Abstract
The resurgence of cannabis (Cannabis sativa L.) has been propelled by changes in the legal framework governing its cultivation and use, increased demand for hemp-derived products, and studies recognizing the industrial and health benefits of hemp. This has led to the creation of novel high-cannabidiol, low-Δ9-tetrahydrocannabinol varieties, enabling hemp crop expansion worldwide. This review elucidates the recent implications for hemp cultivation in Europe, with a focus on the legislative impacts on the cultivation practices, prospective breeding efforts, and dynamic scientific landscape surrounding this crop. We also review the current cultivars’ cannabinoid composition of the European hemp market and its major differences with that of the United States.
1. Introduction
1.1. History and Perspective of Hemp Cultivation in Europe
Hemp is undoubtedly one of the most important crops cultivated in human history and was one of the first plants cultivated in Europe [1]. In Europe, hemp has a rich history of traditional uses that date back centuries. Commonly cultivated for its strong fibers, which were used to produce textiles, ropes, and sails, hemp was a valuable commodity in maritime industries and everyday life. Additionally, hemp seeds were used as a food source and in traditional medicine for their nutritional and therapeutic properties [2].
The history of Cannabis genus plant laws in Europe has been intricate and diverse, as each country adopted distinct approaches and regulations concerning the cultivation, possession, sale, and use of cannabis. In the early 20th century, when international drug control efforts began to take shape, numerous European countries enacted laws to regulate the use of all C. sativa varieties [3]. The International Opium Convention of 1925 was the first international treaty that addressed cannabis control, defining ‘Indian hemp’ as the dried flowering or fruiting tops of the pistillate plant C. sativa from which the resin has not been extracted, regardless of its commercial designation. Many European nations subsequently implemented national laws to comply with this treaty. At that time, low-Δ9-tetrahydrocannabinol (THC) plants (hemp) were regulated to the same extent as high-THC varieties (marijuana, more recently known as ‘medical cannabis’) of cannabis [4].
In 1961, the United Nations’ Single Convention on Narcotic Drugs was introduced, aiming to control the production and distribution of cannabis and other narcotic drugs [3]. This had a significant impact on the development of stricter drug policies in European countries. In the United States, there were already laws in place restricting the cannabis industry, predating the Single Convention on Narcotic Drugs [5]. As global efforts to restrict drugs gained momentum, many European nations adopted stringent drug laws, criminalizing the possession, cultivation, and distribution of cannabis plants [6].
However, in the latter part of the 20th century and into the 21st century, some European countries began to decriminalize possession of small amounts of cannabis for personal use, treating it as an administrative, rather than a criminal, offense [7]. In response to growing evidence of the therapeutic benefits of cannabis, several European countries started legalizing medical cannabis in the 21st century [8,9].
1.2. Past and Future of European Hemp Science and Applications
Cannabis (Cannabis sativa L.) has a long history of use in various parts of the world, including Europe. Its fibers and seeds have been used traditionally for many purposes, including clothing, building materials, paper, nutrients, and human and animal health [1,2]. Because of psychotropic use in the 1920–1930s of the high-Δ9-tetrahydrocannabinol (THC) content-dominant varieties, cannabis was largely banned in most countries in subsequent decades, including in most of Europe [1]. However, legislators now recognize the difference between high THC-containing plants (i.e., marijuana) and hemp, which has a low THC content, and is, therefore, non-euphorigenic and an excellent source of beneficial cannabinoids, such as cannabidiol (CBD) [10]. Taxonomically, it is thought that both marijuana and hemp belong to a single species, C. sativa L., which encompasses all cannabis/hemp varieties [11]. However, based on observations of phenotypic differences, some scientists believe that the Cannabis genus comprises three species, namely C. sativa (hemp), C. indica Lam (marijuana), and C. ruderalis [12,13]. The original classification of cannabis varieties according to their phenotype distinguished three types: Type I—THC-dominant, Type II—THC/CBD-balanced, and Type III—CBD-dominant [14]. Later on, another chemotype was identified and described and has since been called Type IV— CBG-dominant [15]. Only in very recent research was the final type added to the chemotype classification, Type V, which accumulated few if any cannabinoids [16]. In this review, we will focus on low-THC C. sativa hemp varieties, which have enjoyed a noticeable surge in cultivation in recent years because of increasing interest in consumer and medical applications across Europe [2].
1.3. CBD Promotes Revival of Hemp Cultivation
In recent years, hemp has experienced a remarkable revival, primarily because of its emergence as a valuable source of cannabinoids other than the psychotropic THC, particularly CBD. This resurgence marked a significant shift from the historical stigmatization of cannabis, as it became recognized that the low-THC plant (hemp) could be utilized for numerous industrial applications [17] and for the therapeutic potential of its cannabinoid-rich extracts [18,19]. Specifically, the medicinal potential of hemp is primarily associated with its cannabinoid content, particularly CBD [20]. CBD has been widely studied for its potential in alleviating various health conditions, including pain [21,22,23,24], anxiety [25,26], epilepsy [27,28], and inflammation [29,30].
There has been a notable surge in the development of hemp inhalation studies [31,32,33,34], driven by the increasing interest in hemp-derived compounds and the growing acceptance of alternative methods of consumption. These products primarily focus on delivering cannabinoids such as CBD through various inhalation techniques, including vaporization and smoking [35,36,37,38]. Innovations in vaporization technology have led to the creation of portable and user-friendly hemp vaporizers, allowing consumers to inhale CBD-infused vapors without the harmful byproducts associated with traditional smoking. Additionally, hemp pre-rolls, containing high-CBD hemp flowers, have gained popularity for convenient and discreet hemp consumption through smoking. These products offer consumers a quick onset of effects and are favored for their potential therapeutic benefits, making them a prominent segment in the expanding hemp market.
Some hemp varieties are naturally abundant in CBD, making them an appealing option for producing CBD products without the unwanted psychoactive THC effects associated with marijuana. This has led to a surge in hemp cultivation worldwide, with farmers and entrepreneurs seeking to capitalize on the growing demand for CBD-driven health and wellness properties [39,40,41,42]. However, the appeal of hemp extends beyond CBD. The plant can produce over 140 cannabinoids in total [43,44], among them, a variety of non-psychoactive, biologically active cannabinoids, such as CBG [45,46,47,48], cannabichromene [49,50,51], cannabinol (CBN) [52,53,54,55], cannabidivarin (CBDV) [56,57,58,59], and tetrahydrocannabivarin (THCV) [60,61,62,63], with each of them exhibiting potential health benefits. Scientists are increasingly exploring the therapeutic properties of various cannabinoids to better understand their potential health applications.
1.4. Industrial Versatility of Hemp
Hemp fibers have been used for millennia in the production of textiles and fabrics. Hemp fabrics are known for their durability, breathability, and resistance to UV rays. Hemp textiles are not only environmentally friendly but also offer a sustainable alternative to traditional cotton [64,65] and synthetic fibers [66,67].
Hemp-based construction materials, such as hempcrete, have gained popularity as eco-friendly alternatives in the building industry [68]. In several studies, hempcrete performed well as a building material, replacing traditional construction materials while adhering to the thermal, insulating, and acoustic characteristics required in construction [69,70,71,72,73].
Hemp seeds are rich in essential nutrients and have gained recognition as a superfood. They are a complete source of protein, containing all nine essential amino acids, making them an ideal plant-based protein option for vegetarians and vegans [74,75,76]. Hemp seeds are also abundant in healthy fats, particularly omega-3 and omega-6 fatty acids [77], promoting heart health and overall well-being [78].
Hemp seed oil is a prized ingredient in the cosmetic and skincare industry because of its nourishing and moisturizing properties [79]. It is a natural emollient, helping to soothe and hydrate the skin without clogging pores. Hemp seed oil is also rich in antioxidants [80], aiding in the fight against free radicals and supporting skin health [81]. It can also be used as an anti-inflammatory agent, particularly in irritable bowel syndrome and other gastrointestinal conditions [82]. Hemp seed oil can also be used as a biofuel [83,84]. Hemp biodiesel has shown promise as a renewable and environmentally friendly alternative to fossil fuels [83,84]. CBG is a cannabinoid found in cannabis with potential health benefits. Research suggests CBG might have neuroprotective properties, anti-inflammatory effects useful for disorders such as inflammatory bowel disease, and potential pain relief capabilities. Additionally, CBG could aid in stimulating appetite and possibly serving as an antimicrobial agent [47]. In another study beneficial effects on anxiety, chronic pain, depression, and insomnia were reported with few if any side effects [85].
To summarize, hemp exhibits great promise as a sustainable alternative to traditional materials and crops. Its versatility, carbon sequestration capabilities [86,87], and moderate growth cycle make it an attractive option for numerous industries seeking eco-friendly solutions. In Malawi, where hemp is replacing tobacco cultivation, the use of hemp has led to reduced water consumption and pesticide use, contributing to a more sustainable agricultural and industrial landscape [88,89]. As awareness of the environmental benefits of hemp continues to grow, more regions and industries may consider incorporating this versatile plant into their practices, fostering a greener and more sustainable future.
