Polyurethane Nanocomposite Coatings Coupled with Titanium-Based Conversion Layers for Enhanced Anticorrosion, Icephobic Properties, and Surface Protection
Abstract
1. Introduction
2. Results and Discussion
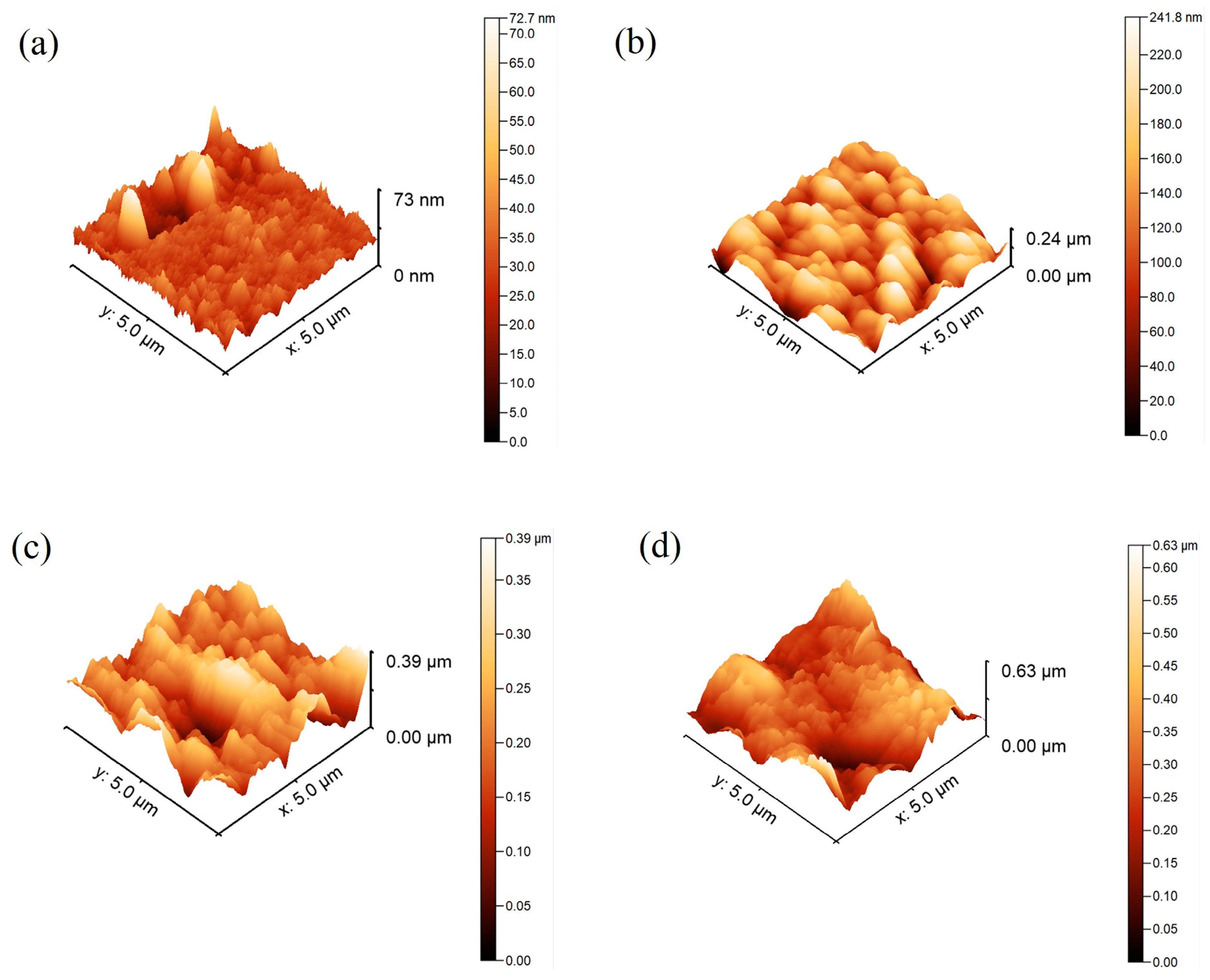
2.1. Surface Characterization
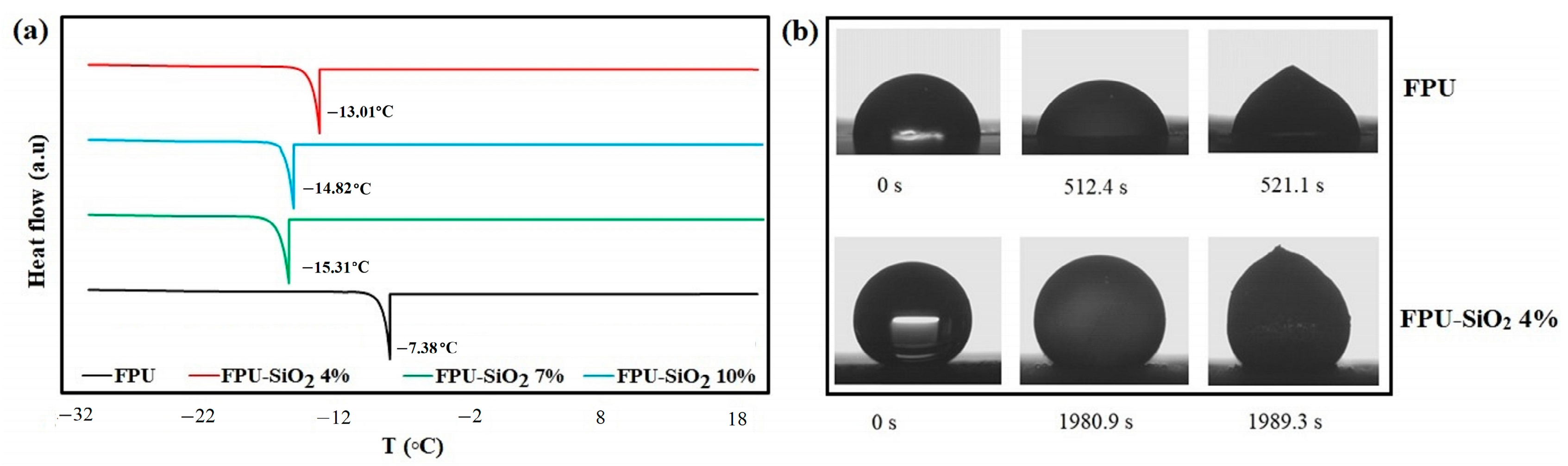
2.2. Ice Nucleation Temperature and Freezing Time Delay
2.3. Ice Adhesion
2.4. Performance of a Polyurethane Nanocomposite When Coupled with a Titanium-Based Conversion Coating
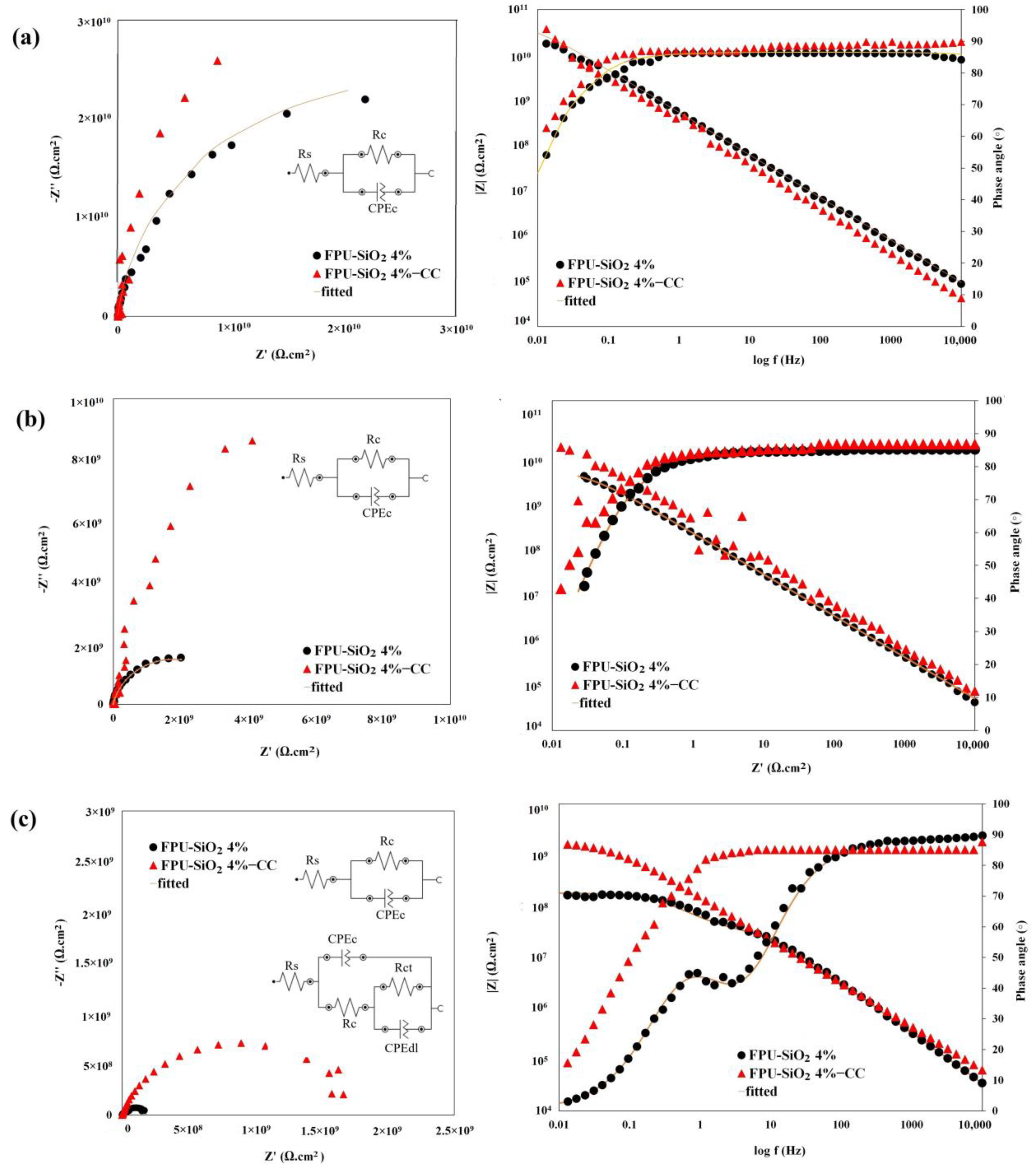
2.4.1. Anticorrosion Performance and Coating Adhesion
2.4.2. Weathering Resistance
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Corrosion: Understanding the Basics; ASM International, Materials Park: Novelty, OH, USA, 2000. [Google Scholar]
- Winston, R.R.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ji, W.G.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Reducing the water absorption in epoxy coatings by silane monomer incorporation. Corros. Sci. 2006, 48, 3731–3739. [Google Scholar] [CrossRef]
- Eivaz Mohammadloo, H.; Sarabi, A.A.; Roshan, S.; Eivaz Mohammadloo, A. 8-Hydroxyquinoline/nanoclay epoxy nanocomposite as a smart coating for early corrosion detection. Corros. Eng. Sci. Technol. 2021, 56, 753–766. [Google Scholar] [CrossRef]
- Wicks, Z.W., Jr.; Jones, F.N.; Pappas, S.; Wicks, D.A. Organic Coatings Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Heidarian, M.; Shishesaz, M.R.; Kassiriha, S.M.; Nematollahi, M. Characterization of structure and corrosion resistivity of polyurethane/organoclay nanocomposite coatings prepared through an ultrasonication assisted process. Prog. Org. Coat. 2010, 68, 180–188. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Mobarakeh, L.F.; Jafari, R.; Farzaneh, M. Robust icephobic, and anticorrosive plasma polymer coating. Cold Reg. Sci. Technol. 2018, 151, 89–93. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lei, J.; He, J.; Lv, R.; Li, N.; Pan, F. A facile approach to fabricate superhydrophobic Zn surface and its effect on corrosion resistance. Corros. Sci. 2014, 85, 174–182. [Google Scholar] [CrossRef]
