Abstract
The emergence of inflammatory diseases is a heavy burden on modern societies. Cannabis has been used for several millennia to treat inflammatory disorders such as rheumatism or gout. Since the characterization of cannabinoid receptors, CB1 and CB2, the potential of cannabinoid pharmacotherapy in inflammatory conditions has received great interest. Several studies have identified the importance of these receptors in immune cell migration and in the production of inflammatory mediators. As the presence of the CB2 receptor was documented to be more predominant in immune cells, several pharmacological agonists and antagonists have been designed to treat inflammation. To better define the potential of the CB2 receptor, three online databases, PubMed, Google Scholar and clinicaltrial.gov, were searched without language restriction. The full texts of articles presenting data on the endocannabinoid system, the CB2 receptor and its role in modulating inflammation in vitro, in animal models and in the context of clinical trials were reviewed. Finally, we discuss the clinical potential of the latest cannabinoid-based therapies in inflammatory diseases.
1. Introduction
Inflammatory diseases are the leading causes of disability and death throughout developing countries [1]. Therefore, it is more and more urgent to find a therapeutic target that could improve the conditions of people suffering from inflammatory diseases [2]. These disorders evolve from a sustained activation of the immune system that can be localized and/or disseminated throughout the body [3]. Chinese traditional healers have used cannabis to treat inflammation since 2000 before Common Era [4]. Since the identification of cannabinoid receptors in immune cells, the potential of cannabinoid pharmacotherapy in pain and inflammatory conditions has received much attention [5]. Here, we are updating the literature review by Turcotte et al. [6], highlighting the studies of the last decade on the role of the CB2 receptor in peripheral inflammation and its potential as a pharmaceutical target in inflammatory diseases.
2. The Type 2 Cannabinoid Receptor (CB2)
Our knowledge of the endocannabinoid system started with the elucidation of the structure, metabolism and function of Δ9-tetrahydrocannabinol (Δ9-THC) [7,8,9]. The first cannabinoid receptor CB1 was isolated and cloned from a rat cerebral cortex cDNA library. The translated sequence of the identified cDNA was a 473 amino acid protein sequence and a member of the G-protein-coupled family of receptors [10]. Two years later, the human cannabinoid receptor CB2 was cloned from the promyeloid cell line HL-60 [11]. The protein encoded by a sequence of 360 amino acids was found to have 44% homology with the CB1 receptor [12,13]. The CB2 receptor is considered a peripheral receptor due to its predominant expression in various immune cells [14,15]. Eosinophils, B cells and natural killer (NK) cells were found to strongly express CB2, whereas monocytes, neutrophils, helper T cells and cytotoxic T cells showed mRNA transcript levels ranging from moderate to low [16,17]. The membrane expression of CB2 was also accessed by flow cytometry, and it was shown that circulating B cells, NK cells and monocytes express higher levels of the receptor compared to CD4+T lymphocytes, CD8+T lymphocytes and neutrophils [18]. Another study confirmed the membrane expression of CB2 on eosinophils and found that it was increased in eosinophils isolated from the blood of allergic patients compared to those isolated from healthy donors [19]. Furthermore, functional CB2 is detected in perivascular microglia [20] and the brain [21]. Therefore, it is suggested that CB2 is also expressed in the healthy central nervous system albeit at lower transcript levels and not necessarily in neurons [21].
To better understand the expression of CB2 in the inflammatory context, Turcotte et al. [6] and others comprehensively reviewed the expression of CB2 in human tissues and animal models and highlighted the role of CB2 as a regulator of inflammation. For instance, in the gastrointestinal tract, CB2 is detected in the esophagus, stomach and ileum [22]. In humans, CB2 is expressed by colonic epithelial cells but only under inflammatory conditions [23]. The expression of CB2 by the liver is specifically high during embryonic development [24], and its expression by hepatic myofibroblasts increases under pathological conditions such as liver fibrosis [25] and liver injury [26]. In the cardiovascular system, CB2 is expressed by cardiomyocytes and endothelial cells [27], and its expression increases in an inflammatory context such as atherosclerosis and acute myocardial infarction [28]. In the musculoskeletal system, the protein expression of CB2 by chondrocytes has been detected and shown to be increased in the joint tissues of rats [29,30] and humans with osteoarthritis [31]. In the brain, CB2 expression is still controversial, as it seems to be detected only in the context of neuroinflammation and is attributed to microglia [32]. However, other studies have reported that CB2 is detected in neurons and may mediate brain functions [33]. Moreover, the expression of this receptor in innate and adaptive immune cells was recently reviewed by Simard and colleagues [17]. In particular, they showed that high CB2 (Cnr2) expression is found in eosinophils and B cells, while low expression is found in T cells and monocytes [17]. Additionally, the expression of CB2 by different tissues is upregulated in an inflammatory context including neuroinflammation [34,35,36] and acute and chronic inflammation [37,38]. Considering the relevance of the CB2 receptor in the regulation of the immune response, CB2 is seen as a potential therapeutic target in inflammatory diseases.
3. CB2 Signaling Pathway
CB1 and CB2 are both G-protein-coupled receptors binding to a trimer of Gα/β/γ proteins and activate in part similar signaling cascades. CB1 can couple to either Gαs or Gαi/o proteins which respectively activate or inhibit the adenylyl cyclases. Conversely, CB2 almost exclusively recruits Gαi/o proteins leading to inhibition of adenylyl cyclase activity which reduces AMPc levels and prevents activation of PKA [39]. Since PKA activation leads to NF-κB and CREB transactivation [40], Gi/o signaling triggered by CB2 is believed to prevent the induction of inflammatory genes. Although CB2 coupling to Gαs has been reported only in rare instances, it was suggested to play an important role in the induction of a pro-inflammatory response dependent on PKA activation [41]. CB2 engagement also leads to the Gβ- and Gγ-dependent activation of p38 and ERK1/2 MAP kinases [41,42]. CB2-dependent MAPK phosphorylation was shown to be responsible for the activation of pro-inflammatory genes through transcription factors NF-κB and CREB [41,43]. Moreover, contrarily to CB1, CB2 cannot trigger potassium channel signaling through Gαs but can initiate Ca2+ signaling via phospholipase C activation by Gαi/o proteins [44]. Finally, the c-terminal tail of CB1 and CB2 can also be phosphorylated by G-Protein-Coupled Receptor Kinases (GRK), allowing the recruitment of β-arrestins. CB2 can bind β-arrestin1 and β-arrestin2 which leads to receptor internalization but also to the phosphorylation of MAPK ERK1/2 [45,46] (Figure 1).
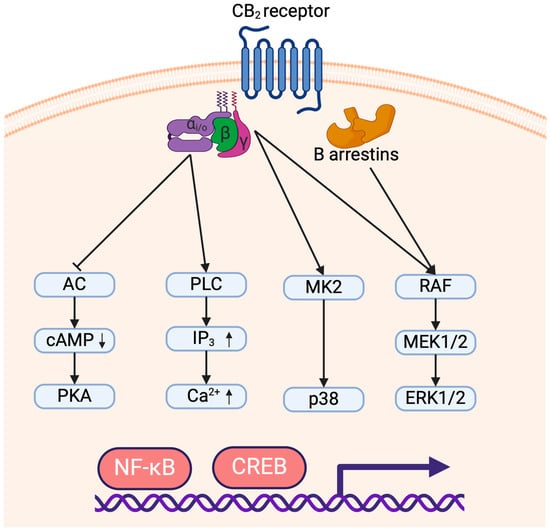
Figure 1.
Signaling of the CB2 receptor leading to pro- and anti-inflammatory responses. The arrows (↑/↓) indicate an increase or decrease in molecule concentration, respectively.
A single GPCR can adopt different conformations depending on its interaction with specific agonists, and some conformations can trigger only part of the signaling events attributed to the receptor [47]. Evidence of this mechanism, termed biased agonism, has been reported for both CB1 and CB2. In the case of CB2, THC strongly potentiates ERK phosphorylation but is less effective on β-arrestin recruitment and cAMP signaling and does not activate G-protein-coupled inwardly rectifying potassium channels (GIRK). Endocannabinoids can activate all signaling pathways, but N-arachidonoylethanolamine (AEA) strongly increases GIRK and ERK activation and is less impactful on cAMP and β-arrestin signaling, while 2-AG favors GIRK signaling to other pathways. As for selective CB2 agonists, JWH-133 induces β-arrestin and cAMP signaling more potently than GIRK and ERK activation, while HU910 and HU308 do not show a strong bias toward a specific signaling pathway [43,48]. Given that CB2 agonists vary greatly in their selectivity, efficacy and capacity to trigger signaling events, studies using different agonists must be compared carefully, and agonists that are both selective and not greatly biased toward signaling pathways should be favored in future studies.
Adenylyl cyclase (AC); cAMP Response Element-Binding Protein (CREB); Cyclic adenosine monophosphate (cAMP); Extracellular signal-regulated kinase (ERK); Inositol trisphosphate (IP3); Mitogen-activated protein kinase kinase (MEK); Mitogen-activated protein kinase-activated protein kinase-2 (MK2); Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB); p38 mitogen-activated protein kinase (p38); Phospholipase C (PLC); Protein kinase A (PKA); Protein kinase C (PKC); Rapidly accelerated fibrosarcoma kinase (RAF).
4. Cnr2-KO Mice and Inflammation
Cnr2c deficient animals provide a powerful tool to understand the roles of CB2 in inflammatory mouse models [49]. A majority of studies showed that Cnr2-deficient mice have a pro-inflammatory profile (Table 1), implying that CB2 is involved in the negative regulation of the inflammatory response [50,51,52,53,54,55]. In inflammatory models such as lipopolysaccharide (LPS)-induced inflammation [54,56] or alcoholic liver disease [50], the genetic deletion of Cnr2 was associated with an increased production of pro-inflammatory cytokines. The secretion of chemokines and the expression of their receptors also increased leading to a modification of cell migration and infiltration. In acute inflammatory conditions, such as the murine dorsal air pouch model, the absence of Cnr2 altered the recruitment of neutrophils to the site of inflammation [54].
On the other hand, some studies have shown that Cnr2 deficiency can also dampen inflammation, notably, inflammatory states involving eosinophils, as we summarize in Table 2. For instance, mouse models of allergic bronchitis are characterized by the infiltration of activated eosinophils in the airways [57]. This could be explained, in part, by the ability of 2-AG, concomitantly with IL-5, to act as a chemoattractant for eosinophils [58]. Eosinophil accumulation often correlates positively with disease severity and immune cell infiltration and is effectively reversed by genetic deletion of Cnr2 [59]. The absence of Cnr2 alters innate immune cell function as it reduces the ability of dendritic cells to express costimulatory molecules that affect T cell activation and proliferation [53]. Furthermore, CB2 signaling affects macrophage functions by steering them toward those typical of an M2 phenotype [60].
These contrasting studies demonstrate that the inflammatory effect of Cnr2 deletion is not completely understood. It is also crucial to take into consideration other receptors and endocannabinoid-like compounds belonging to the endocannabinoidome (see below), which can compensate for the absence of CB2 (Table 1 and Table 2) [61,62,63]. Among these receptors, we highlight PPARγ (peroxisome proliferator-activator receptor gamma), which belongs to the endocannabinoidome [64]. Through its affinity for endocannabinoids, PPARγ can downregulate the activation, proliferation and migration of helper T cells [65,66,67,68]. Considering that the endocannabinoid system is involved in key biological processes, effects seen in Cnr2−/− inflammation mice models cannot be strictly attributed to a direct implication of CB2 in inflammation. Endocannabinoids can also act through other receptors, namely transient receptor potential vanilloid type 1(TRPV1) [69] and PPARs [70], which can counteract the absence of CB2 and explain the variable outcomes in response to the genetic deletion of Cnr2 [64].

