Synthesis and Electrochemical Study of Gold(I) Carbene Complexes
Abstract
1. Introduction
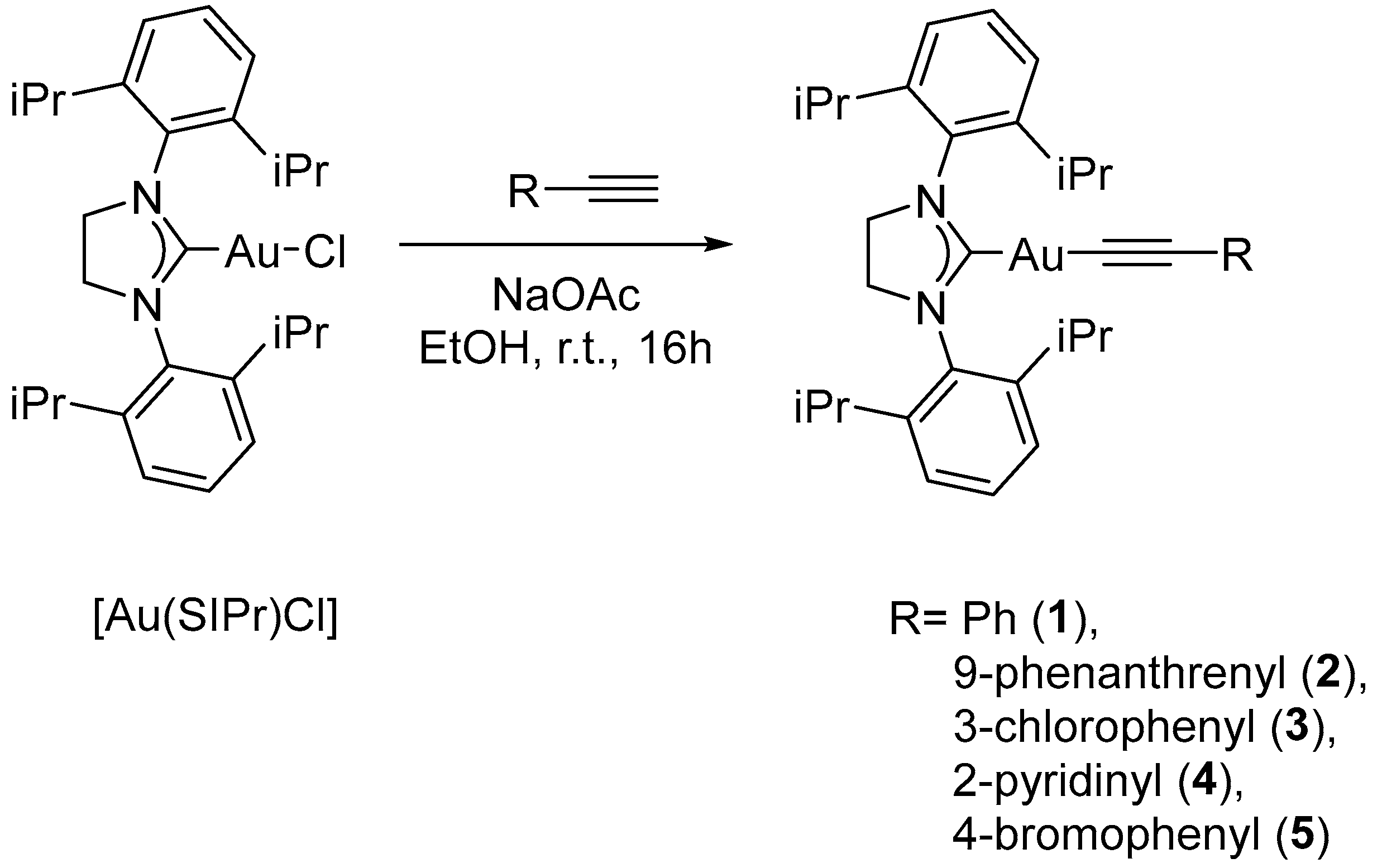
2. Results and Discussion
2.1. X-ray Structure Analyses
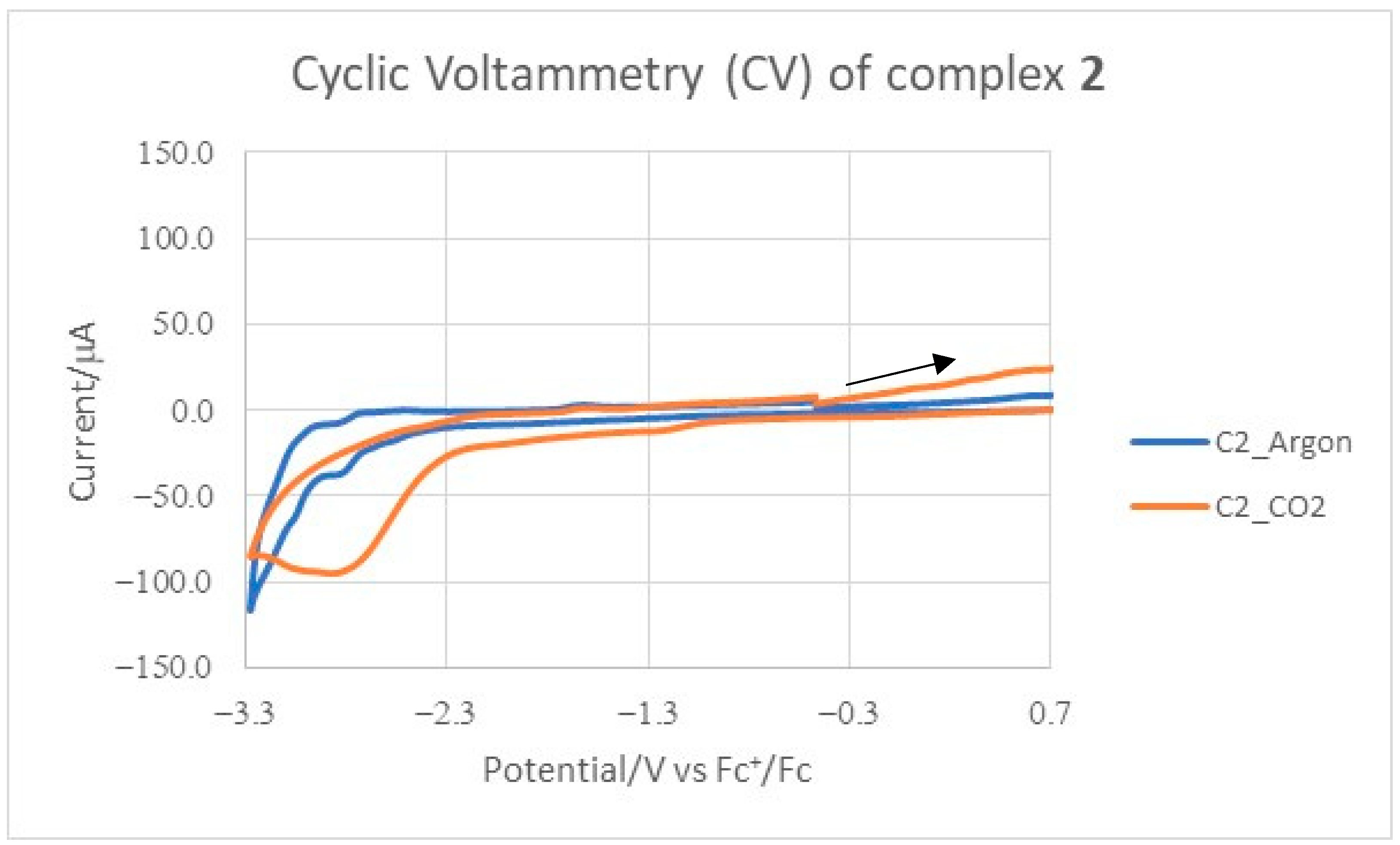
2.2. Electrochemical Measurements
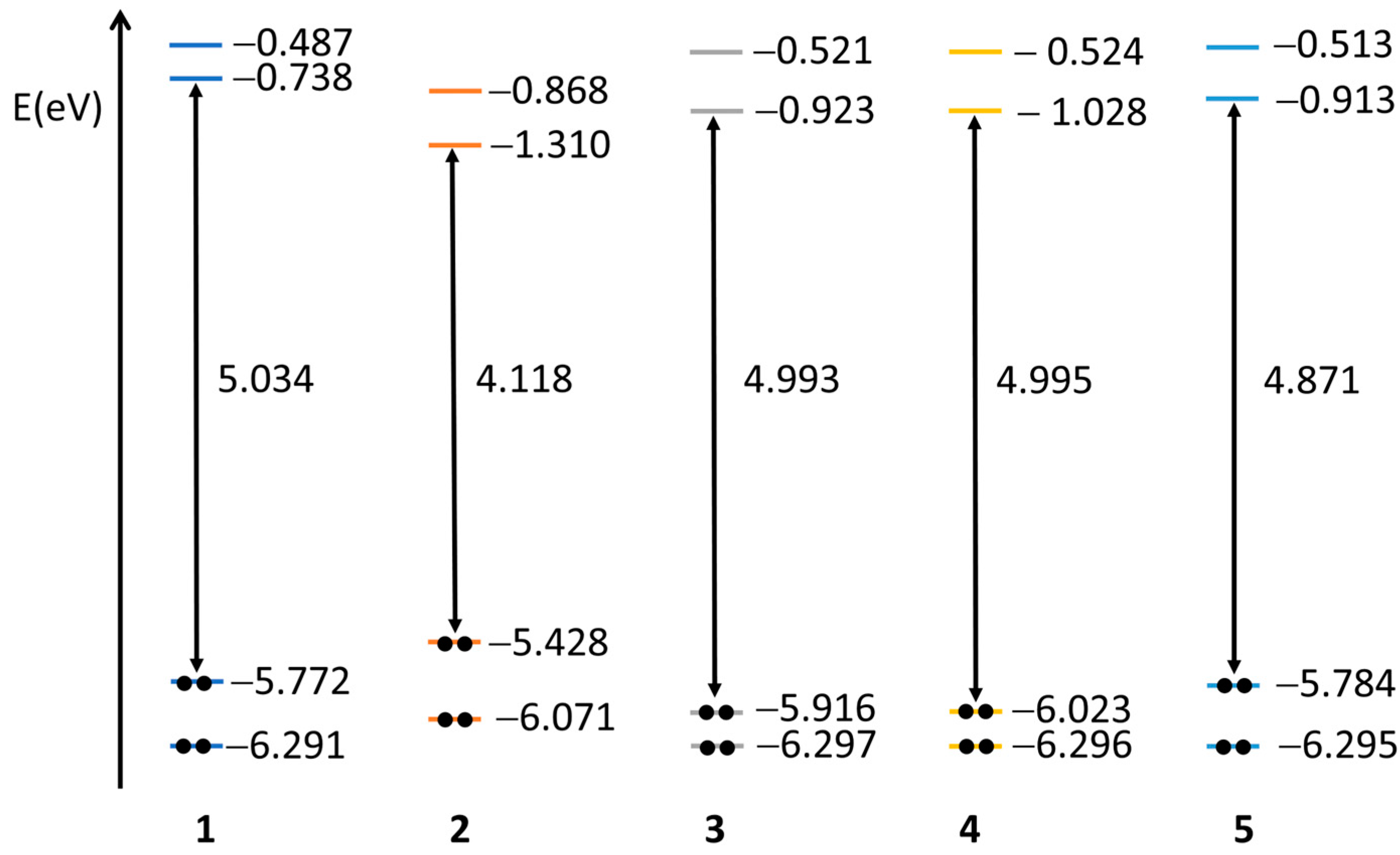
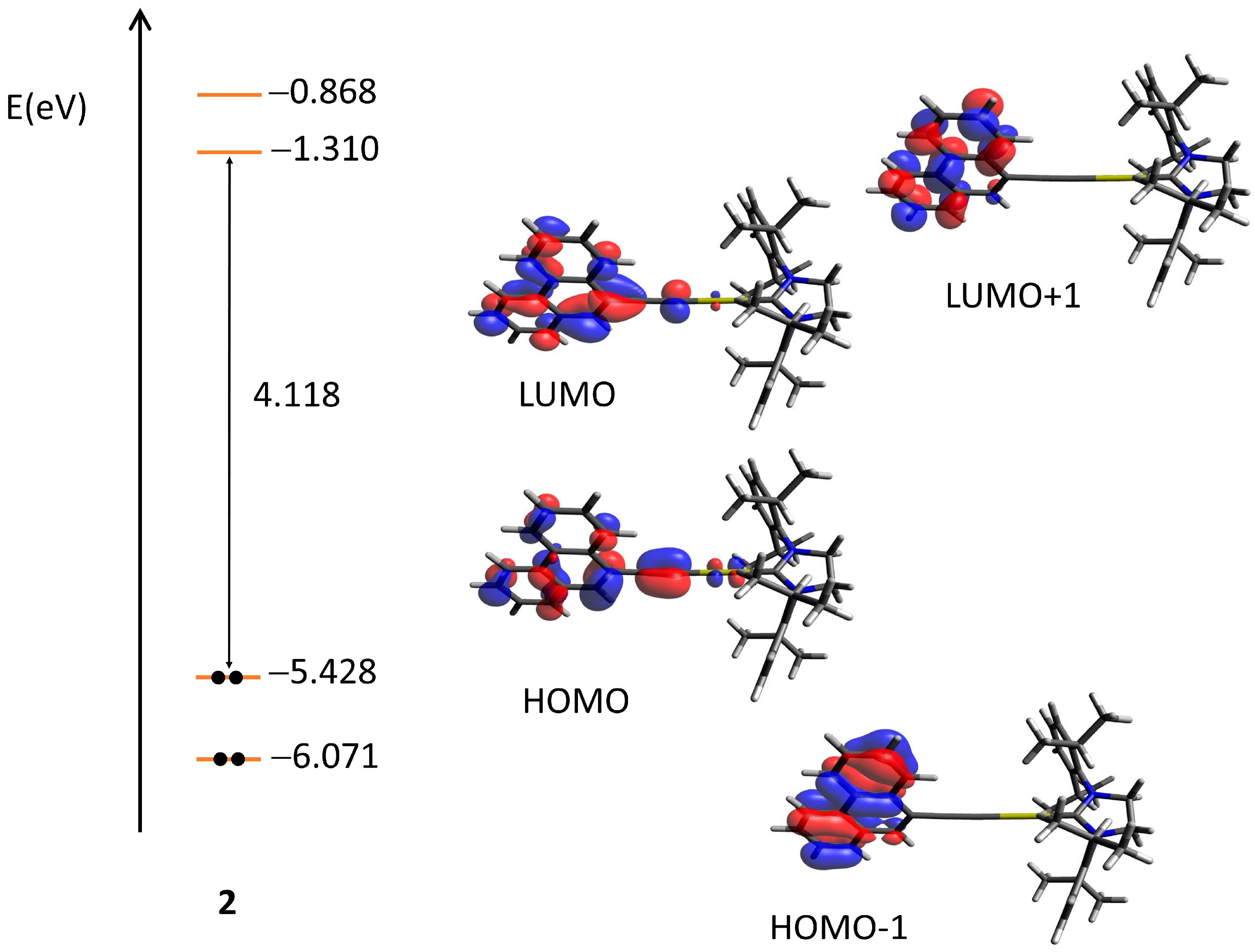
2.3. Quantum Chemical Calculations
3. Materials and Methods
3.1. Electrochemical Measurements
3.2. Quantum Chemical Calculations
3.3. X-ray Crystallography
3.4. Synthesis of the Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulks, F.F. Gold Carbene Complexes and beyond: New Avenues in Gold(I)—Carbon Coordination Chemistry. Gold Bull. 2022, 55, 1–13. [Google Scholar] [CrossRef]
- Lin, I.J.; Vasam, C.S. Review of Gold(I) N−Heterocyclic Carbenes. Can. J. Chem. 2005, 83, 812–825. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent Advances in Gold–NHC Complexes with Biological Properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent Progress in Silver(I)-, Gold(I)/(III)- and Palladium(II)-N-Heterocyclic Carbene Complexes: A Review towards Biological Perspectives. J. Organomet. Chem. 2019, 882, 96–111. [Google Scholar] [CrossRef]
- Mertens, R.T.; Gukathasan, S.; Arojojoye, A.S.; Olelewe, C.; Awuah, S.G. Next Generation Gold Drugs and Probes: Chemistry and Biomedical Applications. Chem. Rev. 2023, 123, 6612–6667. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Yin, Y.-K.; Zhang, H.; Han, Y.-F. Metal N-Heterocyclic Carbene Complexes as Potential Metallodrugs in Antitumor Therapy. Coord. Chem. Rev. 2024, 514, 215941. [Google Scholar] [CrossRef]
- Tialiou, A.; Chin, J.; Keppler, B.K.; Reithofer, M.R. Current Developments of N-Heterocyclic Carbene Au(I)/Au(III) Complexes toward Cancer Treatment. Biomedicines 2022, 10, 1417. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent Development of Gold(I) and Gold(III) Complexes as Therapeutic Agents for Cancer Diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef]
- Yeo, C.I.; Goh, C.H.P.; Tiekink, E.R.T.; Chew, J. Antibiotics: A “GOLDen” Promise? Coord. Chem. Rev. 2024, 500, 215429. [Google Scholar] [CrossRef]
- Scattolin, T.; Tonon, G.; Botter, E.; Guillet, S.G.; Tzouras, N.V.; Nolan, S.P. Gold(I)- N -Heterocyclic Carbene Synthons in Organometallic Synthesis. Chem. Eur. J. 2023, 29, e202301961. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Gold Catalysis. Chem. Soc. Rev. 2008, 37, 1776. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.B.; Urvashi; Patil, N.T. Asymmetric Gold Catalysis Enabled by Specially Designed Ligands. Org. Chem. Front. 2024, 11, 1858–1895. [Google Scholar] [CrossRef]
- Michalak, M.; Kośnik, W. Chiral N-Heterocyclic Carbene Gold Complexes: Synthesis and Applications in Catalysis. Catalysts 2019, 9, 890. [Google Scholar] [CrossRef]
- Jiang, J.; Wong, M. Recent Advances in the Development of Chiral Gold Complexes for Catalytic Asymmetric Catalysis. Chem.An Asian J. 2021, 16, 364–377. [Google Scholar] [CrossRef]
