Effect of Tourmaline Addition on the Anti-Poisoning Performance of MnCeOx@TiO2 Catalyst for Low-Temperature Selective Catalytic Reduction of NOx
Abstract
1. Introduction
2. Results and Discussion
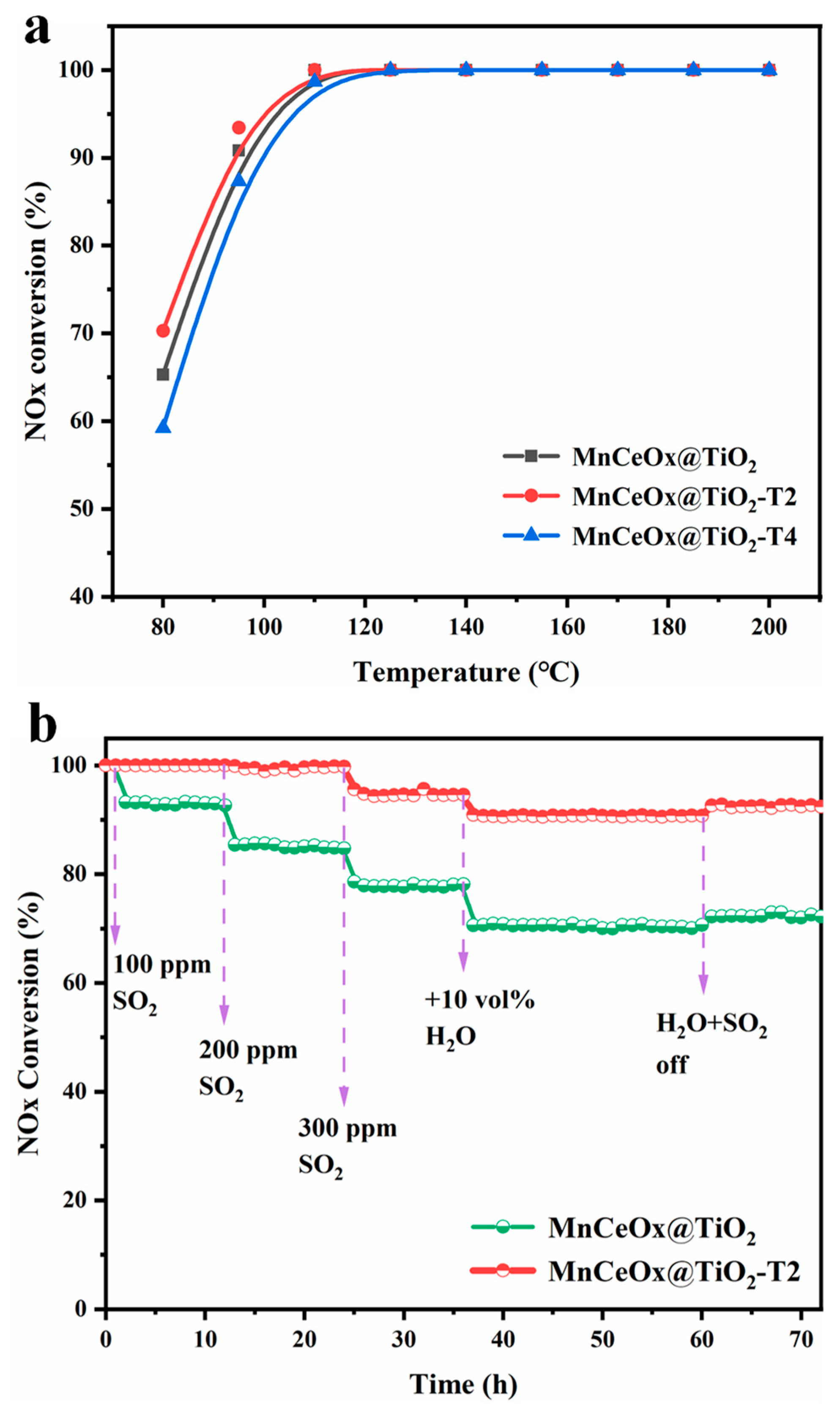
2.1. NOx Reduction and SO2 Tolerance
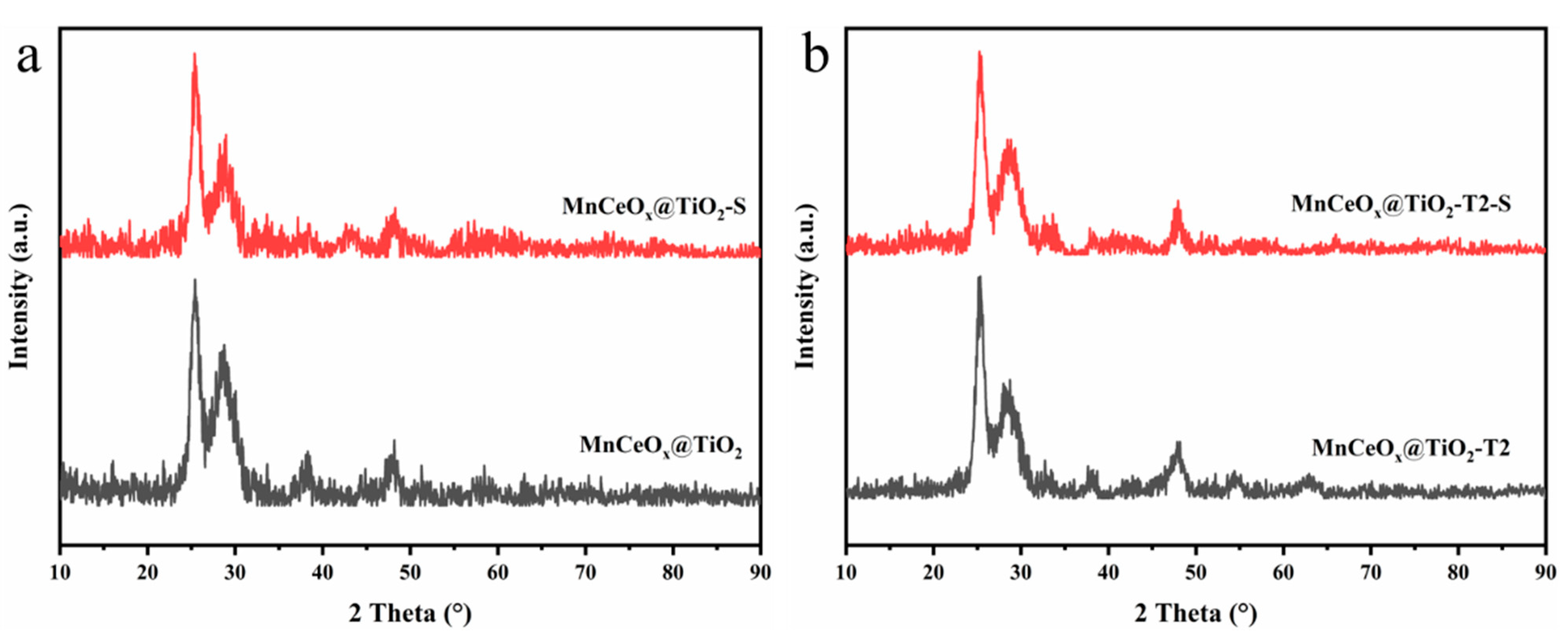
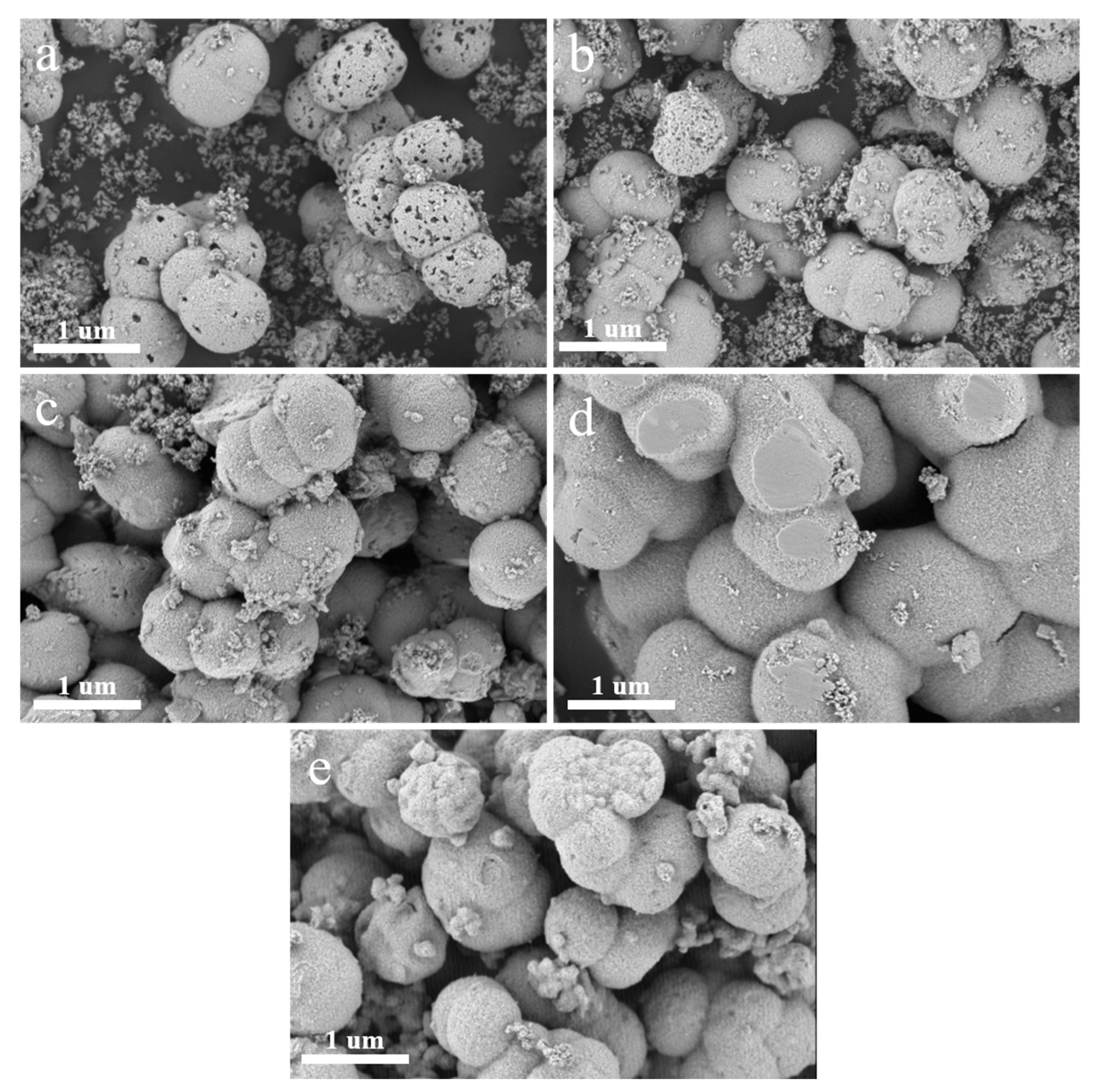
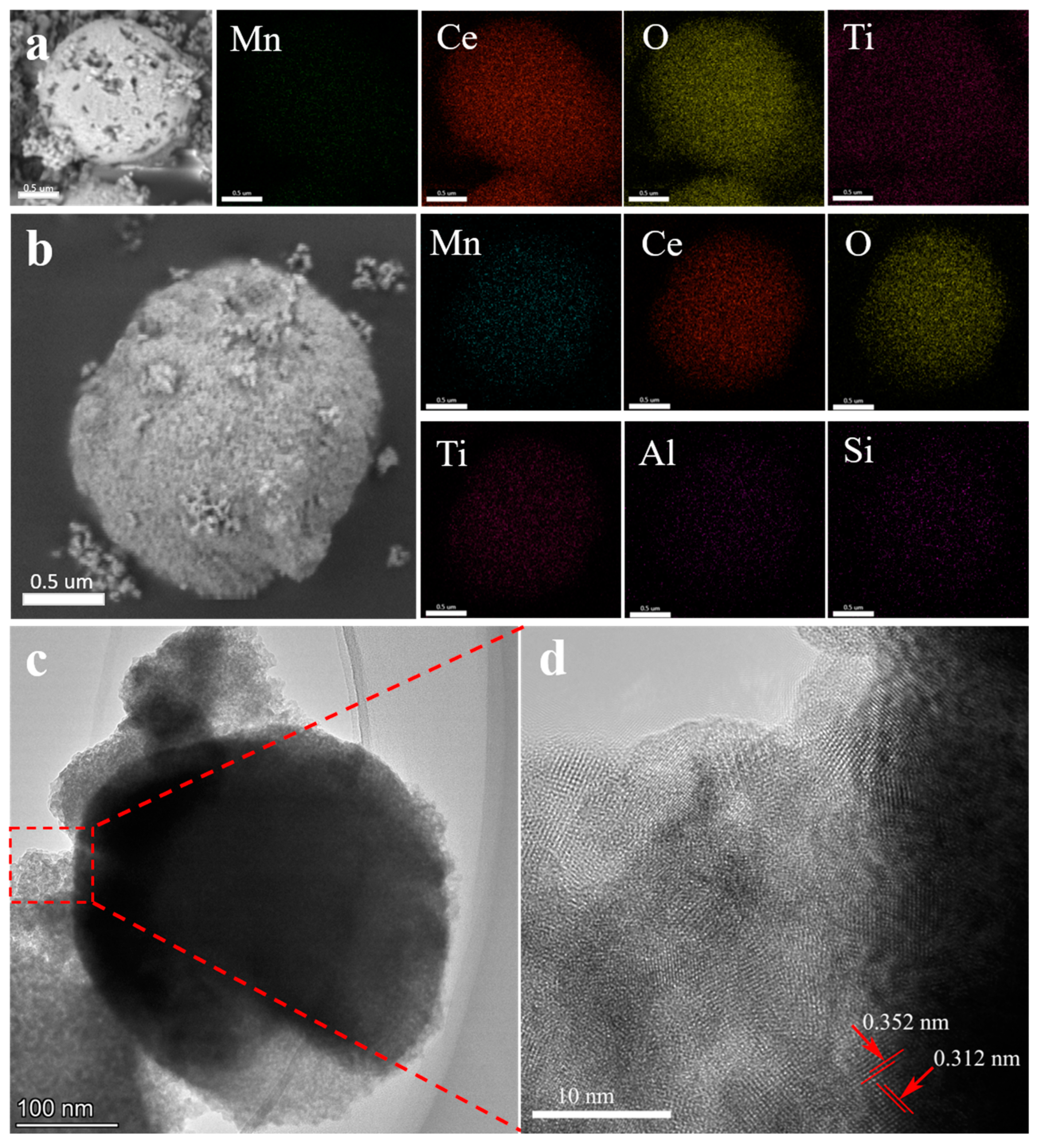
2.2. Research on Crystal Structure and Morphology
2.3. Specific Surface Area and Surface Element Analysis
2.4. Redox Capability
2.5. Study on the Mechanism of Catalyst Anti-Poisoning Reaction
2.5.1. Adsorption of SO2 + O2 on the Catalyst Surface
2.5.2. Adsorption of NH3 + SO2 on the Catalyst Surface
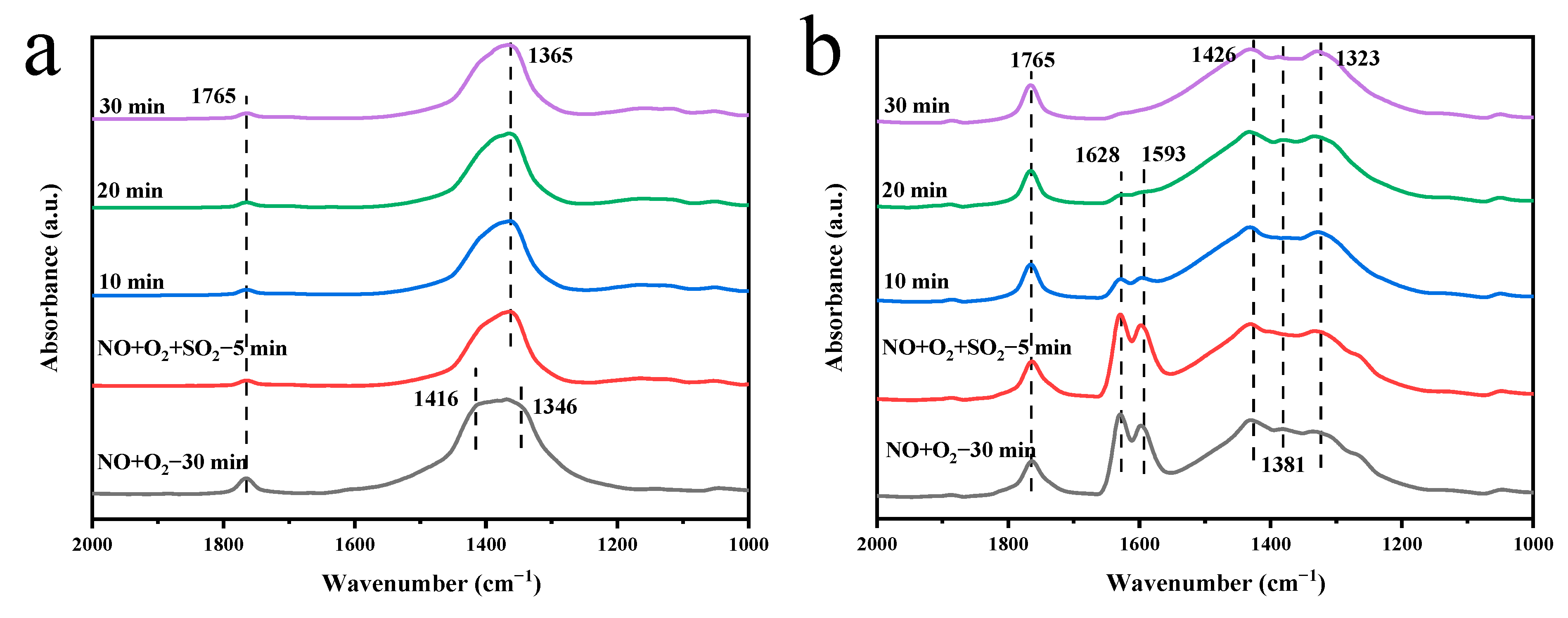
2.5.3. Adsorption of NO + O2 + SO2 on the Catalyst Surface
2.5.4. Adsorption of NH3 on the Catalyst Surface before and after Poisoning
2.5.5. Adsorption of NO + O2 on the Catalyst Surface before and after Poisoning
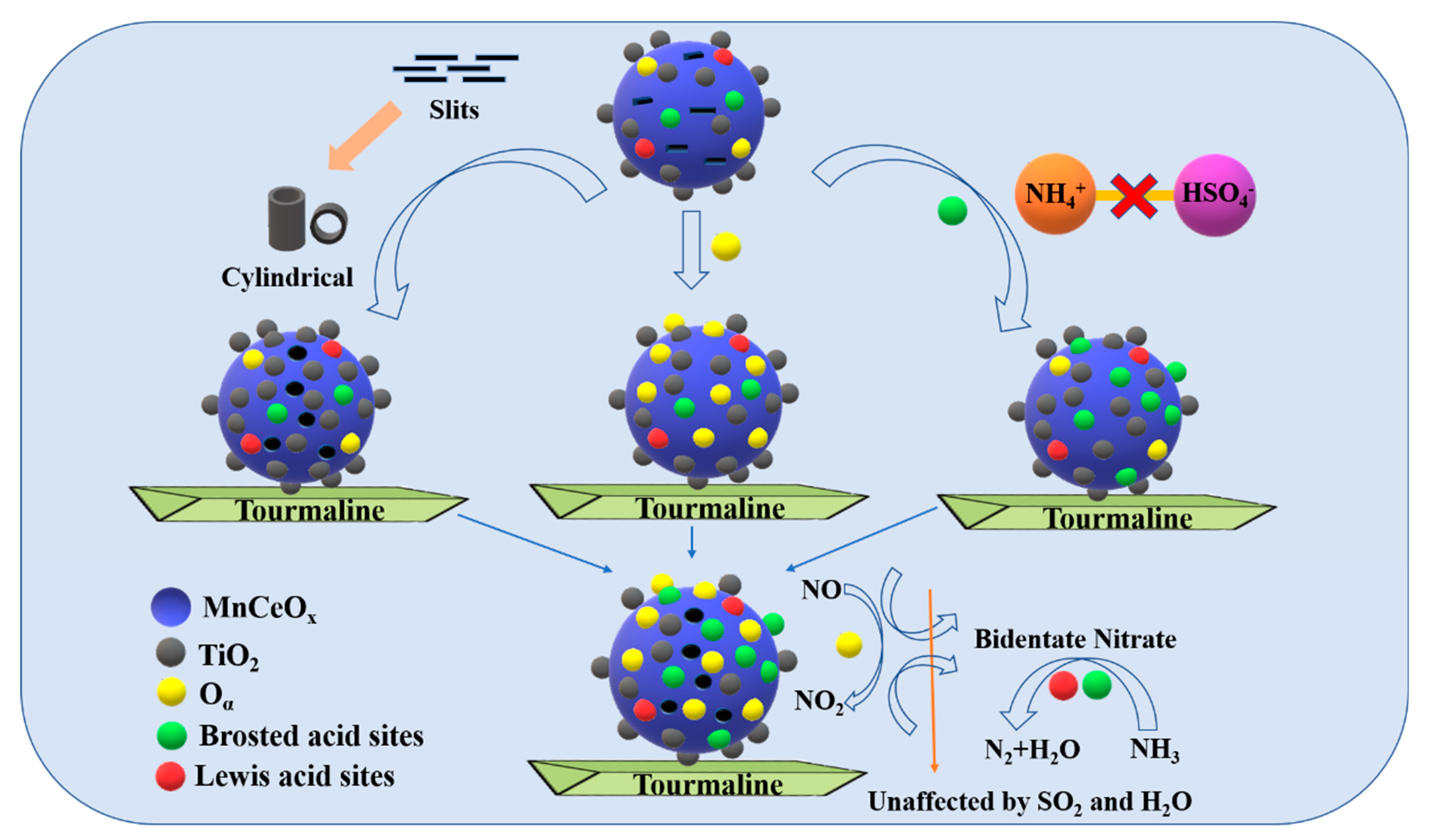
2.5.6. Anti-Poisoning Mechanism of MnCeOx@TiO2-T2 Catalyst
3. Experimental Section
3.1. Preparation of Catalysts
3.2. Catalytic Performance Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zeng, W.L.; Song, M.J.; Wang, F.L.; Hu, X.D.; Guo, Q.J.; Liu, Y.Z. Polyetheramine improves the CO2 adsorption behavior of tetraethylenepentamine-functionalized sorbents. Chem. Eng. J. 2019, 364, 475–484. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Q.J.; Kong, T.T. Tetraethylenepentamine-modified MCM-41/silica gel with hierarchical mesoporous structure for CO2 capture. Chem. Eng. J. 2015, 273, 472–480. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhou, T.T.; Cao, Z.; Yuan, Z.M.; He, H.Y.; Fan, M.H.; Jiang, Z.Y. Advanced 3D Ordered Electrodes for PEMFC Applications: From Structural Features and Fabrication Methods to the Controllable Design of Catalyst Layers. Green Energy Environ. 2024, 9, 1336–1365. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, H.; Huang, B. Review of deNOx catalysts with SO2 and H2O poisoning resistance for low-temperature NH3-SCR. Chem. Ind. Eng. Prog. 2014, 33, 907. [Google Scholar]
