Characterization and Cytotoxic Assessment of Bis(2-hydroxy-3-carboxyphenyl)methane and Its Nickel(II) Complex
Abstract
1. Introduction
2. Results and Discussion
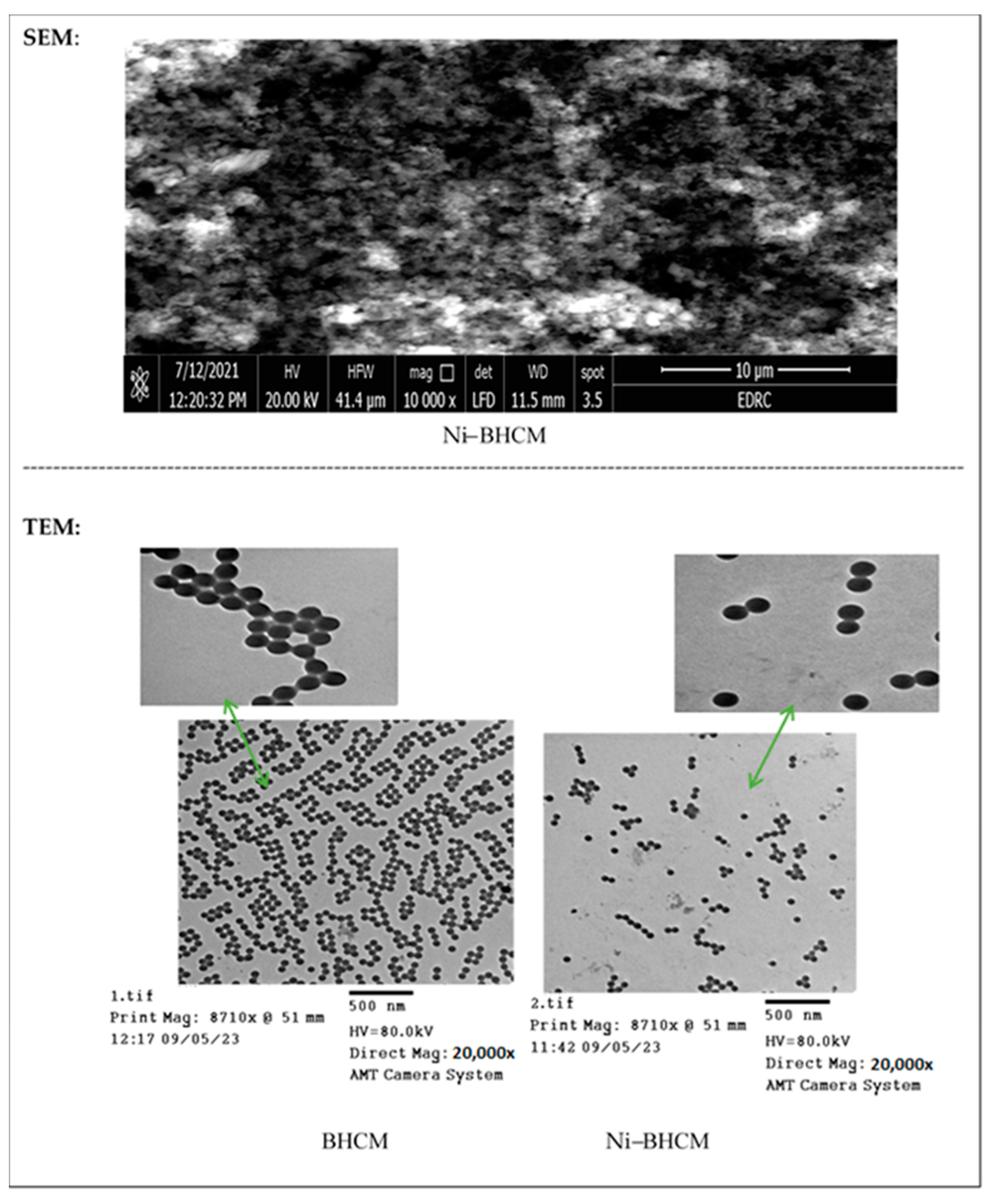
2.1. Morphology (SEM and TEM)
2.2. Powder XRD
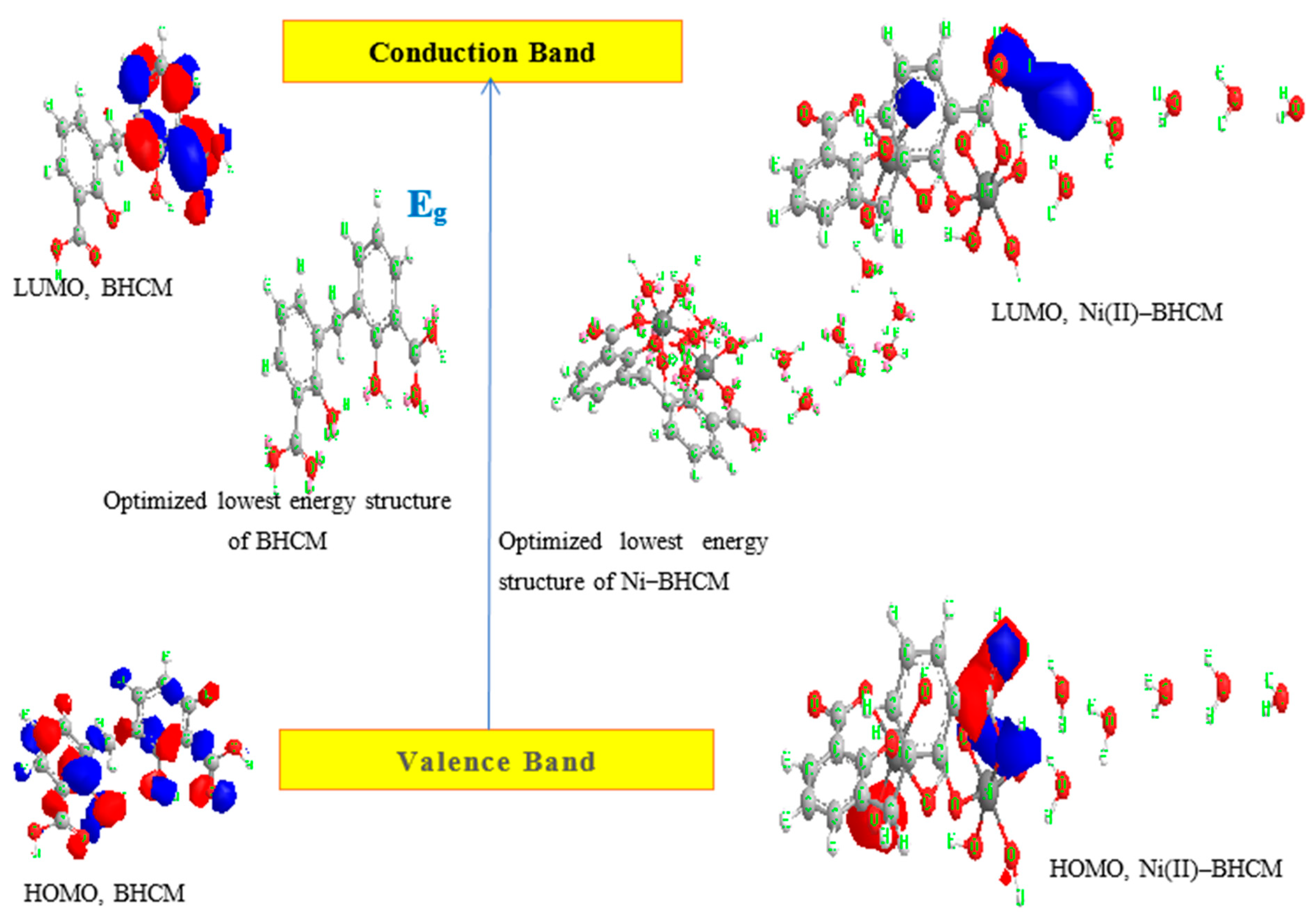
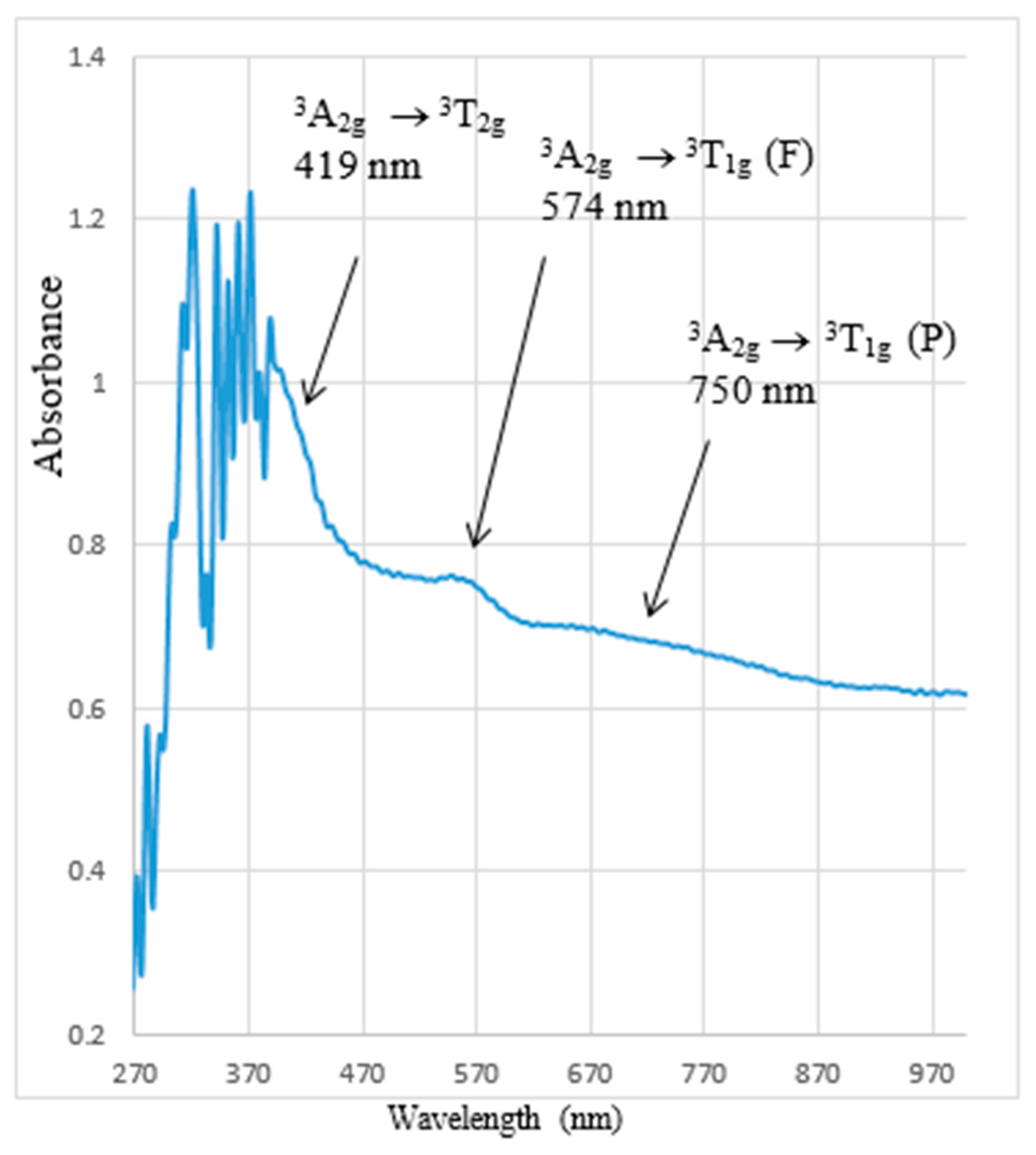
2.3. Optical Properties
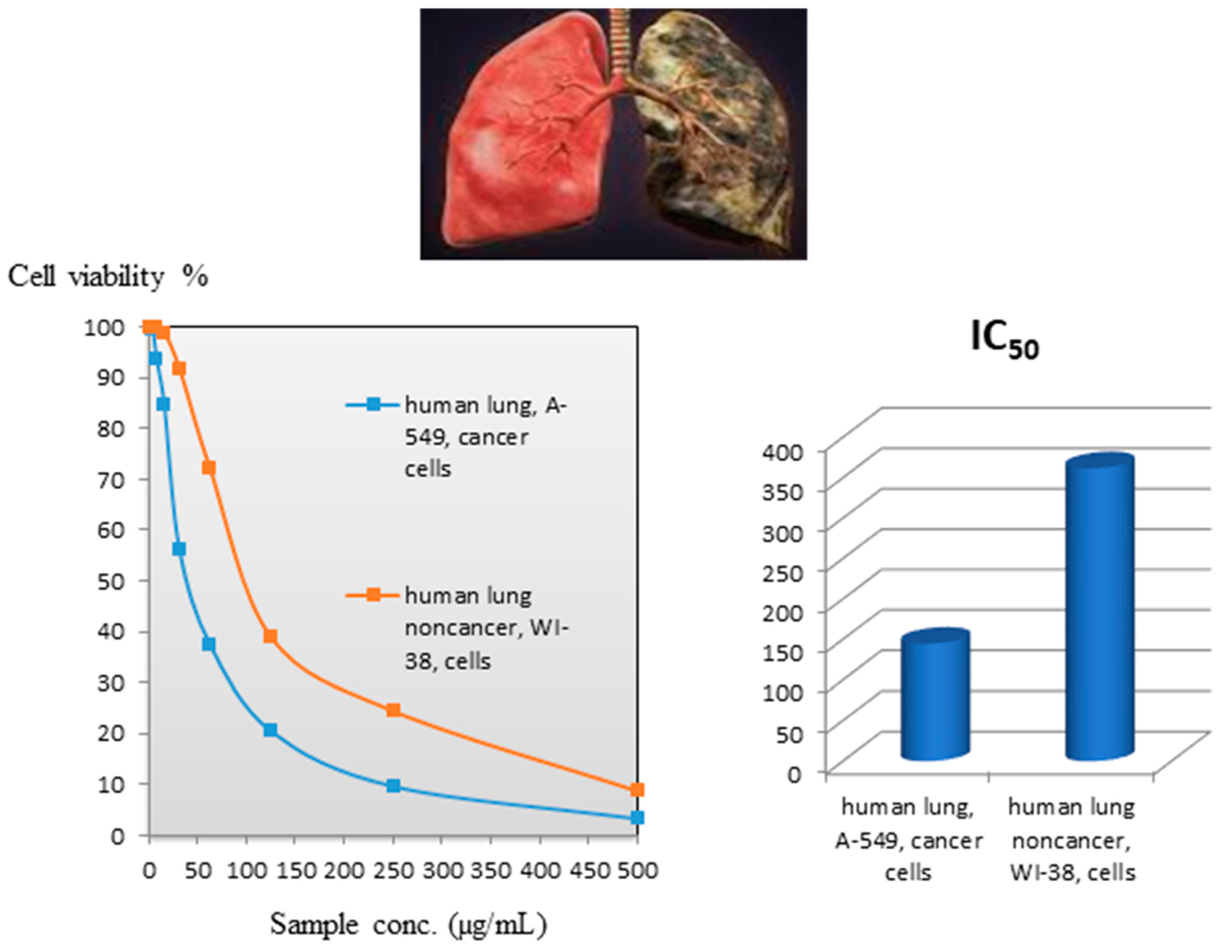
2.4. Cytotoxic Impact
2.4.1. Against Human Lung Cancer Cells (A-549)
2.4.2. Against Human Lung Noncancerous Cells
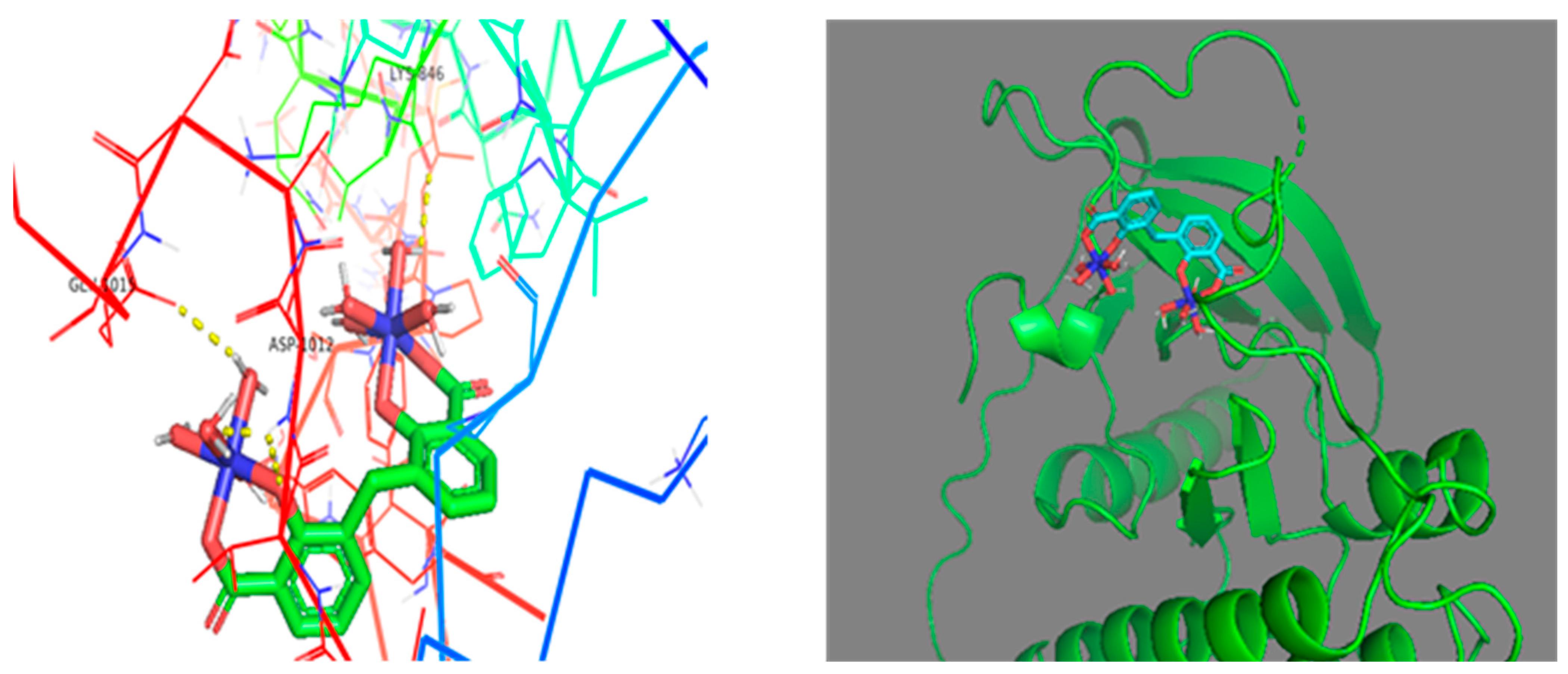
2.5. Molecular Docking Simulation
3. Experimental
3.1. Materials and Techniques
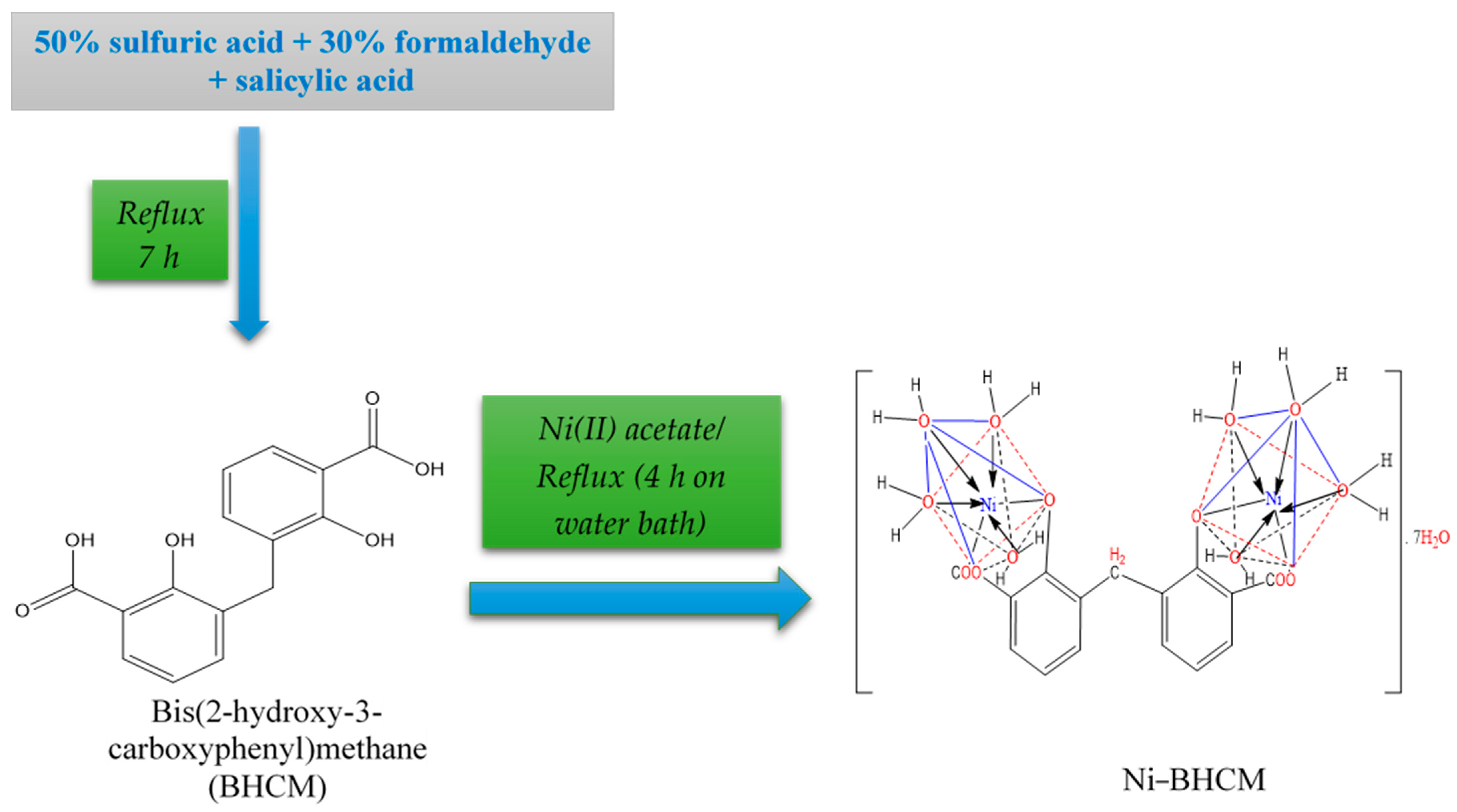
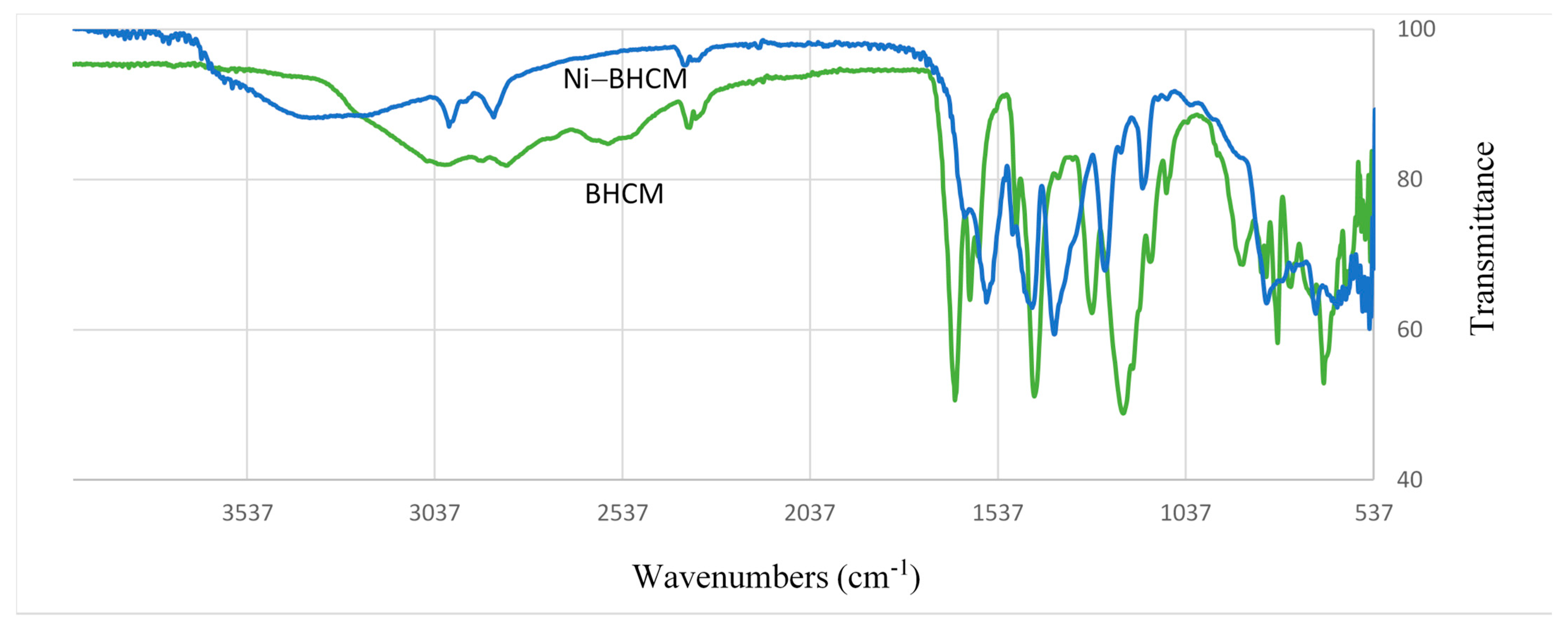
3.2. Syntheses (BHCM and Ni–BHCM)
3.3. Cell Viability Assay
3.3.1. Human Lung Carcinoma Cells (A-549)
3.3.2. Human Lung Fibroblast Normal Cells (WI-38)
3.3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aglan, H.A.; Ahmed, H.H.; El-Toumy, S.A.; Mahmoud, N.S. Gallic acid against hepatocellular carcinoma: An integrated scheme of the potential mechanisms of action from in vivo study. Tumor Biol. 2017, 39, 1010428317699127. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qiu, Y.; Zhang, Y. Research progress on therapeutic targeting of cancer-associated fibroblasts to tackle treatment-resistant NSCLC. Pharmaceuticals 2022, 15, 1411. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, G.; Wang, X.; Yang, Y.; Niu, X. Structure-Activity relationship of MDSA and its derivatives against Staphylococcus aureus Ser/Thr phosphatase Stp1. Comput. Biol. Chem. 2020, 85, 107230. [Google Scholar] [CrossRef] [PubMed]
- Takashi, H.; Takaaki, H.; Hyozo, S.; Alan, P.M.; Kaipenchery, A.K.; Simon, G.B.; Kata, M.M.; Tatjana, S.; Nazar, S.A.E.; Hong-Sik, H.; et al. Molecular design of lipophilic disalicylic acid compounds with varying spacers for selective lead(II) extraction. Talanta 2000, 52, 385–396. [Google Scholar]