- Mato, M.; Franchino, A.; García-Morales, C.; Echavarren, A.M. Gold-Catalyzed Synthesis of Small Rings. Chem. Rev. 2021, 121, 8613–8684. [Google Scholar] [CrossRef]
- Praveen, C.; Dupeux, A.; Michelet, V. Catalytic Gold Chemistry: From Simple Salts to Complexes for Regioselective C–H Bond Functionalization. Chem. A Eur. J. 2021, 27, 10495–10532. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic Carbene Complexes in C–H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Nijamudheen, A.; Datta, A. Gold-Catalyzed Cross-Coupling Reactions: An Overview of Design Strategies, Mechanistic Studies, and Applications. Chem. Eur. J. 2020, 26, 1442–1487. [Google Scholar] [CrossRef]
- Gao, P.; Szostak, M. Hydration Reactions Catalyzed by Transition Metal–NHC (NHC = N-Heterocyclic Carbene) Complexes. Coord. Chem. Rev. 2023, 485, 215110. [Google Scholar] [CrossRef]
- Crochet, P.; Cadierno, V. Gold Complexes with Hydrophilic N-Heterocyclic Carbene Ligands and Their Contribution to Aqueous-Phase Catalysis. Catalysts 2023, 13, 436. [Google Scholar] [CrossRef]
- Dang, H.; Guan, B.; Chen, J.; Ma, Z.; Chen, Y.; Zhang, J.; Guo, Z.; Chen, L.; Hu, J.; Yi, C.; et al. Research Status, Challenges, and Future Prospects of Carbon Dioxide Reduction Technology. Energy Fuels 2024, 38, 4836–4880. [Google Scholar] [CrossRef]
- Almomani, F.; Abdelbar, A.; Ghanimeh, S. A Review of the Recent Advancement of Bioconversion of Carbon Dioxide to Added Value Products: A State of the Art. Sustainability 2023, 15, 10438. [Google Scholar] [CrossRef]
- Akash, S.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Biotechnology to Convert Carbon Dioxide into Biogas, Bioethanol, Bioplastic and Succinic Acid Using Algae, Bacteria and Yeast: A Review. Environ. Chem. Lett. 2023, 21, 1477–1497. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Abu-Reesh, I.M.; Mohanakrishna, G.; Bulut, M.; Pant, D. Advanced Routes of Biological and Bio-Electrocatalytic Carbon Dioxide (CO2) Mitigation Toward Carbon Neutrality. Front. Energy Res. 2020, 8, 94. [Google Scholar] [CrossRef]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef]
- Hakami, O. Thermocatalytic and Solar Thermochemical Carbon Dioxide Utilization to Solar Fuels and Chemicals: A Review. Int. J. Energy Res. 2022, 46, 19929–19960. [Google Scholar] [CrossRef]
- Abanades, S. Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review. Energies 2022, 15, 7061. [Google Scholar] [CrossRef]
- Wei, L.; Pan, Z.; Shi, X.; Esan, O.C.; Li, G.; Qi, H.; Wu, Q.; An, L. Solar-Driven Thermochemical Conversion of H2O and CO2 into Sustainable Fuels. iScience 2023, 26, 108127. [Google Scholar] [CrossRef]
- Ulmer, U.; Dingle, T.; Duchesne, P.N.; Morris, R.H.; Tavasoli, A.; Wood, T.; Ozin, G.A. Fundamentals and Applications of Photocatalytic CO2 Methanation. Nat. Commun. 2019, 10, 3169. [Google Scholar] [CrossRef]