Table 1.
Pro-inflammatory effects of Cnr2 genetic deletion in mouse models of inflammation.
Table 1.
Pro-inflammatory effects of Cnr2 genetic deletion in mouse models of inflammation.
| Model | Species | Effects | References |
|---|---|---|---|
| Alcoholic liver disease | C57BL/6N | ↑ CCL3 ↑ TNF-α, IL-6, IL-1β, IL-1α | [50] |
| Concavalin A-induced acute liver injury | C57BL/6J | ↑ Liver injury ↑ Macrophage proliferation and activation ↑ TNF-α | [51] |
| Corneal injury | BALB/c | ↑ Neutrophil infiltration | [52] |
| DNFB-induced hypersensitivity | C57BL/6J | ↑ Neutrophil recruitment ↑ Ear swelling ↓ CCR7 and CXCR4 ↓ MHC II and CD40/CD86 expression by dendritic cells | [53] |
| Dorsal air pouch model | C57BL/6J and B6.SJL | ↑ Neutrophil migration ↑ MMP-9, CCL2, CCL4, CXCL1, CXCL2, CXCL5, CXCL10 ↑ IL-6 | [54] |
| Endotoxin-induced uveitis | BALB/c | ↑ Endothelial leukocytes adhesion ↑ Neutrophil migration | [71] |
| Excisional skin wound | C57BL/6 | ↑ IL-6 and TNF-α | [72] |
| Hepatic ischemia-reperfusion injury | C57BL/6 | ↑ Neutrophil recruitment ↑ Inflammatory cytokines ↑ Liver damage | [73] |
| LPS-induced inflammation | C57BL/6J | ↑ Neutrophil recruitment to the spleen ↑ Leukocyte mobilization ↑ MMP-9 ↑ CCL2, CXCL10, ↑ IL-6 | [54,56,74] |
| Myocardial ischemia-reperfusion injury | C57BL/6J | ↑ Neutrophil and macrophage infiltration ↓ IL-10 | [75] |
| Obesity | C57BL/6J | ↑ Obesity ↑ Macrophage infiltration in adipose tissue ↑ CCL2 ↑ TNF-α | [55,76] |
| TNBS-induced colitis | C57BL/6 | ↑ Colitis ↑ TNF-α and IL-1β | [77] |
| Traumatic brain injury | C57BL/6 | ↑ TNF-α, iNOS and ICAM mRNA ↑ Blood–brain barrier permeability | [78,79] |
The arrows (↑/↓) indicate whether a process is enhanced or reduced, respectively.

Table 2.
Anti-inflammatory effects of Cnr2 genetic deletion in mouse models of inflammation.
Table 2.
Anti-inflammatory effects of Cnr2 genetic deletion in mouse models of inflammation.
| Model | Species | Effects | References |
|---|---|---|---|
| Cecal-ligation-induced sepsis | C57BL/6J | ↓ Bacterial invasion ↓ Mortality ↓ IL-10 | [80] |
| DSS-induced colitis | C57BL/6J | ↑ Expansion of regulatory CX3CR1hi macrophages (M2 macrophages) ↓ NLRP3 inflammasome activation in macrophages | [81] |
| Obesity | C57BL/6J | ↑ Insulin sensitivity ↓ CCL2 ↓ TNF-α, IL-6 and CCL2 | [82] |
| Plasmodium falciparum infection (Malaria) | C57BL/6J | ↓ CCL17 ↓ IFN-γ and TNF-α | [83] |
| Traumatic brain injury | C57BL/6 | ↓ Neurological deficits ↓ Edema and blood–brain barrier permeability | [78,79] |
| Type-1 diabetes | C57BL/6 and NOD/Lt | ↑ CX3CR1hi macrophages | [60] |
The arrows (↑/↓) indicate whether a process is enhanced or reduced, respectively.
4.1. Endogenous Ligands
Endogenous molecules with similar functions as Δ9-THC were discovered and named “endocannabinoids” [84]. To date, there are two characterized endocannabinoids binding to the cannabinoid receptors [7,13,85,86]. N-arachidonoylethanolamine (AEA), also known as anandamide, was first isolated from the porcine brain [7]. The second molecule, 2-arachidonoylglycerol (2-AG), was next found in canine intestinal tissue [86]. 2-AG has a higher efficacy at CB2 than AEA [64,85]. Moreover, since AEA is a partial agonist at CB2 as compared to 2-AG, when both ligands are present in a competitive way, AEA may act as a competitive antagonist of CB2 in the presence of 2-AG [87].
Other candidate endocannabinoids derived from arachidonic acid have also been identified. The ester form of arachidonic acid coupled to glycerol, also known as Noladin or 2-AGE, was isolated from the porcine brain in 2001 by Hanus et al. [88]. Based on the selective affinity of Noladin for the CB1 receptor [89], it took longer to identify that Noladin can also act as a full agonist at the CB2 receptor [90,91]. However, in areas such as the brain, where endocannabinoids and their receptors are known to be abundant, the concentration of 2-AGE was similar to that of AEA. [92]. In addition, Oka and colleagues showed that in mammals, the ratio of 2-AGE to 2-AG was very low, ranging from 1/500 to 1/4000 [93]. Although 2-AGE can act via the two endocannabinoid receptors, the low presence of Noladin both centrally and peripherally could raise the question of the physiological relevance of Noladin. The endocannabinoid family also includes O-arachidonoyl-ethanolamine, also known as Virodhamine, which is the ester of arachidonic acid and ethanolamine [94]. Discovered in 2002 by Porter et al., Virodhamine is known to be a CB2 agonist [94]. In peripheral tissues where CB2 is expressed, such as the spleen, Virodhamine levels were eight times higher than AEA. Although Virodhamine is known to be a CB2 agonist, it has been shown that it is also an agonist of the GPR55 receptor and that it exerts its effects through this receptor [95]. The third component results from the conjugation of arachidonic acid with dopamine, N-arachidonoyl-dopanime, also known as NADA [96], and has been reported to be present in specific regions of the brain including the striatum and hippocampus [96,97]. NADA is an endogenous ligand for CB1 but also for transient receptor potential vanilloid type 1 (TRPV1) and exerts its role in the inflammatory response through this receptor [98]. Given that these endocannabinoid ligands activate other receptors of the endocannabinoid system and the endocannabinoidome, such as CB1, GPR55 and TRPV1 [96], in this review, we will focus on endogenous ligands that exert an effect through the CB2 receptor.
Both AEA and 2-AG are lipid mediators derived from the cleavage of membrane phospholipid precursors [99]. However, their biosynthesis depends on different enzymatic pathways. Amongst the four known pathways leading to the production of AEA, the most studied is the hydrolysis of N-arachidonoyl-phosphatidylethanolamine (NAPE) by the N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD) [100]. AEA is primarily degraded by fatty acid amide hydrolase 1 (FAAH1), a serine hydrolase active at alkaline pH [101]. N-acylethanolamine-selective acid amidase (NAAA), which is a cysteine hydrolase working in an acidic environment, is another important catabolic enzyme degrading AEA, although with lower affinity/efficacy than with other N-acyl-ethanolamines, such as N-palmitoyl-ethanolamine. Indeed, with varying degrees of selectivity, these enzymes can hydrolyze N-acylethanolamines to fatty acids and ethanolamine [102].
As for 2-AG, it notably arises from the conversion of a phospholipid into 1/2-diacylglycerol (DAG), followed by the hydrolysis of the latter by the DAG lipases (DAGL) α or β [103], but other biosynthetic pathways have also been documented [104]. Like its 2-monoacylglycerol congeners, 2-AG is mainly catabolized by the monoacylglycerol lipase (MAGL) [105]. AEA, 2-AG, their N-acylethanolamine and 2-monoacylglycerol congeners and other long-chain fatty acid amides and their receptors constitute the endocannabinoidome [64].
In an attempt to manipulate the bioavailability of endocannabinoids and enhance their benefits on the immune system response, several inhibitors of FAAH and MAGL were developed [101,106]. Namely, selective inhibition of FAAH with URB597 is associated with increased levels of N-acylethanolamines such as AEA and reduced production of pro-inflammatory cytokines and immune cell infiltration in the ovalbumin-induced allergic asthma model [107]. A study by Genovese et al., confirmed that inhibition of FAAH activity by URB878 significantly reduced the inflammation associated with acute lung injury [108]. The genetic deletion of Mgll, which encodes the MAGL, is linked to increased levels of monoacylglycerols [109] and protects mice from diet-induced obesity and associated inflammatory diseases [110]. Furthermore, the absence of Mgll helps to reduce inflammation and liver damage when mice are exposed to carbon tetrachloride [111]. In addition, using JZL184 to inhibit the activity of MAGL produces antiarthritic effects and reduces the paw inflammation associated with collagen-induced arthritis [112]. These results suggest that endocannabinoids modulate inflammation, although these effects cannot be necessarily attributed to CB2 activation.
The activation of the CB2 receptor by endogenous ligands was specifically examined in inflammatory models as detailed in Table 3. Briefly, treatments that target CB2 either with Δ9-THC [113] or the two endocannabinoids AEA and 2-AG [6,114] revealed a dichotomy regarding inflammatory responses. First, most studies highlighted a reduction in immune cell infiltration and secretion of inflammatory mediators associated with CB2 activation [115,116,117,118]. More importantly, these treatments are accompanied by an improvement in symptoms associated with inflammation. For example, Madig et al. observed reduced edema and improved blood–brain barrier function and neurological recovery after treatment with 2-AG in order to specifically target the CB2 receptor in Theiler’s murine encephalomyelitis virus-induced demyelination [79]. Conversely, other authors have shown that a pro-inflammatory response is associated with cannabinoid treatment as well as selective CB2 receptor agonists. As described by Oka et al. in mice treated with oxazolone to induce dermatitis, the number of infiltrating eosinophils is reduced when CB2 is blocked with SR144528 [119]. However, the pro-inflammatory effects of endocannabinoids in some cases can be attributed to the products of endocannabinoid metabolism [114,120]. Eicosanoid metabolites derived from arachidonic acid oxidation contribute to inflammation (see Dennis et al. for details [121]). In addition, arachidonic acid and its metabolites modulate type 2 immune responses, which are important in the allergic response through actions on eosinophils and neutrophils [122].
Though the large body of evidence supporting endocannabinoids as anti-inflammatory mediators is cohesive and well established, these contradictory results reaffirm the need to investigate further the mechanisms by which endocannabinoids can exert pro-inflammatory effects.

Table 3.
Effects of endocannabinoid treatment in rodent models of inflammation.
Table 3.