- Allahdini, A.; Jafari, R.; Momen, G. Transparent non-fluorinated superhydrophobic coating with enhanced anti-icing performance. Prog. Org. Coat. 2022, 165, 106758. [Google Scholar] [CrossRef]
- Momen, G.; Farzaneh, M.J.A.S.S. Facile approach in the development of icephobic hierarchically textured coatings as corrosion barrier. Appl. Surf. Sci. 2014, 299, 41–46. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, R.; Wang, J.; Yang, W.; Liu, L. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corros. Sci. 2014, 80, 177–183. [Google Scholar] [CrossRef]
- Menini, R.; Farzaneh, M. Elaboration of Al2O3/PTFE icephobic coatings for protecting aluminum surfaces. Surf. Coat. Technol. 2009, 203, 1941–1946. [Google Scholar] [CrossRef]
- Saito, H.; Takai, K.; Yamauchi, G. Water-and ice-repellent coatings. Surf. Coat. Int. 1997, 80, 168–171. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. Ice adhesion on super-hydrophobic surfaces. Appl. Surf. Sci. 2009, 255, 8153–8157. [Google Scholar] [CrossRef]
- Vijayan, S.; John, B.; Sahoo, S.K. Modified cardanol based colorless, transparent, hydrophobic and anti-corrosive polyurethane coating. Prog. Org. Coat. 2022, 162, 106586. [Google Scholar] [CrossRef]
- Huang, R.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Highly stretchable polyurethane coating based on functionalized cerium oxide nanoparticles for anti-corrosive/UV protection. J. Appl. Polym. Sci. 2022, 139, 51927. [Google Scholar] [CrossRef]
- Rabbani, S.; Bakhshandeh, E.; Jafari, R.; Momen, G. Superhydrophobic and icephobic polyurethane coatings: Fundamentals, progress, challenges and opportunities. Prog. Org. Coat. 2022, 165, 106715. [Google Scholar] [CrossRef]
- Roshan, S.; Sarabi, A.A.; Jafari, R.; Momen, G. One-step fabrication of superhydrophobic nanocomposite with superior anticorrosion performance. Prog. Org. Coat. 2022, 169, 106918. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; El-Saeed, A.M.; Wahby, M.H.; Abdallah, M.M. Superhydrophobic organic and inorganic clay nanocomposites for epoxy steel coatings. Prog. Org. Coat. 2020, 140, 105502. [Google Scholar] [CrossRef]
