Changes in Phenolic Composition and Bioactivities of Ayocote Beans under Boiling (Phaseolus coccineus L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content (TPC)
2.2. Total Anthocyanins Content (TAC)
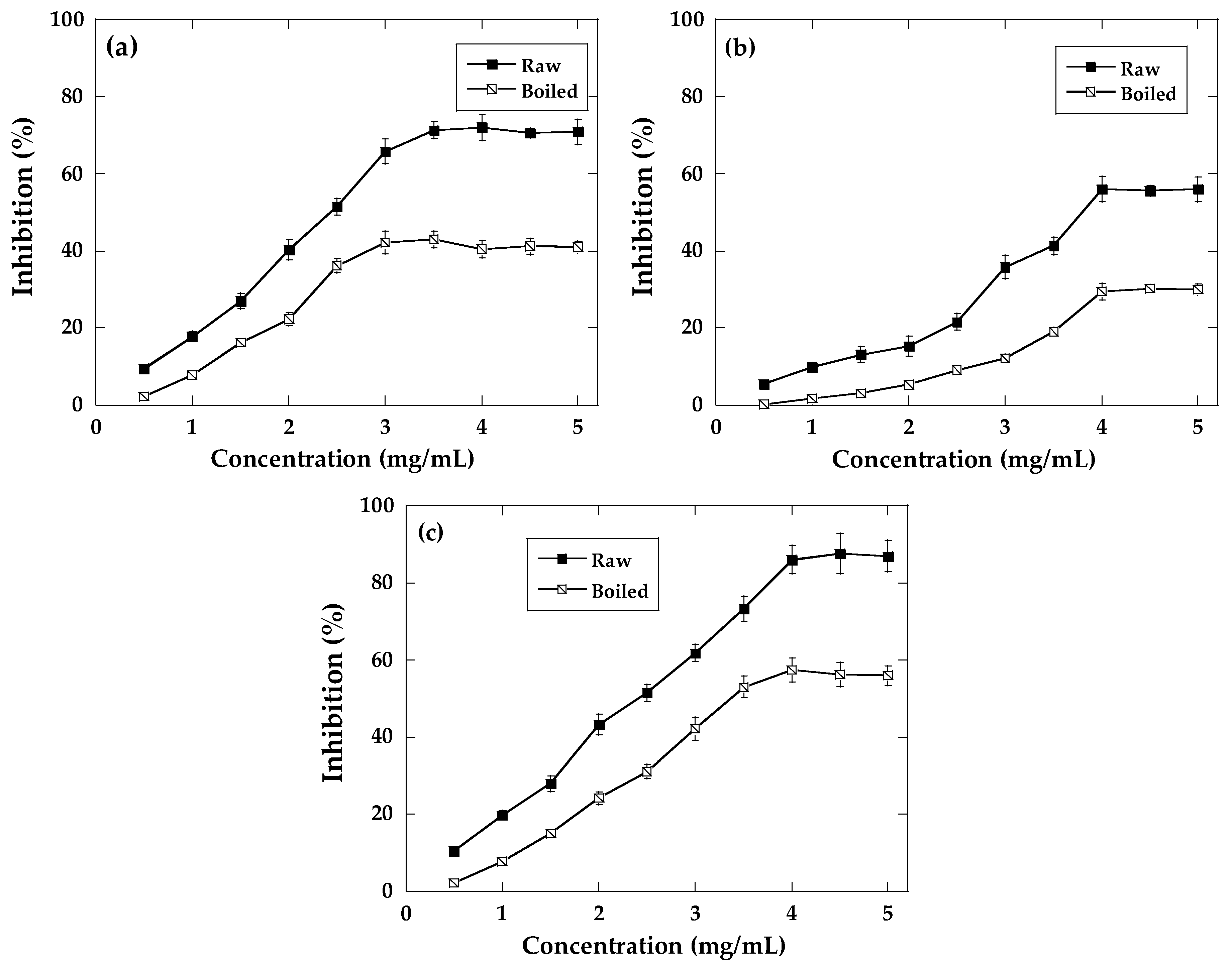
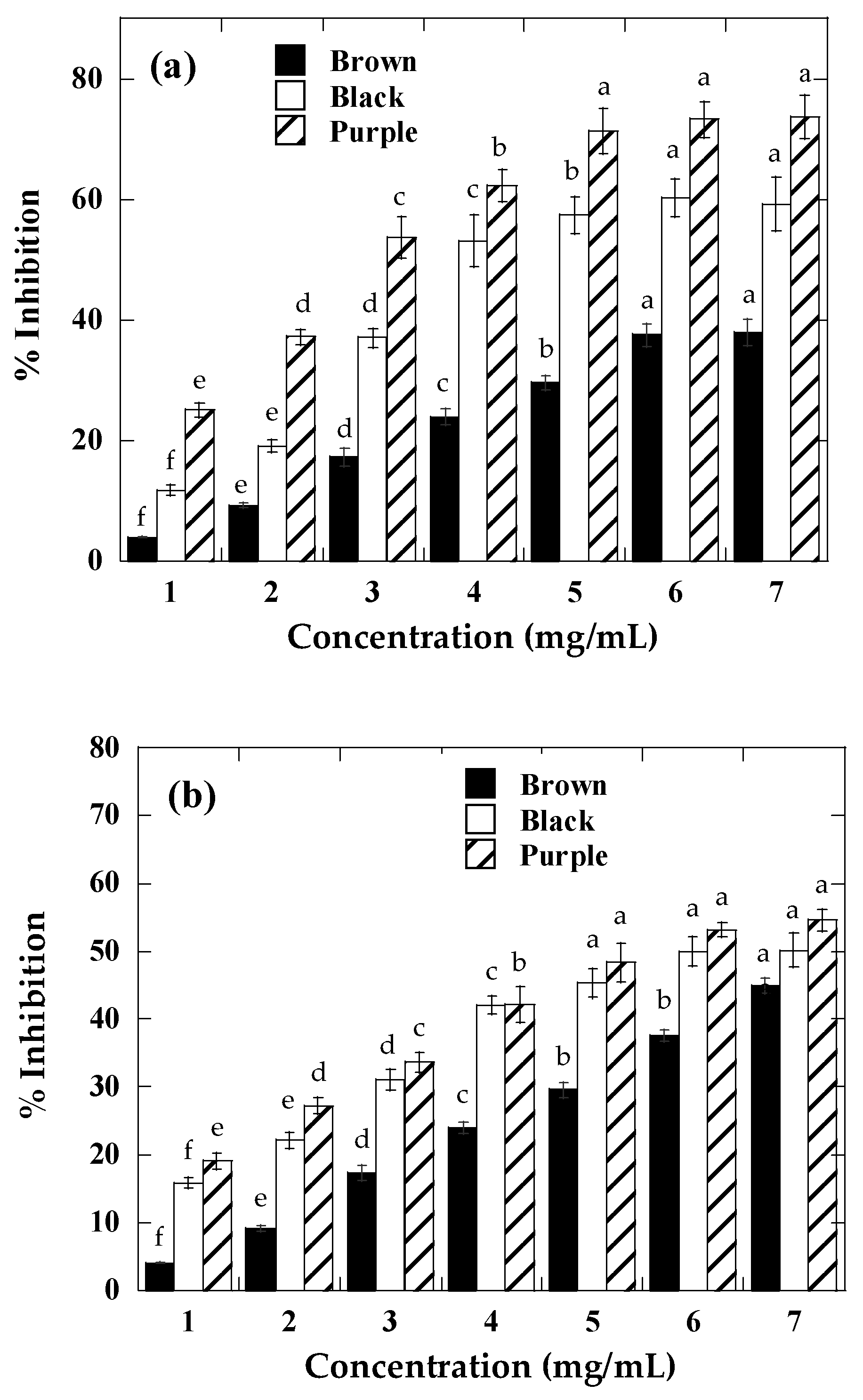
2.3. Antioxidant Activity
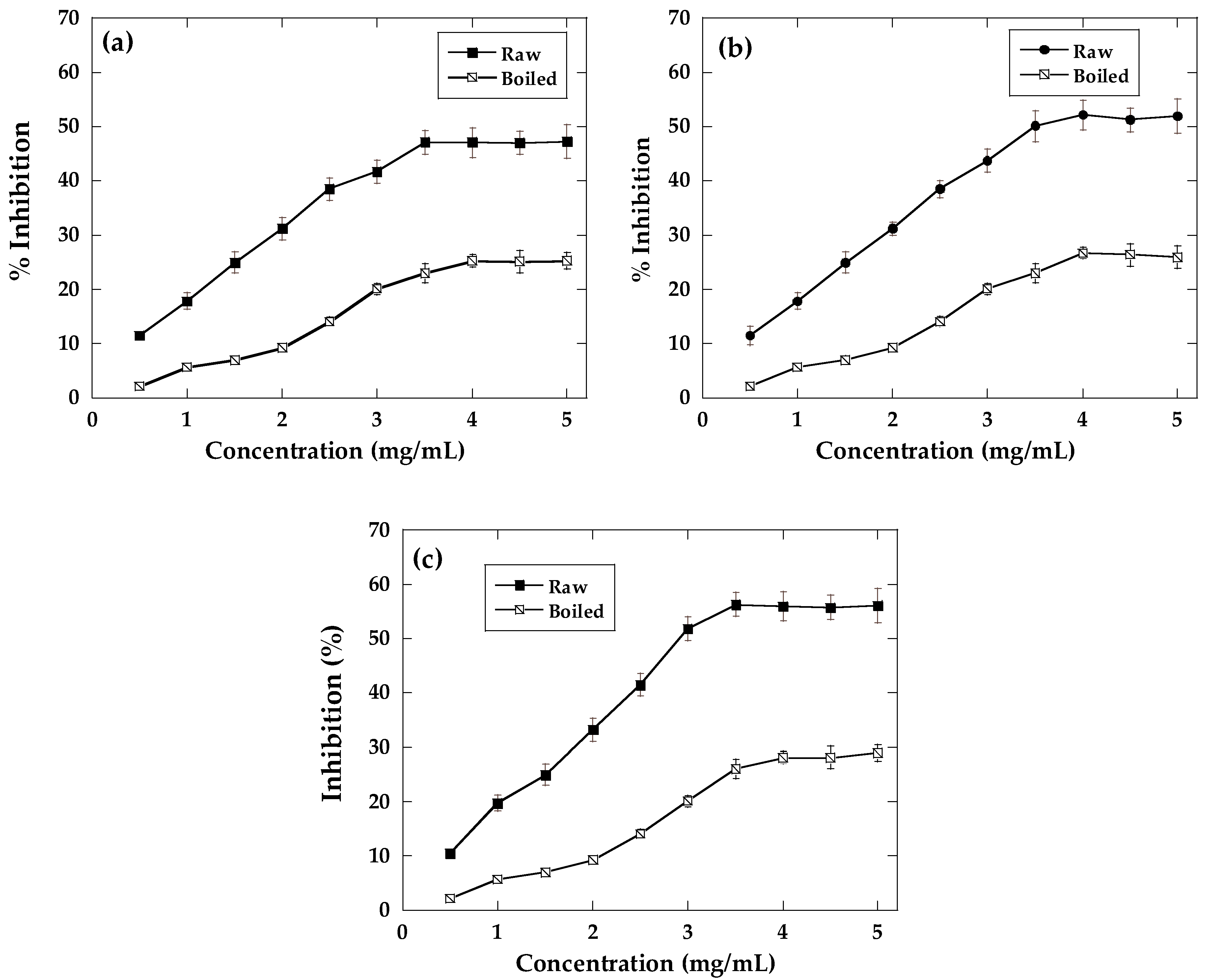
2.4. α-Amylase and α-Glucosidase Inhibition
2.5. AGE Inhibition
2.6. Phenolic Profile
3. Materials and Methods
3.1. Plant Material
3.2. Boiling Treatment
3.3. Extract Preparation
3.4. Phenolic Content Assays
3.4.1. Determination of Total Phenolic Compounds (TPC)
3.4.2. Determination of Total Anthocyanin Compounds (TAC)
3.5. Antioxidant Capacity Assays
3.6. α-Amylase Inhibition
3.7. α-Glucosidase Inhibition
3.8. Antigycation Inhibition Activity
3.9. HPLC-DAD and ESI-MSD Conditions
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawal, V.; Navarro, D.K. (Eds.) The Global Economy of Pulses; FAO: Rome, Italy, 2019. [Google Scholar]
- Alvarado-López, A.N.; Gómez-Oliván, L.M.; Heredia, J.B.; Baeza-Jiménez, R.; Garcia-Galindo, H.S.; López-Martínez, L.X. Nutritional and bioactive characteristics of Ayocote bean (Phaseolus coccienus L.): An underutilized legume, harvested in Mexico. CyTA-J. Food 2019, 17, 199–206. [Google Scholar] [CrossRef]
- Ahmad, B.; Friar, E.P.; Vohra, M.S.; Garrett, M.D.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Mechanisms of action for the anti-obesogenic activities of phytochemicals. Phytochemistry 2020, 180, 112513. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.T.; Sarfaraz, S.; Qi, X.; Ramdath, D.G.; Fougere, G.C.; Ramdath, D.D. A Review of the Relationship between lentil serving and acute postprandial blood glucose response: Effects of dietary fibre, protein and carbohydrates. Nutrients 2022, 14, 849. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Li, M.; Zhou, J.; Imran, A.; de Souza, T.S.; Barrow, C.; Dushea, F.; Suleria, H.A. Influence of processing methods on phytochemical composition of different varieties of beans (Phaseolus vulgaris). Food Rev. Int. 2023, 40, 1941–1979. [Google Scholar] [CrossRef]