Effects of endocannabinoid treatment in rodent models of inflammation.
| Model | Species | Treatment | CB2-Dependent Validation | Application | Effects | References |
|---|---|---|---|---|---|---|
| Anti-inflammatory effects | ||||||
| Atherosclerosis | C57BL/6 NOD/SCID DBA/1J C57BL/6 ABH C57BL/6J C57BL/6 BALB/c C57BL/6 C57BL/6 C57BL/6J C57BL/6 C57BL/6J C57BL/6J C57BL/6 | Δ9-THC 0.1 to 10 mg/kg | SR144528 0.7 mg/kg | Oral administration | ↓ Atherosclerotic lesion ↓ Macrophage infiltration ↓ Leukocyte adhesion | [115] |
| ApoE/MGL-DKO | SR144528 0.01 mg/kg | Oral administration | ↓ Atherosclerotic lesion ↓ Leukocyte infiltration | [109] | ||
| Collagen-induced arthritis | JZL184 8 and 40 mg/kg | SR144528 3 mg/kg | Subcutaneous injection | ↓ Paw inflammation | [112] | |
| Experimental autoimmune encephalomyelitis | Δ9-THC 2.5 to 25 mg/kg | Cnr2-/- | Intraperitoneal injection | ↓ Monocyte recruitment ↓ IFN-γ and IL-2 ↓ T cell proliferation | [116] | |
| Hepatic ischemia-reperfusion injury | HU910 3 and 10 mg/kg | SR144528 3 mg/kg | Intraperitoneal injection | ↓ Hepatic injury ↓ CCL3, CXCL2 and TNF-α ↓ Neutrophil infiltration | [117] | |
| Influenza virus infection | Δ9-THC 75 mg/kg | Cnr2−/− | Oral administration | ↓ Lymphocyte and monocyte recruitment ↓ Viral hemagglutinin | [118] | |
| L. pneumophila infection | Δ9-THC 8 mg/kg | Cnr2−/− | Intravenous injection | ↓ IFN-γ and IL-12 | [123] | |
| ConA-induced hepatitis | AEA 20 mg/kg | SR144528 10 to 20 mg/kg | Intraperitoneal injection | ↓ Inflammatory cytokines | [73] | |
| Theiler’s murine encephalomyelitis virus-induced demyelination disease | HU914 5 and 10 mg/kg | Cnr2−/− | Intraperitoneal injection | ↓ Neurological deficits ↓ Edema and blood–brain barrier permeability | [79] | |
| Carrageenan-induced acute inflammation | URB602 10 and 20 mg/kg | SR144528 1 mg/kg | Intraperitoneal injection | ↓ Edema ↓ Nociception | [124] | |
| Cecal-ligation- and puncture-induced sepsis | HU308 2.5 mg/kg | AM630 2.5 mg/kg | Intraperitoneal injection | ↓ Inflammatory cytokines ↓ Pyroptosis and NLRP3 activity | [125] | |
| Experimental autoimmune encephalomyelitis | WWL70 5 mg/kg | AM630 3 mg/kg | Intraperitoneal injection | ↓ iNOS, COX-2, TNF-α and IL-1β ↓ T cell infiltration ↓ Microglial activation ↓ NF-κB activation | [126] | |
| LPS-induced tactile allodynia | URB597 1 and 10 mg/kg | Cnr2−/− | Intraperitoneal injection | ↓ Leukocyte rolling ↓ Microvascular perfusion | [74] | |
| LPS-induced acute lung injury | JZL184 16 mg/kg | AM630 2.5 and 5 mg/kg | Intraperitoneal injection | ↓ Leukocyte infiltration ↓ BALF cytokines and chemokines | [127] | |
| LPS-induced inflammatory pain | C57BL/6J C57BL/6J | Faah−/− | SR144528 3 mg/kg | Intraperitoneal injection | ↓ Edema ↓ TNF-α and IL-1β | [128] |
| Faah−/− | Cnr2−/− | - | ↓ Allodynia | [74] | ||
| Pro-inflammatory effects | ||||||
| Type-1 diabetes | NOD ICR ICR C57BL/6 | AEA 250 μg to 500 μg/ 100ul | Cnr2−/− | Oral administration | ↓ CX3CR1+ macrophages | [60] |
| Oxazolone-induced dermatitis | 2-AG 30 μg dissolved in acetone | SR144528 30 μg dissolved in acetone | Topical application | ↑ Eosinophil recruitment ↑ Swelling ↑ CCL2, CCL3 and TNF-α | [129] | |
| TPA-induced ear inflammation | 2-AG 30 μg dissolved in acetone | SR144528 30 μg dissolved in acetone | Topical application | ↑ Neutrophil recruitment ↑ Swelling ↑ LTB4 synthesis | [119] | |
| Pancreatic cancer | 2-AG 20 mg/kg | AM630 (ND) | Intraperitoneal injection | ↓ Cancer cells proliferation ↓ Dendritic cell activation | [130] | |
The arrows (↑/↓) indicate whether a process is enhanced or reduced, respectively.
4.2. Synthetic Ligands
Studies on marijuana users prompted the potential therapeutic importance of exogenous, synthetic CB2 receptor agonists even before the identification of endocannabinoid ligands and receptors and inhibitors of endocannabinoid-inactivating enzymes.
In a multivariate study by Tindall et al., conducted on human immunodeficiency virus (HIV)-positive patients who had not yet developed acquired immunodeficiency syndrome, marijuana users were more likely to have a lower percentage of CD4+ cells and a higher percentage of CD8+ cells [131]. In addition, HIV carriers who use marijuana exhibit an increased risk of developing bacterial pneumonia and other opportunistic infections [132]. Indeed, it was demonstrated that alveolar macrophages in the lungs of marijuana smokers were less effective in their ability to clear bacteria and tumor cells [133]. These studies suggest that natural cannabinoids capable of activating CB2 receptors affect the immune system, and this effect could be exploited as a treatment.
Synthetic compounds such as CP55,940 and WIN55,212-2 were available when CB2 was cloned, although these synthetic cannabinoids can also activate CB1 with similar efficiency [11,134]. A highly selective antagonist for CB2 receptors, SR144528, was the first molecule designed in order to investigate CB2-mediated endocannabinoid functions in the immune system [135]. Since then, researchers have developed a wide variety of CB2-specific antagonists or agonists (Table 4) [136].
In 2017, Soethoudt et al. tested 18 pharmacological agonists and antagonists used in preclinical models and concluded that JWH-133, HU308 and HU910 are the agonists with the best affinity for CB2 and least psycho-chemical consequence [48]. JWH-133 (Ki = 3.4 nM) was characterized in 1999 and found to be a potent CB2 agonist, 200 times more selective for CB2 than for CB1 [13,137]. HU308 (Ki = 22.7 nM) is a specific agonist for CB2 and does not appreciably bind to CB1. Of these three specific agonists, HU910 (Ki = 6 nM) is one of the most recently developed as it was first used in 2012 and has specific affinity for CB2. Other CB2 selective agonists were synthesized, such as GW405833 [138], which has anti-inflammatory properties [139,140,141]. However, GW405833 may also act as a non-competitive antagonist for CB1 as Li et al. noted in their study that the anti-allodynic effect of this compound was mediated through CB1 [142].
Further in vitro and in vivo studies revealed that targeting the CB2 receptor has immunomodulatory effects in several ways: the induction of apoptosis and anti-inflammatory cytokine production, as well as, the repression of cell proliferation, immune cell migration and pro-inflammatory cytokine production [6]. We list in Table 5 recent studies targeting CB2 in animal models of inflammation. Interestingly, in in vitro and in vivo studies in mouse models of ovalbumin-induced acute asthma, JWH-133 enhanced the mobilization of eosinophils [19]. Furthermore, in cecal-ligation- and puncture-induced polymicrobial sepsis in rats, JWH-133 induced an anti-inflammatory response by inhibiting apoptosis and NF-κB signaling in the brain, lung, liver and heart [143]. When administered to mice, HU308 reduced blood pressure, slowed defecation and caused anti-inflammatory effects [5]. HU910 was found to reduce the markers associated with liver injury in the ischemia/reperfusion injury mouse model and to attenuate the levels of pro-inflammatory cytokines and chemokines as well as immune cell infiltration [117].
Furthermore, in the context of mouse models of rheumatoid arthritis, JWH-133 induced an anti-inflammatory response by decreasing the production of proinflammatory cytokines and increasing the polarization of macrophages toward an M2 phenotype [144,145]. In addition, Fukuda et al. showed that, in collagen type-II antibody-induced arthritis, JWH-133 reduced arthritis scores and limited bone destruction [146]. Moreover, in the monoiodoacetic acid-induced osteoarthritis model, systemic and chronic administration of JWH-133 was associated with a reduction in pain-related behaviors [147]. In addition, experiments carried out in CB2-overexpressing mice suffering from monosodium iodoacetate-induced joint pain showed a better control of pain manifestation [148]. In the case of rheumatic diseases, CB2 agonists can improve inflammation and reduce pain concomitantly.
Taken together, these findings confirm that targeting CB2 with pharmacological agents can often improve inflammation in a wide range of inflammatory diseases, by modulating, directly or indirectly, the responses of various immune cells such as eosinophils [143], macrophages [149], neutrophils [54,56] or lymphocytes [15,150].

Table 4.
CB2 selective ligands.
Table 4.
CB2 selective ligands.
| Compound | Function | CB2 Ki (nM) | CB1 Ki (nM) | References |
|---|---|---|---|---|
| JWH-133 | CB2 Agonist | 3.4 | 677 | [137] |
| HU308 | CB2 Agonist | 22.7 | 10 μM | [5] |
| HU910 | CB2 Agonist | 6 | 1.37 μM | [117] |
| Gp1a | CB2 Agonist | 0.037 | 363 | [151] |
| JWH-015 | Agonist | 13.8 | 383 | [13] |
| AM1241 | Agonist | 2 | 580 | [152] |
| RO6871304 | Agonist | 17 | 10 μM | [153] |
| RO6871085 | Agonist | 76 | - | [151] |
| GW405833 | Agonist | 12 | 4472 | [138] |
| 4Q3C | Agonist | 8.5 | 10 μM | [154] |
| ABK-5 | Agonist | 28 | - | [155] |
| S-777469 | Agonist | 36 | 4607 | [156] |
| CP 55,940 | CB1 and CB2 Agonist | 0.68 | 0.58 | [157] |
| WIN 55,212-2 | CB1 and CB2 Agonist | 3.30 | 1.9 | [158] |
| SR145298 | CB2 inverse agonist | 0.6 | 10 μM | [135] |
| AM630 | CB2 inverse agonist | 31.2 | 5 μM | [159] |

Table 5.
Anti-inflammatory effects of CB2 activation by agonists in rodent models of inflammation.
Table 5.
