Abstract
Sodium-ion batteries (SIBs) have received considerable attention in recent years. Anode material is one of the key factors that determine SIBs’ electrochemical performance. Current commercial hard carbon anode shows poor rate performance, which greatly limits applications of SIBs. In this study, a novel vanadium-based material, SrV4O9, was proposed as an anode for SIBs, and its Na+ storage properties were studied for the first time. To enhance the electrical conductivity of SrV4O9 material, a microflower structure was designed and reduced graphene oxide (rGO) was introduced as a host to support SrV4O9 microflowers. The microflower structure effectively reduced electron diffusion distance, thus enhancing the electrical conductivity of the SrV4O9 material. The rGO showed excellent flexibility and electrical conductivity, which effectively improved the cycling life and rate performance of the SrV4O9 composite material. As a result, the SrV4O9@rGO composite showed excellent electrochemical performance (a stable capacity of 273.4 mAh g−1 after 200 cycles at 0.2 A g−1 and a high capacity of 120.4 mAh g−1 at 10.0 A g−1), indicating that SrV4O9@rGO composite can be an ideal anode material for SIBs.
1. Introduction
The widespread use of fossil fuels has created several energy and environmental issues that threaten the sustainable development of human society. It is important to develop renewable energy to overcome environmental problems. However, renewable energy—solar, tidal, and wind—is generally unstable, and requires the support of energy storage systems. Among various energy storage systems, lithium-ion batteries (LIBs) are widely used in consumer electronics and power tools due to their high energy density, environmental friendliness, and no memory effect [1,2,3,4,5,6,7,8]. However, due to required expensive rare metals, such as lithium and cobalt, LIBs cannot well meet the demand for widespread energy storage.
In recent years, sodium-ion batteries (SIBs) have received considerable attention from international researchers due to their many advantages: (1) sodium resources are abundant and widely distributed [9,10,11], (2) SIBs have similar working mechanisms to LIBs so they can be manufactured by the same technologies as LIBs [12], (3) to achieve the same ionic conductivity, the concentration of sodium salts in electrolytes is lower than that of lithium salts, resulting in lower cost, (4) aluminum foil can be used as the current collector for the anode in SIBs because Na+ does not react with aluminum, further reducing the cost, and (5) SIBs have high safety performance due to higher internal resistance compared to LIBs. Therefore, SIBs are considered as an alternative to LIBs in some areas.
The anode material is one of the key factors determining the electrochemical performance of batteries [13,14,15,16,17,18]. The main anode materials for SIBs include carbon materials, alloy materials, transition metal oxides, and organic compounds. Among them, hard carbons are the most cost effective, making them promising for industrial application. However, the rate performance of hard carbons is poor, which limits their applications [19,20]. Therefore, it is essential to develop anode materials with high Na+ diffusion coefficient for SIBs. Vanadium-based materials have been widely studied as anode materials for SIBs [21,22] due to several advantages. Firstly, they exhibit high capacity due to the multivalent nature of vanadium. Secondly, they have a high Na+ diffusion coefficient. Finally, vanadium resources are abundant and environmentally friendly. To date, a novel vanadium-based material (SrV4O9) has been prepared and has shown excellent Zn2+ storage performance [23]. However, research into SrV4O9 as an anode material for SIBs has not been reported.
In this work, the Na+ storage performance of SrV4O9 material was studied to enhance the electrical conductivity of SrV4O9 material, and a microflower structure was designed. Reduced graphene oxide (rGO) was then introduced as a host to support SrV4O9 microflowers. The microflower structure effectively reduced electron diffusion distance, thus enhancing the electrical conductivity of the SrV4O9 material. In addition, the rGO showed excellent flexibility and electrical conductivity, which can effectively improve the cycling life and rate performance of SrV4O9 material. As a result, the SrV4O9@rGO anode exhibited excellent cycling performance and remarkable rate performance.
2. Results and Discussion
The preparation scheme for SrV4O9@rGO is shown in Figure 1. The SrV4O9@rGO shows a microflower structure. The mechanism of formation of the SrV4O9 flower-like structure involved a combination of factors, including precursor and growth conditions. First, Sr(OH)2∙8H2O and V2O5 were dissolved in a solvent and heated at 200 °C for 48 h to form a precursor cluster. The precursor was then heated to 450 °C under argon and held for 5 h. During this process, many branches were formed on the cluster and then grew to eventually form hierarchical structures with intricate morphologies resembling flowers.