- Tsai, Y.; Chen, T.E.; Lee, Y.L. Development and characterization of anticorrosion and antifriction properties for high performance polyurethane/graphene composite coatings. Coatings 2018, 8, 250. [Google Scholar] [CrossRef]
- Uzoma, C.; Liu, F.; Xu, L.; Zhang, Z.; Han, E.H.; Ke, W.; Arukalam, I.O. Superhydrophobicity, conductivity and anticorrosion of robust siloxane-acrylic coatings modified with graphene nanosheets. Prog. Org. Coat. 2019, 127, 239–251. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H.; Wang, L.; Wang, Z.; Du, C.; Li, X.; Zhang, D. Superhydrophobic carbon nanotubes/epoxy nanocomposite coating by facile one-step spraying. Surf. Coat. Technol. 2018, 341, 15–23. [Google Scholar] [CrossRef]
- Huang, Y.F.; Huang, C.; Zhong, Y.L.; Yi, S. Preparing superhydrophobic surfaces with very low contact angle hysteresis. Surf. Eng. 2013, 29, 633–636. [Google Scholar] [CrossRef]
- Shen, Y.; Li, K.; Chen, H.; Wu, Z.; Wang, Z. Superhydrophobic F-SiO2@ PDMS composite coatings prepared by a two-step spraying method for the interface erosion mechanism and anti-corrosive applications. Chem. Eng. J. 2021, 413, 127455. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. A novel coating material that uses nano-sized SiO2 particles to intensify hydrophobicity and corrosion protection properties. Electrochim. Acta 2016, 220, 417–426. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W. Morphology and hydrophobicity of a polyurethane film molded on a porous anodic alumina template. Surf. Coat. Technol. 2006, 200, 3492–3495. [Google Scholar] [CrossRef]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple method for preparing superhydrophobic paper: Spray-deposited hydrophobic silica nanoparticle coatings exhibit high water-repellency and transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef]
- Xue, L.; Li, J.; Fu, J.; Han, Y. Super-hydrophobicity of silica nanoparticles modified with vinyl groups. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 15–19. [Google Scholar] [CrossRef]
