Surface Modification of Calcined Kaolinite for Enhanced Solvent Dispersion and Mechanical Properties in Polybutylene Adipate/Terephthalate Composites
Abstract
1. Introduction
2. Results and Discussion
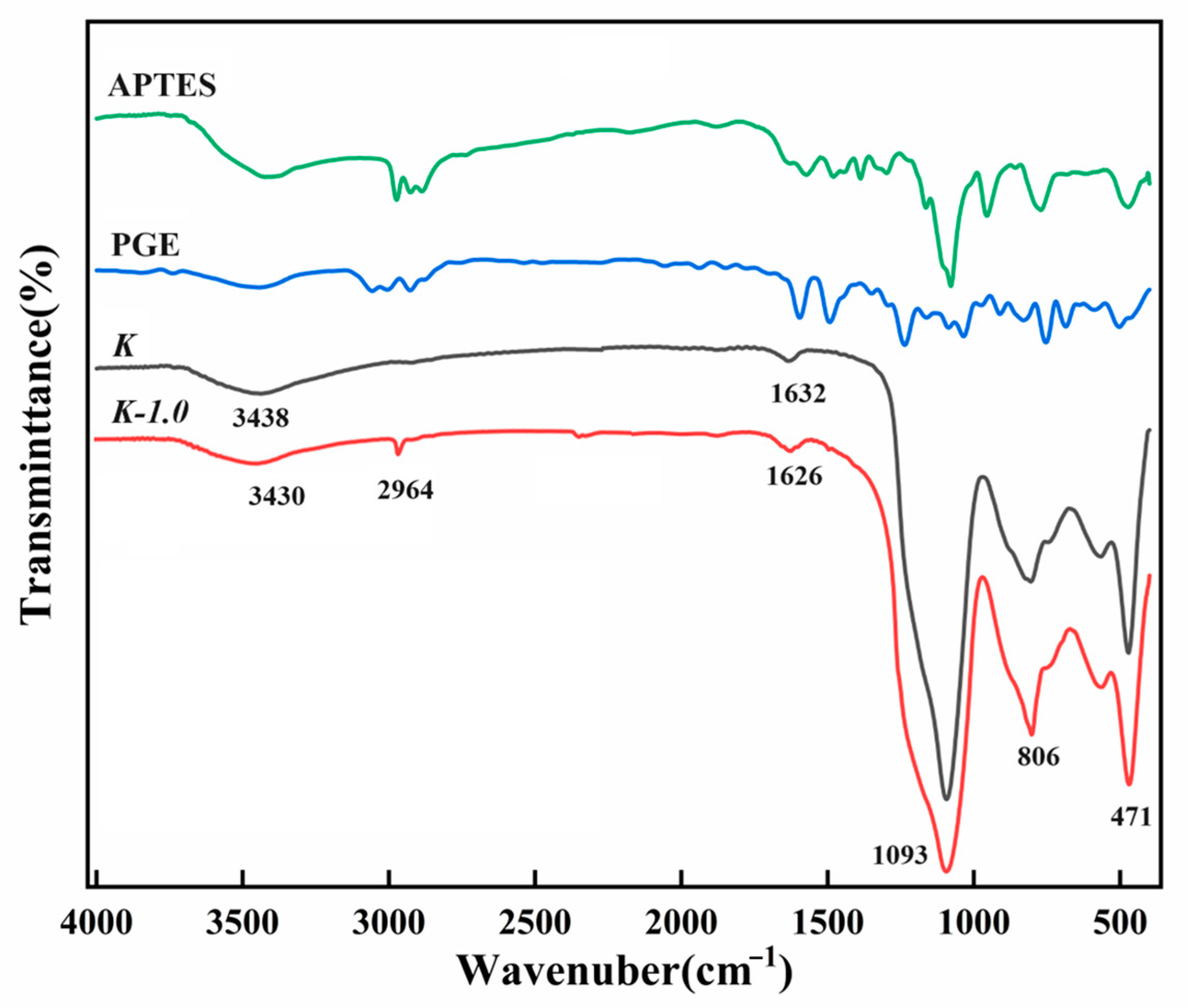
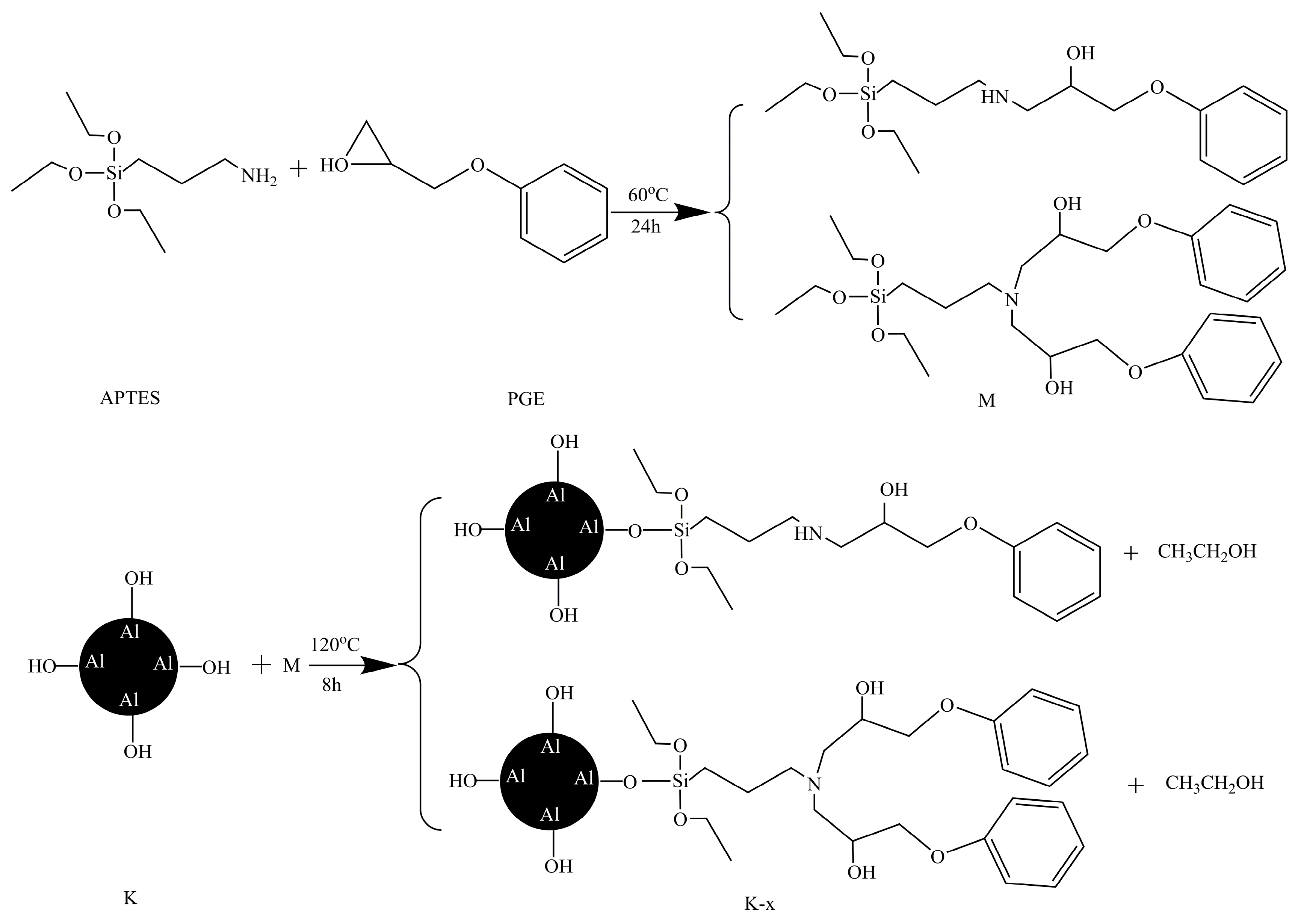
2.1. FT-IR Spectrograms
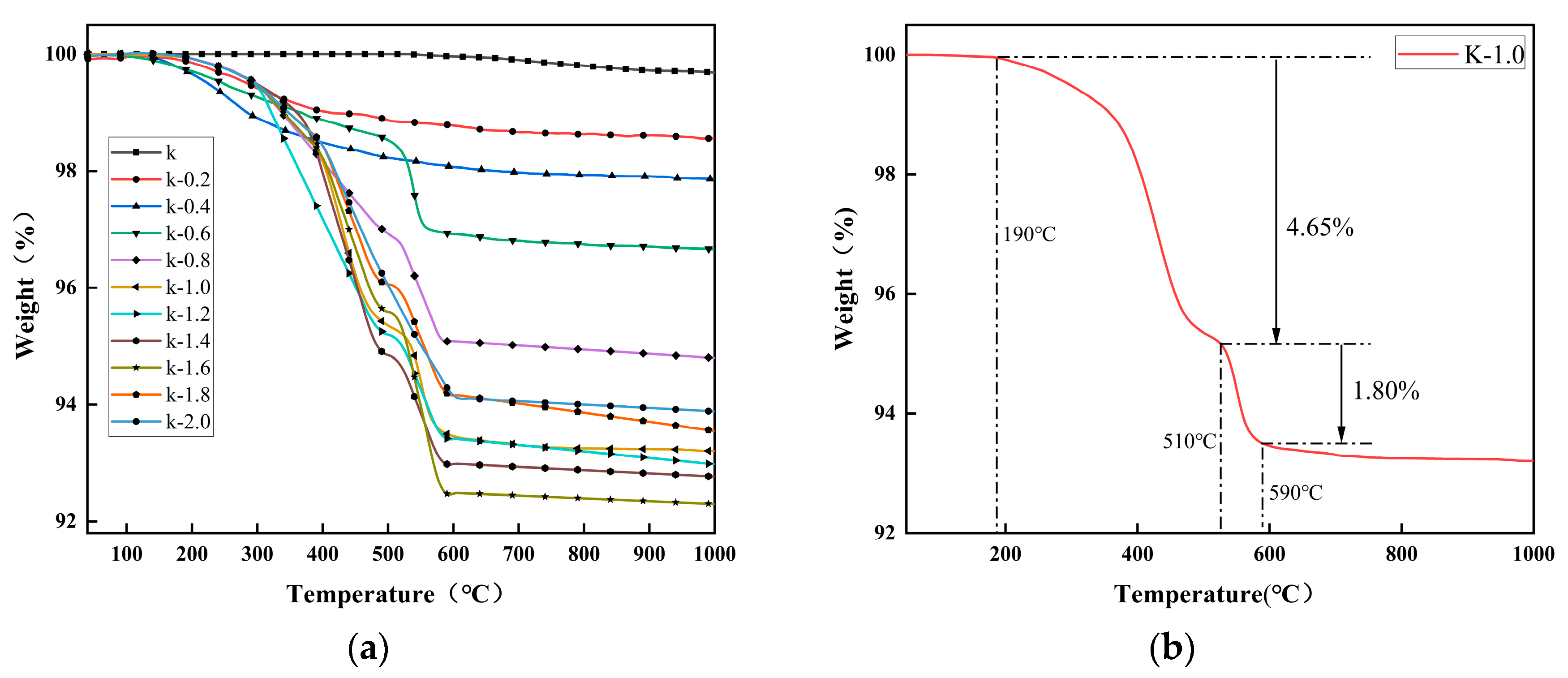
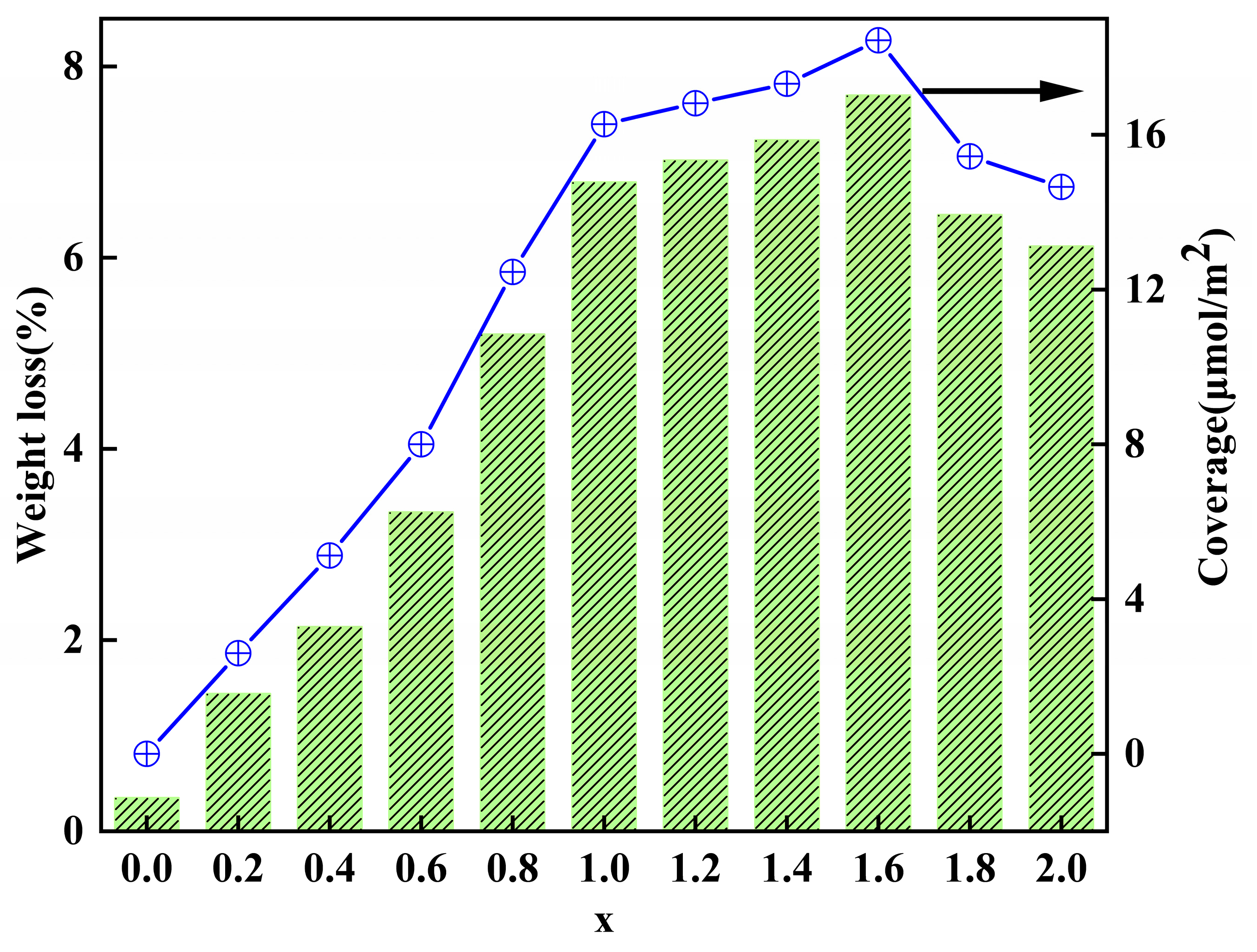
2.2. Thermogravimetric Analysis
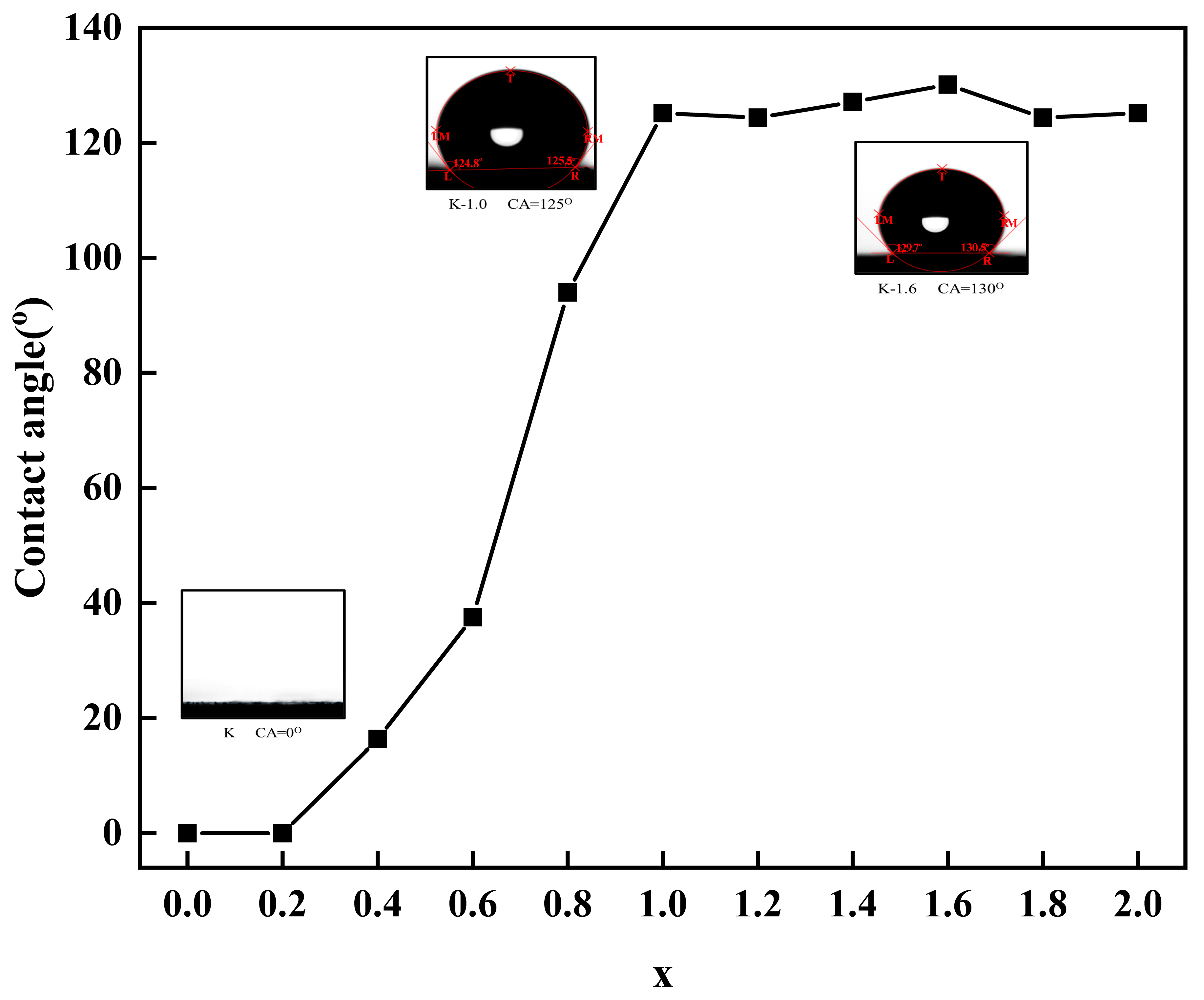
2.3. Contact Angle
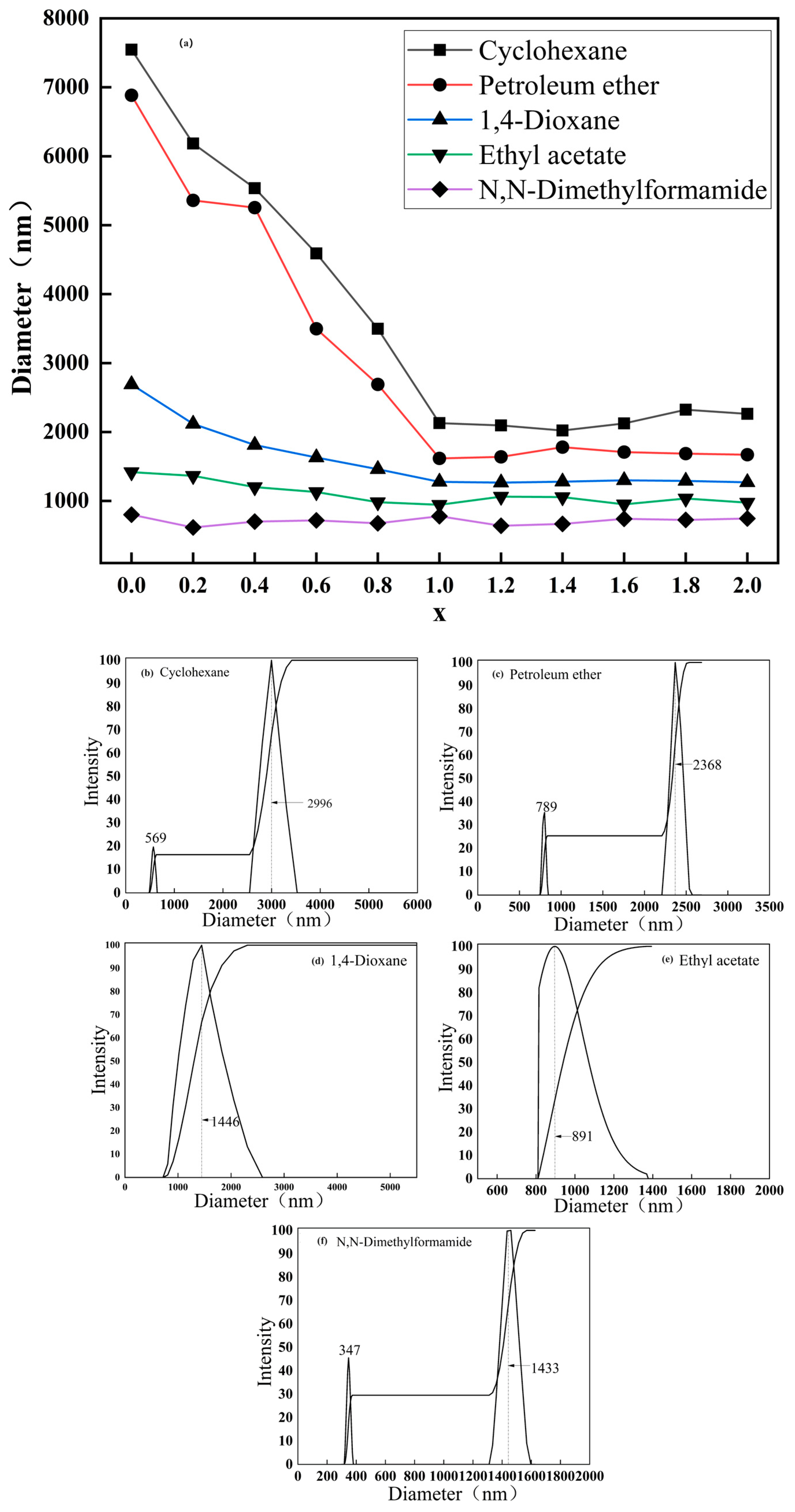
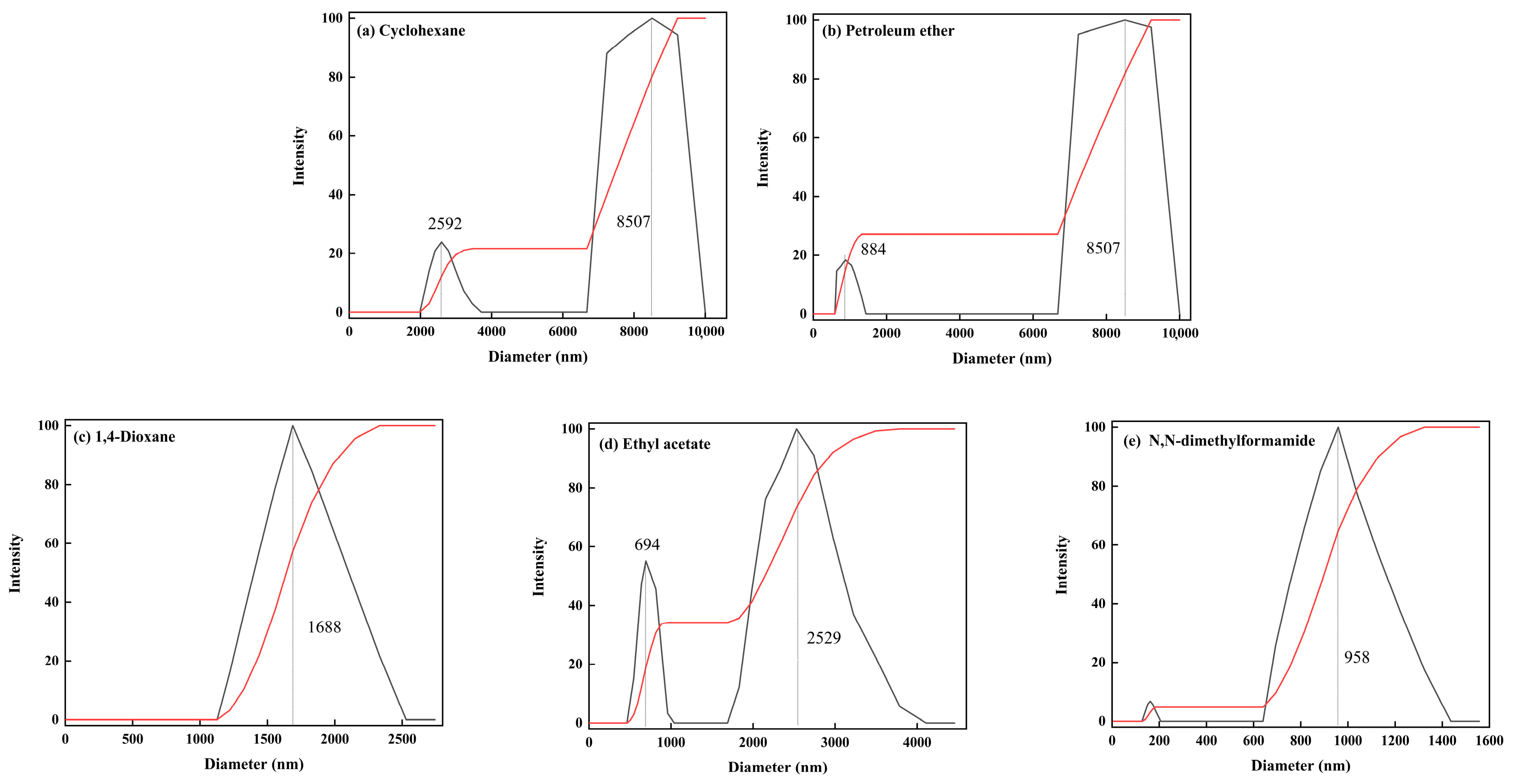
2.4. Dispersion Performance
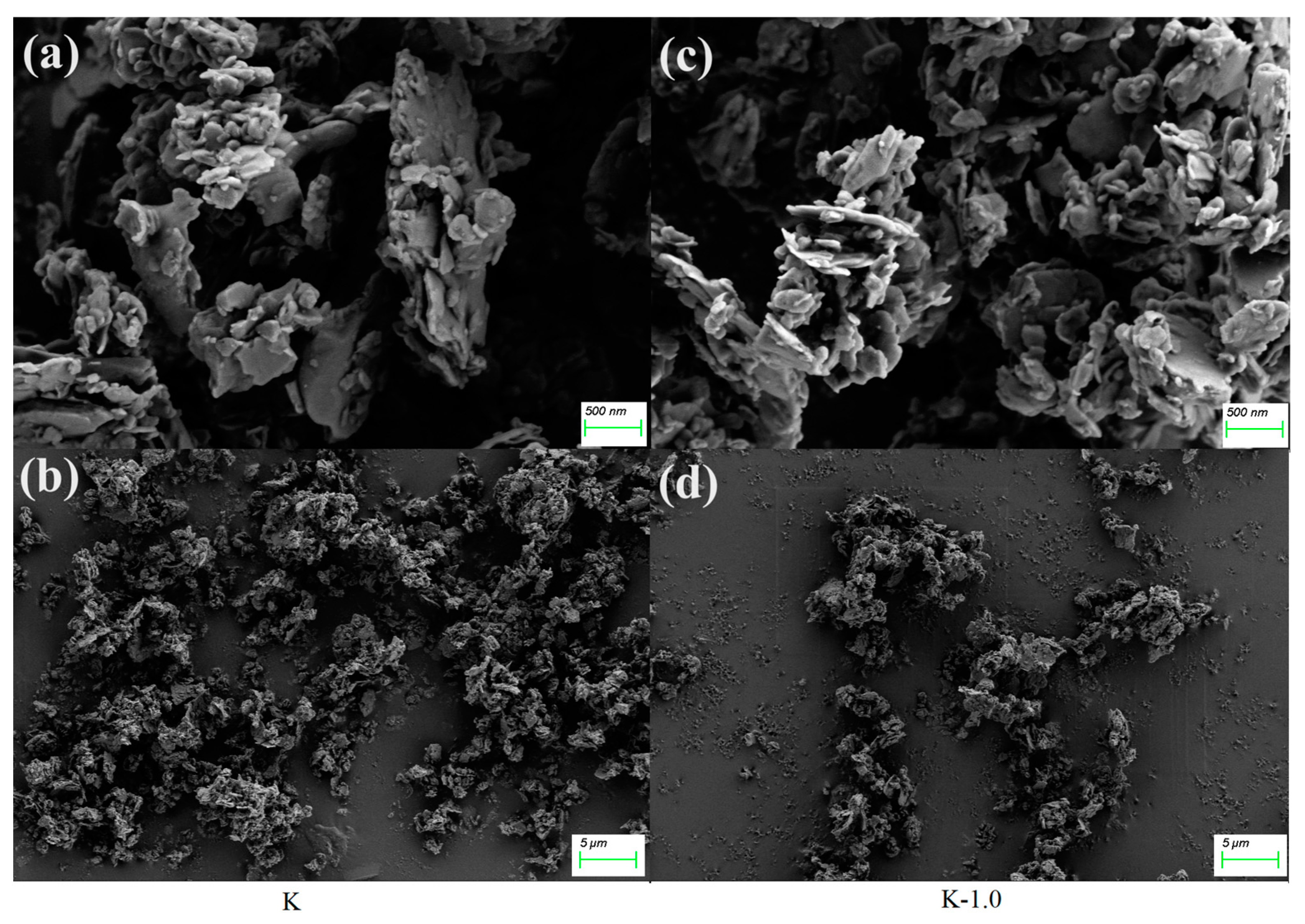
2.5. Scanning Electron Microscopy of Unmodified and Modified Kaolinite
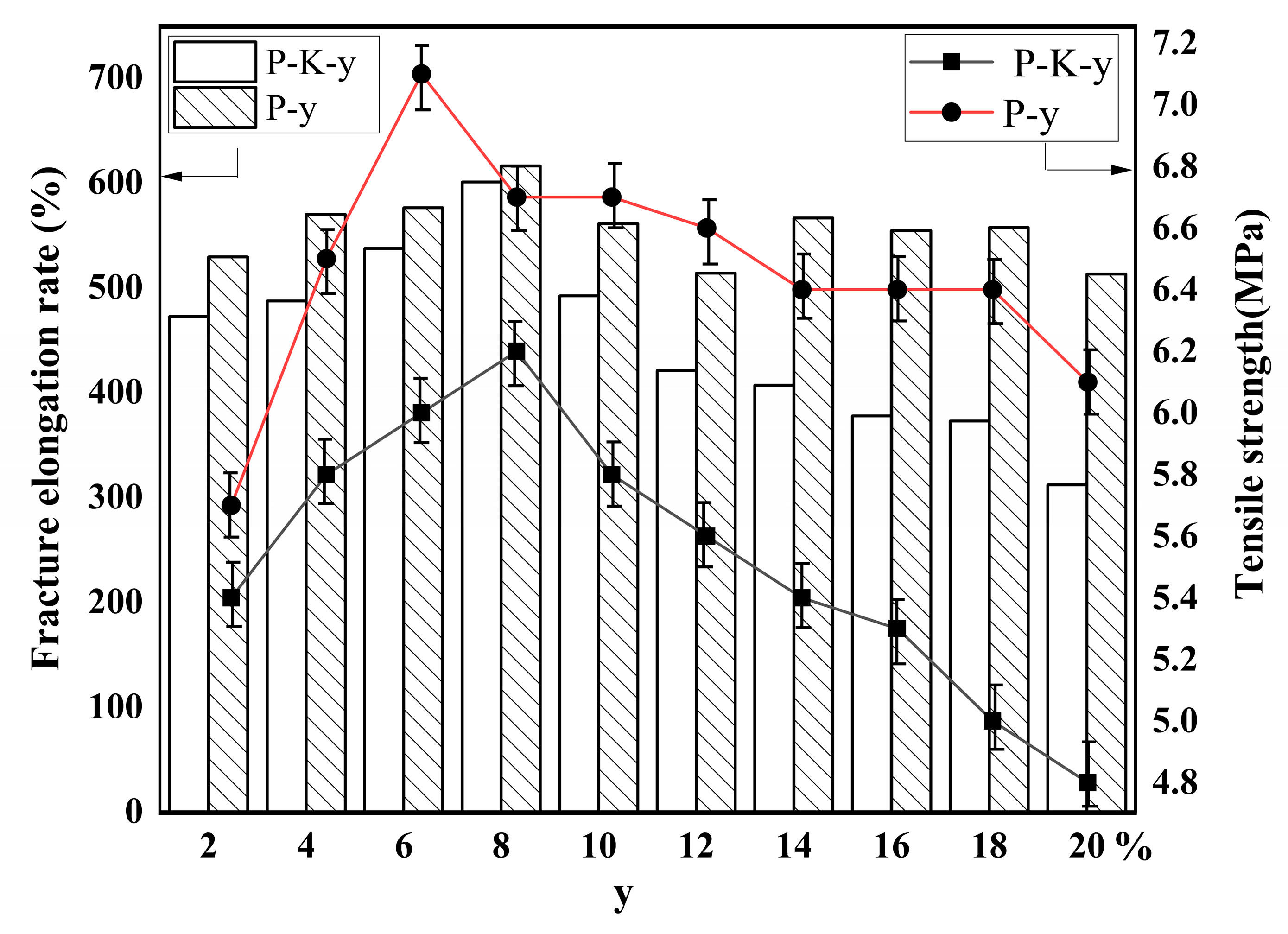
2.6. Mechanical Properties of Composites
3. Experiments and Analysis
3.1. Materials
3.2. Preparation of Modified Calcined Kaolinite/PBAT Composites
3.3. Analytical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novak, B.M. Hybrid nanocomposite materials-between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Huang, X.Y.; Brittain, W.J. Synthesis and characterization of PMMA nanocomposites by suspension and emulsion polymerization. Macromolecules 2001, 34, 3255–3260. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.M.; Zhou, S.X.; You, B. Synthesis of raspberry-like PMMA/SiO2 nanocomposite particles via a surfactant-free method. Macromolecules 2004, 37, 9613–9619. [Google Scholar] [CrossRef]
- Karaman, M.; Kooi, S.E.; Gleason, K.K. Vapor deposition of hybrid organic-inorganic dielectric Bragg mirrors having rapid and reversibly tunable optical reflectance. Chem. Mater. 2008, 20, 2262–2267. [Google Scholar] [CrossRef]