- Shrestha, S.; Bhattarai, B.R.; Chang, K.J.; Leea, K.-H.; Cho, H. Methylenedisalicylic acid derivatives: New PTP1B inhibitors that confer resistance to diet-induced obesity. Bioorganic Med. Chem. Lett. 2007, 17, 2760–2764. [Google Scholar] [CrossRef]
- Trevin, S.; Bedioui, F.; Gomez, M.G.; Charreton, C.B. Electro polymerized nickel Micro cyclic complex-base films design and elctro-catalytic application. J. Mater. Chem. 1997, 7, 923–928. [Google Scholar] [CrossRef]
- Parulerkar, M.H.; Bhatt, H.A.; Potnis, S.P. New intermediates for plastics and coatings: 1. preparation and characterization of methylene-di-salicylic-acid. J. Ind. Chem. Soc. 1972, 49, 1201–1207. [Google Scholar]
- Clemmensen, E.; Heitman, A.H.C. Methylenedisalicylic acid and its reaction with bromine and iodine. J. Am. Chem. Soc. 1911, 33, 733–745. [Google Scholar] [CrossRef][Green Version]
- Sivapullaiah, P.V.; Soundararajan, S. Methylene di salicylates of rare-earths. J. Indian Inst. Sci. 1976, 58, 289–293. [Google Scholar]
- Patel, R.P.; Karampurwala, A.M.; Shah, J.R. Physicochemical studies on square planar Co2+, Ni2+ and Cu2+ chelate polymers. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1980, 87, 87–94. [Google Scholar]
- Du, M.; Zhang, Z.H.; Guo, W.; Fu, X.J. Multi-Component Hydrogen-Bonding Assembly of a Pharmaceutical Agent Pamoic Acid with Piperazine or 4,4′-Bipyridyl: A Channel Hydrated Salt with Multiple-Helical Motifs vs a Bimolecular Cocrystal. Cryst. Growth Des. 2009, 9, 1655–1657. [Google Scholar] [CrossRef]
- Wang, S.; Yun, R.; Peng, Y.; Zhang, Q.; Lu, J.; Dou, J.; Bai, J.; Li, D.; Wang, D. A Series of Four-Connected Entangled Metal–Organic Frameworks Assembled from Pamoic Acid and Pyridine-Containing Ligands: Interpenetrating, Self-Penetrating, and Supramolecular Isomerism. Cryst. Growth Des. 2012, 12, 79–92. [Google Scholar] [CrossRef]
- Wahl, H.; Haynes, D.A.; le Roex, T. Porous salts based on the pamoate ion. Chem. Commun. 2012, 48, 1775–1777. [Google Scholar] [CrossRef]
- Wahl, H.; Haynes, D.A.; le Roex, T. Solvate formation in lutidinium pamoate salts: A systematic study. CrystEngComm 2011, 13, 2227–2236. [Google Scholar] [CrossRef]
