Photoinitiators for Medical Applications—The Latest Advances
Abstract
1. Introduction
- -
- An oligomer or resin (unsaturated or acrylate), which is responsible for the properties of the final polymer, consisting of 1 to 12 unit monomers;
- -
- Monomers, which are used to increase the viscosity and flexibility of the polymer;
- -
- Additives—for example, talc or pigments—which are responsible for lowering prices or giving colour;
- -
- A photoinitiator, whose role is to react at a specific wavelength and form cations, anions, or radicals (depending on the types of particles formed, for which cationic or radical photopolymerization can be distinguished [8,9,10,11,12]) and then initiate the polymerization process. The mechanism of this reaction is based on the absorption of light of a specific wavelength, followed by the excitation of ground-state electrons to the excited singlet state and then the triplet state, from which they undergo decay and form active centers [13]. Photoinitiators play a crucial role in photopolymerization, hence the great interest in the design of new photoinitiator systems. It is also crucial to determine the appropriate concentration of a photoinitiator because it is the most expensive element of the photocurable mixture, and this has economic justification [14]. The requirements for photoinitiators during their design and subsequent use are a low production cost, relatively uncomplicated synthesis, good solubility in the ingredients of the reaction mixture, absorption in the visible spectrum, and high polymerization efficiency. Additionally, when used in biomedical applications, photoinitiators must have good solubility in water and no negative effects on cells [15,16]. In free radical photopolymerization, the following types of photoinitiators (PIs) can be distinguished:
- Type I, where the dissociation of the molecule and the formation of active centers takes place through direct absorption of a quantum of light by the molecule, which is a one-component system;
- Type II, where active centers are produced after multi-component reactions, involving the transfer of electrons or hydrogen between the co-initiator and photoinitiators after the absorption quantum of light, constituting two- or multi-component systems [8].
2. Classification of Photoinitiators
2.1. Photoinitiators of Natural Origin
2.2. Photoinitiators of Synthetic Origin
- Due to the low content of germanium in the earth’s crust, the widespread use of germanium, and therefore, germanium-based photoinitiators, may be limited due to their high price and restricted to critical areas such as medicine [25],
- They are type I photoinitiators, photodissociate at the Ge-C bond [26],
- Absorption capacity is up to 480 nm [26],
- The synthesis method (Corey-Seebach synthesis), on which the production of a commonly used germanium-based compound (IvocerinTM) is based, is complicated and involves several stages, which affects the reaction efficiency. The challenge undertaken by Frűhwirt’s group is to develop a one-pot synthesis method [27],
- Tetraacylgermanates have poor solubility; the perception is to appropriate substituents [27],
- Germanium-based derivatives showed little or no toxicity. It was also demonstrated that there was no effect on the occurrence of gene mutations [24].
| Name of Substances | Chemical Structure | Type of Photoinitiator | Uses in Medicine | Additional Information | Literature |
|---|---|---|---|---|---|
| Riboflavin (7,8-dimethyl-10-ribityl-isoalloxazine) | 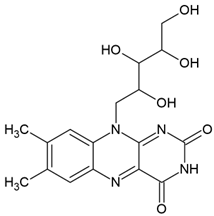 | Type II | hydrogels in drug delivery, tissue crosslinking, dermal filler, cellular scaffolds | yellow pigment, water-soluble and heat-stable, can be found in milk, eggs, fish, vegetables (broccoli, asparagus), no toxic for humans, absorbs in range between 220 and 450 nm with maximum absorbance: 223 nm, 267 nm, 373 nm and 444 nm, used as a photoinitiator in the absence of DMAEMA (co-initiator) and with the use DPIC (accelerator) | [20,22,30,31] |
