Abstract
Orthoflaviviruses, including zika (ZIKV), West Nile (WNV), and dengue (DENV) virus, induce severely debilitating infections and contribute significantly to the global disease burden, yet no clinically approved antiviral treatments exist. This review offers a comprehensive analysis of small-molecule drug development targeting orthoflaviviral infections, with a focus on NS2B-NS3 inhibition. We systematically examined clinical trials, preclinical efficacy studies, and modes of action for various viral replication inhibitors, emphasizing allosteric and orthosteric drugs inhibiting NS2B-NS3 protease with in vivo efficacy and in vitro-tested competitive NS2B-NS3 inhibitors with cellular efficacy. Our findings revealed that several compounds with in vivo preclinical efficacy failed to show clinical antiviral efficacy. NS3-NS4B inhibitors, such as JNJ-64281802 and EYU688, show promise, recently entering clinical trials, underscoring the importance of developing novel viral replication inhibitors targeting viral machinery. To date, the only NS2B-NS3 inhibitor that has undergone clinical trials is doxycycline, however, its mechanism of action and clinical efficacy as viral growth inhibitor require additional investigation. SYC-1307, an allosteric inhibitor, exhibits high in vivo efficacy, while temoporfin and methylene blue represent promising orthosteric non-competitive inhibitors. Compound 71, a competitive NS2B-NS3 inhibitor, emerges as a leading preclinical candidate due to its high cellular antiviral efficacy, minimal cytotoxicity, and favorable in vitro pharmacokinetic parameters. Challenges remain in developing competitive NS2B-NS3 inhibitors, including appropriate biochemical inhibition assays as well as the selectivity and conformational flexibility of the protease, complicating effective antiviral treatment design.
1. Introduction
The Orthoflavivirus (formerly known as Flavivirus) genus comprises more than 50 positive-sense and single-stranded RNA viruses [1,2] and belongs to the Flaviviridae family [3]. Zika virus (ZIKV, O. zikaense), West Nile virus (WNV, O. nilense), and the four serotypes of dengue virus (DENV1-4, O. denguei), all transmitted by mosquitoes, are the most prevalent and clinically relevant members of the genus. Infections with ZIKV and WNV can lead to neurological syndromes such as encephalitis, whereas both ZIKV and DENV infections may result in visceral diseases like hemorrhagic syndromes [1,2]. DENV alone accounts for an estimated 390 million infections annually, with nearly 100 million of these cases being clinically manifested and a significant majority occurring in Asia (70%) [4]. WNV has caused 7 million infections in the United States within a 20-year period, with 51,000 clinical cases and 2300 reported fatalities [5]. Although ZIKV typically causes mild symptoms in its 850,000 recorded cases, it can lead to fetal deformities if transmitted during pregnancy, with 3700 cases of congenital birth defects reported in the Americas before 2018, especially throughout a major outbreak that occurred in 2015–2016 [6].
Despite the global impact of these viral infections, no clinically approved antiviral treatments are currently available. The high infection rates and severe health consequences of orthoflavivirus infections underscore the importance of developing effective therapeutics [1,2,4,5,6]. Research efforts have focused on the structural and biochemical properties of orthoflaviviruses, leading to the development of vaccines for Japanese encephalitis virus (JEV), yellow fever virus (YFV), tick-borne encephalitis virus (TBEV), and DENV [7,8,9,10]. There are as yet no approved vaccines against ZIKV and WNV [11,12], and DENV vaccine development remains challenging due to the existence of four distinct serotypes, which can cause antibody-dependent enhancement (ADE) upon subsequent infections with different serotypes [10,13]. Two approved vaccines, Dengvaxia and Qdenga, are available for preventing severe dengue infections. After the fatalities of numerous children in the Philippines due to ADE following vaccination with Dengvaxia [14], Sanofi modified the application of its vaccine [15] for individuals with documented prior dengue infection and who are living in endemic areas [16,17]. Additionally, in 2023 Takeda withdrew the application for Qdenga to be approved in the U.S. [18,19]. DENV presents high genetic variability also among different strains of the same serotype, complicating further the development of an effective antiviral treatment [10]. Overall, the progress of formulating vaccines, pharmaceuticals, and immunotherapies for the prevention or treatment of dengue has been hindered by the intricate interplay between the dengue vector, virus, host, and disease pathogenesis [10,20].
In addition to vaccine development, therapeutic strategies targeting viral replication have garnered interest. Among these, small molecules targeting various components of the DENV replication machinery have demonstrated potential as antiviral agents. An overview of the DENV life cycle (those of ZIKV and WNV are similar) highlighting therapeutic targets is shown in Figure 1. A prime target for such inhibitors is the NS2B-NS3 protease, a complex responsible for the cleavage of the viral polyprotein, which is essential for viral replication [21]. Several small-molecule inhibitors have been designed to specifically target the active site of this protease, thereby disrupting its catalytic activity and impeding the viral life cycle [22]. Furthermore, the NS2B-NS3 protease is responsible for cleavage of host proteins in infected cells [23,24]. Additionally, NS2B is known to help evade the antiviral innate immune response by interacting with the host-immunomodulating proteins MAVS and IKKε [25]. Similarly, NS3 is reported to modulate host chaperone proteins, silencing the innate immune antiviral response [26]. NS3 also has an RNA helicase domain, which ensures efficient translation of the viral genome [27]. The NS3 helicase domain requires acetylation by host acetyltransferase KAT5γ to exert its activity, representing a drug target distinct from the viral protease [28]. The NS2B-NS3 protease is largely conserved across the four DENV serotypes as well as WNV and ZIKV [29], making it an attractive target for the development of anti-orthoflaviviral inhibitors [30]. Another promising target is the NS3–NS4B interaction, which plays a crucial role in facilitating the viral replication complex assembly [31,32]. Inhibitors targeting this interface have shown potential in disrupting the formation of the replication complex, thus hindering viral RNA synthesis [33]. Another relevant target is NS5, which acts as a viral RNA polymerase (RNA-dependent RNA polymerase, RdRp) [34] and methyltransferase [35] in the replication complex [31] but also migrates in the host cell nucleus to modulate the host’s antiviral response [36]; the developments in NS5-targeting compounds have recently been reviewed [37]. Other viral and host machineries have been successfully targeted to combat orthoflaviviral infections in a preclinical setting, but as none of those have yet yielded a clinical candidate, they are not covered in this work; we refer to reviews on the topic collecting the most recent developments [38,39]. In this review, we look at orthoflaviviral replication inhibitors (i.e., small-molecule compounds exerting a virucide effect, thus directly blocking viral replication) that have entered clinical trials. Compounds modulating the immune response or acting on the symptomatic form of the disease, as well as biologics, have been excluded. Additionally, we review NS2B-NS3 protease inhibitors with reported in vivo efficacy. Lastly, we focus on competitive broad-spectrum anti-orthoflaviviral NS2B-NS3 inhibitors with reported cellular antiviral activity in vitro under 10 µM.
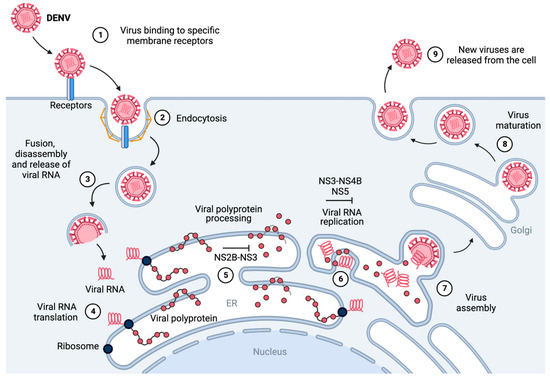
Figure 1.
Schematic representation of the replication cycle of dengue virus [31,40]. (1) The virus binds to host-cell membrane receptors and (2) enters the host cell via clathrin-mediated endocytosis. (3) After internalization, the viral particle disassembles and fuses with the endosome, releasing the viral RNA into the cytoplasm. (4) The viral RNA is translated at the rough endoplasmic reticulum into a viral polyprotein. (5) The viral polyprotein is processed by host and viral proteases (such as NS2B-NS3), releasing seven non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) and three structural proteins (C, prM, and E). (6) The NS proteins (such as NS3-NS4B and NS5) and the ER (endoplasmic reticulum) form a convolution called the replication complex, which replicates the viral RNA [31]. (7) The replicated genome and translated viral structural proteins are assembled, and immature virus particles stem from the surface of the ER. (8) Viral particles undergo trafficking via the trans-Golgi network, maturing into their infectious conformation. (9) The fully developed virions are liberated from the host cell, thereby facilitating the propagation of infection to neighboring cells. Figure generated with BioRender.com.
2. NS2B-NS3 Functional Analysis
The activity of NS2B-NS3 is schematically represented in Figure 2, where eight subsites (from left to right: S4, S3, S2, S1, S1’, S2’, S3’, S4’) are identified in the protease, which recognize as many amino acids (P4, P3, P2, P1, P1’, P2’, P3’, P4’) from the targeted peptide sequence to be cleaved [41,42]. Most relevant for binding to the protease are the S1–S4 subsites, which in the case of NS2B-NS3, accommodate a sequence of cationic amino acids, such as Lys and Arg. Hydrolysis of the amide bond takes place between the so-called P1 and P1’ residues by a nucleophilic serine present at the center of the catalytic site and part of a catalytic triad (Asp-His-Ser). The Asp-His pair deprotonates the side chain -OH of the catalytic serine, increasing its nucleophilicity. As shown in Figure 2, the catalytic serine attacks the carbonyl of the P1 residue, forming a tetrahedral intermediate, whose oxyanion moiety is thought to be stabilized by the so-called oxyanion hole [42]. Collapse of this intermediate results in the release of the P1’ to P4’ peptide fragment and yields an acyl protease intermediate with the P1 to P4 remaining peptide. The active site is then regenerated via hydrolysis of the ester by an adventitious molecule of water and positioned by an intricate web of hydrogen bonds from the protease, releasing the P1–P4 fragment.
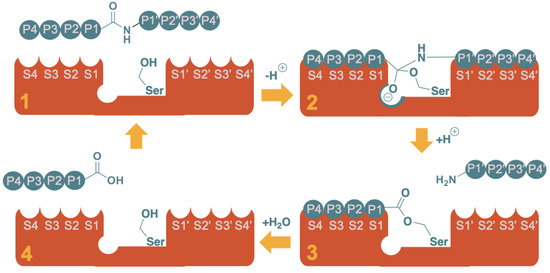
Figure 2.
Schematic representation of the proteolysis of an octapeptide by a serine protease. The mechanism can be rationalized in four major stages: (1) Substrate recognition; (2) Nucleophilic attack and formation of the tetrahedral intermediate (note the oxyanion hole stabilizing the negative charge); (3) Release of the “prime” fragment (H-P1’-P2’-P3’-P4’) as a free amine and formation of the acyl protease intermediate with the non-prime fragment (P4-P3-P2-P1-O-[Ser]); (4) Regeneration of the active site, releasing the non-prime fragment as free carboxylic acid.
3. Mechanisms of Enzyme Inhibition
In the exploration of the structural design and efficacy of inhibitors of viral proteases, kinetic and structural analyses play crucial but distinct roles in understanding their mechanism of inhibition and influence the different stages and strategies in their development as new antiviral therapeutics. In the kinetic analysis, which focuses on how a drug inhibits its target enzyme, including the nature and strength of the inhibition, competitive, non-competitive, and uncompetitive inhibition are distinguished. The activity of a competitive inhibitor depends on the concentration of both the inhibitor and the substrate, while that of a non-competitive inhibitor will not be affected by the substrate concentration. In uncompetitive inhibition, the inhibitor binds only to the enzyme–substrate complex and not to the free enzyme; this form of inhibition is unique in that it requires the enzyme to first bind to its substrate before the inhibitor can bind [43]. In the structural analysis of inhibition, the molecular architecture of the target and its binding interactions with the drug are considered, and binding to the orthosteric site, the active site, is distinguished from binding to an allosteric site [44], a different site that modulates the activity of the enzyme.
The most straightforward type of inhibition is competitive orthosteric, where the inhibitor competes directly with the substrate for the active site [43]. However, in some cases orthosteric enzyme inhibition can also result in non-competitive behavior, for instance in cases where compounds bind to the active site but induce conformational changes affecting the affinity for the natural substrate or the catalytic activity [45,46]. Herein, we refer to such compounds as non-competitive orthosteric inhibitors. It is important to note that this kind of inhibition is distinct from uncompetitive inhibition, a mechanism through which none of the NS2B-NS3 protease inhibitors discussed here were reported to act.
Parallelly, the general kinetic of allosteric inhibition is non-competitive, where the inhibitor binds to a site distinct from the active site, leading to structural changes that reduce enzyme activity without direct competition with the natural substrate [47,48]. However, allosteric inhibition can also show competitive behavior, for instance when binding of the substrate affects the structure of the allosteric site [49,50]. In addition, an inhibitor may bind covalently to the active site, either reversibly or irreversibly. Irreversible competitive covalent inhibitors initially compete with the substrate for binding to the active site. Upon binding, they form a covalent bond with the enzyme, leading to irreversible inactivation. Reversible competitive covalent inhibitors also form a covalent bond with the enzyme, but this bond can be cleaved (often hydrolyzed), re-forming the free enzyme and inhibitor [51].