- Du, M.; Li, C.P.; Zhao, X.J.; Yu, Q. Interplay of coordinative and supramolecular interactions in engineering unusual crystalline architectures of low-dimensional metal–pamoate complexes under co-ligand intervention. CrystEngComm 2007, 9, 1011–1028. [Google Scholar] [CrossRef]
- Shi, X.M.; Li, M.X.; He, X.; Liu, H.-J.; Shao, M. Crystal structures and properties of four coordination polymers constructed from flexible pamoic acid. Polyhedron 2010, 29, 2075–2080. [Google Scholar] [CrossRef]
- Zhang, L.N.; Sun, X.L.; Du, C.X.; Hou, H.W. Structural diversity and fluorescent properties of new metal–organic frameworks constructed from pamoic acid and different N-donor ligands. Polyhedron 2014, 72, 90–95. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Xiong, J.B.; Tan, Y.H.; Wang, Y.; Deng, Y.P.; Xu, Q.; Wen, H.R. Solvothermal syntheses, crystal structures and photoluminescent properties of four coordination polymers with pamoic acid and pyridine mixed ligands. Inorganica Chim. Acta 2014, 410, 82–87. [Google Scholar] [CrossRef]
- Rocha, L.D.; Monteiro, M.C.; Anderson, J.T. Anticancer properties of hydroxycinnamic acids—A Review. Cancer Clin. Oncol. 2012, 1, 109–121. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, M. Potential cancer chemopreventive activity of protocatechuic acid. J. Exp. Clin. Med. 2011, 3, 27–33. [Google Scholar] [CrossRef]
- Gomes, C.A.; Cruz, T.G.; Andrade, J.L.; Milhazes, N.; Borges, F.; Marques, M.P.M. Anticancer activity of phenolic acids of natural or synthetic origin: A structure–activity study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.; Karimi, E.; Ghasemzadeh, A.; Jaafar, H.Z.E.; Karimi, E. Involvement of salicylic acid on antioxidant and anticancer properties, anthocyanin production anthocyanin production and chalcone synthase activity in ginger (Zingiber officinale Roscoe) varieties. Int. J. Mol. Sci. 2012, 13, 14828–14844. [Google Scholar] [CrossRef] [PubMed]
