Synthesis and Characterization of Transition Metal Complexes Supported by Phosphorus Ligands Obtained Using Hydrophosphination of Cyclic Internal Alkenes
Abstract
1. Introduction
2. Results
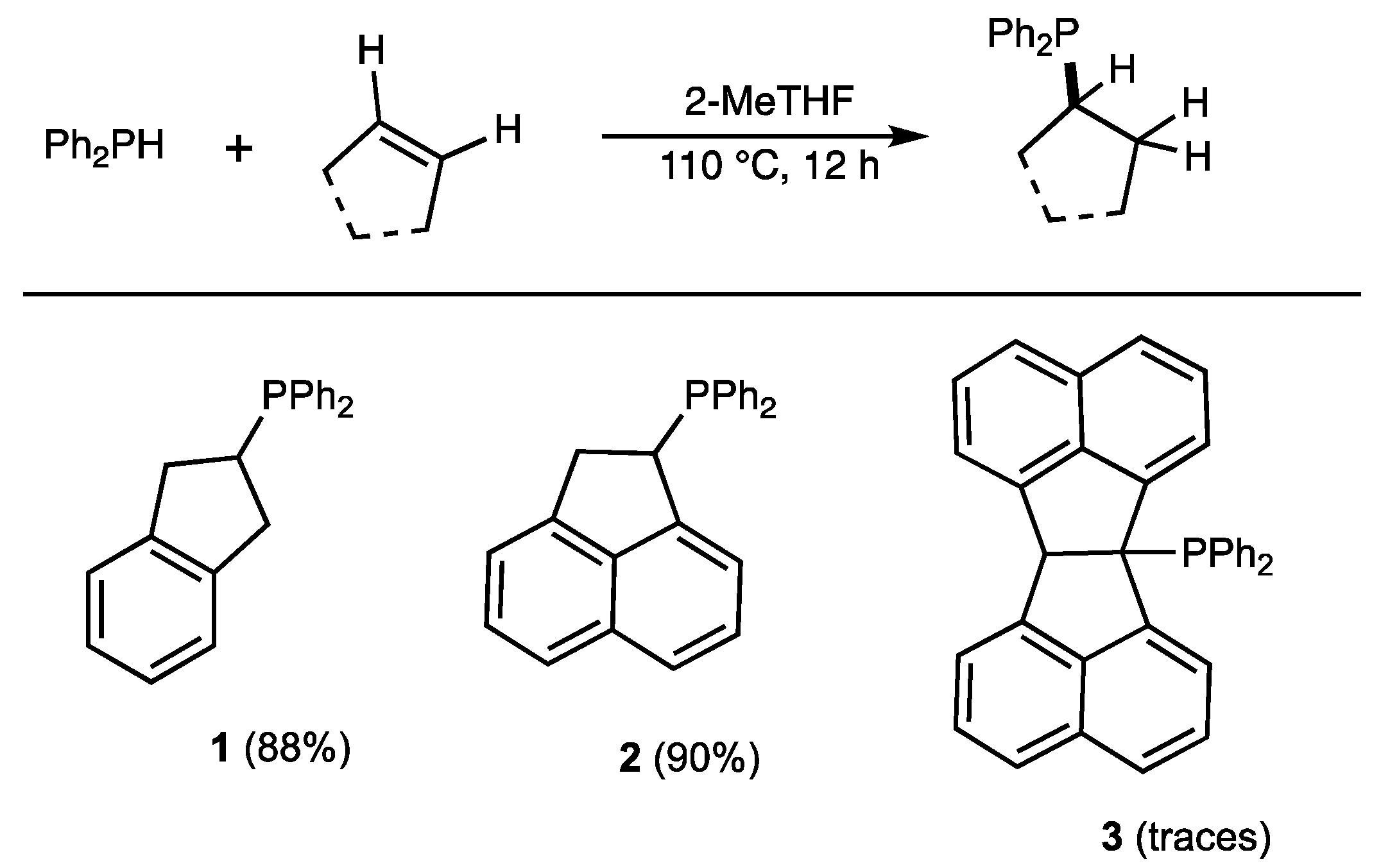
2.1. Phosphine Synthesis
2.2. Synthesis and Characterization of Transition Metal Complexes
2.2.1. Synthesis
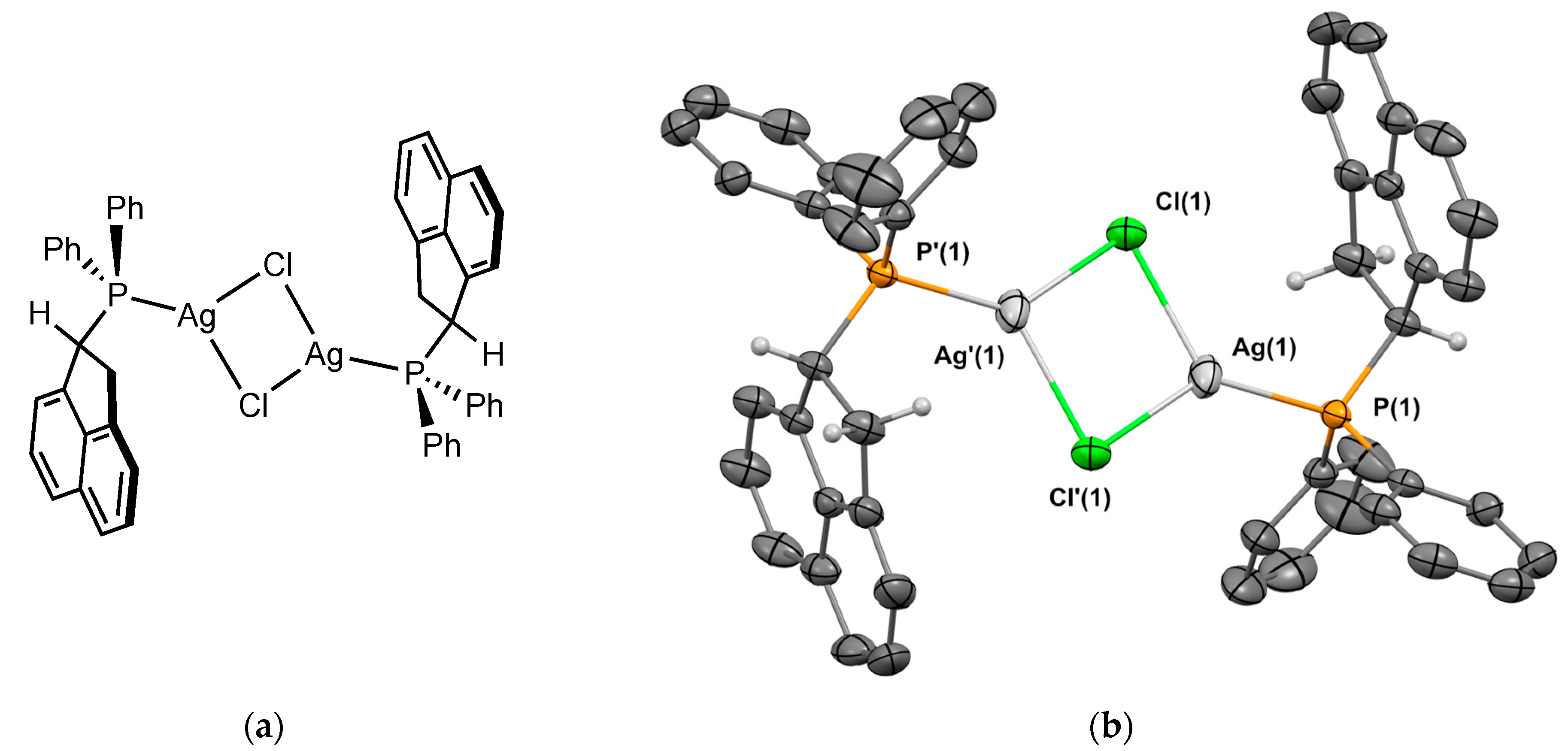
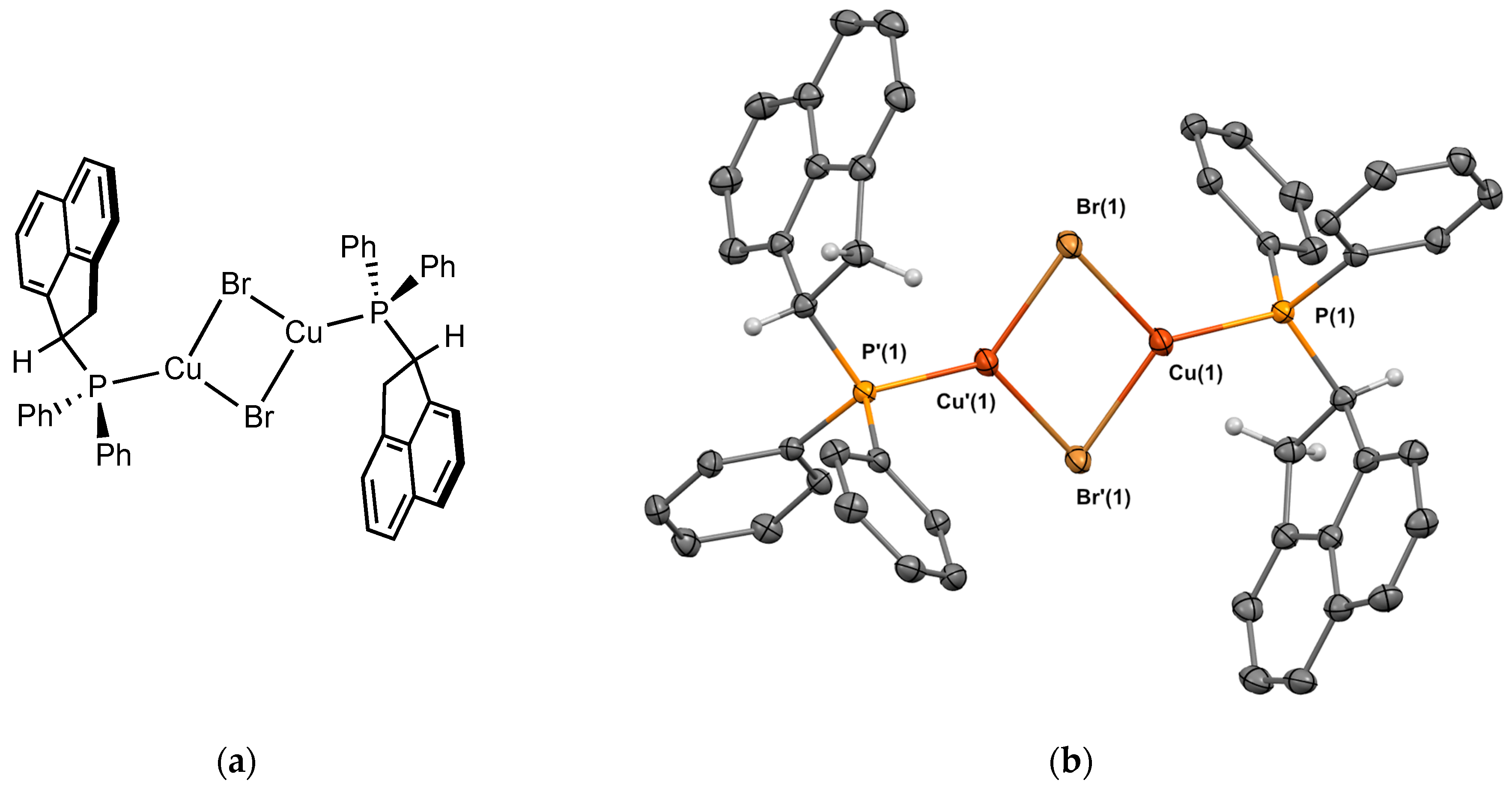
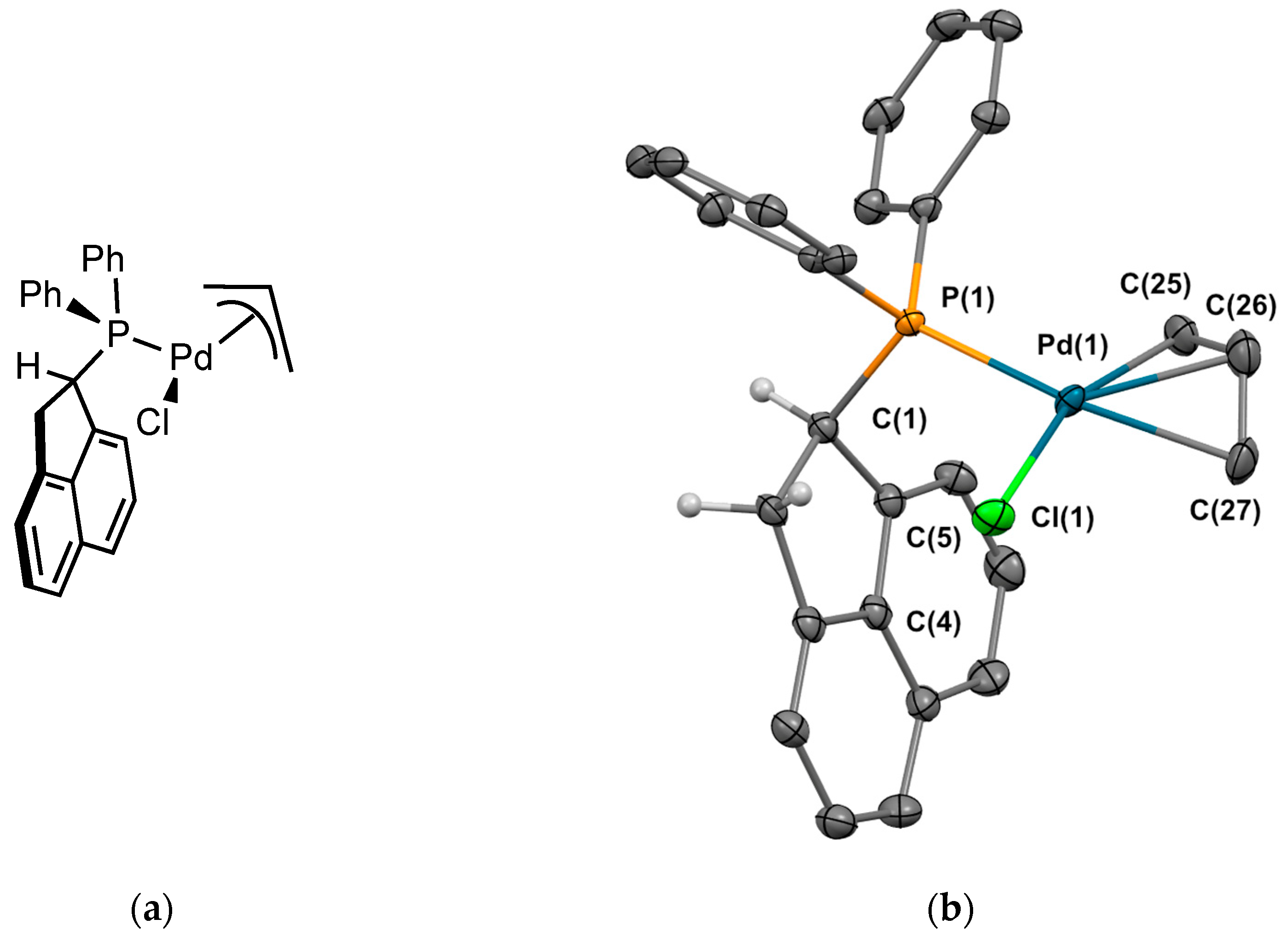
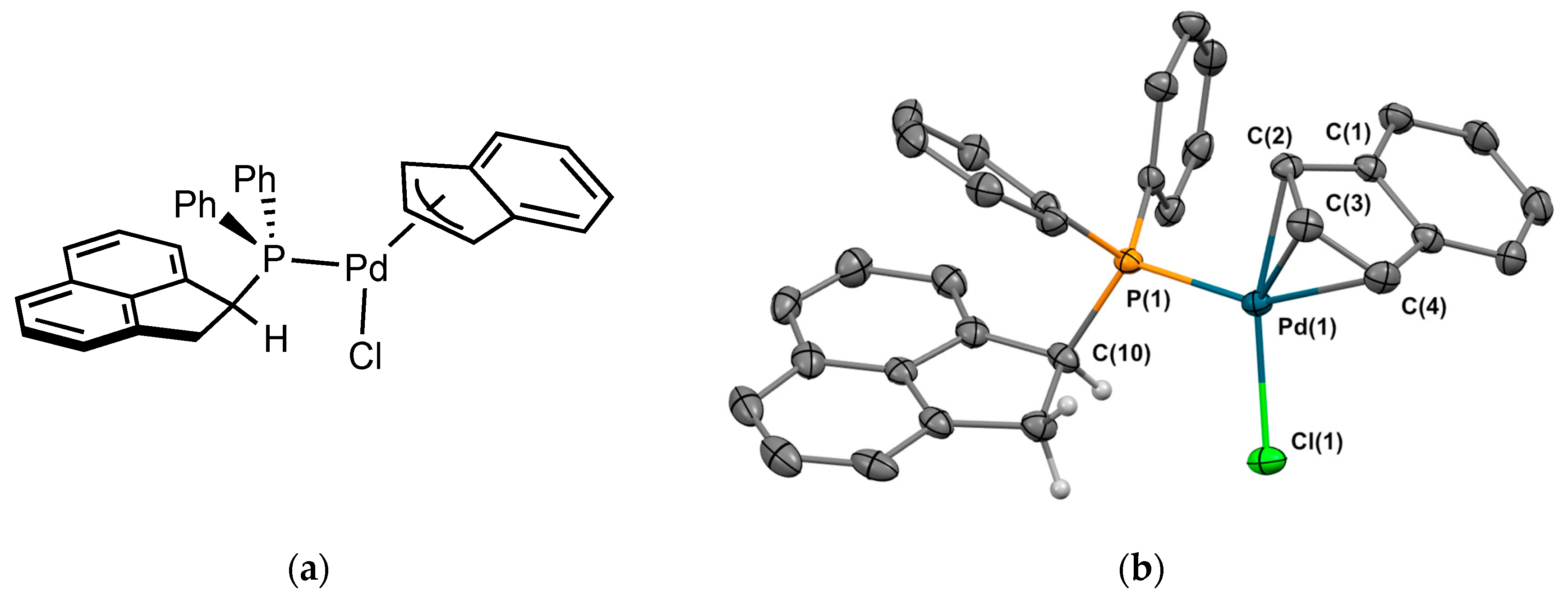
2.2.2. Crystal Structure of the Metal Complexes
Complex 1Au
Complex 2Au
Complex 2Ag
Complex 2Cu
Complex 2Pd1
Complex 2Pd2
2.3. Preliminary Results in Catalysis
2.3.1. Palladium Catalysis
2.3.2. Gold Catalysis
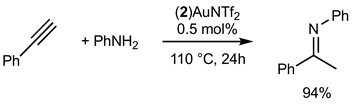
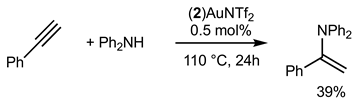
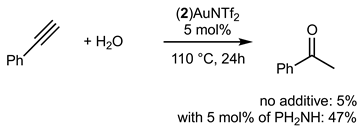
3. Discussion
4. Materials and Methods
4.1. General Remarks
4.2. X-ray Analyses
4.3. Synthesis of Ligand 1
4.4. Synthesis of Ligand 2
4.5. Synthesis of Gold Complexes 1Au and 2Au
4.6. Synthesis of Silver Complex 2Ag
4.7. Synthesis of Copper Complex 2Cu