2. Major Hemp Compounds and Breeding Efforts
2.1. Exploring Major Cannabinoids
The most important compounds of C. sativa are cannabinoids, with THC being the most well-known one because of its strong psychoactive effect, while the second most-known one and target of multiple breeding efforts is CBD [90]. However, the cannabis plant produces more than 140 different cannabinoid compounds [11,12,90]. We will not describe the detailed biosynthesis or the chemical transformations of cannabinoids, as such processes have been extensively described elsewhere [91,92]. Substantial clinical evidence exists for the efficacy of CBD in the settings of anxiety, psychosis, schizophrenia, post-traumatic stress disorder, and substance abuse [20]. This includes uncontrolled and randomized controlled trial (RCT) studies for anxiety, psychosis, schizophrenia, post-traumatic stress disorder, substance abuse, and sleep quality [20].
Regarding biological activity, it is important to consider the stereoisomers of Δ9-THC, which has two stereogenic centers (C-6a and C-10a) and can exist as pairs of enantiomers and diastereomers (two enantiomers of Δ9-trans-THC and two enantiomers of Δ9-cis-THC) [93]. It has been shown that low-THC hemp varieties are rich in Δ9-cis-THC in concentrations comparable with that of Δ9-trans-THC, which is predominantly responsible for psychoactive effects [94]. The enantiomers of Δ9-cis-THC had less CB1/CB2 binding (Ki) and functional activity (EC50 [35S]GTPγS binding) for the inhibition of the endocannabinoid-degrading enzymes (IC50 values) than (−)-Δ9-trans-THC in a comparative in vitro biological evaluation [94].
2.2. Understanding Terpenes
Multiple terpenes are found in cannabis plants, where they are abundant and complex in nature, contributing to the overall aroma and scent of different varieties and are a major breeding focus [95]. Terpenes are responsible for the distinct aromas and flavors associated with various plants, fruits, and herbs, but more importantly, they play essential roles in the plant kingdom, serving as a defense mechanism against herbivores and pathogens [96,97], as well as attracting pollinators [98].
The most prevalent terpenes in cannabis are myrcene, which is recognized for its musky, earthy, and fruity fragrance and linked to relaxant, sedative, anti-inflammatory, and analgesic effects [99]; α- and β-limonene, which emits a citrusy, lemon/orange-like aroma and is associated with mood elevation and stress alleviation [100] and neuroprotective properties [101]; α-pinene, which elicits a piney scent akin to coniferous trees, potentially possesses anti-inflammatory properties [102], and aids brain health [103]; β-caryophyllene, which has a peppery aroma such as that found in black pepper and cloves, with neuroprotective [104] and antioxidant properties [105]; linalool, which is known for its floral, lavender-like aroma that is connected with relaxation and stress reduction [106]; humulene, which exudes an earthy, woody scent, and is being researched for its potential anti-inflammatory capabilities [107]; terpinolene, which features a complex bouquet of floral, herbal, and citrus notes, though it is less common and its effects remain under scrutiny [108]; ocimene, with its sweet, herbal, occasionally fruity fragrance, which is believed to have antiviral and antifungal properties; nerolidol, which is characterized by a woody, citrusy aroma and undergoing exploration for potential anti-parasitic and antimicrobial benefits [109,110]; and α-bisabolol, which emits a sweet floral aroma and is under investigation for potential pharmacological effects [111].
Some studies have indicated that terpenes neither act on cannabinoid receptors directly [112] nor activate transient receptor potential vanilloid 1 and ankyrin 1 channels nor modulate their activation by THC [113]. However, other studies have shown that terpenes can activate the CB1 receptor in vivo [114]. Cannabis terpenes α-humulene, geraniol, linalool, and β-pinene have also been shown to activate the CB1 receptor in vivo and can in fact be multifunctional cannabimimetic ligands [115]. Although the action of terpenes on cannabinoid receptors is unclear, they have gained significant attention in the cannabis industry and the broader field of aromatherapy and natural medicine because of their potential health benefits [116]. Different terpenes may exhibit various effects, such as promoting relaxation, reducing stress, improving focus, or providing anti-inflammatory properties.
Cannabis contains other aromatic compounds, such as aldehydes, ketones, alcohols, esters, nitrogen-containing compounds, and phenols, which contribute to its characteristic aroma and flavor [116,117]. These aromatic compounds work in synergy with terpenes to create the diverse and complex scents found in different cannabis varieties [116]. The presence and concentration of these compounds can vary among varieties, giving rise to the wide range of aromas associated with various cannabis varieties [116].
Phenolic compounds encompass a range of aromatic compounds with pivotal roles in both plant defense mechanisms and potential human health benefits [116]. Some prominent phenolic compounds found in cannabis include flavonoids such as quercetin, kaempferol, luteolin, and apigenin [118]. Quercetin, a flavonol, has been detected in noteworthy concentrations and is recognized for its antioxidant and anti-inflammatory attributes [119]. Similarly, kaempferol, another flavonol, contributes to the antioxidant capacity of cannabis [120] and holds promise for its potential cardioprotective effects [121]. Apigenin, a flavone, is known for its anxiolytic properties and potential as an anti-inflammatory agent [122]. Furthermore, cannabis also contains cannflavins A and B, unique flavonoids with emerging research highlighting their potential anti-inflammatory properties [118]. These phenolic compounds, along with others, underpin the intricate biochemical profile of cannabis, potentially influencing its aroma, flavor, and therapeutic potential. Although the precise roles and interactions of these phenolic compounds are still being elucidated, their presence underscores the multifaceted nature of cannabis and its potential applications in both plant biology and human well-being.
Among the more interesting recent discoveries was the identification of a new family of volatile sulfur compounds containing the prenyl (3-methylbut-2-en-1-yl) functional group that is responsible for skunk scent [123]. Their remarkable similarity to garlic volatile sulfur compounds also marks them as a target for discovery of their additional health benefits [123].
2.3. Advancing Classical Cannabis Breeding
Since the first sequencing effort to produce the draft genome of C. sativa [124], there have been many subsequent studies improving genome assembly and expanding on the number of varieties [125,126], with the most recent and well-regarded one being published in 2021 [127].
Geographical expansion and domestication have had little impact on the Cannabis genome size. Although there are significant differences between male and female genome sizes, they cannot be distinguished via combined flow cytometry [128], which is useful for the hemp industry as it is female plants that produce flowers with desired cannabinoids. and identification is now easily achieved by testing for Y chromosomes using PCR [129].
There have been recent efforts in Europe to develop improved methods, such as rapid generation cycling (speed breeding), to produce hemp varieties adapted to local climate conditions and specific applications [130]. Hemp is inherently a short-day plant requiring 12–14 of daylight hours for optimal growth. While longer days promote yield and reduce flowering, these are suitable for the production of hemp as a fiber source. It remains unclear if the performance of hemp varieties grown in higher-latitude regions will hold true in lower-latitude and tropical regions [131]. On the other hand, longer dark periods can induce early flowering and reduce biomass. Other efforts have focused on developing improved protocols for rapid regeneration, such as modified nodal cutting and shoot-tip protocols [132] or seed priming and pericarp removal [133]. Further studies have been undertaken to measure cannabinoid concentrations and their correlation with climate conditions [134].
It is important to note that cannabis offers remarkable plasticity and is perfectly suitable for breeding efforts that follow Mendelian selection. In addition to classical methods, the combination of breeding and genetic engineering, including CRISPR technology, hold immense potential for optimizing hemp plants to maximize specific cannabinoid and terpene contents. Genetic engineering, particularly the precise and targeted modifications facilitated by CRISPR, offers the ability to directly manipulate the Cannabis genome [135,136]. This advancement allows scientists to enhance the expression of genes responsible for cannabinoid and terpene production or introduce novel pathways to produce rare or valuable compounds [137,138,139]. By harnessing these techniques, hemp cultivators can develop varieties tailored to meet specific market demands for medicinal, recreational, or industrial purposes, unlocking the full potential of this versatile plant. However, ethical considerations, regulatory compliance, and responsible research practices are crucial in exploring the potential of breeding and genetic engineering to ensure the sustainable and safe development of optimized hemp varieties.
In recent years, there has been growing interest in the biosynthesis of minor cannabinoids, which are cannabis compounds present in lower abundance compared with major cannabinoids, such as THC and CBD [140,141,142,143]. Research in this field has sought to elucidate the enzymatic pathways involved in the synthesis of these minor cannabinoids and explore the genetic factors influencing their production [143]. Scientists have identified various biosynthetic pathways responsible for minor cannabinoids, such as CBG [144], CBN, cannabichromene, cannabidivarin, and tetrahydrocannabivarin [145]. This knowledge has led to advancements in biotechnological approaches, including genetic engineering and synthetic biology, to enhance the yield and accessibility of these compounds [137]. The exploration of minor cannabinoids holds promise for their potential therapeutic benefits, as early studies indicate that some of these compounds may possess unique medicinal properties. As the field of cannabis research continues to expand, further understanding of the biosynthesis of minor cannabinoids may unlock exciting opportunities for the development of novel pharmaceuticals and therapeutics.