- Bonta, R.K. Dietary phenolic acids and flavonoids as potential anti-cancer agents: Current state of the art and future perspectives. Anti-Cancer Agents Med. Chem. 2020, 20, 29–48. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Montoro-Bustos, A.R.; Pereira-Reyes, R.; Paniagua, S.A.; Vega-Baudrit, J.R. Novel pathway for the sonochemical synthesis of silver nanoparticles with near-spherical shape and high stability in aqueous media. Sci. Rep. 2022, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Joseyphus, R.S.; Nair, M.S. Synthesis, characterization and biological studies of some Co(II), Ni(II) and Cu(II) complexes derived from indole-3-carboxaldehyde and glycylglycine, as Schiff base ligand. Arab. J. Chem. 2010, 3, 195–204. [Google Scholar] [CrossRef]
- Rashad, M.M.; Hassan, A.M.; Nassar, A.M.; Ibrahim, N.M.; Mourtada, A. Anew nano-structured Ni(II) Schiff base complex: Synthesis, characterization, optical band gaps and biological activity. Appl. Phys. A 2014, 117, 877–890. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Sivagami, M.; Asharani, I.V. Phyto-mediated Ni/NiO NPs and their catalytic applications-a short review. Inorg. Chem. Commun. 2022, 145, 110054. [Google Scholar] [CrossRef]
- Kaya, Y.; Gençkal, H.M.; Irez, G. Uv-Vis Spectra and Fluorescence Properties of Two Iminooxime Ligands and Their Metal Complexes: Optical Band Gaps. Gazi Univ. J. Sci. 2010, 23, 13–18. [Google Scholar]
- Kaya, I.; Koyuncu, S.; Şenol, D. Conductivity and band gap of oligo-2-[(4-chlorophenyl) imino methylene] phenol and its oligomer–metal complexes. Mater. Lett. 2006, 60, 1922–1926. [Google Scholar] [CrossRef]
- Karipcin, F.; Dede, B.; Caglar, Y.; Hur, D.; Ilican, S.; Caglar, M.; Sahin, Y. A new dioxime ligand and its trinuclear copper(II) complex: Synthesis, characterization and optical properties. Opt. Commun. 2007, 272, 131–137. [Google Scholar] [CrossRef]
- Turan, N.; Gündüz, B.; Körkoca, H.; Adigüzel, R.; Çolak, N.; Buldurun, K. Study of structure and spectral characteristics of the zinc(II) and copper(II) complexes with 5,5-dimethyl-2-(2-(3-nitrophenyl)hydrazono)cyclohexane-1,3-dione and their effects on optical properties and the developing of the energy band gap and investigation of antibacterial activity. J. Mex. Chem. Soc. 2014, 58, 65–75. [Google Scholar]
