Energetic Aspects and Molecular Mechanism of 3-Nitro-substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study
Abstract
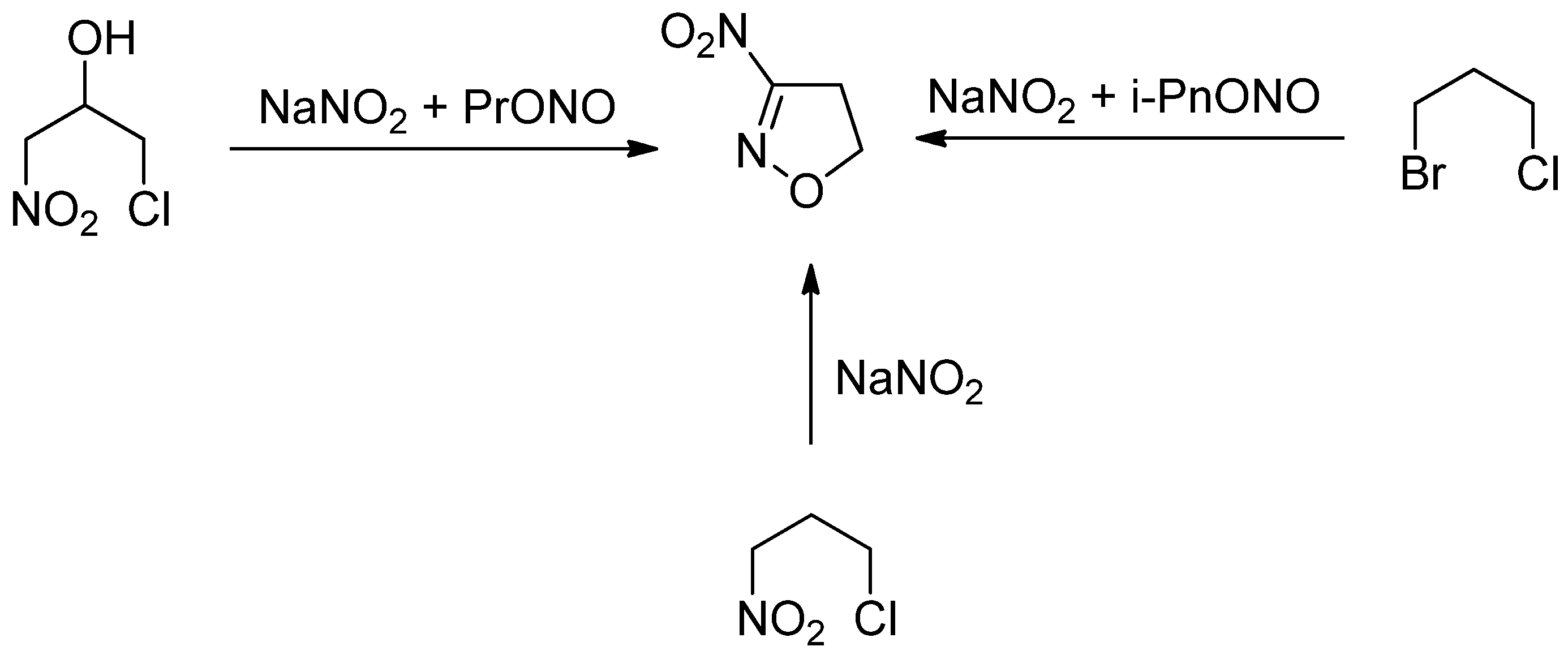
1. Introduction
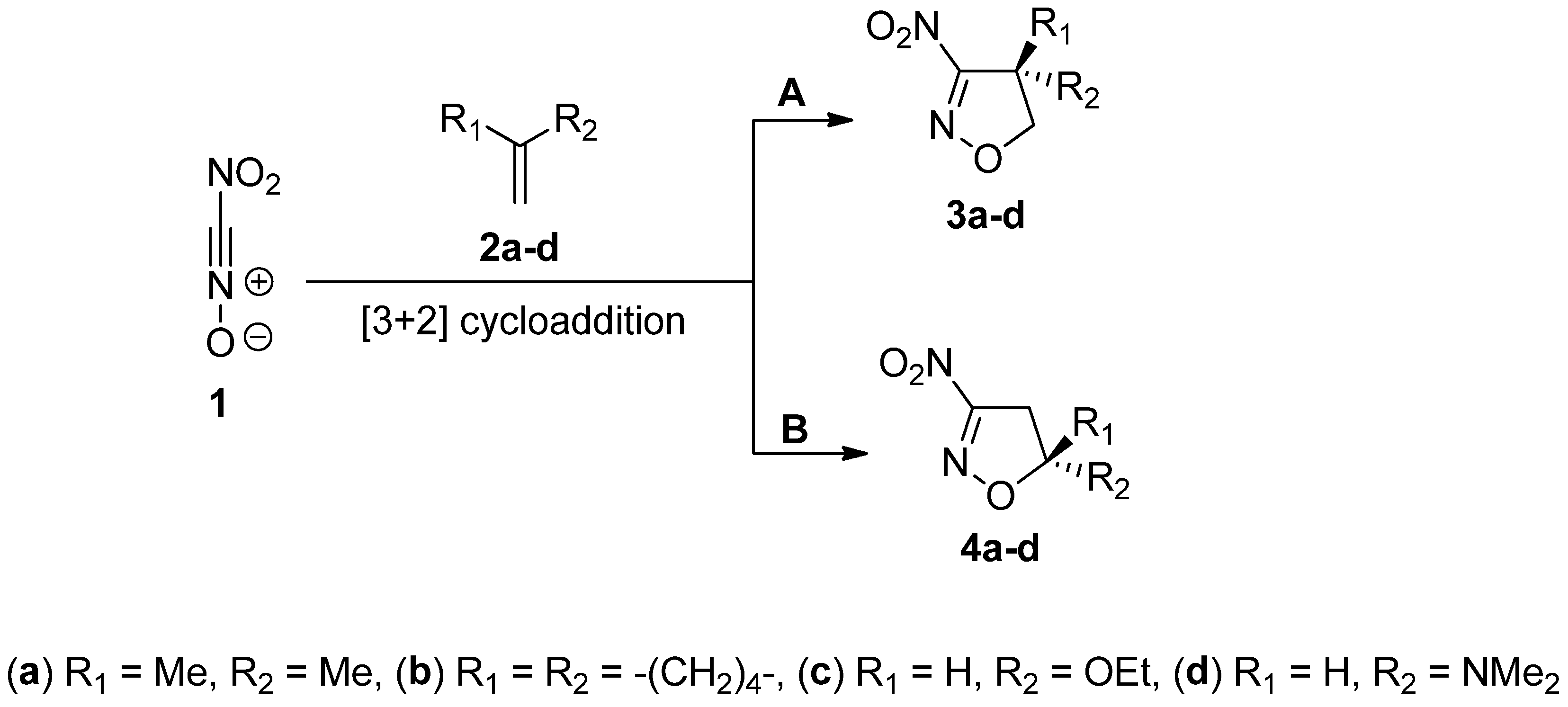
2. Results and Discussion
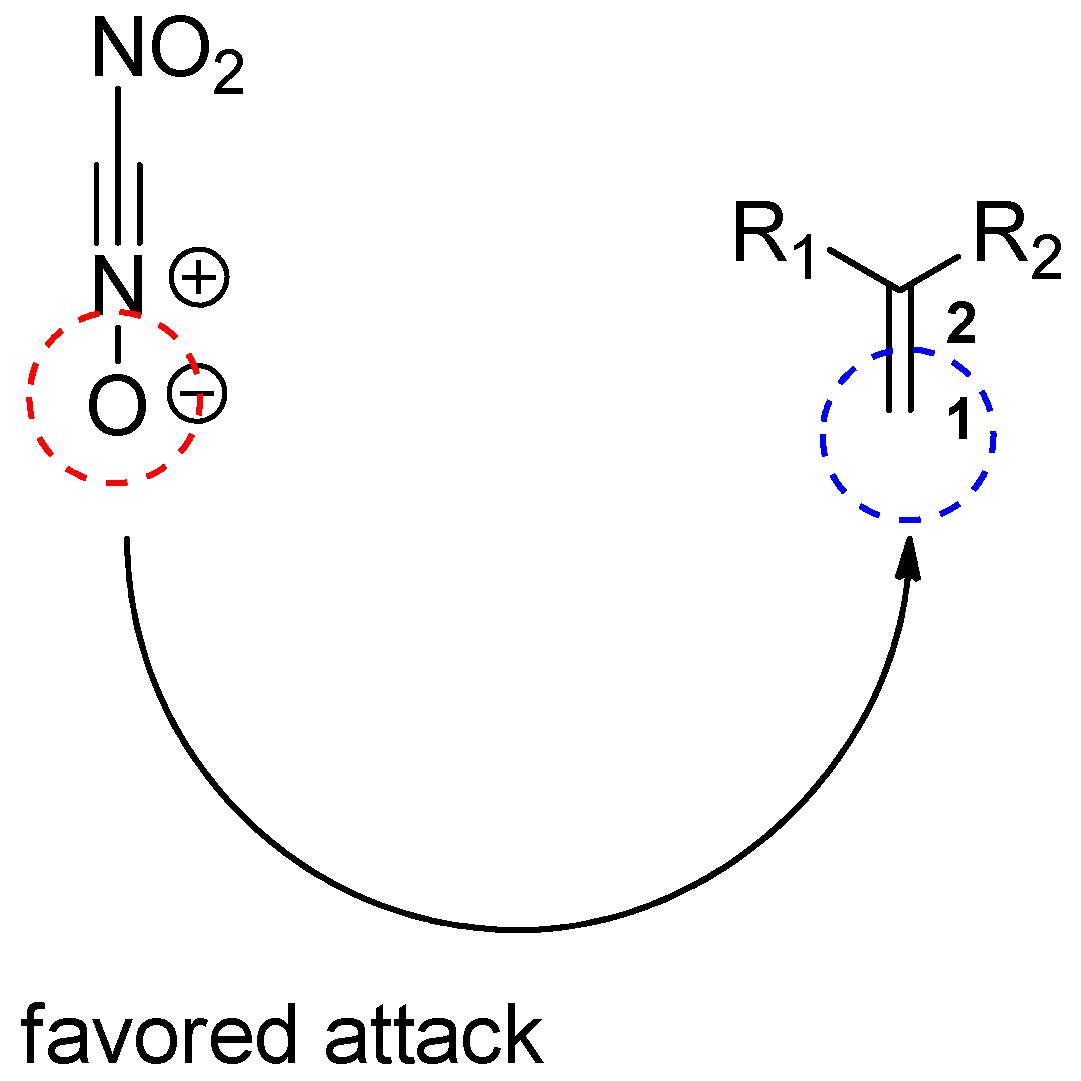
2.1. Power of the Global and Local Interactions in the Context of the CDFT
2.2. Thermodynamic Considerations
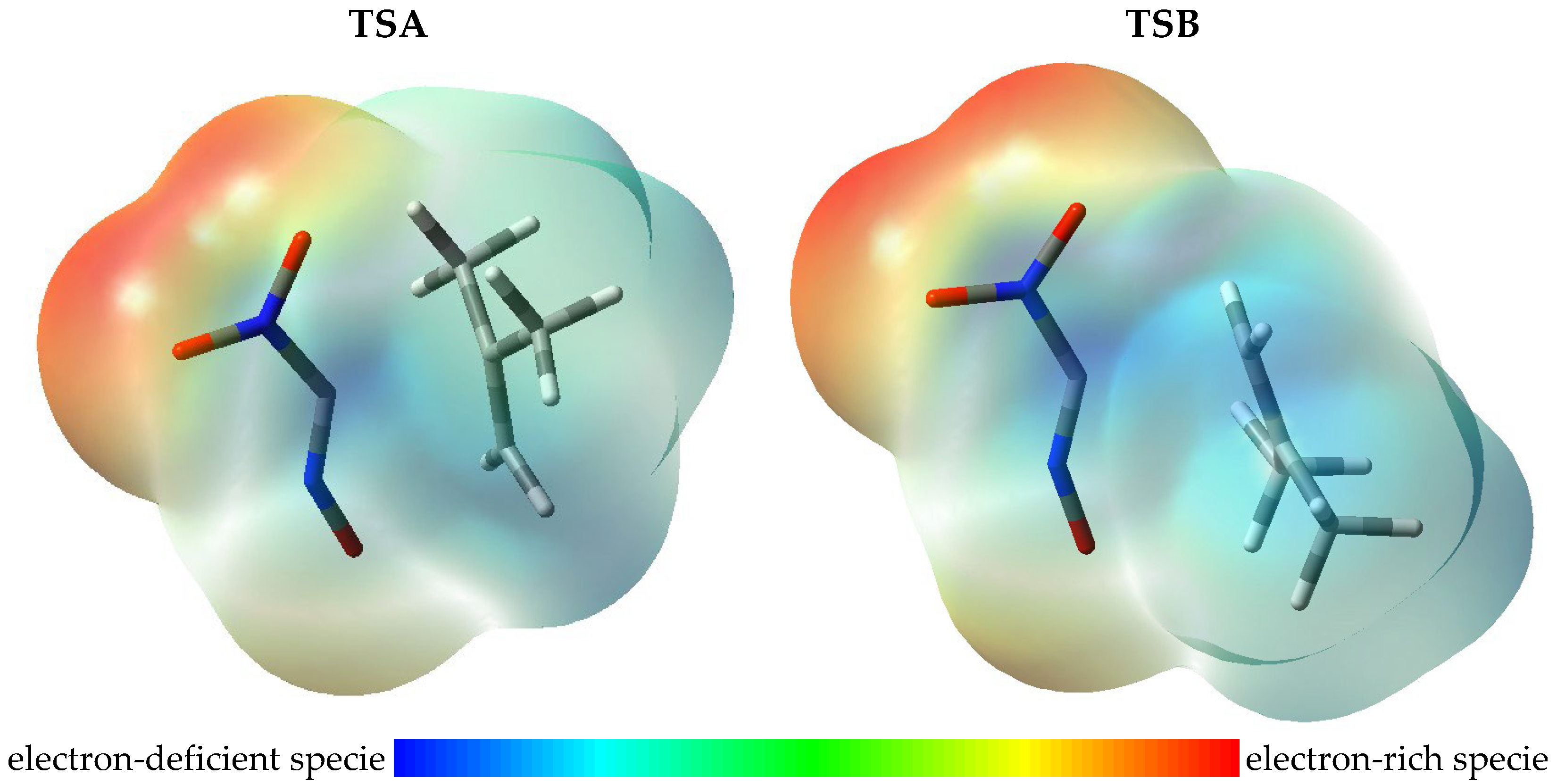
2.3. Exploration of Reaction Profiles
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machado, M.A.; Campos, D.R.; Lopes, N.L.; Bastos, I.P.B.; Alves, M.S.R.; Correia, T.R.; Scott, F.B.; Fernandes, J.I. Efficacy of afoxolaner in the flea control in experimentally infested cats. Rev. Bras. Parasitol. Vet. 2019, 28, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Paarlberg, T.; Karadzovska, D.; Helbig, R. Efficacy of lotilaner (Credelio™) against experimentally induced infestations of the adult cat flea, Ctenocephalides felis, and flea eggs following oral administration to cats. Parasites Vectors 2021, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Six, R.H.; Geurden, T.; Carter, L.; Everett, W.R.; McLoughlin, A.; Mahabir, S.P.; Myers, M.R.; Slootmans, N. Evaluation of the speed of kill of sarolaner (Simparica™) against induced infestations of three species of ticks (Amblyomma maculatum, Ixodes scapularis, Ixodes ricinus) on dogs. Vet. Parasitol. 2016, 222, 37–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujinami, M.; Takahashi, Y.; Tanetani, Y.; Ito, M.; Nasu, M. Development of a rice herbicide, fenoxasulfone. J. Pestic. Sci. 2019, 44, 282–289. [Google Scholar] [CrossRef]
- Kleinman, E.F. Isoxazoline Compoundsas Antnflammatoryagents. U.S. Patent 5,716,967 A, 10 February 1998. [Google Scholar]
- Al-Abed, Y. Isoxazoline Compounds Having MIF Antagonist Activity. U.S. Patent 7,662,843 B2, 16 February 2004. [Google Scholar]
- Sajadikhah, S.S.; Didehban, K. Synthesis of substituted isoxazolidines (microreview). Chem. Heterocycl. Compd. 2023, 59, 640–642. [Google Scholar] [CrossRef]
- Nikol’skiy, V.V.; Starosotnikov, A.M. Synthesis of isoxazolo[4,5-b]pyridine derivatives. Chem. Heterocycl. Comp. 2023, 59, 240–242. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Mei, H.; Soloshonok, V.A.; Han, J. A three-component cycloaddition of alkyl trifluorodiazoethane for the synthesis of trifluoromethylated isoxazolines. Chem. Heterocycl. Comp. 2023, 59, 465–471. [Google Scholar] [CrossRef]
- Kras, J.; Sadowski, M.; Zawadzińska, K.; Nagatsky, R.; Woliński, P.; Kula, K.; Łapczuk, A. Thermal [3+2] cycloaddition reactions as most universal way for the effective preparation of five-membered nitrogen containing heterocycles. Sci. Rad. 2023, 2, 247–267. [Google Scholar] [CrossRef]
- Łapczuk, A. The [3+2] cycloaddition reaction as an attractive way for the preparation of nicotine analogs. Chem. Heterocycl. Comp. 2023, 59, 109–111. [Google Scholar] [CrossRef]
- Dresler, E. The Participation of Oleic Acid and its Esters in [3+2] Cycloaddition Reactions: A Mini-Review. Sci. Rad. 2024, 3, 53–61. [Google Scholar] [CrossRef]
- Digamber, R.; Mukund, S. Recent Advances in Nitrile Oxide Cycloadditions. Synthesis of Isoxazolines. Curr. Org. Synth. 2011, 8, 616–627. [Google Scholar]