4. NS2B-NS3 Protease Structural Analysis
The crystal structure of the DENV3 NS2B-NS3 bound to the peptide-like inhibitor Bz-nKRR-H (Benzoyl-Nle-Lys-Arg-Arg-H) was solved in 2012 (PDB: 3D1I) [52]. More recently, the conformation and assembly of the DENV NS2B-NS3 was studied using NMR and molecular dynamics [53]. It is a serine protease, composed of the co-activator NS2B and the essential subunit NS3. The catalytic triad of amino acids Ser135-His51-Asp75 responsible for the cleavage of the P1-P1’ amide bond is located in the NS3 subunit, while the NS2B co-activator contributes to the formation of the S2 and S3 subsites [31,34]. The specificity and location of the S1-S4 subsites is shown for the crystal structure of DENV3 NS2B-NS3 in Figure 3, and it was determined by the positioning of the P1-P4 residues of the Bz-nKRR-H ligand crystallized within.
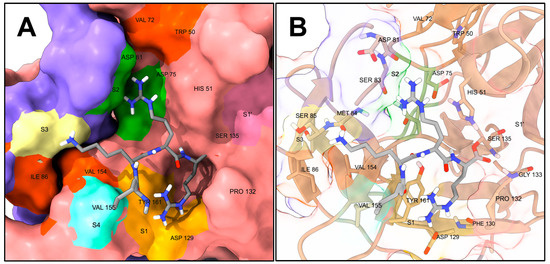
Figure 3.
Snapshot of DENV2 NS2B-NS3 bound to the peptide aldehyde Bz-nKRR-H (PDB: 3D1I) [31]. Benzoyl-Nle-Lys-Arg-Arg-H (Bz-nKRR-H) is in dark gray. Only non-polar hydrogens are shown; the Bz- fragment was hidden for displaying purposes. (A) Representation showing the solvent-excluded surface of the protease functionally colored: the light pink surface is from NS3 and the violet from NS2B; S1 is highlighted in light orange, S2 in green, S3 in light yellow, S4 in aquamarine, and S1’ in dark pink; hydrophobic surfaces (generally not addressed as specificity subsites per se) are highlighted in dark orange. (B) Representation showing only the side chains and backbones of relevant amino acids of NS2B-NS3 as sticks; the protease is colored in tan and the rest of its backbone is represented as ribbon. Figure generated with UCSF ChimeraX v1.4 [54,55].
Most of the binding region of the protease is shallow; however, the S1 subsite is generally considered the best-defined, and it is characterized by the side chain of Tyr161 and the side chain of Asp129, which respectively display cation–pi interaction and form an ion pair with the guanidinium of the P1 Arg of Bz-nKRR-H [52,56]. Additionally, Pro132 encases the side chain of P1, creating an open cavity defining the S1 subsite. The oxyanion hole, located in the vicinity of the catalytic Ser135 and characterized by the backbone N-Hs of Ser135 and Gly133, also represents an appealing druggable volume, being more buried and furnishing a cavity rich in polar interactions [29,52,56]. The S2, S3, and S4 subsites of the protease are more exposed to the solvent than the S1 or the oxyanion hole, and apart from the presence of Asp89 between S2 and S3 and the catalytic triad Asp75-His51-Ser135 between S1 and S2, do not present significantly buried binding surfaces. However, as explained in the literature [52,56,57,58], there is a hydrophobic area between the S3 and S4 subsites created by the interface of NS2B-NS3 (Ile86 and Val154, dark orange surface in Figure 3), which can potentially be targeted by hydrophobic substituents [59,60,61,62]. Similarly, beyond the S2 subsite, away from the catalytic center, the Trp152 and Val72 side chains make for an additional hydrophobic surface. Many orthosteric inhibitors showing non-competitive behavior are present in the literature, and such compounds are likely to bind to some parts of the S1–S4 subsites.
It is important to note that the orthoflaviviral NS2B-NS3 complexes commonly employed for evaluating biochemical protease inhibition and for determining crystal structures are typically fusion constructs in which NS2B and NS3 are covalently linked by a polyglycine chain [52,56,63,64].
5. NS2B-NS3 Inhibition
A successful strategy to produce effective viral protease inhibitors is to generate a mimic of the P1–P4 peptide fragment, which would compete with the natural substrate for the S1–S4 subsites, impeding further cleavage of the viral polyprotein [65]. Additionally, an electrophilic trap (viz., warhead) reacting with the nucleophilic catalytic serine of NS2B-NS3 can be included at the C-terminus of the P1-mimic, generating an inhibitor that will bind covalently to the active site. Covalent viral protease inhibitors have already been successfully marketed, e.g., Narlaprevir, Arlansa® against HCV (hepatitis C virus, from another genus of the Flaviviridae) [66] and Nirmatrelvir, Paxlovid® against SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) [67]. This strategy has been exploited before [68,69], improving the inhibition of DENV NS2B-NS3 by the peptide Bz-nKRR-NH2 3000-fold, by substituting the C-terminal amide with a boronic acid warhead (Figure 4). Formation of the protease-inhibitor covalent complex locks the enzyme in an inactive state amenable to crystallographic studies. For instance, the C-terminal aldehyde (Bz-nKRR-H) made it possible to solve the bound crystal structure of DENV3 NS2B-NS3 (Figure 3) [52], and peptide-hybrid C-terminal boronic acid cn-716 that of WNV [63] and ZIKV [70]. From the previously reported SAR studies of covalent orthoflaviviral protease inhibitors, it emerged that covalent inhibition requires the presence of additional, specific molecular recognition elements [63,71] and that it is possible to achieve comparable inhibition optimizing the peptide sequence without introducing an electrophilic warhead (mb-53 in Figure 4) [63,72]. The inhibition of viral proteases has previously proven effective in treating various viral diseases; nevertheless, NS2B-NS3 inhibitors remain underrepresented in the realm of clinical development. Biochemical optimization for orthoflaviviral protease inhibitory activity of peptide-like inhibitors has often not translated into improvement of antiviral cellular efficacy, hampering the development of effective competitive inhibitors [59]. This has been ascribed principally to both the artificial nature of the fusion protein construct mostly used to assess biochemical viral protease inhibition and the cellular environment, requiring membrane permeability and proteolytic stability features, which are difficult to optimize on small cationic peptides [59]. Extensive work has been performed to improve the protease biochemical assays, both in terms of the type of NS2B-NS3 construct and assay conditions. It has been established that the unlinked NS2B-NS3 construct, as opposed to the linked or self-cleavable constructs, exerts better enzymatic activity [73]. Unfortunately, and despite being closer to the natural form, the unlinked protease is still not the primary choice for most researchers in the drug discovery field, mainly due to the associated time-consuming efforts and extra costs. Recently, it has been reported [74] that variations in assay conditions, viz. pH, salinity, buffers, and temperature, can modify the binding affinity of inhibitors as well as the proteolytic capacity. This shows the importance of choosing the right conditions, particularly in the validation of new hits.
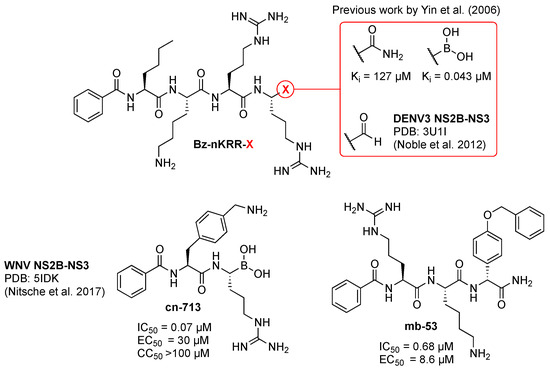
Figure 4.
Previously reported orthoflaviviral protease peptide-like inhibitors. Ki and IC50 values are relative to DENV2 NS2B-NS3 inhibition and EC50 to efficacy of DENV2-infected cells. Data for Bz-nKRR-X are reported in references [52,68,69], for cn-716 in reference [63], and for compound mb-53 in reference [72].
In addition to competitive inhibition, many non-competitive NS2B-NS3 inhibitors are reported as orthosteric (reviewed in the later sections) in the literature, inhibiting the protease by binding to parts of the active site in NS3 and disrupting the functional interactions with the NS2B protein domain. These inhibitors could be classified more accurately as protein–protein interaction (PPI) inhibitors.
Furthermore, NS2B-NS3 exists in two distinct conformations (Figure 5), an open, inactive conformation and a closed, active conformation. Competitive orthosteric inhibitors are studied in the closed, active conformation of NS2B-NS3 (Figure 5B), which is formed upon binding with a substrate-like molecule in the active site. The impact of orthosteric non-competitive inhibitors on conformational changes remains unclear. An allosteric site has been identified in the NS3 subunit behind the active site (Figure 5), around Ala125. Allosteric inhibition hinders the NS2B cofactor to close around the NS3 subunit, impeding the formation of the active site. It is possible that allosteric inhibition may be achieved also by binding to different regions of the open conformation. While targeting the orthosteric site presents challenges due to the shallow surface of the S1–S4 subsites, structural rationalization of allosteric inhibition is also difficult because of the dynamic nature of the drastic conformational change between the inactive and active forms [75,76,77,78].
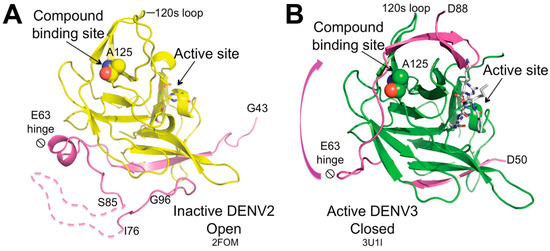
Figure 5.
Crystal structures of NS2B-NS3 in (A) open inactive and (B) closed active conformation. Upon substrate binding, the NS2B region (colored purple) spanning residues 63 to 88 undergoes conformational changes around the hinge at residue 63, subsequently interacting with the region adjacent to Ala125, which is located between the 120 s and 150 s loops. Ala125 is depicted as spheres, while the catalytic triad is illustrated as sticks. NS3 is in yellow in (A) and green in (B). The crystal structure of DENV3 NS2B-NS3 protease (B) is complexed with the substrate-like, active-site inhibitor Bz-nKRR-H (represented as white sticks). Figure taken from Yildiz M. et al. ACS Chem Biol. 2013, 8(12), 2744–2752 [75].
This review aims to provide a comprehensive overview of small-molecule drug development targeting DENV, ZIKV, and WNV, as these represent the orthoflaviviruses that cause the highest health burden to humans. We focus on preclinical and clinical results for compounds inhibiting viral replication to determine the impact of NS2B-NS3 inhibition on anti-orthoflaviviral drug development. We systematically examine clinical trials registered on ClinicalTrials.gov that assess the safety and efficacy of various viral replication inhibitors in human patients and report their preclinical efficacy and mode of action. Additionally, we discuss allosteric and orthosteric drugs inhibiting the NS2B-NS3 protease with proven in vivo efficacy. Because of the limited number of competitive orthosteric viral protease inhibitors reported with in vivo efficacy, we also explore in vitro-tested competitive NS2B-NS3 protease inhibitors exhibiting cellular antiviral activity. This review offers a detailed analysis of the anti-orthoflaviviral drug development pipeline as of June 2024, emphasizing viral NS2B-NS3 protease inhibitors. It should be noted that such inhibitors, including the ones for which no in vivo efficacy has yet been reported and which are not discussed here, have recently been reviewed [79].
6. Small-Molecule Therapeutics in Clinical Trials
A comprehensive search was conducted on ClinicalTrials.gov, the EU Clinical Trials Register, and the international traditional medicine clinical trial registry (ISRCTN) using the terms “Drug NON Biologic” or “NOT vaccine” in conjunction with the target virus (DENV, ZIKV, or WNV) to identify pertinent therapeutic candidates. All retrieved results were evaluated for relevance and subsequently incorporated into the study. A total of eleven compounds were identified that inhibit orthoflaviviral growth and are either registered on ClinicalTrials.gov or announced for clinical trials by their respective sponsors. Figure 6 presents an overview of these agents, and in Table 1 their most significant clinical and preclinical data are reported. Based on the findings from our study, only one small-molecule therapeutic against DENV or orthoflaviviral infections has demonstrated antiviral efficacy in clinical development thus far, although several compounds exhibited antiviral efficacy in preclinical animal models. To assess the in vivo efficacy of potential anti-orthoflavivirals, a variety of infected murine models were employed, including A129 and AG129 mouse strains, Balb/c, ICR suckling, Chinese Kunming, and Atg16/1 HM mice. The AG129 mouse model, which lacks both type I (IFN-α/β) and type II (IFN-γ) interferon receptors, is especially valuable for antiviral drug development against DENV and ZIKV. This is due to its heightened susceptibility to viral infections, making it the most widely used model in this area of research [80,81]. In contrast, the A129 mouse model is deficient solely in the type I interferon (IFN-α/β) receptor and has mostly been used in ZIKV infection models [82]. The in vivo experiments focused on evaluating generally two primary outcomes: survival rates (SR) and viral load reduction in drug-treated groups compared with vehicle control groups. The highest DENV viremia level is measured around days 3 and 4 post-infection in the AG129 model, and it is reported here as peak viremia. The viral load during peak viremia is generally quantified through viral RNA copies or viral pFU per organ. In common animal trials, the treatment is started either the same day as or prior to infection (prophylactic regimen, PR in Table 1), or 24–72 h post infection (therapeutic regimen, TH in Table 1). In most cases, differences in viremia were not observed in treatments started >48 h post infection.