- Kuan, H.C.; CAhiu, S.L.; Chen, C.H.; Kuan, C.F.; Chiang, C.L. Synthesis, characterization, and thermal stability of PMMA/SiO2/TiO2 tertiary nanocomposites via non-hydrolytic sol-gel method. J. Appl. Polym. Sci. 2009, 113, 1959–1965. [Google Scholar] [CrossRef]
- Kos, T.; Anžlovar, A.; Pahovnik, D.; Žagar, E.; Orel, Z.C.; Žigon, M. Zinc-containing block copolymer as a precursor for the in situ formation of nano ZnO and PMMA/ZnO nanocomposites. Macromolecules 2013, 46, 6942–6948. [Google Scholar] [CrossRef]
- Demir, M.M.; Wegner, G. Challenges in the preparation of optical polymer composites with nanosized pigment particles: A review on recent efforts. Macromol. Mater. Eng. 2012, 297, 838–863. [Google Scholar] [CrossRef]
- Magalhães, N.F.; Dahmouche, K.; Lopes, G.K.; Andrade, C.T. Using an organically-modified montmorillonite to compatibilize a biodegradable blend. Appl. Clay Sci. 2013, 72, 1–8. [Google Scholar] [CrossRef]
- Maria, A.; Aurora, A.; Montone, A.; Andrade, C.T. Synthesis and characterization of PMMA/silylated MMTs. J. Nanopart. Res. 2011, 13, 6049–6058. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.W.; Wolcott, M.P. Comparison of polylactide/nano-sized calcium carbonate and polylactide/montmorillonite composites: Reinforcing effects and toughening mechanisms. Polymer 2007, 48, 7632–7644. [Google Scholar] [CrossRef]
- Xiang, D.H.; Gu, C.J. A study on the friction and wear behavior of PTFE filled with ultra-fine kaolinite particulates. Mater. Lett. 2006, 60, 689–692. [Google Scholar] [CrossRef]
