A [3+3] Aldol-SNAr-Dehydration Approach to 2-Naphthol and 7-Hydroxyquinoline Derivatives
Abstract
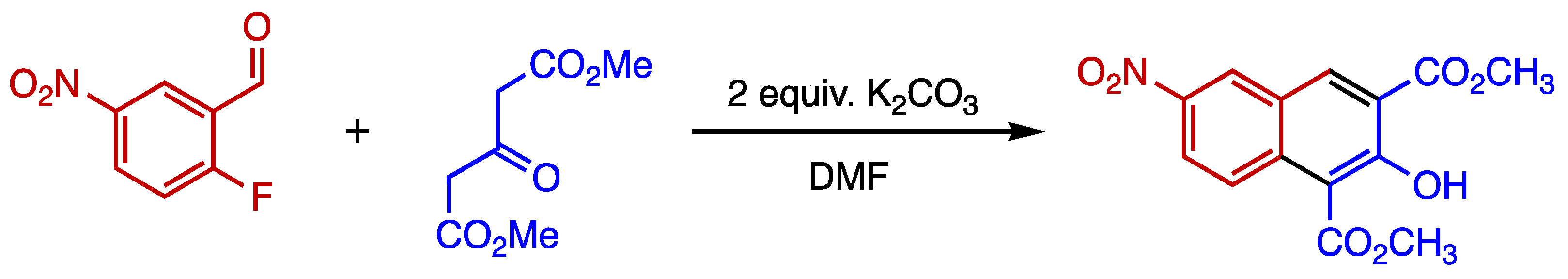
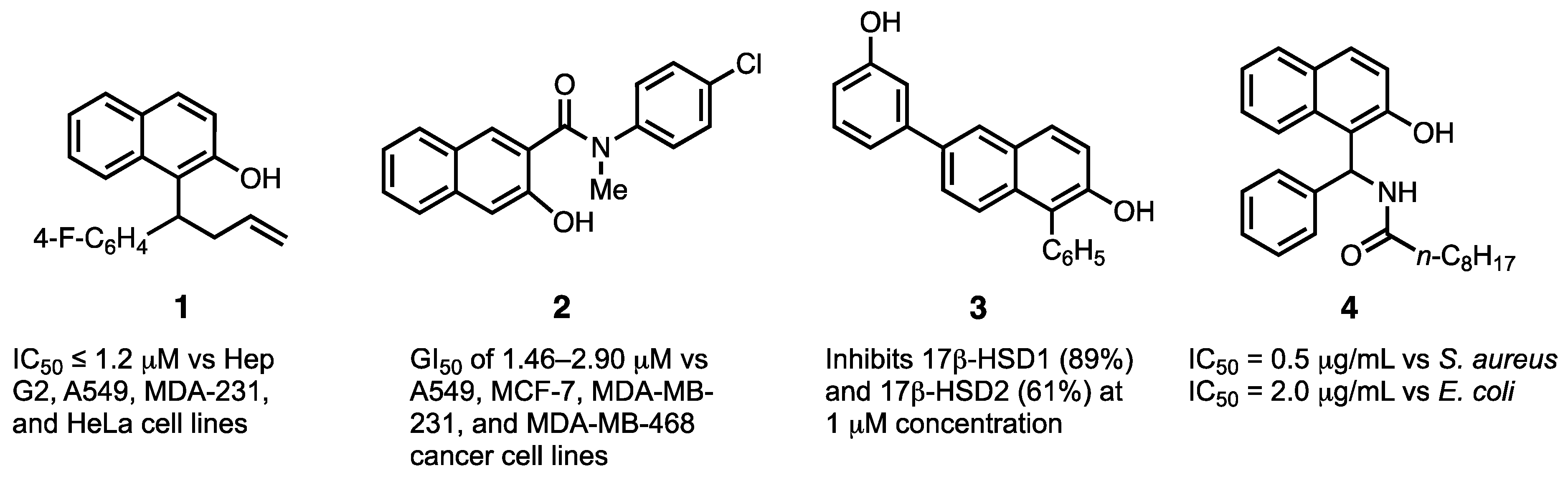
1. Introduction
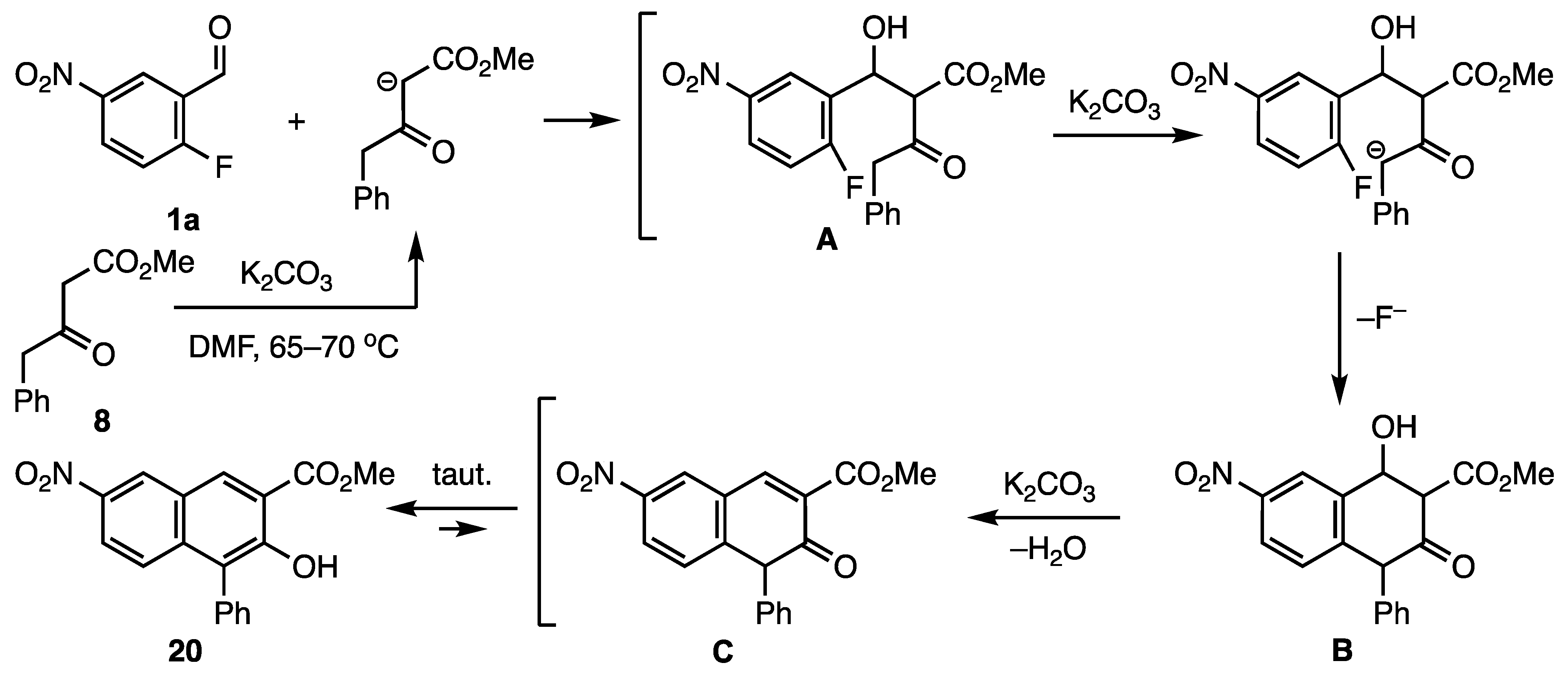
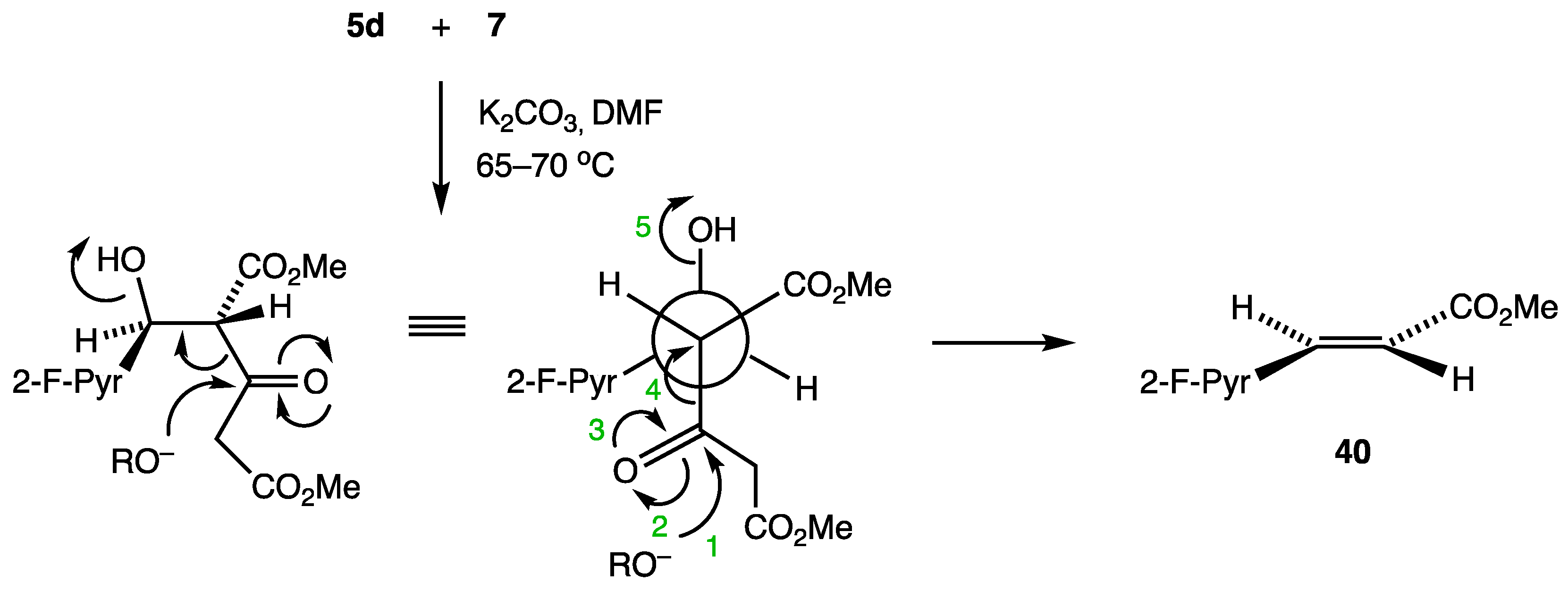
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Methyl 3-Oxo-4-phenylbutanoate (8)
3.3. Methyl 4-(2-Fluorophenyl)-3-oxobutanoate (9)
3.4. Methyl 4-(4-Chlorophenyl)-3-oxobutanoate (10)
3.5. Methyl 4-(4-Methylphenyl)-3-oxobutanoate (11)
3.6. Methyl 4-(4-Methoxyphenyl)-3-oxobutanoate (12)
3.7. Methyl 3-Oxo-4-(3-(trifluoromethyl)phenyl)butanoate (13)
3.8. Methyl 4-(2,5-Dimethylphenyl)-3-oxobutanoate (14)
3.9. 1,3 Dibenzoylpropan-2-One (15)
3.10. 1-Phenyl-3-(Phenylsulfonyl)propan-2-one (16)
3.11. 1,3 Bis(Phenylsulfonyl)propan-2-one (17)
3.12. Representative Procedure for the [3+3] Preparation of Hindered 2-Naphthols
3.12.1. Dimethyl 2-Hydroxy-6-nitronaphthalene-1,3-dicarboxylate (18)
3.12.2. Diethyl 2-Hydroxy-6-nitronaphthalene-1,3-dicarboxylate (19)
3.12.3. Methyl 3-Hydroxy-7-nitro-4-phenyl-2-naphthoate (20)
3.12.4. Methyl 4-(2-Fluorophenyl)-3-hydroxy-7-nitro-2-naphthoate (21)
3.12.5. Methyl 4-(4-Chlorophenyl)-3-hydroxy-7-nitro-3-naphthoate (22)
3.12.6. Methyl 3-Hydroxy-4-(4-methylphenyl)-7-nitro-3-naphthoate (23)
3.12.7. Methyl 3-Hydroxy-4-(4-methoxyphenyl)-7-nitro-3-naphthoate (24)
3.12.8. Methyl 3-Hydroxy-7-nitro-4-(3-(trifluoromethyl)phenyl)-2-naphthoate (25)
3.12.9. Methyl 4-(2,5-Dimethylphenyl)-3-hydroxy-7-nitro-2-naphthoate (26)
3.12.10. 1,3 Dibenzoyl-2-Hydroxy-6-nitronaphthalene (27)
3.12.11. 6-Nitro-1-Phenyl-3-(phenylsulfonyl)-2-naphthol (28)
3.12.12. 5-Nitro-2-(Phenylsulfonyl)benzaldehyde (29)
3.12.13. Dimethyl 6-Cyano-2-hydroxynaphthalene-1,3-dicarboxylate (30)