- Ono, N. Cycloaddition Chemistry of Nitro Compounds. In The Nitro Group in Organic Synthesis; Feuer, H., Ono, N., Eds.; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Jasiński, R. Synthesis of 1,2-oxazine N-oxides via non-catalyzed hetero-Diels–Alder reactions of nitroalkenes (microreview). Chem. Heterocycl. Compd. 2024, 60, 121–123. [Google Scholar]
- Hao, F.; Nishiwaki, N. Recent Progress in Nitro-Promoted Direct Functionalization of Pyridones and Quinolones. Molecules 2020, 25, 673. [Google Scholar] [CrossRef]
- Rocard, L.; Goujon, A.; Hudhomme, P. Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials. Molecules 2020, 25, 1402. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Kula, K. Nitro-functionalized analogues of 1,3-Butadiene: An overview of characteristic, synthesis, chemical transformations and biological activity. Curr. Chem. Lett. 2024, 13, 15–30. [Google Scholar] [CrossRef]
- Kras, J.; Mikulska, M.; Allnajar, R.; Kula, K. Unsuccessful synthesis of individual 1,1-dinitroethene. Sci. Rad. 2024, 3, 9–13. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiołek, Ł.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel functionalized β-nitrostyrenes: Promising candidates for new antibacterial drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska-Czubara, A.; Lapczuk-Krygier, A.; Rykala, K.; Biernasiuk, A.; Wnorowski, A.; Popiolek, L.; Maziarka, A.; Hordyjewska, A.; Jasiński, R. Novel synthesis scheme and in vitro antimicrobial evaluation of a panel of (E)-2-aryl-1-cyano-1-nitroethenes. J. Enzyme Inhib. Med. Chem. 2016, 31, 900–907. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gostyński, B. Nitrosubstituted analogs of isoxazolines and isoxazolidines: A surprising estimation of their biological activity via molecular docking. Sci. Rad. 2023, 2, 25–46. [Google Scholar] [CrossRef]
- Kula, K.; Zawadzińska, K. Local nucleophile-electrophile interactions in [3+2] cycloaddition reactions between benzonitrile N-oxide and selected conjugated nitroalkenes in the light of MEDT computational study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Kula, K. Application of β-Phosphorylated Nitroethenes in [3+2] Cycloaddition Reactions Involving Benzonitrile N-Oxide in the Light of a DFT Computational Study. Organics 2021, 2, 26–37. [Google Scholar] [CrossRef]
- Jasiński, R.; Jasińska, E.; Dresler, E. A DFT computational study of the molecular mechanism of [3+2] cycloaddition reactions between nitroethene and benzonitrile N-oxides. J. Mol. Model. 2017, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, K.; Ríos-Gutiérrez, M.; Kula, K.; Woliński, P.; Mirosław, B.; Krawczyk, T.; Jasiński, R. The Participation of 3,3,3-Trichloro-1-nitroprop-1-ene in the [3+2] Cycloaddition Reaction with Selected Nitrile N-Oxides in the Light of the Experimental and MEDT Quantum Chemical Study. Molecules 2021, 26, 6774. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, K.; Gaurav, G.K.; Jasiński, R. Preparation of conjugated nitroalkenes: Short review. Sci. Rad. 2022, 1, 69–83. [Google Scholar] [CrossRef]
- Halimehjani, A.Z.; Namboothiri, I.N.N.; Hooshmanda, S.E. Nitroalkenes in the synthesis of carbocyclic compounds. RSC Adv. 2014, 4, 31261–31299. [Google Scholar] [CrossRef]