4.8. Synthesis of Palladium Complexes 2Pd1 and 2Pd2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Huser, M.; Youinou, M.T.; Osborn, J.A. Chlorocarbon Activation: Catalytic Carbonylation of Dichloromethane and Chlorobenzene. Angew. Chem. Int. Ed. Engl. 1989, 28, 1386–1388. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef]
- Zuccarello, G.; Zanini, M.; Echavarren, A.M. Buchwald-Type Ligands on Gold(I) Catalysis. Isr. J. Chem. 2020, 60, 360–372. [Google Scholar] [CrossRef]
- Rataboul, F.; Zapf, A.; Jackstell, R.; Harkal, S.; Riermeier, T.; Monsees, A.; Dingerdissen, U.; Beller, M. New Ligands for a General Palladium-Catalyzed Amination of Aryl and Heteroaryl Chlorides. Chem. Eur. J. 2004, 10, 2983–2990. [Google Scholar] [CrossRef]
- Fleckenstein, C.A.; Plenio, H. 9-Fluorenylphosphines for the Pd-Catalyzed Sonogashira, Suzuki, and Buchwald–Hartwig Coupling Reactions in Organic Solvents and Water. Chem. Eur. J. 2007, 13, 2701–2716. [Google Scholar] [CrossRef]
- Fleckenstein, C.A.; Plenio, H. Sterically Demanding Trialkylphosphines for Palladium-Catalyzed Cross Coupling Reactions—Alternatives to PtBu3. Chem. Soc. Rev. 2010, 39, 694–711. [Google Scholar] [CrossRef]
