Application of Photoactive Compounds in Cancer Theranostics: Review on Recent Trends from Photoactive Chemistry to Artificial Intelligence
Abstract
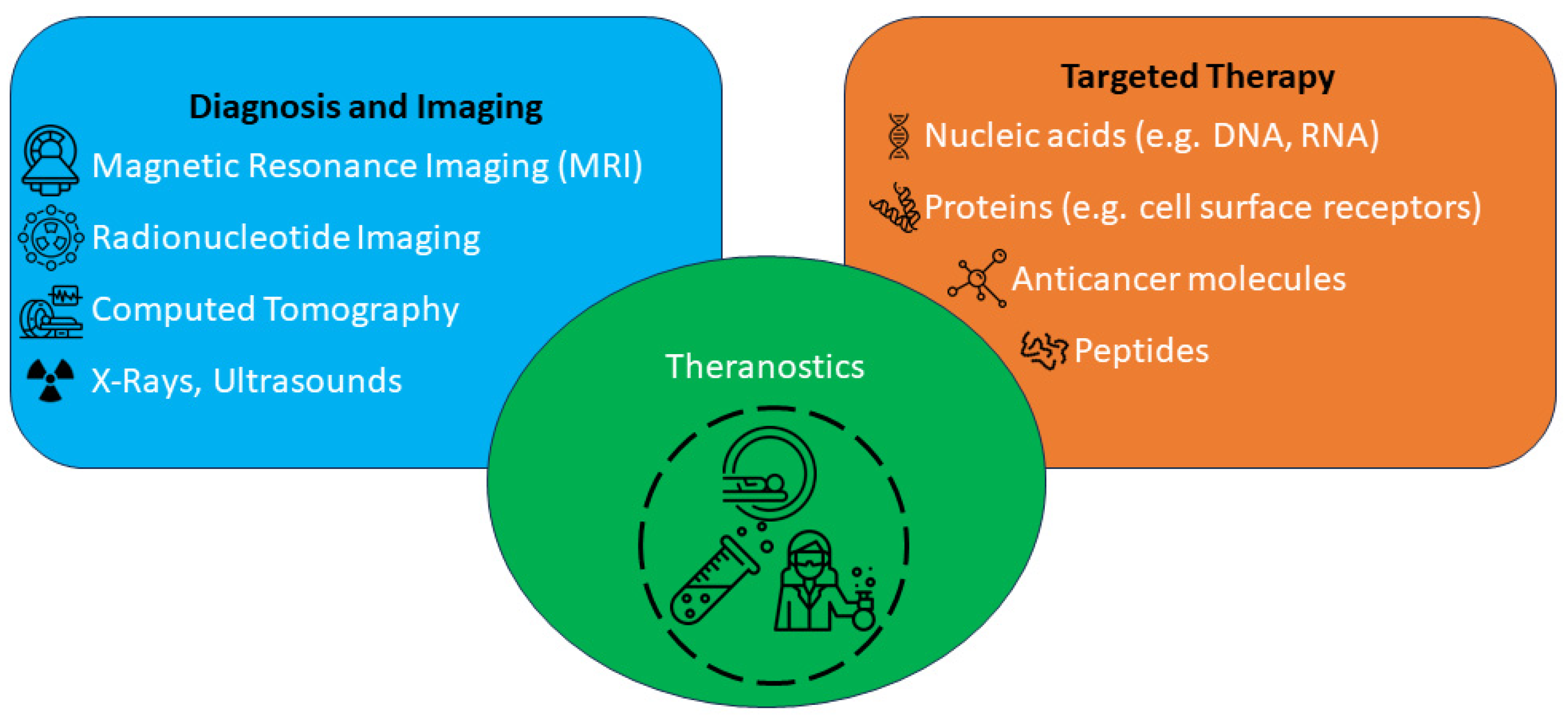
1. Introduction
2. Design of Organic Small Molecule Photosensitizer-Based Theranostic Drugs
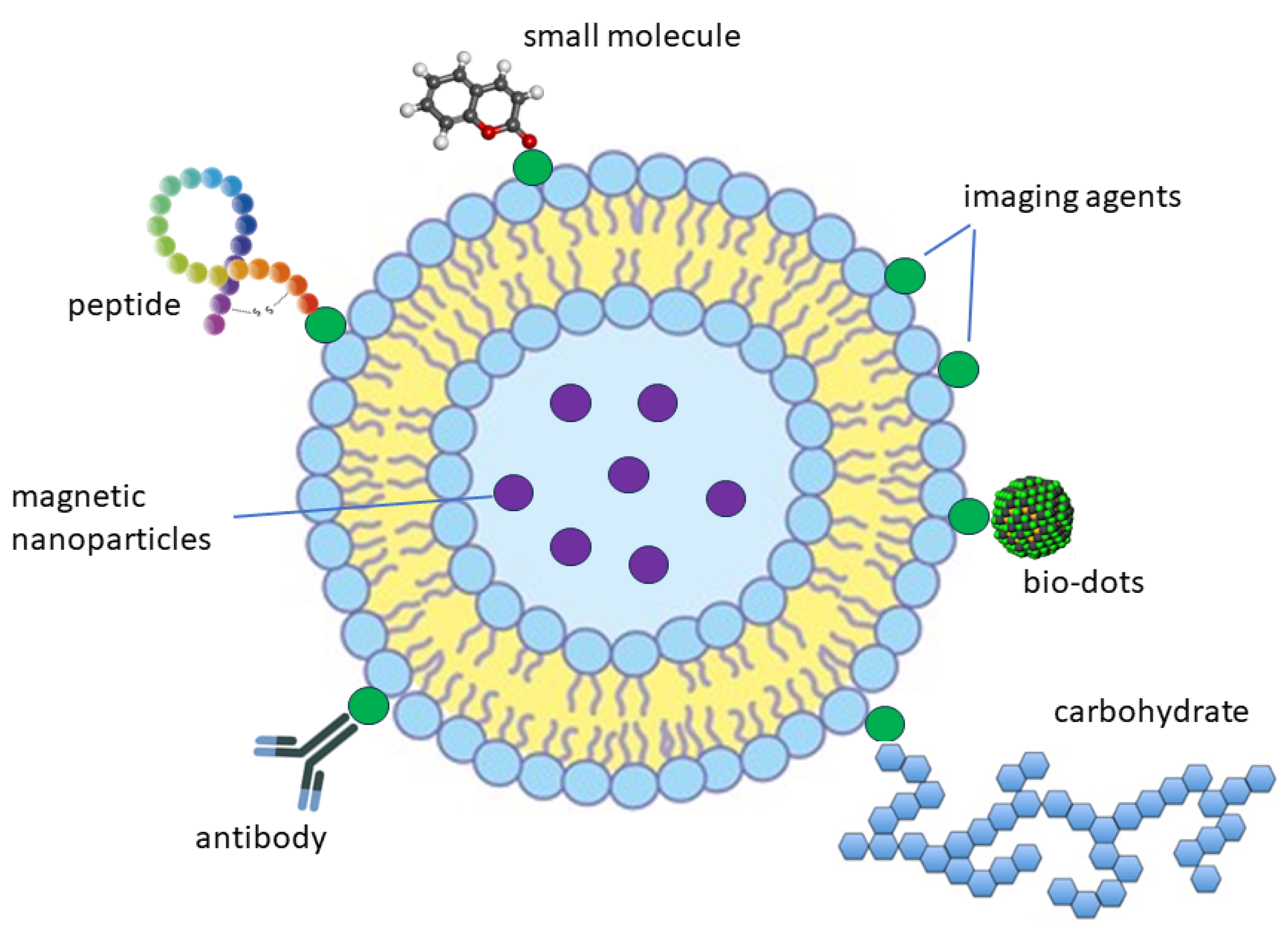
3. Design of Theranostic Agents Using Photosensitisers and Tumour-Targeted Units
4. Application of Photosensitisers in Theranostic Agents
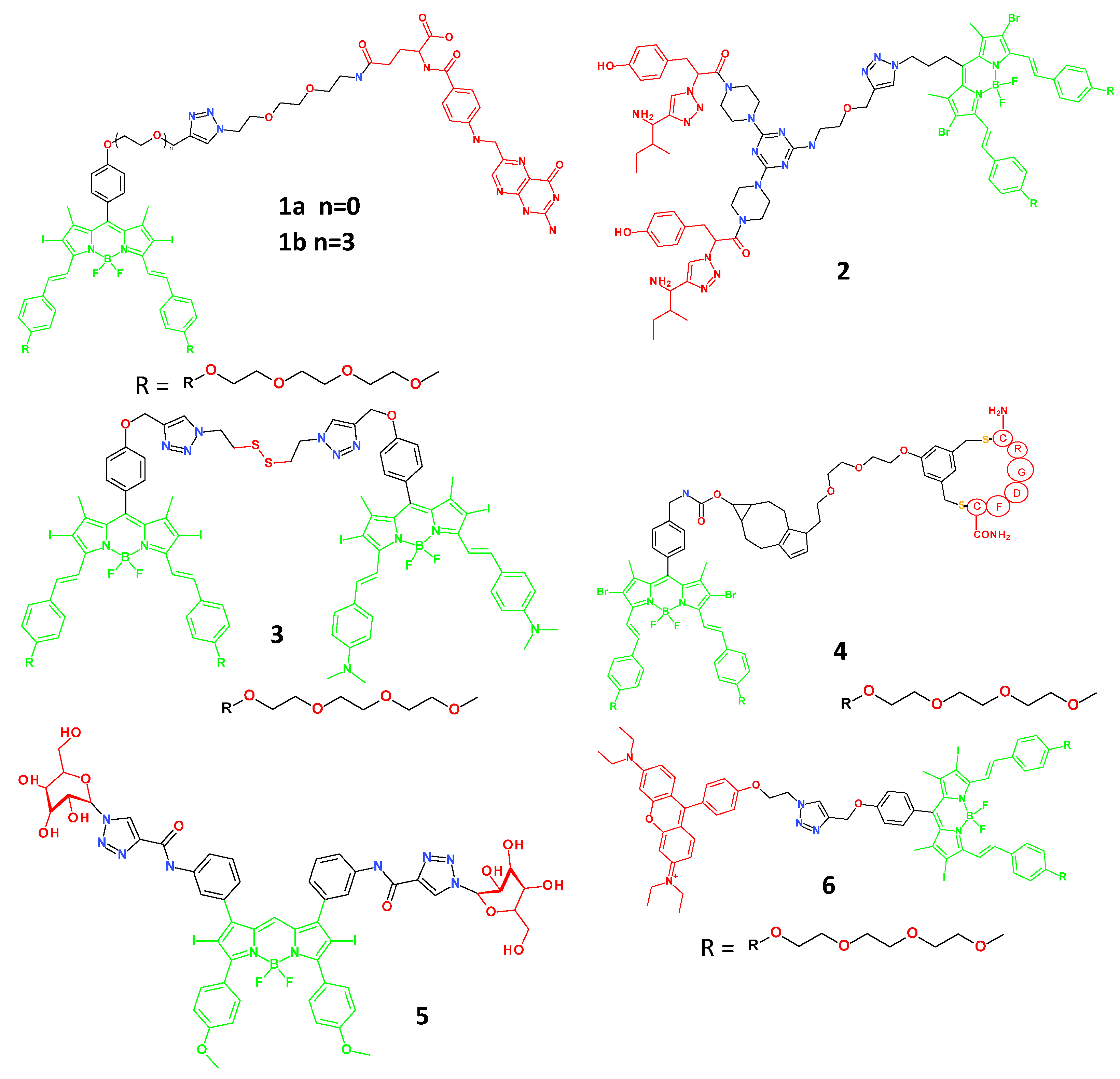
4.1. BODIPY-Based Small Molecule Theranostic Agents
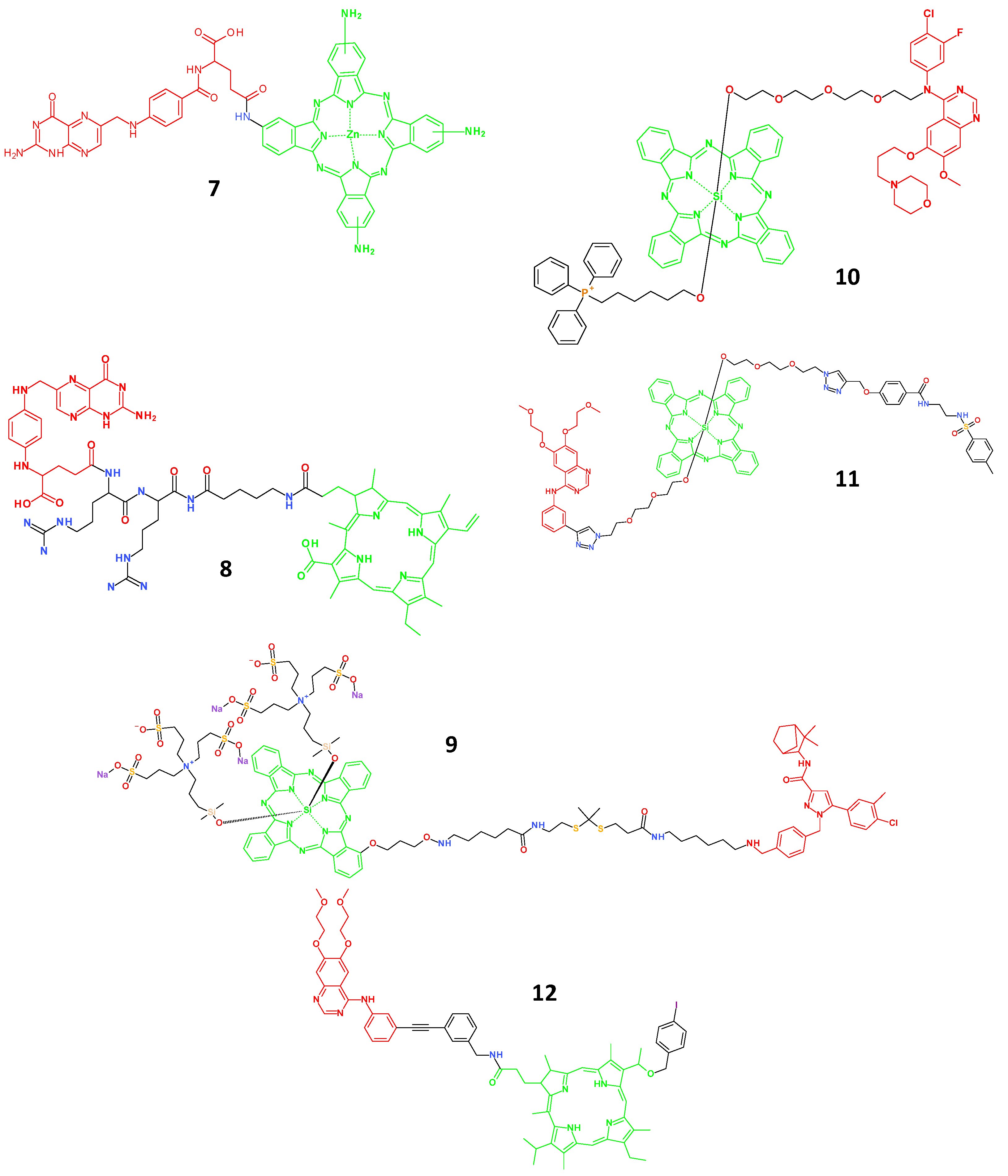
4.2. Porphyrin-Based Small Molecule Theranostic Agents
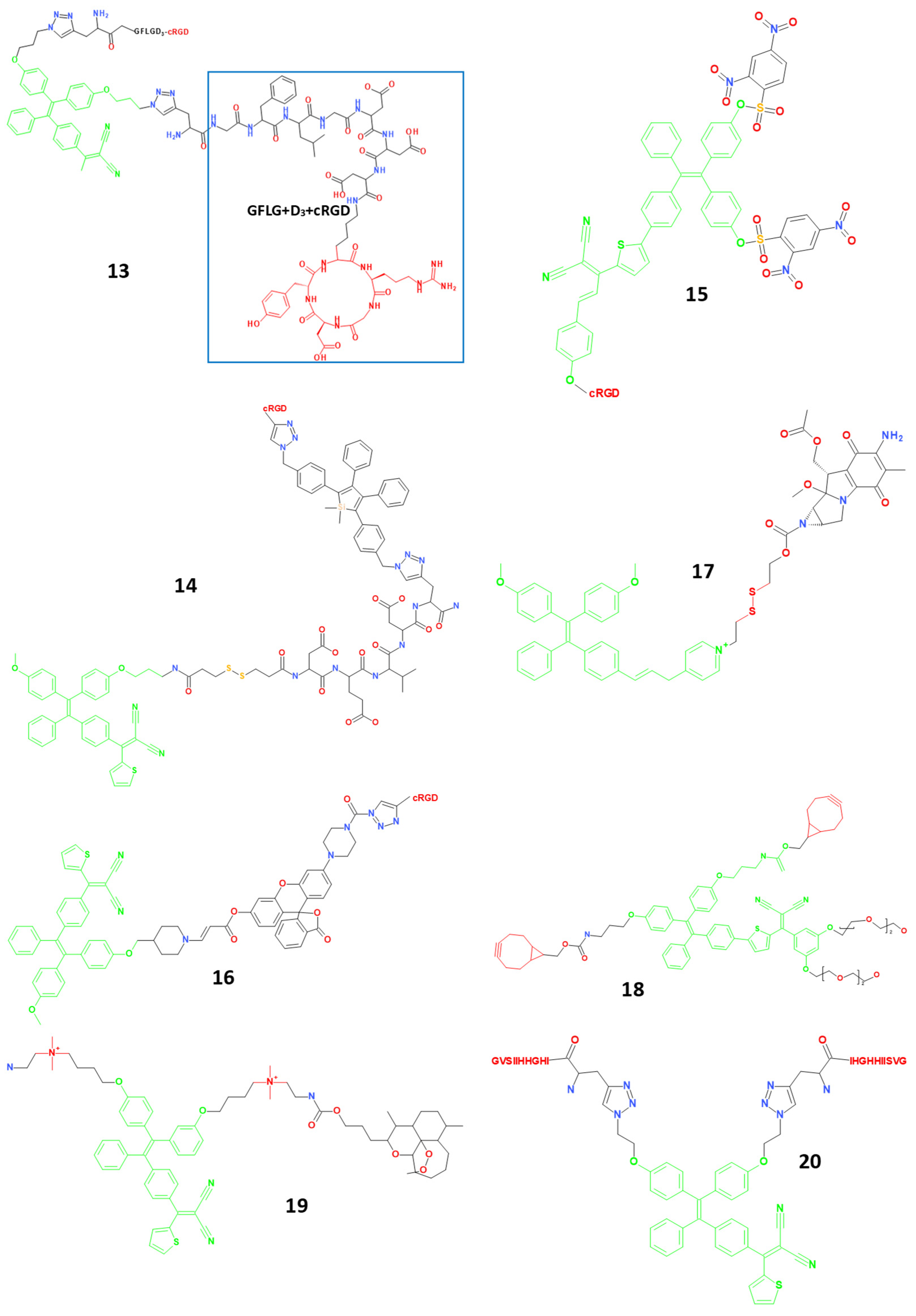
4.3. Small Molecule Theranostic Agents Based on Aggregation-Induced Emission (AIE)
4.4. Theranostic Agents Responding to ROS or H2S
4.5. Application of Complexes (III) in PDT
4.6. Quantum Dots as Theranostic Agents
4.7. Radioisotopes
4.8. Liposomes
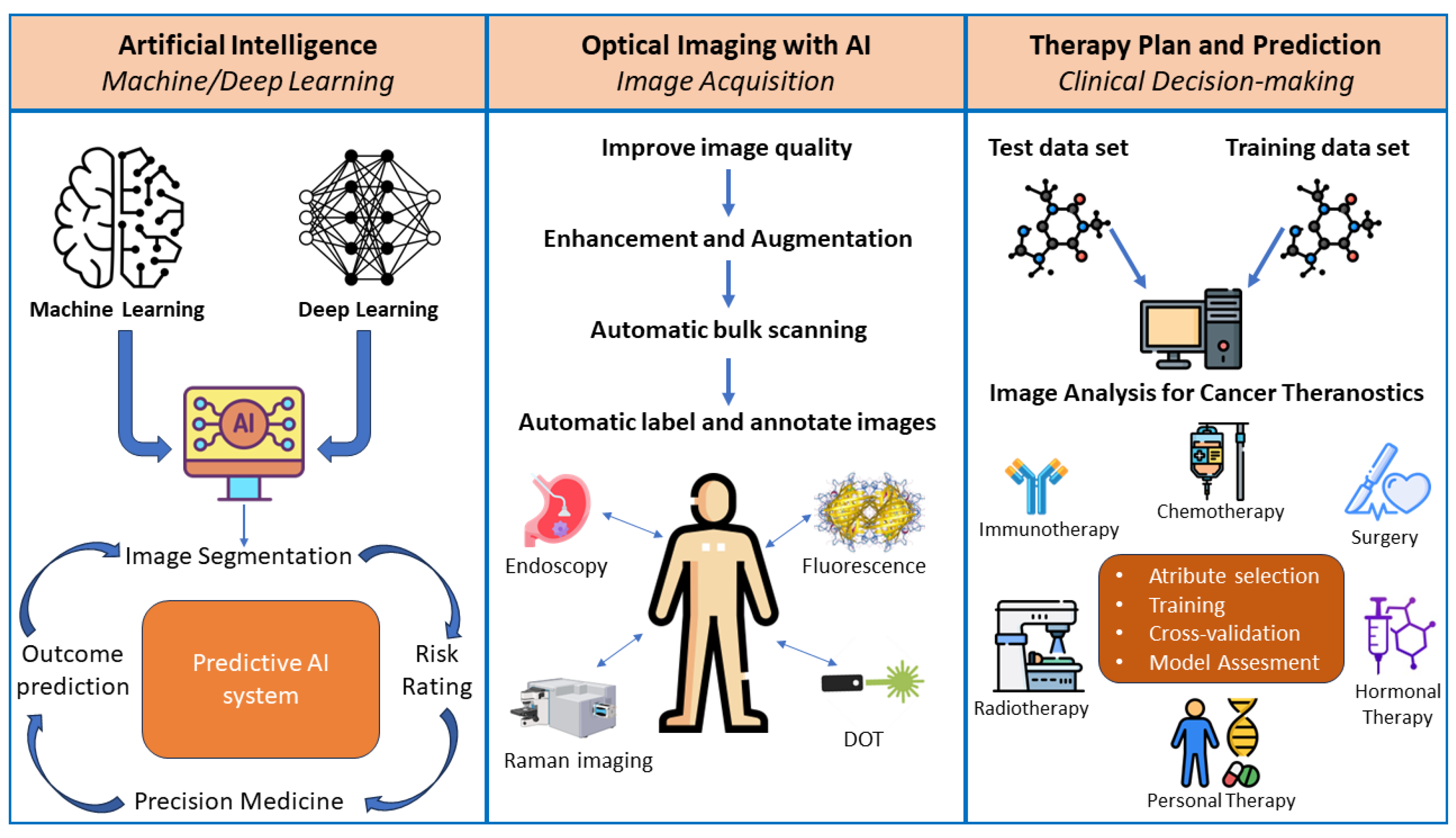
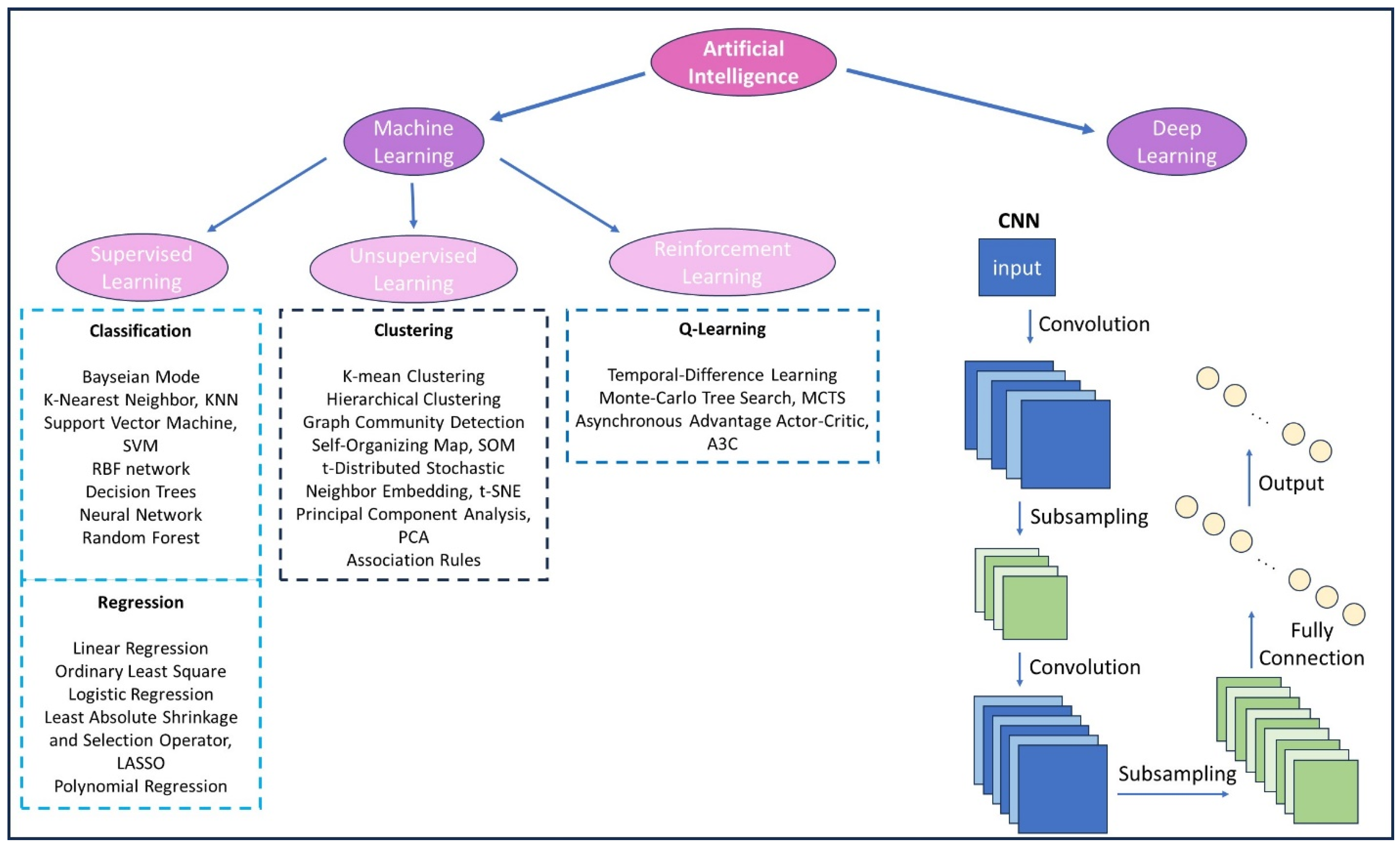
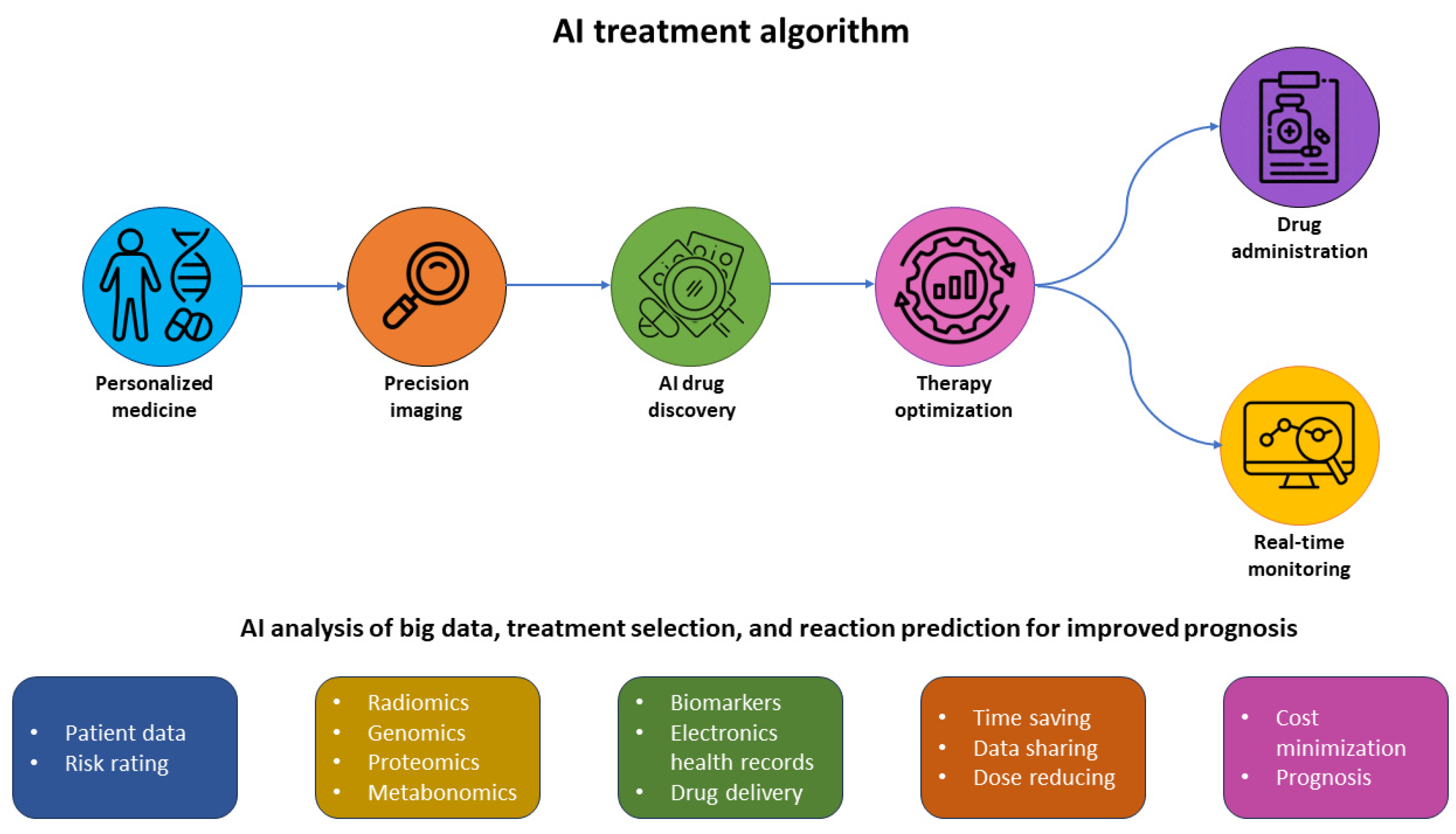
5. Artificial Intelligence for Cancer Theranostics
6. Artificial Intelligence in the Pathology of Cancer
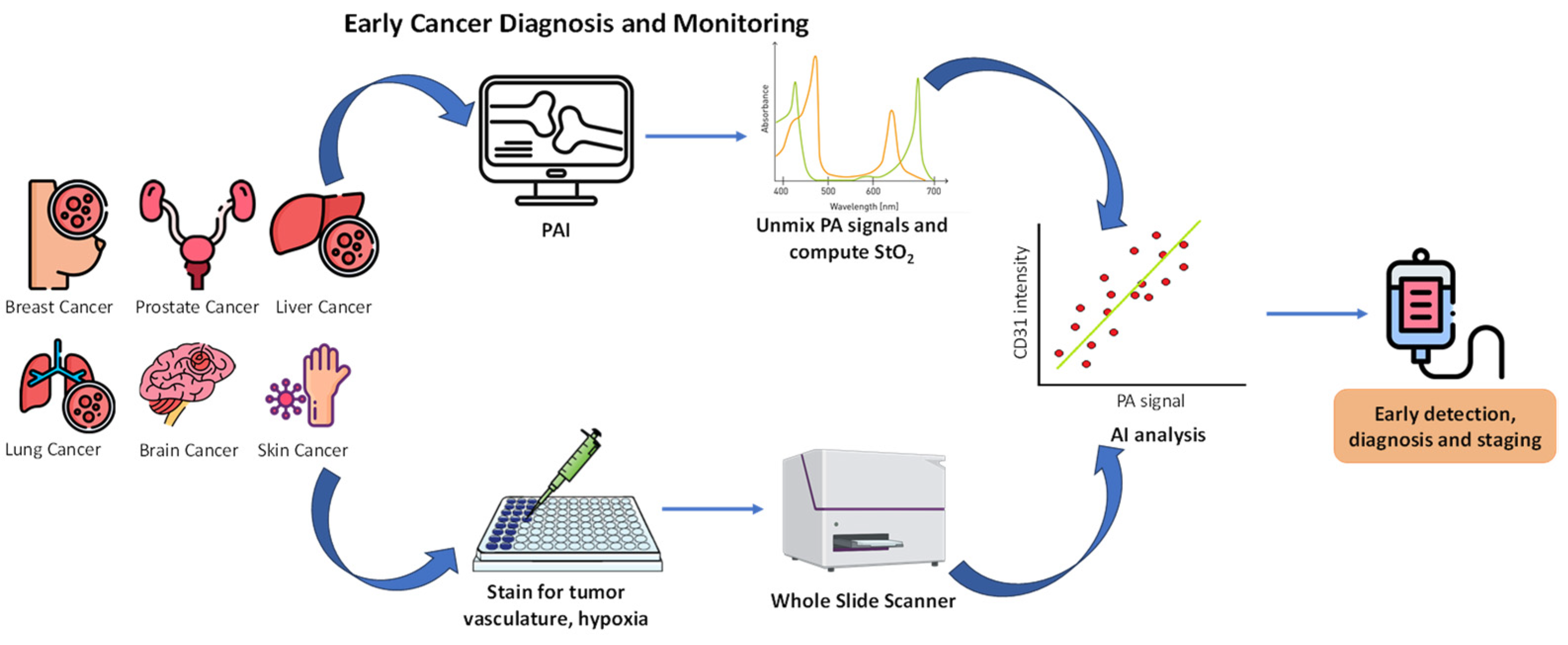
7. Artificial Intelligence for the Detection and Monitoring of Cancer
8. Artificial Intelligence for the Diagnosis and Treatment of Cancer
9. Clinical Trials for Theranostic Agents
10. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Al-Azri, M.H. Delay in cancer diagnosis: Causes and possible solutions. Oman. Med. J. 2016, 31, 325. [Google Scholar] [CrossRef] [PubMed]
- Shyamala, K.; Girish, H.; Murgod, S. Risk of tumour cell seeding through biopsy and aspiration cytology. J. Int. Soc. Prev. Community Dent. 2014, 4, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, A. History of Cancer, Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009, 1, 1. [Google Scholar] [CrossRef]
- Kang, S.J.; Jeong, H.Y.; Kim, M.W.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Park, Y.S. Anti-EGFR lipid micellar nanoparticles co-encapsulating quantum dots and paclitaxel for tumour-targeted theranosis. Nanoscale 2018, 10, 19338–19350. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Basu, S.; Lemke, H.D.; Jankowski, J.; Kratz, K.; Lendlein, A.; Tetali, S.D. Effect of extracts of poly(ether imide) microparticles on cytotoxicity, ROS generation and pro-inflammatory effects on human monocytic (THP-1) cells. Clin. Hemorheol. Microcirc. 2016, 61, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, J.; Tarighatnia, A.; Barar, J.; Aghanejad, A.; Davaran, S. Recent advances and trends in nanoparticles-based photothermal and photodynamic therapy. Photodiagn. Photodyn. Ther. 2022, 37, 102697. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, H.; Chen, P.; Zhang, R.; Liu, N. Photosensitizer-based small molecule theranostic agents for tumour-targeted monitoring and phototherapy. Bioorg. Chem. 2023, 136, 106554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yee, D.; Wang, C. Quantum dots for cancer diagnosis and therapy: Biological and clinical perspectives. Nanomedicine 2008, 3, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Adil, M.; Khan, R.; Dhadi, S.; Ahmad, K.; Rabbani, G.; Bashir, T.; Imran, M.A.; Husain, F.M.; Lee, E.J. Enzyme targeting strategies for prevention and treatment of cancer: Implications for cancer therapy. Semin. Cancer Biol. 2019, 56, 1–11. [Google Scholar] [CrossRef]
- Sani, S.; Messe, M.; Fuchs, Q.; Pierrevelcin, M.; Laquerriere, P.; Entz-Werle, N.; Reita, D.; Etienne-Selloum, N.; Bruban, V.; Choulier, L. Biological Relevance of RGD-Integrin Subtype-Specific Ligands in Cancer. ChemBioChem 2021, 22, 1151–1160. [Google Scholar] [CrossRef]
- Cheng, T.M.; Chang, W.J.; Chu, H.Y.; Luca, R.; Pedersen, J.Z.; Incerpi, S.; Li, Z.L.; Shih, Y.J.; Lin, H.Y.; Wang, K.; et al. Nano-strategies targeting the integrin αvβ3 network for cancer therapy. Cells 2021, 10, 1684. [Google Scholar] [CrossRef]
- Petko, F.; Świeży, A.; Ortyl, J. Photoinitiating systems and kinetics of frontal photopolymerization processes-the prospects for efficient preparation of composites and thick 3D structures. Polym. Chem. 2021, 12, 4593–4612. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers, Part II: Determination of relative quantum efficiency of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test 2018, 67, 144–150. [Google Scholar] [CrossRef]
- Ortyl, J.; Galica, M.; Popielarz, R.; Bogdał, D. Application of a carbazole derivative as a spectroscopic fluorescent probe for real time monitoring of cationic photopolymerization. Pol. J. Chem. Technol. 2014, 16, 75–80. [Google Scholar] [CrossRef]
- Tomal, W.; Chachaj-Brekiesz, A.; Popielarz, R.; Ortyl, J. Multifunctional biphenyl derivatives as photosensitisers in various types of photopolymerization processes, including IPN formation, 3D printing of photocurable multiwalled carbon nanotubes (MWCNTs) fluorescent composites. RSC Adv. 2020, 10, 32162–32182. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 20, 1–20. [Google Scholar] [CrossRef]
- Ke, M.R.; Yeung, S.L.; Ng, D.K.P.; Fong, W.P.; Lo, P.C. Preparation and in vitro photodynamic activities of folate-conjugated distyryl boron dipyrromethene based photosensitizers. J. Med. Chem. 2013, 56, 8475–8483. [Google Scholar] [CrossRef] [PubMed]
- Tomal, W.; Świergosz, T.; Pilch, M.; Kasprzyk, W.; Ortyl, J. New horizons for carbon dots: Quantum nano-photoinitiating catalysts for cationic photopolymerization and three-dimensional (3D) printing under visible light. Polym. Chem. 2021, 12, 3661–3676. [Google Scholar] [CrossRef]
- Petko, F.; Galek, M.; Hola, E.; Topa-Skwarczyńska, M.; Tomal, W.; Jankowska, M.; Pilch, M.; Popielarz, R.; Graff, B.; Morlet-Savary, F.; et al. Symmetric Iodonium Salts Based on Benzylidene as One-Component Photoinitiators for Applications in 3D Printing. Chem. Mater. 2022, 34, 10077–10092. [Google Scholar] [CrossRef]
- Topa-Skwarczyńska, M.; Ortyl, J. Photopolymerization shrinkage: Strategies for reduction, measurement methods and future insights. Polym. Chem. 2023, 14, 2145–2158. [Google Scholar] [CrossRef]
- Kamkaew, A.; Burgess, K. Double-Targeting using a Trkc ligand conjugated to dipyrrometheneboron difluoride (BODIPY) based photodynamic therapy (PDT) agent. J. Med. Chem. 2013, 56, 7608–7614. [Google Scholar] [CrossRef]
- Ko, E.; Kamkaew, A.; Burgess, K. Small molecule ligands for active targeting of TrkC-expressing tumour cells. ACS Med. Chem. Lett. 2012, 3, 1008–1012. [Google Scholar] [CrossRef]
- Cao, J.J.; Zhang, M.S.; Li, X.Q.; Yang, D.C.; Xu, G.; Liu, J.Y. A glutathione-responsive photosensitizer with fluorescence resonance energy transfer characteristics for imaging-guided targeting photodynamic therapy. Eur. J. Med. Chem. 2020, 193, 112203. [Google Scholar] [CrossRef]