- Gittleman, J.I.; Sichel, E.K.; Arie, Y. Composite semiconductors: Selective absorbers of solar energy. Sol. Energy Mater. 1979, 1, 93–104. [Google Scholar] [CrossRef]
- Belmokhtar, A.; Yahiaoui, A.; Hachemaoui, A.I.; Abdelghani, B.; Sahli, N.; Belbachir, M. A NovelPoly{(2,5-diylfuran)(benzylidene)}:Anew synthetic approach and electronic properties. Int. Sch. Res. Not. 2011, 2012, 781879. [Google Scholar]
- Yakuphanoglu, F.; Erten, H. Refractive index dispersion and analysis of the optical constants of an ionomer thin film. Opt. Appl. 2005, 35, 969–976. [Google Scholar]
- Paul, T.C.; Podder, J. Synthesis and characterization of Zn-incorporated TiO2 thin flms: Impact of crystallite size on X-ray line broadening and bandgap tuning. Appl. Phys. A 2019, 125, 818. [Google Scholar] [CrossRef]
- Ahmed, A.H. N,N′-bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells. Open Chem. 2020, 18, 426–437. [Google Scholar] [CrossRef]
- Kavitha, P.; Reddy, K.L. Synthesis, spectral characterization, morphology, biological activity and DNA cleavage studies of metal complexes with chromone Schiff base. Arab. J. Chem. 2016, 9, 596–605. [Google Scholar] [CrossRef]
- Baxter, G.J.; Graham, A.B.; Lawrence, J.R.; Wiles, D.; Paterson, J.R. Salicylic acid in soups prepared from organically and non-organically grown vegetables. Eur. J. Nutr. 2001, 40, 289–292. [Google Scholar] [CrossRef]
- Wang, W.; Hong, L.; Shen, Z.; Zheng, M.; Meng, H.; Ye, T.; Lin, Z.; Chen, L.; Guo, Y.; He, E. Molecular insights into the anti-spoilage effect of salicylic acid in Favorita potato processing. Food Chem. 2024, 461, 140823. [Google Scholar] [CrossRef]