| Coumarins (2H-1-benzopyran-2-one) | 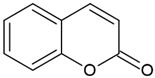 | Type I and II | 3D printing, drug delivery systems, tissue engineering, biomaterials, dental resin | extracted from bacteria (Streptomycces) or fungal (Aspergillus) and plants (green tea, cloudberry, tonka beans), absorption spectra in the range between 270 and 510 mm, maximum absorbance at 330 nm, | [32,33] |
| Flavones | 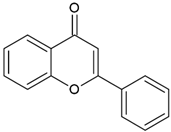 | Type II | 3D printing | found in vegetables (celery, parsley), fruits (citrus fruits), flowers (chamomile), greenish—yellow colour, absorption spectra in the range between 350 and 470 nm, maximum absorbance at 405 nm | [20,21,34] |
| Curcumin (E,E)-1,7-bis(4-hydroxy-3-methoxy-phenyl)-1,6-heptadiene-3,5-dione |  | Type II | hydrogels, photodynamic therapy, antibacterial coatings | orange-yellow pigment, insoluble in water, is extracted from turmeric, absorb light in a range between 350 and 750 nm with maximum absorbance at 436 nm, is used as a photoinitiator in the absence of an electron donor—for example, DPI | [20,35,36,37,38] |
| Chalcones (1,3-diphenyl-2-propen-1-one) | 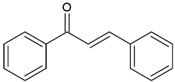 | Type II | 3-D printing dental materials | yellow, orange and brown colour, absorb light in the range between 220 and 270 nm and 340 and 390 nm with maximum absorption at 423 nm, 363 nm, 362 nm and 344 nm | [20,21,39,40] |
| Naphthoquinones (1,4-naphthoquinones) | 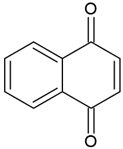 | Type I and II | polymerization of acrylates | orange, yellow, red, purple colour, extracted from a tropical shrub Lawsonia inermis maximum absorption in visible light region—420 nm | [20,41,42,43] |
| Name of Substances | Chemical Structure | Type of Photoinitiator | Uses in Medicine | Additional Information | Literature |
|---|---|---|---|---|---|
| CQ (camphorquinone) |  | Type II | dental resin | curing by using a blue wavelength spectrum of light (from 430 to 510 nm) with maximum absorption peak of 468 nm (molar extinction coefficient—28 L/mol generating two radicals (amino and alkyl), rate of conversion of resin composites is between 35 and 77% Advantage: sensitive to light in the visible range Disadvantage: yellow colour—it negatively affects the appearance of dental resin, unreacted CQ has a toxic effect on pulp cells, a low value of molar extinction coefficient | [17,19,44,45,46,47,48] |
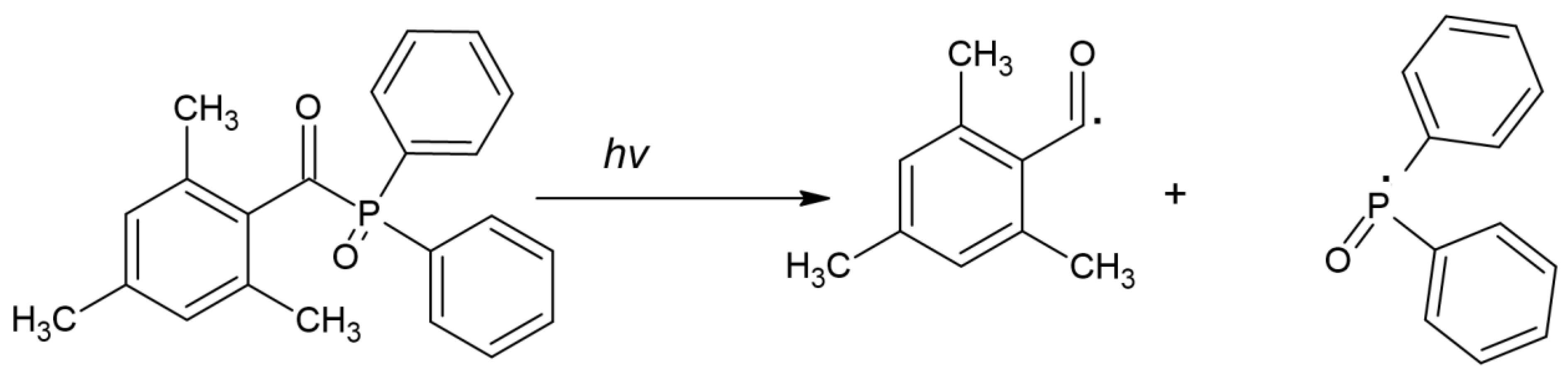
| TPO (2,4,5–trimethylbenzoyldiphenylphosphine oxide) |  | Type I | dental resin, hydrogels | the absorption spectrum is between 380 and 425 nm with maximum absorption peak at 380 nm, Advantage: no yellowing effect and higher colour stability, high molar absorption coefficient (520 L/mol) Disadvantage: it may be polymerized only as thick layers that increase the degree of shrinkage and use a UV light source | [44,47,49] |
| BAPO (bisacylphosphine oxide) | 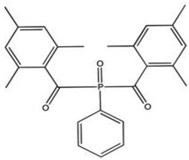 | Type I | dental resin, | absorption spectrum between 365 and 416 nm and maximum absorption peak at 370 nm (molar extinction coefficient—870 L/mol, generate 4 types of radicals, Advantage: white colour which has a positive effect on dental resin, no need for the presence of co-initiator, Disadvantage: need to use a halogen lamp, | [44,50] |
| BP (benzophenone) | 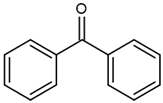 | Type II | dental resin, | absorption spectrum between 320 and 370 nm and 240 and 300 nm and maximum absorption peek at 294 nm, Advantage: low cost and efficiency Disadvantage: need to use a light source with UV spectrum | [17] |
| PPD (1-phenyl-1,2-propanedione) | 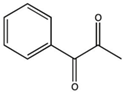 | Type I and II | dental resin | absorption spectrum between 350 and 500 nm, and maximum absorption at 398 nm (molar extinction coefficient—150 L/mol, Advantage: positive effect of colour of dental resin, | [44] |