- Canivet, J.; Wisser, F.M. Metal–Organic Framework Catalysts for Solar Fuels: Light-Driven Conversion of Carbon Dioxide into Formic Acid. ACS Appl. Energy Mater. 2023, 6, 9027–9043. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Senthil Kumar, P.; Vo, D.-V.N.; Hoang, T.K. A Review of Recent Progress on Photocatalytic Carbon Dioxide Reduction into Sustainable Energy Products Using Carbon Nitride. Chem. Eng. Res. Des. 2022, 177, 304–320. [Google Scholar] [CrossRef]
- Van Le, Q.; Nguyen, V.-H.; Nguyen, T.D.; Sharma, A.; Rahman, G.; Nguyen, D.L.T. Light-Driven Reduction of Carbon Dioxide: Altering the Reaction Pathways and Designing Photocatalysts toward Value-Added and Renewable Fuels. Chem. Eng. Sci. 2021, 237, 116547. [Google Scholar] [CrossRef]
- Han, J.; Bai, X.; Xu, X.; Bai, X.; Husile, A.; Zhang, S.; Qi, L.; Guan, J. Advances and Challenges in the Electrochemical Reduction of Carbon Dioxide. Chem. Sci. 2024, 15, 7870–7907. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.-X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic Carbon Dioxide Reduction: From Fundamental Principles to Catalyst Design. Mater. Today Adv. 2020, 7, 100074. [Google Scholar] [CrossRef]
- Liu, B.; Wei, Z.; Ding, J.; Wang, Z.; Wang, A.; Zhang, L.; Liu, Y.; Guo, Y.; Yang, X.; Zhai, Y. Enhanced Electrochemical CO2 Reduction to Formate over Phosphate-Modified In: Water Activation and Active Site Tuning. Angew. Chemie Int. Ed. 2024, 136, e202402070. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Liu, Z. Recent Advances in Electrochemical Reduction of CO2. Curr. Opin. Green Sustain. Chem. 2019, 16, 77–84. [Google Scholar] [CrossRef]
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial Photosynthesis for Sustainable Fuel and Chemical Production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. [Google Scholar] [CrossRef]
- Al-Tamreh, S.A.; Ibrahim, M.H.; El-Naas, M.H.; Vaes, J.; Pant, D.; Benamor, A.; Amhamed, A. Electroreduction of Carbon Dioxide into Formate: A Comprehensive Review. ChemElectroChem 2021, 8, 3207–3220. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Park, K.T.; Lee, W.; Lim, H.; Kwon, Y.; Kang, S. Current Achievements and the Future Direction of Electrochemical CO2 Reduction: A Short Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 769–815. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, W.; Hu, F.X.; Yang, H.; Liu, B. Recent Advances in Single Atom Catalysts for the Electrochemical Carbon Dioxide Reduction Reaction. Chem. Sci. 2021, 12, 6800–6819. [Google Scholar] [CrossRef]
- Duarah, P.; Haldar, D.; Yadav, V.; Purkait, M.K. Progress in the Electrochemical Reduction of CO2 to Formic Acid: A Review on Current Trends and Future Prospects. J. Environ. Chem. Eng. 2021, 9, 106394. [Google Scholar] [CrossRef]
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Feng, D.-M.; Zhu, Y.-P.; Chen, P.; Ma, T.-Y. Recent Advances in Transition-Metal-Mediated Electrocatalytic CO2 Reduction: From Homogeneous to Heterogeneous Systems. Catalysts 2017, 7, 373. [Google Scholar] [CrossRef]