The accumulation of cannabinoids in cannabis plants is influenced not only by genetic factors but also by environmental conditions [145,146,147,148]. The genetic makeup of a cannabis variety determines its potential to produce specific cannabinoids [145,149], but environmental factors play a vital role in whether these traits are expressed to their fullest. Elements such as temperature, light intensity, humidity, soil composition, and nutrient availability impact cannabinoid accumulation [146]. For example, some varieties may produce higher THC levels under specific light conditions [147], whereas others may favor CBD production in different environments. Additionally, stressors such as drought [150] or pest attacks can trigger the production of certain cannabinoids as a defense mechanism [151]. This intricate interplay between genetics and the environment highlights the importance of understanding and optimizing cultivation practices to achieve desired cannabinoid profiles in cannabis plants [152]. By controlling environmental variables, cultivators can maximize the expression of desired cannabinoids, tailoring cannabis varieties to suit various consumer preferences and specific end-use applications.
3. The Growing Hemp Industry in Europe
In the European Union (EU), hemp cultivation is governed by regulations that define hemp as C. sativa having a THC content below 0.3%. Hemp farmers must exclusively use certified seed from varieties listed in the EU Common Catalogue of Varieties of Agricultural Plant Species. The Plant Varietal Portal of the European Union provides a searchable catalog of such EU-approved hemp varieties through its website [153]. Cultivators must also obtain licenses or authorizations, and regular inspections are conducted to ensure compliance with the THC limit. Additionally, regulations cover the processing and trade of hemp-derived products, such as CBD extracts, which may require further licensing or authorization.
In Switzerland, farmers are required to obtain permits for legal hemp cultivation, and the THC limit is set at 1.0%. Switzerland also regulates the processing and trade of hemp-derived products to ensure compliance with the established guidelines [154]. Contrary to the EU, farmers in Switzerland are permitted to cultivate and trade any hemp variety regardless of origin if it possesses a THC content below 1.0% (Figure 1).

Figure 1.
Three-dimensional chart of European Union and Swiss varieties of C. sativa L. Concentrations of THC, CBD, and CBG contents as percentage (%) of dry weight.
Several countries and regions in Europe have become prominent players in hemp cultivation [155,156]. France has a longstanding history of hemp farming and a well-established industry serving both domestic and international markets [157]. Germany has seen notable growth due to increasing demand for hemp products and supportive government policies. Italy’s favorable climate has contributed to its thriving hemp industry. Furthermore, Spain, particularly in regions such as Andalusia and Catalonia, has experienced significant expansion of cultivation [158,159]. The Netherlands, with its progressive approach to cannabis-related industries, has been actively involved in hemp farming for various applications. Moreover, Eastern European countries, including Poland [160], Romania, and Lithuania, are emerging as key contributors to the European hemp sector, witnessing growing cultivation areas and processing facilities [155]. However, it is necessary to consider the critical importance of maceration and the availability of water resources in hemp processing, especially given the diverse environmental conditions in many regions where hemp cultivation takes place.
The higher THC threshold of 1.0% enables the production of a wide range of high-CBD hemp varieties, contributing to a country’s flourishing market for CBD products [161,162]. While Swiss farmers benefit from diverse microclimates [163] that support the cultivation of various hemp varieties [164], ultimately, low humidity, long growing season, and high solar radiation that will best aid CBD-dominant varieties.
Hemp cultivators in Europe encounter a range of challenges and opportunities. One of the major hurdles lies in the diverse regulations across European countries concerning hemp cultivation, THC limits, and hemp-derived product processing, leading to uncertainty and hindering cross-border trade [165]. The stigma associated with cannabis, despite hemp’s legal distinction from marijuana, can impact investment and market acceptance. Competition from other agricultural sectors and imported hemp products further adds pressure to local cultivators.
Nonetheless, there are promising prospects for hemp cultivators in Europe. The increasing demand for hemp-derived products, especially CBD, presents a vast market potential for farmers and processors. As consumers gravitate toward sustainable and eco-friendly alternatives, the appeal of hemp-based goods increases significantly. The industry’s growth is supported by increasing research and innovation, leading to improved cultivation practices and the development of high-performing hemp varieties. Collaborative efforts between European countries can harmonize regulations, facilitate cross-border trade, and expand market reach for hemp cultivators.
4. THC- vs. CBD-Dominant Cannabis sativa L. Varieties
The cannabis flowers from the USA and Europe contrast in their botanical features and chemical composition (Figure 2 and Figure 3). USA varieties have a significantly higher THC concentration than those from Switzerland or the rest of Europe, which have a much lower CBD content on average, particularly when compared with Swiss varieties (Figure 2 and Figure 3).

Figure 2.
Concentrations of THC and CBD in cannabis varieties in the United States, Europe, and Switzerland. Data sourced from producers’ Certificates of Analysis as well as scientific publications. The full data are available as Supporting Table S1. Varieties in Europe are represented by circles, those in the Switzerland by squares, and those in the USA by triangles. Additionally, the THC concentration of each variety is shown in blue-red scale. An enlarged image of the European and Swiss varieties is provided on the right side of the figure.

Figure 3.
CBD concentrations (% of dry weight) in cannabis varieties in the United States, Europe, and Switzerland. Respective total cannabinoid concentrations are represented by red-blue color code (% dry weight). Mean, median, standard deviation, and number of varieties are indicated in boxes next to respective chart cloud bars.
It is worth noting that the legislative limit of 0.3% THC in Europe highly influences the amount of CBD content (mean = 2.542%); the limit of 1.0% THC in Switzerland has no such effect (CBD mean =13.64%) (Figure 3). This relationship between THC content limits and CBD content is particularly evident when comparing CBD content in Swiss varieties with that of CBD-dominant varieties in the USA, where no THC limit is enforced (CBD mean = 12.78%) (Figure 3). Cannabis flowers from the USA, often characterized by their lush and resinous appearance, tend to have higher THC levels, which contributes to their potency and psychoactive effects [166,167]. Cultivated primarily as a source of fiber and seed oil, European cannabis flowers are CBD-dominant, offering potential therapeutic benefits without inducing psychoactive effects. The varying climate and regional cultivation practices in each continent contribute to the unique characteristics of their respective cannabis flowers, offering diverse varieties and effects.
In THC-dominant cannabis varieties, the types and concentrations of terpenes present can differ from those in CBD-dominant varieties (Figure 4 and Figure 5). The number of total terpenes does not significantly differ among CBD-dominant, THC-dominant, or CBD/THC-balanced varieties (Figure 4). This could suggest independent terpene accumulation and biosynthesis from that of determining cannabinoid dominance. When comparing particular terpenes among CBD-dominant, THC-dominant, and CBD/THC-balanced varieties, no clear trend emerges that would favor any terpene pattern in relation to specific cannabinoid accumulation (Figure 5). These terpene variations contribute to the distinct aromatic profiles and potential effects of the two types of cannabis.

Figure 4.
Concentrations (% of dry weight) of total terpenes in THC-dominant, CBD-dominant, and THC/CBD-balanced cannabis varieties in the United States.
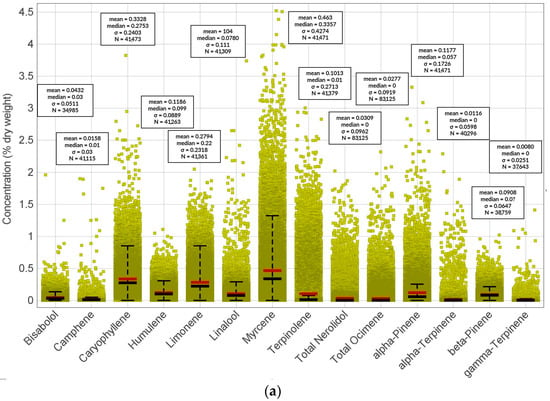

Figure 5.
Concentrations (% of dry weight) of particular terpenes in (a) THC-dominant, (b) CBD-dominant, and (c) THC/CBD-balanced cannabis varieties.
However, the CBD-dominant strains of cannabis exhibit a greater degree of consistency in terpene profiles in comparison with their THC-dominant counterparts (Figure 5 standard deviations). This divergence in terpene variability can be attributed to the fact that a narrower range of CBD-focused varieties have been cultivated in contrast to the broader array of THC-dominant cultivars. This phenomenon is primarily a result of prevailing consumer inclinations within the United States market [167]. Recently, endeavors in the realm of breeding are being channeled toward the augmentation of aromatic and flavor profiles within CBD-dominant cultivars, specifically hemp.