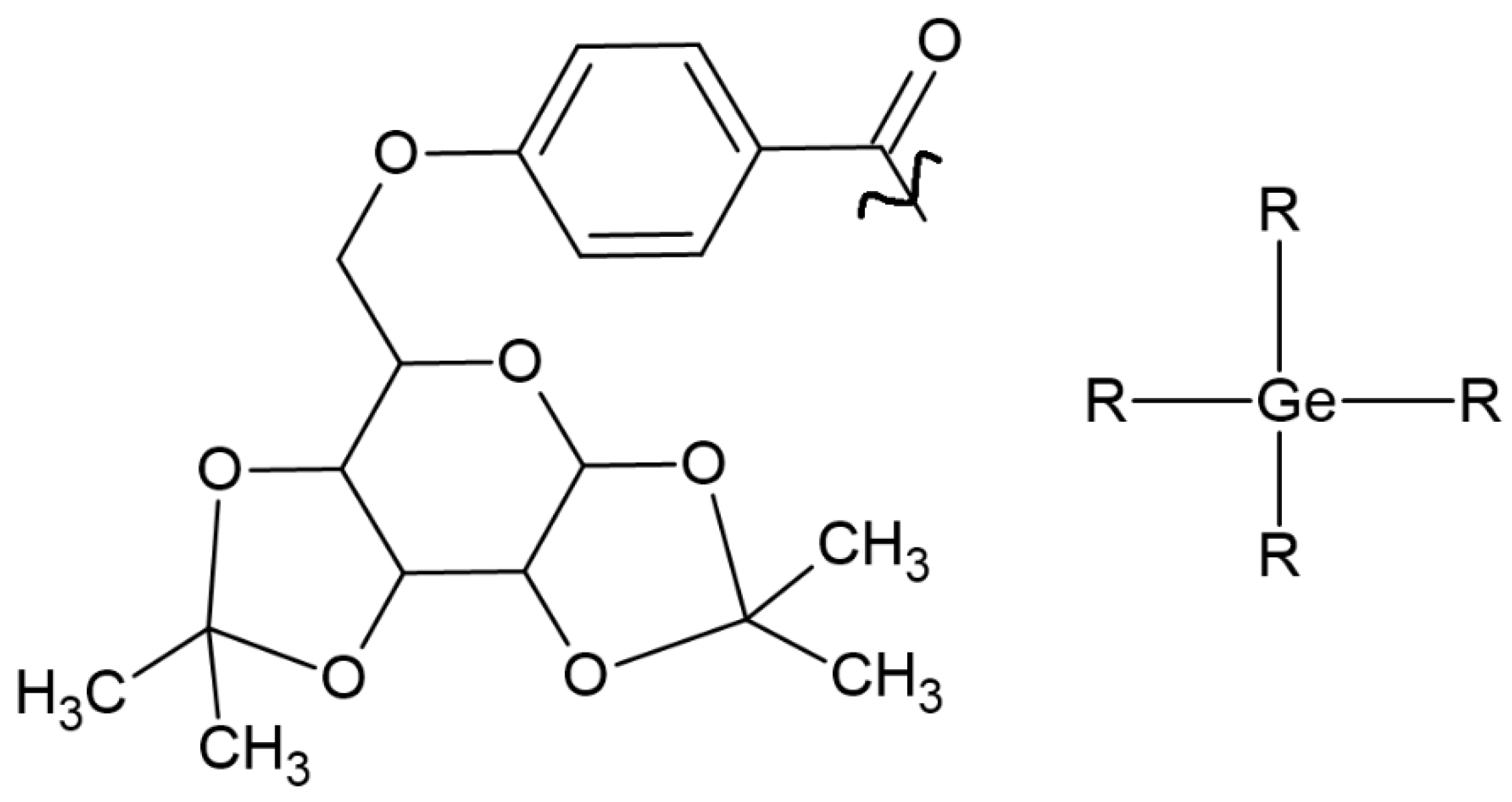
| IVO (Ivocerin®) bis-(4-methoxybenzoyl)diethyl-germane (Ge-3) |  | Type I | dental resin | Absorption spectrum between 390 and 445 nm, with maximum absorption at 418 nm Advantage: no cytotoxicity, White colour, High initiation rate Disadvantage: high cost, low solubility | [17,51,52,53] |
3. Application of Photoinitiators Used in Medicine
3.1. Dentistry
- -
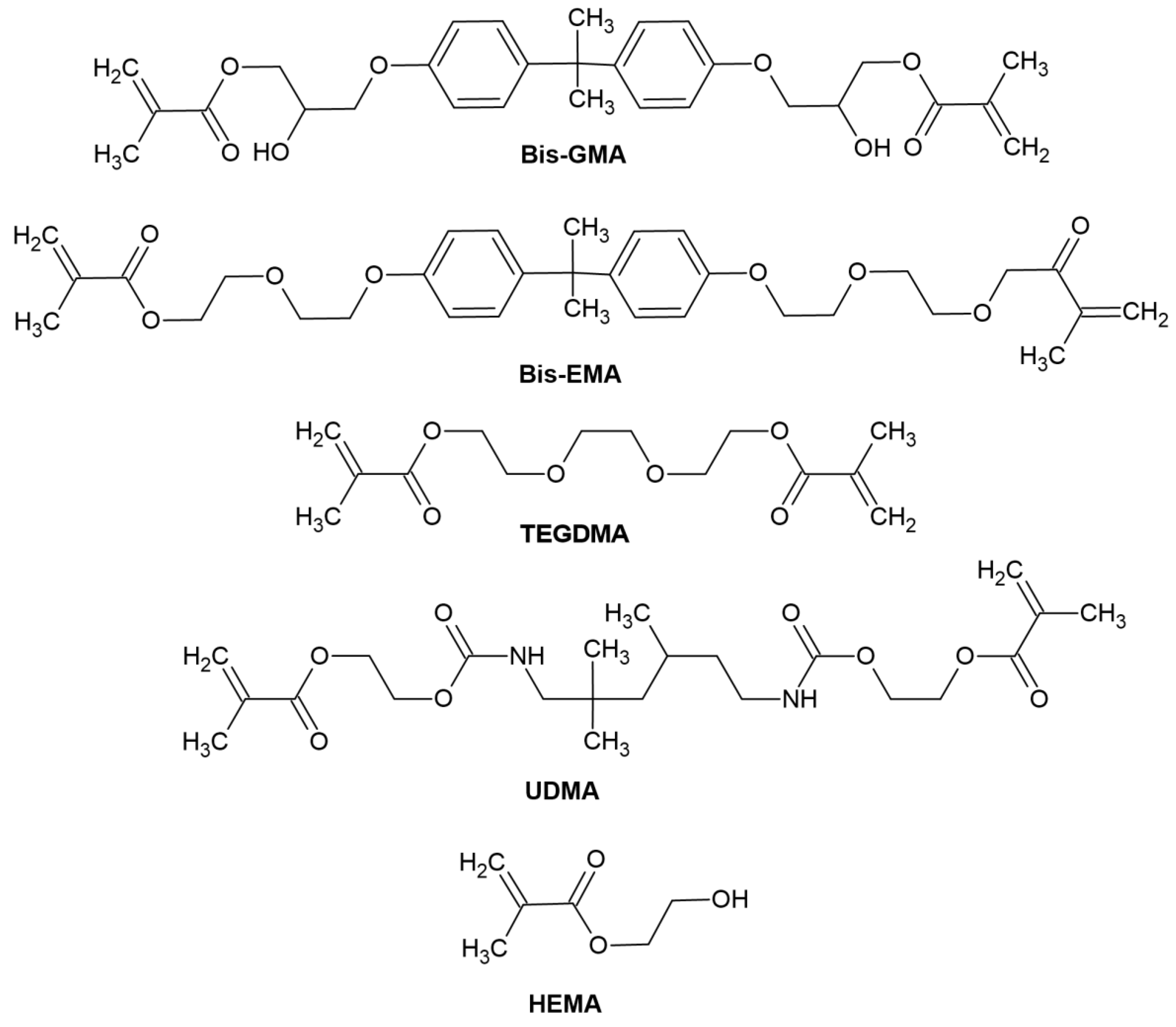
- Organic resin matrix: organic monomers—among which the most frequently used include bis-GMA ((2,2-bis [4-(2-hydroxy-3-methacryloyloxypropoxy)]-phenyl propane) or bisphenol A-glycidyl methacrylate), bis-EMA (ethoxylated bisphenol A glycol dimethacrylate), TEGDMA (triethylene glycol dimethacrylate), UDMA (urethane dimethacrylate), HEMA (hydroxyethyl methacrylate), photoinitiator or photoinitiator system and stabilizers to extend durability [54]. The chemical structure of monomers is shown in Figure 4.
- -
- Inorganic fillers, which increase strength and abrasion resistance. It is most often glass or silica,
- -
- A coupling agent, which combines all the mixture’s ingredients with the organic matrix [17].
- (a)
- (b)
- BAPO—The advantages of this PI reveal a degree of conversion and polymerization rate similar to that of the CQ/amine system. Additionally, the presence of a co-initiator is redundant [36]. Experimental work by Lim et al. [50] confirmed the abovementioned content. However, they indicate the need to conduct additional studies on this photoinitiator, examining the interaction between photoinitiators and monomers in the composites.
- (c)
- BP—A shift in the absorption spectrum towards longer wavelengths was started by Huang’s team. Three new compounds were synthesized based on the BP skeleton. Its chemical structure is presented in Figure 6. The experiment obtained compounds with good solubility in organic solvents and the TMPTMA (trimethylolpropane trimethacrylate) monomer. The modification of the chemical structures of benzophenone resulted in a shift of the absorption spectrum to approximately 380 nm and an increase in the molar extinction coefficient. The effectiveness of new photoinitiators in combination with the abovementioned monomer in the photopolymerization process was also demonstrated. This opens up new possibilities for BP modification [57].
- (d)
- PPD—Connecting with CQ positively reduces the degree of yellowing. Its presence also improves the physicochemical properties of the obtained polymer [44,58]. Dressano’s team studied three-component systems (CQ, PPD and DPI salts—diphenyliodonium hexafluorophosphate) and their effect on the physical and chemical properties of the dental resins. PPD is supposed to reduce the yellowing effect, and DPI is supposed to improve the reaction efficiency by regenerating inactive CQ molecules and replacing them with active phenyl radicals. The best concentration of CQ-PPD is 0.5 mol% and DPI is 1 mol%. This system showed low water absorption, low solubility and good mechanical properties—these features are desirable in dental resin formulations [58].
3.2. Hydrogels
3.3. Drug Delivery
3.4. Photodynamic Therapy
3.5. Individualized Tablets
3.6. 3D Printing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stirbet, A.; Lazar, D.; Guo, Y.; Govindjee, G. Part of a special issue on functional—Structural plant growth modelling. Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef] [PubMed]
- Harb, F.; Hidalgo, M.P.; Martau, B. Lack of exposure to natural light in the workspace is associated with physiological, sleep and depressive symptoms. Chronobiol. Int. 2015, 32, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Pączkowski, J. Fotochemia polimerów. Teoria i zastosowanie. UMK 2003. Available online: https://wydawnictwo.umk.pl/produkt/fotochemia-polimerow-teoria-i-zastosowanie (accessed on 16 July 2024).