- Qin, L.; Wang, H.; Zhang, W.; Pan, M.; Xie, H.; Guo, X. Effects of different drying methods on phenolic substances and antioxidant activities of seedless raisins. LWT-Food Sci. Technol. 2020, 131, 109807. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Assessing the in vitro inhibitory effects on key enzymes linked to type-2 diabetes and obesity and protein glycation by phenolic compounds of Lauraceae plant species endemic to the Laurisilva forest. Molecules 2021, 26, 2023. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nut. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.S.; Ko, S.H. Advanced Glycation End Products and Their Effect on Vascular Complications in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3086. [Google Scholar] [CrossRef]
- Liu, Y.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.S.M. Composition of phenolic acids and antioxidant properties of selected pulses cooked with different heating conditions. Foods 2020, 9, 908. [Google Scholar] [CrossRef]
- Mazı, B.G.; Yıldız, D.; Barutçu Mazı, I. Influence of different soaking and drying treatments on anti-nutritional composition and technological characteristics of red and green lentil (Lens culinaris Medik.) flour. J. Food Meas. Charact. 2023, 17, 3625–3643. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Montesano, D.; Masoero, F.; Trevisan, M. Impact of boiling on free and bound phenolic profile and antioxidant activity of commercial gluten-free pasta. Food Res. Int. 2017, 100, 69–77. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, A.; Alvarado López, A.N.; Redondo-Cuenca, A. Effect of high hydrostatic pressure and thermal treatment on polyphenolic compounds and the antioxidant capacity of Phaseolus coccineus L. Cereal Chem. 2024, 101, 750–758. [Google Scholar] [CrossRef]
- Osuna-Gallardo, E.I.; Cuevas-Rodríguez, E.O.; Sepúlveda-García, C.I.; Reyes-Moreno, C.; León-López, L.; Han, R.; Hernández-Álvarez, A.J. Impact of Cooking and Extrusion Processing on Nutritional, Antinutritional, and Techno-Functional Characteristics of Indigenous Bean (Phaseolus coccineus). ACS Food Sci. Technol. 2023, 3, 1835–1853. [Google Scholar] [CrossRef]