In the hemp industry, significant discrepancies exist between reported and measured cannabinoid concentrations in various hemp products, posing challenges to consumers and businesses alike [168]. This disparity arises from a combination of factors, including the absence of standardized testing methods and regulations for cannabinoid analysis [169]. Different laboratories employ varying testing protocols and equipment, leading to inconsistent results for the same product. The inherent variability in cannabinoid composition within the same hemp product is another key factor influencing reported concentrations [170,171]. Factors such as plant genetics, growing conditions, and extraction methods contribute to this variability.
Additionally, the decarboxylation process, where raw cannabinoids are converted from their acid form in the plant into their pharmacologically active forms, can impact the reported cannabinoid content. Incomplete or non-uniform conversion during decarboxylation may lead to discrepancies between the reported levels of cannabinoids and their actual concentrations in the final product. Furthermore, inaccurate labeling practices and differences in extraction efficiency and sample preparation techniques can also contribute to the variation in reported cannabinoid concentrations [172,173,174,175]. It is also important to consider the degradation of cannabinoids, once formulated, in products and the limited shelf life resulting from this process [176,177,178].
To address these challenges and enhance consumer confidence, the European hemp industry needs standardized testing protocols and clear regulations for cannabinoid analysis. Implementing third-party testing and certification programs can provide reliable information on cannabinoid content, promoting transparency and accountability. By establishing a robust and reliable system for cannabinoid analysis, the industry can build consumer trust, drive market growth, and solidify the European hemp industry’s position in the ever-expanding global market. As the regulatory landscape evolves, efforts to standardize testing and enhance transparency will play a pivotal role in addressing the reported versus measured concentrations of cannabinoids in European hemp products.
5. Concluding Remarks and Future Perspectives
The resurgence of hemp cultivation in Europe has been catalyzed by the increased demand in hemp-derived products as well as the less restrictive legislative landscape (wherein changes in laws were driven by public pressure), leading to a flourishing industry with diverse applications. The historical significance of hemp as a valuable resource for textiles, construction, food, and medicine has been revived, showcasing its potential to revolutionize multiple industries in a sustainable and eco-friendly manner. The increasing interest in hemp-derived compounds, particularly CBD, has allowed for new opportunities in the wellness and pharmaceutical sectors. Moreover, research and innovation in hemp science have advanced significantly, providing insights into cannabinoids, terpenes, and their potential therapeutic applications. Navigating the complexities of Cannabis sativa L. research presents a host of intriguing challenges, each offering a pathway to deeper insights and progress. For instance, understanding the role of terpenes in shaping the unique traits of different cannabis strains raises questions about their potential interaction with cannabinoid receptors. Exploring the precise functions and interactions of other aromatic compounds, including aldehydes, ketones, alcohols, esters, nitrogen-containing compounds, and phenols, remains an intriguing yet elusive endeavor, ripe for further exploration. The interplay of genetics and environmental factors in influencing cannabinoid and terpene profiles highlights the importance of optimizing cultivation practices to achieve desired outcomes. The hemp industry in Europe faces both challenges and opportunities, with diverse regulations and market acceptance being key factors to navigate.
The future of hemp cultivation and scientific progress in Europe hold immense promise. As research in hemp science continues to advance, deeper insights into minor cannabinoids and their potential therapeutic benefits are anticipated. The use of classical plant breeding techniques as well as genetic engineering (including CRISPR technology) offer exciting possibilities to create novel, innovative hemp varieties with elevated and/or unique cannabinoid and terpene profiles, further unlocking the plant’s potential for diverse industrial and medical applications. However, alongside scientific exploration, it is crucial to navigate ethical considerations, regulatory compliance, and responsible research practices, particularly in breeding and genetic engineering. Collaboration among researchers, cultivators, and policymakers will play a pivotal role in establishing standardized testing protocols and regulations to address the reported versus measured concentrations of cannabinoids in hemp products. The sustainability and eco-friendliness of hemp field cultivation make it a compelling option for industries seeking environmentally conscious solutions. Balancing innovation with sustainability is paramount, calling for the incorporation of practices like water-efficient irrigation, organic farming, and responsible land management to mitigate environmental risks associated with hemp cultivation. As the hemp industry in Europe continues to grow and evolve, responsible practices and regulatory frameworks will be essential to maximize the benefits of this versatile and valuable crop for society and the environment. The importance of clear trade rules and the elimination of cross-border restrictions to facilitate hemp production cannot be overstated. Clear trade regulations ensure smooth operations and promote fair competition within the industry. By removing cross-border restrictions, hemp producers gain access to larger markets, fostering growth and innovation in the sector. By embracing innovative research, sustainable practices, and sound policies, Europe’s hemp industry is poised to help shape a more sustainable future.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29102397/s1, Table S1: Cannabinoid data for varieties.
Author Contributions
Conceptualization, J.H., K.P.K., F.G., W.K.S. and D.L.; methodology, K.P.K.; software, D.L.; validation, K.P.K.; formal analysis, K.P.K.; investigation, K.P.K.; resources, K.P.K. and D.L.; data curation, D.L.; writing—original draft preparation, K.P.K.; writing—review and editing, J.H., K.P.K., F.G., W.K.S. and D.L.; visualization, D.L.; supervision, J.H.; project administration, J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data used to generate figures in this review were obtained from multiple publicly available sources, namely, USA data from commercially available varieties—Smith, C.J.; Vergara, D.; Keegan, B.; Jikomes, N. The phytochemical diversity of commercial cannabis in the United States. PLoS ONE 2022, 17, e0267498. PMID: 35588111; Swiss data were obtained from labels of the products and certificates of analysis; European data were obtained from Schafroth, M.A.; Mazzoccanti, G.; Reynoso-Moreno, I.; Erni, R.; Pollastro, F.; Caprioglio, D.; Botta, B.; Allegrone, G.; Grassi, G.; Chicca, A.; et al. Δ9-cis-Tetrahydrocannabinol: Natural occurrence, chirality, and pharmacology. J. Nat. Prod. 2021, 84, 2502–2510. https://doi.org/10.1021/acs.jnatprod.1c00513. PMID: 34304557.
Acknowledgments
The authors would like to thank Jeremy Plumb for reviewing and giving essential directions on the manuscript.
Conflicts of Interest
Julia Hoeng is an employee of Vectura Fertin Pharma, which sponsored this review. The remaining authors are paid consultants for Vectura Fertin Pharma, including Fernando Goffman, who operates through company Seedcraft S.L.
References
- John, F. The history of hemp. In Industrial Hemp as a Modern Commodity Crop; Williams, D.W., Ed.; American Society of Agronomy Crop Science Society of America Soil Science Society of America: Madison, WI, USA, 2019; pp. 1–25. [Google Scholar]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Collins, J. A brief history of cannabis and the drug conventions. Am. J. Int. Law 2020, 114, 279–284. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse. Cannabis (Marijuana) Research Report. Available online: https://nida.nih.gov/publications/research-reports/cannabis-marijuana (accessed on 22 February 2024).
- Patton, D.V. A history of United States cannabis law. J. Law Health 2020, 34, 1. [Google Scholar] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. Cannabis Legislation in Europe—An Overview. Available online: https://www.emcdda.europa.eu/system/files/publications/4135/TD0217210ENN.pdf (accessed on 22 February 2024).
- European Monitoring Centre for Drugs and Drug Addiction. Cannabis Policy: Status and Recent Developments. Available online: https://www.emcdda.europa.eu/publications/topic-overviews/cannabis-policy/html_en (accessed on 22 February 2024).