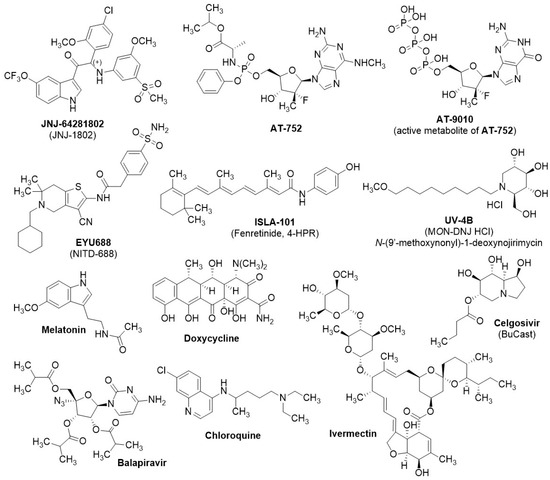
Figure 6.
Chemical structures of viral replication inhibitors of clinical relevance from the literature. References and biological activities are in Table 1.
It is important to note that the pathogenesis of orthoflaviviral infection in those animal models is different from the timeline observed in humans infected through a mosquito bite. For instance, common symptoms of dengue are shown after 4–7 days of incubation, and DENV can be detected only slightly before. The symptomatic (febrile) stage lasts for about 5–7 days, and while it is self-resolving in most cases, it can escalate in some toward a critical phase, resulting in a hemorrhagic fever or shock syndrome that can be life-threatening [83]. An anti-DENV therapeutic treatment (wt-TH in Table 1) should be administered in the early febrile window to show detectable efficacy and potentially prevent internal bleeding and plasma leakage. Most recent studies require the patient to show symptoms for <48 h to be included in a therapeutic regimen. Sampling and fast inclusion in clinical trials make the recruitment of eligible patients with confirmed DENV often difficult. A prophylactic treatment regimen would instead be administered prior to a possible infection and could potentially serve as a preventive treatment in people at risk (wt-PR in Table 1). In some recently developed dengue human infection models (DHIM in Table 1, or dengue human challenge) [84,85], the participants are inoculated with an attenuated virus after the start of a prophylactic regimen of the drug under investigation.

Table 1.
Orthoflaviviral replication inhibitors of clinical relevance.
Table 1.
Orthoflaviviral replication inhibitors of clinical relevance.
| Preclinical Data | Clinical Data | |||||||
|---|---|---|---|---|---|---|---|---|
| Name | Target | Cellular EC50 Virus (Cell Line) | Peak Viremia Reduction or %SR, Drug Regimen [a], Dosage, Route [b] (Animal Infection model) [c] | Trial Identifier | Phase (Study Type) [d] | Year [e] | Status/Outcome | Ref. |
| JNJ-64281802 | NS3–NS4B | 0.06–1.4 nM DENV1-4 (Multiple) | ≤LLOD, PR, 3 mg/kg, SC (DENV1/2, NHP) ≤LLOD, PR, 2 mg/kg, IP (DENV2 RL, AG129) <1-log, TH (4d p.i.) [f] 60 mg/kg, IP (DENV2 RL, AG129) | NCT05201937 NCT04906980 NCT05048875 NCT05201794 NCT04480736 | I II (wt-TH) II (DHIM-DENV3) II (wt-PR) II (DHIM-DENV1) | 2023 2024 2024 2025 2027 | Safe Terminated [g] Recruiting Recruiting Suspended | [86,87] |
| AT-752 | Viral RNA polymerase | 0.49/0.77 μM [h] DENV2/3 (Huh-7) | ≤1-log, PR, 1000–500 mg/kg, PO (DENV D2Y98P, AG129) | NCT04722627 NCT05366439 NCT05466240 | I I (DHIM) II (wt-TH) | 2021 2023 2023 | Safe Terminated [i] Terminated [i] | [88,89] |
| EYU688 | NS4B | 8–38 nM DENV1-4 (Vero) | 4.3-log, PR, 100 mg/kg, PO (DENV2 RL, AG129) ≤-log, TH (2d p.i.), 30 mg/kg, PO (DENV2 RL, AG129) | NCT06006559 | II (wt-TH) | 2026 | Recruiting | [90] |
| ISLA-101 | Host nuclear import inhibitor, NS5 entry | 1.3–2.4 µM DENV1-4 (Huh-7) | 70% SR, PR, 20 mg/kg, PO (DENV2 S221, AG129) [j] | n.a. | I II (TH and PR) | 2022 2023 [k] | Safe [k] Announced | [91,92] |
| UV-4B | Host ER glucosidases | 2.1–87 µM DENV1-4 (Vero) | 100% SR, PR, 40 mg/kg, PO 90% SR, TH (2d p.i.), 40 mg/kg, PO (DENV2 S221AG129) [l] | NCT02061358 NCT02696291 | I I | 2015 2017 | Safe (1000 mg) Terminated [m] | [93,94] |
| Melatonin | Host anti-inflammatory factors/viral proteins (NS3) | 140–200 µM DENV2 (Huh-7 EA.hy.926, A549, U937) | SR 69%, PR, 500 µg/kg (WNV WN-25, CD1) | NCT05034809 | II (wt-TH) | 2022 | Not yet recruiting | [95,96] |
| Doxycycline | NS2B-NS3 | 40 µM DENV2 (Vero) | n.a. | n.a. n.a. CTRI/2021/09/036661 CTRI/2018/01/011548 | II (wt-TH) II (wt-TH) II (wt-TH) II (wt-TH) | 2015 2022 2023 2019 | Reduction in inflammatory markers n.a. | [97,98,99] |
| Celgosivir | Host ER glucosidases | 0.22–0.65 µM DENV1-4 (BHK-21) | 16.5-fold, PR, 50 mg/kg, PO <1 log, TH (2d p.i.), 50 mg/kg, PO (DENV EDEN2, AG129) | NCT01619969 NCT02569827 | I/II (wt-TH) I/II (wt-TH) | 2013 2019 | No efficacy Withdrawn | [100,101,102] |
| Ivermectin | Host nuclear import inhibitor, NS5 entry | 1.2–1.6 µM DENV1-4 (BHK-21) | n.a. | NCT03432442 NCT02045069 | II (wt-TH) [n] II/III (wt-TH) [n] | 2020 2016 | No efficacy Withdrawn | [103,104] |
| Balapiravir | Viral RNA polymerase | 1.9–11 µM DENV1-4 (Huh-7) | n.a. | NCT01096576 | I (wt-TH) | 2011 | No efficacy | [105] |
| Chloroquine | Virus assembly | 1.7–2.7 µM ZIKV MR766 (Vero) [o] | <LLOD [p], PR, 25 mg/kg, PO 75% [p], TH (1d p.i.), PR, 25 mg/kg, PO 25% [p], TH (2d p.i.), PR, 25 mg/kg, PO (DENV2 NGC, NHP) | NCT00849602 ISRCTN38002730 | I/II (wt-TH) [n] I/II (wt-TH) [n] | 2009 2010 | Unknown No efficacy | [106,107,108,109] |
Abbreviations: n.a. = not available; LLOD, lower limit of detection; SR: survival rate; [a] PR: prophylactic; TH: therapeutic (d p.i.: days post-infection). [b] Administration routes: PO, oral administration; SC, intradermal administration, IP: intraperitoneal administration. [c] AG129 is a murine model; NHP, non-human primate; CD1, a murine model. [d] wt-TH: patients with dengue fever (<48 h fever onset) are administered the drug under investigation with a therapeutic treatment; DHIM virus: dengue human infection model, attenuated infection of healthy participants after a prophylactic treatment; wt-PR: prophylactic study, healthy participants are administered the drug, and the protection against wild-type dengue is assessed. [e] Latest relevant trial update/est. completion year. [f] Attenuated viral regimen, peaks through days 4 to 6. [g] Due to the small number of enrolled participants, data collection and analysis was not performed for the efficacy; thus, only safety analysis data were reported. [h] Data relative to the active metabolite AT-9010. [i] Sponsor decision to deprioritize the dengue program. [j] Efficacy proven also against ZIKV: 1-log peak viremia reduction, following 60 mg/kg IP, (ZIKV MR-766, AG129) [110,111]. [k] Details and results of the trial announced but not disclosed in peer-reviewed journals. [l] In vitro DENV-2 S221-infected BHK cells EC50 = 39 µM. [m] Product development halted for business reasons. [n] Administration of treatment < 72 h before symptoms onset. [o] Cellular efficacy against DENV has been proven [106]; however, in the literature only the EC50 in ZIKV-infected cells was reported [109]. This EC50 value is reasonably in the same range of the reported cellular efficacy observed against DENV. [p] Qualitative assessment, % of animals not presenting infection markers.
In this review, only small molecules that showed direct viral growth inhibition were included. Of the eleven small molecules that are in or have completed clinical trials and that we identified as relevant to our study (Figure 6), five (JNJ-64281802, AT-752, EYU688, ISLA-101, and UV-4B) have recently entered or completed phase I clinical trials, and their potential clinical antiviral efficacy remains to be determined. JNJ-64281802 (JNJ-1802, renamed mosnodenvir) has recently shown promising clinical efficacy in a prophylactic treatment against attenuated DENV3 infection (DHIM) in a phase II clinical trial [112] (announcement not peer-reviewed). Four (Ivermectin, Celgosivir, Balapiravir, and chloroquine) completed their clinical trials between 2010 and 2020, but none exhibited clinical efficacy against DENV. For the phase II clinical trial of melatonin, as a coadjutant to standard care in dengue fever, recruitment has not yet begun. Doxycycline is the only NS2B-NS3 inhibitor currently undergoing clinical investigation; however, its mechanism of action and clinical efficacy as a viral growth inhibitor have not yet been established.
JNJ-64281802 [113] is at the cutting edge of antiviral drug development against DENV, showing favorable oral bioavailability, pharmacokinetics, and safety profile in a phase I clinical trial in healthy patients (NCT05201937) [86] and is being registered in four additional phase II efficacy trials (NCT04906980, NCT05048875, NCT05201794, and NCT04480736). Among the phase II clinical trials, two have been halted due to patient recruitment issues deriving from the 2020–2022 COVID pandemic. It is an NS3-NS4B inhibitor, which hinders formation of the viral replication complex (Figure 1, step 6). JNJ-64281802 shows subnanomolar antiviral efficacy against DENV (EC50 = 0.06–1.4 nM, depending on virus strain and cell line) and potent dose-dependent efficacy in vivo (viral RNA levels ≤ LLOD (lower limit of detection) in infected AG129 mice and NHPs (non-human primates), following resp. 6 mg/kg IP (intraperitoneal) and 3 mg/kg SC (subcutaneous)) [87]. Notably, the efficacy of the compound wanes in preclinical murine models after delayed administration post-infection. Preliminary data released by Johnson & Johnson Innovative Medicine (formerly known as Janssen Pharmaceuticals) of the phase IIa clinical trial NCT05048875 showed promising human efficacy data in a prophylactic DENV3 human challenge trial (DHIM): participants received a daily dose of either JNJ-64281802 or a placebo over a span of 26 days. On day five, they were inoculated with attenuated DENV3. Sixty percent of the high-dose antiviral recipients showed no viral RNA in their blood throughout the study. Conversely, detectable viral RNA appeared in all placebo-treated subjects within five days post-exposure. Those administered low to medium doses of the antiviral showed evidence of viral RNA but with a delayed onset relative to the placebo group [112]. In vitro viral kinetic modeling of JNJ-64281802 favorably describes NS3-NS4B inhibition as a therapeutic target against DENV infection in a prophylactic regimen, blocking the transition of infected cells to infectious ones [114].
AT-752 is a protected guanosine nucleotide analog (pro-drug); its metabolite AT-9010 inhibits RNA synthesis by acting as an RNA chain terminator, targeting two NS5-associated enzyme activities, the RNA 2′-O-MTase and the viral RNA polymerase [115]. AT-9010 showed submicromolar efficacy in infected cells (DENV2- or DENV3-infected Huh-7 cells, resp. EC50 = 0.49 μM and 0.77 μM) [89], while AT-752 showed limited efficacy in DENV2-infected AG129 mice (≤1-log peak viremia reduction, following 1000–500 mg/kg PO (oral administration)) [89], especially if compared with JNJ-64281802. AT-752 favorably passed a phase I clinical trial (NCT04722627), where it was shown to be safe in the therapeutic regimen intended to treat DENV infection [88]. Two phase II clinical trials were initiated, but both were prematurely terminated and the development halted.
EYU688 is an NS4B inhibitor developed by Novartis, and it is the second, after JNJ-64281802, most effective compound (DENV1-4 EC50 = 8 to 38 nM in Vero cells, up to 4.2-log peak viremia reduction in AG129 mice, following 100 mg/kg PO), among those in Table 1 still actively in development [90]. EYU688 is now initiating a phase II clinical trial (NCT06006559) in dengue patients, which is scheduled to be completed in 2026.