- Zhao, Y.; Wakeling, M.G.; Meloni, F.; Sum, T.J.; Van Nguyen, H.; Buckley, B.R.; Davies, P.W.; Fossey, J.S. Balancing Bulkiness in Gold(I) Phosphino-triazole Catalysis. Eur. J. Org. Chem. 2019, 2019, 5540–5548. [Google Scholar] [CrossRef]
- Bissessar, D.; Egly, J.; Achard, T.; Steffanut, P.; Bellemin-Laponnaz, S. Catalyst-Free Hydrophosphination of Alkenes in Presence of 2-Methyltetrahydrofuran: A Green and Easy Access to a Wide Range of Tertiary Phosphines. RSC Adv. 2019, 9, 27250–27256. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Galán, P.; Delpont, N.; Herrero-Gómez, E.; Maseras, F.; Echavarren, A.M. Metal-Arene Interactions in Dialkylbiarylphosphane Complexes of Copper, Silver, and Gold. Chem. Eur. J. 2010, 16, 5324–5332. [Google Scholar] [CrossRef]
- Homs, A.; Escofet, I.; Echavarren, A.M. On the Silver Effect and the Formation of Chloride-Bridged Digold Complexes. Org. Lett. 2013, 15, 5782–5785. [Google Scholar] [CrossRef] [PubMed]
- Grirrane, A.; Álvarez, E.; García, H.; Corma, A. Catalytic Activity of Cationic and Neutral Silver(I)-XPhos Complexes with Nitrogen Ligands or Tolylsulfonate for Mannich and Aza-Diels-Alder Coupling Reactions. Chem. Eur. J. 2016, 22, 340–354. [Google Scholar] [CrossRef] [PubMed]