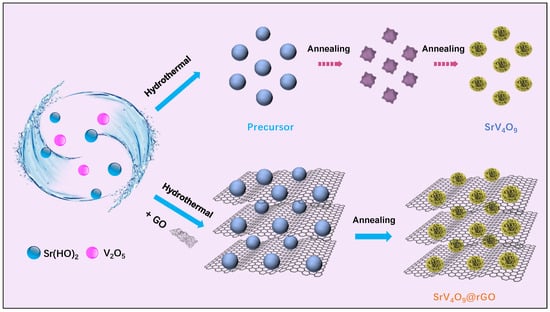
Figure 1.
The preparation scheme for SrV4O9 and the SrV4O9@rGO composite.
The structure of the SrV4O9@rGO composite was investigated by XRD. As shown in Figure 2a, peaks at 16.8°, 29.2°, 33.8°, 35.8°, 48.7°, 52.5°, 57.7°, and 71.1° can be assigned to the (001), (121), (310), (102), (240), (322), (501), and (620) planes of SrV4O9 (JCPDS No. 70-4468) [23], respectively. Note that rGO diffraction peaks were observed at 26.8° and 42.4°. However, the intensity of the rGO diffraction peaks was weak due to the dense coating of SrV4O9 on the rGO. To probe the structure of the SrV4O9@rGO composite, Raman spectroscopy experiments were performed. As seen in Figure 2b, peaks at 148, 292, 380, 524, 682, and 992 cm−1 can be attributed to SrV4O9 [23]. For the SrV4O9@rGO composite, two additional broad peaks at 1348 and 1578 cm−1 corresponded to the D-band and G-band of the rGO, respectively [24,25]. The SrV4O9@rGO composite was further tested by nitrogen adsorption–desorption experiments to characterize its porosity and specific surface area (Figure 2c,d). The SrV4O9@rGO composite exhibited a Type I isotherm, indicating abundant micro/mesopores in its structure [26,27]. The specific surface area of the SrV4O9@rGO composite was 41.4 m2 g−1. Figure 2d reveals that the average pore size of the SrV4O9@rGO composite was 17.2 nm, further confirming its porous structure.
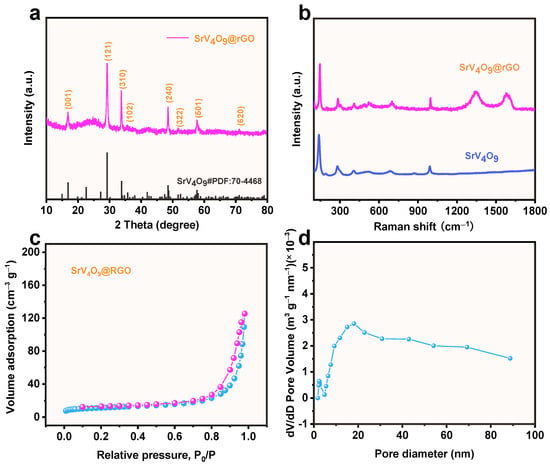
Figure 2.
(a) XRD pattern of the SrV4O9@rGO composite. (b) Raman spectra of SrV4O9 and SrV4O9@rGO. (c) N2 adsorption–desorption isotherms of SrV4O9@rGO. (d) Pore size distribution of SrV4O9@rGO.
To study its morphology, the SrV4O9@rGO composite was characterized using SEM and TEM. As shown in Figure 3a,b, the SrV4O9@rGO composite exhibited a three-dimensional porous spherical structure with a diameter of ~6 μm in which flexible rGO coated SrV4O9 microflowers. Figure 3c shows that SrV4O9 had a regular micrometer-scale flower-like structure with an average diameter of ~1 µm consisting of porous ultrathin nanosheets with a thickness of ~50 nm (Figure 3d). TEM images (Figure 3e,f) confirmed the three-dimensional porous structure of the SrV4O9@rGO composite. The porous nanostructure increases the contact area between the active material and the electrolyte and effectively reduces the ion diffusion distance, thus improving ion transport efficiency [28,29,30,31,32]. In addition, the porous nanostructure provides sufficient buffering space for volume expansion during cycling processes, effectively enhancing cycling stability [33,34,35,36,37,38]. As shown in Figure 3g–j, Sr, V, O, and C were uniformly dispersed in the SrV4O9@rGO composite, indicating that SrV4O9 was uniformly loaded in rGO.