3.12.14. Methyl 7-Cyano-3-hydroxy-4-phenyl-2-naphthoate (31)
3.12.15. Methyl 7-Cyano-4-(2-fluorophenyl)-3-hydroxy-2-naphthoate (32)
3.12.16. Methyl 4-(4-Chlorophenyl)-7-cyano-3-hydroxy-2-naphthoate (33)
3.12.17. Methyl 7-Cyano-3-hydroxy-4-(4-methoxyphenyl)-2-naphthoate (34)
3.12.18. Methyl 7-Cyano-3-hydroxy-4-(2,5-dimethylphenyl)-2-naphthoate (35)
3.12.19. 5,7 Dibenzoyl-6-hydroxy-2-naphthonitrile (36)
3.12.20. Ethyl (E)-3-(2-Fluoro-5-(trifluoromethyl)phenyl)prop-2-enoate (37)
3.12.21. Methyl 3-Hydroxy-4-(4-methylphenyl)-7-trifluoromethyl-2-naphthoate (38)
3.12.22. Methyl 3-Hydroxy-4-(4-methoxylphenyl)-7-(trifluoromethyl)-2-naphthoate (39)
3.12.23. Ethyl (E)-3-(2-Fluoropyridin-3-yl)prop-2-enoate (40)
3.12.24. Methyl 7-Hydroxy-8-phenylquinoline-6-carboxylate (41)
3.12.25. Methyl 8-(4-Chlorophenyl)-7-hydroxyquinoline-6-carboxylate (42)
3.12.26. Methyl 2-Hydroxy-8-(4-methylphenyl)quinoline-1-carboxylate (43)
3.12.27. Methyl 7-Hydroxy-8-(4-methoxyphenyl)quinoline-6-carboxylate (44)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunce, R.A.; Rogers, D.; Nago, T.; Bryant, S.A. 4H-1-Benzopyrans by a tandem SN2-SNAr reaction. J. Heterocycl. Chem. 2008, 45, 547–550. [Google Scholar] [CrossRef]
- Fobi, K.; Ametsetor, E.; Bunce, R.A. Domino aldol-SNAr-dehydration sequence for [3+3] annulations to prepare quinoline-2(1H)-ones and 1,8-naphthyridin-2(1H)-ones. Molecules 2023, 28, 5856. [Google Scholar] [CrossRef] [PubMed]
- Kito, T.; Yoshinaga, K.; Yamaye, M.; Mizobe, H. Base-catalyzed alkylation of 2-naphthol with glyoxal. J. Org. Chem. 1991, 56, 3336–3339. [Google Scholar] [CrossRef]
- Kooli, A.; Shalima, T.; Lopusanskaja, E.; Paju, A.; Lopp, M. Selective C-alkylation of substituted naphthols under non-aqueous conditions. Tetrahedron 2021, 95, 132278. [Google Scholar] [CrossRef]