- Kemme, S.; Pfeffer, K.; Von Rodbertus, L. Cannabis policy reform in Germany: Political and constitutional discourses on decriminalisation and regulation strategies. Bergen J. Crim. Law Crim. Justice 2021, 9, 31. [Google Scholar] [CrossRef]
- Gabri, A.C.; Galanti, M.R.; Orsini, N.; Magnusson, C. Changes in cannabis policy and prevalence of recreational cannabis use among adolescents and young adults in Europe—An interrupted time-series analysis. PLoS ONE 2022, 17, e0261885. [Google Scholar] [CrossRef] [PubMed]
- Kalinová, J.P.; Vrchotová, N.; Tríska, J.; Hellerová, Š. Industrial hemp (Cannabis sativa L.) as a possible source of cannabidiol. J. Cent. Eur. Agric. 2021, 22, 110–118. [Google Scholar]
- Hurgobin, B.; Tamiru-Oli, M.; Welling, M.T.; Doblin, M.S.; Bacic, A.; Whelan, J.; Lewsey, M.G. Recent advances in Cannabis sativa genomics research. New Phytol. 2021, 230, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Henry, P.; Khatodia, S.; Kapoor, K.; Gonzales, B.; Middleton, A.; Hong, K.; Hilyard, A.; Johnson, S.; Allen, D.; Chester, Z.; et al. A single nucleotide polymorphism assay sheds light on the extent and distribution of genetic diversity, population structure and functional basis of key traits in cultivated north American cannabis. J. Cannabis Res. 2020, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Small, E.; Beckstead, H.D. Common cannabinoid phenotypes in 350 stocks of Cannabis. Lloydia 1973, 36, 144–165. [Google Scholar]
- Fournier, G.; Richez-Dumanois, C.; Duvezin, J.; Mathieu, J.P.; Paris, M. Identification of a new chemotype in Cannabis sativa: Cannabigerol-dominant plants, biogenetic and agronomic prospects. Planta Med. 1987, 53, 277–280. [Google Scholar] [CrossRef]
- Mandolino, G.; Carboni, A. Potential of marker-assisted selection in hemp genetic improvement. Euphytica 2004, 140, 107–120. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, X.; Guo, Y.; Qiu, C.; Long, S.; Wang, Y.; Qiu, H. Industrial hemp—An old but versatile bast fiber crop. J. Nat. Fibers 2022, 19, 6269–6282. [Google Scholar] [CrossRef]
- Abate, G.; Uberti, D.; Tambaro, S. Potential and limits of cannabinoids in Alzheimer’s disease therapy. Biology 2021, 10, 542. [Google Scholar] [CrossRef]
- Suraev, A.S.; Marshall, N.S.; Vandrey, R.; McCartney, D.; Benson, M.J.; McGregor, I.S.; Grunstein, R.R.; Hoyos, C.M. Cannabinoid therapies in the management of sleep disorders: A systematic review of preclinical and clinical studies. Sleep Med. Rev. 2020, 53, 101339. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Jensen, S.S.; Nikolajsen, G.N.; Bruun, H.Z.; Bhuller, R.; Hoeng, J. The therapeutic potential of purified cannabidiol. J. Cannabis Res. 2023, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Zurbriggen, L.; Dieterle, M.; Mauermann, E.; Frei, P.; Mercer-Chalmers-Bender, K.; Ruppen, W. Pain response to cannabidiol in induced acute nociceptive pain, allodynia, and hyperalgesia by using a model mimicking acute pain in healthy adults in a randomized trial (CANAB I). Pain 2022, 163, e62–e71. [Google Scholar] [CrossRef] [PubMed]
- Vela, J.; Dreyer, L.; Petersen, K.K.; Arendt-Nielsen, L.; Duch, K.S.; Kristensen, S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: A randomized, double-blind, placebo-controlled trial. Pain 2022, 163, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.R.B.; Joshaghani, N.; Villa, N.; Badla, O.; Goit, R.; Saddik, S.E.; Dawood, S.N.; Rabih, A.M.; Niaj, A.; Raman, A.; et al. Efficacy, safety, and regulation of cannabidiol on chronic pain: A systematic review. Cureus 2022, 14, e26913. [Google Scholar] [CrossRef]
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a treatment for arthritis and joint pain: An exploratory cross-sectional study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef]
- Hutten, N.R.; Arkell, T.; Vinckenbosch, F.; Schepers, J.; Kevin, R.; Theunissen, E.; Kuypers, K.; McGregor, I.; Ramaekers, J. Cannabis containing equivalent concentrations of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) induces less state anxiety than THC-dominant cannabis. Psychopharmacology 2022, 239, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Spinella, T.C.; Stewart, S.H.; Naugler, J.; Yakovenko, I.; Barrett, S.P. Evaluating cannabidiol (CBD) expectancy effects on acute stress and anxiety in healthy adults: A randomized crossover study. Psychopharmacology 2021, 238, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.A.; Whalley, B.J. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord. 2020, 22, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Therapeutic and clinical foundations of cannabidiol therapy for difficult-to-treat seizures in children and adults with refractory epilepsies. Exp. Neurol. 2023, 359, 114237. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Romero-Zerbo, S.Y.; García-Fernández, M.; Espinosa-Jiménez, V.; Pozo-Morales, M.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Lara, E.; Cobo-Vuilleumier, N.; Rafacho, A.; Olveira, G.; et al. The atypical cannabinoid Abn-CBD reduces inflammation and protects liver, pancreas, and adipose tissue in a mouse model of prediabetes and non-alcoholic fatty liver disease. Front. Endocrinol. 2020, 11, 103. [Google Scholar] [CrossRef]
- Hädener, M.; Vieten, S.; Weinmann, W.; Mahler, H. A preliminary investigation of lung availability of cannabinoids by smoking marijuana or dabbing BHO and decarboxylation rate of THC-and CBD-acids. Forensic Sci. Int. 2019, 295, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gulluni, N.; Re, T.; Loiacono, I.; Lanzo, G.; Gori, L.; Macchi, C.; Epifani, F.; Bragazzi, N.; Firenzuoli, F. Cannabis essential oil: A preliminary study for the evaluation of the brain effects. eCAM 2018, 2018, 1709182. [Google Scholar] [CrossRef] [PubMed]
- Javadi-Paydar, M.; Creehan, K.M.; Kerr, T.M.; Taffe, M.A. Vapor inhalation of cannabidiol (CBD) in rats. Pharmacol. Biochem. Behav. 2019, 184, 172741. [Google Scholar] [CrossRef]
- Spindle, T.R.; Cone, E.J.; Kuntz, D.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Vandrey, R. Urinary pharmacokinetic profile of cannabinoids following administration of vaporized and oral cannabidiol and vaporized CBD-dominant cannabis. J. Anal. Toxicol. 2020, 44, 109–125. [Google Scholar] [CrossRef]
- Cleirec, G.; Desmier, E.; Lacatus, C.; Lesgourgues, S.; Braun, A.; Peloso, C.; Obadia, C. Efficiency of inhaled cannabidiol in cannabis use disorder: The pilot study Cannavap. Front. Psychiatry 2022, 13, 899221. [Google Scholar] [CrossRef] [PubMed]
- Rabgay, K.; Waranuch, N.; Chaiyakunapruk, N.; Sawangjit, R.; Ingkaninan, K.; Dilokthornsakul, P. The effects of cannabis, cannabinoids, and their administration routes on pain control efficacy and safety: A systematic review and network meta-analysis. J. Am. Pharm. Assoc. 2020, 60, 225–234.e6. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Gagnier, J.J.; Matallana, L.; Williams, D.A. Cannabidiol product dosing and decision-making in a national survey of individuals with fibromyalgia. J. Pain 2022, 23, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Leas, E.C.; Moy, N.; McMenamin, S.B.; Shi, Y.; Benmarhnia, T.; Stone, M.D.; Trinidad, D.R.; White, M. Availability and promotion of cannabidiol (CBD) products in online Vape shops. Int. J. Environ. Res. Public Health 2021, 18, 6719. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Chye, Y.; Christensen, E.; Solowij, N.; Yücel, M. The endocannabinoid system and cannabidiol’s promise for the treatment of substance use disorder. Front. Psychiatry 2019, 10, 63. [Google Scholar] [CrossRef]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiat. 2019, 6, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Hanus, L.O. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 heteroreceptor complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; Žvar Baškovič, B.; Majc, B.; Mlinar, M.; Bošnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol is a potential therapeutic agent in a novel combined therapy for glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological aspects and biological effects of cannabigerol and its synthetic derivatives. Evid. Based Complement. Alternat. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In vitro and clinical evaluation of cannabigerol (CBG) produced via yeast biosynthesis: A cannabinoid with a broad range of anti-inflammatory and skin health-boosting properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Shin, E.; Jarrahi, A.; Costigliola, V.; Kumar, P.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; Vaibhav, K.; et al. A potential role for cannabichromene in modulating TRP channels during acute respiratory distress syndrome. J. Cannabis Res. 2021, 3, 45. [Google Scholar] [CrossRef]
- Izzo, A.A.; Capasso, R.; Aviello, G.; Borrelli, F.; Romano, B.; Piscitelli, F.; Gallo, L.; Capasso, F.; Orlando, P.; Di Marzo, V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br. J. Pharmacol. 2012, 166, 1444–1460. [Google Scholar] [CrossRef]
- DeLong, G.T.; Wolf, C.E.; Poklis, A.; Lichtman, A.H. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010, 112, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, R.K.; Zou, S.; Kadhim, S.; Padania, S.; Hsu, E.; Kumar, U. Cannabinol modulates neuroprotection and intraocular pressure: A potential multi-target therapeutic intervention for glaucoma. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166325. [Google Scholar] [CrossRef] [PubMed]