- Chang, Z.H.; Guo, F.; Yu, J.H.; Wang, G.Q. Synergistic flame retardant effects of nano-kaolinite and nano-HAO on LDPE/EPDM composites. Polym. Degrad. Stabil. 2007, 92, 1204–1212. [Google Scholar] [CrossRef]
- Buggy, M.; Bradley, G.; Sullivan, A. Polymer-filler interactions in kaolin/nylon 6,6 composites containing a silane coupling agent. Compos. Part A Appl. Sci. Manuf. 2005, 36, 437–442. [Google Scholar] [CrossRef]
- Lungu, A.; Perrin, F.X.; Bélec, L.; Sarbu, A.; Teodorescu, M. Kaolin/poly(acrylic acid) composites as precursors for porous kaolinite ceramics. Appl. Clay Sci. 2012, 62–63, 63–69. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A. Synthesis and properties of polyimide-clay hybrid. J. Polym. Sci. Pol. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Cipolletti, V.; Riccio, P.; Riccò, T.; Pandini, S.; Conzatti, L. Enhancement of mechanical reinforcement due to hybrid filler networking promoted by an organoclay in hydrocarbon-based nanocomposites. Appl. Clay Sci. 2012, 65–66, 57–66. [Google Scholar] [CrossRef]
- Paiva, L.B.; Morales, A.R.; Díaz FR, V. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Martín, Z.; Jiménez, I.; Gómez, M.N.; Ade, H.W.; Kilcoyne, D.A.; Hernádez-Cruz, D. Spectromicroscopy study of intercalation and exfoliation in polypropylene/montmorillonite nanocomposites. J. Phys. Chem. B 2009, 113, 11160–11165. [Google Scholar] [CrossRef]