- Corrigan, N.; Yeow, J.; Judzewitsch, P.; Xu, J.; Boyer, C. Seeing the light: Advancing materials chemistry through photopolymerization. Angew. Chem. Int. Ed. 2019, 58, 5170–5189. [Google Scholar] [CrossRef] [PubMed]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B.; Gibson, I.; Rosen, D.W.; Stucker, B. Photopolymerization processes. Addit. Manuf. Technol. Rapid Prototyp. Direct Digit. Manuf. 2010, 78–119. [Google Scholar]
- Younes, H.M. Photopolymerization of polymeric composites in drug delivery, tissue engineering, and other biomedical applications. Polym. Nanocomposites Biomed. Eng. Springer Cham 2019, 271–297. [Google Scholar]
- Hola, E.; Pilch, M.; Ortyl, J. Thioxanthone derivatives as a new class of organic photocatalysts for photopolymerisation processes and the 3D printing of photocurable resins under visible light. Catalysts 2020, 10, 903. [Google Scholar] [CrossRef]
- Kostrzewska, K.; Balcerak, A.; Dobosz, R.; Kabatc, J. Sole N-alkoksonionowe jako koinicjatory w procesie polimeryzacji rodnikowej sensybilizowanej przez barwnik skwarynowy w zakresie światła UV-Vis. Przemysł Chem. 2017, 96. [Google Scholar] [CrossRef]
- Balcerak, A.; Kwiatkowska, D.; Iwińska, K.; Kabatc, J. Highly efficient UV-Vis light activated three-component photoinitiators composed of tris (trimethylsilyl) silane for polymerization of acrylates. Polym. Chem. 2020, 11, 5500–5511. [Google Scholar] [CrossRef]
- Balcerak, A.; Kwiatkowska, D.; Kabatc, J. Novel photoinitiators based on difluoroborate complexes of squaraine dyes for radical polymerization of acrylates upon visible light. Polym. Chem. 2022, 13, 220–234. [Google Scholar] [CrossRef]
- Balcerak, A.; Kabatc, J. Recent progress in the development of highly active dyeing photoinitiators based on 1, 3-bis (p-substituted phenylamino) squaraines for radical polymerization of acrylates. Polym. Chem. 2022, 13, 1787–1812. [Google Scholar] [CrossRef]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-radical photopolymerization for curing products for refinish coatings market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Nie, J. Low-temperature photopolymerization and post-cure characteristics of acrylates. Polym. Int. 2007, 56, 707–710. [Google Scholar] [CrossRef]
- Deng, L.; Qu, J. Design of novel phenothiazine-based push-pull photoinitiators for visible light with high activity, good solubility and low migration. Prog. Org. Coat. 2023, 183, 107766. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-soluble photoinitiators in biomedical applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The photoinitiators used in resin based dental composite—A review and future perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Balcerak-Woźniak, A.; Kabatc-Borcz, J.; Czech, Z. High potential of new dyeing photoinitiators for fast curing of (meth) acrylate compositions under low intensity UV–Vis light. Polym. Chem. 2023, 14, 3931–3949. [Google Scholar] [CrossRef]
- Chiulan, I.; Heggset, E.B.; Voicu, S.I.; Chinga-Carrasco, G. Photopolymerization of bio-based polymers in a biomedical engineering perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Monem, M.M. Naturally derived photoinitiators for dental and biomaterials applications. Eur. Dent. Res. Biomater. J. 2020, 1, 72–78. [Google Scholar] [CrossRef]
- Vazquez-Martel, C.; Mainik, P.; Blasco, E. Natural and naturally derived photoinitiating systems for light-based 3D printing. Org. Mater. 2022, 4, 281–291. [Google Scholar] [CrossRef]
- Lee, Y.B.; Lim, S.; Lee, Y.; Park, C.H.; Lee, H.J. Green chemistry for crosslinking biopolymers: Recent advances in riboflavin-mediated photochemistry. Materials 2023, 16, 1218. [Google Scholar] [CrossRef] [PubMed]
- Sanay, B.; Strehmel, B.; Strehmel, V. Manufacturing and photocrosslinking of a new bio-based dimethacrylate resulting in hydrophobic crosslinked films. Appl. Res. 2022, 1, e202100003. [Google Scholar] [CrossRef]
- Wiesner, T.; Haas, M. Do germanium-based photoinitiators have the potential to replace the well-established acylphosphine oxides? Dalton Trans. 2021, 50, 12392–12398. [Google Scholar] [CrossRef] [PubMed]
- Püschmann, S.D.; Frühwirt, P.; Pillinger, M.; Knoechl, A.; Mikusch, M.; Radebner, J.; Torvisco, A.; Fischer, R.C.; Moszner, N.; Gescheidt, G.; et al. Synthesis of Mixed-Functionalized Tetraacylgermanes. Chem. –A Eur. J. 2021, 27, 3338–3347. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Drusgala, M.; Fischer, R.C.; Torvisco, A.; Kern, W.; Haas, M.; Bandl, C. Surface-Initiated Polymerizations Mediated by Novel Germanium-Based Photoinitiators. ACS Appl. Mater. Interfaces 2023, 15, 31836–31848. [Google Scholar] [CrossRef] [PubMed]
- Frühwirt, P.; Knoechl, A.; Pillinger, M.; Müller, S.M.; Wasdin, P.T.; Fischer, R.C.; Radebner, J.; Torvisco, A.; Moszner, N.; Kelterer, A.M.; et al. The Chemistry of Acylgermanes: Triacylgermenolates Represent Valuable Building Blocks for the Synthesis of a Variety of Germanium-Based Photoinitiators. Inorg. Chem. 2020, 59, 15204–15217. [Google Scholar] [CrossRef] [PubMed]
- Schuh, L.; Müller, P.; Torvisco, A.; Stueger, H.; Wrodnigg, T.M.; Haas, M. Synthesis of d-Galactose-Substituted Acylsilanes and Acylgermanes. Model Compounds for Visible Light Photoinitiators with Intriguing High Solubility. Organometallics 2021, 40, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Radebner, J.; Eibel, A.; Gescheidt, G.; Stueger, H. Recent advances in germanium-based photoinitiator chemistry. Chem. –A Eur. J. 2018, 24, 8258–8267. [Google Scholar] [CrossRef] [PubMed]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Abdul-Monem, M.M.; Kamoun, E.A.; Ahmed, D.M.; El-Fakharany, E.M.; Al-Abbassy, F.H.; Aly, H.M. Light-cured hyaluronic acid composite hydrogels using riboflavin as a photoinitiator for bone regeneration applications. J. Taibah Univ. Med. Sci. 2021, 16, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 1, 963248. [Google Scholar] [CrossRef] [PubMed]
- Cazin, I.; Rossegger, E.; Guedes de la Cruz, G.; Griesser, T.; Schlögl, S. Recent advances in functional polymers containing coumarin chromophores. Polymers 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y. Flavones in Green Tea: Part I. Isolation and Structures of Flavones Occurring in Green Tea Infusion. Agric. Biol. Chem. 1967, 31, 1029–1034. [Google Scholar] [CrossRef]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photodynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, I.; Chmielarz, P. Comestible curcumin: From kitchen to polymer chemistry as a photocatalyst in metal-free ATRP of (meth) acrylates. J. Ind. Eng. Chem. 2022, 105, 481–490. [Google Scholar] [CrossRef]
- Sun, G.; Huang, Y.; Ma, J.; Li, D.; Fan, Q.; Li, Y.; Shao, J. Photoinitiation mechanisms and photogelation kinetics of blue light induced polymerization of acrylamide with bicomponent photoinitiators. J. Polym. Sci. 2021, 59, 567–577. [Google Scholar] [CrossRef]