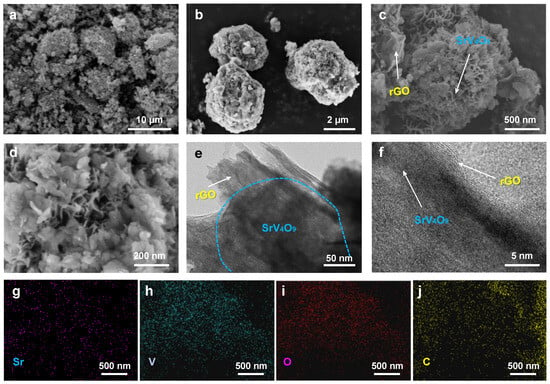
Figure 3.
(a–d) SEM images of the SrV4O9@rGO composite. (e,f) TEM images of the SrV4O9@rGO composite. (g–j) EDS elemental mapping of Sr, V, O, and C.
XPS is a powerful analytical technique for characterizing the chemical composition and oxidation state of materials. The importance of XPS lies in its ability to provide detailed information about the chemical composition of the SrV4O9@rGO composite at the atomic level. Figure 4a shows the XPS survey spectrum of the SrV4O9@rGO composite, which shows the photoemission characteristics of Sr, V, O, and C. The high-resolution spectrum of Sr 3d (Figure 4b) can be deconvoluted into Sr2+ 3d5/2 (135.6 eV) and Sr2+ 3d3/2 (133.2 eV) [23]. The V 2p high-resolution spectrum (Figure 4c) can be deconvoluted into V4+ 2p1/2 (523.6 eV) and V4+ 2p3/2 (516.6 eV) [39,40]. The high-resolution spectrum of O 1s (Figure 4d) exhibits two peaks, at 531.6 eV and 530.4 eV; the peak at 530.4 eV corresponds to the bonding of adsorbed oxygen on the surface of the SrV4O9@rGO composite with Sr and V, denoted as Sr–O–V [23], while the peak at 531.8 eV was likely due to the presence of H2O. As shown in Figure 4e, the high-resolution spectrum of C 1s reveals distinct peaks at 285.6 eV and 284.8 eV, corresponding to C–O and C–C bonds, respectively [41,42].
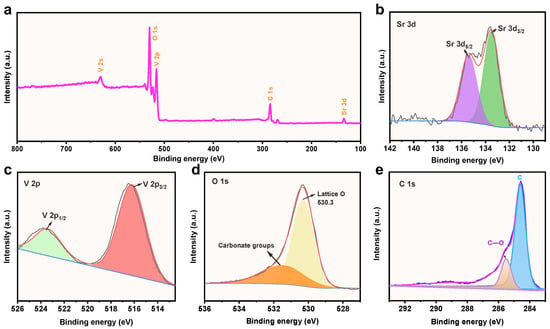
Figure 4.
(a) The XPS survey spectrum of the SrV4O9@rGO composite. (b) The high-resolution spectrum of Sr 3d. (c) The high-resolution spectrum of V 2p. (d) The high-resolution spectrum of O 1s. (e) The high-resolution spectrum of C 1s.
Figure 5a,b compare the CV curves of SrV4O9-based and SrV4O9@rGO-based electrodes for the first three cycles between 0.01 and 3.0 V at a scan rate of 0.1 mV s−1. All CV curves exhibited two reduction peaks and one oxidation peak. Two reduction peaks appeared at ~0.75 V and ~0.45 V, representing the penetration of sodium ions into SrV4O9. A weak and broad oxidation peak at around 1.48 V might correspond to the oxidation reaction of V. And the CV curves of the second and third cycles showed a high degree of overlap, indicating good reversibility of the electrochemical reactions. It is worth noting that the peak current densities of the oxidation–reduction peaks of the SrV4O9@rGO-based electrode (Figure 5b) were higher than those of the SrV4O9-based electrode (Figure 5a), indicating the improved electron/ion transport kinetics of the SrV4O9@rGO composite facilitated by rGO and more dispersed SrV4O9. The cycling performances of the SrV4O9-based and SrV4O9@rGO-based electrodes at 0.2 A g−1 are shown in Figure 5c. The SrV4O9@rGO composite showed a high initial reversible capacity of 284.4 mAh g−1 and maintained a stable capacity of 273.4 mAh g−1 after 200 cycles, with an ultra-high capacity retention of 96.1%. For the SrV4O9 electrode, the capacity after 200 cycles was only 142.5 mAh g−1. The excellent cycling performance can be attributed to the super aspect ratio of the porous microflower structure, which facilitated ion transfer. The introduction of rGO increased the specific surface area involved in the reaction, providing more reaction sites. The galvanostatic charge–discharge (GCD) curves of the SrV4O9@rGO electrode are shown in Figure 5d. These GCD curves show a high degree of overlap, indicating excellent cycling performance of the SrV4O9@rGO electrode. To characterize the positive effect of rGO on SrV4O9, an EIS test was performed. Figure S1 shows Nyquist plots for SrV4O9 and SrV4O9@rGO electrodes in the initial