- McLauchlin, A.; Bao, X.J.; Zhao, F. Organoclay polybutylene terephthalate nanocomposites using dual surfactant modified montmorillonite prepared by the masterbatch method. Appl. Clay Sci. 2011, 53, 749–753. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Yuan, P.; He, H.-P.; Qin, Z.-H.; Zhou, Q.; Zhu, J.-X.; Liu, D. Effect of reaction temperature on grafting of γ-aminopropyl triethoxysilane (APTES) onto kaolinite. Appl. Clay. Sci. 2012, 62–63, 8–14. [Google Scholar] [CrossRef]
- Domka, L. Silane-modified kaolins as fillers in rubber compositions. J. Adhes. Sci. Technol. 1990, 4, 1–6. [Google Scholar] [CrossRef]
- Letaief, S.; Tonle, I.K.; Diaco, T.; Detellier, C. Nanohybrid materials from interlayer functionalization of kaolinite. Application to the electrochemical preconcentration of cyanide. Appl. Clay Sci. 2008, 42, 95–101. [Google Scholar] [CrossRef]
- Moretti, E.; Storaro, L.; Chessa, G.; Talon, A.; Callone, E.; Mueller, K.J.; Enrichi, F.; Lenarda, M. Stepwise dansyl grafting on the kaolinite interlayer surface. J. Colloid Interf. Sci. 2012, 375, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Faria, E.H.; Ciuffi, K.J.; Nassar, E.J.; Vicente, M.A.; Trujillano, R.; Calefi, P.S. Novel reactive amino-compound: Tris(hydroxymethyl)aminomethane covalently grafted on kaolinite. Appl. Clay Sci. 2010, 48, 516–521. [Google Scholar] [CrossRef]
- Liu, Q.F.; Spears, D.A.; Liu, Q.P. MAS NMR study of surface-modified calcined kaolinite. Appl. Clay Sci. 2001, 19, 89–94. [Google Scholar] [CrossRef]
- Iijima, M.; Kamiya, H. Layer-by-layer surface modification of functional nanoparticles for dispersion in organic solvents. Langmuir 2010, 26, 17943–17948. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Misra, M.; Mohanty, A.K. New engineered biocomposites from poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV)/poly (butylene adipate-co-terephthalate)(PBAT) blends and switchgrass: Fabrication and performance evaluation. Ind. Crops Products. 2013, 42, 461–468. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.W.; Lee, K.H. Properties of potentially biodegradable copolyesters of (succinic acid–1, 4-butanediol)/(dimethyl terephthalate–1, 4-butanediol). Polym. Int. 1999, 48, 861–867. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly (butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Dong, W.; Zou, B.; Yan, Y.; Ma, P.; Chen, M. Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly (lactide)/Poly (butylene adipate-co-terephthalate) Blends. Int. J. Mol. Sci. 2013, 14, 20189–20203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Q.; Ren, J.; Wang, L. Preparation and properties of biodegradable poly (lactic acid)/poly (butylene adipate-co-terephthalate) blend with glycidyl methacrylate as reactive processing agent. J. Mater. Sci. 2009, 44, 250–256. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A.; Billon, N.; Combeaud, C. Effect of the simultaneous biaxial stretching on the structural and mechanical properties of PLA, PBAT and their blends at rubbery state. Eur. Polym. J. 2015, 68, 288–301. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Chen, J.H.; Chen, C.C.; Yang, M.C. Characterization of nanocomposites of poly (butylene adipate-co-terephthalate) blending with organoclay. J. Polym. Res. 2011, 18, 2151–2159. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, B.; Yang, Y.; Yang, Y.; Jiang, G.; Tian, Y. Effects of biodegradable (PBAT/PLA) and conventional (LDPE) mulch film residues on bacterial communities and metabolic functions in different agricultural soils. J. Hazard Mater. 2024, 472, 134425. [Google Scholar] [CrossRef]