- Chu, J.C.H.; Yang, C.; Fong, W.P.; Wong, C.T.T.; Ng, D.K.P. Facile one-pot synthesis of cyclic peptide-conjugated photosensitisers for targeted photodynamic therapy. Chem. Commun. 2020, 56, 11941–11944. [Google Scholar] [CrossRef]
- Ortyl, J.; Milart, P.; Popielarz, R. Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polym. Test 2013, 32, 708–715. [Google Scholar] [CrossRef]
- Treekoon, J.; Pewklang, T.; Chansaenpak, K.; Gorantla, J.N.; Pengthaisong, S.; Lai, R.Y.; Ketudat-Cairns, J.; Kamkaew, A. Glucose conjugated aza-BODIPY for enhanced photodynamic cancer therapy. Org. Biomol. Chem. 2021, 19, 5867–5875. [Google Scholar] [CrossRef]
- Li, M.; Long, S.; Kang, Y.; Guo, L.; Wang, J.; Fan, J.; Du, J.; Peng, X. De Novo Design of Phototheranostic Sensitizers Based on Structure-Inherent Targeting for Enhanced Cancer Ablation. J. Am. Chem. Soc. 2018, 140, 15820–15826. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Wang, J.; Chen, J.Y. Conjugates of folic acids with zinc aminophthalocyanine for cancer cell targeting and photodynamic therapy by one-photon and two-photon excitations. J. Mater. Chem. B 2014, 2, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tung, C.H.; Choi, Y. Smart dual-functional warhead for folate receptor-specific activatable imaging and photodynamic therapy. Chem. Commun. 2014, 50, 10600–10603. [Google Scholar] [CrossRef]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Applicability of quinolizino-coumarins for monitoring free radical photopolymerization by fluorescence spectroscopy. Polym. Test 2015, 42, 99–107. [Google Scholar] [CrossRef]
- Hulkower, K.I.; Butler, C.C.; Linebaugh, B.E.; Kalus, J.L.; Keppler, D.; Giranda, V.L.; Sloane, B.F. Fluorescent microplate assay for cancer cell-associated cathepsin b. Eur. J. Biochem. 2000, 267, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Light-activatable cannabinoid prodrug for combined and target-specific photodynamic and cannabinoid therapy. J. Biomed. Opt. 2018, 23, 108001. [CrossRef]
- Zhao, X.; Ma, H.; Chen, J.; Zhang, F.; Jia, X.; Xue, J. An epidermal growth factor receptor-targeted and endoplasmic reticulum-localized organic photosensitizer toward photodynamic anticancer therapy. Eur. J. Med. Chem. 2019, 182, 111625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, Y.; Yuan, G.; Zou, K.; Huang, Y.; Chen, J.; Li, J.; Xue, J. A novel tumour and mitochondria dual-targeted photosensitizer showing ultra-efficient photodynamic anticancer activities. Chem. Commun. 2019, 55, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Cheruku, R.R.; Cacaccio, J.; Durrani, F.A.; Tabaczyński, W.A.; Watson, R.; Marko, A.; Kumar, R.; El-Khouly, M.E.; Fukuzumi, S.; Missert, J.R.; et al. Epidermal Growth Factor Receptor-Targeted Multifunctional Photosensitizers for Bladder Cancer Imaging and Photodynamic Therapy. J. Med. Chem. 2019, 62, 2598–2617. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polym. Test 2016, 55, 310–317. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, C.J.; Gao, M.; Zhang, R.; Tang, B.Z.; Liu, B. Specific light-up bioprobe with aggregation-induced emission and activatable photoactivity for the targeted and image-guided photodynamic ablation of cancer cells. Angew. Chem. Int. Ed. 2015, 54, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, C.J.; Kwok, R.T.K.; Xu, S.; Zhang, R.; Wu, J.; Tang, B.Z.; Liu, B. Light-Up Probe for Targeted and Activatable Photodynamic Therapy with Real-Time in Situ Reporting of Sensitizer Activation and Therapeutic Responses. Adv. Funct. Mater. 2015, 25, 6586–6595. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, S.; Zhang, C.J.; Zhang, R.; Liu, B. Dual-targeted activatable photosensitizers with aggregation-induced emission (AIE) characteristics for image-guided photodynamic cancer cell ablation. J. Mater. Chem. B 2016, 4, 169–176. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, C.J.; Xu, S.; Liu, B. A self-reporting AIE probe with a built-in singlet oxygen sensor for targeted photodynamic ablation of cancer cells. Chem. Sci. 2016, 7, 1862–1866. [Google Scholar] [CrossRef]
- Hu, F.; Yuan, Y.; Mao, D.; Wu, W.; Liu, B. Smart activatable and traceable dual-prodrug for image-guided combination photodynamic and chemo-therapy. Biomaterials 2017, 144, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Mao, D.; Kenry; Cai, X.; Wu, W.; Kong, D.; Liu, B. A Light-Up Probe with Aggregation-Induced Emission for Real-Time Bio-orthogonal Tumour Labeling and Image-Guided Photodynamic Therapy. Angew. Chem. 2018, 130, 10339–10343. [Google Scholar] [CrossRef]
- Feng, G.; Liu, J.; Zhang, C.J.; Liu, B. Artemisinin and AIEgen Conjugate for Mitochondria-Targeted and Image-Guided Chemo- and Photodynamic Cancer Cell Ablation. ACS Appl. Mater. Interfaces 2018, 10, 11546–11553. [Google Scholar] [CrossRef]
- Ortyl, J.; Fiedor, P.; Chachaj-Brekiesz, A.; Pilch, M.; Hola, E.; Galek, M. The applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile sensors for monitoring different types of photopolymerization processes and acceleration of cationic and free-radical photopolymerization under near UV light. Sensors 2019, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Czech, Z.; Kowalczyk, A.; Ortyl, J.; Swiderska, J. Acrylic pressure-sensitive adhesives containing SiO2 nanoparticles. Pol. J. Chem. Technol. 2013, 15, 12–14. [Google Scholar] [CrossRef]
- Hola, E.; Pilch, M.; Ortyl, J. Thioxanthone derivatives as a new class of organic photocatalysts for photopolymerisation processes and the 3D printing of photocurable resins under visible light. Catalysts 2020, 10, 903. [Google Scholar] [CrossRef]
- Petko, F.; Galek, M.; Hola, E.; Popielarz, R.; Ortyl, J. One-Component Cationic Photoinitiators from Tunable Benzylidene Scaffolds for 3D Printing Applications. Macromolecules 2021, 54, 7070–7087. [Google Scholar] [CrossRef]
- Hola, E.; Ortyl, J. Pyrylium salt as a visible-light-induced photoredox catalyst for polymer and organic synthesis—Perspectives on catalyst design and performance. Eur. Polym. J. 2021, 150, 110365. [Google Scholar] [CrossRef]
- Hu, F.; Huang, Y.; Zhang, G.; Zhao, R.; Yang, H.; Zhang, D. Targeted bioimaging and photodynamic therapy of cancer cells with an activatable red fluorescent bioprobe. Anal. Chem. 2014, 86, 7987–7995. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J.; Chachaj-Brekiesz, A.; Kamińska-Borek, I.; Pilch, M.; Popielarz, R. Applicability of samarium(III) complexes for the role of luminescent molecular sensors for monitoring progress of photopolymerization processes and control of the thickness of polymer coatings. Spectrochim Acta. A Mol. Biomol. Spectrosc. 2018, 199, 430–440. [Google Scholar] [CrossRef]