- Corzo-Ríos, L.J.; Sánchez-Chino, X.M.; Cardador-Martínez, A.; Martínez-Herrera, J.; Jiménez-Martínez, C. Effect of cooking on nutritional and non-nutritional compounds in two species of Phaseolus (P. vulgaris and P. coccineus) cultivated in Mexico. Int. J. Gastron. Food Sci. 2020, 20, 100206. [Google Scholar] [CrossRef]
- Bento, J.A.C.; Ribeiro, P.R.V.; e Silva, L.M.A.; Alves Filho, E.G.; Bassinello, P.Z.; de Brito, E.S.; Caliari, M.; Júnior, M.S.S. Chemical profile of colorful bean (Phaseolus vulgaris L.) flours: Changes influenced by the cooking method. Food Chem. 2021, 356, 129718. [Google Scholar] [CrossRef]
- Quiróz-Sodi, M.; Mendoza-Díaz, S.; Hernández-Sandoval, L.; Carrillo-Ángeles, I. Characterization of the secondary metabolites in the seeds of nine native bean varieties (Phaseolus vulgaris and P. coccineus) from Querétaro, Mexico. Bot. Sci. 2018, 96, 650–661. [Google Scholar] [CrossRef]
- Capistrán-Carabarin, A.; Aquino-Bolaños, E.N.; García-Díaz, Y.D.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Complementarity in phenolic compounds and the antioxidant activities of Phaseolus coccineus L. and P. vulgaris L. landraces. Foods 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Aprodu, I.; Milea, Ș.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal degradation kinetics of anthocyanins extracted from purple maize flour extract and the effect of heating on selected biological functionality. Foods 2020, 9, 1593. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Asghari Ghajari, M.; Tavasoli, S. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Montoya, P.; Vio, F.; Speisky, H. Total phenolics and antioxidant capacity of vegetables grown in the Southwestern Andes Region of South America. J. Food Nutr. Res. 2016, 4, 760–772. [Google Scholar] [CrossRef]
- Hussain, F.; Hafeez, J.; Khalifa, A.S.; Naeem, M.; Ali, T.; Eed, E.M. In vitro and in vivo study of inhibitory potentials of α-glucosidase and acetylcholinesterase and biochemical profiling of M. charantia in alloxan-induced diabetic rat models. Am. J. Trans. Res. 2022, 14, 3824. [Google Scholar]