- Kinzel, N.W.; Werlé, C.; Leitner, W. Transition Metal Complexes as Catalysts for the Electroconversion of CO2: An Organometallic Perspective. Angew. Chem. Int. Ed. 2021, 60, 11628–11686. [Google Scholar] [CrossRef]
- Boogaerts, I.I.F.; Nolan, S.P. Carboxylation of C−H Bonds Using N -Heterocyclic Carbene Gold(I) Complexes. J. Am. Chem. Soc. 2010, 132, 8858–8859. [Google Scholar] [CrossRef]
- D’Amato, A.; Sirignano, M.; Russo, S.; Troiano, R.; Mariconda, A.; Longo, P. Recent Advances in N-Heterocyclic Carbene Coinage Metal Complexes in A3-Coupling and Carboxylation Reaction. Catalysts 2023, 13, 811. [Google Scholar] [CrossRef]
- Hase, S.; Kayaki, Y.; Ikariya, T. NHC–Gold(I) Complexes as Effective Catalysts for the Carboxylative Cyclization of Propargylamines with Carbon Dioxide. Organometallics 2013, 32, 5285–5288. [Google Scholar] [CrossRef]
- Wen, D.; Zheng, Q.; Yang, S.; Zhu, H.; Tu, T. Direct Knitting Boosts the Stability and Catalytic Activity of NHC-Au Complexes towards Valorization of SO2 and CO2. J. Catal. 2023, 418, 64–69. [Google Scholar] [CrossRef]
- Collado, A.; Gómez-Suárez, A.; Webb, P.B.; Kruger, H.; Bühl, M.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P. Trapping Atmospheric CO2 with Gold. Chem. Commun. 2014, 50, 11321–11324. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, M.; Ren, Y.; Ma, A.; Yin, Z.; Ma, X.; Wang, S. The Smallest Superatom Au4(PPh3)4I2 with Two Free Electrons: Synthesis, Structure Analysis, and Electrocatalytic Conversion of CO2 to CO. Nanoscale Adv. 2023, 5, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, T.I.; Yi, H.; Aloisio, M.D.; Dang, N.K.; Gao, G.; Sharma, S.; Dinh, C.-T.; Crudden, C.M. Electrocatalytic CO2 Reduction with Atomically Precise Au13 Nanoclusters: Effect of Ligand Shell on Catalytic Performance. ACS Catal. 2024, 14, 4155–4163. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Xiao, K.; Zhu, Y.; Chen, M. Active-Site Tailoring of Gold Cluster Catalysts for Electrochemical CO2 Reduction. ACS Catal. 2021, 11, 11551–11560. [Google Scholar] [CrossRef]
- Gao, Z.-H.; Wei, K.; Wu, T.; Dong, J.; Jiang, D.; Sun, S.; Wang, L.-S. A Heteroleptic Gold Hydride Nanocluster for Efficient and Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2022, 144, 5258–5262. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Shi, Y.; Li, H.; Shan, J.; Sun, C.; Wu, Z.; Ji, Y.; Wang, Z. Efficient CO2 Electroreduction via Au-Complex Derived Carbon Nanotube Supported Au Nanoclusters. ChemSusChem 2021, 14, 4929–4935. [Google Scholar] [CrossRef]
- Seong, H.; Efremov, V.; Park, G.; Kim, H.; Yoo, J.S.; Lee, D. Atomically Precise Gold Nanoclusters as Model Catalysts for Identifying Active Sites for Electroreduction of CO2. Angew. Chem. Int. Ed. 2021, 60, 14563–14570. [Google Scholar] [CrossRef]