- Ji, S.; Gao, H.; Mu, W.; Ni, X.; Yi, X.; Shen, J.; Liu, Q.; Bao, P.; Ding, D. Enzyme-instructed self-assembly leads to the activation of optical properties for selective fluorescence detection and photodynamic ablation of cancer cells. J. Mater. Chem. B 2018, 6, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Ortyl, J.; Topa, M.; Kamińska-Borek, I.; Popielarz, R. Mechanism of interaction of aminocoumarins with reaction medium during cationic photopolymerization of triethylene glycol divinyl ether. Eur. Polym. J. 2019, 116, 45–55. [Google Scholar] [CrossRef]
- Tomal, W.; Krok, D.; Chachaj-Brekiesz, A.; Lepcio, P.; Ortyl, J. Harnessing light to create functional, three-dimensional polymeric materials: Multitasking initiation systems as the critical key to success. Addit. Manuf. 2021, 48, 102447. [Google Scholar] [CrossRef]
- Kim, E.J.; Bhuniya, S.; Lee, H.; Kim, H.M.; Cheong, C.; Maiti, S.; Hong, K.S.; Kim, J.S. An activatable prodrug for the treatment of metastatic tumours. J. Am. Chem. Soc. 2014, 136, 13888–13894. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, G.; Bagheri, M.; Saini, D.K.; Chakrapani, H. A small molecule for theraNOstic targeting of cancer cells. Chem. Commun. 2017, 53, 13352–13355. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Hu, X.X.; Li, K.; Liu, Y.; Rong, Q.; Zhu, L.; Yuan, L.; Qu, F.L.; Zhang, Z.B.; Tan, W. A mitochondrial-targeted prodrug for NIR imaging guided and synergetic NIR photodynamic-chemo cancer therapy. Chem. Sci. 2017, 8, 7689–7695. [Google Scholar] [CrossRef] [PubMed]
- Bobba, K.N.; Saranya, G.; Sujai, P.T.; Manu, M.J.; Velusamy, N.; Podder, A.; Maiti, K.K.; Bhuniya, S. Endogenous H2S-Assisted Cancer-Cell-Specific Activation of Theranostics with Emission Readout. ACS Appl. Bio Mater. 2019, 2, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Badrigilan, S.; Shaabani, B.; Gharehaghaji, N.; Mesbahi, A. Iron oxide/bismuth oxide nanocomposites coated by graphene quantum dots: “Three-in-one” theranostic agents for simultaneous CT/MR imaging-guided in vitro photothermal therapy. Photodiagn. Photodyn. Ther. 2019, 25, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Chen, G.; Li, Y.; Xu, W.; Gong, S. Quantum-Dot-Based Theranostic Micelles Conjugated with an Anti-EGFR Nanobody for Triple-Negative Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 30297–30305. [Google Scholar] [CrossRef]
- Bansal, S.; Singh, J.; Kumari, U.; Kaur, I.P.; Barnwal, R.P.; Kumar, R.; Singh, S.; Singh, G.; Chatterjee, M. Development of biosurfactant-based graphene quantum dot conjugate as a novel and fluorescent theranostic tool for cancer. Int. J. Nanomed. 2019, 14, 809–818. [Google Scholar] [CrossRef]
- Brunetti, J.; Riolo, G.; Gentile, M.; Bernini, A.; Paccagnini, E.; Falciani, C.; Lozzi, L.; Scali, S.; Depau, L.; Pini, A.; et al. Near-infrared quantum dots labelled with a tumour selective tetrabranched peptide for in vivo imaging. J. Nanobiotechnol. 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Yip, G.W.; Smollich, M.; Götte, M. Therapeutic value of glycosaminoglycans in cancer. Mol. Cancer Ther. 2006, 5, 2139–2148. [Google Scholar] [CrossRef]
- Brunetti, J.; Pillozzi, S.; Falciani, C.; Depau, L.; Tenori, E.; Scali, S.; Lozzi, L.; Pini, A.; Arcangeli, A.; Menichetti, S.; et al. Tumour-selective peptide-carrier delivery of Paclitaxel increases in vivo activity of the drug. Sci. Rep. 2015, 5, 17736. [Google Scholar] [CrossRef]
- Brunetti, J.; Piantini, S.; Fragai, M.; Scali, S.; Cipriani, G.; Depau, L.; Pini, A.; Falciani, C.; Menichetti, C.; Bracci, L. A new NT4 peptide-based drug delivery system for cancer treatment. Molecules 2020, 25, 1088. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, X.; Zhao, D.; Hou, X.; Li, C.; Song, X.; Chen, W.; Wang, Q.; Zhao, Y.; Liu, B. An injectable hybrid hydrogel based on a genetically engineered polypeptide for second near-infrared fluorescence/photoacoustic imaging-monitored sustained chemo-photothermal therapy. Nanoscale 2019, 11, 16080–16091. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Arch. Med. Res. 2021, 52, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K.; Thakur, M.; Bahadur, R.; Kaku, T.; Prabhuraj, R.S.; Suchitta, A.; Srivastava, R. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater. Sci. Eng. C 2019, 103, 109774. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Ma, Y.; Shen, H.; Tu, X.; Zhou, X.; Xu, J.; Dai, J.; Fan, S.; Zhang, Z. The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 2014, 35, 5041–5048. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Hou, Y.; Yang, G.; Fei, X.; Zhao, H.; Guo, Y.; Su, C.; Wang, Z.; Zhong, H.; et al. Multifunctional Nanoplatform Based on Black Phosphorus Quantum Dots for Bioimaging and Photodynamic/Photothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 25098–25106. [Google Scholar] [CrossRef]
- Wu, C.; Guan, X.; Xu, J.; Zhang, Y.; Liu, Q.; Tian, Y.; Li, S.; Qin, X.; Yang, H.; Liu, Y. Highly efficient cascading synergy of cancer photo-immunotherapy enabled by engineered graphene quantum dots/photosensitizer/CpG oligonucleotides hybrid nanotheranostics. Biomaterials 2019, 205, 106–119. [Google Scholar] [CrossRef]
- McBean, R.; O’Kane, B.; Parsons, R.; Wong, D. Lu177-PSMA therapy for men with advanced prostate cancer: Initial 18 months experience at a single Australian tertiary institution. J. Med. Imaging Radiat. Oncol. 2019, 63, 538–545. [Google Scholar] [CrossRef]
- Müller, C.; Singh, A.; Umbricht, C.A.; Kulkarni, H.; Jhonston, K.; Benesova, M.; Senftleben, S.; Muller, D.; Vermeulen, C.; Schibli, R.; et al. Preclinical investigations and first-in-human application of 152Tb-PSMA-617 for PET/CT imaging of prostate cancer. EJNMMI Res. 2019, 9, 55. [Google Scholar] [CrossRef]
- Park, B.N.; Lee, S.J.; Roh, J.H.; Lee, K.H.; An, Y.S.A.; Yoon, J.K. Radiolabeled Anti-Adenosine Triphosphate Synthase Monoclonal Antibody as a Theragnostic Agent Targeting Angiogenesis. Mol. Imaging 2017, 16, 1536012117737399. [Google Scholar] [CrossRef] [PubMed]
- Even, A.J.G.; Hamming-Vrieze, O.; van Elmpt, W.; Winnepenninckx, V.J.L.; Heukelom, J.; Yesselaar, M.E.T.; Vogel, W.V.; Hoeben, A.; Zegers, C.M.L.; Vugts, D.; et al. Quantitative assessment of Zirconium-89 labeled cetuximab using PET/CT imaging in patients with advanced head and neck cancer: A theragnostic approach. Oncotarget 2017, 8, 3870–3880. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, H.; Palanisamy, S.; Subbiah, L. Theranostic Liposomes in Cancer: Current Status and Applications. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Šimečková, P.; Hubatka, F.; Kotouček, J.; Knötigová, P.T.; Mašek, J.; Slavík, J.; Kováč, O.; Neča, J.; Kulich, P.; Hrebík, D.; et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: In vitro safety study in liver cells and macrophages. Sci. Rep. 2020, 10, 4780. [Google Scholar] [CrossRef]