- Jin, M.; Ren, W.; Qian, D.-W.; Yang, S.-D. Direct allylic C(sp3)-H alkylation with 2-naphthols via cooperative palladium and copper catalysis: Construction of cyclohexadienones with quaternary carbon centers. Org. Lett. 2018, 20, 7015–7019. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, N.; Filippini, G.; Mazzanti, A.; Righi, P.; Bencivenni, G. Controlling the C(sp3)-C(sp3) axial conformation in the enantioselective Friedel-Crafts type alkylation of b-naphthols with inden-1-ones. Org. Lett. 2017, 19, 6692–6695. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-T.; Gu, Q.; You, S.-L. Enantioselective annulation of enals with 2-naphthols by triazolium salts derived from L-phenylalanine. Chem. Sci. 2015, 6, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Duvedi, R. Environment-friendly green chemistry approaches for an efficient synthesis of 1-amidoalkyl-2-naphthols catalyzed by tannic acid. Arab. J. Chem. 2018, 11, 91–98. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kiyani, H.; Pourmousavi, S.A.; Ajloo, D. Solvent-free synthesis of 1-amidoalkyl-2-naphthols using magnetic nanoparticle-supported 2-(((4-(1-iminoethyl)phenyl)imino)methyl)phenol Cu(II) or Zn(II) Schiff base complexes. Res. Chem. Intermed. 2020, 46, 3145–3164. [Google Scholar] [CrossRef]
- Rahimizadeh, R.; Mobinikhaledi, A.; Moghanian, H.; Kashaninejad, S.-S. Design and synthesis of some new biologically active amidoalkyl naphthols in the presence of sulfonic acid functionalized silica coated Fe3O4 nanoparticles. Res. Chem Intermed. 2022, 48, 607–627. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.; Xu, D.; Xia, C.; Sun, W. Direct halogenative dearomatization of 2-naphthols by NXS (X = Cl, Br) in water. Green Chem. 2016, 18, 5485–5492. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, L.; Li, D.; Wang, K.; Liu, Y.; Zhu, H.; Liu, X.; Yang, D.; Wang, R. Asymmetric dearomative halogenation of β-naphthols: The axial chirality transfer reaction. Adv. Synth. Catal. 2018, 360, 401–405. [Google Scholar] [CrossRef]
- Jia, L.; Tang, Q.; Luo, M.; Zeng, X. Direct ortho-selective amination of 2-naphthol and its analogues with hydrazines. J. Org. Chem. 2018, 83, 5082–5091. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Vidal, H.D.A.; Januário, M.A.P.; Corrêa, A.G. Advances in the asymmetric synthesis of BINOL derivatives. Molecules 2023, 28, 12. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.C.; Dugan, E.C.; DiVirgilio, E.S.; Maksimenka, K.; Bringmann, G. Asymmetric total synthesis of nigerone and ent-nigerone: Enantioselective oxidative biaryl coupling of highly hindered naphthols. Adv. Synth. Catal. 2007, 349, 583–594. [Google Scholar] [CrossRef]
- Petkov, H.; Simeonov, S.P. Amidoalkyl naphthols as important bioactive substances and building blocks: A review on the current catalytic Mannich-type synthetic approaches. Appl. Sci. 2023, 13, 6616. [Google Scholar] [CrossRef]
- Chaudhary, A. Recent development in the synthesis of heterocycles by naphthol-based multicomponent reactions. Mol. Divers. 2021, 25, 1211–1245. [Google Scholar] [CrossRef] [PubMed]
- Olyaei, A.; Sadeghpour, M. Recent advances in the synthesis and synthetic applications of Betti base (aminoalkylnaphthol) and bis-Bette base derivatives. RSC Adv. 2019, 9, 18467–18497. [Google Scholar] [CrossRef]
- Juteau, H.; Gareau, Y.; Lachance, H. Synthesis of polysubstituted-2-naphthols. Tetrahedron Lett. 2005, 46, 4547–4549. [Google Scholar] [CrossRef]