ISLA-101 is an NS5 nuclear transport inhibitor in development by Island Pharmaceuticals, and, compared with EYU688, showed significantly lower preclinical efficacy against DENV [91,92]. Island Pharmaceuticals announced that ISLA-101 entered phase II trials in DENV-infected patients in 2023; however, the absence of trial registration makes it challenging to understand its full potential. Recently, Island Pharmaceuticals announced that the compound will be trialed also in a prophylactic regimen [116]. UV-4B is a host glucosidase inhibitor, which showed good preclinical efficacy (90–100% SR in AG129 mice following 20 mg/kg PO) and favorably passed a phase I clinical trial (NCT02061358), deeming it as safe [93,94]. Notably, UV-4B shows significant efficacy also after delayed administration in the survival model. UV-4B was scheduled to initiate an additional phase I trial (NCT02696291); however, its development was halted and the trial terminated.
Melatonin is a hormone primarily produced by the pineal gland in the brain. Its production increases in response to darkness and decreases with light exposure, playing a crucial role in regulating circadian rhythms [117]. Melatonin is currently undergoing one phase II clinical trial (NCT05034809) as a coadjutant to standard care in dengue fever with warning signs. Although the trial was expected to be completed in 2022, patient recruitment has not yet begun. Melatonin exhibits antiviral properties against several orthoflaviviruses in vitro, such as DENV, ZIKV, and JEV, and WNV also in vivo. In vitro antiviral activity is often reported in the high-micromolar range (> 100 µM). Melatonin exerts its antiviral activity by inhibiting various host anti-inflammatory mechanisms and possibly viral proteins. It inhibits DENV production via the activation of the sirtuin 1-mediated interferon pathway, modulating the transcription of antiviral genes and suppressing viral replication, showing EC50 ranging from 140 to 200 µM in Huh-7, EA.hy.926, A549, and U937 cells [95]. Notably, a parallel screening study showed limited (EC50 > 500 µM) antiviral activity of melatonin in HEK293T/17 and HepG2 cells [118]. Melatonin inhibits ZIKV replication in Vero and SK-N-SH cells, possibly by interfering with NS3 or NS3 production [119]. For JEV, melatonin inhibits viral replication and reduces neurotoxicity by modulating the calcineurin-autophagy pathway [120]. In WNV infections, melatonin reduces viremia and delays the onset of disease in stressed mice by modulating immune responses and reducing inflammation [96].
Doxycycline is a tetracycline antibiotic with broad-spectrum antimicrobial properties and anti-inflammatory activity. It has demonstrated significant antiviral activity against dengue virus (DENV) in vitro, with an EC50 = 40 µM in Vero cells infected with DENV2. It inhibits DENV2 NS2B-NS3 with an IC50 value of 52.3 ± 6.2 µM at 37 °C (normal human temperature) and 26.7 ± 5.3 µM at 40 °C (high fever temperature) [121]. Doxycycline seems to exert its antiviral activity during the early to mid-stages of infection, interfering with viral entry and replication processes. Despite showing NS2B-NS3 inhibition, further studies are needed to understand its exact mode of action. It has undergone three phase II clinical trials, but no viral load reduction was recorded in any of them. It appears to improve the prognosis of severe DENV patients and to reduce the inflammation markers associated with the disease; further investigation of its mode of action and clinical efficacy is required [97,98,99]. Currently, it is registered in an additional phase II trial in India (CTRI/2018/01/011548), which should have been concluded in 2019, but to date no public results are available. Doxycycline inhibited NS2B-NS3 of ZIKV even more strongly than that of DENV, with IC50 values of 9.9 and 5.3 µM at 37 and 40 °C, respectively; at 20 µM it gave approximately 50% reduction in ZIKV replication in human skin fibroblasts, and at 40 µM it almost eliminated the cytopathic effect [122].
Among the compounds that did not show clinical efficacy, celgosivir is worth noting, because it drew particular attention due to its promising early preclinical efficacy [80]. It exerts its antiviral activity through host ER (endoplasmic reticulum) α-glucosidase inhibition, similarly to UV-4B, resulting in misfolding of several viral proteins such as E, prM, and NS1, which are essential for viral RNA replication [100]. Celgosivir showed submicromolar pan-serotype (DENV1-4) antiviral efficacy in infected cells (EC50 = 0.22–0.68 µM) [100] and high antiviral efficacy in DENV2-infected AG129 mice (from 2.4- to 16.5-fold peak viremia reduction, following 50 mg/kg PO) [101]. However, it did not show clinical efficacy in humans against DENV in a phase I/II trial in 2013 (NCT01619969) [102], and a subsequent phase I/II trial, scheduled to conclude in 2019 (NCT02569827), where it should have been compared with treatment with modipafant, was withdrawn. No in vivo preclinical data are available for ivermectin and balapiravir, but, being repurposed drugs, they are safe for humans. They showed promising antiviral efficacy in cells (resp. EC50 = 1.2–1.6 µM [103] and 1.9–11 µM [114]) but no clinical efficacy (resp. NCT03432442 and NCT01096576). Ivermectin is an antiparasitic drug used to treat intestinal roundworms, lice, and scabies, and its antiviral efficacy against DENV was ascribed to inhibition of the nuclear transport of NS5 [103,123], similarly to ISLA-101. Although ivermectin did not show clinical efficacy in the treatment of DENV in humans (NCT03432442), its antiviral pharmacology is still being researched [124], and it seems to show promise in combination with the cholesterol-lowering drug atorvastatin [125,126]. Balapiravir is a nucleoside analog that was initially developed against HCV, and it supposedly exerts antiviral activity in vitro through inhibition of the viral RNA polymerase [104], as established for AT-9010/AT-752. Since the mechanism through which balapiravir exerts anti-orthoflaviviral activity (as observed in cells) is solely inferred from its known action against HCV, and there is no direct evidence confirming that it would function through the same mechanism in DENV, it is crucial not to dismiss the potential for RNA polymerase inhibition to yield clinical efficacy. Chloroquine is an approved antimalarial drug that showed pan-serotype micromolar cellular efficacy, and it is thought to interfere with host machinery, resulting in the inhibition of viral assembly [105]. Despite showing very potent antiviral efficacy in NHPs at very low dosing (peak viremia ≤ LLOD, 5 mg/kg, PO), it failed to show clinical antiviral efficacy in a phase II clinical trial [106]. It is plausible that the specific antiviral mechanisms of melatonin, ivermectin, celgosivir, and chloroquine that account for their observed antiviral efficacy in animal models do not translate into clinical antiviral efficacy in human patients, possibly because they are characterized by inhibition of and/or interaction with host pathways, which may vary between humans and the animal models. On the contrary, JNJ-64281802, AT-752, and EYU688 exert their antiviral activity through inhibition of the viral machinery (resp. NS3-NS4B, viral RNA polymerase, and NS4B), directly hindering viral RNA replication. While host pathways may vary among different species, inhibition of viral machinery may hopefully allow the translation of preclinical antiviral efficacy in animals to clinical antiviral efficacy in humans. Conversely, the in vivo anti-orthoflaviviral preclinical efficacy of ISLA-101, which inhibits NS5 host nuclear transfer in a manner similar to ivermectin, and of UV-4B, which exerts activity through host ER glucosidases’ inhibition, akin to celgosivir, might not translate to clinical efficacy in humans. Several compounds scrutinized herein (AT-752, UV-4B, ivermectin, celgosivir, chloroquine, and balapiravir) either did not complete phase II efficacy trials (studies terminated or withdrawn) or did not show clinical efficacy in dengue patients after a therapeutic regimen, even though they performed favorably in preclinical orthoflaviviral infection animal models. It is important to note that most of those compounds showed already waning efficacy after delayed administration post-infection in the same animal models. It cannot be ruled out that the animal models employed in anti-orthoflaviviral drug development (e.g., DENV-infected AG129 mice or NHPs) may not accurately represent DENV infection in humans, especially in a therapeutic application. The preliminary data from the phase IIa clinical trial of JNJ-64281802 suggest that current animal models may approximate human DENV infection at least in a prophylactic regimen of the drug under investigation. Nevertheless, a conclusive assessment hinges on the release of more comprehensive data, particularly because viral RNA was still detectable in 40% of participants receiving the highest dose, contrary to what was observed in the animal models. These results highlight the relevance of developing novel anti-orthoflavivirals based on the inhibition of diverse viral targets, such as NS2B-NS3 inhibition, which is so far underrepresented in the clinical settings.
7. Small-Molecule NS2B-NS3 Protease Inhibitors with In Vivo Efficacy
In order to understand the preclinical developmental status of NS2B-NS3 viral protease inhibitors, we performed a systematic search using PubMed, Reaxys, Google Scholar, and ChemBL. Compounds were selected cross-referencing orthoflaviviral NS2B-NS3 protease inhibition with demonstrated in vivo anti-orthoflaviviral efficacy in an animal model, identifying 12 relevant compounds meeting these criteria (Figure 7). Notably, the in vivo antiviral efficacy of the vast majority of those viral protease inhibitors is reported against ZIKV infection models, although they also show in vitro potency against other orthoflaviviruses. Only for niclosamide was the antiviral efficacy against DENV in vivo reported.
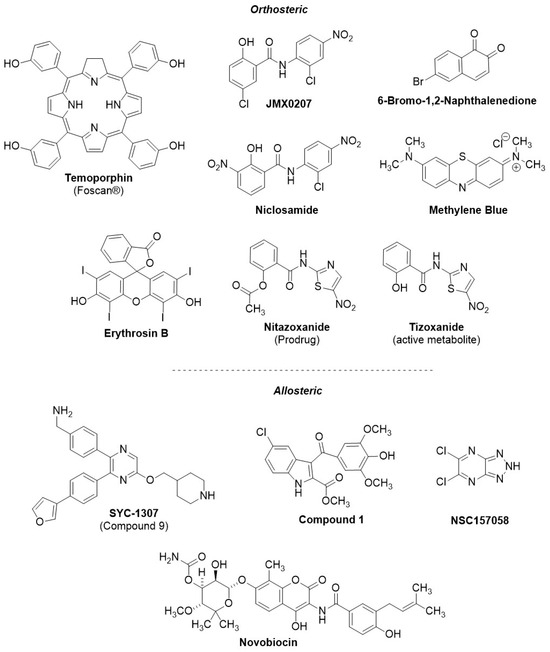
The identified NS2B-NS3 inhibitors were further classified based on their reported mode of inhibition, either orthosteric or allosteric (resp. Table 2 and Table 3, structures of both categories in Figure 7). In the animal studies reported for both sets of compounds, the drug under investigation was administered at the same time as the viral infection, so we define the drug regimen as prophylactic in all cases.

Table 2.
Orthosteric NS2B-NS3 inhibitors with in vivo efficacy.

Table 3.
Allosteric NS2B-NS3 inhibitors with in vivo efficacy.
The orthosteric inhibitors identified in Table 2 were principally discovered in libraries of repurposed approved drugs, which were screened for in vitro NS2B-NS3 protease inhibition. Interestingly, among the orthosteric NS2B-NS3 inhibitors with demonstrated in vivo efficacy, only one compound, 6-bromo-1,2-naphthalenedione, was reported to be a competitive inhibitor. All other compounds listed in Table 2 have demonstrated binding to the active site of the viral protease; however, they are reported to exhibit non-competitive inhibition behavior. 6-Bromo-1,2-naphthalenedione is a selective ZIKV NS2B-NS3 protease inhibitor (IC50 = 67 µM), identified after in silico structure-based screening, and is approved for the treatment of influenza. The binding of the compound to the protease has been rationalized through in silico docking. Its specificity for ZIKV protease, compared with other orthoflaviviruses, appears to result from its interaction with specific residues in the S3–S4 subsites of the ZIKV protease. 6-Bromo-1,2-naphthalenedione showed antiviral efficacy against ZIKV in infected cells (EC50 = 1 µM) and in ZIKV-infected AG129 (20–60% SR, up to 3.7-fold peak viremia reduction following 50 µg IV) [135]. Various aspects of 6-bromo-1,2-naphthalenedione are still unclear; for instance, there is a significant discrepancy between its low potency against the protease and its high efficacy against the virus as well as its reported competitive mechanism combined with high selectivity against ZIKV. The remaining identified orthosteric inhibitors showing in vivo efficacy (viz. temoporfin, JMX0207, niclosamide, methylene blue, and erythrosin B) inhibit the NS2B-NS3 protease via a non-competitive mechanism. Preclinical data suggest that temoporfin is the most promising orthosteric NS2B-NS3 protease inhibitor (NS2B-NS3 IC50 = 1.1 µM, cellular infection EC50 = 0.010–0.024 µM), exhibiting a strong reduction in peak viremia at low doses (2-log peak viremia reduction in ZIKV-infected Balb/c mice following 1.6 mg/kg IP). Temoporfin is an already-approved pharmaceutical in oncology treatment, and, while it is safe in humans, it is not orally bioavailable [127]. JMX0207, niclosamide, methylene blue, erythrosin B, and nitazoxanide display relatively limited in vivo efficacy compared with temoporfin and 6-bromo-1,2-naphthalenedione, exhibiting < 2-log peak viremia reduction in vivo. Among those, methylene blue demonstrates the most significant reduction in peak viremia in vivo (1.7-log reduction in peak viremia following 100 mg/kg PO), while inhibiting in vitro the DENV protease in the micromolar range (IC50 = 9 µM) and DENV infection in cells in the submicromolar range (EC50 = 0.36 µM). Although niclosamide had been previously identified as an NS2B-NS3 inhibitor [127], later studies involving a replicon assay revealed that it may instead exert its antiviral activity through endosomal acidification, retarding the DENV infection rather than inhibiting NS2B-NS3 activity [131]. Considering the preclinical findings, 6-bromo-1,2-naphthalenedione, temoporfin, and methylene blue appear to show the most significant antiviral efficacy in vivo among orthosteric NS2B-NS3 inhibitors. Notably, the temoporfin study was published in 2017 [127], and no following studies have been disclosed until now, suggesting that it is unlikely to show promising efficacy. 6-Bromo-1,2-naphthalenedione, temoporfin, and methylene blue show significantly lower in vivo antiviral efficacy compared with JNJ-64281802 and EYU688, and hence, their clinical relevance may be limited; however, they may represent viable scaffolds for further development of analogs with improved efficacy.