state and the SrV4O9@rGO electrode after 200 cycles. The SrV4O9@rGO electrode exhibited a smaller charge transfer resistance than the SrV4O9 electrode in the initial state, indicating that rGO improved the rate of Na+ transfer. A comparison between the initial and cycled electrodes revealed a significant decrease in impedance for the SrV4O9@rGO electrode after 200 cycles at 0.2 A g−1 due to the considerably enhanced ion and electron transfer in the electrode. The SEM image of the SrV4O9@rGO electrode after 200 cycles (Figure S2) revealed that the SrV4O9@rGO electrode had good structural stability after electrochemical testing. The SrV4O9@rGO electrode also demonstrated excellent rate performance (Figure 5e). At 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 A g−1, the specific capacities of the SrV4O9@rGO electrode remained at 287.1, 268.5, 250.4, 228.7, 209.6, 168.2, and 120.4 mAh g−1, respectively, which were significantly higher than those of the SrV4O9 electrode (149.4, 119.0, 85.6, 69.2, 47.3, 23.1, and 7.7 mAh g−1, respectively). The cycling performance of the SrV4O9@rGO composite at a high rate (1 A g−1) was also tested, and results are shown in Figure S3. The SrV4O9@rGO composite delivered an initial reversible discharge capacity of 278.3 mAh g−1 at 1.0 A g−1. The capacity decreased slightly to 265.7 mAh g−1 after 310 cycles, demonstrating good cycling performance at a high rate. The improved electrochemical performance of the SrV4O9@rGO composite can be attributed to the large aspect ratio of the composite and improved electrical conductivity [43,44] (Figure 5f).
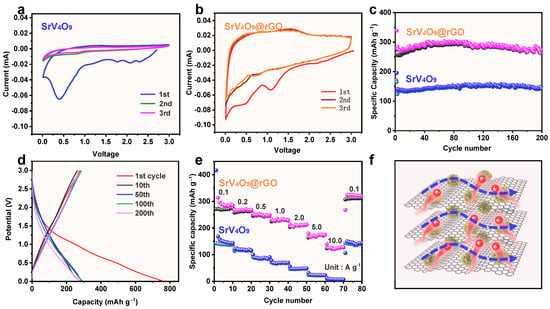
Figure 5.
(a) CV curves of the SrV4O9-based electrode for the initial three cycles between 0.01 and 3.0 V at a scan rate of 0.1 mV s−1. (b) CV curves of SrV4O9@rGO-based electrodes for the initial three cycles between 0.01 and 3.0 V at a scan rate of 0.1 mV s−1. (c) Cycling performance of the SrV4O9-based electrode and the SrV4O9@rGO-based electrode at 0.2 A g−1. (d) Voltage profiles of the SrV4O9@rGO-based electrode at 0.2 A g−1. (e) Rate performance of the SrV4O9-based electrode and the SrV4O9@rGO-based electrode. (f) Schematic illustration of the reaction mechanism of the SrV4O9@rGO-based electrode.
3. Experimental Section
3.1. Materials
All reagents were directly used after purchase. Sr(OH)2∙8H2O, C3H8O3, V2O5, and H2O2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). GO was provided by Nanjing XFNANO Materials Tech Co., Ltd. (Nanjing, China).
3.2. Synthesis of SrV4O9@rGO Composite and SrV4O9
The SrV4O9@rGO composite was prepared using the hydrothermal annealing method. First, 1 mmol of Sr(OH)2∙8H2O was dissolved in a mixed solution consisting of 10 mL of deionized water and 10 mL of C3H8O3. Next, 2 mmol (0.364 g) of V2O5 and 0.05 g of GO were added to a mixed solution consisting of 10 mL of deionized water and 5 mL of H2O2 (30%) and stirred for 1 h, and was then slowly added to the Sr(OH)2∙8H2O solution, followed by stirring for 2 h. The mixed solution was transferred to a hydrothermal reactor and heated in an oven at 200 °C for 48 h. After cooling to room temperature, the precipitate was collected after several washes with deionized water and ethanol. The collected material was then dried in a vacuum oven at 80 °C for 24 h. Finally, the collected material was heated to 450 °C at a heating rate of 5 °C min−1 in a tube furnace under argon and then held for 5 h to obtain SrV4O9@rGO. For comparison, SrV4O9 was prepared using the same procedures without the addition of GO.
