Selective Oxidation of Benzo[d]isothiazol-3(2H)-Ones Enabled by Selectfluor
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
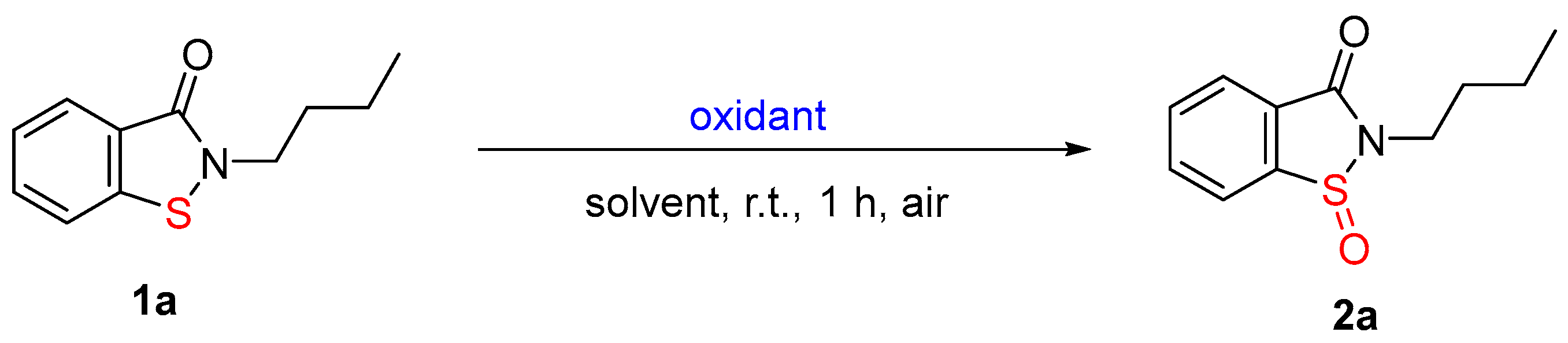
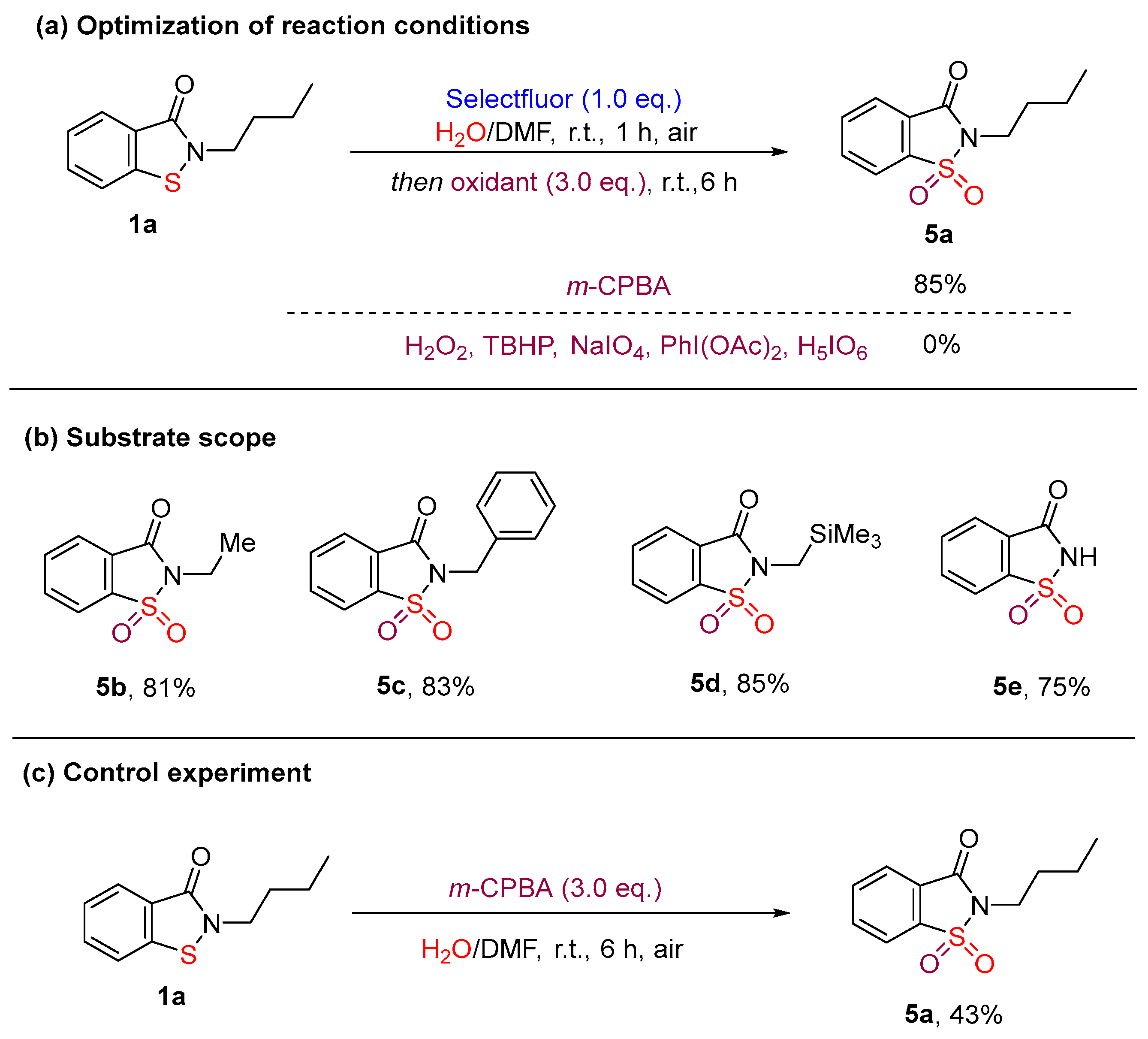
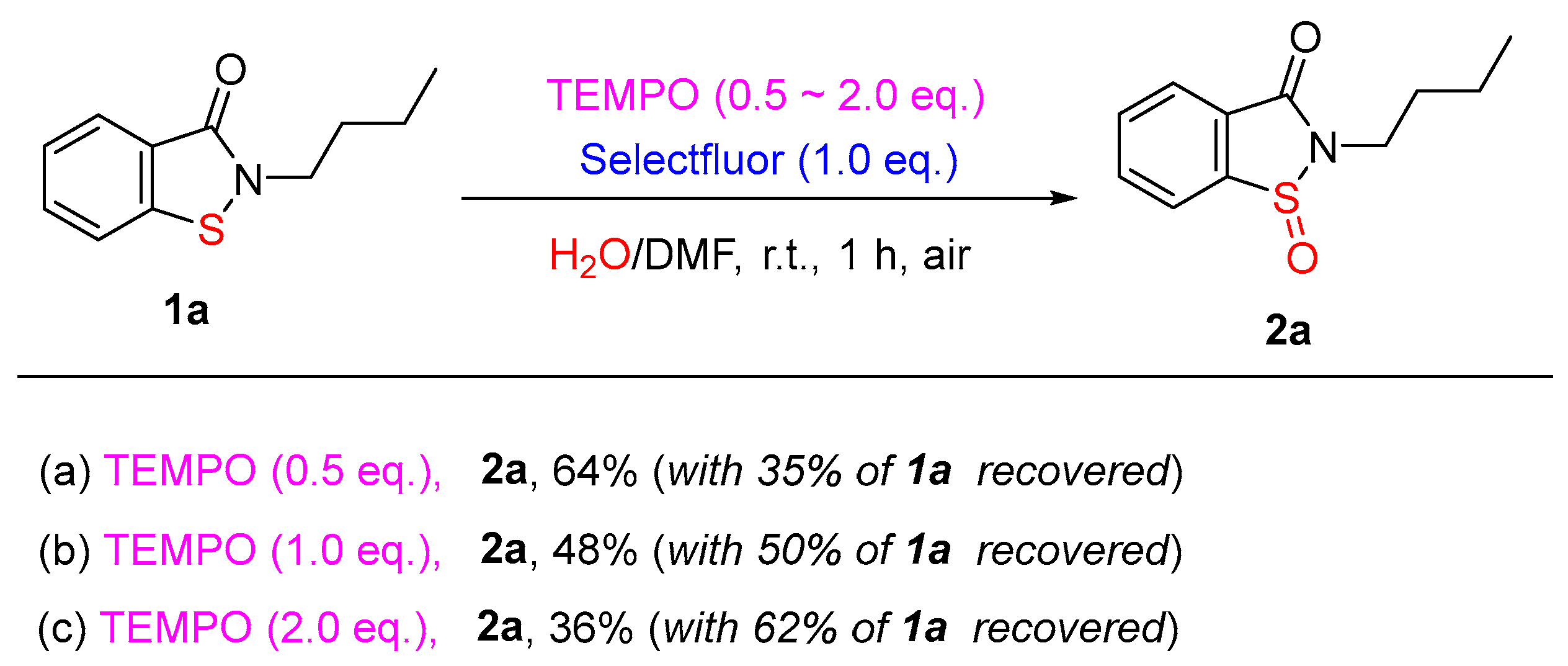
3.2. Optimization of the Reaction Conditions
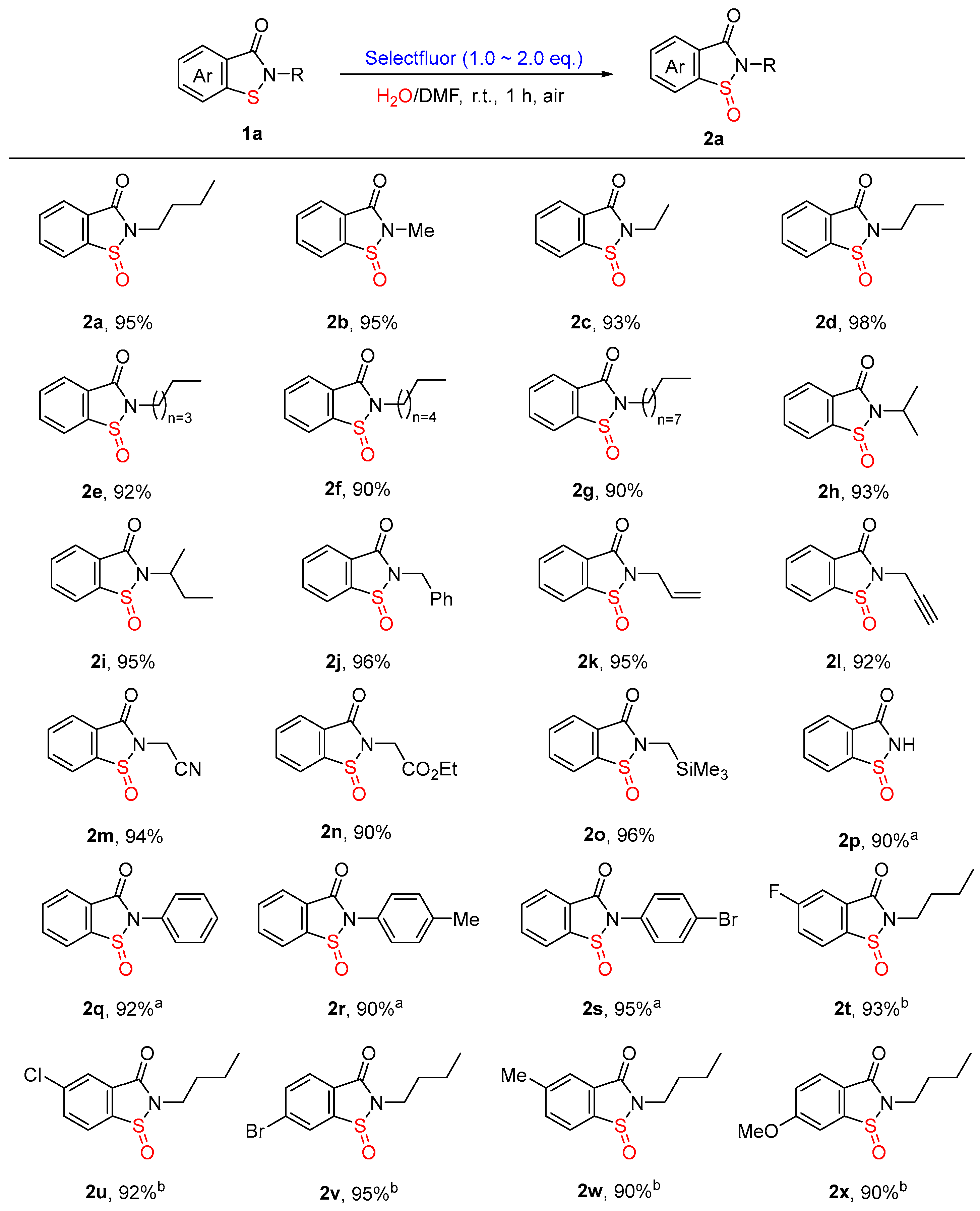
3.3. Synthetic Procedures for the Synthesis of Compounds 2
- The details for 2-Butylbenzo[d]isothiazol-3(2H)-one-1-oxide (2a). Colorless oil, 42.4 mg, 95%. 1H NMR (300 MHz, CDCl3) δ 7.94–7.91 (m, 1H), 7.83 (d, J = 7.3 Hz, 1H), 7.76–7.66 (m, 2H), 3.93–3.83 (m, 1H), 3.75–3.65 (m, 1H), 1.75–1.66 (m, 2H), 1.39–1.32 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.38, 145.56, 134.10, 133.21, 128.43, 126.11, 125.07, 41.07, 31.36, 20.09, 13.67. HRMS (ESI, m/z): calcd. for C11H14NO2S [M + H]+, 224.0740; found, 224.0737.