- Jha, A.; Viswanadh, M.K.; Burande, A.S.; Mehamat, A.K.; Poddar, S.; Yadav, K.; Mahto, S.K.; Parmar, A.S.; Muthu, M.S. DNA biodots based targeted theranostic nanomedicine for the imaging and treatment of non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 150, 413–425. [Google Scholar] [CrossRef]
- Wang, X.; Tong, J.; He, Z.; Yang, X.; Meng, F.; Liang, H.; Zhang, X.; Luo, L. Paclitaxel-Potentiated Photodynamic Theranostics for Synergistic Tumour Ablation and Precise Anticancer Efficacy Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 5476–5487. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Harmon, S.A.; Klyuzhin, I.S.; Rahmim, A.; Turkbey, B. Clinical Application of Artificial Intelligence in Positron Emission Tomography: Imaging of Prostate Cancer. PET Clin. 2022, 17, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical imaging modalities: Principles and applications in preclinical research and clinical settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Luker, G.D.; Luker, K.E. Optical imaging: Current applications and future directions. J. Nucl. Med. 2008, 49, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 11, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Li, D.; Ren, Y.; Qu, C.; Shi, X.; Liu, R.; Liu, H.; Tian, J.; Hu, Z.; Sun, T.; et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng. 2022, 6, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 2014, 5, 4206. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Zhu, S.; Cui, R.; Zhang, M. Noninvasive in vivo imaging in the second near-infrared window by inorganic nanoparticle-based fluorescent probes. Anal. Chem. 2019, 92, 535–542. [Google Scholar] [CrossRef]
- Huang, Y.T.; Tsai, Y.S.; Lin, P.C.; Yeh, Y.M.; Hsu, Y.T.; Wu, P.Y.; Shen, M.R. The Value of Artificial Intelligence-Assisted Imaging in Identifying Diagnostic Markers of Sarcopenia in Patients with Cancer. Dis. Markers 2022, 2022, 1819841. [Google Scholar] [CrossRef]
- Jiang, M.; Lei, S.; Zhang, J.; Hou, L.; Zhang, M.; Luo, Y. Multimodal Imaging of Target Detection Algorithm under Artificial Intelligence in the Diagnosis of Early Breast Cancer. J. Healthc. Eng. 2022, 2022, 9322937. [Google Scholar] [CrossRef]
- Kaneko, M.; Fukuda, N.; Nagano, H.; Yamada, K.; Yamada, K.; Konishi, E.; Sato, Y.; Ukimura, O. Artificial intelligence trained with integration of multiparametric MR-US imaging data and fusion biopsy trajectory-proven pathology data for 3D prediction of prostate cancer: A proof-of-concept study. Prostate 2022, 82, 793–803. [Google Scholar] [CrossRef]
- Kwan, J.M.; Oikonomou, E.K.; Henry, M.L.; Sinusas, A.J. Multimodality Advanced Cardiovascular and Molecular Imaging for Early Detection and Monitoring of Cancer Therapy-Associated Cardiotoxicity and the Role of Artificial Intelligence and Big Data. Front. Cardiovasc. Med. 2022, 9, 829553. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Laudicella, R.; Balma, M.; Nicolotti, D.G.; Buschiazzo, A.; Grimaldi, S.; Lorenzon, L.; Bianchi, A.; Peano, S.; Bartolotta, T.V.; et al. Radiomics and artificial intelligence in prostate cancer: New tools for molecular hybrid imaging and theragnostics. Eur. Radiol. Exp. 2022, 6, 27. [Google Scholar] [CrossRef]
- Qin, Y.; Deng, Y.; Jiang, H.; Hu, N.; Song, B. Artificial Intelligence in the Imaging of Gastric Cancer: Current Applications and Future Direction. Front. Oncol. 2021, 11, 631686. [Google Scholar] [CrossRef]
- Horvat, N.; Veeraraghavan, H.; Nahas, C.S.R.; Bates, D.D.B.; Ferreira, F.R.; Zheng, J.; Capanu, M.; Fuqua, J.L.; Fernandes, M.C.; Sosa, R.E.; et al. Combined artificial intelligence and radiologist model for predicting rectal cancer treatment response from magnetic resonance imaging: An external validation study. Abdom. Radiol. 2022, 47, 2770–2782. [Google Scholar] [CrossRef]
- Adamson, A.S.; Welch, H.G. Machine Learning and the Cancer-Diagnosis Problem—No Gold Standard. N. Engl. J. Med. 2019, 381, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed]
- Kehl, K.L.; Xu, W.; Gusev, A.; Bakouny, Z.; Choueiri, T.K.; Riaz, I.B.; Elmarakeby, H.; Allen, E.M.; Schrag, D. Artificial intelligence-aided clinical annotation of a large multi-cancer genomic dataset. Nat. Commun. 2021, 12, 7304. [Google Scholar] [CrossRef]
- Oren, O.; Gersh, B.J.; Bhatt, D.L. Artificial intelligence in medical imaging: Switching from radiographic pathological data to clinically meaningful endpoints. Lancet Digit. Health 2020, 2, e486–e488. [Google Scholar] [CrossRef]
- Varoquaux, G.; Cheplygina, V. Machine learning for medical imaging: Methodological failures and recommendations for the future. NPJ Digit. Med. 2022, 5, 48. [Google Scholar] [CrossRef]
- Gharavi, S.M.H.; Faghihimehr, A. Clinical Application of Artificial Intelligence in PET Imaging of Head and Neck Cancer. PET Clin. 2022, 17, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Combalia, M.; Codella, N.; Rotemberg, V.; Carrera, C.; Dusza, S.; Gutman, D.; Helba, B.; Kittler, H.; Kurtansky, N.R.; Liopyris, K.; et al. Validation of artificial intelligence prediction models for skin cancer diagnosis using dermoscopy images: The 2019 International Skin Imaging Collaboration Grand Challenge. Lancet. Digit. Health 2022, 4, e330–e339. [Google Scholar] [CrossRef] [PubMed]
- Corradini, D.; Brizi, L.; Gaudiano, C.; Bianchi, L.; Marcelli, E.; Golfieri, R.; Schiavina, R.; Testa, C.; Remondini, D. Challenges in the use of artificial intelligence for prostate cancer diagnosis from multiparametric imaging data. Cancers 2021, 13, 3944. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Chou, K.; Yeung, S.; Naik, N.; Madani, A.; Liu, Y.; Topol, E.; Dean, J.; Sccher, R. Deep learning-enabled medical computer vision. NPJ Digit. Med. 2021, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Ciompi, F.; Wolterink, J.M.; Vos, B.D.; Leiner, T.; Teuwen, J.; Isgum, I. State-of-the-Art Deep Learning in Cardiovascular Image Analysis. JACC Cardiovasc. Imaging 2019, 12, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Sounderajah, V.; Martin, G.; Ting, D.S.; Karthinkesalingam, A.; King, D.; Ashrafian, H.; Darzi, A. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. NPJ Digit. Med. 2021, 4, 65. [Google Scholar] [CrossRef]
- de Rooij, M.; van Poppel, H.; Barentsz, J.O. Risk Stratification and Artificial Intelligence in Early Magnetic Resonance Imaging–based Detection of Prostate Cancer. Eur. Urol. Focus 2022, 8, 1187–1191. [Google Scholar] [CrossRef]