- Ma, J.; Cao, Y.; Fan, L.; Xie, Y.; Zhou, X.; Ren, Q.; Feng, Y. Degradation characteristics of polybutylene adipate terephthalic acid (PBAT) and its effect on soil physicochemical properties: A comparative study with several polyethylene (PE) mulch films. J. Hazard Mater. 2023, 456, 131661. [Google Scholar] [CrossRef]
- Almeida, T.G.; Neto, J.E.S.; Costa, A.R.M.; da Silva, A.S.; Carvalho, L.H.; Canedo, E.L. Degradation during processing in poly (butylene adipate-co-terephthalate)/vegetable fiber compounds estimated by torque rheometry. Polym. Test. 2016, 55, 204–211. [Google Scholar] [CrossRef]
- Fukushima, K.; Wu, M.H.; Bocchini, S.; Rasyida, A.; Yang, M.C. PBAT based nanocomposites for medical and industrial applications. Mater. Sci. Eng. C 2012, 32, 1331–1351. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.S.; Zhang, P.; Li, Y. Synthesis and characterization of kaolin/TiO2 nano-photocatalysts. Appl. Clay Sci. 2011, 53, 646–649. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Mako, E.; Martens, W.N. Modification of the hydroxyl surface of kaolinite through mechanochemical treatment followed by intercalation with potassium acetate. Langmuir 2002, 18, 6491–6498. [Google Scholar] [CrossRef]