- The details for 2-Methylbenzo[d]isothiazol-3(2H)-one-1-oxide (2b). White solid, 34.4 mg, 95%, m.p. = 114–115 °C. 1H NMR (300 MHz, CDCl3) δ 8.01–7.99 (m, 1H), 7.93–7.90 (m, 1H), 7.84–7.73 (m, 2H), 3.39 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 165.45, 145.47, 134.18, 133.27, 128.10, 126.07, 125.09, 26.98. HRMS (ESI, m/z): calcd. for C8H8NO2S [M + H]+, 182.0270; found, 182.0264.
- The details for 2-Ethylbenzo[d]isothiazol-3(2H)-one-1-oxide (2c). White solid, 36.3 mg, 93%, m.p. = 80–81 °C. 1H NMR (300 MHz, CDCl3) δ 8.02–7.99 (m, 1H), 7.93–7.89 (m, 1H), 7.83–7.73 (m, 2H), 4.09–3.97 (m, 1H), 3.91–3.79 (m, 1H), 1.42 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.14, 145.54, 134.12, 133.21, 128.47, 126.05, 125.07, 36.39, 14.81. HRMS (ESI, m/z): calcd. for C9H10NO2S [M + H]+, 196.0427; found, 196.0422.
- The details for 2-Propylbenzo[d]isothiazol-3(2H)-one-1-oxide (2d). White solid, 41.0 mg, 98%, m.p. = 55–56 °C. 1H NMR (300 MHz, CDCl3) δ 8.01–7.98 (m, 1H), 7.92–7.89 (m, 1H), 7.83–7.73 (m, 2H), 3.96–3.86 (m, 1H), 3.80–3.70 (m, 1H), 1.91–1.77 (m, 2H), 1.01 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.40, 145.55, 134.12, 133.21, 128.38, 126.10, 125.07, 42.93, 22.67, 11.37. HRMS (ESI, m/z): calcd. for C10H12NO2S [M + H]+, 210.0583; found, 210.0581.
- The details for 2-Pentylbenzo[d]isothiazol-3(2H)-one-1-oxide (2e). Colorless oil, 43.7 mg, 92%. 1H NMR (300 MHz, CDCl3) δ 7.91 (d, J = 7.1 Hz, 1H), 7.83 (d, J = 7.3 Hz, 1H), 7.75–7.65 (m, 2H), 3.91–3.82 (m, 1H), 3.73–3.63 (m, 1H), 1.78–1.68 (m, 2H), 1.37–1.23 (m, 4H), 0.83 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.34, 145.59, 134.07, 133.17, 128.43, 126.08, 125.05, 41.29, 28.99, 28.93, 22.23, 13.93. HRMS (ESI, m/z): calcd. for C12H15NNaO2S [M + Na]+, 260.0716; found, 260.0716.
- The details for 2-Hexylbenzo[d]isothiazol-3(2H)-one 1-oxide (2f). Colorless oil, 45.2 mg, 90%. 1H NMR (300 MHz, CDCl3) δ 7.92 (d, J = 7.2 Hz, 1H), 7.83 (d, J = 7.3 Hz, 1H), 7.75–7.65 (m, 2H), 3.92–3.79 (m, 1H), 3.73–3.63 (m, 1H), 1.78–1.67 (m, 2H), 1.35–1.18 (m, 6H), 0.81 (t, J = 6.7 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.35, 145.58, 134.08, 133.19, 128.44, 126.10, 125.06, 41.32, 31.37, 29.29, 26.51, 22.51, 14.02. HRMS (ESI, m/z): calcd. for C13H17NNaO2S [M + Na]+, 274.0872; found, 274.0866.
- The details for 2-Nonylbenzo[d]isothiazol-3(2H)-one-1-oxide (2g). Colorless oil, 52.8 mg, 90%. 1H NMR (300 MHz, CDCl3) δ 8.00 (dd, J = 6.9, 1.7 Hz, 1H), 7.91 (dd, J = 6.8, 1.6 Hz, 1H), 7.83–7.72 (m, 2H), 3.99–3.90 (m, 1H), 3.81–3.71 (m, 1H), 1.88–1.73 (m, 2H), 1.42–1.25 (m, 12H), 0.87 (t, J = 6.8 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.35, 145.60, 134.07, 133.18, 128.46, 126.11, 125.05, 41.33, 31.83, 29.44, 29.33, 29.22, 29.17, 26.85, 22.66, 14.12. HRMS (ESI, m/z): calcd. for C16H23NNaO2S [M + Na]+, 316.1342; found, 316.1337.
- The details 2-Isopropylbenzo[d]isothiazol-3(2H)-one-1-oxide (2h). White solid, 39.0 mg, 93%, m.p. = 47–48 °C. 1H NMR (300 MHz, CDCl3) δ 7.90 (d, J = 7.2 Hz, 1H), 7.81 (d, J = 7.3 Hz, 1H), 7.75–7.61 (m, 2H), 4.67–4.54 (m, 1H), 1.52–1.48 (m, 6H). 13C NMR (75 MHz, CDCl3) δ 165.29, 145.59, 134.07, 133.07, 128.74, 125.96, 124.87, 46.96, 22.49, 21.87. HRMS (ESI, m/z): calcd. for C10H12NO2S [M + H]+, 210.0583; found, 210.0583.
- The details for 2-(sec-Butyl)benzo[d]isothiazol-3(2H)-one-1-oxide (2i). Yellow oil, 42.4 mg, 95%. 1H NMR (300 MHz, CDCl3) δ 8.00–7.96 (m, 1H), 7.90–7.87 (m, 1H), 7.82–7.71 (m, 2H), 4.52–4.40 (m, 1H), 2.11–1.77 (m, 2H), 1.57–1.52 (m, 3H), 1.04–0.93 (m, 3H). 13C NMR (75 MHz, CDCl3) δ 165.56 (d, J = 10.3 Hz), 145.67 (d, J = 6.9 Hz), 134.05, 133.05 (d, J = 1.7 Hz), 128.68 (d, J = 7.7 Hz), 126.00 (d, J = 3.3 Hz), 124.87, 52.72 (d, J = 13.2 Hz), 29.00 (d, J = 38.6 Hz), 20.23 (d, J = 35.2 Hz), 11.14 (d, J = 3.9 Hz). HRMS (ESI, m/z): calcd. for C11H14NO2S [M + H]+, 224.0740; found, 224.0738.
- The details for 2-Benzylbenzo[d]isothiazol-3(2H)-one-1-oxide (2j). White solid, 49.4 mg, 96%, m.p. = 92–93 °C (known compound [30]). 1H NMR (300 MHz, CDCl3) δ 7.93–7.90 (m, 1H), 7.79 (d, J = 7.2 Hz, 1H), 7.72–7.62 (m, 2H), 7.35–7.18 (m, 5H), 5.20 (d, J = 15.3 Hz, 1H), 4.65 (d, J = 15.3 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 165.22, 145.64, 135.83, 134.28, 133.29, 128.89, 128.65, 128.24, 128.22, 126.31, 125.24, 44.33.