- Li, X.; Niu, Y.; Li, J.; Yang, M.; Chen, R.; Shao, D.; Zheng, X.; Zhang, C.; Qi, Y. Trace Co doping improves NH3-SCR performance and poisoning resistance of Ce-Mn-based catalysts. Chem. Eng. J. 2023, 454, 140180. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Li, J.; Ma, L.; Arandiyan, H.; Du, Y.; Xu, J.; Hao, J. Enhancement of Activity and Sulfur Resistance of CeO2 Supported on TiO2–SiO2 for the Selective Catalytic Reduction of NO by NH3. Environ. Sci. Technol. 2012, 46, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Su, Z.; Ren, S.; Long, H.M.; Kong, M.; Jiang, L.J. Low-temperature SCR of NO with NH3 over biomass char supported highly dispersed MnCe mixed oxides. J. Energy Inst. 2019, 92, 883–891. [Google Scholar] [CrossRef]
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B-Environ. 2008, 79, 347–355. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-Temperature Selective Catalytic Reduction (SCR) of NO with NH3 by Using Mn, Cr, and Cu Oxides Supported on Hombikat TiO2. Angew. Chem. Int. Ed. 2001, 40, 2479–2482. [Google Scholar] [CrossRef]
- Yu, J.; Si, Z.; Zhu, M.; Wu, X.; Chen, L.; Weng, D.; Zou, J. NH3-SCR activity, hydrothermal stability and poison resistance of a zirconium phosphate/Ce0.5Zr0.5O2 catalyst in simulated diesel exhaust. RSC Adv. 2015, 5, 83594–83599. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B-Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T. A superior catalyst for low-temperature NO reduction with NH3. Chem. Commun. 2003, 7, 848–849. [Google Scholar] [CrossRef]
- Tang, X.L.; Hao, J.M.; Xu, W.G.; Li, J.H. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2007, 8, 329–334. [Google Scholar] [CrossRef]
- Tang, X.F.; Li, J.H.; Sun, L.A.; Hao, J.M. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3. Appl. Catal. B-Environ. 2010, 99, 156–162. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Feng, C.; Shi, L.; Zhang, D.S. Fe2O3-CeO2@Al2O3 Nano-arrays on Al-Mesh as SO2-tolerant monolith catalysts for NOx reduction by NH3. Environ. Sci. Technol. 2019, 53, 5946–5956. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B-Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Hasegawa, J.; Li, S.; Shen, Y.J.; Li, H.R.; Shi, L.; Zhang, D. SO2-tolerant selective catalytic reduction of NOx over meso-TiO2@Fe2O3@Al2O3 metal-based monolith catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, D.; Zhang, J.; Cai, S.; Fang, C.; Huang, L.; Li, H.; Gao, R.; Shi, L. Design of meso-TiO2@MnOx-CeOx/CNTs with a core-shell structure as DeNOx catalysts: Promotion of activity, stability and SO2-tolerance. Nanoscale 2013, 5, 9821–9829. [Google Scholar] [CrossRef]