- Lam, P.Y.S.; Adams, J.J.; Clark, C.G.; Calhoun, W.J.; Luettgen, J.M.; Knabb, R.M.; Wexler, R.R. Discovery of 3-Amino-4-Chlorophenyl P1 as a novel and potent benzamidine mimic via solid-phase synthesis of an isoxazoline library. Bioorg. Med. Chem. Lett. 2003, 13, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.L.; Marcuccio, S.M.; Savage, G.P. Aryl nitrile oxide cycloaddition reactions in the presence of pinacol boronic acid ester. Beilstein J. Org. Chem. 2012, 8, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Toczko, M.A.; Heathcock, C.H. Total Synthesis of (±)-Aspidospermidine. J. Org. Chem. 2000, 65, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Groundwater, P.W.; Nyerges, M.; Fejes, I.; Hibbs, D.E.; Bendell, D.; Anderson, R.J.; McKillop, A.; Sharif, T.; Zhang, W. Preparation and reactivity of some stable nitrile oxides and nitrones. ARKIVOC 2000, V, 684–697. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Skripnichenko, L.N.; Pavlikov, V.V.; Rozantsev, E.G. Synthesis of nitroxyl radicals based on 4-ethynyl-4-hydroxy-2,2,6,6-tetramethylpiperidine. Russ. Chem. Bull. 1979, 28, 140–148. [Google Scholar] [CrossRef]
- Shitov, O.P.; Baranin, S.V.; Tartakovsky, V.A. Esters of nitromethane nitronic acid. Russ. Chem. Bull. 2022, 71, 350–353. [Google Scholar] [CrossRef]
- Kacka-Zych, A. Push-pull nitronates in the [3+2] cycloaddition with nitroethylene: Molecular Electron Density Theory study. J. Mol. Graph. Model. 2020, 97, 1075492. [Google Scholar] [CrossRef] [PubMed]
- Wade, P.A.; D’Ambrosio, S.G.; Price, D.T. 2-Amino-2-deoxytetrose Derivatives. Preparation from 4,5-Dihydroisoxazoles via Reductive Cleavage. J. Org. Chem. 1995, 60, 6302–6308. [Google Scholar] [CrossRef]
- Kloeckner, J.; Schmitz, J.; Holzgrabe, U. Convergent, short synthesis of the muscarinic superagonist iperoxo. Tetrahedron Lett. 2010, 51, 3470–3472. [Google Scholar] [CrossRef]
- Wade, P.A. Synthesis of 3-substituted 2-isoxazolines and 5,6-dihydro-4H-1,2-oxazines. J. Org. Chem. 1978, 43, 2020–2023. [Google Scholar] [CrossRef]
- Diamantini, G.; Duranti, E.; Tontini, A. Nitroisoxazoles by Manganese(IV) Oxide Oxidation of Nitro-4,5-dihydroisoxazoles. Synthesis 1993, 1993, 1104–1108. [Google Scholar] [CrossRef]
- Baum, K.; Tzeng, D. Synthesis and reactions of tetranitroethylene. J. Org. Chem. 1985, 50, 2736–2739. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Ekaterina, M.; Budynina, E.M.; Averina, E.B.; Kuznetsova, T.S.; Zefirov, N.S. Application of a Thermal b-Elimination Reaction to N-Alkoxy-3,3-dinitroisoxazolidines: Synthesis of 3-Nitroisoxazolines. Synthesis 2006, 2006, 706–710. [Google Scholar] [CrossRef]
- Ovchinnikov, I.V.; Makhova, N.N.; Khmel’nitskii, L.I. Generation of Nitro Formonitrile Oxide as an Intermediate for the Preparation of Dinitrofuroxan. Mendeleev Commun. 1993, 3, 210–211. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Khakimov, D.V.; Makhova, N.N. Dinitrofuroxan cycloreversion as a novel general approach for the synthesis of nitroazoles. Russ. Chem. Bull. 2015, 64, 415–422. [Google Scholar] [CrossRef]
- Rodriguez-Betancourtt, V.-M.; Quezada-Navarro, V.-M.; Neff, M.; Rauhut, G. Anharmonic frequencies of [F,C,N,X] isomers (X = O,S) obtained from explicitly correlated coupled-cluster calculations. Chem. Phys. 2011, 387, 1–4. [Google Scholar] [CrossRef]