- The details for 2-Allylbenzo[d]isothiazol-3(2H)-one-1-oxide (2k). Colorless oil, 39.4 mg, 95% (known compound [30]). 1H NMR (300 MHz, CDCl3) δ 7.94 (d, J = 7.2 Hz, 1H), 7.84 (d, J = 7.4 Hz, 1H), 7.77–7.66 (m, 2H), 5.95–5.82 (m, 1H), 5.31 (d, J = 17.0 Hz, 1H), 5.24 (d, J = 10.1 Hz, 1H), 4.62–4.54 (m, 1H), 4.22 (dd, J = 15.9, 6.8 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 165.11, 145.68, 134.26, 133.26, 131.73, 128.20, 126.24, 125.19, 119.34, 43.17.
- The details for 2-(Prop-2-yn-1-yl)benzo[d]isothiazol-3(2H)-one-1-oxide (2l). White solid, 37.8 mg, 92%, m.p. = 142–143 °C. 1H NMR (300 MHz, CDCl3) δ 7.99–7.96 (m, 1H), 7.85–7.82 (m, 1H), 7.75–7.65 (m, 2H), 5.29–5.16 (m, 2H), 2.67 (t, J = 2.5 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.98, 154.81, 132.73, 132.39, 129.33, 125.05, 124.27, 77.00, 76.30, 57.79. HRMS (ESI, m/z): calcd. for C10H7NNaO2S [M + Na]+, 228.0090; found, 228.0090.
- The details for 2-(1-Oxido-3-oxobenzo[d]isothiazol-2(3H)-yl)acetonitrile (2m). White solid, 38.7 mg, 94%, m.p. = 120–121 °C. 1H NMR (300 MHz, CDCl3) δ 7.98 (d, J = 7.4 Hz, 1H), 7.90 (d, J = 7.5 Hz, 1H), 7.85–7.72 (m, 2H), 4.84 (d, J = 17.9 Hz, 1H), 4.50 (d, J = 17.9 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 164.57, 145.53, 135.26, 133.86, 126.79, 126.61, 125.69, 114.17, 28.04. HRMS (ESI, m/z): calcd. for C9H7N2O2S [M + H]+, 207.0223; found, 207.0223.
- The details for Ethyl 2-(1-oxido-3-oxobenzo[d]isothiazol-2(3H)-yl)acetate (2n). Colorless oil, 45.5 mg, 90%, (known compound [31]). 1H NMR (300 MHz, CDCl3) δ 7.89 (d, J = 6.8 Hz, 1H), 7.79 (d, J = 7.9 Hz, 1H), 7.69–7.58 (m, 2H), 5.13 (d, J = 15.7 Hz, 1H), 4.97 (d, J = 15.7 Hz, 1H), 4.22 (q, J = 7.2 Hz, 2H), 1.23 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 170.23, 166.54, 154.91, 132.75, 132.38, 129.12, 125.02, 124.37, 65.61, 61.90, 14.11.
- The details for 2-((Trimethylsilyl)methyl)benzo[d]isothiazol-3(2H)-one-1-oxide (2o). Colorless oil, 48.7 mg, 96%. 1H NMR (300 MHz, CDCl3) δ 7.96–7.93 (m, 1H), 7.88–7.83 (m, 1H), 7.78–7.69 (m, 2H), 3.43 (d, J = 15.6 Hz, 1H), 3.19 (d, J = 15.6 Hz, 1H), 0.16 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 165.22, 145.49, 133.86, 133.21, 128.40, 125.93, 124.97, 32.13, -1.63. HRMS (ESI, m/z): calcd. for C11H16NO2SSi [M + H]+, 254.0666; found, 254.0666.
- The details for Benzo[d]isothiazol-3(2H)-one-1-oxide (2p). White solid, 30.1 mg, 90%, m.p. = 158–159 °C (known compound [30]). 1H NMR (300 MHz, DMSO-d6) δ 11.52 (br, 1H), 8.13 (d, J = 7.5 Hz, 1H), 7.95–7.82 (m, 3H). 13C NMR (75 MHz, DMSO-d6) δ 167.94, 148.57, 135.18, 133.69, 127.73, 126.33, 125.91.
- The details for 2-Phenylbenzo[d]isothiazol-3(2H)-one-1-oxide (2q). White solid, 44.8 mg, 92%, m.p. = 136–137 °C (known compound [30]). 1H NMR (400 MHz, CDCl3) δ 8.11–8.09 (m, 1H), 7.98–7.95 (m, 1H), 7.89–7.79 (m, 2H), 7.53–7.43 (m, 5H). 13C NMR (101 MHz, CDCl3) δ 164.54, 145.51, 134.65, 133.90, 133.48, 129.79, 128.94, 128.25, 127.42, 126.80, 125.26.
- The details for 2-(p-Tolyl)benzo[d]isothiazol-3(2H)-one-1-oxide (2r). White solid, 46.3 mg, 90%, m.p. = 169–170 °C (known compound [30]). 1H NMR (400 MHz, CDCl3) δ 8.10–8.07 (m, 1H), 7.97–7.94 (m, 1H), 7.88–7.78 (m, 2H), 7.41–7.38 (m, 2H), 7.32–7.30 (m, 2H), 2.41 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.66, 145.57, 139.19, 134.57, 133.42, 131.04, 130.42, 128.29, 127.45, 126.73, 125.24, 21.31.
- The details for 2-(4-Bromophenyl)benzo[d]isothiazol-3(2H)-one-1-oxide (2s). White solid, 61.2 mg, 95%, m.p. = 100–101 °C. 1H NMR (400 MHz, CDCl3) δ 8.11–8.09 (m, 1H), 7.98–7.96 (m, 1H), 7.91–7.81 (m, 2H), 7.66–7.62 (m, 2H), 7.45–7.41 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 164.37, 145.39, 134.81, 133.60, 133.10, 132.94, 128.74, 128.02, 126.87, 125.30, 122.81. HRMS (ESI, m/z): calcd. for C13H9BrNO2S [M + H]+, 321.9532; found, 321.9529.
- The details for 2-Butyl-5-fluorobenzo[d]isothiazol-3(2H)-one-1-oxide (2t). White solid, 45.0 mg, 93%, m.p. = 82–83 °C. 1H NMR (300 MHz, CDCl3) δ 7.83 (dd, J = 8.5, 4.3 Hz, 1H), 7.58 (dd, J = 7.4, 2.4 Hz, 1H), 7.44–7.37 (m, 1H), 3.91–3.82 (m, 1H), 3.74–3.64 (m, 1H), 1.76–1.66 (m, 2H), 1.41–1.29 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.65 (d, J = 256.5 Hz), 164.12 (d, J = 3.0 Hz), 141.09 (d, J = 3.1 Hz), 131.59 (d, J = 9.2 Hz), 127.33 (d, J = 9.3 Hz), 121.45 (d, J = 23.9 Hz), 113.32 (d, J = 24.5 Hz), 41.33, 31.25, 20.04, 13.60. 19F NMR (282 MHz, CDCl3) δ -103.42. HRMS (ESI, m/z): calcd. for C11H13FNO2S [M + H]+, 242.0646; found, 242.0648.