- Das, A.P.; Mathur, P.; Agarwal, S.M. Machine learning, molecular docking and dynamics-based computational identification of potential inhibitors against lung cancer. ACS Omega 2024, 9, 4528–4539. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Wang, X.; Tian, B.; Zhang, S.; Wang, T.; Ma, Y.; Fan, Y. Design, synthesis and biological evaluation of potent epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitors against resistance mutation for lung cancer treatment. Bioorganic Chem. 2014, 143, 107004. [Google Scholar] [CrossRef]
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.H.; Thabet, M.S. Metallo-hydrazone complexes immobilized in zeoliteY: Synthesis, identification and acid violet-1 degredation. J. Mol. Struct. 2011, 1006, 527–535. [Google Scholar] [CrossRef]
- Drago, R.S. Physical Methods in Inorganic Chemistry PB, 1st ed.; Affiliated East-West Press Pvt. Ltd.: New Delhi, India, 2012. [Google Scholar]
- Lewetegn, K.; Birhanu, H.; Liu, Y.; Taddesse, P. Synthesis of Zn0.87(Fe, Al)0.065O—MO (MO = NiO, Fe2O3, CuO, and CoO) nanocomposites: Structural, vibrational, optical and antibacterial studies. J. Mol. Struct. 2025, 1319, 139401. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M. Physicochemical studies on some selected oxaloyldihydrazones and their novel palladium(II) complexes along with using oxaloyldihydrazones as corrosion resistants. Inorg. Nano-Met. Chem. 2017, 47, 1652–1663. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hassan, A.M.; Gumaa, H.A.; Mohamed, B.H.; Eraky, A.M.; Omran, A.A. Copper(II)-oxaloyldihydrazone complexes: Physico-chemicalstudies; energy band gap and inhibition evaluation of free oxaloyldihydrazones toward the corrosion of copper metal in acidic medium. Arab. J. Chem. 2019, 12, 4287–4302. [Google Scholar] [CrossRef]
- Haque, R.A.; Iqbal, M.A.; Khadeer, M.B. Design, synthesis and structural studies of meta-xylyl linked bis-benzimidazolium salts: Potential anticancer agents against human colon cancer. Chem. Cent. J. 2012, 6, 68. [Google Scholar] [CrossRef]
- Lal, R.A.; Basumatary, D.; Arjun, K.D.; Kumar, A. Synthesis and spectral characterization of zinc(II), copper(II), nickel(II) and manganese(II) complexes derived from bis(2-hydroxy-1-naphthaldehyde) malonoyldihydrazone. Transit. Met. Chem. 2007, 32, 481–493. [Google Scholar] [CrossRef]