- Condat, M.; Mazeran, P.E.; Malval, J.P.; Lalevée, J.; Morlet-Savary, F.; Renard, E.; Langlois, V.; Abbad Andalloussi, S.; Versace, D.L. Photoinduced curcumin derivative-coatings with antibacterial properties. RSC Adv. 2015, 5, 85214–85224. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Abbo, H.S.; Lai, C.H.; Titinchi, S.J. Substituent and solvent effects on UV-visible absorption spectra of chalcones derivatives: Experimental and computational studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123180. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.; Polonik, S. 1, 4-Naphthoquinones: Some biological properties and application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F. Recent advances on naphthoquinone-based photoinitiators of polymerization. Eur. Polym. J. 2023, 193, 112120. [Google Scholar] [CrossRef]
- Borjigin, T.; Feng, J.; Schmitt, M.; Zhu, D.; Morlet-Savary, F.; Xiao, P.; Lalevée, J. Photoinitiators from bio-sourced naphthoquinone–the application of naphthoquinone-based vitamins K1 and K3 in free radical photopolymerization. Green Chem. 2024, 26, 277–286. [Google Scholar] [CrossRef]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.; Rocha, M.G.; Menezes, L.R.D.; Correr, A.B.; Sinhoreti, M.A.C.; Oliveira, D. Effect of combining photoinitiators on cure efficiency of dental resin-based composites. J. Appl. Oral Sci. 2021, 29, e20200467. [Google Scholar] [CrossRef] [PubMed]
- Maciel, D.D.S.A.; Caires-Filho, A.B.; Fernandez-Garcia, M.; Anauate-Netto, C.; Alonso, R.C.B. Effect of Camphorquinone Concentration in Physical-Mechanical Properties of Experimental Flowable Resin Composites. BioMed Res. Int. 2018, 1, 7921247. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.F.J.; Cavalcante, L.M.; Prahl, S.A.; Pfeifer, C.S.; Ferracane, J.L. Curing efficiency of dental resin composites formulated with camphorquinone or trimethylbenzoyl-diphenyl-phosphine oxide. Dent. Mater. 2012, 28, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Lin, L.D.; Wu, M.T.; Chan, C.P.; Chang, H.H.; Lee, M.S.; Sun, M.S.; Jeng, P.Y.; Yeung, S.Y.; Lin, H.J.; et al. Effects of camphorquinone on cytotoxicity, cell cycle regulation and prostaglandin E2 production of dental pulp cells: Role of ROS, ATM/Chk2, MEK/ERK and hemeoxygenase-1. PLoS ONE 2015, 10, e0143663. [Google Scholar] [CrossRef] [PubMed]
- Hadis, M.A.; Shortall, A.C.; Palin, W.M. Competitive light absorbers in photoactive dental resin-based materials. Dent. Mater. 2012, 28, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.D.R.; da Silva, D.B.; Vitti, R.P.; Miranda, M.E.; Brandt, W.C. Mechanical properties of experimental resin cements containing different photoinitiators and co-initiators. Clin. Cosmet. Investig. Dent. 2019, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, L.M.; Borges, M.G.; Soares, C.J.; Menezes, M.S.; Huynh, V.; Logan, M.G.; Fugolin, A.P.P.; Pfeifer, C.S. Effect of the photoinitiator system on the polymerization of secondary methacrylamides of systematically varied structure for dental adhesive applications. Dent. Mater. 2020, 36, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Topa, M.; Ortyl, J. Moving towards a finer way of light-cured resin-based restorative dental materials: Recent advances in photoinitiating systems based on iodonium salts. Materials 2020, 13, 4093. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Sokolowski, J.; Gozdek, T.; Krasowski, M.; Kopacz, K.; Bociong, K. The influence of various photoinitiators on the properties of commercial dental composites. Polymers 2021, 13, 3972. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, B.; Ni, K.; Wang, Z. Optimal photoinitiator concentration for light-cured dental resins. Polym. Test. 2021, 94, 107039. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokołowski, J.; Szynkowska-Jóźwik, M.I.; Gozdek, T.; Kopacz, K.; Bociong, K. Can TPO as Photoinitiator Replace “Golden Mean” Camphorquinone and Tertiary Amines in Dental Composites? Testing Experimental Composites Containing Different Concentration of Diphenyl (2, 4, 6-trimethylbenzoyl) phosphine Oxide. Int. J. Mol. Sci. 2022, 23, 11594. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Chen, Y.C. Ketone Number and Substitution Effect of Benzophenone Derivatives on the Free Radical Photopolymerization of Visible-Light Type-II Photoinitiators. Polymers 2021, 13, 1801. [Google Scholar] [CrossRef] [PubMed]
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Lima, A.F. Effect of diphenyliodonium hexafluorophosphate on the physical and chemical properties of ethanolic solvated resins containing camphorquinone and 1-phenyl-1, 2-propanedione sensitizers as initiators. Dent. Mater. 2016, 32, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Pyszka, I.; Skowroński, Ł.; Jędrzejewska, B. Study on New Dental Materials Containing Quinoxaline-Based Photoinitiators in Terms of Exothermicity of the Photopolymerization Process. Int. J. Mol. Sci. 2023, 24, 2752. [Google Scholar] [CrossRef] [PubMed]
- Topa-Skwarczyńska, M.; Jankowska, M.; Gruchała-Hałat, A.; Petko, F.; Galek, M.; Ortyl, J. High-performance photoinitiating systems for new generation dental fillings. Dent. Mater. 2023, 39, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Zeuner, F.; Liska, R. Polymerisierbare Zusammensetzungen mit Acylgermanium-Verbindungen als Initiatoren. EU Patent Application EP190 5415B1 1 July 2009. [Google Scholar]
- Moszner, N.; Fischer, U.K.; Ganster, B.; Liska, R.; Rheinberger, V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent. Mater. 2008, 24, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Nikitas, N.F.; Gkizis, P.L.; Kokotos, C.G. Thioxanthone: A powerful photocatalyst for organic reactions. Org. Biomol. Chem. 2021, 19, 5237–5253. [Google Scholar] [CrossRef] [PubMed]
- Ely, C.; Schneider, L.F.J.; Ogliari, F.A.; Schmitt, C.C.; Corrêa, I.C.; Lima, G.D.S.; Samuel, S.M.W.; Piva, E. Polymerization kinetics and reactivity of alternative initiators systems for use in light-activated dental resins. Dent. Mater. 2012, 28, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Breloy, L.; Rios De Anda, A.; Hayek, H.; Chiappone, A.; Malval, J.P.; Grande, D.; Versace, D.L. Thioxanthone-Based Siloxane Photosensitizer for Cationic/Radical Photopolymerization and Photoinduced Sol–Gel Reactions. Molecules 2024, 29, 255. [Google Scholar] [CrossRef] [PubMed]
- Fattah-alhosseini, A.; Chaharmahali, R.; Alizad, S.; Kaseem, M.; Dikici, B. A review of smart polymeric materials: Recent developments and prospects for medicine applications. Hybrid Adv. 2024, 100178. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Tomal, W.; Petko, F.; Galek, M.; Swiezy, A.; Tyszka-Czochara, M.; Sroda, P.; Mokrzyński, K.; Ortyl, J. Water-Soluble Type I Radical Photoinitiators Dedicated to Obtaining Microfabricated Hydrogels. Chem. Mater. 2024. [Google Scholar] [CrossRef]
- Substance Information—ECHA. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.071.211 (accessed on 18 April 2024).