- Molina, O.M.; Domínguez-Avila, J.A.; Pareek, S.; Madera Santana, T.J.; González Aguilar, G.A.; Lopez-Martínez, L.X. Valorization of tropical fruit peel powders: Physico chemical composition, techno-functional properties, and in vitro antioxidant and antidiabetic activities. EJFA 2023, 35, 577–587. [Google Scholar]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Neves, N.; Stringheta, P.C.; da Silva, I.F.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Identification and quantification of phenolic composition from different species of Jabuticaba (Plinia spp.) by HPLC-DAD-ESI/MSn. Food Chem. 2021, 355, 129605. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Biological potential of sixteen legumes in China. Int. J. Mol. Sci. 2011, 12, 7048–7058. [Google Scholar] [CrossRef]
- Azizan, A.; Lee, A.X.; Abdul Hamid, N.A.; Maulidiani, M.; Mediani, A.; Abdul Ghafar, S.Z.; Zulaikha Zolkefle, N.K.; Abas, F. Potentially bioactive metabolites from pineapple waste extracts and their antioxidant and α-glucosidase inhibitory activities by 1H NMR. Foods 2020, 9, 173. [Google Scholar] [CrossRef]
- Salahuddin, M.A.H.; Ismail, A.; Kassim, N.K.; Hamid, M.; Ali, M.S.M. Phenolic profiling and evaluation of in vitro antioxidant, α-glucosidase and α-amylase inhibitory activities of Lepisanthes fruticosa (Roxb) Leenh fruit extracts. Food Chem. 2020, 331, 127240. [Google Scholar] [CrossRef]
- Lan, M.Y.; Li, H.M.; Tao, G.; Lin, J.; Lu, M.W.; Yan, R.A.; Huang, J.Q. Effects of four bamboo derived flavonoids on advanced glycation end products formation in vitro. J. Funct. Foods 2020, 71, 103976. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.L.R.; de Oliveira, L.L.; Freitas, V.V.; Júnior, B.R.D.C.L.; Nascimento, A.L.A.A.; Castro, G.A.D.; Stringheta, P.C. Digestive enzymes inhibition, antioxidant and antiglycation activities of phenolic compounds from jabuticaba (Plinia cauliflora) peel. Food Biosci. 2022, 50, 102195. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and antiglycation effects of polyphenol compounds extracted from hazelnut skin on advanced glycation end-products (AGEs) formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-amylase, α-glucosidase, and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyperglycaemia-related damage linked with aldose reductase activity and protein glycation. LWT-Food Sci. Technol. 2020, 118, 108727. [Google Scholar] [CrossRef]