- Farrimond, J.A.; Whalley, B.J.; Williams, C.M. Cannabinol and cannabidiol exert opposing effects on rat feeding patterns. Psychopharmacol. 2012, 223, 117–129. [Google Scholar] [CrossRef]
- Wong, H.; Cairns, B.E. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch. Oral. Biol. 2019, 104, 33–39. [Google Scholar] [CrossRef]
- Maioli, C.; Mattoteia, D.; Amin, H.I.M.; Minassi, A.; Caprioglio, D. Cannabinol: History, syntheses, and biological profile of the greatest "minor" cannabinoid. Plants 2022, 11, 2896. [Google Scholar] [CrossRef]
- Huizenga, M.N.; Sepulveda-Rodriguez, A.; Forcelli, P.A. Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology 2019, 148, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Gabaglio, M.; Woolley-Roberts, M.; Bingham, S.; Rubino, T.; Parolaro, D. Cannabidivarin treatment ameliorates autism-like behaviors and restores hippocampal endocannabinoid system and glia alterations induced by prenatal valproic acid exposure in rats. Front. Cell. Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E.; Rubino, T.; Parolaro, D. Therapeutic potential of cannabidivarin for epilepsy and autism spectrum disorder. Pharmacol. Ther. 2021, 226, 107878. [Google Scholar] [CrossRef]
- Russo, C.; Lavorgna, M.; Nugnes, R.; Orlo, E.; Isidori, M. Comparative assessment of antimicrobial, antiradical and cytotoxic activities of cannabidiol and its propyl analogue cannabidivarin. Sci. Rep. 2021, 11, 22494. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020, 177, 4330–4352. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A commentary on potential therapeutic benefit for the management of obesity and diabetes. J. Cannabis Res. 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Tudge, L.; Williams, C.; Cowen, P.J.; McCabe, C. Neural effects of cannabinoid CB1 neutral antagonist tetrahydrocannabivarin on food reward and aversion in healthy volunteers. Int. J. Neuropsychopharmacol. 2014, 18, pyu094. [Google Scholar] [CrossRef]
- Zimniewska, M. Hemp fibre properties and processing target textile: A review. Materials 2022, 15, 1901. [Google Scholar] [CrossRef]
- Ahirwar, M.; Behera, B. Development of hemp-blended cotton fabrics and analysis on handle behavior, low-stress mechanical and aesthetic properties. J. Text. I. 2022, 113, 934–942. [Google Scholar] [CrossRef]
- Väisänen, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of hemp fibers (Cannabis Sativa L.) for composite applications. Ind. Crops Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
- Shah, N.; Fehrenbach, J.; Ulven, C.A. Hybridization of hemp fiber and recycled-carbon fiber in polypropylene composites. Sustainability 2019, 11, 3163. [Google Scholar] [CrossRef]
- Yadav, M.; Saini, A. Opportunities & challenges of hempcrete as a building material for construction: An overview. Mater. Today Proc. 2022, 65, 2021–2028. [Google Scholar]
- Di Capua, S.E.; Paolotti, L.; Moretti, E.; Rocchi, L.; Boggia, A. Evaluation of the environmental sustainability of hemp as a building material, through life cycle assessment. Environ. Clim. Technol. 2021, 25, 1215–1228. [Google Scholar] [CrossRef]
- Abdellatef, Y.; Kavgic, M. Thermal, microstructural and numerical analysis of hempcrete-microencapsulated phase change material composites. Appl. Therm. Eng. 2020, 178, 115520. [Google Scholar] [CrossRef]
- Arrigoni, A.; Pelosato, R.; Melià, P.; Ruggieri, G.; Sabbadini, S.; Dotelli, G. Life cycle assessment of natural building materials: The role of carbonation, mixture components and transport in the environmental impacts of hempcrete blocks. J. Clean. Prod. 2017, 149, 1051–1061. [Google Scholar] [CrossRef]
- Shang, Y.; Tariku, F. Hempcrete building performance in mild and cold climates: Integrated analysis of carbon footprint, energy, and indoor thermal and moisture buffering. Build. Environ. 2021, 206, 108377. [Google Scholar] [CrossRef]
- Birjukovs, M.; Sinka, M.; Jakovics, A.; Bajare, D. Combined in situ and in silico validation of a material model for hempcrete. Constr. Build. Mater. 2022, 321, 126051. [Google Scholar] [CrossRef]
- Liu, M.; Toth, J.A.; Childs, M.; Smart, L.B.; Abbaspourrad, A. Composition and functional properties of hemp seed protein isolates from various hemp cultivars. J. Food Sci. 2023, 88, 942–951. [Google Scholar] [CrossRef]
- Schultz, C.J.; Lim, W.L.; Khor, S.F.; Neumann, K.A.; Schulz, J.M.; Ansari, O.; Skewes, M.A.; Burton, R.A. Consumer and health-related traits of seed from selected commercial and breeding lines of industrial hemp, Cannabis sativa L. J. Agric. Food Res. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Mookerjee, A.; Borugadda, V.B.; Dalai, A.K.; Meda, V. Valorization of hemp hearts oils by advanced extraction techniques and their comparative physicochemical characterization. Appl. Food Res. 2022, 2, 100051. [Google Scholar] [CrossRef]
- Senila, L.; Neag, E.; Cadar, O.; Kovacs, M.H.; Becze, A.; Senila, M. Chemical, nutritional and antioxidant characteristics of different food seeds. Appl. Sci. 2020, 10, 1589. [Google Scholar] [CrossRef]
- Uzunlar, E.A.; Kahveci, B. Nutritional properties and health effects of hemp seeds. Res. Rev. Healthc. Open Access J. 2022, 7, 706–712. [Google Scholar]
- Şeker, M.; Özlem, E. The effect of hemp seed oil on skin and soap performance. Int. J. Life Sci. Biotechnol. 2021, 4, 420–438. [Google Scholar] [CrossRef]
- Kitamura, M.; Kiba, Y.; Suzuki, R.; Tomida, N.; Uwaya, A.; Isami, F.; Deng, S. Cannabidiol content and in vitro biological activities of commercial cannabidiol oils and hemp seed oils. Medicines 2020, 7, 57. [Google Scholar] [CrossRef]
- Huang, Y.; Pei, L.; Gu, X.; Wang, J. Study on the oxidation products of hemp seed oil and its application in cosmetics. Tenside Surfact. Det. 2020, 57, 230–236. [Google Scholar] [CrossRef]
- van Orten-Luiten, A.-C.B.; De Roos, N.M.; Majait, S.; Witteman, B.J.; Witkamp, R.F. Effects of cannabidiol chewing gum on perceived pain and well-being of irritable bowel syndrome patients: A placebo-controlled crossover exploratory intervention study with symptom-driven dosing. Cannabis Cannabinoid Res. 2022, 7, 436–444. [Google Scholar] [CrossRef]
- John, C.B.; Raja, S.A. Analysis of combustion, emission and performance attributes of hemp biodiesel on a compression ignition engine. World Rev. Sci. Technol. Sustain. Dev. 2020, 16, 169–183. [Google Scholar] [CrossRef]
- Ji, A.; Jia, L.; Kumar, D.; Yoo, C.G. Recent advancements in biological conversion of industrial hemp for biofuel and value-added products. Fermentation 2021, 7, 6. [Google Scholar] [CrossRef]
- Russo, E.B.; Cuttler, C.; Cooper, Z.D.; Stueber, A.; Whiteley, V.L.; Sexton, M. Survey of patients employing cannabigerol-predominant cannabis preparations: Perceived medical effects, adverse events, and withdrawal symptoms. Cannabis Cannabinoid Res. 2022, 7, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Senthil, R.; Sundaram, P. Recent trends in the carbon capture and storage technologies. Technology 2017, 8, 885–891. [Google Scholar]
- Lawson, L.; Degenstein, L.M.; Bates, B.; Chute, W.; King, D.; Dolez, P.I. Cellulose textiles from hemp biomass: Opportunities and challenges. Sustainability 2022, 14, 15337. [Google Scholar] [CrossRef]
- Bandawe, G. Medical cannabis and cannabidiol: A new harvest for Malawi. Malawi Med. J. 2022, 34, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Sowoya, L.; Akamwaza, C.; Matola, A.M.; Klein, A. Goodbye Nicky hello Goldie–exploring the opportunities for transitioning tobacco farmers into cannabis production in Malawi. Drugs Alcohol Today 2020, 20, 295–303. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, F.; Stehle, F.; Kayser, O. The biosynthesis of cannabinoids. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 13–23. [Google Scholar]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G. The early history of cannabinoid research. Rend. Lincei Sci. Fis. Nat. 2020, 31, 919–929. [Google Scholar] [CrossRef]
- Schafroth, M.A.; Mazzoccanti, G.; Reynoso-Moreno, I.; Erni, R.; Pollastro, F.; Caprioglio, D.; Botta, B.; Allegrone, G.; Grassi, G.; Chicca, A.; et al. Δ9-cis-Tetrahydrocannabinol: Natural occurrence, chirality, and pharmacology. J. Nat. Prod. 2021, 84, 2502–2510. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The cannabis terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Huang, A.C.; Osbourn, A. Plant terpenes that mediate below-ground interactions: Prospects for bioengineering terpenoids for plant protection. Pest Manag. Sci. 2019, 75, 2368–2377. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.; Li, C.; Lee, I.H.; Lam, H.-M.; Chan, T.-F.; Hui, J.H. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective potential of limonene and limonene containing natural products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Santos, E.S.; Coelho, G.L.A.; Loula, Y.K.S.F.; Landim, B.L.S.; Lima, C.N.F.; de Sousa Machado, S.T.; Lopes, M.J.P.; Gomes, A.D.S.; da Costa, J.G.M.; de Menezes, I.R.A.; et al. Hypoglycemic, hypolipidemic, and anti-Inflammatory effects of beta-pinene in diabetic rats. eCAM 2022, 2022, 8173307. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: Discovering novel therapeutics in the flavours and fragrances of cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- da Conceição Machado, K.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of oxidative stress and mitochondrial dysfunction by β-caryophyllene: A focus on the nervous system. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- de Lacerda Leite, G.M.; de Oliveira Barbosa, M.; Lopes, M.J.P.; de Araújo Delmondes, G.; Bezerra, D.S.; Araújo, I.M.; de Alencar, C.D.C.; Coutinho, H.D.M.; Peixoto, L.R.; Barbosa-Filho, J.M.; et al. Pharmacological and toxicological activities of α-humulene and its isomers: A systematic review. Trends Food Sci. Technol. 2021, 115, 255–274. [Google Scholar] [CrossRef]