- Enriquez, J.S.; Chu, Y.; Pudakalakatti, S.; Hsieh, K.L.; Salmon, D.; Dutta, P.; Millward, N.Z.; Lurie, E.; Millward, S.; McAllister, F. Hyperpolarized magnetic resonance and artificial intelligence: Frontiers of imaging in pancreatic cancer. JMIR Med. Inform. 2021, 9, e26601. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Wang, Y.; He, Y.; Liu, K.; Raghunathan, R.; Shen, S.S.; He, T.; Yu, X.; Danforth, R.; et al. Artificial intelligence-augmented, label-free molecular imaging method for tissue identification, cancer diagnosis, and cancer margin detection. Biomed. Opt. Express 2021, 12, 5559–5582. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Feldman, M.D.; Abels, E.; Ashfaq, R.; Beltaifa, S.; Cacciabeve, N.; Cathro, H.P.; Cheng, L.; Cooper, K.; Dickey, G.E.; et al. Whole Slide Imaging Versus Microscopy for Primary Diagnosis in Surgical Pathology: A Multicenter Blinded Randomized Noninferiority Study of 1992 Cases (Pivotal Study). Am. J. Surg. Pathol. 2018, 42, 39–52. [Google Scholar] [CrossRef]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet. Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.E.; Misawa, M.; Saito, Y.; Ikematsu, H.; Hotta, K.; Ohtsuka, K.; Urushibara, F.; Kataoka, S.; Ogawa, Y.; et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy a prospective study. Ann. Intern. Med. 2018, 169, 357. [Google Scholar] [CrossRef]
- Steiner, D.F.; Macdonald, R.; Liu, Y.; Truszkowski, P.; Hipp, J.D.; Hammage, C.; Thing, F.; Peng, L.; Stumpe, M.C. Impact of Deep Learning Assistance on the Histopathologic Review of Lymph Nodes for Metastatic Breast Cancer. Am. J. Surg. Pathol. 2018, 42, 1636–1646. [Google Scholar] [CrossRef]
- Niazi, M.K.K.; Parwani, A.V.; Gurcan, M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019, 20, e253–e261. [Google Scholar] [CrossRef]
- Acs, B.; Rantalainen, M.; Hartman, J. Artificial intelligence as the next step towards precision pathology. J. Intern. Med. 2020, 288, 62–81. [Google Scholar] [CrossRef]
- Saldanha, O.L.; Quirke, P.; West, N.P.; James, J.A.; Loughrey, M.B.; Grabsch, H.I.; Salto-Tellez, M.; Alwers, E.; Cifci, D.; Laleh, N.G.; et al. Swarm learning for decentralized artificial intelligence in cancer histopathology. Nat. Med. 2022, 28, 1232–1239. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—new tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Ardlie, K.G.; DeLuca, D.S.; Segrè, A.V.; Segre, A.V.; Sullivan, T.J.; Young, T.R.; Gelfand, E.T.; Trowbridge, C.A.; Maller, J.B.; Tukiainen, T.; et al. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Mobadersany, P.; Yousefi, S.; Amgad, M.; Gutman, D.A.; Barnholtz-Sloan, J.S.; Velázquez Vega, J.E.; Brat, D.J.; Cooper, L.A.D. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc. Natl. Acad. Sci. USA 2018, 115, E2970–E2979. [Google Scholar] [CrossRef]
- Liu, Y.; Kohlberger, T.; Norouzi, M.; Dahl, G.E.; Smith, J.L.; Mohtashamian, A.; Olson, N.; Peng, L.H.; Hipp, J.D.; Stumpe, M.C. Artificial intelligence–based breast cancer nodal metastasis detection insights into the black box for pathologists. Arch. Pathol. Lab. Med. 2019, 143, 859–868. [Google Scholar] [CrossRef]
- Goldenberg, S.L.; Nir, G.; Salcudean, S.E. A new era: Artificial intelligence and machine learning in prostate cancer. Nat. Rev. Urol. 2019, 16, 391–403. [Google Scholar] [CrossRef]
- Ström, P.; Kartasalo, K.; Olsson, H.; Solorzano, L.; Delahunt, B.; Berney, D.M.; Bostwick, D.; Evans, A.J.; Grignon, D.J.; Humphrey, P.A.; et al. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: A population-based, diagnostic study. Lancet. Oncol. 2020, 21, 222–232. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Fine, S.W.; Algaba, F.; Aron, M.; Baydar, D.E.; Beltran, A.L.; Brimo, F.; Cheville, J.C.; Colecchia, M.; et al. The 2019 genitourinary pathology society (GUPS) white paper on contemporary grading of prostate cancer. Arch. Pathol. Lab. Med. 2021, 145, 461–493. [Google Scholar] [CrossRef]
- Yu, G.; Sun, K.; Xu, C.; Shi, X.H.; Wu, C.; Xie, T.; Meng, R.Q.; Meng, X.H.; Wang, K.S.; Xiao, H.M.; et al. Accurate recognition of colorectal cancer with semi-supervised deep learning on pathological images. Nat. Commun. 2021, 12, 6311. [Google Scholar] [CrossRef]
- Jayapandian, C.P.; Chen, Y.; Janowczyk, A.R.; Palmer, M.B.; Cassol, C.A.; Sekulic, M.; Hodgin, J.B.; Zee, J.; Hewitt, S.M.; O’Toole, J.; et al. Development and evaluation of deep learning–based segmentation of histologic structures in the kidney cortex with multiple histologic stains. Kidney Int. 2020, 99, 86–101. [Google Scholar] [CrossRef]
- van der Laak, J.; Litjens, G.; Ciompi, F. Deep learning in histopathology: The path to the clinic. Nat. Med. 2021, 27, 775–784. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, D.Y. Artificial intelligence and computational pathology. Lab. Investig. 2021, 101, 412–422. [Google Scholar] [CrossRef]
- Mahmood, H.; Shaban, M.; Rajpoot, N.; Khurram, S.A. Artificial Intelligence-based methods in head and neck cancer diagnosis: An overview. Br. J. Cancer 2021, 124, 1934–1940. [Google Scholar] [CrossRef]
- Polymeri, E.; Kjölhede, H.; Enqvist, O.; Ulén, J.; Poulsen, M.H.; Simonsen, J.A.; Borrello, P.; Trägårdh, E.; Johnsson, Å.A.; Høilund-Carlsen, P.F.; et al. Artificial intelligence-based measurements of PET/CT imaging biomarkers are associated with disease-specific survival of high-risk prostate cancer patients. Scand J. Urol. 2021, 55, 427–433. [Google Scholar] [CrossRef]
- Penzkofer, T.; Padhani, A.R.; Turkbey, B.; Haider, M.A.; Huisman, H.; Walz, J.; Salomon, G.; Schoots, I.G.; Richenberg, J.; Villeirs, G.; et al. ESUR/ESUI position paper: Developing artificial intelligence for precision diagnosis of prostate cancer using magnetic resonance imaging. Eur. Radiol. 2021, 31, 9567–9578. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Z.; Zheng, J.; Zhao, Q.; Yuan, Z. Artificial intelligence-aided optical imaging for cancer theranostics. Semin. Cancer Biol. 2023, 94, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Teare, P.; Fishman, M.; Benzaquen, O.; Toledano, E.; Elnekave, E. Malignancy Detection on Mammography Using Dual Deep Convolutional Neural Networks and Genetically Discovered False Color Input Enhancement. J. Digit. Imaging 2017, 30, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Bejnordi, B.E.; Veta, M.; Van Diest, P.J.; Ginneken, B.; Karssemeijer, N.; Litjens, G.; Laak, J.A.W.M. CAMELYON16 Consortium Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA J. Am. Med. Assoc. 2017, 318, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.M. Intelligent Imaging: Artificial Intelligence Augmented Nuclear Medicine. J. Nucl. Med. Technol. 2019, 47, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Van Ginneken, B.; Schaefer-Prokop, C.M.; Prokop, M. Computer-aided diagnosis: How to move from the laboratory to the clinic. Radiology 2011, 261, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Quinn, S.J.; Abbott, T.; Cairney, S. The role of Aboriginal literacy in improving English literacy in remote Aboriginal communities: An empirical systems analysis with the Interplay Wellbeing Framework. Educ. Res. Policy Pract. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, Y.; Li, C.; Kim, J.; Cai, W.; Han, Z.; Feng, D.D. Deepgene: An advanced cancer type classifier based on deep learning and somatic point mutations. BMC Bioinform. 2016, 17, 243–256. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, J.; Lin, Z.; Zhao, X. A deep learning-based multi-model ensemble method for cancer prediction. Comput. Methods Programs Biomed. 2018, 153, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Chen, T.Y.; Williamson, D.F.K.; Zhao, M.; Shady, M.; Lipkova, J.; Mahmodd, F. AI-based pathology predicts origins for cancers of unknown primary. Nature 2021, 594, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Zhang, L.; Zhang, Z. Vomiting Management and Effect Prediction after Early Chemotherapy of Lung Cancer with Diffusion-Weighted Imaging under Artificial Intelligence Algorithm and Comfort Care Intervention. Comput. Math. Methods Med. 2022, 2022, 1056910. [Google Scholar] [CrossRef] [PubMed]