Additionally, we identified four relevant allosteric inhibitors of the NS2B-NS3 protease with proven in vivo efficacy (Table 3). Three (SYC-1307, compound 1, and NSC157058) are novel compounds, and one (novobiocin) is a repurposed approved drug. SYC-1307, the most promising among these, belongs to a novel series of 2,5,6-trisubstituted pyrazine derivatives, and it is a submicromolar allosteric inhibitor of the orthoflaviviral NS2B-NS3 protease (determined in DENV, ZIKV, WNV, IC50 = 0.2–0.8 μM), with submicromolar anti-orthoflaviviral efficacy in cells (EC68 = 0.3–0.6 μM), correlating with significant reduction in ZIKV peak viremia in vivo (96–98% reduced viral RNA following 15–30 mg/kg IP) [140,141]. NSC157058 also showed similar efficacy in vivo against ZIKV (10-fold peak viremia reduction, 100% SR, following 30 mg/kg PO) and submicromolar orthoflaviviral NS2B-NS3 inhibition (IC50 = 0.8 µM); however, it demonstrated limited cellular antiviral efficacy (EC50 = 50 µM) [78]. Compound 1 and novobiocin also exhibited in vivo efficacy against ZIKV (100% SR, resp. 1 mg/kg IP and 100 mg/kg SC); however, they showed limited in vitro inhibitory potency against NS2B-NS3 (resp. IC50 = 158 and 14 µM) and limited antiviral efficacy in infected cells (EC50 = 14 and 43 µM) [142,143,144] compared with SYC-1307. Difficulties in correlating the in vitro potency and efficacy with the in vivo efficacy may hamper the structure-optimization process. SYC-1307, bearing strong correlation between potency and efficacy (both in vitro and in vivo), an extensive SAR, broad-spectrum anti-orthoflaviviral potency in vitro, and demonstrated potent antiviral efficacy against ZIKV in vivo, represents the most promising compound among the NS2B-NS3 protease inhibitors (orthosteric and allosteric) with reported in vivo efficacy in the literature. Furthermore, from our study a substantial disparity between non-competitive and competitive inhibitors (1 competitive against 11 non-competitive) emerges, underscoring the challenges in developing competitive NS2B-NS3 inhibitors.
8. Small-Molecule NS2B-NS3 Orthosteric Competitive Inhibitors with Cellular Efficacy
In the following section, we summarize the recent advancements in designing competitive NS2B-NS3 protease inhibitors that have undergone in vitro testing against DENV. Until now, no competitive viral protease inhibitors with in vivo efficacy against orthoflaviviral infections have been reported, with the exception the aforementioned case of 6-bromo-1,2-naphthalenedione against ZIKV. To encompass all pertinent drug development, we will examine seven competitive NS2B-NS3 protease inhibitors with EC50 values below 20 μM (structures in Figure 8 and biological data in Table 4). These compounds were primarily identified through a comprehensive search in the EMBL-EBI ChEMBL database, which included all compounds demonstrating competitive NS2B-NS3 inhibition and exhibiting cellular efficacy below the 20 µM threshold. Additional competitive inhibitors meeting the established criteria were identified; however, they are less-potent analogs of the compounds discussed in Figure 8. For instance, mb-53, shown in Figure 4 in the introduction, is an analog developed parallelly to compound 104 and having slightly lower viral protease inhibitory potency and cellular efficacy. Moreover, numerous competitive NS2B-NS3 peptide-like inhibitors are known but not discussed in this section due to their low (EC50 > 20 μM), absent, or unreported cellular efficacy data. As previously mentioned in the introduction, challenges in structure-based drug design approaches for NS2B-NS3 inhibition can be attributed to the majority of the peptide-like competitive NS2B-NS3 inhibitors reported in the literature. These inhibitors often possess positive charges that negatively affect cellular efficacy [59,60,72]. Furthermore, biochemical assay conditions do not precisely replicate the viral proteolytic processes that occur within cells, often resulting in a weak correlation between biochemical potency and cellular efficacy.
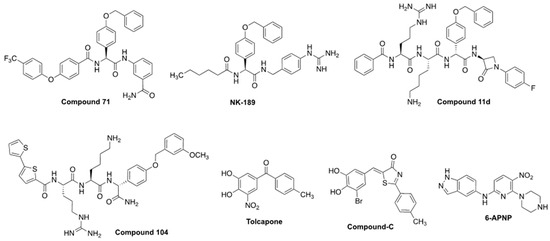
Figure 8.
Competitive NS2B-NS3 inhibitors with potent cellular efficacy (EC50 < 20 µM). References and biological data in Table 4.

Table 4.
Orthosteric competitive NS2B-NS3 inhibitors with relevant (<20 µM) antiviral cellular efficacy.
Nevertheless, relentless medicinal and synthetic chemistry efforts, principally driven by reducing the cationic and peptidic character of substrate-like inhibitors such as Bz-nKRR-NH2 and optimizing assay conditions to better replicate NS2B-NS3 inhibition in vitro, allowed the development of compounds such as NK-189 [59] followed by its optimized scaffold compound 71 [61], both showing submicromolar cellular efficacy (resp. EC50 = 0.89 and 0.24 µM). Between Bz-nKRR-NH2 and NK-189, several iterations of peptide-like derivatives, such as cn-716, mb-53 (both shown in Figure 4), and compound 11d were explored, also to structurally characterize the viral protease. Notably, compound 11d is the only reported competitive covalent NS2B-NS3 protease inhibitor showing relevant cellular efficacy [145]. However, proteolytic studies revealed that the fragment bearing the covalent warhead is excised by the protease, resulting in an analog of mb-53, which is thought to be actually responsible for its antiviral efficacy [145].
The development of NK-189 and compound 71 was facilitated by the establishment of a replicon assay that enabled the selection of viable antivirals in a virus-free cellular environment while specifically targeting the NS2B-NS3 inhibition pathway [59,61]. Compound-C and tolcapone were identified in a high-throughput screening of 120,000 commercially available compounds as potential inhibitors of DENV, which were evaluated using replicon, plaque, and cytotoxicity assays [146]. They are repurposed, commercially available drugs, which lack peptide features, and, together with 6-bromo-1,2-naphthalenedione, they are the only reported small-molecule NS2B-NS3 competitive inhibitors with relevant cellular efficacy (resp. EC50 = 8.97 and 2.03 µM). The activities of Compound-C and tolcapone correlate with potent pan-serotype viral protease inhibition (resp. IC50 = 2.94–4.06 and 0.64–1.15 µM) [146]. The research identifying Compound-C and tolcapone was conducted in 2016; considering the time that has passed, it is unlikely that these molecules exhibited adequate efficacy in animal models. 6-APNP was identified as an orthosteric inhibitor, notably, after a structure-based HTS screening in silico aimed to identify NS2B-NS3 inhibitors [147]. Its binding to the protease could be well-rationalized in silico, and the compound showed micromolar to submicromolar antiviral efficacy against DENV4 and ZIKV (respectively, EC50 = 7.1 and 0.69–5.0 µM), and similar viral protease inhibitory potency (IC50 = 1.5 µM) [147]. Tolcapone, compound-C, and 6-APNP are less potent than peptide derivatives such as compound 71; however, they present more drug-like features, which can ease their development and lead to an improved pharmacokinetic profile in vivo. Although direct analogs with cellular efficacy have not yet been documented, tolcapone, compound-C, and 6-APNP may serve as scaffolds in the development of future non-peptide NS2B-NS3 inhibitors.
9. Conclusions
In this study, we systematically analyzed the scientific literature and publicly available clinical data to assess the current state of drug development against orthoflaviviral infections, with a particular focus on viral replication inhibitors. Moreover, we sought to understand the role of NS2B-NS3 inhibition in this context. Our analysis began with clinical trials involving small molecules, followed by a review of NS2B-NS3 protease inhibitors exhibiting in vivo antiviral efficacy, and concluded with a focus on competitive NS2B-NS3 inhibitors. It is concerning that numerous compounds with demonstrated in vivo efficacy failed to exhibit clinical antiviral efficacy, raising doubts regarding the accuracy of the animal models employed in anti-orthoflaviviral drug development in representing orthoflaviviral infections in humans. This may be explained by differences in the pathogenesis of dengue between humans and animal models and by possible variations in immunological response and drug metabolism (pharmacokinetics) between species. More importantly, the assessment of the efficacy of a therapeutic treatment against wild-type dengue in a clinical setting (wt-TH in Table 1, treatment administered < 48 h of the symptoms’ onset) may be hampered by the fact that most compounds already do not show significant antiviral effect in preclinical models after a delayed administration post-infection (therapeutic regimen). At the time of writing, the preliminary results for JNJ-64281802 in a phase II (DHIM, prophylactic regimen) clinical trial look favorable and make it the most promising compound, followed by EYU688. These compounds inhibit the viral machinery by targeting, respectively, NS3-NS4B and NS4B, and they show cellular antiviral efficacy ranging from nanomolar to subnanomolar, unlike the majority of the compounds that did not demonstrate clinical antiviral efficacy. This emphasizes the importance of developing novel NS2B-NS3 inhibitors, of which only doxycycline is currently in clinical trials. However, its precise mechanism of action and clinical efficacy as a viral growth inhibitor have yet to be established and demand further investigation.
To date, only one competitive NS2B-NS3 protease inhibitor, 6-bromo-1,2-naphthalenedione, has been reported with in vivo efficacy. However, it exhibits limited broad-spectrum orthoflaviviral efficacy, primarily demonstrating selectivity toward ZIKV. SYC-1307, an allosteric inhibitor, is the most promising NS2B-NS3 inhibitor, exhibiting high in vivo efficacy that correlates with high potency and efficacy in vitro. Temoporfin and methylene blue represent the most promising orthosteric non-competitive inhibitors, although their in vivo efficacy is limited compared with SYC-1307.
Among the competitive inhibitors with potent cellular efficacy, Compound 71, a non-polycationic NS2B-NS3 inhibitor, emerges as the leading preclinical candidate due to its high cellular antiviral efficacy, minimal cytotoxicity, and favorable in vitro pharmacokinetic parameters. These attributes position it as an ideal candidate for potential advancement to in vivo studies. The development of competitive NS2B-NS3 inhibitors faces several challenges, including the dynamic nature of the mechanism of action of NS2B-NS3 and the use of artificial constructs in the biochemical inhibition assays and in X-ray crystallography and other techniques for structure elucidation. Furthermore, the very shallow binding area, which exhibits a preference for binding cationic residues in the natural substrate, contributes to the difficulty in developing competitive inhibitors.
Author Contributions
Conceptualization, L.C. and M.C.F.; methodology, M.J.B. and L.C.; data curation, M.J.B. and L.C.; writing—original draft preparation, M.J.B. and L.C.; writing—review and editing, L.C., D.G., and M.C.F.; visualization, M.J.B. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge support by the European Union and the provinces of Gelderland and Overijssel in the EFRO (Europees Fonds voor Regionale Ontwikkeling) project “Tropinhi” PROJ-00672.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author Daniel Gironés was employed by the company Protinhi Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADE, antibody-dependent enhancement; DENV, dengue virus; DHIM, dengue human infection models; ER, endoplasmic reticulum; HCV, hepatitis C virus; IP, intraperitoneal; IV, intravenous; ISRCTN, international traditional medicine clinical trial registry; JEV, Japanese encephalitis virus; LLOD, lower limit of detection; NHPs, non-human primates; NS, non-structural (protein); PO, per os, oral administration; PR, prophylactic regimen; SC, subcutaneous, intradermal; SR, survival rate; TBEV, tick-borne encephalitis virus; TH, therapeutic regimen; WNV, West Nile virus; YFV, yellow fever virus; ZIKV, zika virus.
References
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Best, S.M. Flaviviruses. Curr. Biol. 2016, 26, R1258–R1260. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Neglected Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Japanese encephalitis vaccines: WHO position paper-February 2015. Wkly. Epidemiol. Rec. 2015, 90, 69–88. [Google Scholar]
- World Health Organization. Detection and Investigation of Serious Adverse Events Following Yellow Fever Vaccination. Guidance from an Informal Consultation of Experts; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Kubinski, M.; Beicht, J.; Gerlach, T.; Volz, A.; Sutter, G.; Rimmelzwaan, G.F. Tick-borne encephalitis virus: A quest for better vaccines against a virus on the rise. Vaccines 2020, 8, 451. [Google Scholar] [CrossRef]
- Waickman, A.T.; Newell, K.; Endy, T.P.; Thomas, S.J. Biologics for dengue prevention: Up-to-date. Expert Opin. Biol. Ther. 2023, 23, 73–87. [Google Scholar] [CrossRef]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Ahsan, O.; Khan, M.; Sah, R.; Waheed, Y. Advances in Zika virus vaccines and therapeutics: A systematic review. Asian Pac. J. Trop. Med. 2024, 17, 97–109. [Google Scholar] [CrossRef]
- Khetarpal, N.; Khanna, I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J. Immunol. Res. 2016, 2016, 6803098. [Google Scholar] [CrossRef]
- Arkin, F. Dengue Vaccine Fiasco Leads to Criminal Charges for Researcher in the Philippines. Available online: https://www.science.org/content/article/dengue-vaccine-fiasco-leads-criminal-charges-researcher-philippines (accessed on 23 August 2024).