- Ma, Q. Preparation of tourmaline fine stone particles and introduction of related research. China Powder Ind. 2020, 96, 11–15. [Google Scholar]
- Li, H.; Liu, J. Research status and application of high-efficiency far-infrared radiation ceramics. Mod. Tech. Ceram. 2005, 26, 24–26. [Google Scholar]
- Zhu, D.; Liang, J.; Li, F.; Ding, Y.; Wang, L.; Liang, G.; Xu, G. Preparation and characterization of rare earth composite materials radiating far infrared for activating liquefied petroleum gas. J. Rare Earths 2006, 24, 277–280. [Google Scholar]
- Hu, Y.; Chen, X.; Tang, M. Research progress and prospects of tourmaline functional composite materials. Front. Earth Sci.-Prc. 2014, 21, 331–337. [Google Scholar]
- Luo, G.; Chen, A.; Zhu, M.; Zhao, K.; Zhang, X.; Hu, X. Improving the electrocatalytic performance of Pd for formic acid electrooxidation by introducing tourmaline. Electrochim. Acta 2020, 360, 137023. [Google Scholar] [CrossRef]
- Helian, Y.; Cui, S.; Ma, X. The effect of tourmaline on SCR denitrification activity of MnOx/TiO2 at low temperature. Catalysts 2020, 10, 1020. [Google Scholar] [CrossRef]
- Tan, C.; Cui, D.; Liu, Y.; Ji, Y. Influence of tourmaline on the anaerobic ammonium oxidation process in sequencing batch reactors. J. Environ. Eng. 2017, 143, 04017053. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Hongmanorom, P.; Wang, Z.; Zhang, S.; Chen, J.; Zhong, Q.; Kawi, S. Active sites adjustable phosphorus promoted CeO2/TiO2 catalysts for selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2021, 409, 128242. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. Influence of tungsten on the activity of a Mn/Ce/W/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. A-Gen. 2015, 497, 160–166. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Liu, C.; Song, L.; Guo, Q.; Peng, T.; Chai, Q.; Zheng, X. Mn MOF-derivatives synthesized based upon a mechanochemistry method for low-temperature NH3- SCR: From amorphous precursor to amorphous catalyst. Mol. Catal. 2024, 554, 113767. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on γ-Al2O3. Appl. Catal. B-Environ. 2011, 103, 275–286. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Li, C.; Liu, J.; He, S.; Zhao, Y.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Catalytic behavior of supported Ru nanoparticles on the (101) and (001) facets of anatase TiO2. RSC Adv. 2014, 4, 10834–10840. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Li, W.; Zhong, L.; Zhang, C.; Fang, Q.Y.; Chen, G. Effect of preferential exposure of anatase TiO2 {001} facets on the performance of Mn-Ce/TiO2 catalysts for low-temperature selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2019, 369, 26–34. [Google Scholar] [CrossRef]