3.3. Characterization
X-ray diffraction (XRD, Rigaku MiniFlexll, Rigaku Corporation, Tokyo, Japan) patterns were collected from 10° to 80° using Cu Kα radiation (λ = 1.5408 Å). X-ray photoelectron spectra (XPS, Thermo Scientific K-Alpha, Waltham, MA, USA) surveys and specific patterns were obtained with an Al Kα X-ray source. The structures of SrV4O9 and SrV4O9@rGO were further examined by Raman spectroscopy (Thermo Fisher DXR2xi). Morphologies of SrV4O9 and the SrV4O9@rGO composite were analyzed by scanning electron microscopy (SEM, JEOL JSM-7800F Field Emission, Manufacturer JEOL Ltd., Tokyo, Japan) with EDS mapping (JEOL JSM-7100F, Manufacturer JEOL Ltd., Tokyo, Japan) and transmission electron microscopy (TEM, JEOL JEM, 1011, Manufacturer JEOL Ltd., Tokyo, Japan). N2 adsorption–desorption isotherms were measured on an Autosorb-iQ instrument (Quantachrome, Anton Paar acquired Quantachrome Instruments, Inc., Boynton Beach, FL, USA).
3.4. Electrochemical Measurements
To prepare the working electrode, SrV4O9 or the SrV4O9@rGO composite (70 wt%) was combined with acetylene black (20 wt%) and polyvinylidene difluoride (10 wt%) in 1-methyl-2-pyrrolidone to form a uniform slurry. The slurry was then coated onto copper foil and dried at 80 °C for 12 h. The loading density of the active materials was about 1.2–1.5 mg cm−2. For electrochemical performance testing, a CR2025 coin cell was assembled with SrV4O9 or the SrV4O9@rGO composite as the working electrode, Na foil as the counter electrode, and glass fiber membrane (Whatman GF/A) as the separator. The electrolyte was 1 M NaClO4 dissolved in ethylene carbonate/dimethyl carbonate (EC/DMC, 1:1 by volume) with 5 wt% of fluorodimethylene carbonate (FEC) as an additive. Galvanostatic charge–discharge curves and cycling performance evaluations were performed on a LAND battery test system in the range of 0.01–3.0 V. Cyclic voltammetry (CV) tests were performed using the CHI660e electrochemical workstation.
4. Conclusions
In summary, an rGO-supported SrV4O9 composite was synthesized as an anode material for SIBs. The SrV4O9 had a regular micrometer-scale flower-like structure consisting of porous ultrathin nanosheets with a thickness of ~50 nm, which was coated with rGO to form a three-dimensional porous spherical structure. The porous nanostructure increased the contact area between SrV4O9@rGO and the electrolyte and effectively reduced the ion diffusion distance, thus improving ion transport efficiency. In addition, the porous nanostructure reduced SrV4O9 aggregation and provided sufficient buffering space for volume expansion during cycling processes, effectively improving cycling stability. Therefore, the SrV4O9@rGO composite showed a high initial reversible capacity of 284.4 mAh g−1 at 0.2 A g−1 and retained a stable capacity of 273.4 mAh g−1 after 200 cycles with an ultra-high capacity retention of 96.1%. At 0.1, 0.2, 0.5, 1.0, 2.0, 5.0, and 10.0 A g−1, the specific capacities of the SrV4O9@rGO electrode remained at 287.1, 268.5, 250.4, 228.7, 209.6, 168.2, and 120.4 mAh g−1, respectively, demonstrating excellent rate performance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29112704/s1, Figure S1: Nyquist plots of the SrV4O9 and SrV4O9@rGO electrodes in the initial state and the SrV4O9@rGO electrode after 200 cycles; Figure S2: SEM image of the SrV4O9@rGO electrode after 200 cycles at 0.2 A g−1; Figure S3: Cycling performance of the SrV4O9@rGO composite after 200 cycles at 1 A g−1.
Author Contributions
Writing—original draft preparation, G.L.; Conceptualization, Y.L.; Investigation, Y.Z. and S.L.; Funding acquisition, J.H.; Project administration, H.L.; Writing—review and editing, B.F. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Foundation of Key Laboratory of Flexible Electronics of Zhejiang Province (No. 2023FE011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the corresponding authors.