- Jacobs, J.; Jülicher, B.; Schatte, G.; Willner, H.; Mack, H.G. CFNO-Isomere—Theoretische und experimentelle Studien. Chem. Ber. 1993, 126, 2167–2176. [Google Scholar] [CrossRef]
- Zana, A.; Galbiati, A. Synthesis and Reactivity of 3-Halo-4,5-dihydroisoxazoles: An Overview. ChemistrySelect 2021, 6, 8249–8261. [Google Scholar] [CrossRef]
- Lichau, H.; Gillies, C.W.; Gillies, J.Z.; Ross, S.C.; Winnewisser, B.P.; Winnewisser, M. On the Anharmonic XCN Bending Modes of the Quasilinear Molecules BrCNO and ClCNO. J. Phys. Chem. A 2001, 105, 10065–10079. [Google Scholar] [CrossRef]
- Pasinszki, T.; Westwood, N.P.C. Substituted oximes and furoxans as precursors to unstable nitrile oxides. electronic and geometric structures by ultraviolet photoelectron spectroscopy, infrared spectroscopy and ab initio calculations. J. Mol. Struct. 1997, 408–409, 161–169. [Google Scholar] [CrossRef]
- Yazdani, H.; Hooshmand, S.E.; Varma, R.S. Gold Nanoparticle-Catalyzed Multicomponent Reactions. ACS Sustain. Chem. Eng. 2021, 9, 16556–16569. [Google Scholar] [CrossRef]
- Tamborini, L.; Mastronardi, F.; Presti, L.L.; Nielsen, B.; De Micheli, C.; Conti, P.; Pinto, A. Synthesis of l-Tricholomic Acid Analogues and Pharmacological Characterization at Ionotropic Glutamate Receptors. ChemistrySelect 2017, 2, 10295–10299. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Progress in 1,3-dipolar cycloadditions in the recent decade: An update to strategic development towards the arsenal of organic synthesis. Tetrahedron 2016, 72, 1603–1644. [Google Scholar] [CrossRef]
- Cao, W.-L.; Tariq, Q.-u.-N.; Li, Z.-M.; Yang, J.-Q.; Zhang, J.-G. Recent advances on the nitrogen-rich 1,2,4-oxadiazole-azoles-based energetic materials. Def. Technol. 2022, 18, 344–367. [Google Scholar] [CrossRef]
- Escapa, C.; Torres, T.; Neuparth, T.; Coimbra, R.N.; García, A.I.; Santos, M.M.; Otero, M. Zebrafish embryo bioassays for a comprehensive evaluation of microalgae efficiency in the removal of diclofenac from water. Sci. Total Environ. 2018, 640–641, 1024–1033. [Google Scholar] [CrossRef]
- Robertson, E.G.; McNaughton, D. IR Spectroscopy of OP−X and Derivatives: Mistaken Identity on a Large Scale. J. Phys. Chem. A 2003, 107, 642–650. [Google Scholar] [CrossRef]
- Jasiński, R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Jasiński, R. First example of stepwise, zwitterionic mechanism for bicyclo[2.2.1]hept-5-ene (norbornene) formation process catalyzed by the 1-butyl-3-methylimidazolium cations. Monatsh. Chem. 2016, 147, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Kącka, A.; Jasiński, R. A dramatic change of kinetic conditions and molecular mechanism of decomposition processes of nitroalkyl carboxylates catalyzed by ethylammonium cations. Comput. Theor. Chem. 2017, 1104, 37–42. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Mirosław, B.; Wielgus, E.; Olszewska, A.; Jasiński, R. Green, one-pot synthesis of 1,2-oxazine-type herbicides via non-catalyzed Hetero Diels-Alder reactions comprising (2E)-3-aryl-2-nitroprop-2-enenitriles. J. Clean. Prod. 2022, 356, 131878. [Google Scholar] [CrossRef]