- Leder, G.; Ladwig, T.; Valter, V.; Frahn, S.; Meyer, J. New effects of fumed silica in modern coatings. Prog. Org. Coat. 2002, 45, 139–144. [Google Scholar] [CrossRef]
- Lazauskas, A.; Guobienė, A.; Prosyčevas, I.; Baltrušaitis, V.; Grigaliūnas, V.; Narmontas, P.; Baltrusaitis, J. Water droplet behavior on superhydrophobic SiO2 nanocomposite films during icing/deicing cycles. Mater. Charact. 2013, 82, 9–16. [Google Scholar] [CrossRef]
- Król, P.; Król, B.; Kozakiewicz, J.; Zapotoczny, S.; Pilch-Pitera, B.; Kozdra, S. Composites prepared from polyurethanes modified with silicone-acrylic nanopowders. Prog. Org. Coat. 2015, 81, 72–79. [Google Scholar] [CrossRef]
- Przybyszewski, B.; Boczkowska, A.; Kozera, R.; Mora, J.; Garcia, P.; Aguero, A.; Borras, A. Hydrophobic and icephobic behaviour of polyurethane-based nanocomposite coatings. Coatings 2019, 9, 811. [Google Scholar] [CrossRef]
- Lei, Y.; Jiang, B.; Liu, H.; Zhang, F.; An, Y.; Zhang, Y.; Yuan, Y.; Xu, J.; Li, X.; Liu, T. Mechanically robust superhydrophobic polyurethane coating for anti-icing application. Prog. Org. Coat. 2023, 183, 107795. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Dodiuk, H.; Kenig, S.; Ratto, J.A.; Barry, C.; Turkoglu, S.; Mead, J. The effect of superhydrophobic coating composition on the topography and ice adhesion. Cold Reg. Sci. Technol. 2022, 201, 103623. [Google Scholar] [CrossRef]
- Ejenstam, L.; Swerin, A.; Pan, J.; Claesson, M. Corrosion protection by hydrophobic silica particle-polydimethylsiloxane composite coatings. Corros. Sci. 2015, 99, 89–97. [Google Scholar] [CrossRef]
- Roshan, S.; Sarabi, A.A. Comprehensive study on the electrochemical, morphological, and adhesion properties of Cr-free thin film: With and without polyurethane coating. J. Coat. Technol. Res. 2021, 18, 761–776. [Google Scholar] [CrossRef]
- Salmasifar, A.; Sarabi, A.A.; Eivaz Mohammadloo, H. Anticorrosive performance of epoxy/clay nanocomposites pretreated by hexafluorozirconic acid based conversion coating on St12. Corros. Eng. Sci. Technol. 2015, 50, 372–379. [Google Scholar] [CrossRef]
- Troconis, B.R.; Frankel, G.S. Effect of roughness and surface topography on adhesion of PVB to AA2024-T3 using the blister test. Surf. Coat. Technol. 2013, 236, 531–539. [Google Scholar] [CrossRef]