- Ho, D. Artificial intelligence in cancer therapy. Science 2020, 367, 982–983. [Google Scholar] [CrossRef]
- Elemento, O.; Leslie, C.; Lundin, J.; Tourassi, G. Artificial intelligence in cancer research, diagnosis and therapy. Nat. Rev. Cancer 2021, 21, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xue, B.; Wang, Y.; Wang, D.; Gao, D.; Yang, S.; Zhao, Q.; Zhou, C.; Ruan, S.; Yuan, Z. Temperature-Feedback Nanoplatform for NIR-II Penta-Modal Imaging-Guided Synergistic Photothermal Therapy and CAR-NK Immunotherapy of Lung Cancer. Small 2021, 17, 2101397. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; He, T.; Li, H.; Guo, X.; Zheng, Z. Improve individual treatment by comparing treatment benefits: Cancer artificial intelligence survival analysis system for cervical carcinoma. J. Transl. Med. 2022, 20, 293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xue, Y.; Li, M.; Yang, C.; Zhang, D.; Wang, Q.; Wnag, J.; Chen, J.; You, J.; Yuan, Z. Artificial Intelligence-Based Automated Treatment Planning of Postmastectomy Volumetric Modulated Arc Radiotherapy. Front. Oncol. 2022, 12, 871871. [Google Scholar] [CrossRef]
- Lang, Q.; Zhong, C.; Liang, Z.; Zhang, Y.; Wu, B.; Xu, F.; Cong, L.; Wu, S.; Tian, Y. Six application scenarios of artificial intelligence in the precise diagnosis and treatment of liver cancer. Artif. Intell. Rev. 2021, 54, 5307–5346. [Google Scholar] [CrossRef]
- Chen, Z.H.; Lin, L.; Wu, C.F.; Li, C.F.; Xu, R.H.; Sun, Y. Artificial intelligence for assisting cancer diagnosis and treatment in the era of precision medicine. Cancer Commun. 2021, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2020, 32, 1901989. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, Y.; Lang, J.; Li, L.; Zhang, Y. Triboelectric nanogenerator and artificial intelligence to promote precision medicine for cancer. Nano Energy 2022, 92, 106783. [Google Scholar] [CrossRef]
- Fu, Y.; Jung, A.W.; Torne, R.V.; Gonzalez, S.; Vohringer, H.; Shmatko, A.; Yates, L.R.; Jimenez-Linan, M.; Moore, L.; Gerstung, M. Pan-cancer computational histopathology reveals mutations, tumour composition and prognosis. Nat Cancer 2020, 1, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Raccuglia, P.; Elbert, K.C.; Adler, P.D.F.; Falk, C.; Wenny, M.B.; Mollo, A.; Zeller, M.; Friedler, S.A.; Schrier, J.; Norquist, A.J. Machine-learning-assisted materials discovery using failed experiments. Nature 2016, 533, 73–76. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target Ther. 2022, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Kuenzi, B.M.; Park, J.; Fong, S.H.; Sanchez, K.S.; Lee, J.; Kreisberg, J.F.; Ma, J.; Ideker, T. Predicting Drug Response and Synergy Using a Deep Learning Model of Human Cancer Cells. Cancer Cell 2020, 38, 672–684.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luan, Y.; Yue, F. EagleC: A deep-learning framework for detecting a full range of structural variations from bulk and single-cell contact maps. Sci. Adv. 2022, 8, eabn9215. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, W.; Ong, Y.; Busch, T.M.; Zhu, T.C. Fractionated Photofrin-Mediated Photodynamic Therapy Significantly Improves Long-Term Survival. Cancers 2023, 15, 5682. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic therapy—An up-to-date review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Henderson, B.W.; Busch, T.M.; Vaughan, L.A.; Frawley, N.P.; Babich, D.; Sosa, T.A.; Zollo, J.D.; Dee, A.S.; Cooper, M.T.; Bellnier, D.A.; et al. Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res. 2000, 60, 525–529. [Google Scholar] [PubMed]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; Destanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.; Mohan, P.; Ye, Y.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Hubner, R.A.; Cubillo, A.; Blanc, J.F.; Melisi, D.; Von Hoff, D.D.; Wang-Gillam, A.; Chen, L.T.; Becker, C.; Mamlouk, K.; Belanger, B.; et al. Quality of life in metastatic pancreatic cancer patients receiving liposomal irinotecan plus 5-fluorouracil and leucovorin. Eur. J. Cancer 2019, 106, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Siveke, J.T.; Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.P.; Shan, Y.S.; Jameson, G.S.; Macarulla, T.; Lee, K.H.; et al. Survival with nal-IRI (liposomal irinotecan) plus 5-fluorouracil and leucovorin versus 5-fluorouracil and leucovorin in per-protocol and non-per-protocol populations of NAPOLI-1: Expanded analysis of a global phase 3 trial. Eur. J. Cancer 2018, 105, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.; Mishoe, A.; Feng, F.; Nguyen, F.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68 Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Correlations between pretherapeutic imaging and whole-body tumour dosimetry with treatment outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Tsuji, A.; Yamaguchi, K.; Takeda, K.; Uetake, H.; Esaki, T.; Takeda, K.; Uetake, H.; Esaki, T.; Amagai, K.; et al. A phase II study of NK012, a polymeric micelle formulation of SN-38, in unresectable, metastatic or recurrent colorectal cancer patients. Cancer Chemother. Pharmacol. 2018, 82, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, H.S.; Lee, K.Y.; Kim, H.J.; Jeon, Y.J.; Jang, T.W.; Lee, K.H.; Kim, Y.C.; Kim, K.S.; Oh, I.J.; et al. YPaclitaxel-loaded polymeric micelle (230 mg/m2) and cisplatin (60 mg/m2) vs. paclitaxel (175 mg/m2) and cisplatin (60 mg/m2) in advanced non-small-cell lung cancer: A multicenter randomized phase IIB trial. Clin. Lung Cancer 2013, 14, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vincenzo Canzonieri, M.P. The Theranostic Value of STARD3 in Colorectal Cancer: The STAR Study (STAR). Available online: https://clinicaltrials.gov/study/NCT06136949 (accessed on 18 June 2024).