- Yuan, Y.B.; Chen, H.L.; Lin, J.B.; Ji, Y. Surface modification of calcined kaolinite with toluene diisocyanate based on high energy ball milling. Appl. Surf. Sci. 2013, 284, 214–221. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.F.; Mark, J.E.; Noda, I. A novel biodegradable nanocomposite based on poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and silylated kaolinite/silica core–shell nanoparticles. Appl. Clay Sci. 2009, 46, 51–56. [Google Scholar] [CrossRef]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; Imprint CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Chaimberg, M.; Cohen, Y. Note on the silylation of inorganic oxide supports. J. Colloid. Interf. Sci. 1990, 134, 576–579. [Google Scholar] [CrossRef]
- Lin, J.B.; Chen, H.L.; Yao, L.C. Surface tailoring of SiO2 nanoparticles by mechanochemical method based on simple milling. Appl. Surf. Sci. 2010, 256, 5978–5984. [Google Scholar] [CrossRef]
- Beruto, D.T.; Lagazzo, A.; Botter, R. Silica–paraffin and kaolin–paraffin dispersions: Use of rheological and calorimetric methods to investigate the nature of their dispersed microstructure units. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 153–160. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X-type zeolites synthesised from kaolinite at low temperature. Appl. Clay Sci. 2013, 80, 162–168. [Google Scholar] [CrossRef]
- Ming, M.; Zhou, Y.; Wang, L.; Zhou, F.; Zhang, Y. Effect of polycarbodiimide on the structure and mechanical properties of PLA/PBAT blends. J. Polym. Res. 2022, 29, 371. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, S.; Zhang, Y.; Zhao, G. Mechanical properties and structure of injection molded poly (hydroxybutyrate-co-hydroxyvalerate)/poly (butylene adipate-co-terephthalate)(PHBV/PBAT) blends. J. Appl. Polym. Sci. 2023, 140, e53880. [Google Scholar] [CrossRef]