- Roshan, S.; Sarabi, A.A. Improved performance of Ti-based conversion coating in the presence of Ce/Co ions: Surface characterization, electrochemical and adhesion study. Surf. Coat. Technol. 2021, 410, 126931. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Egorkin, V.S.; Mashtalyar, D.V.; Emel’Yanenko, A.M.; Alpysbaeva, D.A.; Boinovich, L.B. Features of the occurrence of electrochemical processes in contact of sodium chloride solutions with the surface of superhydrophobic coatings on titanium. Russ. J. Electrochem. 2012, 48, 336–345. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Vazirinasab, E.; Momen, G.; Jafari, R. Icephobicity and durability assessment of superhydrophobic surfaces: The role of surface roughness and the ice adhesion measurement technique. J. Mater. Process. Technol. 2021, 288, 116883. [Google Scholar] [CrossRef]
- Davis, A.; Yeong, Y.H.; Steele, A.; Bayer, I.S.; Loth, E. Superhydrophobic nanocomposite surface topography and ice adhesion. ACS Appl. Mater. Interfaces 2014, 6, 9272–9279. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Iwasaki, T.; Matsutani, A.; Hatano, M. Effect of radical fluorination on mono-and bi-layer graphene in Ar/F2 plasma. Appl. Phys. Lett. 2012, 101, 163105. [Google Scholar] [CrossRef]
- Kassis, C.M.; Steehler, J.K.; Betts, D.E.; Guan, Z.; Romack, T.J.; DeSimone, J.M.; Linton, R.W. XPS studies of fluorinated acrylate polymers and block copolymers with polystyrene. Macromolecules 1996, 29, 3247–3254. [Google Scholar] [CrossRef]
- Farzaneh, M. Ice accretions on high–voltage conductors and insulators and related phenomena. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2000, 358, 2971–3005. [Google Scholar] [CrossRef]
- Ru, Y.; Fang, R.; Gu, Z.; Jiang, L.; Liu, M. Reversibly thermosecreting organogels with switchable lubrication and anti-icing performance. Angew. Chem. 2020, 132, 11974–11978. [Google Scholar] [CrossRef]
- Shamshiri, M.; Jafari, R.; Momen, G. Icephobic properties of aqueous self-lubricating coatings containing PEG-PDMS copolymers. Prog. Org. Coat. 2021, 161, 106466. [Google Scholar] [CrossRef]