- Wen, C.; Dechsupa, N.; Yu, Z.; Zhang, X.; Liang, S.; Lei, X.; Xu, T.; Gao, X.; Hu, Q.; Innuan, P.; et al. Pentagalloyl Glucose: A Review of Anticancer Properties, Molecular Targets, Mechanisms of Action, Pharmacokinetics, and Safety Profile. Molecules 2023, 28, 4856. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R.; Kubik, M.; Łapczuk-Krygier, A.; Kącka, A.; Dresler, E.; Boguszewska-Czubara, A. An experimental and theoretical study of the hetero Diels–Alder reactions between (E)-2-aryl-1-cyano-1-nitroethenes and ethyl vinyl ether: One-step or zwitterionic, two-step mechanism? React. Kinet. Mech. Catal. 2014, 113, 333–345. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Regioselectivity and the Molecular Mechanism of [3+2] Cycloaddition Reactions between Nitrous Oxide and Conjugated Nitroalkenes: A DFT Computational Study. Molecules 2022, 27, 8441. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials 2022, 15, 7584. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Kras, J.; Zawadzińska, K.; Demchuk, O.M.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-Ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef]
- Dresler, E.; Woliński, P.; Wróblewska, A.; Jasiński, R. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions between Aryl Azides and Ethyl Propiolate. Molecules 2023, 28, 8152. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos Gutiérrez, M.; Castellanos Soriano, J. Understanding the Origin of the Regioselectivity in Non-Polar [3+2] Cycloaddition Reactions through the Molecular Electron Density Theory. Organics 2020, 1, 19–35. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. A DFT analysis of the participation of zwitterionic TACs in polar [3+2] cycloaddition reactions. Tetrahedron 2014, 70, 4519–4525. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Molecular Mechanism of Thermal and LA-Catalysed Diels–Alder Reactions between Cyclopentadiene and Isopropyl 3-Nitroprop-2-Enate. Molecules 2023, 28, 5289. [Google Scholar] [CrossRef] [PubMed]
- Woliński, P.; Kącka-Zych, A.; Wróblewska, A.; Wielgus, E.; Dolot, R.; Jasiński, R. Fully Selective Synthesis of Spirocyclic-1,2-oxazine N-Oxides via Non-Catalysed Hetero Diels-Alder Reactions with the Participation of Cyanofunctionalysed Conjugated Nitroalkenes. Molecules 2023, 28, 4586. [Google Scholar] [CrossRef]
- Sadowski, M.; Utnicka, J.; Wójtowicz, A.; Kula, K. The global and local Reactivity of C,N-diarylnitryle imines in [3+2] cycloaddition processes with trans-β-nitrostyrene according to Molecular Electron Density Theory: A computational study. Curr. Chem. Lett. 2023, 12, 421–430. [Google Scholar] [CrossRef]
- Kącka-Zych, A. Understanding the uniqueness of the stepwise [4+1] cycloaddition reaction between conjugated nitroalkenes and electrophilic carbene systems with a molecular electron density theory perspective. Int. J. Quantum. Chem. 2021, 121, e26440. [Google Scholar] [CrossRef]
- Aitouna, A.O.; Barhoumi, A.; Zeroual, A. A Mechanism Study and an Investigation of the Reason for the Stereoselectivity in the [4+2] Cycloaddition Reaction between Cyclopentadiene and Gem-substituted Ethylene Electrophiles. Sci. Rad. 2023, 2, 217–228. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Based on the Flux of the Electron Density. Sci. Rad. 2023, 2, 1–24. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Saz Sousa, A.; Domingo, L.R. Electrophilicity and nucleophilicity scales at different DFT computational levels. J. Phys. Org. Chem. 2023, 36, e4503. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R.; Ghodsi, F. Unveiling the Different Reactivity of Bent and Linear Three-Atom-Components Participating in [3+2] Cycloaddition Reactions. Organics 2021, 2, 274–286. [Google Scholar] [CrossRef]