- Kim, H.Y.; Oh, K. A facile access to 4-substituted-2-naphthols via a tandem Friedel-Crafts reaction: A β-chlorovinyl ketone pathway. Org. Lett. 2014, 16, 5934–5936. [Google Scholar] [CrossRef]
- Zhang, X.; Sarkar, S.; Larock, R.C. Synthesis of naphthalenes and 2-naphthols by the electrophilic cyclization of alkynes. J. Org. Chem. 2006, 71, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Feng, X.; Liu, H.; Jiang, H.; Bao, M. Synthesis of 2-naphthols via carbonylative Stille coupling reactions of 2-bromobenzyl bromides with tributylallylstannane followed by the Heck reaction. J. Org. Chem. 2011, 76, 10068–10077. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Pandey, A.K.; Lee, H.; Kim, S.; Kang, D.; Jung, Y.H.; Kim, H.S.; Hong, S.; Kim, I.S. One-pot synthesis of 2-naphthols from nitrones and MBH adducts via decarboxylative N–O bond cleavage. Org. Chem. Front. 2018, 5, 3210–3218. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, Y.; Zhang, Y.; Yu, S. De novo synthesis of polysubstituted naphthols and furans using photoredox neutral coupling of alkynes with 2-bropmo-1,3-dicarbonyl compounds. Org. Lett. 2013, 15, 4884–4887. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Reddy, C.R.; Kashanna, J.; Mamidyala, S.K.; Kumar, C.G. Multicomponent one-pot synthesis of 2-naphthol derivatives and evaluation of their anticancer activity. Med. Chem. Res. 2012, 21, 3321–3325. [Google Scholar] [CrossRef]
- Li, B.X.; Yamanaka, K.; Xiao, X. Structure-activity relationship studies of naphthol AS-E and its derivatives as anticancer agents by inhibiting CREB-mediated gene transcription. Bioorg. Med. Chem. 2012, 20, 6811–6820. [Google Scholar] [CrossRef] [PubMed]