Anti-inflammatory effects of CB2 activation by agonists in rodent models of inflammation.
| Model | Species | Treatment | Dose/Route of Administration | Effects | References |
|---|---|---|---|---|---|
| Neuro-inflammation | |||||
| Brain ischemia | Mouse | JWH-133 | 1.5 mg/kg Intraperitoneal injection | ↓ Microglia and macrophage infiltration ↓ CCL2, CCL3 and CCL5 ↓ IL-6, TNF-α ↓ iNOS | [160] |
| Hypoxic-ischemic encephalopathy | 1.5 mg/kg Intraperitoneal injection | ↑ Neuroprotection ↓ CCL2 ↓ TNF-α | [161] | ||
| Neuroinflammation in the rostral ventrolateral medulla | 1 mmol/L in 10 μL Intracerebroventricular injection | ↓ Blood pressure, heart rate ↓ Pro-inflammatory cytokines | [162] | ||
| Pentylenetetrazole-induced epilepsy | |||||
| Pentylenetetrazole-induced seizures | 3 mg/kg Intraperitoneal injection | ↓ Susceptibility to pentylenetetrazole-induced seizures | [163] | ||
| Postoperative cognitive dysfunction | 3 mg/kg Intraperitoneal injection | ↓ Surgery memory loss ↓ Pro-inflammatory cytokines | [164] | ||
| Stress-induced neuroinflammation | 2 mg/kg Intraperitoneal injection | ↓ CCL2 and TNF-α ↓ COX-2, iNOS and NF-κB | [165] | ||
| Subarachnoid hemorrhage | 2 mg/kg Intraperitoneal injection | ↓ Edema ↑ Expression of ZO-1 and blood–brain barrier integrity ↑ Expression of TGF-β1 ↓ Pro-inflammatory cytokines | [164] | ||
| L-dopa-induced dyskinesia (Parkinson’s disease model) | HU308 | 2 mg/kg Intraperitoneal injection | ↓ Dyskinesia ↓ Microglia proliferation and cytokine release | [166] | |
| Traumatic brain injury | HU910 | 5 to 10 mg/kg Intraperitoneal injection | ↑ Neurobehavioral recovery ↑ Recovery of the cortical spinal tract ↓ TNF-α | [79] | |
| O-1966 | 5 mg/kg Intraperitoneal injection | ↓ Microglia and macrophage infiltration ↓ Blood–brain barrier disruption | [167] | ||
| Gp1a | 1 to 5 mg/kg Intraperitoneal injection | ↓ Edema and neurovascular injury ↑ Blood flow and improved neurobehavioral ↑ Macrophage polarization into M2 phenotype | [168] | ||
| Retrovirus-infection-induced neuropathic pain | 5 mg/kg Intraperitoneal injection | ↓ Allodynia ↔ Neuroinflammation | [169] | ||
| Germinal matrix hemorrhage-induced neuroinflammation | Rat | JWH-133 | 1 mg/kg Intraperitoneal injection | ↑ Macrophage polarization into M2 phenotype ↓ Microglia accumulation ↑ Anti-inflammatory cytokines release ↓ TNF-α | [170] [171] |
| Intracerebral hemorrhage | Rat Mouse | 1.5 mg/kg 1 mg/kg Intraperitoneal injection | ↓ Edema ↑ Expression of ZO-1 and blood–brain barrier integrity ↑ Expression of TGF-β1 ↓ Pro-inflammatory cytokines | [164] [172] | |
| Meningitis induced by S.pneumonae | Rat | 1 mg/kg Intraperitoneal injection | ↓ Microglia activation ↔ Hippocampal injury | [173] | |
| Inflammation | |||||
| Cecal-ligation- and puncture-induced sepsis | Mouse | HU308 | 2.5 mg/kg Intraperitoneal injection | Protection against sepsis ↓ Activity of NLRP3 and Caspase-1 ↓ Cell pyroptosis | [125] |
| Corneal injury | 0.5 to 1.5% w/v Topical application | ↓ Neutrophil infiltration | [52] | ||
| Pneumonia-induced lung acute injury | 3 mg/kg Intravenous injection | ↑ IL-10 ↓ CXCL2 and TNF-α ↓ Acute lung injury score | [174] | ||
| LPS-induced interstitial cystitis | 5 mg/kg Intraperitoneal injection | ↓ Bladder inflammation | [175] | ||
| Sepsis | 2.5 mg/kg Intravenous injection | ↓ Adherent leukocyte in submucosal venules | [176] | ||
| LPS-induced interstitial cystitis | JWH-015 | 5 mg/kg Intraperitoneal injection | ↓ Leukocyte infiltration ↓ Myeloperoxidase ↓ TNF-α, IL-1α and IL-1β | [177] | |
| Trinitrobenzene sulfonic acid (TNBS)-induced colitis | AM1241 | 10 to 20 mg/kg Intraperitoneal injection | ↓ Macroscopic damage and colitis ↓ Inflammation and MPO production | [178] | |
| Rheumatoid arthritis | 4Q3C | 10 mg /kg Intraperitoneal injection | ↓ Rheumatoid arthritis severity ↓ TNF-α and IL-1β | [144] | |
| JWH-133 | 1 mg /kg Intraperitoneal injection | ↑ M2 polarization of macrophages ↓ TNF-α, IL1-β and IL-6 ↑ IL-10 | [145] | ||
| Spinal cord injury | O-1966 | 5 mg/kg Intravenous injection | ↓ Leukocyte infiltration ↓ CXCL9 and CXCL11 ↓ IL-23p19 and IL-23R ↓ TLR expression | [179] | |
| Cecal-ligation- and puncture-induced sepsis (CLP) and sepsis | Rat | JWH-133 | 0.2 to 5 mg/kg Intravenous injection | ↓ TNF-α, IL-1β, IL-6 ↑ IL-10 | [143] |
| Intestinal ischemia/reperfusion | AM1241 | 0.1 to 10 mg/kg Intravenous injection | ↓ TNF-α and IL-1β | [180] | |
| Bile duct ligation | 3mg/kg Intraperitoneal injection | ↓ Apoptosis ↓ Pro-inflammatory cytokines ↑ IL-10 | [181] | ||
| Endotoxin-induced uveitis | HU910 RO6871304 and RO6871085 | 1.5% w/v Topical application | ↓ Eye inflammation ↓ Neutrophils migration | [153] | |
| Carrageenan-induced paw oedema | GW405833 | 3 mg/kg Intravenous injection | ↓ MPO activity ↓ Leukocyte recruitment | [140] | |
| Concanavalin A-induced acute liver injury | 20 mg/kg Intraperitoneal injection | ↓ Liver damage and hepatocyte apoptosis ↓ Jurkat-T cell apoptosis | [139] | ||
| Acrolein-induced cystitis | GP1a | 10 mg/kg Intraperitoneal injection | ↓ Severity of cystitis ↓ Bladder inflammation | [182] | |
| Pulmonary inflammation induced by Mycobacterium bovis | 10 mg/kg Intraperitoneal injection | ↓ Pulmonary inflammation ↓ Neutrophil accumulation ↓ CCL2, CXCL1, TNF-α and LTB4 | [183] | ||
| Chronic ileitis model TNF RE/+ | 5 mg/kg Retro-orbital injection | ↓ Ileitis ↑ T-regulatory lymphocytes and IL-10 | [184] | ||
| Incised skin wound model | 3 mg/kg Intraperitoneal injection | ↑ Keratinocyte migration and re-epithelialization ↓ Pro-inflammatory cytokines ↑ M2 macrophages | [185] [186] | ||
| Injection of complete Freund’s adjuvant (CFA) into the hind paw | ABK-5 | 5 to 20 mg/kg Intraperitoneal injection | ↑ Jurka-T cell migration ↓ IL-2 and TNF-α ↓ CXCL12 chemotaxis | [187] | |
| Allergy | |||||
| Antigen-induced dermatitis | Mouse | S-777469 | 10 to 30 mg/kg Oral administration | ↓ Mast cell infiltration ↓ Eosinophil accumulation ↓ Block 2-AG activity ↓ Swelling | [188] |
| Intranasal-LPS inflammation | Mouse | URB597 JZL184 | 0.3 mg/kg Intraperitoneal injection −1 and 5 mg/kg intranasal administration | ↓ Neutrophil in broncho alveolar ↓ TNF-α | [189] |
| Ovalbumin-induced asthma | Guinea pig | CP 55,940 | 0.1 mg/kg Intraperitoneal injection | ↓ Myeloperoxidase ↓ Mast cell degranulation ↓ TNF-α and PGD2 | [190] |
| Metabolic syndrome | |||||
| Diet-induced obesity | Mouse | JWH-133 | 5 to 10 mg/kg Intraperitoneal injection | ↓ Weight gain ↑ Glucose tolerance and insulin sensitivity ↓ M1-associated markers and cytokine production | [191] |
| HU308 | 4 mg/kg Intraperitoneal injection | ↔ Weight gain ↓ M1 polarization of adipose tissue macrophages | [76] | ||
| Myocardial infarction | -JWH-133 -HU308 | −1 to 10 mg/kg −2 mg/kg Intraperitoneal injection | ↓ The severity of myocardial infraction and myocardial enzymes ↑ Myocardial viability ↓ NLRP3 activation ↓ Pro-inflammatory cytokines | [192] | |
| Hepatorenal syndrome induced by bile duct ligation | HU910 10 mg/kg | 10 mg/kg Intraperitoneal injection | ↓ Liver and kidney fibrosis ↓ Inflammatory markers ↓ Oxidative damage | [193] | |
| Inflammatory diabetic retinopathy | 5 mg/kg Intraperitoneal injection | ↓ Vascular permeability ↓ TNF-α, IL-1β, IL-6 | [194] | ||
| Atherosclerosis | WIN 55,212-2 | 0.5 to 1 mg/kg Intraperitoneal injection | ↓ Atherosclerotic lesion ↓ Macrophage infiltration ↓ CCL2, IL-6 and TNF-α | [195] | |
| Myocardial ischemia-reperfusion injury | 3.5 mg/kg Intraperitoneal injection | ↓ Myeloperoxidase ↓ IL-1β and IL-8 | [196] | ||
| Wistar Kyoto and spontaneous hypertensive line rats | Rat | JWH-133 | 1mmol/l Intracerebroventricular injection | ↓ Blood pressure ↓ Pro-inflammatory cytokines | [162] |
The arrows (↑/↓/↔) indicate whether a process is enhanced, reduced or unchanged respectively.
5. The Effect of CB2 Activation in Airway Inflammation
The recruitment of circulating immune cells is an amplification factor for pathologies with an inflammatory component [197]. This is the case in chronic allergic airway inflammation where the infiltration of the airways by eosinophils, mast cells and T helper lymphocytes is an important step of the pathogenesis [198]. Activation of both recruited and airway resident immune cells, namely innate lymphoid type 2 cells (ILC2), leads to the production of pro-inflammatory Th2 cytokines such as IL-4, IL-5 and IL-13 [198,199,200]. IL-4 production by Th2 lymphocytes also leads to the isotypic switch of B cells from IgM to IgE antibodies in the lungs [198]. These responses enhance mucus secretion by goblet cells, airway hyperresponsiveness and bronchoconstriction [201,202]. Since circulating immune cells express high levels of CB2 [17], their recruitment during an inflammatory pathology causes an influx of such receptors that have the potential to influence disease development.
Studies on healthy volunteers or asthmatic patients treated with Δ9-THC revealed a beneficial increase in bronchodilatation and specific airway conductance in treated individuals [203,204]. In the context of an ovalbumin-induced allergic reaction in mice, treatment with Δ9-THC attenuated the Th2 allergic response. Notably, it decreased the gene expression of IL-2, IL-4, IL-5 and IL-13, reduced serum IgE levels and diminished mucus secretion [205].
In order to understand the specific implication of CB1 and CB2 in the beneficial effects elicited by Δ9-THC, Newton and Klein studied the effect of this plant cannabinoid in a Th1-driven inflammatory reaction, i.e., that following Legionella pneumophila infection. They found that Δ9-THC decreased the serum levels of the Th1 cytokines, IL-12 and IFNγ by acting specifically through CB1. Δ9-THC also induced an increase in GATA3 signaling and IL-4 production by activating CB2 exclusively [123]. Furthermore, these authors demonstrated that, in ovalbumin-sensitized mice, CB2 activation by a selective agonist reduced IgE levels [206]. These results emphasize how, although Δ9-THC can tune down the inflammatory reaction of both Th1- and Th2-driven pathologies, this is the sum of independent effects mediated differently through CB1 and CB2 activation.