- Tang, L.; Luo, Y.; Ma, X.; Wang, B.; Ding, M.; Wang, R.; Wang, P.; Pei, Y.; Wang, S. Poly-Hydride [AuI7(PPh3)7H5](SbF6)2 Cluster Complex: Structure, Transformation, and Electrocatalytic CO2 Reduction Properties. Angew. Chem. Int. Ed. 2023, 62, e202402070. [Google Scholar] [CrossRef]
- Albright, E.L.; Malola, S.; Jacob, S.I.; Yi, H.; Takano, S.; Mimura, K.; Tsukuda, T.; Häkkinen, H.; Nambo, M.; Crudden, C.M. Enantiopure Chiral Au13 Nanoclusters Stabilized by Ditopic N -Heterocyclic Carbenes: Synthesis, Characterization, and Electrocatalytic Reduction of CO2. Chem. Mater. 2024, 36, 1279–1289. [Google Scholar] [CrossRef]
- Scattolin, T.; Tzouras, N.V.; Falivene, L.; Cavallo, L.; Nolan, S.P. Using Sodium Acetate for the Synthesis of [Au(NHC)X] Complexes. Dalt. Trans. 2020, 49, 9694–9700. [Google Scholar] [CrossRef]
- Danovich, D.; Shaik, S.; Neese, F.; Echeverría, J.; Aullón, G.; Alvarez, S. Understanding the Nature of the CH···HC Interactions in Alkanes. J. Chem. Theory Comput. 2013, 9, 1977–1991. [Google Scholar] [CrossRef]
- Hamze, R.; Shi, S.; Kapper, S.C.; Muthiah Ravinson, D.S.; Estergreen, L.; Jung, M.-C.; Tadle, A.C.; Haiges, R.; Djurovich, P.I.; Peltier, J.L.; et al. “Quick-Silver” from a Systematic Study of Highly Luminescent, Two-Coordinate, d10 Coinage Metal Complexes. J. Am. Chem. Soc. 2019, 141, 8616–8626. [Google Scholar] [CrossRef]
- Hamze, R.; Idris, M.; Muthiah Ravinson, D.S.; Jung, M.C.; Haiges, R.; Djurovich, P.I.; Thompson, M.E. Highly Efficient Deep Blue Luminescence of 2-Coordinate Coinage Metal Complexes Bearing Bulky NHC Benzimidazolyl Carbene. Front. Chem. 2020, 8, 401. [Google Scholar] [CrossRef]
- Ruiz-Zambrana, C.; Poyatos, M.; Peris, E. A Redox-Switchable Gold(I) Complex for the Hydroamination of Acetylenes: A Convenient Way for Studying Ligand-Derived Electronic Effects. ACS Catal. 2022, 12, 4465–4472. [Google Scholar] [CrossRef]
- Huynh, H.V.; Guo, S.; Wu, W. Detailed Structural, Spectroscopic, and Electrochemical Trends of Halido Mono- and Bis(NHC) Complexes of Au(I) and Au(III). Organometallics 2013, 32, 4591–4600. [Google Scholar] [CrossRef]
- McCall, R.; Miles, M.; Lascuna, P.; Burney, B.; Patel, Z.; Sidoran, K.J.; Sittaramane, V.; Kocerha, J.; Grossie, D.A.; Sessler, J.L.; et al. Dual Targeting of the Cancer Antioxidant Network with 1,4-Naphthoquinone Fused Gold(I) N-Heterocyclic Carbene Complexes. Chem. Sci. 2017, 8, 5918–5929. [Google Scholar] [CrossRef]
- Klenk, S.; Rupf, S.; Suntrup, L.; van der Meer, M.; Sarkar, B. The Power of Ferrocene, Mesoionic Carbenes, and Gold: Redox-Switchable Catalysis. Organometallics 2017, 36, 2026–2035. [Google Scholar] [CrossRef]
- Pažický, M.; Loos, A.; Ferreira, M.J.; Serra, D.; Vinokurov, N.; Rominger, F.; Jäkel, C.; Hashmi, A.S.K.; Limbach, M. Synthesis, Reactivity, and Electrochemical Studies of Gold(I) and Gold(III) Complexes Supported by N-Heterocyclic Carbenes and Their Application in Catalysis. Organometallics 2010, 29, 4448–4458. [Google Scholar] [CrossRef]