- Bent, H.A. Structural chemistry of donor-acceptor interactions. Chem. Rev. 1968, 68, 587–648. [Google Scholar] [CrossRef]
- Foster, R. Electron donor-acceptor complexes. J. Phys. Chem. 1980, 84, 2135–2141. [Google Scholar] [CrossRef]
- Ameur, S.; Barhoumi, A.; Ríos-Gutiérrez, M.; Aitouna, A.O.; El Alaoui El Abdallaoui, H.; Mazoir, N.; Belghiti, M.E.; Syed, A.; Zeroual, A.; Domingo, L.R. A MEDT study of the mechanism and selectivity of the hetero-Diels–Alder reaction between 3-benzoylpyrrolo[1,2-c][1,4]-benzoxazine-1,2,4-trione and vinyl acetate. Chem. Heterocycl. Comp. 2023, 59, 165–170. [Google Scholar] [CrossRef]
- Krishna, C.; Seetharam, K.; Satyadev, T. Synthesis of β-amino alcohols by ring opening of epoxides with amines catalyzed by sulfated tin oxide under mild and solvent-free conditions. Curr. Chem. Lett. 2024, 13, 343–350. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 49–69. [Google Scholar] [CrossRef]
- Siadati, S.A. Beyond the Alternatives that switch the Mechanism of the 1,3-Dipolar Cycloadditions from Concerted to Stepwise or Vice Versa: A Literature Review. Prog. React. Kinet. Mech. 2016, 41, 331–344. [Google Scholar] [CrossRef]
- Siadati, S.A.; Rezazadeh, S. The extraordinary gravity of three atom 4π-components and 1,3-dienes to C20-nXn fullerenes; a new gate to the future of Nano technology. Sci. Rad. 2022, 1, 46–68. [Google Scholar] [CrossRef]
- Mohtat, B.; Siadati, S.A.; Khalilzadeh, M.A.; Zareyee, D. The concern of emergence of multi-station reaction pathways that might make stepwise the mechanism of the 1,3-dipolar cycloadditions of azides and alkynes. J. Mol. Struct. 2018, 1155, 58–64. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Mondal, A.; Acharjee, N. Unveiling the exclusive stereo and site selectivity in [3+2] cycloaddition reactions of a tricyclic strained alkene with nitrile oxides from the molecular electron density theory perspective. Chem. Heterocycl. Comp. 2023, 59, 145–154. [Google Scholar] [CrossRef]
- Abdoul-Hakim, M.; Idrissi, K.E.; Zeroual, A.; Garmes, H. Investigation of the solvent effect, regioselectivity, and the mechanism of the cycloaddition reaction between 2-chlorobenzimidazole and benzonitrile oxide. Chem. Heterocycl. Comp. 2023, 59, 155–164. [Google Scholar] [CrossRef]
- Yousfi, Y.; Benchouk, W.; Mekelleche, S.M. Prediction of the regioselectivity of the ruthenium-catalyzed [3+2] cycloadditions of benzyl azide with internal alkynes using conceptual DFT indices of reactivity. Chem. Heterocycl. Comp. 2023, 59, 118–127. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Liu, S.; Rong, C.; Zhong, A.; Chattaraj, P.K.; Shubin, L. Is It Possible to Determine Oxidation States for Atoms in Molecules Using Density-Based Quantities? An Information-Theoretic Approach and Conceptual Density Functional Theory Study. J. Phys. Chem. A 2019, 123, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]