Some studies investigated the role of AEA and 2-AG in asthma. Zoerner et al. found that the bronchoalveolar fluids of allergic asthma patients challenged with an allergen contained much greater levels of AEA than patients given saline. Moreover, patients with higher AEA levels also had more severe symptoms of airway inflammation [207]. A study by Larose et al. showed that IL-3, IL-5 and GM-CSF greatly potentiate the CB2-dependant chemoattractant effect of 2-AG on eosinophils isolated from healthy donors [58]. In ovalbumin-sensitized guinea pigs, the inhibition of FAAH with URB597 reduced Th2 cytokine production and infiltration of immune cells but had marginal effects on airway hyperreactivity. Conversely, the inhibition of MAGL with JZL184 attenuated cytokine production, immune infiltration and airway hyperreactivity [107].
In a mouse model of ovalbumin-induced asthma, Frei et al. showed that the CB2 agonist JWH-133 increased the chemotaxis, activation and reactive oxygen species production of eosinophils. These effects were reduced in both Cnr2−/− and eosinophil-deficient mice confirming that CB2 activation on eosinophils is a key event in allergic airway inflammation [19]. Furthermore, Hurrel et al. showed that CB2 signaling induced the proliferation and activation of ILC2 isolated from mouse lungs [208]. They confirmed, using Cnr2−/− mice, that these animals presented reduced lung inflammation and airway hyperreactivity, which was a consequence of reduced ILC2 activation [208]. Ferrini et al. demonstrated that the absence of Cnr2 in a model of common house dust mite sensitization increased NK cell infiltration and activation that prompted the secretion of Th1 cytokines in the airways. This activation of NK cells led to a drastically reduced number of ILC2 cells, which was accompanied by a reduction in Th2 cytokine levels [59].
The studies cited above, in addition to those we summarize in Table 6, suggest that the putative beneficial effect of CB2 activation is highly context-dependent. Nevertheless, we can emphasize that in a Th2 inflammatory context, including allergy, antagonism of CB2 is strongly beneficial since it prevents the inflammatory response possibly by switching it toward a Th1-type response.

Table 6.
CB2 modulates the inflammatory response in vivo and in vitro.
Table 6.
CB2 modulates the inflammatory response in vivo and in vitro.
| Model | Treatment | Dose/Route of Administration | Effects | References |
|---|---|---|---|---|
| In vivo | ||||
| House dust mite inhalation (allergy) | Cnr2−/− | - | ↓ Th2 cytokine production ↑ NK cell number ↓ ILC2 | [59,209] |
| Mice challenged with IL-33 | Cnr2−/− | - | ↓ ILC2 | [208] |
| Mice challenged with antigen/adjuvant OVA/Alum combination | Cnr2−/− | - | ↑ Serum level of IgE | [206] |
| Delayed-type hypersensitivity induced by methylated BSA | AEA or 2-AG | 40 mg/kg Intraperitoneal injection | ↑ IL-10 and Th2 cytokines ↓ Th1 and Th17 cytokines | [210,211] |
| Legionella. pneumophilia infection | Δ9-THC | 4 mg/kg Intravenous injection | ↓ Th1 cytokine production ↓ IFNγ production by splenocytes ↑ IL-4 production by splenocytes | [212] |
| Diet-induced obesity | JWH-133 | 5 to 10 mg/kg Intraperitoneal injection | ↑ M2 macrophages polarization | [191] |
| In vitro | ||||
| Mouse-bone-marrow-derived macrophages | HU308 | 1 μM | ↑ Macrophage polarization to M2 | [213] |
| Mouse splenic B cells | SR144528 | 0.1 μM | ↑ Class switch from IgM to IgE | [214] |
| Mouse dendritic cells | -Δ9-THC -2-AG | −5 μM −1 to 10 μM | ↑ Apoptosis ↑ Th1 inflammatory response ↑ Dendritic cell migration | [215,216] |
| Human B cell line SKW 6.4 | -SR144528 -AM630 | −2.5 to 10 μM 0.63 to 2.5 μM | ↓ IL-6-stimulated IgM secretion | [217] |
| Human T lymphocytes | SR144528 | 1 μM | ↓ Th2 cytokine response | [218] |
| Primary-human-fibroblast-like synoviocyte osteoarthritis | HU308 | 1 μM | ↓ CCL2, MMP1, MMP3 and IL-6 | [213] |
| Human peripheral blood mononuclear cells | COR167 | 10−3 to 10 μM | Shift of Th1 phenotype toward Th2 phenotype ↓ Th17 ↓ IL-4 and IL-5 ↓ Chemokines | [219] |
| Human keratinocyte (HaCaT) cell | 2 (1-adamantanylcarboxamido) thiophene derivatives | 10 μM | ↓ CCL2 | [220] |
| Human bronchial epithelial cells (16HBE140-) | Virodhamine | ↓ IL-8 | [94] | |
The arrows (↑/↓) indicate whether a process is enhanced or reduced, respectively.
6. The Effect of Cannabinoid-Based Treatments in Inflammatory Diseases
Tissue alterations in endocannabinoid concentrations and the expression of endocannabinoid receptors and metabolic enzymes have been associated with several inflammatory conditions, such as neuroinflammation and chronic inflammatory diseases (Figure 2). Based on accumulated findings, several clinical studies on cannabinoid therapies were instigated that we bring to light in Table 7.
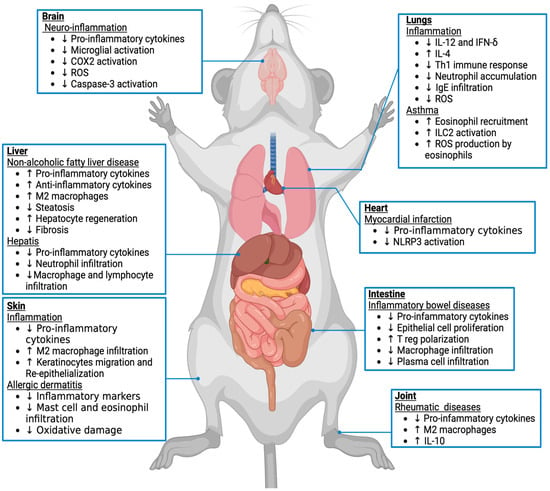
Figure 2.
Effects of CB2 activation on inflammation in different mouse tissues and pathological condition. The endocannabinoid system via the CB2 receptor has been implicated in inflammatory processes. Based on previous studies in animal models, the arrows (↑/↓) indicate whether a process associated with each pathology is enhanced or reduced, respectively.

Table 7.
List of clinical trials based on endocannabinoids targeting inflammation.
Table 7.
List of clinical trials based on endocannabinoids targeting inflammation.
| Title | Compound | Phase | Intervention | Completed | Primary Outcomes | Outcomes Met | References |
|---|---|---|---|---|---|---|---|
| A study of The Effects Of CB2 Compound Of GW842166 In Patients With Osteoarthritis (NCT00479427) | GW842166 (CB2 agonist) | II | 100 mg per os for 14 days. | YES | Change in pain scores from baseline to the end of treatment in Western Ontario and McMasters University Osteoarthritis Index (WOMAC) using the pain subscore for 6–8 weeks. | Unknown | - |
| Dental Pain 3rd Molar Tooth Extraction GW842166 (NCT00444769) | GW842166 (CB2 agonist) | IIa | Single dose of 100–800 mg per os, preoperative or postoperative. | YES | Decrease in Visual Analog Scale (VAS) pain intensity 10 h post-surgery. | NO | [221] |
| A Randomized, Double-Blind Study to Evaluate the Safety and Efficacy of 2 Doses of S-777469 in Patients With Atopic Dermatitis (NCT00703573) | S-777469 (CB2 agonist) | II | 200–800 mg per os twice per day for 12 weeks. | YES | (1) Efficacy assessed by Physician’s Global Assessment (PGA) and Numerical Rating Scale (NRS). (2) Safety, determined by adverse event frequency and changes in laboratory values. | Unknown | - |
| A Phase Ib/IIa, Double-Blind, Randomized Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of S-777469 in Subjects With Atopic Dermatitis (NCT00697710) | S-777469 (CB2 agonist) | Ib/IIa | 50–800 mg per os twice per day for 1, 7 and 14 days. | YES | (1) Safety (adverse event monitoring, vital sign measurements, physical examination measurements, 12-lead electrocardiogram assessments and standard clinical laboratory safety tests (hematology, blood chemistry and urinalysis)). (2) Pharmacokinetic endpoints included Cmax, Tmax, T1/2,12hr, T1/2,z and AUC0-12h for each dose level of S-777469 based on the sampling schedule. | Unknown | - |
| Safety, tolerability, and efficacy of JBT-101 in subjects with dermatomyositis (NCT02466243) | JBT-101 (CB2 agonist) | II | 20 mg per os daily for 28 days followed by 20 mg p.o. twice per day for 56 days. | YES | (1) Change in Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) from baseline. (2) Safety and tolerability measured by the number of participants with treatment-emergent adverse events. | YES | [222] |
| Safety, Tolerability, Pharmacokinetics, and Efficacy of JBT-101 (Lenabasum) in Cystic Fibrosis (NCT02465450) | JBT-101 (CB2 agonist) | II | 20 mg per os twice per day for 5 to 12 weeks. | YES | Number of participants with treatment-emergent adverse events. | YES | [223] |
| Trial to Evaluate Efficacy and Safety of Lenabasum in Cystic Fibrosis (NCT03451045) | JBT-101 (CB2 agonist) | II | 5 and 20 mg per os twice per day for 28 weeks. | YES | Rate of pulmonary exacerbation (PEx) over 28 weeks. | NO | [223] Available at: https://clinicaltrials.gov Reference: NCT03451045 |
| Safety, tolerability, efficacy, and pharmacokinetics of JBT-101 in systemic sclerosis (NCT02465437) | JBT-101 (CB2 agonist) | II | 5 and 20 mg per os twice per day for 28 days followed by 20 mg until day 84. | YES | (1) Number of participants with treatment-emergent adverse events from baseline at day 113. (2) Combined response index in diffuse Cutaneous Systemic Sclerosis (CRISS) at days 85 and 113. | YES | [224] |
| JBT-101 in Systemic Lupus Erythematosus (SLE) (NCT03093402) | JBT-101 (CB2 agonist) | II | 5 or 10 or 20 mg per os daily for 12 weeks. | YES | Improvement in the maximum daily numeric rating scale for pain (NRS-Pain) score at day 84. | YES | [225] |
| Trial to Evaluate Efficacy and Safety of Lenabasum in Diffuse Cutaneous Systemic Sclerosis (RESOLVE-1) (NCT03398837) | JBT-101 (CB2 agonist) Hki= | III | 5 or 20 mg per os daily for 52 weeks. | YES | Efficacy of Lenabasum compared to placebo for the American College of Rheumatology Combined Response Index in diffuse cutaneous Systemic Sclerosis score (ACR-CRISS). | NO | [226] [227] |
| Tolerability, Pharmacokinetics, and Efficacy of APD371 in Participants With Crohn’s Disease Experiencing Abdominal Pain (NCT03155945) | APD371 (CB2 agonist) | IIa | 25 mg per os three times a day for 8 weeks. | YES | Number of participants with treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs). | YES | [228] |
| Olorinab in Irritable Bowel Syndrome With Predominant Constipation (IBS-C) and Irritable Bowel Syndrome With Predominant Diarrhea (IBS-D) (CAPTIVATE) (NCT04043455) | APD371 (CB2 agonist) | II | 10, 20 or 50 mg per os three times a day for 12 weeks. | YES | Change in patient-reported average abdominal pain score (AAPS) from baseline to week 12. | NO | [229] |
| Effect of Olorinab on Gastrointestinal Transit in Patients With Irritable Bowel Syndrome (NCT04655599) | APD371 (CB2 agonist) | Ib | Olorinab per os, three times a day for 4 days with a final dose on day 5. | NO | (1) Colonic transit geometric center after consumption of radiolabeled meal, based on the delivery of activated charcoal in a methacrylate-coated capsule. (2) Gastric emptying half-life (t½) as determined by scintigraphic imaging of radiolabeled meal. | Terminated | - |
| A Study of LY2828360 in Patients With Osteoarthritic Knee Pain (NCT01319929) | LY2828360 (CB2 agonist) | II | 80 mg per os LY2828360 for 4 weeks. | YES | Change from baseline to 4-week endpoint in weekly mean of daily 24 h Average Pain Scores (APS). | NO | [230] |
| Study to Investigate the Analgesic Efficacy of a Single Dose of AZD1940 (NCT00659490) | AZD1940 (dual CB1/CB2 agonist) | II | Single dose per os of 100 or 800 μg. | YES | Pain Area Under the Curve 0–8 h (AUC0-8h). | NO | [231] |
| Study to Investigate the Safety, Tolerability and Pharmacokinetics of AZD1940 (NCT00689780) | AZD1940 (dual CB1/CB2 agonist) | I | Single dose per os of 100 or 800 μg. | YES | Assessment of adverse events (AEs) occurring during the study, blood pressure (supine and standing), pulse rate, respiratory rate, body temperature, laboratory variables and ECG. | NO | [231] |
| TT-816 as Monotherapy or in Combination With a PD-1 Inhibitor in Patients With Advanced Cancers (SEABEAM) (MK3475-E88) (NCT05525455) | TT-816 (CB2 antagonist) | I and II | - | NO | Incidence of adverse events (AEs) and serious adverse events (SAEs) (Phase 1). Incidence and nature of dose-limiting toxicities (DLTs) (Phase 1). The Maximum Tolerated Dose (MTD) or Recommended Phase 2 Dose (RP2D) of oral TT-816 as monotherapy (Phase 1m). The Maximum Tolerated Dose (MTD) or Recommended Phase 2 Dose (RP2D) of oral TT-816 in combination with a PD-1 inhibitor (Phase 1p). Overall Response Rate. Scale: confirmed Complete response (CR) or Partial response (PR), Duration of Response (DOR) and Disease Control Rate (DCR). Changes from baseline in clinical safety laboratory values and vital signs. | - | [232] |
Source: clinicaltrials.gov accessed on mars 2024.