- McGarry, K.R.; McDaniel, M.; Chan, B.C.; O’Connor, A.R. Synthesis and Characterization of (π-Allyl)Palladium(II) Complexes Containing Dialkylbiaryl Phosphine Ligands. Polyhedron 2016, 114, 101–109. [Google Scholar] [CrossRef]
- DeAngelis, A.J.; Gildner, P.G.; Chow, R.; Colacot, T.J. Generating Active “L-Pd(0)” via Neutral or Cationic π-Allylpalladium Complexes Featuring Biaryl/Bipyrazolylphosphines: Synthetic, Mechanistic, and Structure–Activity Studies in Challenging Cross-Coupling Reactions. J. Org. Chem. 2015, 80, 6794–6813. [Google Scholar] [CrossRef] [PubMed]
- Melvin, P.R.; Nova, A.; Balcells, D.; Dai, W.; Hazari, N.; Hruszkewycz, D.P.; Shah, H.P.; Tudge, M.T. Design of a Versatile and Improved Precatalyst Scaffold for Palladium-Catalyzed Cross-Coupling: (η3-1-tBu-Indenyl)2(μ-Cl)2Pd2. ACS Catal. 2015, 5, 3680–3688. [Google Scholar] [CrossRef]
- Topchiy, M.A.; Asachenko, A.F.; Nechaev, M.S. Solvent-Free Buchwald–Hartwig Reaction of Aryl and Heteroaryl Halides with Secondary Amines. Eur. J. Org. Chem. 2014, 2014, 3319–3322. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef]