- Zhao, G.; Li, M.; Wang, L.; Wang, D.; Liang, G.; Xue, G. Environmentally-friendly tourmaline modified CeMnFeOx catalysts for low-temperature selective catalytic reduction of NOx with NH3. Catal. Today 2020, 355, 389–396. [Google Scholar] [CrossRef]
- Zheng, C.; Cheng, T.; Yang, L.; Wu, H.; Fan, H. Effect of SiO2 addition on NH4HSO4 decomposition and SO2 poisoning over V2O5-MoO3/TiO2-CeO2 catalyst. J. Environ. Sci. 2020, 91, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tang, Y.; Wan, Y.; Li, L.; Yao, S.; Li, X.; Gu, J.; Li, Y.; Shi, J. Promotion effects of SiO2 or/and Al2O3 doped CeO2/TiO2 catalysts for selective catalytic reduction of NO by NH3. J. Hazard. Mater. 2014, 278, 350–359. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.; DeLaRiva, A.; Wang, M.; EngeIhard, M.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef]
- Lin, F.; Chen, Y.; Zhang, L.; Mei, D.; Kovarik, L.; Sudduth, B.; Wang, H.; Gao, F.; Wang, Y. Single-Facet Dominant Anatase TiO2 (101) and (001) Model Catalysts to Elucidate the Active Sites for Alkanol Dehydration. ACS Catal. 2020, 10, 4268–4279. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, Q.; Zheng, C.; Cheng, S.; Kang, T.; Dai, H. Effect of ceria loading on Zr-pillared clay catalysts for selective catalytic reduction of NO with NH3. New J. Chem. 2019, 43, 10850–10858. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Tsang, S. Cr-MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, S.; Zhang, X.; Xiao, K.; Wu, X. Improvement of the activity and SO2 tolerance of Sb-modified Mn/PG catalysts for NH3-SCR at a low temperature. J. Environ. Sci. 2021, 101, 1–15. [Google Scholar] [CrossRef]
- Tang, X.; Wang, C.; Gao, F.; Han, W.; Yi, H.; Zhao, S.; Zhou, Y.; Liu, Y. Mn-Fe-Ce multiple oxides with Al2O3 coating supported onto honeycomb cordierite monoliths for NO catalytic oxidation. Colloid Surf. A 2021, 611, 125790. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, X.; Tang, Z. New insight into the synergistic promotion effect of phosphorus and molybdenum on the ceria-titanium catalysts for superior SCR performance. Mol. Catal. 2019, 478, 110562. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Ning, P.; Wang, H.; Ma, Y.; Xu, S.; Liu, M.; Zhang, Q.; Xia, F. The property tuning of NH3-SCR over iron-tungsten catalyst: Role of calcination temperature on surface defect and acidity. Appl. Surf. Sci. 2021, 538, 147999. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Li, Q.; Liu, Y.; Bai, Y.; Zeng, Z.; Huang, Z. Environmental-friendly production of FeNbTi catalyst with significant enhancement in SCR activity and SO2 resistance for NOx removal. Fuel 2021, 285, 119133. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Guo, X.; Cao, L.; Xu, T.; Chen, Z.; Wu, X.; Si, Z.; Weng, D. Nb-modified Mn/Ce/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A-Gen. 2017, 545, 64–71. [Google Scholar] [CrossRef]
- Ma, D.; Yang, L.; Huang, B.; Wang, L.; Wang, X.; Sheng, Z.; Dong, F. MnOx-CeO2@TiO2 core-shell composites for low temperature SCR of NOx. New J. Chem. 2019, 43, 15161–15168. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; Shan, W.; He, H. Improvement of Nb doping on SO2 resistance of VOx/CeO2 catalyst for the selective catalytic reduction of NOx with NH3. J. Phys. Chem. C 2017, 121, 7803–7809. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Wu, P.; Zhuang, K.; Shen, K.; Wang, S.; Huang, T. Effect of SO2 on the low-temperature denitrification performance of Ho-modified Mn/Ti catalyst. Chem. Eng. J. 2019, 400, 122597. [Google Scholar] [CrossRef]
- Yu, C.; Hou, D.; Huang, B.; Lu, M.; Peng, R.; Zhong, Z. A MnOx@Eu-CeOx nanorod catalyst with multiple protective effects: Strong SO2-tolerance for low temperature DeNOx processes. J. Hazard. Mater. 2020, 399, 123011. [Google Scholar] [CrossRef]
- Xu, D.; Wu, W.; Wang, P.; Deng, J.; Yan, T.; Zhang, D. Boosting the alkali/heavy metal poisoning resistance for no removal by using iron-titanium pillared montmorillonite catalysts. J. Hazard. Mater. 2020, 399, 122947. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Li, K.; Niu, H.; Peng, Y.; Chen, J.; Huang, Y.; Li, J. Simultaneous removal of NOx and chlorobenzene on V2O5/TiO2 granular catalyst: Kinetic study and performance prediction. Front. Environ. Sci. Eng. 2021, 15, 70. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B-Environ. 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B-Environ. 2014, 156–157, 371–377. [Google Scholar] [CrossRef]
- Zhan, S.; Zhang, H.; Zhang, Y.; Shi, Q.; Li, Y.; Li, X.J. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3(x)-CeO2 at low temperatures. Appl. Catal. B-Environ. 2017, 203, 199–209. [Google Scholar] [CrossRef]