- Teixeira-Guedes, C.I.; Oppolzer, D.; Barros, A.I.; Pereira-Wilson, C. Impact of cooking method on phenolic composition and antioxidant potential of four varieties of Phaseolus vulgaris L. and Glycine max L. LWT-Food Sci. Technol. 2019, 103, 238–246. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Effect of different cooking conditions on phenolic compounds and antioxidant capacity of some selected Brazilian bean (Phaseolus vulgaris L.) cultivars. J. Agric. Food Chem. 2009, 57, 5734–5742. [Google Scholar] [CrossRef]
- López-García, G.; Baeza-Jiménez, R.; Garcia-Galindo, H.S.; Dublán-García, O.; López-Martínez, L.X. Cooking treatments effect on bioactive compounds and antioxidant activity of quintonil (Amaranthus hybridus) harvested in spring and fall seasons. CyTA-J. Food. 2018, 16, 707–714. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, Y.; Okuno, R.; Kameda, K.; Isobe, M.; Kondo, T. Structural analysis, and measurement of anthocyanins from colored seed coats of Vigna, Phaseolus, and Glycine legumes. Biosci. Biotechnol. Biochem. 1996, 60, 589–593. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Phaseolus vulgaris L.) as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 4754–4764. [Google Scholar] [CrossRef]
- Yang, Z.W.; Tang, C.E.; Zhang, J.L.; Zhou, Q.; Zhang, Z.C. Stability and antioxidant activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) subjected to simulated in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2019, 54, 2604–2614. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Enachi, E.; Stănciuc, N.; Aprodu, I. Optimization of ultrasound assisted extraction of phenolic compounds from cornelian cherry fruits using response surface methodology. CyTA-J. Food 2019, 17, 814–823. [Google Scholar] [CrossRef]
- Fontes-Zepeda, A.; Domínguez-Avila, J.A.; Lopez-Martinez, L.X.; Cruz-Valenzuela, M.R.; Robles-Sánchez, R.M.; Salazar-López, N.J.; González-Aguilar, G.A. The addition of mango and papaya peels to corn extrudates enriches their phenolic compound profile and maintains their sensory characteristics. Waste Biomass Valorization 2023, 14, 751–764. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheat. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Worthington, V. Alpha Amylase Worthington Enzyme Manual; Worthington Biochemical: Lakewood, NJ, USA, 1993; pp. 36–41. [Google Scholar]