- Marchais-Oberwinkler, S.; Krutchen, P.; Frotscher, M.; Ziegler, E.; Neugebauer, A.; Bhoga, U.; Bey, E.; Müller-Vieira, U.; Messinger, J.; Thole, H.; et al. Substituted 6-phenyl-2-naphthols. Potent and selective nonsteroidal inhibitors of 17β-hydroxysteroid dehydrogenase Type 1 (17β-HSD1): Design, synthesis, biological evaluation, and pharmacokinetics. J. Med. Chem. 2008, 51, 4685–4698. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, Y.; Yoshioka, T.; Sugano, K.; Yonemitsu, O. Methyl phenylacetylacetate from phenylacetyl chloride and Meldrum’s acid. Org. Synth. 1985, 63, 198–202. [Google Scholar] [CrossRef]
- Miles, M.; Harris, T.H.; Hauser, C.R. Aroylations at the methyl group of benzoylacetone and related β-diketones with esters to form 1,3,5-triketones by sodium hydride. Other terminal condensations. J. Org. Chem. 1965, 30, 1007–1011. [Google Scholar] [CrossRef]
- Nájera, C.; Yus, M. Substituted lithium (E)-3-lithio-3-tosyl-2-propenolates: Useful intermediates in organic synthesis. J. Org. Chem. 1989, 54, 1491–1499. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M. Synthesis of β- and γ-hydroxy sulfones by regioselective opening of β,γ-epoxy sulfones. Tetrahedron 1990, 46, 3993–4002. [Google Scholar] [CrossRef]
- Liu, Y.; Oble, J.; Poli, G. Switchable selectivity in Pd-catalyzed [3+2] annulations of γ-oxy-2-cycloalkenones with 3-oxoglutarates: C–C/C–C vs C–C/C–O bond formation. Beilstein J. Org. Chem. 2019, 15, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Ripin, D.H.; Evans, D.A. pKa’s of Inorganic and Oxo-Acids, Organic Division of the ACS. Available online: https://organicchemistrydata.org/hansreich/resources/pka/pka_data/evans_pKa_table.pdf (accessed on 25 September 2023).