- Méndez-Hernández, D.D.; Tarakeshwar, P.; Gust, D.; Moore, T.A.; Moore, A.L.; Mujica, V. Simple and Accurate Correlation of Experimental Redox Potentials and DFT-Calculated HOMO/LUMO Energies of Polycyclic Aromatic Hydrocarbons. J. Mol. Model. 2013, 19, 2845–2848. [Google Scholar] [CrossRef]
- Conradie, J. A Frontier Orbital Energy Approach to Redox Potentials. J. Phys. Conf. Ser. 2015, 633, 012045. [Google Scholar] [CrossRef]
- Athanasopoulos, E.; Conradie, J. Substituent Effect on the Oxidation and Reduction of Electronically Altered Iron(II)-Terpyridine Derivatives–DFT Study. Inorg. Chim. Acta 2023, 555, 121599. [Google Scholar] [CrossRef]
- Chiyindiko, E.; Langner, E.H.G.; Conradie, J. Electrochemical Behaviour of Copper(II) Complexes Containing 2-Hydroxyphenones. Electrochim. Acta 2022, 424, 140629. [Google Scholar] [CrossRef]
- Liyanage, N.P.; Dulaney, H.A.; Huckaba, A.J.; Jurss, J.W.; Delcamp, J.H. Electrocatalytic Reduction of CO2 to CO With Re-Pyridyl-NHCs: Proton Source Influence on Rates and Product Selectivities. Inorg. Chem. 2016, 55, 6085–6094. [Google Scholar] [CrossRef]
- Stanton, C.J.; Machan, C.W.; Vandezande, J.E.; Jin, T.; Majetich, G.F.; Schaefer, H.F.; Kubiak, C.P.; Li, G.; Agarwal, J. Re(I) NHC Complexes for Electrocatalytic Conversion of CO2. Inorg. Chem. 2016, 55, 3136–3144. [Google Scholar] [CrossRef]
- Agarwal, J.; Shaw, T.W.; Stanton, C.J.; Majetich, G.F.; Bocarsly, A.B.; Schaefer, H.F. NHC-Containing Manganese(I) Electrocatalysts for the Two-Electron Reduction of CO2. Angew. Chem. Int. Ed. 2014, 53, 5152–5155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Chang, X.; Zhang, Y.-Q.; Liao, R.-Z.; Duan, L. Highly Active Manganese-Based CO2 Reduction Catalysts with Bulky NHC Ligands: A Mechanistic Study. Inorg. Chem. 2020, 59, 10234–10242. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Franco, F.; Martínez Belmonte, M.; Friães, S.; Royo, B.; Luis, J.M.; Lloret-Fillol, J. Decoding the CO2 Reduction Mechanism of a Highly Active Organometallic Manganese Electrocatalyst: Direct Observation of a Hydride Intermediate and Its Implications. ACS Catal. 2023, 13, 10375–10385. [Google Scholar] [CrossRef]
- Gonell, S.; Lloret-Fillol, J.; Miller, A.J.M. An Iron Pyridyl-Carbene Electrocatalyst for Low Overpotential CO2 Reduction to CO. ACS Catal. 2021, 11, 615–626. [Google Scholar] [CrossRef]
- Gonell, S.; Assaf, E.A.; Lloret-Fillol, J.; Miller, A.J.M. An Iron Bis(Carbene) Catalyst for Low Overpotential CO2 Electroreduction to CO: Mechanistic Insights from Kinetic Zone Diagrams, Spectroscopy, and Theory. ACS Catal. 2021, 11, 15212–15222. [Google Scholar] [CrossRef]
- Gonell, S.; Assaf, E.A.; Duffee, K.D.; Schauer, C.K.; Miller, A.J.M. Kinetics of the Trans Effect in Ruthenium Complexes Provide Insight into the Factors That Control Activity and Stability in CO2 Electroreduction. J. Am. Chem. Soc. 2020, 142, 8980–8999. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Nayak, A.; Wang, Y.; Shan, B.; Quan, X.; Meyer, T.J. Steering CO2 Electroreduction toward Ethanol Production by a Surface-Bound Ru Polypyridyl Carbene Catalyst on N-Doped Porous Carbon. Proc. Natl. Acad. Sci. USA 2019, 116, 26353–26358. [Google Scholar] [CrossRef]