6.1. Potential in Neuroinflammation: Alzheimer’s Disease
Mentioned for the first time in 1995 [233], neuroinflammation is characterized by an inflammatory response within the brain or spinal cord and reported as an indicator and modulator of neurodegeneration [234]. Several studies have highlighted the importance of the CB2 receptor in diseases associated with neuroinflammation [235,236]. The CB2 receptor is expressed by several cells in the brain such as astrocytes, reactive microglia, perivascular microglia, oligodendrocytes, neuronal progenitor cells and cells involved in blood–brain barrier integrity [237]. In 2003, Benito et al. found that the CNR2 gene was highly expressed in post mortem brain microglia from subjects with Alzheimer’s disease, whereas the expression of the CNR1 gene remained unchanged [238]. In vitro studies demonstrated that CB2 activation by JWH-015 suppressed the production of IFN-γ, TNF-α and nitrous oxide by microglia after stimulation with the amyloid β peptide [239]. In addition, JWH-133 blocked the activation of microglia by amyloid β peptide, and this treatment with the CB2 agonist reduced the production of pro-inflammatory cytokines [240]. Furthermore, when neurons and microglia were co-cultured, treatment with JWH-133 prevented the microglia-mediated toxicity of neurons after amyloid β peptide exposure [241]. These in vitro results suggest that the beneficial effects of CB2 activation are associated with the presence of this cannabinoid receptor on microglia and are related to the suppression of their pro-inflammatory activation.
Interestingly, the levels of AEA were found to be lower in the frontal and temporal cortex tissues of post mortem patients with Alzheimer’s disease compared to control subjects [242]. Furthermore, higher 2-AG plasma levels correlated with better memory and attention in patients with Alzheimer’s disease. This suggests a protective mechanism associated with the modulation of the endocannabinoid system [243].
Since it is possible to modulate endocannabinoid levels by inhibiting their hydrolysis, JZL184, which is an irreversible inhibitor of MAGL, was used in an Alzheimer’s disease mouse model. Pihlaja et al. observed a decrease in the production of pro-inflammatory mediators by microglia and astrocytes isolated from adult mice treated with JZL184 [244]. The inhibition of MAGL has been associated with several positive effects on Alzheimer’s disease in animal models, such as amelioration of inflammation and neuronal disorders [245]. The inhibition of FAAH activity with a selective irreversible inhibitor, UBR597, increased the availability of AEA [246]. Chiurchiu et al. demonstrated that macrophages from patients with Alzheimer’s disease treated with URB597 produced less pro-inflammatory cytokines [247]. Moreover, the treatment of aged rats with URB597 reduced the expression of IL-1β and TNF-α and restored aged-related disorders [248].
In a transgenic Tg APP 2576 mouse model of Alzheimer’s disease that overexpresses the amyloid precursor protein, the authors observed that chronic treatment with JWH-133 was able to decrease microglia activation and reduce COX-2 activation and TNF-α production. More importantly, CB2 activation helped to reduce cognitive impairment [241]. In the APP/PSI mouse model expressing a chimeric human/mouse amyloid precursor protein directly in neurons, the administration of JWH-133 was found to be effective at reducing Tau-hyperphosphorylation [249].
These results support the idea that targeting CB2 and endocannabinoid metabolism is a therapeutic option for some neuroinflammatory diseases.
6.2. Potential in Chronic Inflammation: Inflammatory Bowel Diseases (IBDs)
Crohn’s disease and ulcerative colitis are the two major chronic idiopathic IBDs [250]. Though several clinical and pathological features differ between these two IBDs [251], both diseases are characterized by intestinal inflammation and alteration of the epithelial barrier [252]. This leads to the translocation of bacteria and microbial products from the gut lumen through the intestinal wall. Consequently, an acute inflammatory reaction occurs, which is driven by immune cell infiltration and cytokine production [253]. As the disease progresses, an increasingly uncontrolled chronic inflammation develops that leads to tissue destruction [254,255].
Two noteworthy independent studies on IBD patients compared users versus nonusers of cannabis. Storr et al. showed that patients who used cannabis for more than six months were more susceptible to undergoing surgical treatment associated with Crohn’s disease [256]. On the other hand, Mbachi et al. compared two groups of patients with Crohn’s disease, one group of non-cannabis users and a second group of cannabis users. They concluded that cannabis users developed fewer complications, such as fistulizing disease, colectomy or intra-abdominal abscess [257]. Furthermore, mucosal tissue from the inflamed region of the colon of IBD patients incubated with the CB2 agonist JWH-133 for 6 h showed increased epithelial cell proliferation accompanied by decreased MMP-9 and IL-8 secretion [258]. This suggests that, in the context of IBD, the protective effect induced by plant cannabinoids might be mediated by the CB2 receptor.
Even though CB2 is highly expressed by infiltrating macrophages and plasma cells [23], the receptor is also detected in the esophagus, stomach, ileum and intestine [259]. It is expressed by epithelial cells, goblet cells and Paneth cells and increased during the acute phase of IBDs [260]. In addition, the Q63R functional variant of the CB2 protein has recently been significantly associated with Crohn’s disease and ulcerative colitis [261].
It is demonstrated that plasma AEA levels are higher in patients with IBD [262]. Regarding 2-AG, its levels are higher in patients with Crohn’s disease and are associated with increased expression of the 2-AG synthesizing enzyme DAGL-α [263].
Several IBD mouse models such as 2,4,6-Trinitrobenzenesulfonic acid- or Dinitrobenzene sulfonic acid-treated mice are used to mimic colitis [264]. First, in Tnfα overexpressing mice treated with TNBS/DSS, it was demonstrated that expression of Cnr2 was increased in immune cells. Furthermore, GP-1a treatment induced the polarization of T lymphocytes into regulatory phenotype secreting IL-10 [184]. In the DNBS-induced IBD model, JTE907, a CB2-specific inverse agonist, induced phenotypic differentiation of inflammatory T cells into foxp3 positive regulatory T cells secreting TGF-β and IL-10. In these studies, a CB2 receptor agonist reduced the severity of the disease [265].
In mice with DSS-induced IBD, treatment with N-arachidonoyl-serotonin, an inhibitor of FAAH, helped to improve clinical scores and pathogenesis [262]. FAAH blockade decreased the number of macrophages, neutrophils, NK and NKT cells in the Peyer’s patches and colonic lamina propria. This treatment reduced systemic and colonic inflammatory cytokine levels [266]. The FAAH inhibitor PF-3845 ameliorated TNBS-induced colitis [267]. Moreover, URB597, another FAAH inhibitor, attenuated TNBS-induced colitis, and this anti-inflammatory effect was abolished when Cnr1 and Cnr2 were genetically deficient [268]. Increasing the availability of 2-AG by using the MAGL selective inhibitor JZL184 attenuated TNBS-induced murine colitis [269].
These results suggest that targeting CB2 and manipulating pharmacologically the availability of endocannabinoids are potential therapeutic avenues to improve the quality of life of patients with IBD in terms of pain and disease symptoms but also to improve inflammation associated with these disorders.
6.3. Potential in Metabolic Disease: Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD encompasses a number of liver diseases ranging from isolated hepatic steatosis to steatohepatitis and irreversible cirrhosis [270]. NAFLD reflects the inability of the adipose tissue to perform its function as fat-storage tissue, leading to increased triglyceride uptake by hepatocytes [271]. The prevalence of NAFLD increases dramatically with obesity [272], dyslipidemia and type 2 diabetes [273] and is becoming the most common liver disease in developed countries [274]. Steatosis without any signs of fibrosis is considered an early condition, whereas the presence of fibrosis predicts chronic progression to severe liver disease [275].
A study with hepatitis-C-positive patients revealed that cannabis users had decreased prevalence of liver cirrhosis, although this did not improve mortality [276]. Furthermore, a study comparing the evolution of liver diseases in obese patients suggested that cannabis reduced the prevalence and progression of steatohepatitis [277].
It was established that Cnr2 mRNA is expressed in the liver of morbidly obese women at different stages of NAFLD. Its expression was correlated with the expression of anti-inflammatory and pro-inflammatory mediators, indicating that CB2 may have a dual role in NAFLD and NAFLD-related complications [278]. Indeed, because of the high expression of Cnr2 in the damaged liver, it was proposed that this phenomenon could predict the progression of liver disease from chronic hepatitis to irreversible cirrhosis and hepatocellular carcinoma [279]. Cnr2 was expressed by hepatocytes but only in patients with non-alcoholic steatosis [280]. In addition, Cnr2 was also expressed by Kupffer cells which are key players in immune control in the liver. Kupffer cells recognize many pathogens through various pattern recognition receptors and respond by producing pro-inflammatory cytokines [281]. Furthermore, the depletion of Kupffer cells in rats fed with a high-fat diet to induce steatosis protects against hepatic steatosis and insulin resistance, highlighting the importance of these cell types in liver disease [282]. These cells are very plastic in response to their environment as they can switch from a pro-inflammatory M1 to an anti-inflammatory M2 phenotype [283]. Kupffer cells isolated from Cnr2 knock-out mice are more polarized toward an M1 phenotype compared to Kupffer cells form wild-type mice [284]. The absence of Cnr2, or treatment with the CB2 antagonist, AM630, also confers to mice protection against steatosis induced with a high-fat diet [55]. On the other hand, rats treated with the agonist AM1241 displayed significant expression of hepatic progenitor cell markers, which indicates that stimulating CB2 enhances hepatocyte regeneration [181]. Furthermore, chronic activation of CB2 with JWH-133 in cirrhotic rats decreased the arterial pressure, immune cell infiltration and the number of activated stellate cells but more importantly, decreased fibrosis [285].