- Kürti, L.; Czákó, B. Strategic Applications of Named Reactions in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2005; pp. 190–191. [Google Scholar]
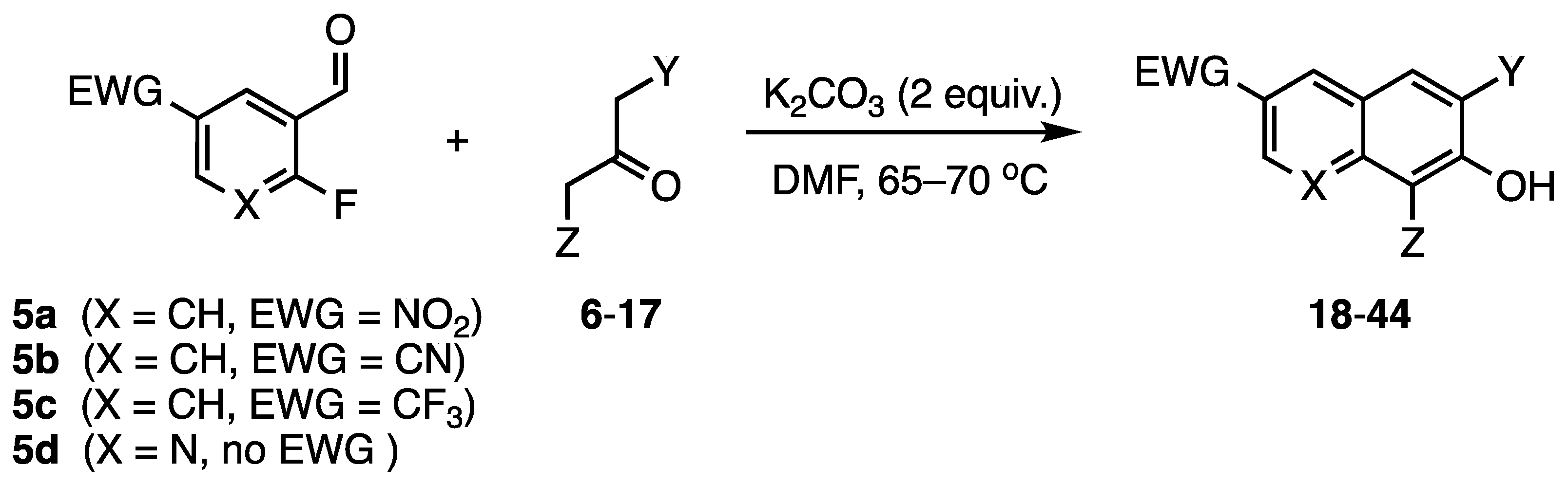
| Aldehyde | Dinucleophile | Product | Yield (%) |
|---|---|---|---|
| 5a entry 1 |  6 |  18 | 89 |
| 5a entry 2 | 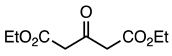 7 | 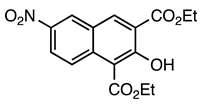 19 | 88 |
| 5a entry 3 | 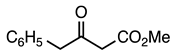 8 | 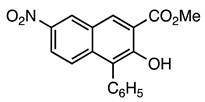 20 | 89 |
| 5a entry 4 | 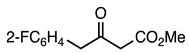 9 | 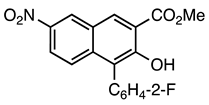 21 | 93 |
| 5a entry 5 |  10 |  22 | 94 |
| 5a entry 6 | 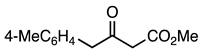 11 | 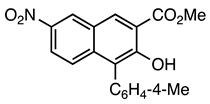 23 | 91 |
| 5a entry 7 | 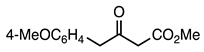 12 | 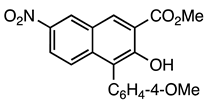 24 | 96 |
| 5a entry 8 |  13 | 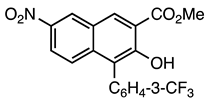 25 | 85 |
| 5a entry 9 | 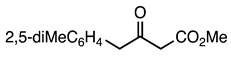 14 | 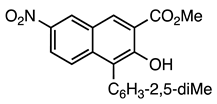 26 | 96 |
| 5a entry 10 | 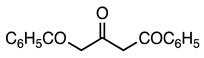 15 | 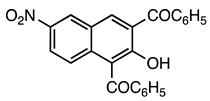 27 | 87 |
| 5a entry 11 |  16 |  28 | 89 |
| 5a entry 12 | 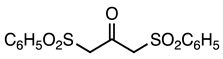 17 a | 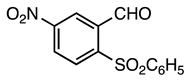 29 b | 82 |
| Aldehyde | Dinucleophile | Product | Yield (%) |
|---|---|---|---|
| 5b entry 1 | 6 |  30 | 84 |
| 5b entry 2 | 8 | 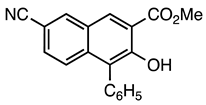 31 | 83 |
| 5b entry 3 | 9 |  32 | 92 |
| 5b entry 4 | 10 | 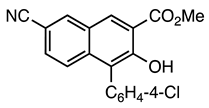 33 | 92 |