- Chen, H.; Borjigin, T.; Regeard, C.; Xiao, P.; Dumur, F.; Lalevée, J. 5, 12-Dihydroindolo [3, 2-a] Carbazole Derivatives-Based Water Soluble Photoinitiators for 3D Antibacterial Hydrogels Preparation. Small 2023, 19, 2300772. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, B.; Zhang, J.; He, X.; Liu, F.; Cui, J.; Lu, Z.; Hu, G.; Yang, J.; Wang, R.; et al. General one-pot method for preparing highly water-soluble and biocompatible photoinitiators for digital light processing-based 3D printing of hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 55507–55516. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Materials Science and Engineering: C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Gencoglu, T.; Eren, T.N.; Lalevée, J.; Avci, D. A Water Soluble, Low Migration, and Visible Light Photoinitiator by Thioxanthone-Functionalization of Poly (ethylene glycol)-Containing Poly (β-amino ester). Macromol. Chem. Phys. 2022, 223, 2100450. [Google Scholar] [CrossRef]
- Ding, X.; Cheng, D.; Zhao, L.; Luo, X.; Yue, L.; Zhang, Y.; Wang, Z. Carboxymethyl chitosan modified with curcumin: A photodynamic antibacterial agent with good solubility and stability. Food Biosci. 2024, 57, 103525. [Google Scholar] [CrossRef]