Taken together, these findings from various human studies and mouse models reveal contradictions in the effects of AEA and 2-AG in liver injury and cast doubt on the value of CB2 as a therapeutic target for liver diseases. Further studies are warranted to confirm whether (1) CB2 has a beneficial role in NAFLD and (2) the anti-inflammatory effect of CB2 is enough to counterbalance its proposed effect of increasing lipid accumulation. It is perhaps more interesting to explore the possibility that endocannabinoids and CB2 expression are biomarkers of the evolution of liver disease.
7. Conclusions and Future Direction
In recent years, we have witnessed an upsurge in research aimed at better understanding the role of CB2 in different inflammatory contexts. Many animal models demonstrate that CB2 stimulation by endogenous and exogenous ligands leads to an anti-inflammatory response and improves the symptoms of inflammatory diseases. However, it is important to consider the inflammatory context in which the CB2 receptor is targeted. This is demonstrated in allergic airway inflammation, a Th2-driven response, for which CB2 activation is detrimental.
Specific agonists and antagonists were developed and used in several clinical studies to target the endocannabinoid system in inflammatory conditions (Table 6). Although only a fraction of these studies have reached successfully their primary outcome, it establishes CB2 as a promising therapeutic target. Therefore, future efforts should focus on developing CB2 ligands activating specific signaling pathways and establishing which ligands are effective in the inflammatory context of each disease.
Author Contributions
Writing—original draft preparation, V.R., T.Z.M. and M.S.; writing—review and editing, V.R., T.Z.M., M.S., N.F. and V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Canada Research Excellence Chair in the Microbiome-Endocannabinoidome Axis in Metabolic Health (CERC-MEND) (to V.D.), the Institut universitaire de cardiologie et de pneumologie de Québec (to V.R.) and the FRQS funded Research Network on Cardiometabolism, Diabetes and Obesity CMDO-2021 (to V.R.). Canadian Institutes of Health Research (CIHR) Fellowship (472448) (to M.S.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nugent, R. Chronic diseases in developing countries: Health and economic burdens. Ann. N. Y. Acad. Sci. 2008, 1136, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.B.; Hartman, M.; Lassman, D.; Catlin, A.; Team, N.H.E.A. National Health Care Spending In 2019: Steady Growth For The Fourth Consecutive Year: Study examines national health care spending for 2019. Health Aff. 2021, 40, 14–24. [Google Scholar] [CrossRef]
- Feehan, K.T.; Gilroy, D.W. Is resolution the end of inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Codere, H. The Social and Cultural Context of Cannabis Use in Rwanda; De Gruyter Mouton: Berlin, Germany, 2011. [Google Scholar]
- Hanus, L.; Breuer, A.; Tchilibon, S.; Shiloah, S.; Goldenberg, D.; Horowitz, M.; Pertwee, R.G.; Ross, R.A.; Mechoulam, R.; Fride, E. HU-308: A specific agonist for CB(2), a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 14228–14233. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Devane, W.; Hanus, L.; Breuer, A.; Pertwee, R.; Stevenson, L.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 1971, 93, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Montero, C.; Campillo, N.E.; Goya, P.; Páez, J.A. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur. J. Med. Chem. 2005, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999, 6, 635–664. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Newton, C.; Larsen, K.; Lu, L.; Perkins, I.; Nong, L.; Friedman, H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003, 74, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Schatz, A.R.; Lee, M.; Condie, R.B.; Pulaski, J.T.; Kaminski, N.E. Cannabinoid Receptors CB1 and CB2: A Characterization of Expression and Adenylate Cyclase Modulation within the Immune System. Toxicol. Appl. Pharmacol. 1997, 142, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Rakotoarivelo, V.; Di Marzo, V.; Flamand, N. Expression and Functions of the CB2 Receptor in Human Leukocytes. Front. Pharmacol. 2022, 13, 826400. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.; Angel, C.; Schwarcz, L.; Dunbar, P.; Glass, M. Detailed characterisation of CB2 receptor protein expression in peripheral blood immune cells from healthy human volunteers using flow cytometry. Int. J. Immunopathol. Pharmacol. 2010, 23, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Frei, R.B.; Luschnig, P.; Parzmair, G.P.; Peinhaupt, M.; Schranz, S.; Fauland, A.; Wheelock, C.E.; Heinemann, A.; Sturm, E.M. Cannabinoid receptor 2 augments eosinophil responsiveness and aggravates allergen-induced pulmonary inflammation in mice. Allergy 2016, 71, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Núñez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; González, S.; Tolón, R.M.; Romero, J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the Presence and Functional Expression of Cannabinoid CB2 Receptors in Brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Wright, K.; Duncan, M.; Sharkey, K. Cannabinoid CB2 receptors in the gastrointestinal tract: A regulatory system in states of inflammation. Br. J. Pharmacol. 2008, 153, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; Rooney, N.; Feeney, M.; Tate, J.; Robertson, D.; Welham, M.; Ward, S. Differential Expression of Cannabinoid Receptors in the Human Colon: Cannabinoids Promote Epithelial Wound Healing. Gastroenterology 2005, 129, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.; Hansson, S.; Harta, G.; Mezey, E. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 1997, 82, 1131–1149. [Google Scholar] [CrossRef] [PubMed]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Vargas, A.; Pastor, A.; Boronat, A.; López-Gambero, A.J.; Sánchez-Marín, L.; Medina-Vera, D.; Serrano, A.; Pavón, F.J.; de la Torre, R. Differential hepatoprotective role of the cannabinoid CB1 and CB2 receptors in paracetamol—Induced liver injury. Br. J. Pharmacol. 2020, 177, 3309–3326. [Google Scholar] [CrossRef] [PubMed]
- Puhl, S.-L. Cannabinoid-sensitive receptors in cardiac physiology and ischaemia. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2020, 1867, 118462. [Google Scholar] [CrossRef] [PubMed]
- More, S.A.; Deore, R.S.; Pawar, H.D.; Sharma, C.; Nakhate, K.T.; Rathod, S.S.; Ojha, S.; Goyal, S.N. CB2 cannabinoid receptor as a potential target in myocardial infarction: Exploration of molecular pathogenesis and therapeutic strategies. Int. J. Mol. Sci. 2024, 25, 1683. [Google Scholar] [CrossRef]
- Bryk, M.; Starowicz, K. Cannabinoid-based therapy as a future for joint degeneration. Focus on the role of CB2 receptor in the arthritis progression and pain: An updated review. Pharmacol. Rep. 2021, 73, 681–699. [Google Scholar] [CrossRef]
- Pajak, A.; Kostrzewa, M.; Malek, N.; Korostynski, M.; Starowicz, K. Expression of matrix metalloproteinases and components of the endocannabinoid system in the knee joint are associated with biphasic pain progression in a rat model of osteoarthritis. J. Pain Res. 2017, 10, 1973–1989. [Google Scholar] [CrossRef][Green Version]
- Dunn, S.L.; Wilkinson, J.M.; Crawford, A.; Bunning, R.A.; Le Maitre, C.L. Expression of cannabinoid receptors in human osteoarthritic cartilage: Implications for future therapies. Cannabis Cannabinoid Res. 2016, 1, 3–15. [Google Scholar] [CrossRef]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-J.; Gao, M.; Gao, F.-F.; Su, Q.-X.; Wu, J. Brain cannabinoid receptor 2: Expression, function and modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hoffert, C.; Vu, H.K.; Groblewski, T.; Ahmad, S.; O’Donnell, D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003, 17, 2750–2754. [Google Scholar] [CrossRef]
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci. Lett. 2006, 396, 113–116. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Creoli, M.; Tortora, C.; Martinelli, M.; Miele, E.; Paino, S.; Luongo, L.; Rossi, F. Increased expression of CB2 receptor in the intestinal biopsies of children with inflammatory bowel disease. Pediatr. Res. 2022, 93, 1–6. [Google Scholar] [CrossRef]
- Merriam, F.V.; Wang, Z.-y.; Guerios, S.D.; Bjorling, D.E. Cannabinoid receptor 2 is increased in acutely and chronically inflamed bladder of rats. Neurosci. Lett. 2008, 445, 130–134. [Google Scholar] [CrossRef]
- Bayewitch, M.; Avidor-Reiss, T.; Levy, R.; Barg, J.; Mechoulam, R.; Vogel, Z. The peripheral cannabinoid receptor: Adenylate cyclase inhibition and G protein coupling. FEBS Lett. 1995, 375, 143–147. [Google Scholar] [CrossRef]
- Gerlo, S.; Kooijman, R.; Beck, I.M.; Kolmus, K.; Spooren, A.; Haegeman, G. Cyclic AMP: A selective modulator of NF-κB action. Cell. Mol. Life Sci. 2011, 68, 3823–3841. [Google Scholar] [CrossRef]
- Saroz, Y.; Kho, D.T.; Glass, M.; Graham, E.S.; Grimsey, N.L. Cannabinoid receptor 2 (CB2) signals via G-alpha-s and induces IL-6 and IL-10 cytokine secretion in human primary leukocytes. ACS Pharmacol. Transl. Sci. 2019, 2, 414–428. [Google Scholar] [CrossRef]
- Bouaboula, M.; Poinot-Chazel, C.; Marchand, J.; Canat, X.; Bourrié, B.; Rinaldi-Carmona, M.; Calandra, B.; Le Fur, G.; Casellas, P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor: Involvement of both mitogen—Activated protein kinase and induction of Krox—24 expression. Eur. J. Biochem. 1996, 237, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Derocq, J.-M.; Jbilo, O.; Bouaboula, M.; Ségui, M.; Clere, C.; Casellas, P. Genomic and functional changes induced by the activation of the peripheral cannabinoid receptor CB2 in the promyelocytic cells HL-60: Possible involvement of the CB2 receptor in cell differentiation. J. Biol. Chem. 2000, 275, 15621–15628. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, C.; Kipmen-Korgun, D.; Osibow, K.; Malli, R.; Graier, W.F. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 2003, 140, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, C.; Qian, J.; W Sutton, S.; Wang, Z.; Lv, J.; Liu, C.; Zhou, N. Involvement of β-arrestin-2 and clathrin in agonist-mediated internalization of the human cannabinoid CB2 receptor. Curr. Mol. Pharmacol. 2014, 7, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.M.; Carrasco, G.A. G-protein receptor kinase 5 regulates the cannabinoid receptor 2-induced up-regulation of serotonin 2A receptors. J. Biol. Chem. 2013, 288, 15712–15724. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Soethoudt, M.; Grether, U.; Fingerle, J.; Grim, T.W.; Fezza, F.; de Petrocellis, L.; Ullmer, C.; Rothenhäusler, B.; Perret, C.; van Gils, N.; et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 2017, 8, 13958. [Google Scholar] [CrossRef]