- Julie, L. Sutcliffe PhD Molecularly Targeted Theranostic Approach for the Detection and Treatment of Metastatic Carcinomas. Available online: https://clinicaltrials.gov/study/NCT06389123 (accessed on 18 June 2024).
- Rudolf, A.; Werner, M.A. Molecular Imaging-Derived Biomarker of PSMA Expression-Revealing Theranostic Potential in Gastrointestinal Tumours (Focusing on Neuroendocrine Neoplasms). Available online: https://clinicaltrial.be/nl/details/23026?per_page=100&only_recruiting=0&only_eligible=0 (accessed on 18 June 2024).

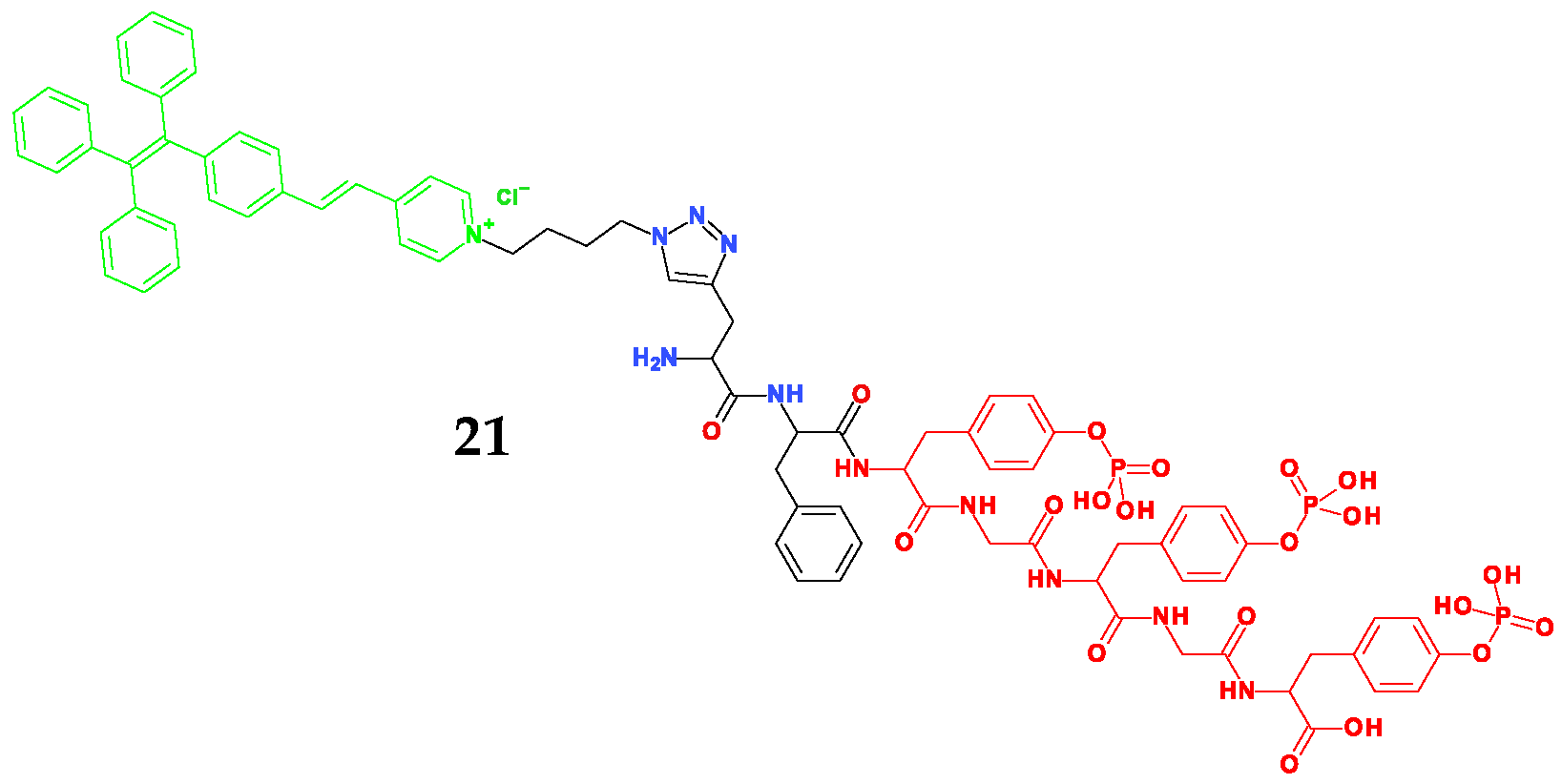
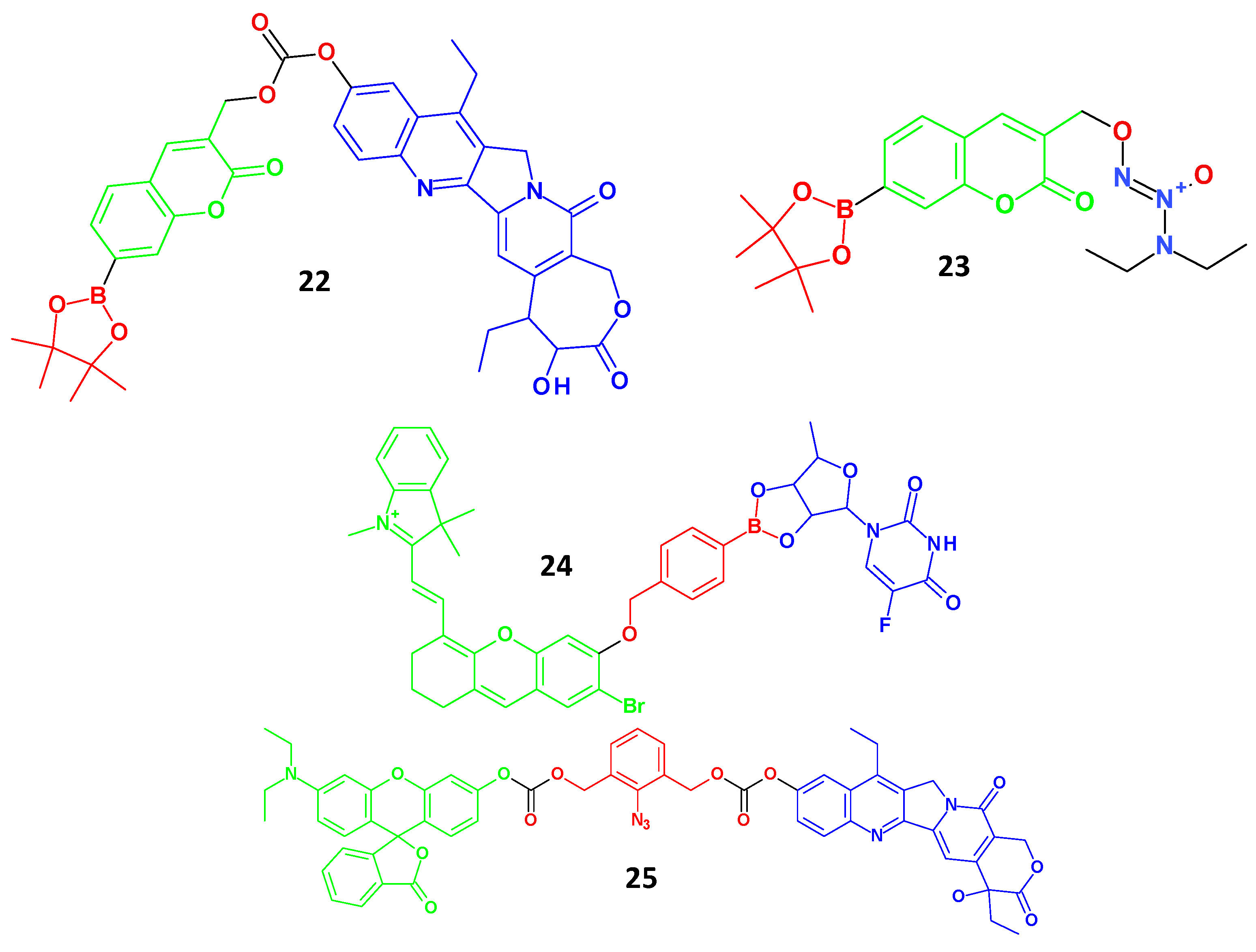
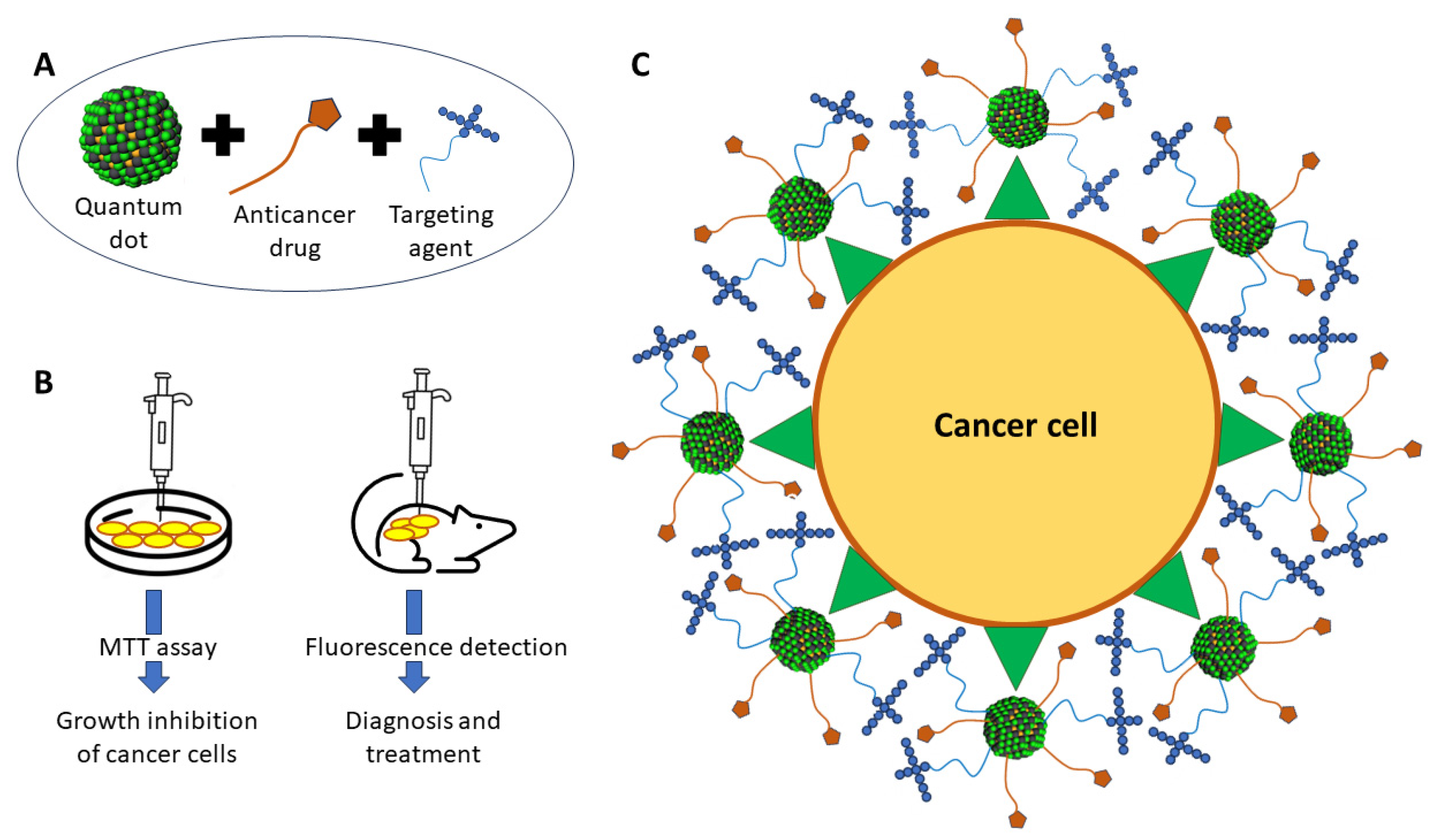
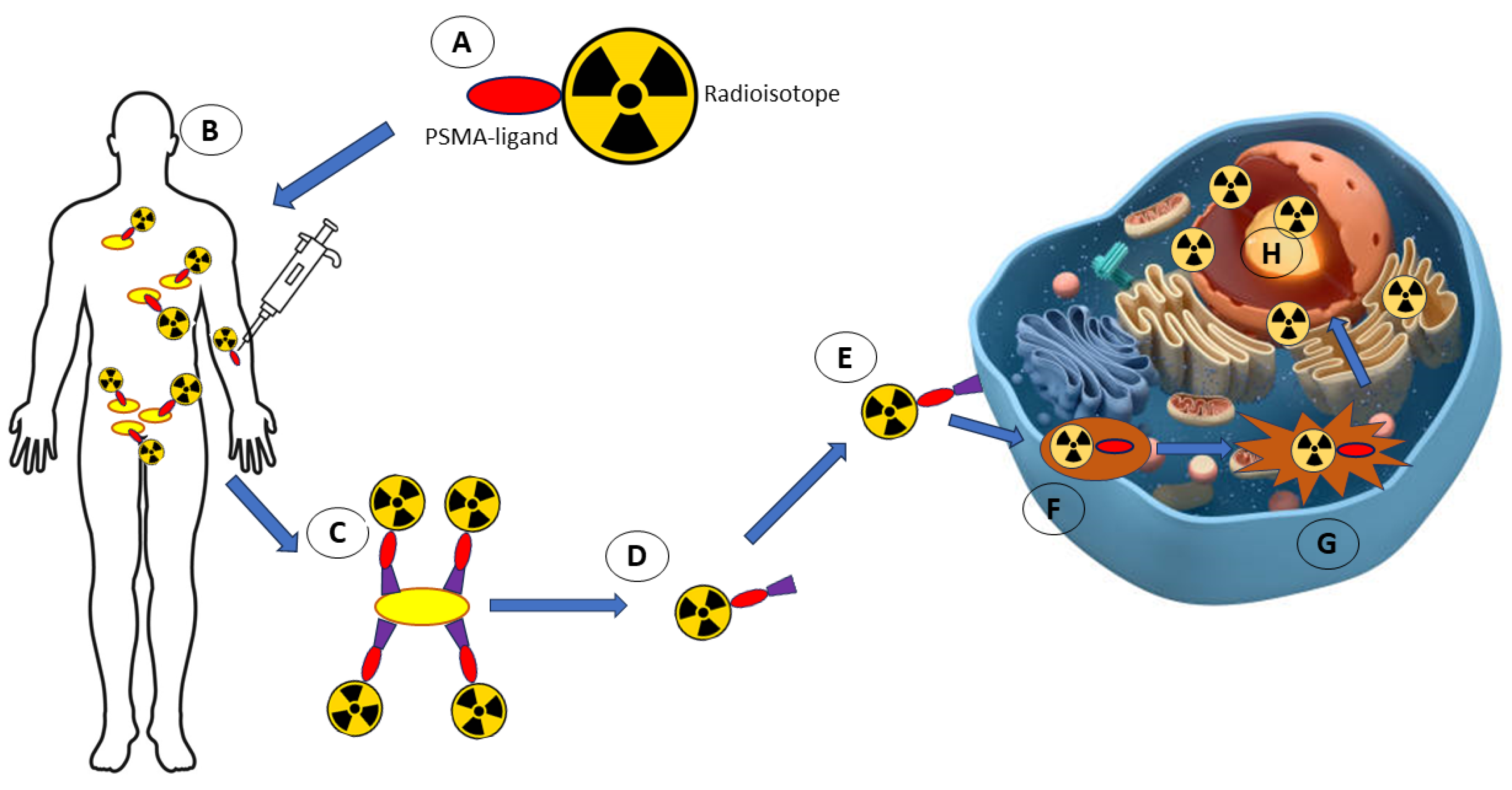
| Quantum Dots Agent | Applications | References | |
|---|---|---|---|
| Pegylated Black Phosphorus Quantum Dots (PEGylated BPQDs) | Suppressed the growth of tumours in 4T1-tumour-bearing mice without the presence of adverse effects | In vivo | [69] |
| Quantum dot-based micelle conjugated with EGFR antibody and loaded with aminoflavone | Induced regression of MDA-MB-468 triple-negative breast cancer cells by targeting EGFR | In vitro; In vivo | [59] |
| Lipid micelles co-loaded with paclitaxel/quantum dots and EGFR antibodies/EGFR aptamers | Induced cell cycle arrest at the G2/M phase and triggered apoptosis in treated S174T human colorectal cancer cells by targeting EGFR | In vitro; In vivo | [4] |
| Iron oxide-bismuth oxide-graphene quantum dots (GQDsFe/Bi) | Induced apoptosis in treated MCF-7 breast cancer cells upon laser irradiation | In vitro | [58] |
| Graphene quantum dots-Candida parapsilosis biosurfactant conjugates | Decreased the viability of MCF-7 breast cancer cells in a dose- and time-dependent manner | In vitro | [60] |
| Graphene quantum dots/photosensitiser/CpG oligonucleotides hybrid attached to dual magnetic resonance/fluorescence imaging probes (PC@GCpD [Gd]) | Hindered the growth of EMT6 murine mammary cancer cells via the upregulation of relevant immune response for the destruction of cancer cells | In vitro; In vivo | [70] |
| Name | Application | References |
|---|---|---|
| Photofrin | Image Analysis: AI analyses images from computed tomography (CT) or magnetic resonance imaging (MRI) to precisely determine the location of tumours and Photofrin accumulation. This enables more accurate therapy planning and optimisation of lighting parameters. Progress Monitoring: AI algorithms track changes in tumour size and response to treatment based on sequential images, allowing dynamic adjustment of the therapy plan. | [159] |
| Aluminium Phthalocyanine (AlPc) | Light Dose Optimization: AI helps simulate and optimise light delivery parameters such as wavelength, intensity, and duration based on individual patient characteristics and photosensitiser distribution. Predictive Modelling: AI-based predictive models can forecast patient response to treatment using AlPc, allowing personalised therapy. | [160] |
| Temoporfin (mTHPC) | Therapy Planning: AI uses genetic, metabolic, and imaging data to predict how temoporfin will distribute and accumulate in cancer tissues. This allows for more precise therapy planning and execution. Dosimetry Automation: AI automatically calculates light dosimetry, ensuring the correct amount of energy is delivered to activate the photosensitiser without excessive damage to surrounding tissues | [161] |
| Theranostic Agents Used in Clinical Trials | Outcomes | Clinical Trial | References |
|---|---|---|---|
| “C dots” (Cornell dots) labelled with 124I (PET imaging) and conjugated with cRGDY peptides (targeting agent) (124IcRGDYePEGeC dots) | Since cRGDY targets integrin anb3, which is overexpressed on endothelial cells involved in angiogenesis, vascular remodelling and solid tumour cells, the accumulation of 124I-cRGDYePEGeC dots on cancer cells could be observed using PET. This method can be used for the selection of patients who require integrin-targeted treatments, imaging and diagnosis of tumour cells and neovasculature and to monitor the progress and efficiency of a particular treatment | NCT01266096 | [163] |