- Pyteraf, J.; Pacławski, A.; Jamróz, W.; Mendyk, A.; Paluch, M.; Jachowicz, R. Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products. Pharmaceutics 2022, 14, 843. [Google Scholar] [CrossRef] [PubMed]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Flavones as natural photoinitiators for light mediated free-radical polymerization via light emitting diodes. J. Polym. Sci. 2020, 58, 254–262. [Google Scholar] [CrossRef]


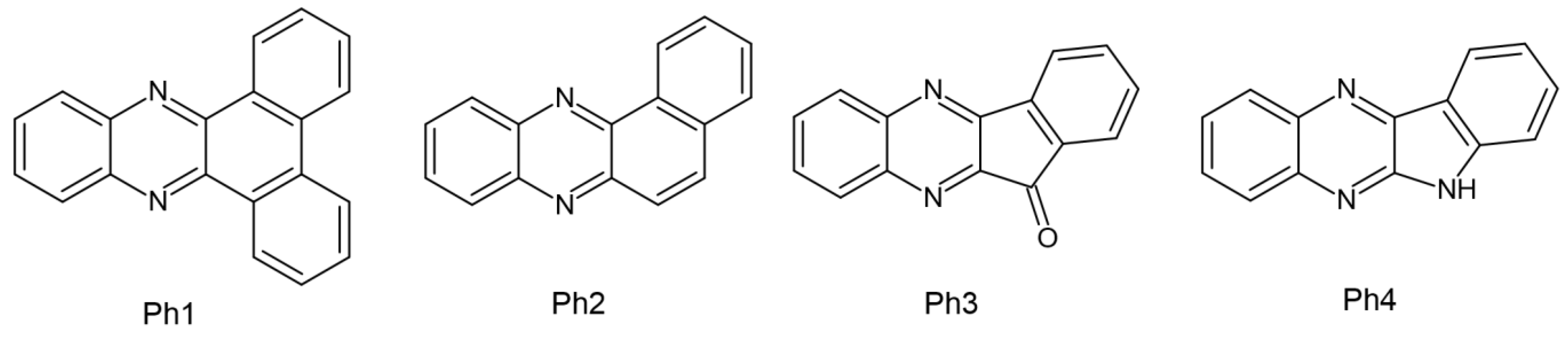
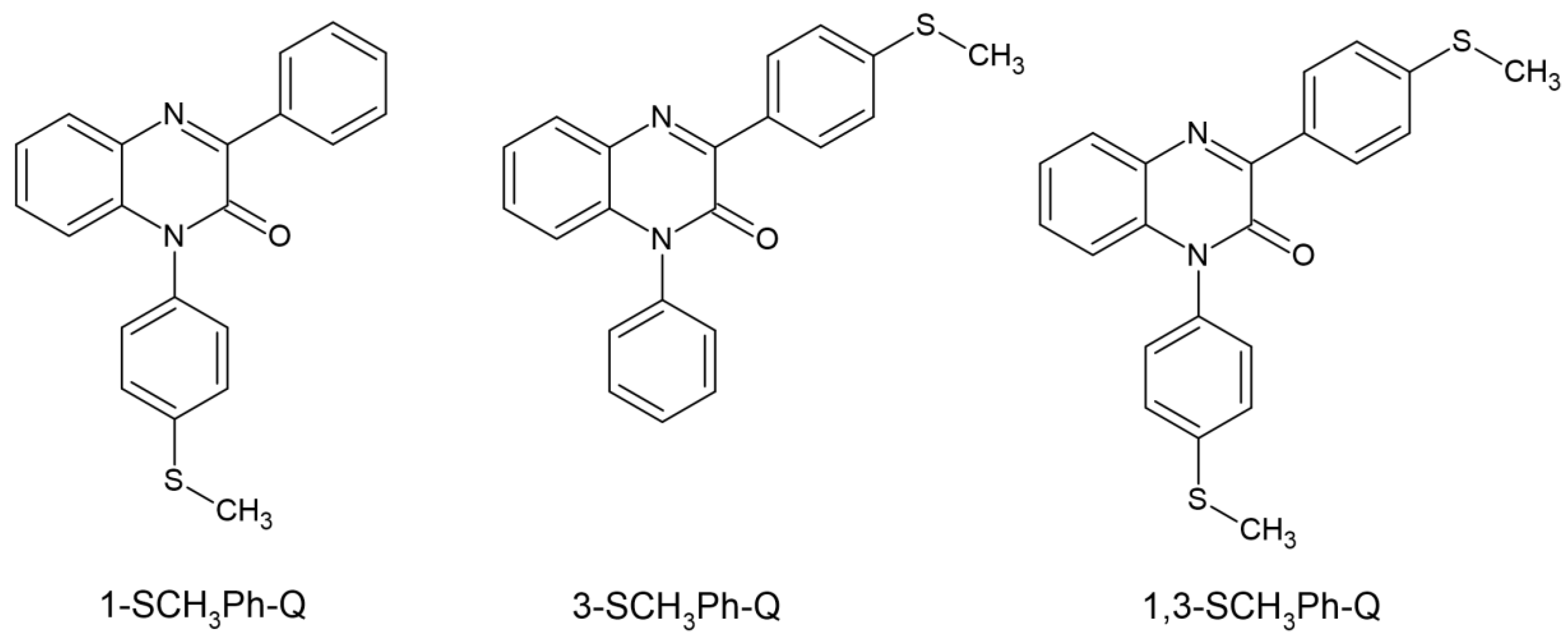
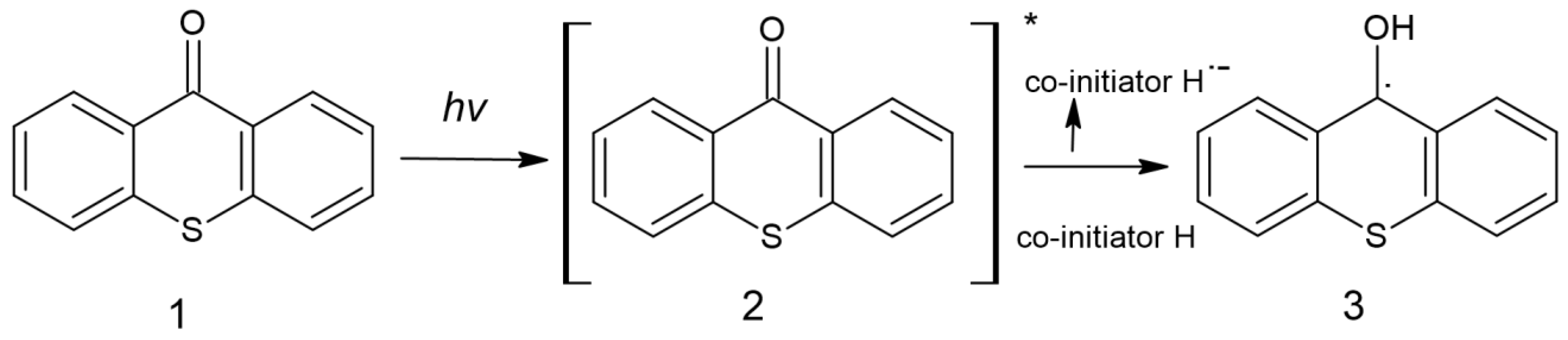
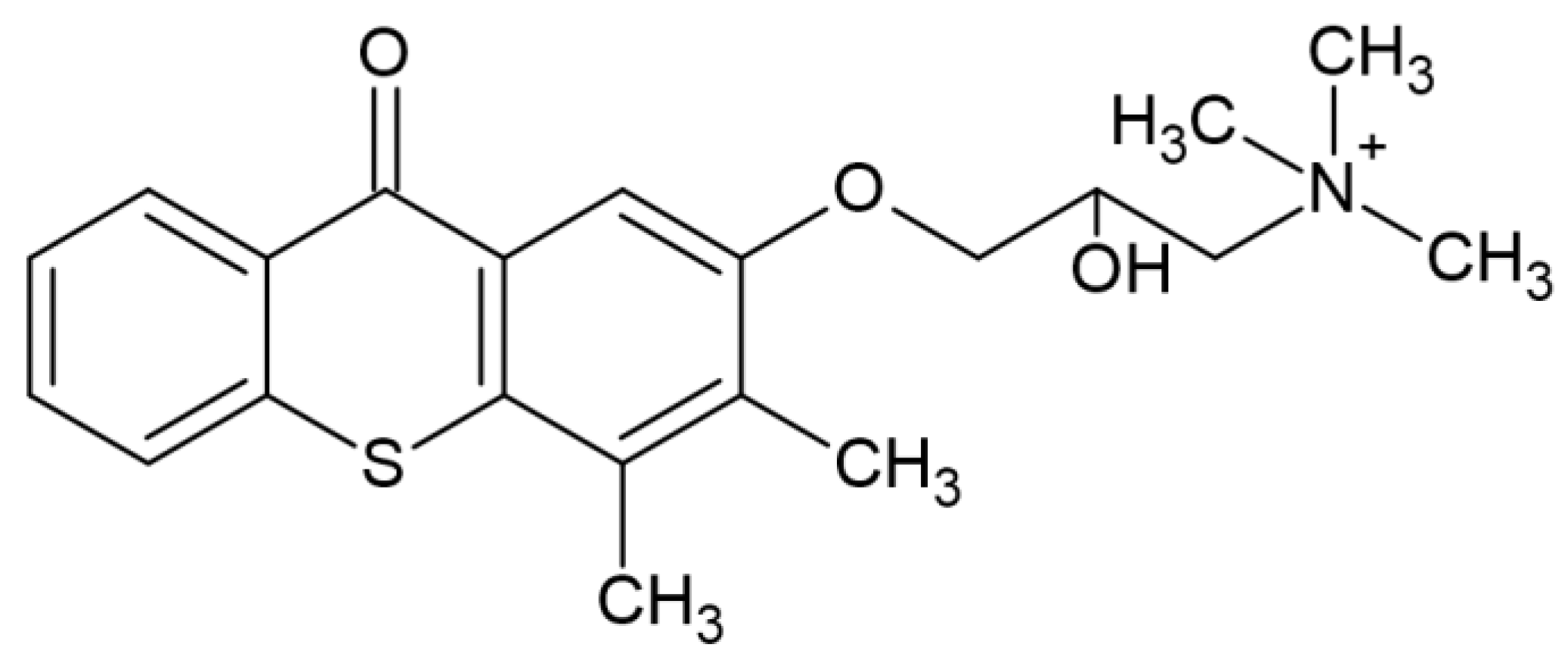
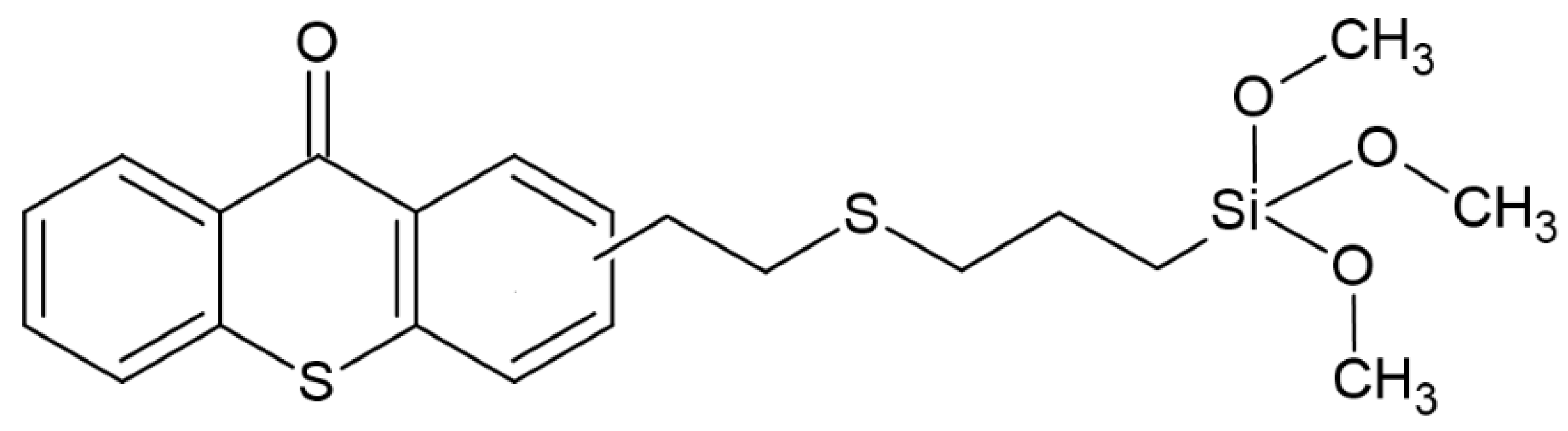
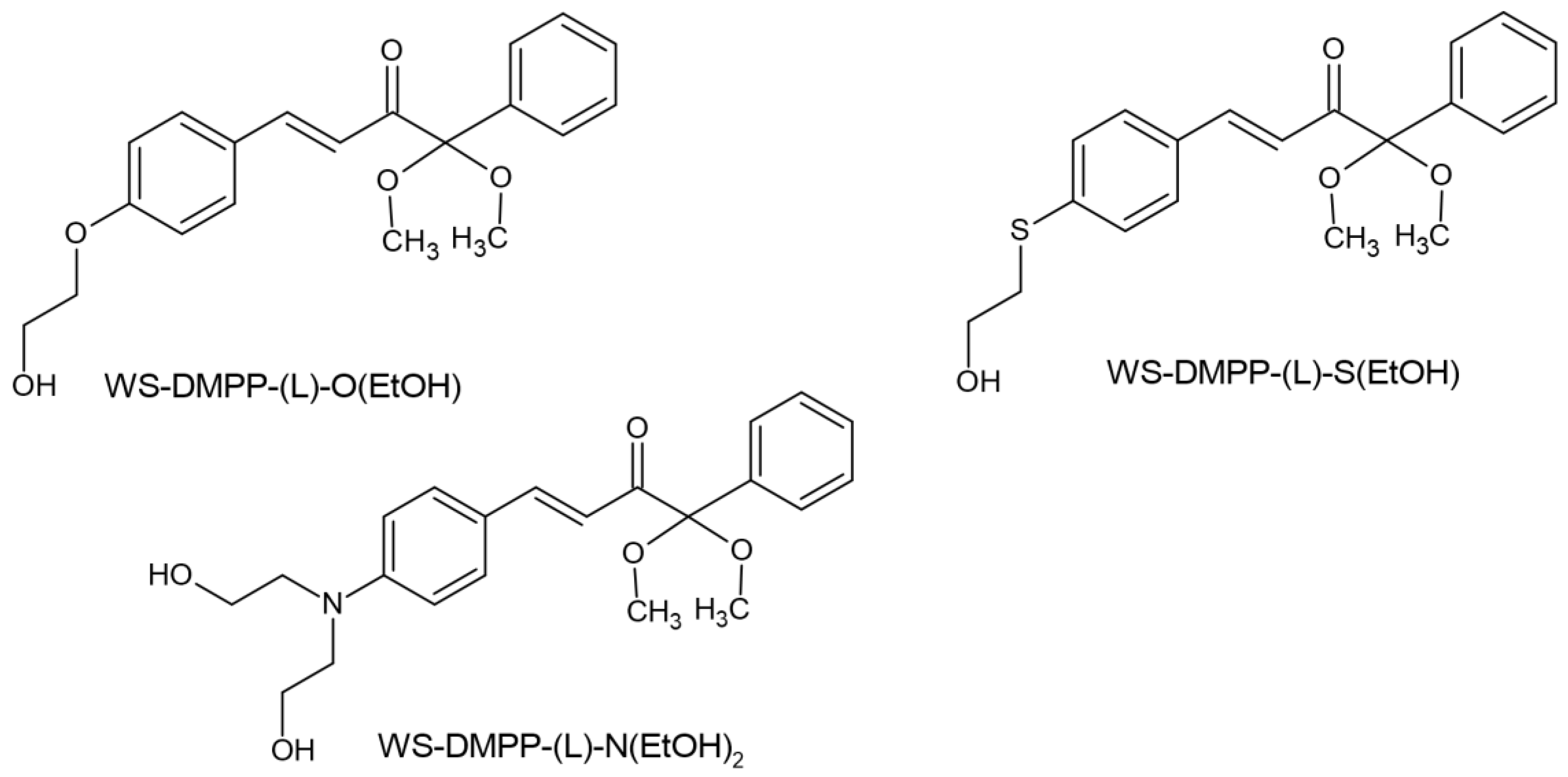
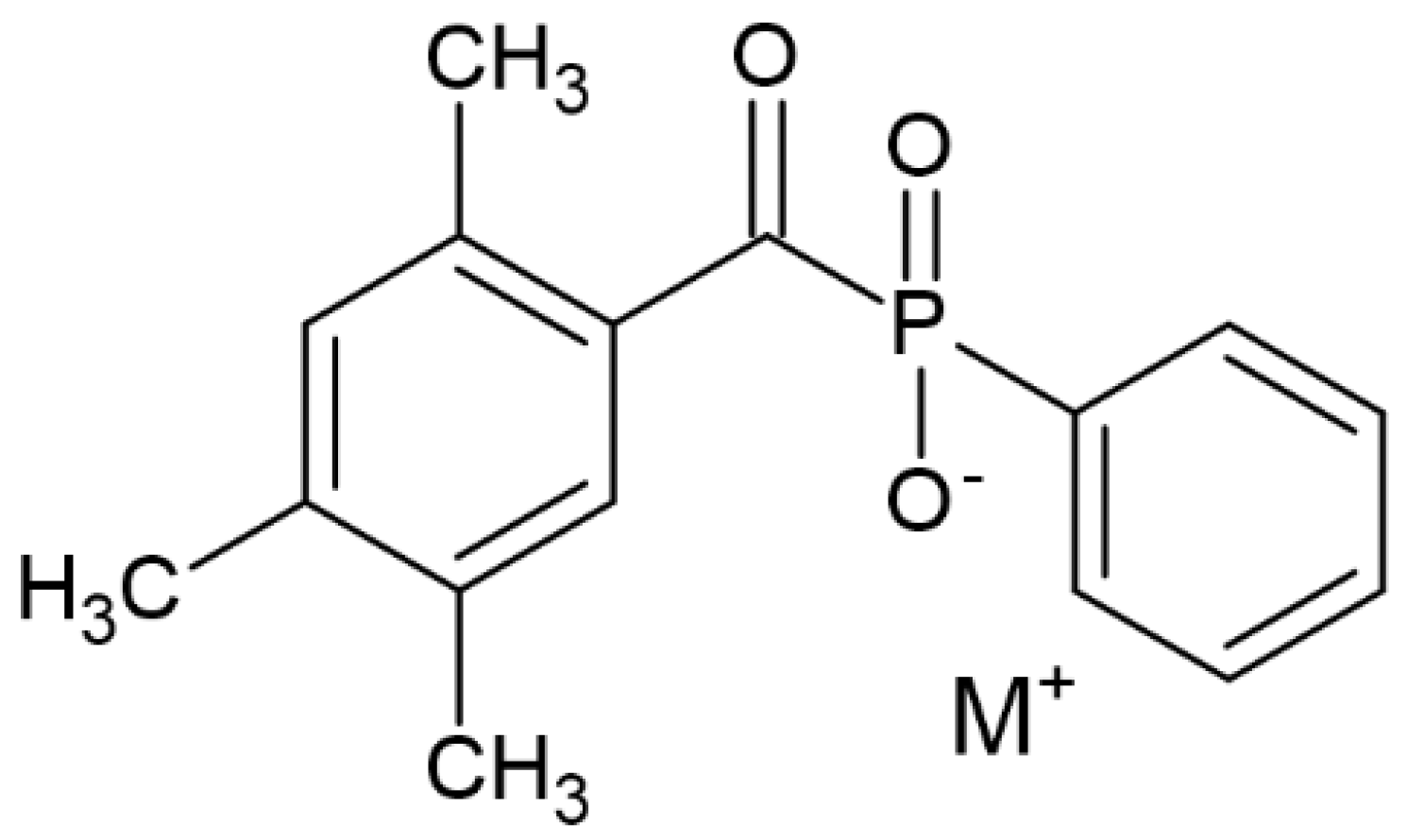
| Parameter | Thermal Polymerization | Photopolymerization |
|---|---|---|
| Chemical resistance | × | √ |
| Variety in formulation | √ | ± |
| No substrate damage | ± | √ |
| Low curing temperature | × | ± |
| Operational cost | × | √ |
| Formulation cost | √ | × |
| Capital cost | × | √ |
| Cure rate | × | √ |