- Dengvaxia®. Vaccine Approved for Prevention of Dengue in Europe. Available online: https://www.sanofi.com/en/media-room/press-releases/2018/2018-12-19-12-00-00-1669374 (accessed on 23 August 2024).
- Mallapaty, S. Dengue vaccine poised for roll-out—But concerns linger. Indonesia will be using the jab from next year, although some scientists say the safety data are insufficient. Nature 2022, 611, 434–435. [Google Scholar] [CrossRef]
- Lenharo, M. Dengue is spreading. Can new vaccines and antivirals halt its rise? Nature 2023, 623, 470. [Google Scholar] [CrossRef]
- Takeda Announces Voluntary Withdrawal of, U.S. Biologics License Application (BLA) for Dengue Vaccine Candidate TAK-003. Available online: https://www.takeda.com/newsroom/statements/2023/takeda-announces-voluntary-withdrawal-of-US-biologics-license-application-for-dengue-vaccine-candidate-TAK-003/ (accessed on 23 August 2024).
- Lambach, P.; Orenstein, W.; Wu, J.S. Report from the World Health Organization’s immunization and vaccines related implementation research advisory committee (IVIR-AC) meeting, Geneva, 11–13 September 2023. Vaccine 2023, 42, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Hassan, A.; Farooq, M.; Afzal, S.; Khan, M.A.; Amin, I.; Shahid, M.; Idrees, M.; Shahid, A.A. Dengue Vaccines: Ongoing Challenges and Current Status in the Advancement of Different Candidates. Crit. Rev. Eukaryot. Gene Expr. 2021, 31, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Schroder, K.; White, H.; Fang, N.X.; Stoermer, M.J.; Abbenante, G.; Martin, J.L.; Young, P.R.; Fairlie, D.P. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J. Biol. Chem. 2001, 276, 45762–45771. [Google Scholar] [CrossRef]
- Nitsche, C.; Holloway, S.; Schirmeister, T.; Klein, C.D. Biochemistry and Medicinal Chemistry of the Dengue Virus Protease. Chem. Rev. 2014, 114, 11348–11381. [Google Scholar] [CrossRef]
- Lennemann, N.J.; Coyne, C.B. Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B. Autophagy 2017, 13, 322–332. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Yuan, L.; Ross, D.; Johansen, A.; Sands, D.; Stanley, V.; Guemez-Gamboa, A.; Gregor, A.; Evans, T.; et al. Zika virus protease cleavage of host protein septin-2 mediates mitotic defects in neural progenitors. Neuron 2019, 101, 1089–1098. [Google Scholar] [CrossRef]
- Nie, Y.; Deng, D.Q.; Mou, L.; Long, Q.; Chen, J.; Wu, J.H. Dengue Virus 2 NS2B Targets MAVS and IKKε to Evade the Antiviral Innate Immune Response. J. Microbiol. Biotechnol. 2023, 33, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kakumani, P.K.; Rajgokul, K.S.; Ponia, S.S.; Kaur, I.; Mahanty, S.; Medigeshi, G.R.; Banerjea, A.C.; Chopra, A.P.; Malhotra, P.; Mukherjee, S.K.; et al. Dengue NS3, an RNAi suppressor, modulates the human miRNA pathways through its interacting partner. Biochem. J. 2015, 471, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, C.M.D.; Basavannacharya, C.; Chan, K.W.K.; Chan, S.-A.; Singh, D.; Wei, N.; Phoo, W.W.; Luo, D.; Lescar, J.; Vasudevan, S.G. NS3 helicase from dengue virus specifically recognizes viral RNA sequence to ensure optimal replication. Nucleic Acids Res. 2017, 45, 12904–12920. [Google Scholar] [CrossRef]
- Serman, T.; Chiang, C.; Liu, G.Q.; Sayyad, Z.; Pandey, S.; Volcic, M.; Lee, H.; Muppala, S.; Acharya, D.; Goins, C.; et al. Acetylation of the NS3 helicase by KAT5γ is essential for flavivirus replication. Cell Host Microbe 2023, 31, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C. Proteases from dengue, West Nile and Zika viruses as drug targets. Biophys. Rev. 2019, 11, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Vasudevan, S.G.; Lescar, J. The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug development. Antivir. Res. 2015, 118, 148–158. [Google Scholar] [CrossRef]
- van den Elsen, K.; Quek, J.P.; Luo, D. Molecular Insights into the Flavivirus Replication Complex. Viruses 2021, 13, 956. [Google Scholar] [CrossRef]
- Miller, S.; Sparacio, S.; Bartenschlager, R. Subcellular Localization and Membrane Topology of the Dengue Virus Type 2 Non-structural Protein 4B*. J. Biol. Chem. 2006, 281, 8854–8863. [Google Scholar] [CrossRef]
- Umareddy, I.; Chao, A.; Sampath, A.; Gu, F.; Vasudevan, S.G. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 2006, 87 Pt 9, 2605–2614. [Google Scholar] [CrossRef]
- Yap, T.L.; Xu, T.; Chen, Y.L.; Malet, H.; Egloff, M.P.; Canard, B.; Vasudevan, S.G.; Lescar, J. Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J. Virol. 2007, 81, 4753–4765. [Google Scholar] [CrossRef]
- Dong, H.; Chang, D.C.; Xie, X.; Toh, Y.X.; Chung, K.Y.; Zou, G.; Lescar, J.; Lim, S.P.; Shi, P.Y. Biochemical and genetic characterization of dengue virus methyltransferase. Virology 2010, 405, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Pryor, M.J.; Rawlinson, S.M.; Butcher, R.E.; Barton, C.L.; Waterhouse, T.A.; Vasudevan, S.G.; Bardin, P.G.; Wright, P.J.; Jans, D.A.; Davidson, A.D. Nuclear localization of dengue virus nonstructural protein 5 through its importin alpha/beta-recognized nuclear localization sequences is integral to viral infection. Traffic 2007, 8, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Maldonado, T.; Moreno-Herrera, A.; Pujadas, G.; Vázquez-Jiménez, L.K.; González-González, A.; Rivera, G. Recent advances in the development of methyltransferase (MTase) inhibitors against (re)emerging arboviruses diseases dengue and Zika. Eur. J. Med. Chem. 2023, 252, 115290. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, K.; Ravi Kumar, Y.S.; Roy, R.; Phadnis, S.; Meena, V.; Bhattacharyya, S.; Verma, B. Dengue virus pathogenesis and host molecular machineries. J. Biomed. Sci. 2024, 31, 43. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, K.; Chew, B.L.A.; Ho, J.S.; Luo, D. Flavivirus nonstructural proteins and replication complexes as antiviral drug targets. Curr. Opin. Virol. 2023, 59, 101305. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell. Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Serine Protease. In Biochemistry, 4th ed.; Wiley: Hoboken, NJ, USA, 2011; p. 525. [Google Scholar]
- Radisky, E.S.; Lee, J.M.; Lu, C.-J.K.; Koshland, D.E. Insights into the serine protease mechanism from atomic resolution structures of trypsin reaction intermediates. Proc. Natl. Acad. Sci. USA 2006, 103, 6835–6840. [Google Scholar]
- Nussinov, R.; Tsai, C.-J. The different ways through which specificity works in orthosteric and allosteric drugs. Curr. Pharm. Des. 2012, 18, 1311. [Google Scholar] [CrossRef]
- Hauske, P.; Ottmann, C.; Meltzer, M.; Ehrmann, M.; Kaiser, M. Allosteric Regulation of Proteases. ChemBioChem 2008, 9, 2920–2928. [Google Scholar] [CrossRef]
- Pesaresi, A. Mixed and non-competitive enzyme inhibition: Underlying mechanisms and mechanistic irrelevance of the formal two-site model. J. Enzym. Inhib. Med. Chem. 2023, 38, 2245168. [Google Scholar] [CrossRef]
- Pedersen, C.N.; Yang, F.Y.; Ita, S.; Xu, Y.B.; Akunuri, R.; Trampari, S.; Neumann, C.M.T.; Desdorf, L.M.; Schiott, B.; Salvino, J.M.; et al. Cryo-EM structure of the dopamine transporter with a novel atypical non-competitive inhibitor bound to the orthosteric site. J. Neurochem. 2024. early view. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.-R.; Kalodimos, C.G. Allosteric inhibition through suppression of transient conformational states. Nat. Chem. Biol. 2013, 9, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Q.; Yin, M.-M.; Song, P.-J.; He, X.-H.; Liu, Y.; Jiang, F.-L. Thermodynamics, Kinetics and Mechanisms of Noncompetitive Allosteric Inhibition of Chymotrypsin by Dihydrolipoic Acid-Coated Gold Nanoclusters. Langmuir 2020, 36, 6447–6457. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Ekka, M.K.; Kaushik, A.; Pandya, V.; Singh, R.P.; Banerjee, S.; Mittal, M.; Singh, V.; Kumaran, S. Substrate-Induced Facilitated Dissociation of the Competitive Inhibitor from the Active Site of O-Acetyl Serine Sulfhydrylase Reveals a Competitive-Allostery Mechanism. Biochemistry 2017, 56, 5011–5025. [Google Scholar] [CrossRef]
- Alphey, M.S.; Pirrie, L.; Torrie, L.S.; Boulkeroua, W.A.; Gardiner, M.; Sarkar, A.; Maringer, M.; Oehlmann, W.; Brenk, R.; Scherman, M.S. Allosteric Competitive Inhibitors of the Glucose-1-phosphate Thymidylyltransferase (RmlA) from Pseudomonas aeruginosa. ACS Chem. Biol. 2013, 8, 387–396. [Google Scholar] [CrossRef]
- Tuley, A.; Fast, W. The Taxonomy of Covalent Inhibitors. Biochemistry 2018, 57, 3326–3337. [Google Scholar] [CrossRef]
- Noble, C.G.; Seh, C.C.; Chao, A.T.; Shi, P.Y. Ligand-bound structures of the dengue virus protease reveal the active conformation. J. Virol. 2012, 86, 438–446. [Google Scholar] [CrossRef]
- Agback, T.; Lesovoy, D.; Han, X.; Lomzov, A.; Sun, R.; Sandalova, T.; Orekhov, V.Y.; Achour, A.; Agback, P. Combined NMR and molecular dynamics conformational filter identifies unambiguously dynamic ensembles of Dengue protease NS2B/NS3pro. Commun. Biol. 2023, 6, 1193. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Noble, C.G.; Shi, P.-Y. Structural biology of dengue virus enzymes: Towards rational design of therapeutics. Antivir. Res. 2012, 96, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Samrat, S.K.; Xu, J.; Li, Z.; Zhou, J.; Li, H. Antiviral Agents against Flavivirus Protease: Prospect and Future Direction. Pathogens 2022, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.B.; Behnam, M.A.M.; El Sherif, Y.; Nitsche, C.; Vechi, S.M.; Klein, C.D. Dual inhibitors of the dengue and West Nile virus NS2B–NS3 proteases: Synthesis, biological evaluation and docking studies of novel peptide-hybrids. Bioorg. Med. Chem. 2015, 23, 5748–5755. [Google Scholar] [CrossRef]
- Kühl, N.; Graf, D.; Bock, J.; Behnam, M.A.M.; Leuthold, M.-M.; Klein, C.D. A New Class of Dengue and West Nile Virus Protease Inhibitors with Submicromolar Activity in Reporter Gene DENV-2 Protease and Viral Replication Assays. J. Med. Chem. 2020, 63, 8179–8197. [Google Scholar] [CrossRef]
- Dražić, T.; Kühl, N.; Gottscheber, N.; Hacker, C.N.; Klein, C.D. The spectrum between substrates and inhibitors: Pinpointing the binding mode of dengue protease ligands with modulated basicity and hydrophobicity. Bioorg. Med. Chem. 2021, 48, 116412. [Google Scholar] [CrossRef] [PubMed]
- Kühl, N.; Leuthold, M.M.; Behnam, M.A.M.; Klein, C.D. Beyond Basicity: Discovery of Nonbasic DENV-2 Protease Inhibitors with Potent Activity in Cell Culture. J. Med. Chem. 2021, 64, 4567–4587. [Google Scholar] [CrossRef]
- Nitsche, C.; Schreier, V.N.; Behnam, M.A.M.; Kumar, A.; Bartenschlager, R.; Klein, C.D. Thiazolidinone–Peptide Hybrids as Dengue Virus Protease Inhibitors with Antiviral Activity in Cell Culture. J. Med. Chem. 2013, 56, 8389–8403. [Google Scholar] [CrossRef]
- Nitsche, C.; Zhang, L.; Weigel, L.F.; Schilz, J.; Graf, D.; Bartenschlager, R.; Hilgenfeld, R.; Klein, C.D. Peptide–Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural Biology. J. Med. Chem. 2017, 60, 511–516. [Google Scholar] [CrossRef]
- Nitsche, C.; Klein, C.D. Fluorimetric and HPLC-Based Dengue Virus Protease Assays Using a FRET Substrate. In Antiviral Methods and Protocols; Gong, E.Y., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 221–236. [Google Scholar]
- Leung, D.; Abbenante, G.; Fairlie, D.P. Protease Inhibitors: Current Status and Future Prospects. J. Med. Chem. 2000, 43, 305–341. [Google Scholar] [CrossRef]