- Wei, L.; Li, X.; Mu, J.; Wang, X.; Fan, S.; Yin, Z.; Tadé, M.O.; Liu, S. Rationally tailored redox properties of a mesoporous Mn-Fe spinel nanostructure for boosting low-temperature selective catalytic reduction of NOx with NH3. ACS Sustain. Chem. Eng. 2020, 8, 17727–17739. [Google Scholar] [CrossRef]
- Shu, D.; Chen, T.; Xie, H.; Sun, F.; Chen, D.; Zou, X.; Liu, H. The decomposition of Mn-substituted siderite during coaling enhanced the transformation of NO before catalyst bed: Effect of Mn substitution. Fuel 2021, 290, 120056. [Google Scholar] [CrossRef]
- Gutierrez-Alejandre, A.; Ramirez, J.; Busca, G. A vibrational and spectroscopic study of WO3/TiO2-Al2O3 catalyst precursors. Langmuir 1998, 14, 630–639. [Google Scholar] [CrossRef]
- Tang, X.; Wang, C.; Gao, F.; Ma, Y.; Yi, H.; Zhao, S.; Zhou, Y. Effect of hierarchical element doping on the low-temperature activity of manganese-based catalysts for NH3-SCR. J. Environ. Chem. Eng. 2020, 8, 104399. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B-Environ. 2011, 10, 369–377. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, J.; Ning, P.; Song, Z.; Liu, X.; Wang, L.; Wang, J.; Wang, H.; Long, K. In situ DRIFTS investigation of NH3-SCR reaction over CeO2/zirconium phosphate catalyst. Appl. Surf. Sci. 2018, 435, 1037–1045. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B-Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, J.; Zheng, R.; Guo, L.; Yuan, J.; Zhang, S.; Gu, M. Insight into the reaction mechanism over PMoA for low temperature NH3-SCR: A combined In-situ DRIFTs and DFT transition state calculations. J. Hazard. Mater. 2021, 412, 125258. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, H.; Su, W.; Li, K.; Zhang, J.; Shi, J.; Tian, J.; Wang, J. Mineral-derived catalysts optimized for selective catalytic reduction of NOx with NH3. J. Clean. Prod. 2021, 289, 125756. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. DRIFT study on Cerium-Tungsten/Titiania catalyst for selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar] [CrossRef]