- The details for 2-Butyl-5-chlorobenzo[d]isothiazol-3(2H)-one-1-oxide (2u). Colorless oil, 47.4 mg, 92%. 1H NMR (300 MHz, CDCl3) δ 7.88 (dd, J = 1.9, 0.5 Hz, 1H), 7.77 (dd, J = 8.2, 0.6 Hz, 1H), 7.68 (dd, J = 8.2, 1.9 Hz, 1H), 3.91–3.81 (m, 1H), 3.74–3.64 (m, 1H), 1.76–1.65 (m, 2H), 1.41–1.29 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 164.14, 143.64, 140.08, 134.12, 130.37, 126.29, 126.27, 41.31, 31.26, 20.05, 13.61. HRMS (ESI, m/z): calcd. for C11H13ClNO2S [M + H]+, 258.0350; found, 258.0343.
- The details for 6-Bromo-2-butylbenzo[d]isothiazol-3(2H)-one-1-oxide (2v). White solid, 57.4 mg, 95%, m.p. = 97–98 °C. 1H NMR (300 MHz, CDCl3) δ 8.04 (s, 1H), 7.90–7.83 (m, 1H), 3.98–3.88 (m, 1H), 3.81–3.72 (m, 1H), 1.86–1.73 (m, 2H), 1.48–1.36 (m, 2H), 0.97 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 164.61, 147.12, 136.50, 128.84, 128.31, 127.35, 127.20, 41.24, 31.27, 20.06, 13.61. HRMS (ESI, m/z): calcd. for C11H13BrNO2S [M + H]+, 301.9845; found, 301.9840.
- The details for 2-Butyl-5-methylbenzo[d]isothiazol-3(2H)-one-1-oxide (2w). White solid, 42.7 mg, 90%, m.p. = 100–101 °C. 1H NMR (300 MHz, CDCl3) δ 7.71–7.68 (m, 2H), 7.51 (d, J = 7.8 Hz, 1H), 3.91–3.81 (m, 1H), 3.72–3.62 (m, 1H), 2.45 (s, 3H), 1.76–1.65 (m, 2H), 1.41–1.29 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.50, 144.33, 142.77, 134.75, 128.68, 126.37, 124.84, 41.03, 31.35, 21.67, 20.08, 13.64. HRMS (ESI, m/z): calcd. for C12H15NNaO2S [M + Na]+, 260.0716; found, 260.0716.
- The details for 2-Butyl-6-methoxybenzo[d]isothiazol-3(2H)-one-1-oxide (2x). White solid, 45.6 mg, 90%, m.p. = 139–140 °C. 1H NMR (300 MHz, CDCl3) δ 7.89 (d, J = 8.4 Hz, 1H), 7.36 (s, 1H), 7.21 (dd, J = 8.5, 2.2 Hz, 1H), 3.95–3.87 (m, 4H), 3.79–3.70 (m, 1H), 1.83–1.72 (m, 2H), 1.46– 1.39 (m, 2H), 0.97 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.21, 164.54, 147.86, 127.43, 120.44, 119.56, 109.51, 56.19, 41.08, 31.42, 20.07, 13.64. HRMS (ESI, m/z): calcd. for C12H16NO3S [M + H]+, 254.0845; found, 254.0666.
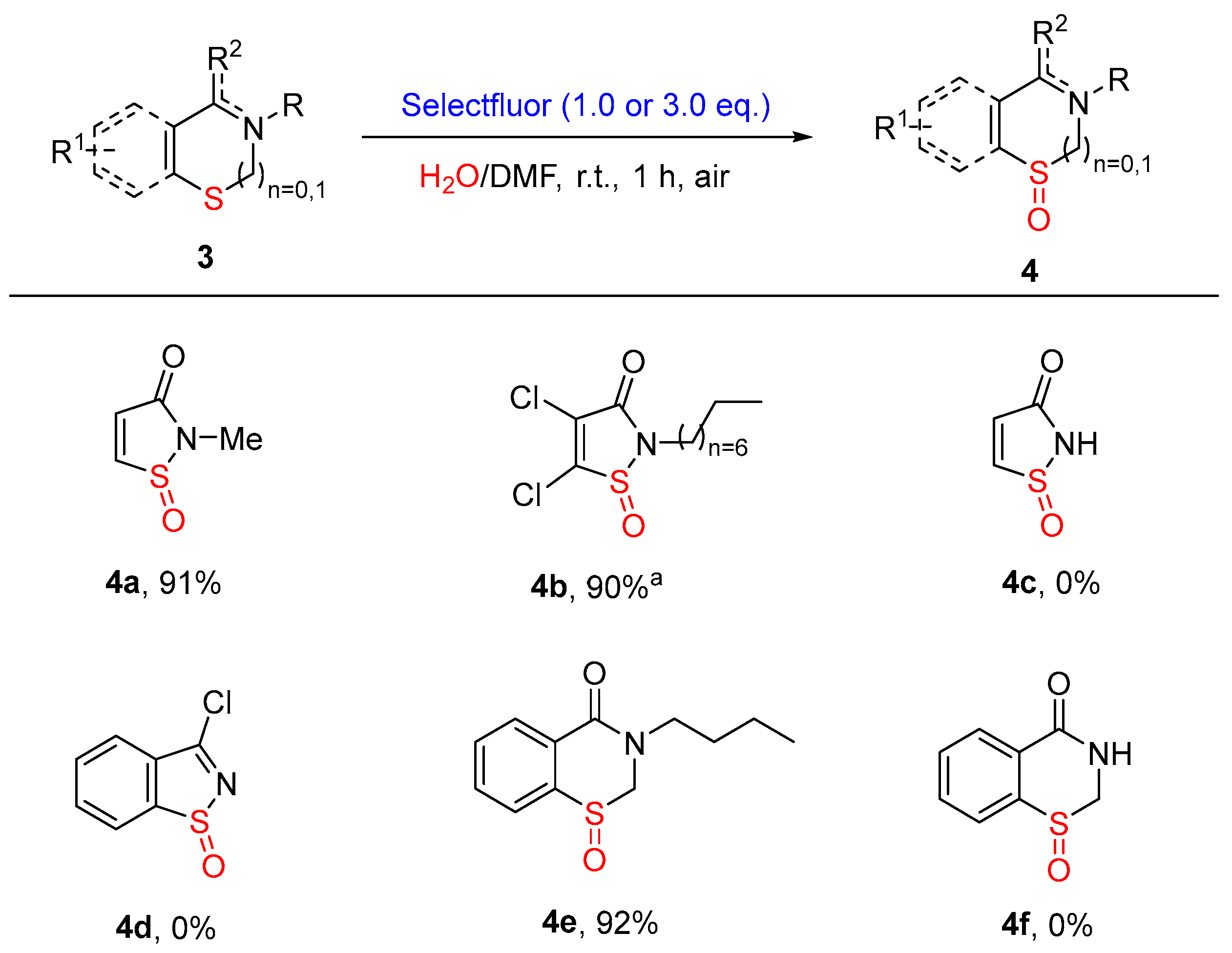
3.4. Synthetic Procedures for the Synthesis of Compounds 4
- The details for 2-Methylisothiazol-3(2H)-one-1-oxide (4a). White solid, 24.0 mg, 91%, m.p. = 81–82 °C. 1H NMR (300 MHz, CDCl3) δ 7.60 (d, J = 6.5 Hz, 1H), 6.83 (d, J = 6.4 Hz, 1H), 3.25 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 166.06, 148.19, 130.66, 26.57. HRMS (ESI, m/z): calcd. for C4H6NO2S [M + H]+, 132.0114; found, 132.0111.