- Cuevas-Juárez, E.; Yuriar-Arredondo, K.Y.; Pío-León, J.F.; Montes-Avila, J.; López-Angulo, G.; Díaz-Camacho, S.P.; Delgado-Vargas, F. Antioxidant and α-glucosidase inhibitory properties of soluble melanins from the fruits of Vitex mollis Kunth, Randia echinocarpa Sessé et Mociño and Crescentia alata. J. Funct. Foods 2014, 9, 78–88. [Google Scholar] [CrossRef]
- Peng, X.F.; Zheng, Z.P.; Cheng, K.W.; Shan, F.; Ren, G.X.; Chen, F.; Wang, M.F. Inhibition effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation end products. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, L.X.; Parkin, K.L.; Garcia, H.S. Antioxidant and quinone reductase inducing activities of ethanolic fractions from purple maize. LWT-Food Sci. Technol. 2014, 59, 270–275. [Google Scholar] [CrossRef]
| Sample | TPC (mg GAE/100 g dw) | TAC (mg C3GE/100 g dw) | DPPH (µmol TE/100 g dw) | ORAC (µmol TE/100 g dw) |
|---|---|---|---|---|
| Brown ayocote | ||||
| Raw | 152.73 ± 10.19 cA | 21.42 ± 0.98 cA | 1630.27 ± 22.11 cA | 3096.62 ± 20.16 cA |
| Cooked | 45.49 ± 1.25 cB | 5.50 ± 0.34 bB | 958.44 ± 19.36 bB | 1061.61 ± 26.15 bB |
| Black ayocote | ||||
| Raw | 218.29 ± 7.56 bB | 75.47 ± 4.16 bA | 1976.09 ± 16.97 bA | 4234.29 ± 10.48 bA |
| Cooked | 86.87 ± 3.33 bA | 14.61 ± 0.05 aB | 1049.13 ± 9.44 aB | 2049.26 ± 21.33 aB |
| Purple ayocote | ||||
| Raw | 263.20 ± 9.25 aA | 86.92 ± 4.25 aA | 2022.51 ± 23.16 aA | 6162.65 ± 14.52 aA |
| Cooked | 110.17 ± 6.19 aB | 12.43 ± 1.19 aB | 827.01 ± 10.28 cB | 2113.56 ± 16.25 aB |
| Ayocote Beans | ||||||
|---|---|---|---|---|---|---|
| Black | Purple | Brown | ||||
| Phenolic acids | ||||||
| Compounds | Raw | Boiled | Raw | Boiled | Raw | Boiled |
| GA | 8.46 ± 0.81 a | 3.32 ± 1.23 b | 40.21 ± 6.61 a | 7.46 ± 0.36 b | 60.27 ± 1.61 a | 10.17 ± 0.97 b |
| SA | 20.12 ± 1.56 a | 4.12 ± 1.16 b | 40.46 ± 4.55 a | 5.29 ± 0.32 b | 60.03 ± 3.41 a | 5.23 ± 0.51 b |
| FA | 21.06 ± 1.64 a | 0.27 ± 0.11 b | 130.38 ± 5.80 a | 1.42 ± 0.37 b | 80.45 ± 5.11 a | 1.39 ± 0.05 b |
| 4 HBA | 16.30 + 1.34 a | 4.29 + 0.53 b | ND | ND | ND | ND |