- Golovin, K.; Kobaku, S.; Lee, D.H.; DiLoreto, E.T.; Mabry, J.M.; Tuteja, A. Designing durable icephobic surfaces. Sci. Adv. 2016, 2, e1501496. [Google Scholar] [CrossRef]
- Yin, L.; Xia, Q.; Xue, J.; Yang, S.; Wang, Q.; Chen, Q. In situ investigation of ice formation on surfaces with representative wettability. Appl. Surf. Sci. 2010, 256, 6764–6769. [Google Scholar] [CrossRef]
- Mangini, D.; Antonini, C.; Marengo, M.; Amirfazli, A. Runback ice formation mechanism on hydrophilic and superhydrophobic surfaces. Cold Reg. Sci. Technol. 2015, 109, 53–60. [Google Scholar] [CrossRef]
- Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A.A.; Asemani, H.R.; Ahmadi, P. A comparative study of eco-friendly hybrid thin films: With and without organic coating application. Prog. Org. Coat. 2018, 125, 432–442. [Google Scholar] [CrossRef]
- Hosseini, R.M.; Sarabi, A.A.; Mohammadloo, H.E.; Sarayloo, M. The performance improvement of Zr conversion coating through Mn incorporation: With and without organic coating. Surf. Coat. Technol. 2014, 258, 437–446. [Google Scholar] [CrossRef]
- Mills, D.J.; Jamali, S.S.; Paprocka, K. Investigation into the effect of nano-silica on the protective properties of polyurethane coatings. Surf. Coat. Technol. 2012, 209, 137–142. [Google Scholar] [CrossRef]
- Fockaert, L.I.; Taheri, P.; Abrahami, S.T.; Boelen, B.; Terryn, H.; Mol, J.M.C. Zirconium-based conversion film formation on zinc, aluminium and magnesium oxides and their interactions with functionalized molecules. Appl. Surf. Sci. 2017, 423, 817–828. [Google Scholar] [CrossRef]
- Jalili, M.M.; Moradian, S. Deterministic performance parameters for an automotive polyurethane clearcoat loaded with hydrophilic or hydrophobic nano-silica. Prog. Org. Coat. 2009, 66, 359–366. [Google Scholar] [CrossRef]
- ASTM 3359B; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM G154-23; Standard Practice for Operating Fluorescent Ultraviolet (UV) Lamp Apparatus for Exposure of Materials. ASTM International: West Conshohocken, PA, USA, 2023.