- The details for 4,5-Dichloro-2-octylisothiazol-3(2H)-one-1-oxide (4b). Colorless oil, 53.7 mg, 90%. 1H NMR (300 MHz, CDCl3) δ 3.80–3.59 (m, 2H), 1.71–1.62 (m, 2H), 1.31–1.18 (m, 10H), 0.81 (d, J = 6.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 159.92, 148.68, 130.86, 42.85, 31.72, 29.08, 29.07, 29.00, 26.66, 22.60, 14.07. HRMS (ESI, m/z): calcd. for C11H18Cl2NO2S [M + H]+, 298.0430; found, 298.0416.
- The details for 3-Butyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one-1-oxide (4e). Colorless oil, 43.7 mg, 92%. 1H NMR (300 MHz, CDCl3) δ 8.14–8.11 (m, 1H), 7.70–7.56 (m, 3H), 4.67 (d, J = 13.0 Hz, 1H), 4.48 (d, J = 13.0 Hz, 1H), 3.61 (t, J = 7.4 Hz, 2H), 1.64–1.54 (m, 2H), 1.39–1.28 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 162.05, 140.88, 132.84, 132.56, 130.42, 127.51, 127.00, 65.22, 48.87, 29.80, 19.95, 13.77. HRMS (ESI, m/z): calcd. for C12H15NNaO2S [M + Na]+, 260.0716; found, 260.0714.
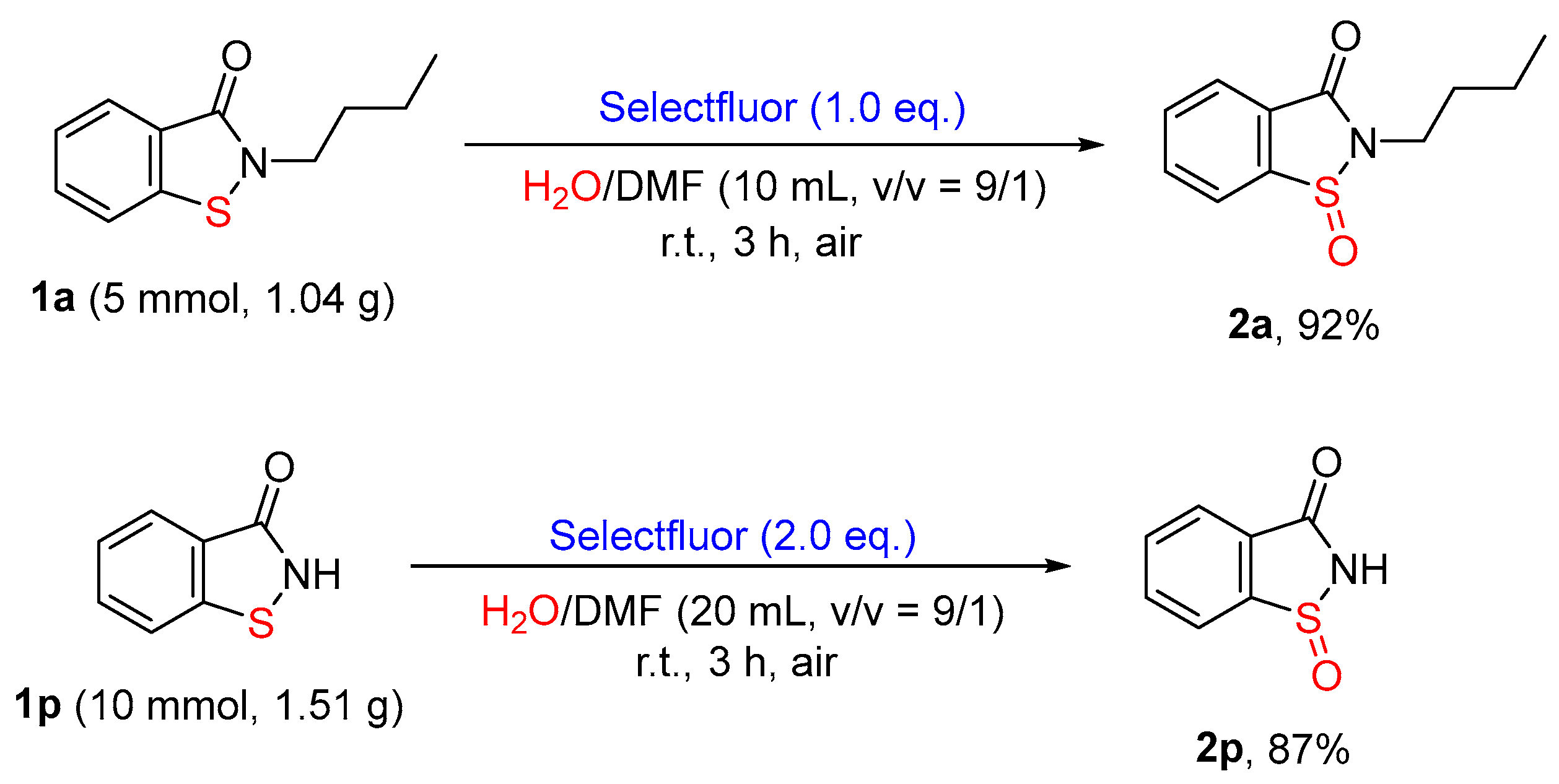
3.5. Synthetic Procedures for Gram-Scale Reactions
3.6. Synthetic Procedures for the Synthesis of Compounds 5
- The details for 2-Butylbenzo[d]isothiazol-3(2H)-one-1,1-dioxide (5a). White solid, 40.7 mg, 85%, m.p. = 42–43 °C (known compound [32]). 1H NMR (300 MHz, CDCl3) δ 8.00–7.96 (m, 1H), 7.87–7.72 (m, 3H), 3.70 (t, J = 7.4 Hz, 2H), 1.82–1.72 (m, 2H), 1.44–1.31 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 158.96, 137.74, 134.65, 134.27, 127.48, 125.09, 120.88, 39.23, 30.43, 20.05, 13.52.
- The details for 2-Ethylbenzo[d]isothiazol-3(2H)-one-1,1-dioxide (5b). White solid, 34.2 mg, 81%, m.p. = 93–94 °C (known compound [32]). 1H NMR (300 MHz, CDCl3) δ 7.99–7.96 (m, 1H), 7.86–7.72 (m, 3H), 3.78 (q, J = 7.2 Hz, 2H), 1.38 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 158.71, 137.78, 134.67, 134.29, 127.50, 125.08, 120.88, 34.50, 13.98.
