedűs, D.; Szemerédi, N.; Petrinca, K.; Berkecz, R.; Spengler, G.; Szatmári, I. Synthesis of Tumor Selective Indole and 8-Hydroxyquinoline Skeleton Containing Di-, or Triarylmethanes with Improved Cytotoxic Activity. Molecules 2024, 29, 4176. https://doi.org/10.3390/molecules29174176
Hegedűs D, Szemerédi N, Petrinca K, Berkecz R, Spengler G, Szatmári I. Synthesis of Tumor Selective Indole and 8-Hydroxyquinoline Skeleton Containing Di-, or Triarylmethanes with Improved Cytotoxic Activity. Molecules. 2024; 29(17):4176. https://doi.org/10.3390/molecules29174176
Chicago/Turabian StyleHegedűs, Dóra, Nikoletta Szemerédi, Krisztina Petrinca, Róbert Berkecz, Gabriella Spengler, and István Szatmári. 2024. "Synthesis of Tumor Selective Indole and 8-Hydroxyquinoline Skeleton Containing Di-, or Triarylmethanes with Improved Cytotoxic Activity" Molecules 29, no. 17: 4176. https://doi.org/10.3390/molecules29174176
APA StyleHegedűs, D., Szemerédi, N., Petrinca, K., Berkecz, R., Spengler, G., & Szatmári, I. (2024). Synthesis of Tumor Selective Indole and 8-Hydroxyquinoline Skeleton Containing Di-, or Triarylmethanes with Improved Cytotoxic Activity. Molecules, 29(17), 4176. https://doi.org/10.3390/molecules29174176