Conflicts of Interest
Guangming Li was employed by CNG Wind Energy Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gu, S.; Kong, J.; Fang, B. Comprehensive recycling of spent lithium-ion batteries cathodes and anodes via targeted electrochemical redox process. Green Chem. 2024, 26, 4484–4492. [Google Scholar] [CrossRef]
- Li, J.; Xie, Q.; Zhao, Y.; Zhao, P.; Zhang, S.; Huang, W. Unveiling morphology evolution and performance enhancement of tin-doped Co3O4 porous nanoarrays anchored on stainless-steel mesh for advanced lithium-ion battery anodes. J. Energy Storage 2024, 88, 111605. [Google Scholar] [CrossRef]
- Sun, R.; Qin, Z.; Li, Z.; Fan, H.; Lu, S. Binary zinc-cobalt metal-organic framework derived mesoporous ZnCo2O4@NC polyhedron as a high-performance lithium-ion battery anode. Dalton Trans. 2020, 49, 14237–14242. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Jin, S.; Xia, Q.; Chang, Y.; Wang, L.; Zhou, A. Synthesis of Mo2C MXene with high electrochemical performance by alkali hydrothermal etching. J. Adv. Ceram. 2023, 12, 1889–1901. [Google Scholar] [CrossRef]
- Fang, B.; Wang, Y.; Wang, H. Does an LaCl3-based lithium superionic conductor work well for anode-free lithium metal batteries? Matter 2023, 6, 2508–2510. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Quan, K.; Wu, L.; Feng, X.; Wang, W. One-pot cocrystallization of mononuclear and 1D cobalt (II) complexes based on flexible triclopyr and 2, 2′-bipyridine coligands: Structural analyses, conformation comparison, non-covalent interactions and magnetic properties. J. Mol. Struct. 2024, 1297, 136830. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Hong, J.; Wang, X.; Huang, X. Boosting bidirectional conversion of polysulfide driven by the built-in electric field of MoS2/MoP Mott–Schottky heterostructures in lithium–sulfur batteries. J. Adv. Ceram. 2023, 12, 1872–1888. [Google Scholar] [CrossRef]
- Zheng, S.; Mo, L.; Chen, K.; Chen, A.-L.; Zhang, X.; Fan, X.; Lai, F.; Wei, Q.; Miao, Y.-E.; Liu, T.; et al. Precise Control of Li+ Directed Transport via Electronegative Polymer Brushes on Polyolefin Separators for Dendrite-Free Lithium Deposition. Adv. Funct. Mater. 2022, 32, 2201430. [Google Scholar] [CrossRef]
- Li, Z.; Sun, R.; Qin, Z.; Liu, X.; Wang, C.; Lu, S.; Zhang, Y.; Fan, H. Coupling of ReS2 nanosheet arrays with hollow NiCoS4 nanocubes enables ultrafast Na+ diffusion kinetics and super Na+ storage of a NiCoS4@ReS2 heterostructure. Mater. Chem. Front. 2021, 5, 7540–7547. [Google Scholar] [CrossRef]
- Zhu, J.; He, Q.; Liu, Y.; Key, J.; Nie, S.; Wu, M.; Shen, P.K. Three-dimensional, hetero-structured, Cu3P@C nanosheets with excellent cycling stability as Na-ion battery anode material. J. Mater. Chem. A 2019, 7, 16999–17007. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, P.; Zeng, Q.; Wang, G.; Wu, K.; Ma, S.; Shen, P.K.; Wu, X.-L. MnS@N,S Co-Doped Carbon Core/Shell Nanocubes: Sulfur-Bridged Bonds Enhanced Na-Storage Properties Revealed by In Situ Raman Spectroscopy and Transmission Electron Microscopy. Small 2020, 16, 2003001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, F.; Chen, X.; Xiong, P.; Lin, C.; Wang, H.-E.; Wei, M.; Qian, Q.; Chen, Q.; Zeng, L. Extraordinarily stable and wide-temperature range sodium/potassium-ion batteries based on 1D SnSe2-SePAN composite nanofibers. InfoMat 2023, 5, e12467. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, L.; Xiang, S.; Yu, S.; Johnson, H.M.; Wang, S.; Yin, J.; Zhao, J.; Luo, Y.; Chu, P.K. Unleashing the Potential of MXene-Based Flexible Materials for High-Performance Energy Storage Devices. Adv. Sci. 2024, 11, 2304874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-L.; Cheng, W.-N.; Bai, Y.-Z.; Hou, C.; Li, K.; Huang, Y.-A. Rise of flexible high-temperature electronics. Rare Met. 2023, 42, 1773–1777. [Google Scholar] [CrossRef]