- Leung, C.H.; Baron, M.; Biffis, A. Gold-Catalyzed Intermolecular Alkyne Hydrofunctionalizations—Mechanistic Insights. Catalysts 2020, 10, 1210. [Google Scholar] [CrossRef]
- Mézailles, N.; Ricard, L.; Gagosz, F. Phosphine Gold(I) Bis-(Trifluoromethanesulfonyl)Imidate Complexes as New Highly Efficient and Air-Stable Catalysts for the Cycloisomerization of Enynes. Org. Lett. 2005, 7, 4133–4136. [Google Scholar] [CrossRef] [PubMed]
- Egly, J.; Bissessar, D.; Achard, T.; Heinrich, B.; Steffanut, P.; Mauro, M.; Bellemin-Laponnaz, S. Copper(I) Complexes with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Variety, Photophysics and Effect onto the Optical Properties. Inorg. Chim. Acta 2021, 514, 119971. [Google Scholar] [CrossRef]
- Bissessar, D.; Egly, J.; Achard, T.; Steffanut, P.; Mauro, M.; Bellemin-Laponnaz, S. A Stable and Photoreactive Copper-Iodide Cubane Suitable for Direct Post-Functionalization. Eur. J. Inorg. Chem. 2022, 2022, e202200101. [Google Scholar] [CrossRef]