- Shirley, H.; Figgins, M.T.; Boudreaux, C.M.; Liyanage, N.P.; Lamb, R.W.; Webster, C.E.; Papish, E.T.; Delcamp, J.H. Impact of the Dissolved Anion on the Electrocatalytic Reduction of CO2 to CO with Ruthenium CNC Pincer Complexes. ChemCatChem 2020, 12, 4879–4885. [Google Scholar] [CrossRef]
- Mudge, M.N.; Bhadbhade, M.; Ball, G.E.; Colbran, S.B. Ruthenium(II) Complexes of a Xanthene-Spanned Dicarbene Ligand. Inorg. Chem. 2023, 62, 18901–18914. [Google Scholar] [CrossRef]
- Assaf, E.A.; Gonell, S.; Chen, C.-H.; Miller, A.J.M. Accessing and Photo-Accelerating Low-Overpotential Pathways for CO2 Reduction: A Bis-Carbene Ruthenium Terpyridine Catalyst. ACS Catal. 2022, 12, 12596–12606. [Google Scholar] [CrossRef]
- Su, X.; McCardle, K.M.; Chen, L.; Panetier, J.A.; Jurss, J.W. Robust and Selective Cobalt Catalysts Bearing Redox-Active Bipyridyl-N-Heterocyclic Carbene Frameworks for Electrochemical CO2 Reduction in Aqueous Solutions. ACS Catal. 2019, 9, 7398–7408. [Google Scholar] [CrossRef]
- Su, X.; McCardle, K.M.; Panetier, J.A.; Jurss, J.W. Electrocatalytic CO2 Reduction with Nickel Complexes Supported by Tunable Bipyridyl-N-Heterocyclic Carbene Donors: Understanding Redox-Active Macrocycles. Chem. Commun. 2018, 54, 3351–3354. [Google Scholar] [CrossRef]
- DeLuca, E.E.; Xu, Z.; Lam, J.; Wolf, M.O. Improved Electrocatalytic CO2 Reduction with Palladium Bis(NHC) Pincer Complexes Bearing Cationic Side Chains. Organometallics 2019, 38, 1330–1343. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for K to Au Including the Outermost Core Orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XXIII. A Polarization-Type Basis Set for Second-Row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- SAINT+. Area-Detector Integration Program; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- SADABS-2016/2; Bruker AXS Inc.: Madison, WI, USA, 2016.
- SHELXTL-NT; Structure Determination Package. Bruker AXS Inc.: Madison, WI, USA, 2001.
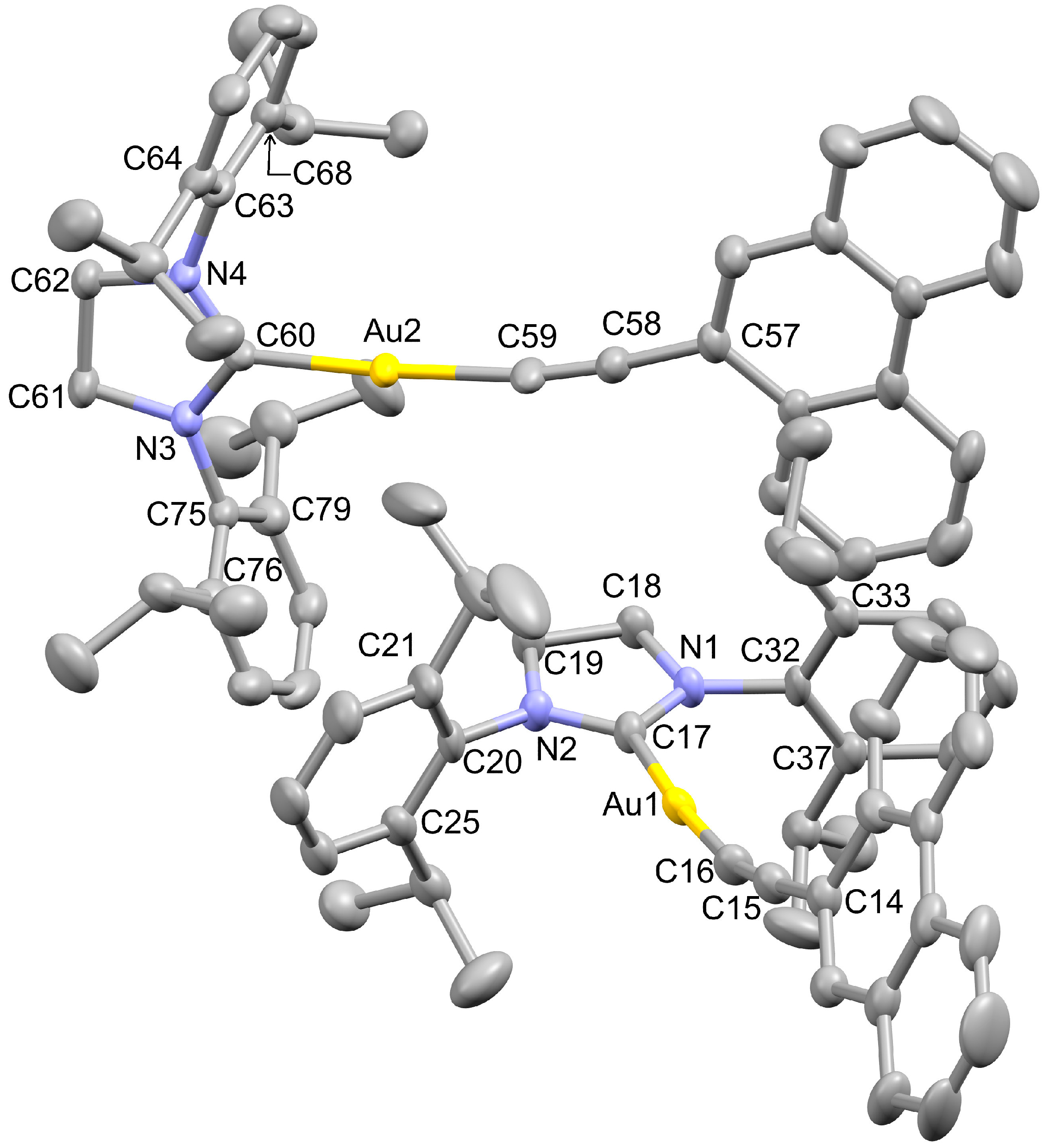
| Empirical Formula | C43H47AuN2 | Formula Weight | 788.79 |
|---|---|---|---|
| temperature/K | 240 | crystal system | orthorhombic |
| space group | P212121 | ||
| a/Å | 13.1684 (4) | α/deg | 90 |
| b/Å | 16.4356 (5) | β/deg | 90 |
| c/Å | 38.3544 (12) | γ/deg | 90 |
| volume/Å3 | 8301.1 (4) | Z | 8 |
| ρcalc g/cm3 | 1.262 | μ/mm−1 | 6.856 |
| F (000) | 3184.0 | radiation | Cu Kα (λ = 1.54178) |
| 2Θ range for data collection/deg | 4.07 to 72.23 | index ranges | −15 ≤ h ≤ 16−20 ≤ k ≤ 20−47 ≤ l ≤ 45 |
| reflections collected | 16064 | goodness-of-fit on F2 | 1.035 |
| final R indexes [I ≥ 2σ (I)] | R1 = 0.0288 | final [all data] | R1 = 0.0328 |
| wR2 = 0.0700 | wR2 = 0.0730 | ||