| Skill level required | √ | ± |
| Non-solvent-releasing | √ | ± |
| Energy consumption | × | √ |
| Radiation hazard | × | √ |
| Fire hazard | × | √ |
| CQ Concentration (wt.%) | Max. DC (%) | Max. DC Rate (%s−1) |
|---|---|---|
| 0.1 | 54.177 | 5.081 |
| 0.2 | 62.912 | 10.464 |
| 0.5 | 69.577 | 15.753 |
| 1.0 | 71.825 | 16.258 |
| 2.0 | 75.450 | 12.643 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzwonkowska-Zarzycka, M.; Sionkowska, A. Photoinitiators for Medical Applications—The Latest Advances. Molecules 2024, 29, 3898. https://doi.org/10.3390/molecules29163898
Dzwonkowska-Zarzycka M, Sionkowska A. Photoinitiators for Medical Applications—The Latest Advances. Molecules. 2024; 29(16):3898. https://doi.org/10.3390/molecules29163898
Chicago/Turabian StyleDzwonkowska-Zarzycka, Monika, and Alina Sionkowska. 2024. "Photoinitiators for Medical Applications—The Latest Advances" Molecules 29, no. 16: 3898. https://doi.org/10.3390/molecules29163898
APA StyleDzwonkowska-Zarzycka, M., & Sionkowska, A. (2024). Photoinitiators for Medical Applications—The Latest Advances. Molecules, 29(16), 3898. https://doi.org/10.3390/molecules29163898