| Element | Fe2O3 | TiO2 | CaO | K2O | P2O5 | MgO | Na2O | Al2O3 | SiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Con. (%) | 0.419 | 0.678 | 0.177 | 0.263 | 0.382 | 0.206 | 0.424 | 49.48 | 47.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Tang, X.; Sun, H.; Shi, J.; Zhou, C.; Northwood, D.O.; Waters, K.E.; Ma, H. Surface Modification of Calcined Kaolinite for Enhanced Solvent Dispersion and Mechanical Properties in Polybutylene Adipate/Terephthalate Composites. Molecules 2024, 29, 3897. https://doi.org/10.3390/molecules29163897
Yuan Y, Tang X, Sun H, Shi J, Zhou C, Northwood DO, Waters KE, Ma H. Surface Modification of Calcined Kaolinite for Enhanced Solvent Dispersion and Mechanical Properties in Polybutylene Adipate/Terephthalate Composites. Molecules. 2024; 29(16):3897. https://doi.org/10.3390/molecules29163897
Chicago/Turabian StyleYuan, Yongbing, Xinyu Tang, Honghong Sun, Junkang Shi, Congshan Zhou, Derek O Northwood, Kristian E Waters, and Hao Ma. 2024. "Surface Modification of Calcined Kaolinite for Enhanced Solvent Dispersion and Mechanical Properties in Polybutylene Adipate/Terephthalate Composites" Molecules 29, no. 16: 3897. https://doi.org/10.3390/molecules29163897
APA StyleYuan, Y., Tang, X., Sun, H., Shi, J., Zhou, C., Northwood, D. O., Waters, K. E., & Ma, H. (2024). Surface Modification of Calcined Kaolinite for Enhanced Solvent Dispersion and Mechanical Properties in Polybutylene Adipate/Terephthalate Composites. Molecules, 29(16), 3897. https://doi.org/10.3390/molecules29163897