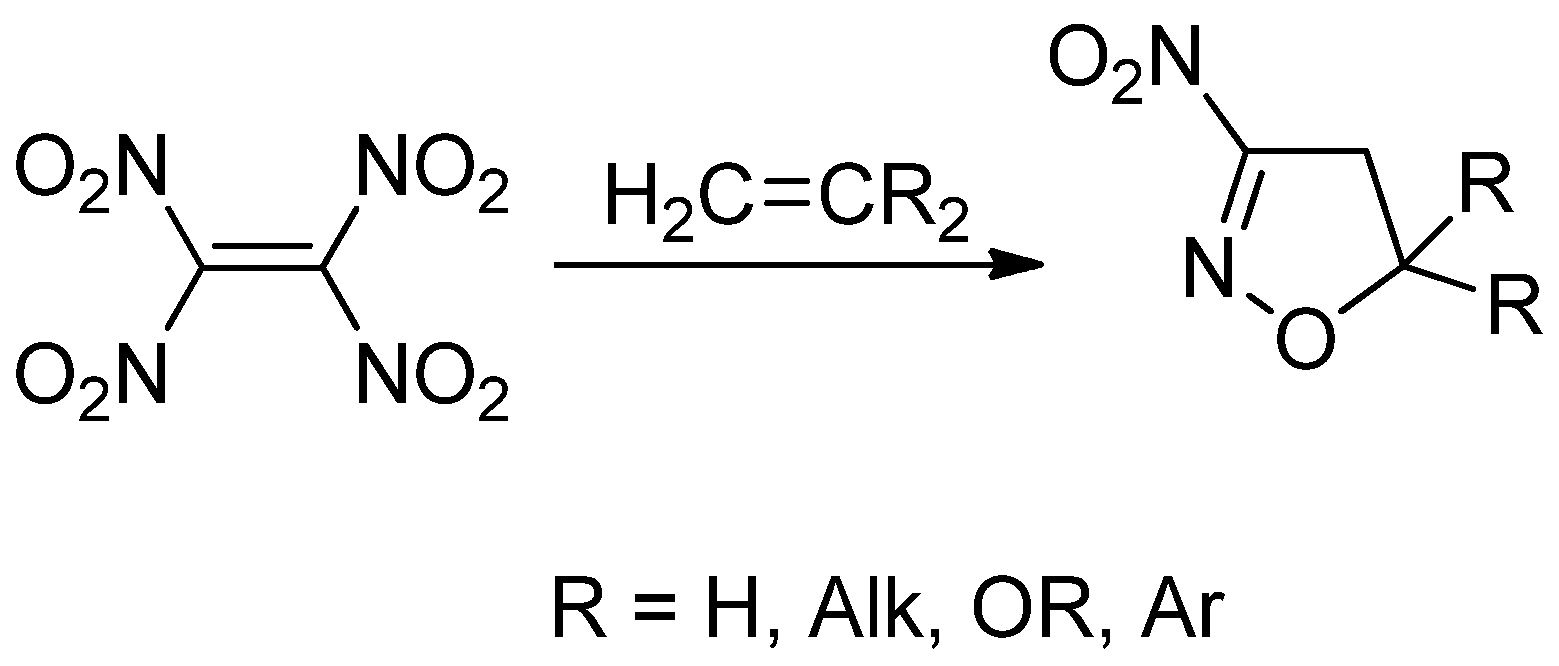



| Global Properties | Local Properties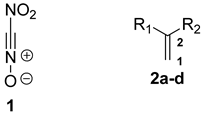 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μ [eV] | η [eV] | ω [eV] | N [eV] | P−2 | P−1 | N2 [eV] | N1 [eV] | P+C | P+O | ωC [eV] | ωO [eV] | |
| 1 | −5.98 | 4.85 | 3.68 | 0.72 | 0.048 | 0.091 | 0.18 | 0.34 | ||||
| 2a | −2.83 | 7.37 | 0.55 | 2.60 | 0.295 | 0.646 | 0.77 | 1.68 | ||||
| 2b | −2.87 | 6.95 | 0.59 | 2.77 | 0.266 | 0.636 | 0.74 | 1.76 | ||||
| 2c | −2.39 | 6.97 | 0.41 | 3.25 | 0.057 | 0.577 | 0.18 | 1.87 | ||||
| 2d | −1.87 | 6.50 | 0.27 | 3.99 | 0.110 | 0.557 | 0.44 | 2.23 | ||||
| Reaction | Transition | Toluene | Nitromethane | ||||
|---|---|---|---|---|---|---|---|
| ΔH | ΔG | ΔS | ΔH | ΔG | ΔS | ||
| 1+2a | 1+2a→MCA | −2.3 | 5.9 | −27.4 | −1.8 | 6.3 | −27.4 |
| 1+2a→TSA | 7.7 | 19.6 | −39.8 | 8.5 | 20.4 | −39.9 | |
| 1+2a→3a | −63.5 | −47.9 | −52.2 | −64.7 | −49.2 | −51.9 | |
| 1+2a→MCB | −2.5 | 6.6 | −30.4 | −3.4 | 5.7 | −30.7 | |
| 1+2a→TSB | 3.6 | 13.6 | −33.3 | 4.1 | 13.8 | −32.7 | |
| 1+2a→4a | −70.0 | −54.6 | −51.7 | −71.4 | −55.7 | −52.6 | |
| 1+2b | 1+2b→MCA | −4.4 | 4.3 | −29.1 | −3.3 | 4.9 | −27.3 |
| 1+2b→TSA | 8.4 | 20.5 | −40.9 | 9.1 | 21.6 | −41.8 | |
| 1+2b→3b | −64.3 | −49.6 | −49.3 | −65.4 | −50.7 | −49.2 | |
| 1+2b→MCB | −3.9 | 4.7 | −28.7 | −2.9 | 5.4 | −27.9 | |
| 1+2b→TSB | 2.0 | 12.6 | −35.6 | 2.3 | 13.0 | −35.8 | |
| 1+2b→4b | −69.2 | −56.6 | −42.1 | −70.4 | −58.2 | −41.0 | |
| 1+2c | 1+2c→MCA | −7.0 | 4.6 | −38.9 | −5.5 | 5.5 | −37.0 |
| 1+2c→TSA | 5.7 | 18.2 | −41.8 | 6.5 | 18.9 | −41.4 | |
| 1+2c→3c | −59.9 | −45.1 | −49.4 | −60.6 | −45.7 | −49.8 | |
| 1+2c→MCB | −5.0 | 3.7 | −29.2 | −1.9 | 3.8 | −19.1 | |
| 1+2c→TSB | 0.5 | 11.5 | −37.1 | 1.4 | 12.0 | −35.6 | |
| 1+2c→4c | −70.5 | −55.9 | −49.1 | −71.0 | −56.4 | −48.9 | |
| 1+2d | 1+2d→MCA | −4.3 | 3.1 | −24.8 | −3.5 | 4.1 | −25.3 |
| 1+2d→TSA | 5.1 | 15.9 | −36.0 | 5.7 | 16.5 | −36.2 | |
| 1+2d→3d | −58.5 | −44.8 | −46.2 | −58.9 | −44.8 | −47.2 | |
| 1+2d→MCB | −4.6 | 3.1 | −25.8 | −3.7 | 4.2 | −26.5 | |
| 1+2d→TSB | −4.0 | 6.6 | −35.7 | −3.6 | 6.8 | −35.2 | |
| 1+2d→4d | −67.4 | −53.5 | −46.5 | −68.3 | −54.3 | −46.8 | |
| Reaction | Structure | Interatomic Distances [Å] | GEDT [e] | ||||
|---|---|---|---|---|---|---|---|
| O1–N2 | N2–C3 | C3–C4 | C4–C5 | C5–O1 | |||
| 1+2a | 1 | 1.181 | 1.162 | ||||
| 2a | 1.332 | ||||||
| MCA | 1.179 | 1.163 | 3.299 | 1.332 | 3.344 | 0.00 | |
| TSA | 1.195 | 1.215 | 2.360 | 1.364 | 2.434 | 0.16 | |
| 3a | 1.347 | 1.271 | 1.510 | 1.546 | 1.455 | ||
| MCB | 1.184 | 1.157 | 3.216 | 1.333 | 3.416 | 0.00 | |
| TSB | 1.196 | 1.199 | 2.292 | 1.357 | 2.865 | 0.13 | |
| 4a | 1.343 | 1.271 | 1.487 | 1.542 | 1.489 | ||
| 1+2b | 2b | 1.329 | |||||
| MCA | 1.179 | 1.167 | 3.596 | 1.330 | 3.565 | 0.00 | |
| TSA | 1.195 | 1.213 | 2.342 | 1.361 | 2.461 | 0.20 | |
| 3b | 1.356 | 1.272 | 1.503 | 1.534 | 1.450 | ||
| MCB | 1.179 | 1.167 | 3.519 | 1.330 | 3.556 | 0.00 | |
| TSB | 1.199 | 1.198 | 2.268 | 1.354 | 3.037 | 0.14 | |
| 4b | 1.344 | 1.271 | 1.488 | 1.532 | 1.489 | ||
| 1+2c | 2c | 1.329 | |||||
| MCA | 1.179 | 1.168 | 3.854 | 1.331 | 5.153 | 0.00 | |
| TSA | 1.201 | 1.217 | 2.264 | 1.365 | 2.484 | 0.12 | |