| Sample Code | Contact Angle (°) | Contact Angle Hysteresis (°) | |
|---|---|---|---|
| FPU |  | 91 ± 1 | 75 ± 3 |
| FPU-SiO2 4% |  | 164 ± 3 | 3 ± 0.5 |
| FPU-SiO2 7% |  | 165 ± 2 | 9 ± 1 |
| FPU-SiO2 10% |  | 169 ± 3 | 25 ± 1 |
| Coating | Average Roughness (nm) |
|---|---|
| PU | 28.2 ± 7.1 |
| PU-SiO2 4% | 132.1 ± 9.5 |
| PU-SiO2 7% | 201.3 ± 11.3 |
| PU-SiO2 10% | 283.1 ± 8.1 |
| Sample Code | |Z|@0.01 Hz (Ω·cm2) 1 Day | |Z|@0.01 Hz (Ω·cm2) 60 Days | |Z|@0.01 Hz (Ω·cm2) 90 Days |
|---|---|---|---|
| FPU-SiO2 4% | 1.7 × 1010 | 2.2 × 109 | 1.6 × 108 |
| FPUSiO2 4%-CC | 3 × 1010 | 9 × 109 | 1.6 × 109 |
| Sample Code | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|
| FPU | 0.74 | −1.00 | 3.05 | 3.3 |
| FPU-SiO2 4% | −0.13 | −1.07 | 1.05 | 2.1 |
| FPU-SiO2 4%-CC | −0.75 | −0.95 | 1.59 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshan, S.; Jafari, R.; Momen, G. Polyurethane Nanocomposite Coatings Coupled with Titanium-Based Conversion Layers for Enhanced Anticorrosion, Icephobic Properties, and Surface Protection. Molecules 2024, 29, 3901. https://doi.org/10.3390/molecules29163901
Roshan S, Jafari R, Momen G. Polyurethane Nanocomposite Coatings Coupled with Titanium-Based Conversion Layers for Enhanced Anticorrosion, Icephobic Properties, and Surface Protection. Molecules. 2024; 29(16):3901. https://doi.org/10.3390/molecules29163901
Chicago/Turabian StyleRoshan, Shamim, Reza Jafari, and Gelareh Momen. 2024. "Polyurethane Nanocomposite Coatings Coupled with Titanium-Based Conversion Layers for Enhanced Anticorrosion, Icephobic Properties, and Surface Protection" Molecules 29, no. 16: 3901. https://doi.org/10.3390/molecules29163901
APA StyleRoshan, S., Jafari, R., & Momen, G. (2024). Polyurethane Nanocomposite Coatings Coupled with Titanium-Based Conversion Layers for Enhanced Anticorrosion, Icephobic Properties, and Surface Protection. Molecules, 29(16), 3901. https://doi.org/10.3390/molecules29163901