- Lin, H.; Lin, C.; Xiao, F.; He, L.; Xiong, P.; Luo, Y.; Hu, X.; Qian, Q.; Chen, Q.; Wen, Z.; et al. High-Performance Wide-pH Zn-Based Batteries via Electrode Interface Regulation with Valine Additive. Adv. Funct. Mater. 2024, 34, 2310486. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, S.; Shao, X.; Chen, J.; Fang, D.-L.; Li, S.; Mao, A.; Li, C. Synergetic effect of lattice distortion and oxygen vacancies on high-rate lithium-ion storage in high-entropy perovskite oxides. J. Adv. Ceram. 2023, 12, 1214–1227. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Yin, J.; Wang, J.; Manshaii, F.; Xiao, X.; Zhang, T.; Bao, H.; Jiang, S.; Chen, J. Flexible Metasurfaces for Multifunctional Interfaces. ACS Nano 2024, 18, 2685–2707. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; He, L.; Xiong, P.; Lin, H.; Lai, W.; Yang, X.; Xiao, F.; Sun, X.-L.; Qian, Q.; Liu, S.; et al. Adaptive Ionization-Induced Tunable Electric Double Layer for Practical Zn Metal Batteries over Wide pH and Temperature Ranges. ACS Nano 2023, 17, 23181–23193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, M.; You, X.; Wang, J.; Feng, X. One-pot cocrystallization of 1D linear and zigzag cobalt (II) polymers assembled by triclopyr and 4, 4′-bipyridine: Structural comparison, conformational analysis, non-covalent interactions as well as the magnetic property of the latter. Polyhedron 2024, 249, 116791. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Guo, Z.; Song, Z.; Lin, Y.; Lin, W.; Zheng, L.; Huang, Z.; Hong, Z.; Titirici, M.-M. Sustainable and scalable fabrication of high-performance hard carbon anode for Na-ion battery. J. Power Sources 2023, 557, 232534. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Z.; Ren, J.; Xu, Y.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Liu, S.; Wang, Z.; et al. Fe2VO4 nanoparticles on rGO as anode material for high-rate and durable lithium and sodium ion batteries. Chem. Eng. J. 2023, 451, 138882. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhao, D.; Ren, J.; Liu, S.; Tang, H.; Xu, P.; Gao, F.; Yue, X.; Yang, H.; et al. Core–Shell Co2VO4/Carbon Composite Anode for Highly Stable and Fast-Charging Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 55020–55028. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Du, Y.; Wang, Z.; Song, B.; Wang, X. SrV4O9 microflowers as high performance cathode for aqueous zinc-ion battery. Mater. Lett. 2023, 331, 133535. [Google Scholar] [CrossRef]
- Xia, P.; Li, S.; Yuan, L.; Jing, S.; Peng, X.; Lu, S.; Zhang, Y.; Fan, H. Encapsulating CoRu alloy nanocrystals into nitrogen-doped carbon nanotubes to synergistically modify lithium-sulfur batteries separator. J. Membr. Sci. 2024, 694, 122395. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Liu, Z.; Li, Y.; Fan, H.; Zhang, Y.; Zeng, L. Organic polymer coating induced multiple heteroatom-doped carbon framework confined Co1-xS@NPSC core-shell hexapod for advanced sodium/potassium ion batteries. J. Colloid Interface Sci. 2024, 660, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yue, X.-A.; Xu, X.-Y.; Xu, P.; Zhang, F.; Fan, H.-S.; Wang, Z.-L.; Wu, Y.-T.; Liu, X.; Zhang, Y. A N/Co co-doped three-dimensional porous carbon as cathode host for advanced lithium–selenium batteries. Rare Met. 2023, 42, 2670–2678. [Google Scholar] [CrossRef]
- Xu, F.; Li, S.; Jing, S.; Peng, X.; Yuan, L.; Lu, S.; Zhang, Y.; Fan, H. Cobalt-vanadium sulfide yolk-shell nanocages from surface etching and ion-exchange of ZIF-67 for ultra-high rate-capability sodium ion battery. J. Colloid Interface Sci. 2024, 660, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, S.; Yin, Y.; Bi, L. Taking advantage of Li-evaporation in LiCoO2 as cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 1849–1859. [Google Scholar] [CrossRef]
- Qu, Y.-P.; Zhou, Y.-L.; Luo, Y.; Liu, Y.; Ding, J.-F.; Chen, Y.-L.; Gong, X.; Yang, J.-L.; Peng, Q.; Qi, X.-S. Universal paradigm of ternary metacomposites with tunable epsilon-negative and epsilon-near-zero response for perfect electromagnetic shielding. Rare Met. 2024, 43, 796–809. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, W.; Wang, F.; Wu, H.; Zhong, S.; Li, B. Meliorative dielectric properties in core@double-shell structured Al@Al2O3@PDA/PVDF nanocomposites via decoupling the intra-particle polarization and inter-particle polarization. Mater. Today Energy 2024, 41, 101543. [Google Scholar] [CrossRef]