- Menezes, I.O.; Scherf, J.R.; Martins, A.O.B.P.B.; Ramos, A.G.B.; de Sousa Siqueira Quintans, J.; Coutinho, H.D.M.; Ribeiro-Filho, J.; de Menezes, I.R.A. Biological properties of terpinolene evidenced by in silico, in vitro and in vivo studies: A systematic review. Phytomedicine 2021, 93, 153768. [Google Scholar] [CrossRef] [PubMed]
- Thakre, A.D.; Mulange, S.V.; Kodgire, S.S.; Zore, G.B.; Karuppayil, S.M. Effects of cinnamaldehyde, ocimene, camphene, curcumin and farnesene on Candida albicans. Adv. Microbiol. 2016, 6, 627–643. [Google Scholar] [CrossRef]
- de Sousa, J.M.S.; de Lima Nunes, T.A.; Rodrigues, R.R.L.; de Sousa, J.P.A.; da Conceição Albuquerque Val, M.; da Rocha Coelho, F.A.; dos Santos, A.L.S.; Maciel, N.B.; de Souza, V.M.R.; Machado, Y.A.A.; et al. Cytotoxic and antileishmanial effects of the monoterpene β-ocimene. Pharmaceuticals 2023, 16, 183. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef] [PubMed]
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front. Pharmacol. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Heblinski, M.; Santiago, M.; Fletcher, C.; Stuart, J.; Connor, M.; McGregor, I.S.; Arnold, J.C. Terpenoids commonly found in Cannabis sativa do not modulate the actions of phytocannabinoids or endocannabinoids on TRPA1 and TRPV1 channels. Cannabis Cannabinoid Res. 2020, 5, 305–317. [Google Scholar] [CrossRef] [PubMed]
- LaVigne, J.; Hecksel, R.; Streicher, J.M. In defense of the “entourage effect”: Terpenes found in Cannabis sativa activate the cannabinoid receptor 1 in vivo. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- LaVigne, J.E.; Hecksel, R.; Keresztes, A.; Streicher, J.M. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crops Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, bioactivities, and biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M.; et al. Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
- Kamisah, Y.; Jalil, J.; Yunos, N.M.; Zainalabidin, S. Cardioprotective properties of kaempferol: A review. Plants 2023, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Oswald, I.W.H.; Ojeda, M.A.; Pobanz, R.J.; Koby, K.A.; Buchanan, A.J.; Del Rosso, J.; Guzman, M.A.; Martin, T.J. Identification of a new family of prenylated volatile sulfur compounds in cannabis revealed by comprehensive two-dimensional gas chromatography. ACS Omega 2021, 6, 31667–31676. [Google Scholar] [CrossRef] [PubMed]
- Van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011, 12, R102. [Google Scholar] [CrossRef]
- Grassa, C.J.; Weiblen, G.D.; Wenger, J.P.; Dabney, C.; Poplawski, S.G.; Timothy Motley, S.; Michael, T.P.; Schwartz, C. A new Cannabis genome assembly associates elevated cannabidiol (CBD) with hemp introgressed into marijuana. New Phytol. 2021, 230, 1665–1679. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.; Xie, S.; Xu, X.; Zhang, J.; Pei, L.; Yu, Y.; Yang, W.; Zhang, Y. A high-quality reference genome of wild Cannabis sativa. Hortic. Res. 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, X.; Li, Y.; Ridout, K.; Serrano-Serrano, M.L.; Yang, Y.; Liu, A.; Ravikanth, G.; Nawaz, M.A.; Mumtaz, A.S.; et al. Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa. Sci. Adv. 2021, 7, eabg2286. [Google Scholar] [CrossRef] [PubMed]
- Balant, M.; Rodríguez González, R.; Garcia, S.; Garnatje, T.; Pellicer, J.; Vallès, J.; Vitales, D.; Hidalgo, O. Novel insights into the nature of intraspecific genome size diversity in Cannabis sativa L. Plants 2022, 11, 2736. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Pauli, C.; Givens, R.; Argyris, J.; Allen, K.; Monfort, A.; Gaudino, R.J. High-throughput methods to identify male Cannabis sativa using various genotyping methods. J. Cannabis Res. 2022, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Melzer, R.; Dowling, C.A.; Shi, J.; Muldoon, S.; McCabe, P.F. A protocol for rapid generation cycling (speed breeding) of hemp (Cannabis sativa) for research and agriculture. Plant J. 2023, 113, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Sunoj Valiaparambil Sebastian, J.; Dong, X.; Trostle, C.; Pham, H.; Joshi, M.V.; Jessup, R.W.; Burow, M.D.; Provin, T.L. Hemp agronomy: Current advances, questions, challenges, and opportunities. Agronomy 2023, 13, 475. [Google Scholar] [CrossRef]
- Wróbel, T.; Dreger, M.; Wielgus, K.; Słomski, R. Modified nodal cuttings and shoot tips protocol for rapid regeneration of Cannabis sativa L. J. Nat. Fibers 2022, 19, 536–545. [Google Scholar] [CrossRef]
- Tan, J.W.; Kester, S.T.; Su, K.; Hildebrand, D.F.; Geneve, R.L. Seed priming and pericarp removal improve germination in low-germinating seed lots of industrial hemp. Crops 2022, 2, 407–414. [Google Scholar] [CrossRef]
- Mostafaei Dehnavi, M.; Ebadi, A.; Peirovi, A.; Taylor, G.; Salami, S.A. THC and CBD fingerprinting of an elite cannabis collection from Iran: Quantifying diversity to underpin future cannabis breeding. Plants 2022, 11, 129. [Google Scholar] [CrossRef]
- Li, L.; Yu, S.; Chen, J.; Cheng, C.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Releasing the full potential of cannabis through biotechnology. Agronomy 2022, 12, 2439. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Establishment of an Agrobacterium-mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in Hemp (Cannabis Sativa L.). Plant Biotechnol. J. 2021, 19, 1979–1987. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2023, 62, 108074. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Lee, S.; Miller, R. Identifying the repellent genes in Cannabis (C. sativa) through CRISPR screening. In Proceedings of the Hidden Use of Marijuana, Inquiry@ Queen’s Undergraduate Research Conference Proceedings, Kingston, ON, Canada, 15 April 2020. [Google Scholar]
- Matchett-Oates, L.; Braich, S.; Spangenberg, G.; Rochfort, S.; Cogan, N. In silico analysis enabling informed design for genome editing in medicinal cannabis; gene families and variant characterisation. PLoS ONE 2021, 16, e0257413. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.J.; Kayser, O. Minor cannabinoids of Cannabis sativa L. J. Med. Sci. 2019, 88, 141–149. [Google Scholar] [CrossRef]
- Rodriguez, C.E.B.; Ouyang, L.; Kandasamy, R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis. Behav. Pharmacol. 2022, 33, 130–157. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Frei, P.; Weber, S.; Scheurer, E.; Mercer-Chalmers-Bender, K. Beyond Δ9-tetrahydrocannabinol and cannabidiol: Chemical differentiation of cannabis varieties applying targeted and untargeted analysis. Anal. Bioanal. Chem. 2022, 414, 3847–3862. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor cannabinoids: Biosynthesis, molecular pharmacology and potential therapeutic uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef] [PubMed]
- Anokwuru, C.P.; Makolo, F.L.; Sandasi, M.; Tankeu, S.Y.; Elisha, I.L.; Agoni, C.; Combrinck, S.; Viljoen, A. Cannabigerol: A bibliometric overview and review of research on an important phytocannabinoid. Phytochem. Rev. 2022, 21, 1523–1547. [Google Scholar] [CrossRef]
- Zager, J.J.; Lange, I.; Srividya, N.; Smith, A.; Lange, B.M. Gene networks underlying cannabinoid and terpenoid accumulation in cannabis. Plant Physiol. 2019, 180, 1877–1897. [Google Scholar] [CrossRef]
- Zandkarimi, F.; Decatur, J.; Casali, J.; Gordon, T.; Skibola, C.; Nuckolls, C. Comparison of the cannabinoid and terpene profiles in commercial cannabis from natural and artificial cultivation. Molecules 2023, 28, 833. [Google Scholar] [CrossRef]
- Desaulniers Brousseau, V.; Wu, B.-S.; MacPherson, S.; Morello, V.; Lefsrud, M. Cannabinoids and terpenes: How production of photo-protectants can be manipulated to enhance Cannabis sativa L. phytochemistry. Front. Plant Sci. 2021, 12, 620021. [Google Scholar] [CrossRef]
- Islam, M.J.; Ryu, B.R.; Rana, M.S.; Cheong, E.J.; Wang, M.-H.; Lim, J.-D.; Hossain, M.A.; Lim, Y.-S. Cannabinoid accumulation in hemp depends on ROS generation and interlinked with morpho-physiological acclimation and plasticity under indoor LED environment. Front. Plant Sci. 2022, 13, 984410. [Google Scholar] [CrossRef]
- Kim, A.L.; Yun, Y.J.; Choi, H.W.; Hong, C.-H.; Shim, H.J.; Lee, J.H.; Kim, Y.-C. Profiling cannabinoid contents and expression levels of corresponding biosynthetic genes in commercial cannabis (Cannabis sativa L.) cultivars. Plants 2022, 11, 3088. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Increasing inflorescence dry weight and cannabinoid content in medical cannabis using controlled drought stress. HortScience 2019, 54, 964–969. [Google Scholar] [CrossRef]
- Punja, Z.K. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag. Sci. 2021, 77, 3857–3870. [Google Scholar] [CrossRef]
- Campbell, B.J.; Berrada, A.F.; Hudalla, C.; Amaducci, S.; McKay, J.K. Genotype × environment interactions of industrial hemp cultivars highlight diverse responses to environmental factors. Agrosystems Geosci. Environ. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- European Commission EUPVP—Common Catalogue. Available online: https://ec.europa.eu/food/plant-variety-portal/ (accessed on 22 February 2024).