- Jin, S.; Haug, G.C.; Nguyen, V.T.; Flores-Hansen, C.; Arman, H.D.; Larionov, O.V. Decarboxylative Phosphine Synthesis: Insights into the Catalytic, Autocatalytic, and Inhibitory Roles of Additives and Intermediates. ACS Catal. 2019, 9, 9764–9774. [Google Scholar] [CrossRef]
- Barder, T.E.; Walker, S.D.; Martinelli, J.R.; Buchwald, S.L. Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure. J. Am. Chem. Soc. 2005, 12, 4685–4696. [Google Scholar] [CrossRef]
- Barder, T.E.; Biscoe, M.R.; Buchwald, S.L. Structural Insights into Active Catalyst Structures and Oxidative Addition to (Biaryl)phosphine−Palladium Complexes via Density Functional Theory and Experimental Studies. Organometallics 2007, 26, 2183–2192. [Google Scholar] [CrossRef]
- Herrero-Gómez, E.; Nieto-Oberhuber, C.; López, S.; Benet-Buchholz, J.; Echavarren, A.M. Cationic η 1/η 2 -Gold(I) Complexes of Simple Arenes. Angew. Chem. Int. Ed. 2006, 45, 5455–5459. [Google Scholar] [CrossRef]
- Woodhouse, S.S.; Buchanan, J.K.; Dais, T.N.; Ainscough, E.W.; Brodie, A.M.; Freeman, G.H.; Plieger, P.G. Structural Trends in a Series of Bulky Dialkylbiarylphosphane Complexes of CuI. Acta Crystallogr. C Struct. Chem. 2021, 77, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, E.; Sato, K.; Hayashi, T.; Tanaka, M. Highly Efficient AuI-Catalyzed Hydration of Alkynes. Angew. Chem. Int. Ed. 2002, 41, 4563–4565. [Google Scholar] [CrossRef]
- Navarro, M.; Holzapfel, M.; Campos, J. Shape Selectivity in the Gold-Catalyzed Hydration of Alkynes Using a Cavity-Shaped Phosphine. ChemPlusChem 2023, 88, e202300231. [Google Scholar] [CrossRef]



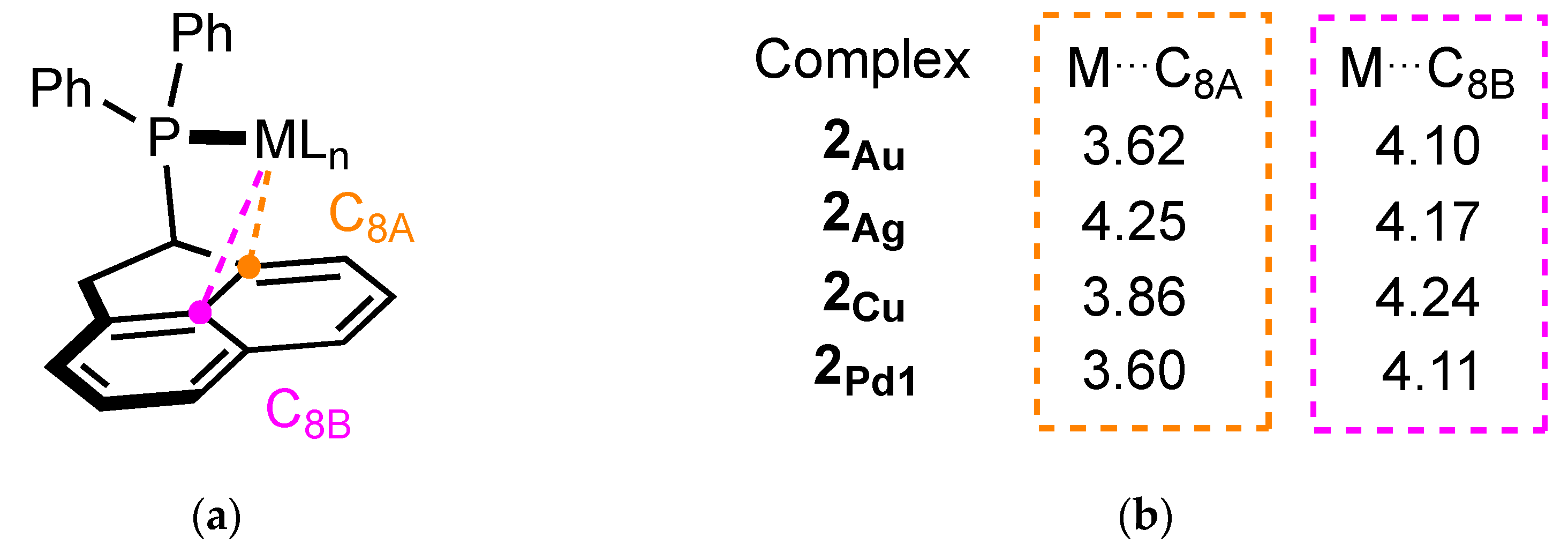
| Entry * | Substrate 1 | Substrate 2 | Product | Yield (%) |
|---|---|---|---|---|
| 1 |  | 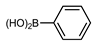 |  | 96 |
| 2 | 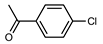 | 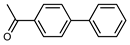 | 99 | |
| 3 | 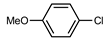 | 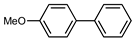 | 98 | |
| 4 | 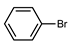 | 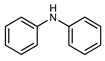 | 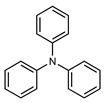 | 30 |
| 5 |  | 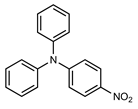 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechrouk, V.; Bissessar, D.; Egly, J.; Parmentier, J.; Bellemin-Laponnaz, S. Synthesis and Characterization of Transition Metal Complexes Supported by Phosphorus Ligands Obtained Using Hydrophosphination of Cyclic Internal Alkenes. Molecules 2024, 29, 3946. https://doi.org/10.3390/molecules29163946
Mechrouk V, Bissessar D, Egly J, Parmentier J, Bellemin-Laponnaz S. Synthesis and Characterization of Transition Metal Complexes Supported by Phosphorus Ligands Obtained Using Hydrophosphination of Cyclic Internal Alkenes. Molecules. 2024; 29(16):3946. https://doi.org/10.3390/molecules29163946
Chicago/Turabian StyleMechrouk, Victoria, Damien Bissessar, Julien Egly, Jordan Parmentier, and Stéphane Bellemin-Laponnaz. 2024. "Synthesis and Characterization of Transition Metal Complexes Supported by Phosphorus Ligands Obtained Using Hydrophosphination of Cyclic Internal Alkenes" Molecules 29, no. 16: 3946. https://doi.org/10.3390/molecules29163946
APA StyleMechrouk, V., Bissessar, D., Egly, J., Parmentier, J., & Bellemin-Laponnaz, S. (2024). Synthesis and Characterization of Transition Metal Complexes Supported by Phosphorus Ligands Obtained Using Hydrophosphination of Cyclic Internal Alkenes. Molecules, 29(16), 3946. https://doi.org/10.3390/molecules29163946