| 3c | 1.349 | 1.272 | 1.510 | 1.531 | 1.453 | ||
| MCB | 1.179 | 1.168 | 3.739 | 1.328 | 3.475 | 0.00 | |
| TSB | 1.200 | 1.192 | 2.357 | 1.351 | 3.045 | 0.13 | |
| 4c | 1.360 | 1.269 | 1.488 | 1.524 | 1.467 | ||
| 1+2d | 2d | 1.341 | |||||
| MCA | 1.187 | 1.164 | 3.271 | 1.347 | 4.557 | 0.00 | |
| TSA | 1.204 | 1.227 | 2.680 | 1.376 | 2.227 | 0.22 | |
| 3a | 1.351 | 1.272 | 1.520 | 1.533 | 1.453 | ||
| MCB | 1.192 | 1.164 | 2.890 | 1.349 | 4.070 | 0.00 | |
| TSB | 1.206 | 1.186 | 2.370 | 1.365 | 3.643 | 0.13 | |
| 4d | 1.346 | 1.272 | 1.487 | 1.533 | 1.515 | ||
| Reaction | Structure | Interatomic Distances [Å] | GEDT [e] | ||||
|---|---|---|---|---|---|---|---|
| O1–N2 | N2–C3 | C3–C4 | C4–C5 | C5–O1 | |||
| 1+2a | 1 | 1.183 | 1.158 | ||||
| 2a | 1.333 | ||||||
| MCA | 1.180 | 1.161 | 3.308 | 1.333 | 3.334 | 0.00 | |
| TSA | 1.195 | 1.214 | 2.369 | 1.364 | 2.458 | 0.18 | |
| 3a | 1.346 | 1.271 | 1.509 | 1.547 | 1.459 | ||
| MCB | 1.181 | 1.164 | 3.416 | 1.333 | 4.015 | 0.00 | |
| TSB | 1.198 | 1.197 | 2.283 | 1.357 | 2.995 | 0.15 | |
| 4a | 1.342 | 1.272 | 1.485 | 1.543 | 1.495 | ||
| 1+2b | 2b | 1.330 | |||||
| MCA | 1.181 | 1.164 | 3.586 | 1.331 | 3.602 | 0.00 | |
| TSA | 1.196 | 1.212 | 2.352 | 1.360 | 2.484 | 0.21 | |
| 3b | 1.354 | 1.272 | 1.502 | 1.535 | 1.455 | ||
| MCB | 1.181 | 1.164 | 3.529 | 1.331 | 3.711 | 0.00 | |
| TSB | 1.201 | 1.196 | 2.271 | 1.353 | 3.166 | 0.16 | |
| 4b | 1.341 | 1.272 | 1.486 | 1.534 | 1.495 | ||
| 1+2c | 2c | 1.330 | |||||
| MCA | 1.180 | 1.166 | 3.813 | 1.332 | 4.933 | 0.00 | |
| TSA | 1.200 | 1.216 | 2.283 | 1.364 | 2.513 | 0.13 | |
| 3c | 1.346 | 1.273 | 1.510 | 1.531 | 1.457 | ||
| MCB | 1.182 | 1.161 | 3.417 | 1.330 | 3.381 | 0.00 | |
| TSB | 1.200 | 1.190 | 2.361 | 1.351 | 3.146 | 0.15 | |
| 4c | 1.357 | 1.270 | 1.487 | 1.525 | 1.475 | ||
| 1+2d | 2d | 1.343 | |||||
| MCA | 1.186 | 1.153 | 3.290 | 1.348 | 4.536 | 0.00 | |
| TSA | 1.202 | 1.226 | 2.756 | 1.375 | 2.257 | 0.25 | |
| 3a | 1.349 | 1.273 | 1.521 | 1.532 | 1.457 | ||
| MCB | 1.191 | 1.164 | 2.892 | 1.350 | 4.082 | 0.00 | |
| TSB | 1.205 | 1.179 | 2.443 | 1.363 | 3.737 | 0.23 | |
| 4d | 1.343 | 1.273 | 1.486 | 1.533 | 1.526 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresler, E.; Wróblewska, A.; Jasiński, R. Energetic Aspects and Molecular Mechanism of 3-Nitro-substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study. Molecules 2024, 29, 3042. https://doi.org/10.3390/molecules29133042
Dresler E, Wróblewska A, Jasiński R. Energetic Aspects and Molecular Mechanism of 3-Nitro-substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study. Molecules. 2024; 29(13):3042. https://doi.org/10.3390/molecules29133042
Chicago/Turabian StyleDresler, Ewa, Aneta Wróblewska, and Radomir Jasiński. 2024. "Energetic Aspects and Molecular Mechanism of 3-Nitro-substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study" Molecules 29, no. 13: 3042. https://doi.org/10.3390/molecules29133042
APA StyleDresler, E., Wróblewska, A., & Jasiński, R. (2024). Energetic Aspects and Molecular Mechanism of 3-Nitro-substituted 2-Isoxazolines Formation via Nitrile N-Oxide [3+2] Cycloaddition: An MEDT Computational Study. Molecules, 29(13), 3042. https://doi.org/10.3390/molecules29133042