| Paclitaxel-loaded polymeric micelle and cisplatin | Paclitaxel-loaded polymeric micelle at a dose of 230 mg/m2 in combination with cisplatin was well tolerated with minimal toxicity in non-small cell lung cancer patients when given in a 3-week cycle | Phase II trial | [166] |
| NK012 polymeric micelle | The anticancer activity of NK012 was tested against unresectable, metastatic, and recurrent colorectal cancer patients. Treated patients with a history of oxaliplatin-based therapy demonstrated side effects such as diarrhoea and neutropenia. | Phase II trial | [167] |
| 177Lu-PSMA-617 and 68Ga-PSMA-11 | Accumulation and prolonged retention of 177Lu-PSMA-617 in tumour tissues compared to normal cells in treated metastatic prostate cancer patients resulted in decreased prostate-specific antigen (PSA) levels, which are overexpressed in prostate cancer patients. The accumulation of 177Lu-PSMA-617 in tumour cells was observed using 68Ga-PSMA-11 via PET. | ANZCTR12615000912583 | [168] |
| 68Ga-PSMA-11 | Accurate and precise diagnosis of recurrent prostate cancer in prostate cancer patients using 68Ga-PSMA-11 using positron emission tomography (PET) were achieved, suggesting the importance of this theranostic agent to aid in the diagnosis of prostate cancer patients. | (NCT02940262; NCT03353740) | [169] |
| 177Lu-PSMA-617 | This study is still ongoing. However, it has been hypothesised that metastatic castration-resistant prostate cancer (mCRPC) patients treated with 177Lu-PSMA-617 would have a longer lifespan and less cytotoxicity towards the surrounding normal cells compared to cabazitaxel chemotherapy. | Phase II trial (NCT03392428) | [163] |
| STARD3 | This study aims to verify the overexpression of STARD3 in both early and advanced CRC patients’ derived tissues to identify the pathways underpinning tumourigenesis and cancer progression in which STARD3 is involved. Moreover, its role as a dynamic biomarker of treatment response and its role in treatment sensitivity will be explored. | NCT06136949 | [170] |
| [68Ga]Ga DOTA-5G and [177Lu]Lu DOTA-ABM-5G | This is a Phase I study to evaluate the safety and efficacy of the [68Ga]Ga DOTA-5G and [177Lu]Lu DOTA-ABM-5G theranostics pair in patients with metastatic cancer. [68Ga]Ga DOTA-5G PET/CT will be used to identify and stratify patients eligible for (and most likely to respond to) the [177Lu]Lu DOTA-ABM-5G therapy. | Phase I trial NCT06389123 | [171] |
| 18F-PSMA PET/CT | The aim is to evaluate whether PSMA-directed in vivo imaging can also be applied to GEP-NEN patients to determine if (i) biopsy-derived tissue of newly diagnosed patients exhibits a PSMA expression profile, (ii) PSMA-PET shows upregulated PSMA expression in vivo, (iii) such a molecular imaging approach identifies more disease sites relative to conventional imaging, and (iv) if the PSMA PET signal predicts further clinical course and outcome under guideline-compatible treatment. | NCT05547919 | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymaszek, P.; Tyszka-Czochara, M.; Ortyl, J. Application of Photoactive Compounds in Cancer Theranostics: Review on Recent Trends from Photoactive Chemistry to Artificial Intelligence. Molecules 2024, 29, 3164. https://doi.org/10.3390/molecules29133164
Szymaszek P, Tyszka-Czochara M, Ortyl J. Application of Photoactive Compounds in Cancer Theranostics: Review on Recent Trends from Photoactive Chemistry to Artificial Intelligence. Molecules. 2024; 29(13):3164. https://doi.org/10.3390/molecules29133164
Chicago/Turabian StyleSzymaszek, Patryk, Małgorzata Tyszka-Czochara, and Joanna Ortyl. 2024. "Application of Photoactive Compounds in Cancer Theranostics: Review on Recent Trends from Photoactive Chemistry to Artificial Intelligence" Molecules 29, no. 13: 3164. https://doi.org/10.3390/molecules29133164
APA StyleSzymaszek, P., Tyszka-Czochara, M., & Ortyl, J. (2024). Application of Photoactive Compounds in Cancer Theranostics: Review on Recent Trends from Photoactive Chemistry to Artificial Intelligence. Molecules, 29(13), 3164. https://doi.org/10.3390/molecules29133164