- Ren, W. Research on Denitration Mechanism and Preparation of Ferrous Sulfate SCR Catalyst. Ph.D. Thesis, Tsinghua University, Beijing, China, 2010. [Google Scholar]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Kang, M.; Yeon, T.H.; Park, E.D.; Yie, J.E.; Kim, J.M. Novel MnOx Catalysts for NO Reduction at Low Temperature with Ammonia. Catal. Lett. 2006, 106, 77–80. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B-Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Park, T.S.; Jeong, S.K.; Hong, S.H.; Hong, S.C. Selective Catalytic Reduction of Nitrogen Oxides with NH3 over Natural Manganese Ore at Low Temperature, Ind. Eng. Chem. Res. 2001, 40, 4491–4495. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Cu–Mn mixed oxides for low temperature NO reduction with NH3. Catal. Today 2006, 111, 236–241. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B-Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T.; Chang, R. Low temperature selective catalytic reduction (SCR) of NO with NH3 over Fe–Mn based catalysts. Chem. Commun. 2002, 5, 452–453. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Ding, Y.; Zhang, C. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B-Environ. 2009, 93, 194–204. [Google Scholar] [CrossRef]
- Liu, F.D.; He, H. Selective catalytic reduction of NO with NH3 over manganese substituted iron titanate catalyst: Reaction mechanism and H2O/SO2 inhibition mechanism study. Catal. Today 2010, 153, 70–76. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Wei, L.; Hao, J. MnOx-SnO2 Catalysts Synthesized by a Redox Coprecipitation Method for Selective Catalytic Reduction of NO by NH3. Chin. J. Catal. 2008, 29, 531–536. [Google Scholar] [CrossRef]
- Singoredjo, L.; Korver, R.; Kapteijn, F.; Moulijn, J. Alumina supported manganese oxides for the low-temperature selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B-Environ. 1992, 1, 297–316. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Sreekanth, P.M.; Pena, D.A.; Jenkins, R.G. Manganese Oxide Catalysts Supported on TiO2, Al2O3, and SiO2: A Comparison for Low-Temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 2006, 45, 6436–6443. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Ke, R.; Luo, C.; Hao, J. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts. Catal. Commun. 2007, 8, 1896–1900. [Google Scholar] [CrossRef]
- Wu, Z.B.; Jin, R.B.; Liu, Y.; Wang, H.Q. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Casapu, M.; Kröcher, O.; Elsener, M. Screening of doped MnOx–CeO2 catalysts for low-temperature NO-SCR. Appl. Catal. B-Environ. 2009, 88, 413–419. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Yi, H.; Li, J. Low-temperature SCR of NO with NH3 over AC/C supported manganese-based monolithic catalysts. Catal. Today 2007, 126, 406–411. [Google Scholar] [CrossRef]