| largest diff. peak/hole/e Å−3 | 0.732/−0.584 | CCDC number | 2,363,526 |
| XRD | DFT | XRD | DFT | ||
|---|---|---|---|---|---|
| Au2–C60 | 2.036 (6) | 2.045 | Au2–C59 | 2.003 (7) | 2.008 |
| Au1–C17 | 2.033 (6) | 2.045 | Au1–C16 | 1.983 (6) | 2.008 |
| N3–C60 | 1.311 (8) | 1.336 | N3–C61 | 1.471 (8) | 1.479 |
| N2–C17 | 1.329 (8) | 1.337 | N2–C19 | 1.469 (8) | 1.479 |
| N4–C60 | 1.331 (8) | 1.336 | N4–C62 | 1.463 (8) | 1.479 |
| N1–C17 | 1.320 (8) | 1.337 | N1–C18 | 1.470 (8) | 1.479 |
| C61–C62 | 1.527 (10) | 1.542 | C14–C15 | 1.433 (9) | 1.426 |
| C16–C15 | 1.207 (9) | 1.228 | C19–C18 | 1.502 (10) | 1.539 |
| C57–C58 | 1.442 (9) | 1.426 | C58–C59 | 1.192 (9) | 1.228 |
| C59–Au2–C60 | 176.1 (3) | 179.1 | C16–Au1–C17 | 173.8 (3) | 179.0 |
| C60–N3–C61 | 113.4 (5) | 113.1 | C17–N2–C19 | 111.7 (5) | 112.8 |
| N3–C60–Au2 | 126.7 (4) | 125.0 | N3–C60–N4 | 108.7 (5) | 109.1 |
| N4–C60–Au2 | 124.5 (4) | 126.0 | C60–N4–C62 | 112.7 (5) | 113.0 |
| N2–C17–Au1 | 126.5 (5) | 125.7 | N1–C17–Au1 | 123.5 (5) | 125.3 |
| N1–C17–N2 | 109.5 (5) | 109.0 | C17–N1–C18 | 112.7 (5) | 112.8 |
| C58–C59–Au2 | 171.1 (6) | 178.6 | N3–C61–C62 | 101.6 (5) | 102.3 |
| C15–C16–Au1 | 168.2 (6) | 178.3 | N2–C19–C18 | 103.5 (5) | 102.2 |
| N4–C62–C61 | 102.2 (5) | 102.4 | N1–C18–C19 | 102.5 (5) | 102.2 |
| C60–N3–C75–C76 | 91.7 (8) | 87.7 | C60–N3–C75–C79 | 88.5 (8) | 91.6 |
| C60–N4–C63–C68 | 84.2 (8) | 87.2 | C60–N4–C63–C64 | 97.1 (8) | 92.1 |
| C17–N2–C20–C25 | 96.6 (8) | 95.7 | C17–N2–C20–C21 | 82.3 (9) | 83.9 |
| C17–N1–C32–C33 | 82.2 (9) | 96.9 | C17–N1–C32–C37 | 98.8 (8) | 82.6 |
| Compound | (V) Ar b | iAr(μA) c | (V) CO2d | iCO2(μA) e | |
|---|---|---|---|---|---|
| 1 | −2.99 | −92.74 | |||
| 2 | −2.87(ir) | −38.36 | −2.88 | −95.58 | 2.49 |
| 3 | −2.90(ir) | −29.11 | −3.04 | −56.50 | 1.94 |
| 4 | −2.91(ir) | −46.51 | −1.40/−3.00 | −35.5/−82.49 | 1.77 |
| 5 | −2.79(ir) | −26.46/ | −1.37/−3.04 | −18.49/−68.94 | 2.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rubio, A.; Yuste, Á.; Torroba, T.; García-Herbosa, G.; Cuevas-Vicario, J.V. Synthesis and Electrochemical Study of Gold(I) Carbene Complexes. Molecules 2024, 29, 4081. https://doi.org/10.3390/molecules29174081
Rodríguez-Rubio A, Yuste Á, Torroba T, García-Herbosa G, Cuevas-Vicario JV. Synthesis and Electrochemical Study of Gold(I) Carbene Complexes. Molecules. 2024; 29(17):4081. https://doi.org/10.3390/molecules29174081
Chicago/Turabian StyleRodríguez-Rubio, Andrea, Álvaro Yuste, Tomás Torroba, Gabriel García-Herbosa, and José V. Cuevas-Vicario. 2024. "Synthesis and Electrochemical Study of Gold(I) Carbene Complexes" Molecules 29, no. 17: 4081. https://doi.org/10.3390/molecules29174081
APA StyleRodríguez-Rubio, A., Yuste, Á., Torroba, T., García-Herbosa, G., & Cuevas-Vicario, J. V. (2024). Synthesis and Electrochemical Study of Gold(I) Carbene Complexes. Molecules, 29(17), 4081. https://doi.org/10.3390/molecules29174081