- Chch Cannabis. Available online: https://www.ch.ch/en/health/medicines-and-narcotic-substances/cannabis/ (accessed on 22 February 2024).
- Raman, A. The cannabis plant: Botany, cultivation and processing for use. In Cannabis: The Genus Cannabis, 1st ed.; Brown, D.T., Ed.; CRC Press: London, UK, 1998; Volume 4, pp. 32–57. [Google Scholar]
- Teirumnieka, Ē.; Blumberga, D.; Teirumnieks, E.; Stramkale, V. Product-oriented production of industrial hemp according to climatic conditions. Agron. Res. 2021, 19, 2026–2036. [Google Scholar]
- Carus, M.; Sarmento, L. The European hemp industry: Cultivation, processing and applications for fibres, shivs, seeds and flowers. Eur. Ind. Hemp Assoc. 2016, 5, 1–9. [Google Scholar]
- García-Tejero, I.; Zuazo, V.D.; Sánchez-Carnenero, C.; Hernández, A.; Ferreiro-Vera, C.; Casano, S. Seeking suitable agronomical practices for industrial hemp (Cannabis sativa L.) cultivation for biomedical applications. Ind. Crops Prod. 2019, 139, 111524. [Google Scholar] [CrossRef]
- Aznar, F.; Negral, L.; Moreno-Grau, S.; Elvira-Rendueles, B.; Costa-Gómez, I.; Moreno, J.M. Cannabis, an emerging aeroallergen in southeastern Spain (Region of Murcia). Sci. Total Environ. 2022, 833, 155156. [Google Scholar] [CrossRef]
- Poniatowska, J.; Wielgus, K.; Szalata, M.; Szalata, M.; Ozarowski, M.; Panasiewicz, K. Contribution of Polish agrotechnical studies on Cannabis sativa L. to the global industrial hemp cultivation and processing economy. Herba Pol. 2019, 65, 37–50. [Google Scholar] [CrossRef]
- Duchateau, C.; Kauffmann, J.M.; Canfyn, M.; Stévigny, C.; De Braekeleer, K.; Deconinck, E. Discrimination of legal and illegal Cannabis spp. according to European legislation using near infrared spectroscopy and chemometrics. Drug Test. Anal. 2020, 12, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the polyphenol and terpene content, antioxidant activity and plant morphology of eight different fiber-type cultivars of Cannabis sativa L. cultivated at three sowing densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef]
- Torriani, D.S.; Calanca, P.; Schmid, S.; Beniston, M.; Fuhrer, J. Potential effects of changes in mean climate and climate variability on the yield of winter and spring crops in Switzerland. Clim. Res. 2007, 34, 59–69. [Google Scholar] [CrossRef]
- Möhring, N.; Finger, R. Pesticide-free but not organic: Adoption of a large-scale wheat production standard in Switzerland. Food Policy 2022, 106, 102188. [Google Scholar] [CrossRef]
- Brunetti, P.; Faro, A.F.L.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and legal status of cannabidiol. Ann. I. Super. Sanita 2020, 56, 285–291. [Google Scholar]
- Plumb, J.; Demirel, S.; Sackett, J.L.; Russo, E.B.; Wilson-Poe, A.R. The nose knows: Aroma, but not THC mediates the subjective effects of smoked and vaporized cannabis flower. Psychoactives 2022, 1, 70–86. [Google Scholar] [CrossRef]
- Smith, C.J.; Vergara, D.; Keegan, B.; Jikomes, N. The phytochemical diversity of commercial cannabis in the United States. PLoS ONE 2022, 17, e0267498. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Kilgore, M.; Babalonis, S. Label accuracy of unregulated cannabidiol (CBD) products: Measured concentration vs. label claim. J. Cannabis Res. 2022, 4, 28. [Google Scholar] [CrossRef]
- Oldfield, K.; Ryan, J.; Doppen, M.; Kung, S.; Braithwaite, I.; Newton-Howes, G. A systematic review of the label accuracy of cannabinoid-based products in regulated markets: Is what’s on the label what’s in the product? Australas. Psychiatry 2021, 29, 88–96. [Google Scholar] [CrossRef]
- Spindle, T.R.; Sholler, D.J.; Cone, E.J.; Murphy, T.P.; ElSohly, M.; Winecker, R.E.; Flegel, R.R.; Bonn-Miller, M.O.; Vandrey, R. Cannabinoid content and label accuracy of hemp-derived topical products available online and at national retail stores. JAMA Netw. Open 2022, 5, e2223019. [Google Scholar] [CrossRef] [PubMed]
- Grafinger, K.E.; Krönert, S.; Broillet, A.; Weinmann, W. Cannabidiol and tetrahydrocannabinol concentrations in commercially available CBD E-liquids in Switzerland. Forensic Sci. Int. 2020, 310, 110261. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Murphy, T.P.; Khan, I.; Walker, L.W.; Gul, W. Analysis of Cannabidiol, Δ9-tetrahydrocannabinol, and their acids in CBD oil/hemp oil products. Med. Cannabis Cannabinoids 2020, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Duchateau, C.; Canfyn, M.; Desmedt, B.; Kauffmann, J.-M.; Stevigny, C.; De Braekeleer, K.; Deconinck, E. CBD oils on the Belgian market: A validated MRM GC-MS/MS method for routine quality control using QuEChERS sample clean up. J. Pharm. Biomed. Anal. 2021, 205, 114344. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Miller, O.S.; Elder, E.J., Jr.; Jones, K.J.; Gidal, B.E. Analysis of cannabidiol (CBD) and THC in nonprescription consumer products: Implications for patients and practitioners. Epilepsy Behav. 2022, 127, 108514. [Google Scholar] [CrossRef] [PubMed]
- Reason, D.A.; Grainger, M.N.; Lane, J.R. Optimal storage conditions of commercial cannabis crops. Ind. Eng. Chem. Res. 2022, 61, 14691–14701. [Google Scholar] [CrossRef]
- García-Valverde, M.T.; Sánchez-Carnerero Callado, C.; Díaz-Liñán, M.C.; Sánchez de Medina, V.; Hidalgo-García, J.; Nadal, X.; Hanuš, L.; Ferreiro-Vera, C. Effect of temperature in the degradation of cannabinoids: From a brief residence in the gas chromatography inlet port to a longer period in thermal treatments. Front. Chem. 2022, 10, 1038729. [Google Scholar] [CrossRef]
- Meija, J.; McRae, G.; Miles, C.O.; Melanson, J.E. Thermal stability of cannabinoids in dried cannabis: A kinetic study. Anal. Bioanal. Chem. 2022, 414, 377–384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).