- Sutanto, F.; Konstantinidou, M.; Dömling, A. Covalent inhibitors: A rational approach to drug discovery. RSC Med. Chem. 2020, 11, 876–884. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Patel, S.J.; Wang, W.-L.; Wang, G.; Chan, W.-L.; Rao, K.R.R.; Alam, J.; Jeyaraj, D.A.; Ngew, X.; Patel, V.; et al. Peptide inhibitors of dengue virus NS3 protease. Part 1: Warhead. Bioorg. Med. Chem. Lett. 2006, 16, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Patel, S.J.; Wang, W.L.; Chan, W.L.; Ranga Rao, K.R.; Wang, G.; Ngew, X.; Patel, V.; Beer, D.; Knox, J.E.; et al. Peptide inhibitors of dengue virus NS3 protease. Part 2: SAR study of tetrapeptide aldehyde inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 40–43. [Google Scholar] [CrossRef]
- Lei, J.; Hansen, G.; Nitsche, C.; Klein, C.D.; Zhang, L.L.; Hilgenfeld, R. Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science 2016, 353, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Jöst, C.; Nitsche, C.; Scholz, T.; Roux, L.; Klein, C.D. Promiscuity and Selectivity in Covalent Enzyme Inhibition: A Systematic Study of Electrophilic Fragments. J. Med. Chem. 2014, 57, 7590–7599. [Google Scholar] [CrossRef]
- Behnam, M.A.M.; Graf, D.; Bartenschlager, R.; Zlotos, D.P.; Klein, C.D. Discovery of Nanomolar Dengue and West Nile Virus Protease Inhibitors Containing a 4-Benzyloxyphenylglycine Residue. J. Med. Chem. 2015, 58, 9354–9370. [Google Scholar] [CrossRef]
- Kang, C.B.; Keller, T.H.; Luo, D. Zika Virus Protease: An Antiviral Drug Target. Trends Microbiol. 2017, 25, 797–808. [Google Scholar] [CrossRef]
- Behnam, M.A.M.; Klein, C.D. Alternate recognition by dengue protease: Proteolytic and binding assays provide functional evidence beyond an induced-fit. Biochimie 2024, in press. [Google Scholar] [CrossRef]
- Yildiz, M.; Ghosh, S.; Bell, J.A.; Sherman, W.; Hardy, J.A. Allosteric Inhibition of the NS2B-NS3 Protease from Dengue Virus. ACS Chem. Biol. 2013, 8, 2744–2752. [Google Scholar] [CrossRef]
- Aleshin, A.E.; Shiryaev, S.A.; Strongin, A.Y.; Liddington, R.C. Structural evidence for regulation and specificity of flaviviral proteases and evolution. Prot. Sci. 2007, 16, 795–806. [Google Scholar] [CrossRef]
- Santos, N.P.; Santos, L.H.; Torquato Quezado de Magalhães, M.; Lei, J.; Hilgenfeld, R.; Salgado Ferreira, R.; Bleicher, L. Characterization of an Allosteric Pocket in Zika Virus NS2B-NS3 Protease. J. Chem. Inf. Model. 2022, 62, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, S.A.; Farhy, C.; Pinto, A.; Huang, C.-T.; Simonetti, N.; Ngono, A.E.; Dewing, A.; Shresta, S.; Pinkerton, A.B.; Cieplak, P.; et al. Characterization of the Zika virus two-component NS2B-NS3 protease and structure-assisted identification of allosteric small-molecule antagonists. Antivir. Res. 2017, 143, 218–229. [Google Scholar] [CrossRef]
- Starvaggi, J.; Previti, S.; Zappalà, M.; Ettari, R. The Inhibition of NS2B/NS3 Protease: A New Therapeutic Opportunity to Treat Dengue and Zika Virus Infection. Int. J. Mol. Sci. 2024, 25, 4376. [Google Scholar] [CrossRef] [PubMed]
- Schul, W.; Liu, W.; Xu, H.Y.; Flamand, M.; Vasudevan, S.G. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J. Infect. Dis. 2007, 195, 665–674. [Google Scholar] [CrossRef]
- Tan, G.K.; Ng, J.K.; Trasti, S.L.; Schul, W.; Yip, G.; Alonso, S. A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice. PLoS Neglected Trop. Dis. 2010, 4, e672. [Google Scholar] [CrossRef]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Neglected Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef]
- Simmons, C.P.; Farrar, J.J.; Nguyen, V.V.C.; Wills, B. Current Concepts: Dengue. N. Engl. J. Med. 2012, 36, 1423–1432. [Google Scholar] [CrossRef]
- Lyke, K.E.; Chua, J.; Koren, M.; Friberg, H.; Gromowski, G.D.; Rapaka, R.R.; Waickman, A.T.; Joshi, S.; Strauss, K.; McCracken, M.K.; et al. Efficacy and immunogenicity following dengue virus-1 human challenge after a tetravalent prime-boost dengue vaccine regimen: An open-label, phase 1 trial. Lancet Infect. Dis. 2024, 24, 896–908. [Google Scholar] [CrossRef]
- Waickman, A.T.; Lu, J.Q.; Fang, H.S.; Waldran, M.J.; Gebo, C.; Currier, J.R.; Ware, L.; Van Wesenbeeck, L.; Verpoorten, N.; Lenz, O.; et al. Evolution of inflammation and immunity in a dengue virus 1 human infection model. Sci. Transl. Med. 2022, 14, abo5019. [Google Scholar] [CrossRef]
- Ackaert, O.; Vanhoutte, F.; Verpoorten, N.; Buelens, A.; Lachau-Durand, S.; Lammens, L.; Hoetelmans, R.; Van Loock, M.; Herrera-Taracena, G. Safety, tolerability, and pharmacokinetics of JNJ-1802, a pan-serotype dengue direct antiviral small molecule, in a phase 1, double-blind, randomized, dose-escalation study in healthy volunteers. Clin. Infect. Dis. 2023, 77, 857–865. [Google Scholar] [CrossRef]
- Goethals, O.; Kaptein, S.J.F.; Kesteleyn, B.; Bonfanti, J.-F.; Van Wesenbeeck, L.; Bardiot, D.; Verschoor, E.J.; Verstrepen, B.E.; Fagrouch, Z.; Putnak, J.R.; et al. Blocking NS3–NS4B interaction inhibits dengue virus in non-human primates. Nature 2023, 615, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-J.; Lickliter, J.; Montrond, M.; Ishak, L.; Pietropaolo, K.; James, D.; Belanger, B.; Horga, A.; Hammond, J. First-in-human trial evaluating safety and pharmacokinetics of AT-752, a novel nucleotide prodrug with pan-serotype activity against dengue virus. Antimicrob. Agents Chemother. 2024, 68, e0161523. [Google Scholar] [CrossRef] [PubMed]
- Good, S.S.; Shannon, A.; Lin, K.; Moussa, A.; Julander, J.G.; Colla, P.L.; Collu, G.; Canard, B.; Sommadossi, J.-P. Evaluation of AT-752, a Double Prodrug of a Guanosine Nucleotide Analog with In Vitro and In Vivo Activity against Dengue and Other Flaviviruses. Antimicrob. Agents Chemother. 2021, 65, e00988-21. [Google Scholar] [CrossRef] [PubMed]
- Moquin, S.A.; Simon, O.; Karuna, R.; Lakshminarayana, S.B.; Yokokawa, F.; Wang, F.; Saravanan, C.; Zhang, J.; Day, C.W.; Chan, K.; et al. NITD-688, a pan-serotype inhibitor of the dengue virus NS4B protein, shows favorable pharmacokinetics and efficacy in preclinical animal models. Sci. Transl. Med. 2021, 13, eabb2181. [Google Scholar] [CrossRef]
- Fraser, J.E.; Wang, C.; Chan, K.W.K.; Vasudevan, S.G.; Jans, D.A. Novel dengue virus inhibitor 4-HPR activates ATF4 independent of protein kinase R–like Endoplasmic Reticulum Kinase and elevates levels of eIF2α phosphorylation in virus infected cells. Antivir. Res. 2016, 130, 1–6. [Google Scholar] [CrossRef]
- Fraser, J.E.; Watanabe, S.; Wang, C.; Chan, W.K.K.; Maher, B.; Lopez-Denman, A.; Hick, C.; Wagstaff, K.M.; Mackenzie, J.M.; Sexton, P.M.; et al. A Nuclear Transport Inhibitor That Modulates the Unfolded Protein Response and Provides In Vivo Protection Against Lethal Dengue virus Infection. J. Infect. Dis. 2014, 210, 1780–1791. [Google Scholar] [CrossRef]
- Warfield, K.L.; Plummer, E.M.; Sayce, A.C.; Alonzi, D.S.; Tang, W.; Tyrrell, B.E.; Hill, M.L.; Caputo, A.T.; Killingbeck, S.S.; Beatty, P.R.; et al. Inhibition of endoplasmic reticulum glucosidases is required for in vitro and in vivo dengue antiviral activity by the iminosugar UV-4. Antivir. Res. 2016, 129, 93–98. [Google Scholar] [CrossRef]
- Callahan, M.; Treston, A.M.; Lin, G.; Smith, M.; Kaufman, B.; Khaliq, M.; Evans DeWald, L.; Spurgers, K.; Warfield, K.L.; Lowe, P.; et al. Randomized single oral dose phase 1 study of safety, tolerability, and pharmacokinetics of Iminosugar UV-4 Hydrochloride (UV-4B) in healthy subjects. PLoS Neglected Trop. Dis. 2022, 16, e0010636. [Google Scholar] [CrossRef]
- Morchang, A.; Malakar, S.; Poonudom, K.; Noisakran, S.; Yenchitsomanus, P.-t.; Limjindaporn, T. Melatonin Inhibits Dengue Virus Infection via the Sirtuin 1-Mediated Interferon Pathway. Viruses 2021, 13, 659. [Google Scholar] [CrossRef]
- Bennathan, D.; Maestroni, G.J.M.; Lustig, S.; Conti, A. Protective Effects of Melatonin in Mice Infected with Encephalitis Viruses. Arch. Virol. 1995, 140, 223–230. [Google Scholar] [CrossRef]
- Chong Teoh, T.; Al-Harbi, S.J.; Abdulrahman, A.Y.; Rothan, H.A. Doxycycline Interferes with Zika Virus Serine Protease and Inhibits Virus Replication in Human Skin Fibroblasts. Molecules 2021, 26, 4321. [Google Scholar] [CrossRef]
- Fredeking, T.M.; Zavala-Castro, J.E.; González-Martínez, P.; Moguel-Rodríguez, W.; Sanchez, E.C.; Foster, M.J.; Diaz-Quijano, F.A. Dengue Patients Treated with Doxycycline Showed Lower Mortality Associated to a Reduction in IL-6 and TNF Levels. Recent Pat. Anti-Infect. Drug Discov. 2015, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Kamboj, K.; Pannu, A.K.; Yadav, A.K.; Bhatia, M.; Saroch, A. Role of doxycycline in the treatment of dengue infection: An open-label, randomized, controlled, pilot trial. Asian Pac. J. Trop. Med. 2024, 17, 160–165. [Google Scholar] [CrossRef]
- Rathore, A.P.; Paradkar, P.N.; Watanabe, S.; Tan, K.H.; Sung, C.; Connolly, J.E.; Low, J.; Ooi, E.E.; Vasudevan, S.G. Celgosivir treatment misfolds dengue virus NS1 protein, induces cellular pro-survival genes and protects against lethal challenge mouse model. Antivir. Res. 2011, 92, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Chan, K.W.; Dow, G.; Ooi, E.E.; Low, J.G.; Vasudevan, S.G. Optimizing celgosivir therapy in mouse models of dengue virus infection of serotypes 1 and 2: The search for a window for potential therapeutic efficacy. Antivir. Res. 2016, 127, 10–19. [Google Scholar] [CrossRef]
- Low, J.G.; Sung, C.; Wijaya, L.; Wei, Y.; Rathore, A.P.S.; Watanabe, S.; Tan, B.H.; Toh, L.; Chua, L.T.; Hou, Y.; et al. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): A phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Tay, M.Y.; Fraser, J.E.; Chan, W.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Suputtamongkol, Y.; Avirutnan, P.; Mairiang, D.; Angkasekwinai, N.; Niwattayakul, K.; Yamasmith, E.; Saleh-Arong, F.A.; Songjaeng, A.; Prommool, T.; Tangthawornchaikul, N.; et al. Ivermectin Accelerates Circulating Nonstructural Protein 1 (NS1) Clearance in Adult Dengue Patients: A Combined Phase 2/3 Randomized Double-blinded Placebo Controlled Trial. Clin. Infect. Dis. 2021, 72, e586–e593. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Tran, C.N.; Phung, L.K.; Duong, K.T.; Huynh Hle, A.; Farrar, J.; Nguyen, Q.T.; Tran, H.T.; Nguyen, C.V.; Merson, L.; et al. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J. Infect. Dis. 2013, 207, 1442–1450. [Google Scholar] [CrossRef]
- Farias, K.J.; Machado, P.R.; de Almeida Junior, R.F.; de Aquino, A.A.; da Fonseca, B.A. Chloroquine interferes with dengue-2 virus replication in U937 cells. Microbiol. Immunol. 2014, 58, 318–326. [Google Scholar] [CrossRef]
- Tricou, V.; Minh, N.N.; Van, T.P.; Lee, S.J.; Farrar, J.; Wills, B.; Tran, H.T.; Simmons, C.P. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Neglected Trop. Dis. 2010, 4, e785. [Google Scholar] [CrossRef]
- Farias, K.J.; Machado, P.R.; Muniz, J.A.; Imbeloni, A.A.; da Fonseca, B.A. Antiviral activity of chloroquine against dengue virus type 2 replication in Aotus monkeys. Viral Immunol. 2015, 28, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, X.; Ji, X.; Quanquin, N.; Deng, Y.-Q.; Tian, M.; Aliyari, R.; Zuo, X.; Yuan, L.; Afridi, S.K.; et al. Chloroquine, a FDA-approved Drug, Prevents Zika Virus Infection and its Associated Congenital Microcephaly in Mice. EBioMedicine 2017, 24, 189–194. [Google Scholar] [CrossRef]
- Pitts, J.D.; Li, P.C.; de Wispelaere, M.; Yang, P.L. Antiviral activity of N-(4-hydroxyphenyl) retinamide (4-HPR) against Zika virus. Antivir. Res. 2017, 147, 124–130. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.N.Y.; Smith, K.; Forwood, J.K.; Jans, D.A. Nuclear import inhibitor N-(4-hydroxyphenyl) retinamide targets Zika virus (ZIKV) nonstructural protein 5 to inhibit ZIKV infection. Biochem. Biophys. Res. Commun. 2017, 493, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Janssen Announces Promising Antiviral Activity against Dengue in a Phase 2a Human Challenge Model. Available online: https://www.jnj.com/janssen-announces-promising-antiviral-activity-against-dengue-in-a-phase-2a-human-challenge-model (accessed on 28 November 2023).