- Sun, G.; Yang, D.; Zhang, Z.; Wang, Y.; Lu, W.; Feng, M. Oxygen vacancy-rich MoO3 nanorods as photocatalysts for photo-assisted Li–O2 batteries. J. Adv. Ceram. 2023, 12, 747–759. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, W.; He, Y.; Lv, Y.; Wang, Y.; Wang, Z. Synergetic improvement of dielectric properties and thermal conductivity in Zn@ZnO/carbon fiber reinforced silicone rubber dielectric elastomers. Compos. Part A Appl. Sci. Manuf. 2024, 181, 108129. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Yu, S.; Zhang, Y.; Hou, J.; Yu, N.; Fang, B. MOF-Derived Nitrogen-Doped Porous Carbon Polyhedrons/Carbon Nanotubes Nanocomposite for High-Performance Lithium–Sulfur Batteries. Nanomaterials 2023, 13, 2416. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; He, Y.; Wang, H.; Fang, B.; Tsubaki, N.; Li, C. Carbon neutrality enabled by structure-tailored zeolite-based nanomaterials. Device 2023, 1, 100173. [Google Scholar] [CrossRef]
- Fang, B.; Daniel, L.; Bonakdarpour, A.; Govindarajan, R.; Sharman, J.; Wilkinson, D.P. Dense Pt Nanowire Electrocatal. Improv. Fuel Cell Perform. Using A Graph. Carbon Nitride-Decor. Hierarchical Nanocarbon Support. Small 2021, 17, 2102288. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.; Peng, W.; Yao, T.; Zhang, Y.; Wang, B.; Cai, H.; Li, B. Core@Double–Shell Engineering of Zn Particles toward Elevated Dielectric Properties: Multiple Polarization Mechanisms in Zn@Znch@PS/PVDF Composites. Macromol. Rapid Commun. 2024, 45, 2300585. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Kim, J.H.; Kim, M.-S.; Yu, J.-S. Hierarchical Nanostructured Carbons with Meso–Macroporosity: Design, Characterization, and Applications. Acc. Chem. Res. 2013, 46, 1397–1406. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, N.; Jiang, Q.; Qi, K.; Zhu, X.; Luo, Z.; Kong, X.; Zang, D.; Liu, H.; Fang, B. Progress in polyacrylate-based electrically conductive adhesives: Featured properties, preparation, applications, and perspectives. Polym. Compos. 2024, 45, 5781–5803. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Zhou, X.; Wang, C.; Pan, Z.; Xu, X.; Liu, X.; Wang, Z.; Wu, Y.; Jiang, S.; et al. Graphene oxide-supported MnV2O6 nanoribbons with enhanced electrochemical performance for sodium-ion batteries. J. Power Sources 2024, 597, 234117. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yu, S.; Johnson, H.M.; Zhao, D.-C.; Tan, S.-C.; Pan, Z.-D.; Wang, Z.-L.; Wu, Y.-T.; Liu, X. Three-dimensional nanostructured Co2VO4-decorated carbon nanotubes for sodium-ion battery anode materials. Rare Met. 2023, 42, 4060–4069. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Kong, Y.; Deng, C.; Li, X.; Hu, Q.; Yang, H.; He, C. A hierarchically structured tin-cobalt composite with an enhanced electronic effect for high-performance CO2 electroreduction in a wide potential range. J. Energy Chem. 2023, 76, 462–469. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhao, H.; Yang, X.; Xiao, S.; Liu, N.; Zhao, N.; Cao, Y.; Yu, X.; Li, X. Dual-Phase engineering of Ni3S2/NiCo-MOF nanocomposites for enhanced ion storage and electron migration. Chem. Eng. J. 2024, 489, 151069. [Google Scholar] [CrossRef]
- Ding, S.; An, J.; Gao, Y.; Ding, D.; Lu, X.; Zhao, L. Electrochemical performance of all-solid-state asymmetric supercapacitors based on Cu/Ni-Co (OH)2/Co4S3 self-supported electrodes. Chem. Eng. J. 2023, 453, 139714. [Google Scholar] [CrossRef]
- Senokos, E.; Anthony, D.B.; Rubio, N.; Ribadeneyra, M.C.; Greenhalgh, E.S.; Shaffer, M.S. Robust single-walled carbon nanotube-infiltrated carbon fiber electrodes for structural supercapacitors: From reductive dissolution to high performance devices. Adv. Funct. Mater. 2023, 33, 2212697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).