- The details for 2-Benzylbenzo[d]isothiazol-3(2H)-one-1,1-dioxide (5c). White solid, 45.4 mg, 83%, m.p. = 110–111 °C (known compound [32]). 1H NMR (300 MHz, CDCl3) δ 7.97–7.94 (m, 1H), 7.85–7.69 (m, 3H), 7.42 (dd, J = 7.6, 1.9 Hz, 2H), 7.30–7.17 (m, 3H), 4.82 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 158.93, 137.75, 134.85, 134.51, 134.38, 128.75, 128.72, 128.30, 127.30, 125.26, 121.06, 42.69.
- The details for 2-((Trimethylsilyl)methyl)benzo[d]isothiazol-3(2H)-one-1,1-dioxide (5d). Colorless oil, 45.8 mg, 85% (known compound [33]). 1H NMR (300 MHz, CDCl3) δ 8.03–8.00 (m, 1H), 7.92–7.89 (m, 1H), 7.86–7.77 (m, 2H), 3.13 (s, 2H), 0.19 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 159.02, 137.75, 134.45, 134.33, 127.79, 124.98, 120.99, 29.12, -1.54.
- The details for Benzo[d]isothiazol-3(2H)-one-1,1-dioxide (5e). White solid, 27.5 mg, 75%, m.p. = 227–228 °C (known compound [34]). 1H NMR (300 MHz, DMSO-d6) δ 12.36 (br, 1H), 8.19–8.15 (m, 1H), 8.04–7.92 (m, 3H). 13C NMR (75 MHz, DMSO-d6) δ 161.30, 139.74, 136.01, 135.22, 127.92, 125.31, 121.64.
3.7. Procedures for Free Radical Trapping Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obermeyer, L.; Dicke, K.; Skudlik, C.; Brans, R. Occupational allergic contact dermatitis from 2-butyl-1,2-benzisothiazol-3-one in cutting fluids: A case series. Contact Dermat. 2024, 90, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Yadav, R.K.; Shukla, P.K.; Srivastava, K.; Puri, S.K.; Muraleedharan, K.M. Broad spectrum anti-infective properties of benzisothiazolones and the parallels in their anti-bacterial and anti-fungal effects. Bioorg. Med. Chem. Lett. 2017, 27, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.; Isaksson, M. Occupational contact dermatitis caused by N-butyl-1,2-benzisothiazolin-3-one in a cutting fluid. Contact Dermat. 2015, 73, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Dou, D.; Aravapalli, S.; Teramoto, T.; Lushington, G.H.; Mwania, T.M.; Alliston, K.R.; Eichhorn, D.M.; Padmanabhan, R.; Groutas, W.C. Design, synthesis and characterization of novel 1,2-benzisothiazol-3(2H)-one and 1,3,4-oxadiazole hybrid derivatives: Potent inhibitors of dengue and west nile virus NS2B/NS3 proteases. Bioorg. Med. Chem. 2013, 21, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.M.; Nelson, M.L.; Unice, K.M.; Keenan, J.J.; Paustenbach, D.J. Estimation of the safe use concentrations of the preservative 1,2-benzisothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem. Toxicol. 2013, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Magid, A.-G.; Moyer, J.A.; Patel, U.; Webb, M.; Schiehser, G.; Andree, T.; Thomas Haskins, J. Synthesis and structure-activity relationship of substituted tetrahydro- and hexahydro-1,2-benzisothiazol-3-one 1,1-dioxides and thiadiazinones: Potential anxiolytic agents. J. Med. Chem. 1989, 32, 1024–1033. [Google Scholar]
- Dakova, B.; Martens, T.; Evers, M. Electrochemical oxidation of [2H] benziso-1,2-thiazol-3-one mediated by chloride anions. Application to a new and expedient electrochemical synthesis of [2H] benziso-1,2-thiazol-3-one S-oxide. Electrochim. Acta 2000, 45, 4525–4530. [Google Scholar] [CrossRef]
- Serebryakov, E.A.; Kislitsin, P.G.; Semenov, V.V.; Zlotin, S.G. Selective synthesis of 1,2-benzisothiazol-3-one-1-oxide nitro derivatives. Synthesis 2001, 2001, 1659–1664. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yang, X.; Zhou, P.; Shu, C. Recent advances in the synthesis of cyclic sulfinic acid derivatives (sultines and cyclic sulfinamides). Chem. Commun. 2023, 59, 6272–6285. [Google Scholar] [CrossRef]
- Dobrydnev, A.V.; Popova, M.V.; Volovenko, Y.M. Cyclic Sulfinamides. Chem. Rec. 2024, 24, e202300221. [Google Scholar] [CrossRef]
- Kim, Y.; Li, C.-J. Perspectives on green synthesis and catalysis. Green Synth. Catal. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Song, H.-X.; Han, Q.-Y.; Zhao, C.-L.; Zhang, C.-P. Fluoroalkylation reactions in aqueous media: A review. Green Chem. 2018, 20, 1662–1731. [Google Scholar] [CrossRef]
- Norcott, P.; Spielman, C.; McErlean, C.S.P. An in-water, on-water domino process for synthesis. Green Chem. 2012, 14, 605–609. [Google Scholar] [CrossRef]
- Nyffeler, P.T.; Duron, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.-H. Selectfluor: Mechanistic insight and applications. Angew. Chem. Int. Ed. 2005, 44, 192–212. [Google Scholar] [CrossRef]
- Stavber, S. Recent advances in the application of SelectfluorTMF-TEDA-BF4 as a versatile mediator or catalyst in organic synthesis. Molecules 2011, 16, 6432–6464. [Google Scholar] [CrossRef]
- Campbell, M.G.; Ritter, T. Modern carbon–fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 2015, 115, 612–633. [Google Scholar] [CrossRef]
- Champagne, P.A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Monofluorination of organic compounds: 10 Years of innovation. Chem. Rev. 2015, 115, 9073–9174. [Google Scholar] [CrossRef]
- Yang, K.; Song, M.; Ali, A.; Mudassir, S.; Ge, H. Recent advances in the application of selectfluor as a “fluorine-free” functional reagent in organic synthesis. Chem. Asian J. 2020, 15, 729–741. [Google Scholar] [CrossRef]