- Kesteleyn, B.; Bonfanti, J.-F.; Bardiot, D.; De Boeck, B.; Goethals, O.; Kaptein, S.J.F.; Stoops, B.; Coesemans, E.; Fortin, J.; Muller, P.; et al. Discovery of JNJ-1802, a First-in-Class Pan-Serotype Dengue Virus NS4B Inhibitor. J. Med. Chem. 2024, 67, 4063–4082. [Google Scholar] [CrossRef]
- McCormack, C.P.; Goethals, O.; Goeyvaerts, N.; Woot de Trixhe, X.D.; Geluykens, P.; Borrenberghs, D.; Ferguson, N.M.; Ackaert, O.; Dorigatti, I. Modelling the impact of JNJ-1802, a first-in-class dengue inhibitor blocking the NS3-NS4B interaction, on in-vitro DENV-2 dynamics. PLoS Comput. Biol. 2023, 19, e1011662. [Google Scholar] [CrossRef] [PubMed]
- Feracci, M.; Eydoux, C.; Fattorini, V.; Lo Bello, L.; Gauffre, P.; Selisko, B.; Sutto-Ortiz, P.; Shannon, A.; Xia, H.; Shi, P.-Y.; et al. AT-752 targets multiple sites and activities on the Dengue virus replication enzyme NS5. Antivir. Res. 2023, 212, 105574. [Google Scholar] [CrossRef]
- ASX Announcement. Island Provides ISLA-101 Clinical Program Update. Available online: https://www.islandpharmaceuticals.com/site/pdf/67d20e62-2ff1-4788-b2b9-991ca9daed72/ISLA101-Clinical-Program-Update.pdf (accessed on 23 August 2024).
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Paemanee, A.; Hitakarun, A.; Roytrakul, S.; Smith, D.R. Screening of melatonin, α-tocopherol, folic acid, acetyl-L-carnitine and resveratrol for anti-dengue 2 virus activity. BMC Res. Notes 2018, 11, 307. [Google Scholar] [CrossRef]
- Wongchitrat, P.; Yasawong, M.; Jumpathong, W.; Chanmanee, T.; Samutpong, A.; Dangsakul, W.; Govitrapong, P.; Reiter, R.J.; Puthavathana, P. Melatonin inhibits Zika virus replication in Vero epithelial cells and SK-N-SH human neuroblastoma cells. Melatonin Res. 2022, 5, 171–185. [Google Scholar] [CrossRef]
- Moon, J.H.; Hong, J.-M.; Seol, J.W.; Park, B.Y.; Eo, S.K.; Park, S.Y. Melatonin inhibits Japanese encephalitis virus replication and neurotoxicity via calcineurin- autophagy pathways. BMC Neurosci. 2023, 24, 59. [Google Scholar] [CrossRef]
- Rothan, H.A.; Mohamed, Z.; Paydar, M.; Abd Rahman, N.; Yusof, R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch. Virol. 2014, 159, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Pambhar, V.; Mathur, N.; Mehta, A.; Mathur, M.; Kumawat, D.C.; Mangalia, R.; Verma, A.; Patyal, A. Effect of doxycycline and doxycycline with carica papaya on thrombocytopenia and leucopenia in acute dengue fever patients. J. Fam. Med. Prim. Care 2022, 11, 3270–3275. [Google Scholar]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef]
- Denolly, S.; Guo, H.B.; Martens, M.; Płaszczyca, A.; Scaturro, P.; Prasad, V.; Kongmanas, K.; Punyadee, N.; Songjaeng, A.; Mairiang, D.; et al. Dengue virus NS1 secretion is regulated via importin-subunit β1 controlling expression of the chaperone GRp78 and targeted by the clinical drug ivermectin. mBio 2023, 14, e01441-23. [Google Scholar] [CrossRef]
- Palacios-Rápalo, S.N.; Farfan-Morales, C.N.; Cordero-Rivera, C.D.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Meraz-Ríos, M.A.; Del Ángel, R.M. An ivermectin—Atorvastatin combination impairs nuclear transport inhibiting dengue infection in vitro and in vivo. iScience 2023, 26, 108294. [Google Scholar] [CrossRef]
- Osuna-Ramos, J.; Farfan-Morales, C.; Cordero-Rivera, C.D.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Hurtado-Monzón, A.M.; Palacios-Rápalo, S.N.; Ricardo Jiménez-Camacho, R.; Meraz-Ríos, M.A.; Del Ángel, R.M. Cholesterol-Lowering Drugs as Potential Antivirals: A Repurposing Approach against Flavivirus Infections. Viruses 2023, 15, 1465. [Google Scholar] [CrossRef]
- Li, Z.; Brecher, M.; Deng, Y.-Q.; Zhang, J.; Sakamuru, S.; Liu, B.; Huang, R.; Koetzner, C.A.; Allen, C.A.; Jones, S.A.; et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res. 2017, 27, 1046–1064. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Lang, Y.; Fan, X.; Kuo, L.; D’Brant, L.; Hu, S.; Samrat, S.K.; Trudeau, N.; Tharappel, A.M.; et al. JMX0207, a Niclosamide Derivative with Improved Pharmacokinetics, Suppresses Zika Virus Infection Both In Vitro and In Vivo. ACS Infect. Dis. 2020, 6, 2616–2628. [Google Scholar] [CrossRef]
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Boorgu, D.; Levin, M.; Kaplan, D.L. Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells. Biol. Open 2018, 7, bio031807. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.C.; HuangFu, W.C.; Tsai, T.T.; Ho, M.R.; Jhan, M.K.; Shen, T.J.; Tseng, P.C.; Wang, Y.T.; Lin, C.F. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. PLoS Neglected Trop. Dis. 2018, 12, e0006715. [Google Scholar] [CrossRef]
- Li, Z.; Lang, Y.; Sakamuru, S.; Samrat, S.; Trudeau, N.; Kuo, L.; Rugenstein, N.; Tharappel, A.; D’Brant, L.; Koetzner, C.A.; et al. Methylene blue is a potent and broad-spectrum inhibitor against Zika virus in vitro and in vivo. Emerg. Microbes Infect. 2020, 9, 2404–2416. [Google Scholar] [CrossRef]
- Li, Z.; Sakamuru, S.; Huang, R.; Brecher, M.; Koetzner, C.A.; Zhang, J.; Chen, H.; Qin, C.F.; Zhang, Q.Y.; Zhou, J.; et al. Erythrosin B is a potent and broad-spectrum orthosteric inhibitor of the flavivirus NS2B-NS3 protease. Antivir. Res. 2018, 150, 217–225. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Lang, Y.; Wu, X.; Hu, S.; Samrat, S.K.; Tharappel, A.M.; Kuo, L.; Butler, D.; Song, Y.; et al. In vitro and in vivo characterization of erythrosin B and derivatives against Zika virus. Acta Pharm. Sin. B 2022, 12, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Yuan, H.; Rao, J.; Zou, J.; Yang, K.; Peng, G.; Cao, S.; Chen, H.; Song, Y. Identification of a small compound that specifically inhibits Zika virus in vitro and in vivo by targeting the NS2B-NS3 protease. Antivir. Res. 2022, 199, 105255. [Google Scholar] [CrossRef]
- Shi, Z.; Wei, J.; Deng, X.; Li, S.; Qiu, Y.; Shao, D.; Li, B.; Zhang, K.; Xue, F.; Wang, X.; et al. Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol. J. 2014, 11, 10. [Google Scholar] [CrossRef]
- Rossignol, J.F. Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antivir. Res. 2014, 110, 94–103. [Google Scholar] [CrossRef]
- Cao, R.Y.; Xu, Y.F.; Zhang, T.H.; Yang, J.J.; Yuan, Y.; Hao, P.; Shi, Y.; Zhong, J.; Zhong, W. Pediatric Drug Nitazoxanide: A Potential Choice for Control of Zika. Open Forum Infect. Dis. 2017, 4, ofx009. [Google Scholar] [CrossRef]
- Yamamoto, K.A.; Blackburn, K.; Goshe, M.B.; Brown, D.T.; Migoswski, E.; Campanhon, I.B.; Moreira, M.F.; Ferreira, D.F.; Soares, M.R. Tizoxanide Antiviral Activity on Dengue Virus Replication. Viruses 2023, 15, 696. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Yao, Y.; Wu, F.; Wu, X.; Zhao, J.; Hua, Y.; Wu, J.; Huo, T.; Lin, Y.-L.; Kneubehl, A.R.; et al. Synthesis, Structure–Activity Relationships, and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B–NS3 Protease. J. Med. Chem. 2021, 64, 2777–2800. [Google Scholar] [CrossRef]
- Yao, Y.; Huo, T.; Lin, Y.-L.; Nie, S.; Wu, F.; Hua, Y.; Wu, J.; Kneubehl, A.R.; Vogt, M.B.; Rico-Hesse, R.; et al. Discovery, X-ray Crystallography and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J. Am. Chem. Soc. 2019, 141, 6832–6836. [Google Scholar] [CrossRef]
- Coluccia, A.; Puxeddu, M.; Nalli, M.; Wei, C.-K.; Wu, Y.-H.; Mastrangelo, E.; Elamin, T.; Tarantino, D.; Bugert, J.J.; Schreiner, B.; et al. Discovery of Zika Virus NS2B/NS3 Inhibitors That Prevent Mice from Life-Threatening Infection and Brain Damage. ACS Med. Chem. Lett. 2020, 11, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chan, J.F.; den-Haan, H.; Chik, K.K.; Zhang, A.J.; Chan, C.C.; Poon, V.K.; Yip, C.C.; Mak, W.W.; Zhu, Z.; et al. Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antivir. Res. 2017, 145, 33–43. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Puerta-Guardo, H.; Espinosa, D.A.; Harris, E.; Caicedo-Torres, W.; Olivero-Verbel, J.; Martínez-Romero, E. In silico drug repurposing for the identification of potential candidate molecules against arboviruses infection. Antivir. Res. 2020, 173, 104668. [Google Scholar] [CrossRef]
- Dražić, T.; Kopf, S.; Corridan, J.; Leuthold, M.M.; Bertoša, B.; Klein, C.D. Peptide-β-lactam Inhibitors of Dengue and West Nile Virus NS2B-NS3 Protease Display Two Distinct Binding Modes. J. Med. Chem. 2020, 63, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Manzano, M.; Teramoto, T.; Pilankatta, R.; Padmanabhan, R. High-throughput screening for the identification of small-molecule inhibitors of the flaviviral protease. Antivir. Res. 2016, 134, 6–16. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, M.H.; Lee, J.Y.; Hwang, I.; Yoon, G.Y.; Kim, H.S.; Kwon, Y.C.; Ahn, D.G.; Kim, K.D.; Kim, B.T.; et al. Structure-Based Virtual Screening: Identification of a Novel NS2B-NS3 Protease Inhibitor with Potent Antiviral Activity against Zika and Dengue Viruses. Microorganisms 2021, 9, 545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).