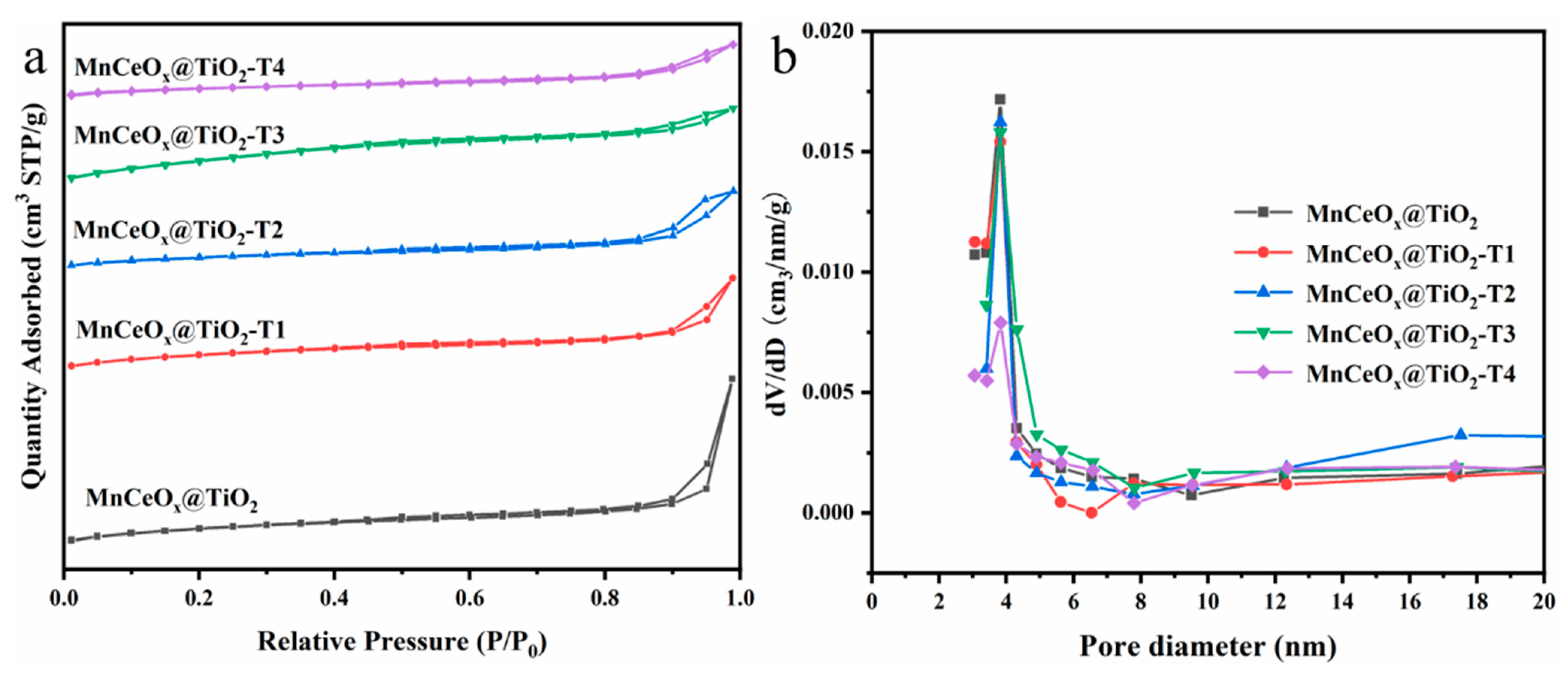
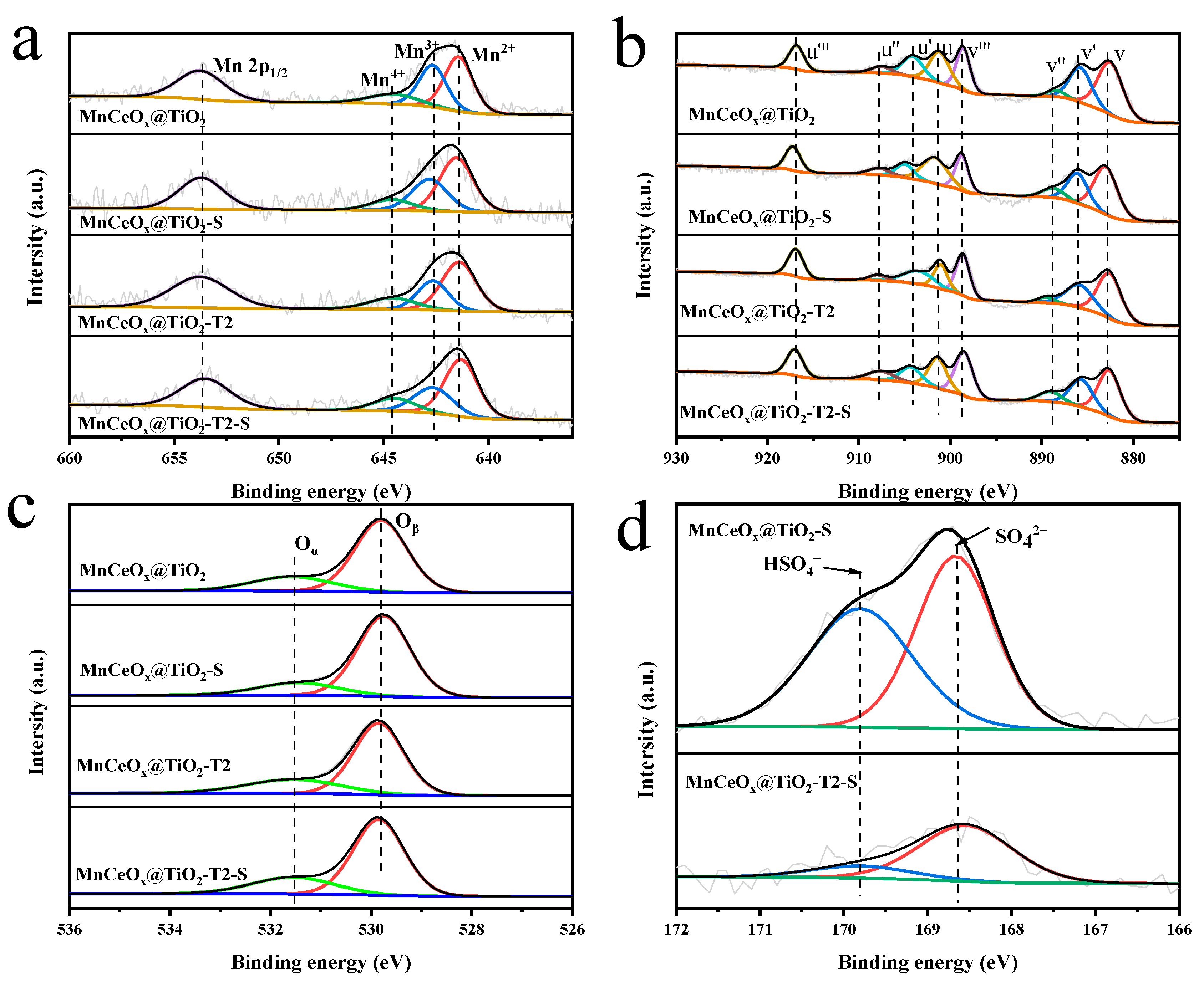
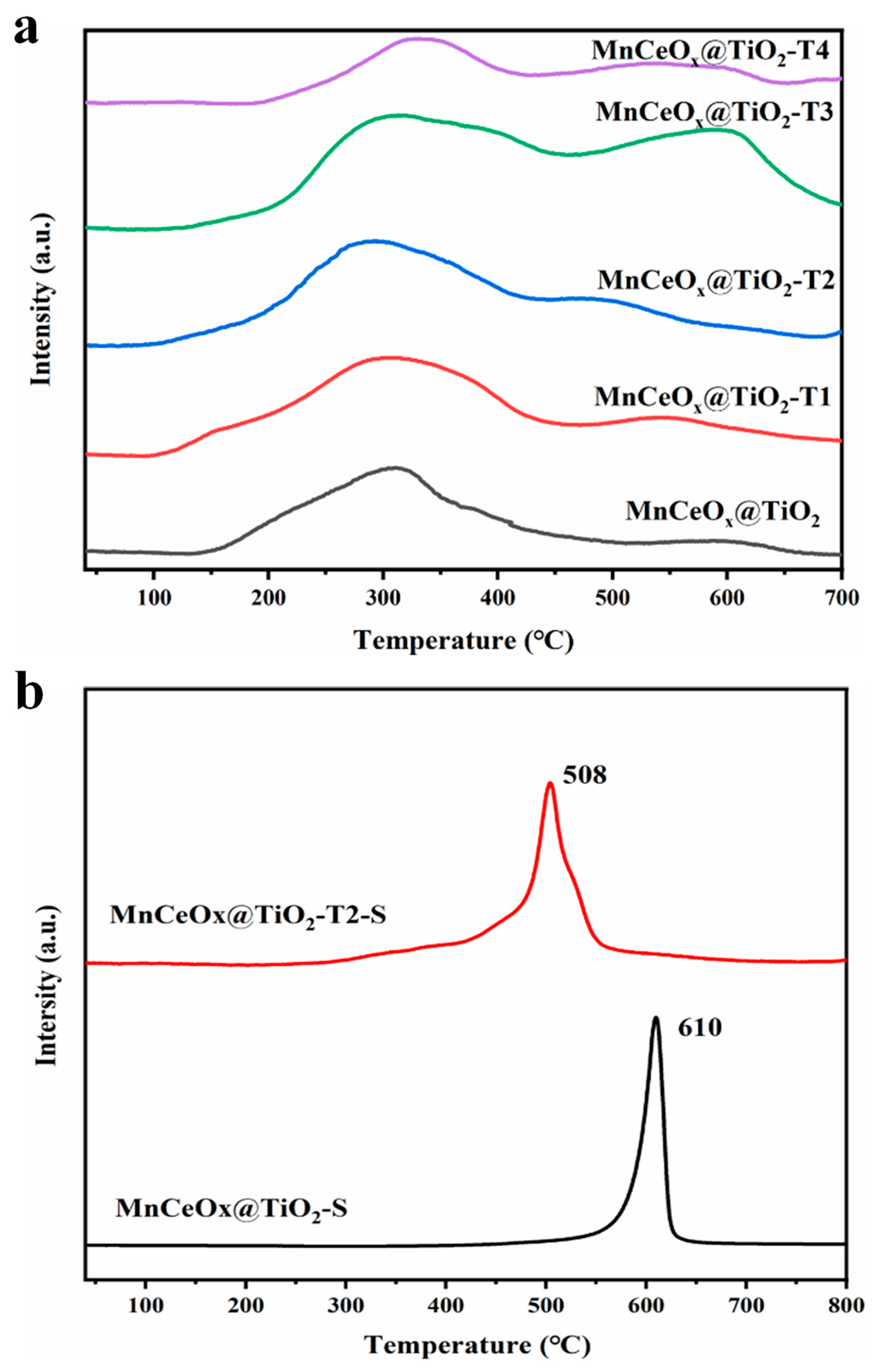

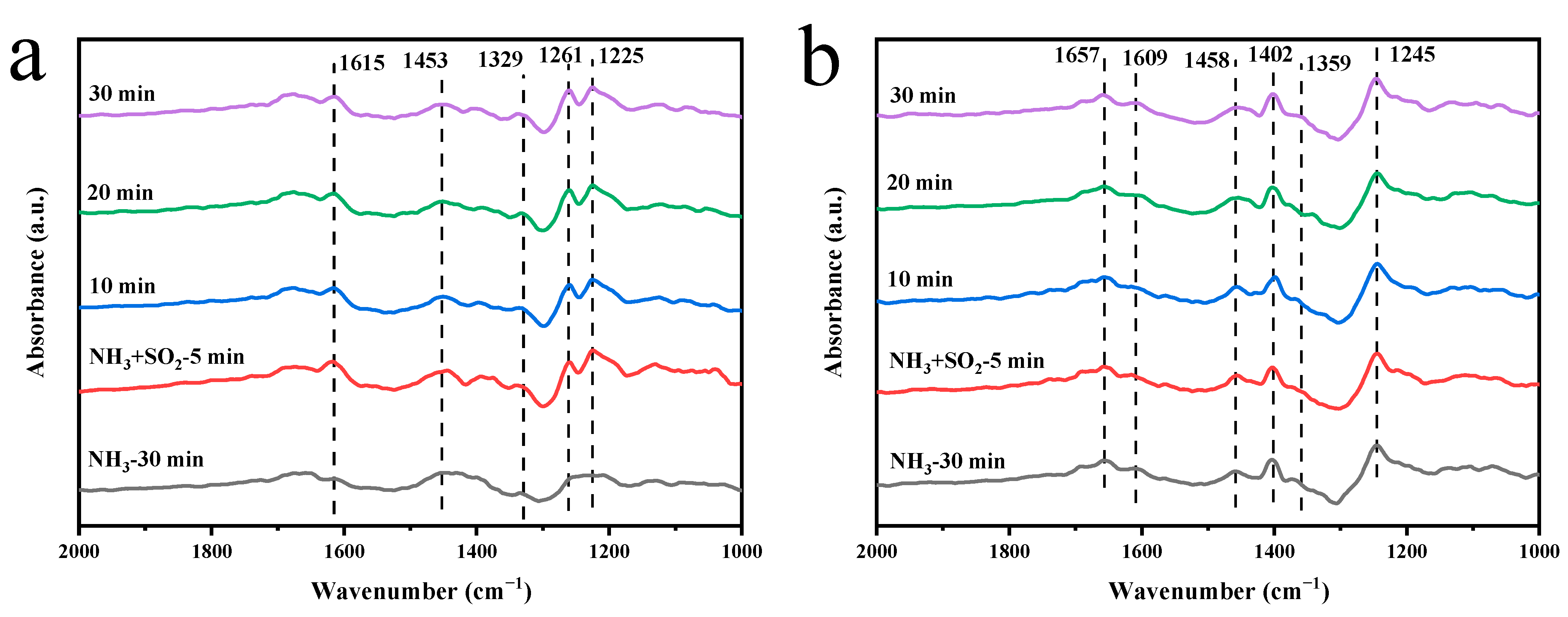


| Catalyst | Specific Surface Area (m2 g–1) | Pore Volume (cm3 g–1) | Pore Size (nm) |
|---|---|---|---|
| MnCeOx@TiO2 | 84.00 | 0.14 | 6.70 |
| MnCeOx@TiO2-T1 | 81.55 | 0.16 | 7.73 |
| MnCeOx@TiO2-T2 | 66.77 | 0.14 | 8.17 |
| MnCeOx@TiO2-T3 | 56.66 | 0.14 | 9.64 |
| MnCeOx@TiO2-T4 | 42.15 | 0.15 | 12.81 |
| Catalyst | Surface Atom Concentration (%) | Relative Concentration (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Ce | Ti | O | S | Mn4+ | Mn2+ | Ce3+ | Oα | |
| MnCeOx@TiO2 | 2.32 | 2.80 | 17.04 | 51.27 | - | 7.46 | 24.37 | 13.93 | 23.07 |
| MnCeOx@TiO2-S | 1.35 | 2.39 | 15.85 | 54.95 | 5.25 | 6.11 | 26.73 | 11.28 | 20.38 |
| MnCeOx@TiO2-T2 | 2.26 | 2.96 | 18.06 | 53.12 | - | 8.46 | 27.18 | 14.25 | 26.16 |
| MnCeOx@TiO2-T2-S | 2.20 | 2.89 | 18.76 | 54.01 | 1.18 | 7.71 | 27.81 | 11.52 | 25.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Wang, L.; Lin, X.; Xue, G.; Hu, H.; Ma, H.; Wang, Z.; Su, X.; Gao, Y. Effect of Tourmaline Addition on the Anti-Poisoning Performance of MnCeOx@TiO2 Catalyst for Low-Temperature Selective Catalytic Reduction of NOx. Molecules 2024, 29, 4079. https://doi.org/10.3390/molecules29174079
Zhao Z, Wang L, Lin X, Xue G, Hu H, Ma H, Wang Z, Su X, Gao Y. Effect of Tourmaline Addition on the Anti-Poisoning Performance of MnCeOx@TiO2 Catalyst for Low-Temperature Selective Catalytic Reduction of NOx. Molecules. 2024; 29(17):4079. https://doi.org/10.3390/molecules29174079
Chicago/Turabian StyleZhao, Zhenzhen, Liyin Wang, Xiangqing Lin, Gang Xue, Hui Hu, Haibin Ma, Ziyu Wang, Xiaofang Su, and Yanan Gao. 2024. "Effect of Tourmaline Addition on the Anti-Poisoning Performance of MnCeOx@TiO2 Catalyst for Low-Temperature Selective Catalytic Reduction of NOx" Molecules 29, no. 17: 4079. https://doi.org/10.3390/molecules29174079
APA StyleZhao, Z., Wang, L., Lin, X., Xue, G., Hu, H., Ma, H., Wang, Z., Su, X., & Gao, Y. (2024). Effect of Tourmaline Addition on the Anti-Poisoning Performance of MnCeOx@TiO2 Catalyst for Low-Temperature Selective Catalytic Reduction of NOx. Molecules, 29(17), 4079. https://doi.org/10.3390/molecules29174079