| 5b entry 5 | 12 | 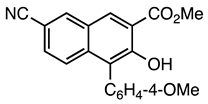 34 | 94 |
| 5b entry 6 | 14 | 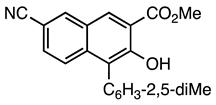 35 | 95 |
| 5b entry 7 | 15 | 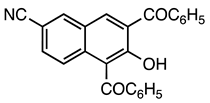 36 | 87 |
| Aldehyde | Dinucleophile | Product | Yield (%) |
|---|---|---|---|
| 5c entry 1 | 7 |  37 | 63 |
| 5c entry 1 | 11 | 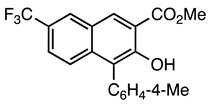 38 | 90 |
| 5c entry 3 | 12 |  39 | 89 |
| Aldehyde | Dinucleophile | Product | Yield (%) |
|---|---|---|---|
| 5d entry 1 | 7 | 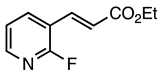 40 | 72 |
| 5d entry 2 | 8 | 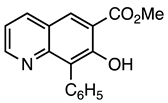 41 | 90 |
| 5d entry 3 | 10 |  42 | 89 |
| 5d entry 4 | 11 | 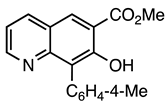 43 | 90 |
| 5d entry 5 | 12 |  44 | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fobi, K.; Ametsetor, E.; Bunce, R.A. A [3+3] Aldol-SNAr-Dehydration Approach to 2-Naphthol and 7-Hydroxyquinoline Derivatives. Molecules 2024, 29, 3406. https://doi.org/10.3390/molecules29143406
Fobi K, Ametsetor E, Bunce RA. A [3+3] Aldol-SNAr-Dehydration Approach to 2-Naphthol and 7-Hydroxyquinoline Derivatives. Molecules. 2024; 29(14):3406. https://doi.org/10.3390/molecules29143406
Chicago/Turabian StyleFobi, Kwabena, Ebenezer Ametsetor, and Richard A. Bunce. 2024. "A [3+3] Aldol-SNAr-Dehydration Approach to 2-Naphthol and 7-Hydroxyquinoline Derivatives" Molecules 29, no. 14: 3406. https://doi.org/10.3390/molecules29143406
APA StyleFobi, K., Ametsetor, E., & Bunce, R. A. (2024). A [3+3] Aldol-SNAr-Dehydration Approach to 2-Naphthol and 7-Hydroxyquinoline Derivatives. Molecules, 29(14), 3406. https://doi.org/10.3390/molecules29143406