- Aguilar Troyano, F.-J.; Merkens, K.; Adrian, G.-S. Selectfluor radical dication (TEDA2+•)—A versatile species in modern synthetic organic chemistry. Asian J. Org. Chem. 2020, 9, 992–1007. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H.; Niu, B.; Tang, T.; Ge, H. Benzisothiazol-3-ones through a metal-free intramolecular N–S bond formation. Eur. J. Org. Chem. 2018, 2018, 5520–5523. [Google Scholar] [CrossRef]
- Dai, S.; Yang, K.; Luo, Y.; Xu, Z.; Li, Z.; Li, Z.-Y.; Li, B.; Sun, X. Metal-free and Selectfluor-mediated diverse transformations of 2-alkylthiobenzamides to access 2,3-dihydrobenzothiazin-4-ones, benzoisothiazol-3-ones and 2-alkylthiobenzonitriles. Org. Chem. Front. 2022, 9, 4016–4022. [Google Scholar] [CrossRef]
- Roberto, C.-M.; Mariela, C.-D.; Rafael, C.-A.; David, L.-N.; Mariana, C.-M.; Valeria Fernanda, V.-C.; Carlos Eduardo, H.-T.; Emilia, G.-C.; Ahmad, M.Z. Natural sweeteners: Sources, extraction and current uses in foods and food industries. Food Chem. 2022, 370, 130991. [Google Scholar]
- Xu, L.; Cheng, J.; Trudell, M.L. Chromium(VI) oxide catalyzed oxidation of sulfides to sulfones with periodic acid. J. Org. Chem. 2003, 68, 5388–5391. [Google Scholar] [CrossRef]
- Vincent, S.P.; Burkart, M.D.; Tsai, C.-Y.; Zhang, Z.; Wong, C.-H. Electrophilic fluorination-nucleophilic addition reaction mediated by selectfluor: Mechanistic studies and new applications. J. Org. Chem. 1999, 64, 5264–5279. [Google Scholar] [CrossRef]
- Guo, X.; Sun, X.; Jiang, M.; Zhao, Y. Switchable synthesis of sulfoxides, sulfones and thiosulfonates through selectfluor-promoted oxidation with H2O as O-source. Synthesis 2022, 54, 1996–2004. [Google Scholar] [CrossRef]
- Waldner, A. Synthesis and application of a highly efficient, homochiral dienophile. Tetrahedron Lett. 1989, 30, 3061–3064. [Google Scholar] [CrossRef]
- Zhang, Y.; Wong, Z.R.; Wu, X.; Lauw, S.J.L.; Huang, X.; Webster, R.D.; Chi, Y.R. Sulfoxidation of alkenes and alkynes with NFSI as a radical initiator and selective oxidant. Chem. Commun. 2017, 53, 184–187. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, H.; Chen, N.; Ren, D.; Xu, J.; Yang, Z. Fluorium-initiated dealkylative cyanation of thioethers to thiocyanates. J. Org. Chem. 2019, 84, 9044–9050. [Google Scholar] [CrossRef]
- Xu, R.; Xiao, G.; Li, Y.; Liu, H.; Song, Q.; Zhang, X.; Yang, Z.; Zheng, Y.; Tan, Z.; Deng, Y. Multifunctional 5,6-dimethoxybenzo[d]isothiazol-3(2H)-one-Nalkylbenzylamine derivatives with acetylcholinesterase, monoamine oxidases and β-amyloid aggregation inhibitory activities as potential agents against Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Yoshida, T. Method for the Preparation of Benzoisothiazolinone-1-oxide Compound. JP2011162465A, 25 August 2011. [Google Scholar]
- Sivaramakrishnan, S.; Cummings, A.H.; Gates, K.S. Protection of a single-cysteine redox switch from oxidative destruction: On the functional role of sulfenyl amide formation in the redox-regulated enzyme PTP1B. Bioorg. Med. Chem. Lett. 2010, 20, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Carta, F.; Vullo, D.; Leitans, J.; Kazaks, A.; Tars, K.; Zalubovskis, R.; Supuran, C.T. N-Substituted and ring opened saccharin derivatives selectively inhibit transmembrane, tumor-associated carbonic anhydrases IX and XII. Bioorg. Med. Chem. 2017, 25, 3583–3589. [Google Scholar] [CrossRef]
- Cho, D.-W.; Oh, S.-W.; Kim, D.-U.; Park, H.-J.; Xue, J.-Y.; Yoon, U.-C.; Mariano, P.S. Studies of silyl-transfer photochemical reactions of N-[(trimethylsilyl)alkyl]saccharins. Bull. Korean Chem. Soc. 2010, 31, 2453–2458. [Google Scholar] [CrossRef]
- Fu, S.; Lian, X.; Ma, T.; Chen, W.; Zheng, M.; Zeng, W. TiCl4-promoted direct N-acylation of sulfonamide with carboxylic ester. Tetrahedron Lett. 2010, 51, 5834–5837. [Google Scholar] [CrossRef]


| Entry | Oxidant (Eq.) | Solvent (v/v, mL) | Yield (%) |
|---|---|---|---|
| 1 | Selectfluor (1.0) | H2O | 87 |
| 2 | Selectfluor (1.0) | MeOH | trace |
| 3 | Selectfluor (1.0) | EtOH | 0 |
| 4 | Selectfluor (1.0) | DMC | 0 |
| 5 | Selectfluor (1.0) | DMF | >99 |
| 6 | Selectfluor II (1.0) | H2O | 82 |
| 7 | NFSI (1.0) | H2O | 80 |
| 8 | NFTP (1.0) | H2O | 0 |
| 9 | NIS (1.0) | H2O | 0 |
| 10 | NaIO4 (1.0) | H2O | 0 |
| 11 | K2S2O8 (1.0) | H2O | 0 |
| 12 | Selectfluor (1.0) | H2O/DMF (v/v = 4/1) | >99 |
| 13 | Selectfluor (1.0) | H2O/DMF (v/v = 9/1) | >99[95] b |
| 14 | Selectfluor (1.0) | H2O/DMF (v/v = 19/1) | 90 |
| 15 | Selectfluor (0.5) | H2O/DMF (v/v = 9/1) | 48 |
| 16 | Selectfluor (2.0) | H2O/DMF (v/v = 9/1) | >99 |
| 17 | Selectfluor (3.0) | H2O/DMF (v/v = 9/1) | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yuan, D.; Liu, C.; Herington, F.; Yang, K.; Ge, H. Selective Oxidation of Benzo[d]isothiazol-3(2H)-Ones Enabled by Selectfluor. Molecules 2024, 29, 3899. https://doi.org/10.3390/molecules29163899
Li Q, Yuan D, Liu C, Herington F, Yang K, Ge H. Selective Oxidation of Benzo[d]isothiazol-3(2H)-Ones Enabled by Selectfluor. Molecules. 2024; 29(16):3899. https://doi.org/10.3390/molecules29163899
Chicago/Turabian StyleLi, Qin, Dan Yuan, Chong Liu, Faith Herington, Ke Yang, and Haibo Ge. 2024. "Selective Oxidation of Benzo[d]isothiazol-3(2H)-Ones Enabled by Selectfluor" Molecules 29, no. 16: 3899. https://doi.org/10.3390/molecules29163899
APA StyleLi, Q., Yuan, D., Liu, C., Herington, F., Yang, K., & Ge, H. (2024). Selective Oxidation of Benzo[d]isothiazol-3(2H)-Ones Enabled by Selectfluor. Molecules, 29(16), 3899. https://doi.org/10.3390/molecules29163899