- Ferraro, J.R. Low Frequency Vibration of Inorganic and Coordination Compounds; Plenum: New York, NY, USA, 1971. [Google Scholar]
- Nicholls, D. Complexes and First-Row Transition Elements, London and Basingstoke; Macmillan Press LTD.: London, UK, 1974. [Google Scholar]
- Ahmed, A.H.; Moustafa, M.G. Spectroscopic, morphology and electrical conductivity studies on Co(II), Ni(II), Cu(II) and Mn(II)- oxaloyldihydrazone complexes. J. Saudi Chem. Soc. 2020, 24, 381–392. [Google Scholar] [CrossRef]
- Lee, J.D. Consize Inorganic Chemistry, 5th ed.; Blackwell Science Ltd.: Chichester, UK, 1996; p. 669. [Google Scholar]
- Abo-Ashour, M.F.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Bua, S.; Ibrahim, H.S.; Elaasser, M.M.; Kryštof, V.; Jorda, R.; Gratteri, P.; et al. 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: Synthesis, in vitro biological evaluation and in silico insights. Eur. J. Med. Chem. 2019, 184, 111768. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activities of thiazoles, 1,3-thiazines, and thiazolidine using chitosan-grafted-poly(vinylpyridine) as basic catalyst. Heterocycles 2015, 91, 1227–1243. [Google Scholar]
- Albqmi, M.; Elkanzi, N.A.A.; Ali, A.M.; Abdou, A. Design, Characterization, and DFT exploration of new mononuclear Fe(III) and Co(II) complexes based on Isatin-hydrazone derivative: Anti-inflammatory profiling and molecular docking insights. J. Mol. Struct. 2025, 1319, 139494. [Google Scholar] [CrossRef]
- Mert, S.; Demir, Y.; Sert, Y.; Kasımoğulları, R.; Gülçin, İ. Synthesis, biological evaluation and molecular docking of novel pyrazole derivatives as multitarget acetylcholinesterase and carbonic anhydrase inhibitors. J. Mol. Struct. 2025, 1319, 139472. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Chem. Biol. Methods Protoc. 2015, 1263, 243–250. [Google Scholar]
- Coumar, M.S. Molecular Docking for Computer-Aided Drug Design: Fundamentals, Techniques, Resources and Applications, 1st ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 463–477. [Google Scholar]
- Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Wang, S. Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking. Curr. Issues Mol. Biol. 2023, 45, 6564–6582. [Google Scholar] [CrossRef]






| Compound | Receptor | Binding Affinity (kcal/mol) | Interacting Amino Acids |
|---|---|---|---|
| L: Bis(2-hydroxy-3-carboxyphenyl)methane (BHCM) | 6CAO (implicated in the pathophysiology of lung cancer) | −7.8 | Phe-997 and Asp-1012 |
| Complex: (Ni–BHCM) | −10.3 | Glu-1015, Lys-846 and Asp-1014 |
| Compound | BHCM | Ni–BHCM | ||||
|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |
| Center coordinates of the grid box | −60.3605 | −7.1502 | −22.3618 | −60.3605 | −7.1502 | −25.2943 |
| Dimensions of the grid box (Ǻ) | 51.453 | 65.0735 | 67.1262 | 51.4252 | 65.0735 | 61.2672 |
| Exhaustiveness | 20 | 20 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.H.; Althobaiti, I.O.; Alenezy, E.K.; Asiri, Y.M.; Ghalab, S.; Hussein, O.A. Characterization and Cytotoxic Assessment of Bis(2-hydroxy-3-carboxyphenyl)methane and Its Nickel(II) Complex. Molecules 2024, 29, 4239. https://doi.org/10.3390/molecules29174239
Ahmed AH, Althobaiti IO, Alenezy EK, Asiri YM, Ghalab S, Hussein OA. Characterization and Cytotoxic Assessment of Bis(2-hydroxy-3-carboxyphenyl)methane and Its Nickel(II) Complex. Molecules. 2024; 29(17):4239. https://doi.org/10.3390/molecules29174239
Chicago/Turabian StyleAhmed, Ayman H., Ibrahim O. Althobaiti, Ebtsam K. Alenezy, Yazeed M. Asiri, Sobhy Ghalab, and Omar A. Hussein. 2024. "Characterization and Cytotoxic Assessment of Bis(2-hydroxy-3-carboxyphenyl)methane and Its Nickel(II) Complex" Molecules 29, no. 17: 4239. https://doi.org/10.3390/molecules29174239
APA StyleAhmed, A. H., Althobaiti, I. O., Alenezy, E. K., Asiri, Y. M., Ghalab, S., & Hussein, O. A. (2024). Characterization and Cytotoxic Assessment of Bis(2-hydroxy-3-carboxyphenyl)methane and Its Nickel(II) Complex. Molecules, 29(17), 4239. https://doi.org/10.3390/molecules29174239