| ChA | 45.59 ± 3.50 a | 15.33 ± 3.87 b | 170.61 ± 5.12 a | 50.21 ± 5.17 b | 70.77 ± 3.63 a | 20.51 ± 1.45 b |
| pCA | 12.06 ± 1.22 a | ND | 60.42 ± 3.65 a | ND | 70.70 ± 2.63 a | ND |
| Flavonoids | ||||||
| Cat | 22.11 ± 1.51 a | 10.02 ± 6.45 b | 20.22 ± 1.46 a | ND | 10.17 ± 0.39 a | ND |
| ECat | 21.19 ± 1.98 a | 14.07 ± 7.88 b | 3.06 ± 0.21 a | ND | 2.88 ± 0.01 a | ND |
| K 3 gluc | 30.93 ± 1.33 a | ND | 140.90 ± 4.61 a | 3.23 ± 0.11 b | 110.05 ± 9.01 a | 4.43 ± 0.61 b |
| Myrc | 98.12 + 4.78 a | ND | ND | ND | ND | ND |
| Quer | 3.30 + 0.11 a | ND | ND | ND | ND | ND |
| Anthocyanins | ||||||
| Delp 3 gluc | 80.58 ± 6.21 a | 1.73 ± 0.01 b | 1100.56 ± 14.01 a | 30.79 ± 2.01 b | 809.26 ± 7.01 | ND |
| Cy 3 gluc | 26.10 ± 1.33 a | 2.62 ± 1.66 b | 400.77 ± 9.52 a | 45.89 ± 3.49 b | 3.41 ± 0.22 | ND |
| PT 3 glu | 63.71 ± 5.12 a | 4.55 ± 2.01 b | 2.99 ± 0.11 a | 170.71 ± 11.03 b | 2100.20 ± 10.46 a | 90.8 ± 4.51 b |
| Malv 3,5 di | 500.28 + 4.98 a | 130.54 ± 8.52 b | 140.17 ± 5.77 a | 39.18 ± 2.44 b | 33.88 ± 1.71 a | 4.05 + 0.12 b |
| Malv 3 gal | 25.84 + 1.49 | ND | 190.33 + 6.33 | ND | 46.26 ± 2.51 | ND |
| Malv 3 glu | 40.02 ± 2.29 a | 3.37 ± 1.66 b | 3100.22 ± 9.01 a | 250.64 ± 6.60 b | 730.19 ± 5.46 a | 65.8 ± 0.95 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeza-Jiménez, R.; López-Martínez, L.X. Changes in Phenolic Composition and Bioactivities of Ayocote Beans under Boiling (Phaseolus coccineus L.). Molecules 2024, 29, 3744. https://doi.org/10.3390/molecules29163744
Baeza-Jiménez R, López-Martínez LX. Changes in Phenolic Composition and Bioactivities of Ayocote Beans under Boiling (Phaseolus coccineus L.). Molecules. 2024; 29(16):3744. https://doi.org/10.3390/molecules29163744
Chicago/Turabian StyleBaeza-Jiménez, Ramiro, and Leticia X. López-Martínez. 2024. "Changes in Phenolic Composition and Bioactivities of Ayocote Beans under Boiling (Phaseolus coccineus L.)" Molecules 29, no. 16: 3744. https://doi.org/10.3390/molecules29163744
APA StyleBaeza-Jiménez, R., & López-Martínez, L. X. (2024). Changes in Phenolic Composition and Bioactivities of Ayocote Beans under Boiling (Phaseolus coccineus L.). Molecules, 29(16), 3744. https://doi.org/10.3390/molecules29163744