- Buckley, N.E.; McCoy, K.L.; Mezey, É.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur. J. Pharmacol. 2000, 396, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Denaës, T.; Lodder, J.; Chobert, M.-N.; Ruiz, I.; Pawlotsky, J.-M.; Lotersztajn, S.; Teixeira-Clerc, F. The cannabinoid receptor 2 protects against alcoholic liver disease via a macrophage autophagy-dependent pathway. Sci. Rep. 2016, 6, 28806. [Google Scholar] [CrossRef]
- Wu, Y.; Long, C.; Shu, Y.; He, P.; Zhou, Y.; Gu, J.; Yang, L.; Wang, Y. Cannabinoid receptor 2 deletion promotes proliferation and activation of hepatic macrophages in mice with acute liver injury induced by concanavalin A. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2019, 35, 13–18. [Google Scholar]
- Thapa, D.; Toguri, J.T.; Szczesniak, A.M.; Kelly, M.E.M. The Non-psychoactive Phytocannabinoid, Cannabidiol (CBD), and the Synthetic Derivatives, HU308 and CBD-DMH, Reduces Hyperalgesia and Inflammation in a Mouse Model of Corneal injury. FASEB J. 2017, 31, 811.7. [Google Scholar] [CrossRef]
- Gaffal, E.; Kemter, A.M.; Scheu, S.; Leite Dantas, R.; Vogt, J.; Baune, B.; Tüting, T.; Zimmer, A.; Alferink, J. Cannabinoid Receptor 2 Modulates Maturation of Dendritic Cells and Their Capacity to Induce Hapten-Induced Contact Hypersensitivity. Int. J. Mol. Sci. 2020, 21, 475. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Taylor, L.; Feuerborn, A.; Valaris, S.; Hussain, M.T.; Rainger, G.E.; Greaves, D.R.; Iqbal, A.J. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. 2019, 33, 6154–6167. [Google Scholar] [CrossRef] [PubMed]
- Deveaux, V.; Cadoudal, T.; Ichigotani, Y.; Teixeira-Clerc, F.; Louvet, A.; Manin, S.; Nhieu, J.T.-V.; Belot, M.P.; Zimmer, A.; Even, P.; et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE 2009, 4, e5844. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Recio, C.; Greaves, D.R.; Iqbal, A.J. Cannabinoid Receptor 2 Modulates Neutrophil Recruitment in a Murine Model of Endotoxemia. Mediat. Inflamm. 2017, 2017, 4315412. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Engel, T.; Aguilar-Pimentel, J.A.; Zimmer, A.; Jakob, T.; Behrendt, H.; Mempel, M. Beneficial effects of cannabinoids (CB) in a murine model of allergen-induced airway inflammation: Role of CB1/CB2 receptors. Immunobiology 2011, 216, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Larose, M.-C.; Turcotte, C.; Chouinard, F.; Ferland, C.; Martin, C.; Provost, V.; Laviolette, M.; Flamand, N. Mechanisms of human eosinophil migration induced by the combination of IL-5 and the endocannabinoid 2-arachidonoyl-glycerol. J. Allergy Clin. Immunol. 2014, 133, 1480–1482.e3. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, M.E.; Hong, S.; Stierle, A.; Stierle, D.; Stella, N.; Roberts, K.; Jaffar, Z. CB2 receptors regulate natural killer cells that limit allergic airway inflammation in a murine model of asthma. Allergy 2017, 72, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Penukonda, S.; Shcheglova, T.; Hagymasi, A.T.; Basu, S.; Srivastava, P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc. Natl. Acad. Sci. USA 2017, 114, 5005–5010. [Google Scholar] [CrossRef]
- Lowin, T.; Apitz, M.; Anders, S.; Straub, R.H. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res. Ther. 2015, 17, 321. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti—Inflammatory mediator palmitoylethanolamide enhances the levels of 2—Arachidonoyl—Glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. The endocannabinoidome as a substrate for noneuphoric phytocannabinoid action and gut microbiome dysfunction in neuropsychiatric disorders. Dialogues Clin. Neurosci. 2020, 22, 259–269. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, D.-H.; Choi, J.-Y.; Kim, W.-J.; Kim, J.Y.; Senejani, A.G.; Hwang, S.S.; Kim, L.K.; Tobiasova, Z.; Lee, G.R.; et al. PPARγ Negatively Regulates T Cell Activation to Prevent Follicular Helper T Cells and Germinal Center Formation. PLoS ONE 2014, 9, e99127. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Hwang, S.G.; Han, S.H.; Kaminski, N.E. Suppression of Interleukin-2 by the Putative Endogenous Cannabinoid 2-Arachidonyl-Glycerol Is Mediated through Down-regulation of the Nuclear Factor of Activated T Cells. Mol. Pharmacol. 1998, 53, 676. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, C.E.; Snider, N.T.; Thompson, J.T.; Vanden Heuvel, J.P.; Kaminski, N.E. Interleukin-2 Suppression by 2-Arachidonyl Glycerol Is Mediated through Peroxisome Proliferator-Activated Receptor γ Independently of Cannabinoid Receptors 1 and 2. Mol. Pharmacol. 2006, 70, 101. [Google Scholar] [CrossRef]
- Coopman, K.; Smith, L.D.; Wright, K.L.; Ward, S.G. Temporal variation in CB2R levels following T lymphocyte activation: Evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int. Immunopharmacol. 2007, 7, 360–371. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The endocannabinoid system and PPARs: Focus on their signalling crosstalk, action and transcriptional regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Toguri, J.T.; Leishman, E.; Szczesniak, A.M.; Laprairie, R.B.; Oehler, O.; Straiker, A.J.; Kelly, M.E.M.; Bradshaw, H.B. Inflammation and CB2 signaling drive novel changes in the ocular lipidome and regulate immune cell activity in the eye. Prostaglandins Other Lipid Mediat. 2018, 139, 54–62. [Google Scholar] [CrossRef]
- Ruhl, T.; Lippold, E.F.; Christer, T.; Schaefer, B.; Kim, B.-S.; Beier, J.P. Genetic deletion of the cannabinoid receptors CB1 and CB2 enhances inflammation with diverging effects on skin wound healing in mice. Life Sci. 2021, 285, 120018. [Google Scholar] [CrossRef]
- Hegde, V.L.; Hegde, S.; Cravatt, B.F.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory T cells. Mol. Pharmacol. 2008, 74, 20–33. [Google Scholar] [CrossRef]
- Booker, L.; Kinsey, S.G.; Abdullah, R.A.; Blankman, J.L.; Long, J.Z.; Ezzili, C.; Boger, D.L.; Cravatt, B.F.; Lichtman, A.H. The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS—Induced tactile allodynia in mice. Br. J. Pharmacol. 2012, 165, 2485–2496. [Google Scholar] [CrossRef] [PubMed]
- Duerr, G.D.; Heinemann, J.C.; Gestrich, C.; Heuft, T.; Klaas, T.; Keppel, K.; Roell, W.; Klein, A.; Zimmer, A.; Velten, M.; et al. Impaired border zone formation and adverse remodeling after reperfused myocardial infarction in cannabinoid CB2 receptor deficient mice. Life Sci. 2015, 138, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.; Mangels, N.; Häussler, A.; Ferreirós, N.; Fleming, I.; Tegeder, I. Pro-inflammatory obesity in aged cannabinoid-2 receptor-deficient mice. Int. J. Obes. 2016, 40, 366–379. [Google Scholar] [CrossRef]
- Engel, M.A.; Kellermann, C.A.; Burnat, G.; Hahn, E.G.; Rau, T.; Konturek, P.C. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. J. Physiol. Pharmacol. 2010, 61, 89–97. [Google Scholar] [PubMed]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Hooper, D.C.; Elliott, M.B. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J. Neuroinflamm. 2014, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Magid, L.; Heymann, S.; Elgali, M.; Avram, L.; Cohen, Y.; Liraz-Zaltsman, S.; Mechoulam, R.; Shohami, E. Role of CB2 Receptor in the Recovery of Mice after Traumatic Brain Injury. J. Neurotrauma 2018, 36, 1836–1846. [Google Scholar] [CrossRef]
- Csoka, B.; Nemeth, Z.H.; Mukhopadhyay, P.; Spolarics, Z.; Rajesh, M.; Federici, S.; Deitch, E.A.; Batkai, S.; Pacher, P.; Hasko, G. CB2 cannabinoid receptors contribute to bacterial invasion and mortality in polymicrobial sepsis. PLoS ONE 2009, 4, e6409. [Google Scholar] [CrossRef]
- Ke, P.; Shao, B.-Z.; Xu, Z.-Q.; Wei, W.; Han, B.-Z.; Chen, X.-W.; Su, D.-F.; Liu, C. Activation of Cannabinoid Receptor 2 Ameliorates DSS-Induced Colitis through Inhibiting NLRP3 Inflammasome in Macrophages. PLoS ONE 2016, 11, e0155076. [Google Scholar] [CrossRef]
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 2010, 53, 2629–2640. [Google Scholar] [CrossRef] [PubMed]
- Alferink, J.; Specht, S.; Arends, H.; Schumak, B.; Schmidt, K.; Ruland, C.; Lundt, R.; Kemter, A.; Dlugos, A.; Kuepper, J.M.; et al. Cannabinoid Receptor 2 Modulates Susceptibility to Experimental Cerebral Malaria through a CCL17-dependent Mechanism. J. Biol. Chem. 2016, 291, 19517–19531. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A. Anandamide, an endogenous cannabinomimetic eicosanoid:‘killing two birds with one stone’. Prostaglandins Leukot. Essent. Fat. Acids 1995, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylgylcerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gonsiorek, W.; Lunn, C.; Fan, X.; Narula, S.; Lundell, D.; Hipkin, R.W. Endocannabinoid 2-Arachidonyl Glycerol Is a Full Agonist through Human Type 2 Cannabinoid Receptor: Antagonism by Anandamide. Mol. Pharmacol. 2000, 57, 1045. [Google Scholar] [PubMed]
- Hanuš, L.; Abu-Lafi, S.; Fride, E.; Breuer, A.; Vogel, Z.; Shalev, D.E.; Kustanovich, I.; Mechoulam, R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 3662–3665. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kodaka, T.; Nakane, S.; Miyashita, T.; Kondo, S.; Suhara, Y.; Takayama, H.; Waku, K.; Seki, C.; Baba, N.; et al. Evidence That the Cannabinoid CB1 Receptor Is a 2-arachidonoylglycerol Receptor: STRUCTURE-ACTIVITY RELATIONSHIP OF 2-ARACHIDONOYLGLYCEROL, ETHER-LINKED ANALOGUES, AND RELATED COMPOUNDS*. J. Biol. Chem. 1999, 274, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.L.; Ruckle, M.B.; Mayeux, P.R.; Prather, P.L. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 2005, 315, 828–838. [Google Scholar] [CrossRef]
- Shoemaker, J.L.; Joseph, B.K.; Ruckle, M.B.; Mayeux, P.R.; Prather, P.L. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J. Pharmacol. Exp. Ther. 2005, 314, 868–875. [Google Scholar] [CrossRef]
- Fezza, F.; Bisogno, T.; Minassi, A.; Appendino, G.; Mechoulam, R.; Di Marzo, V. Noladin ether, a putative novel endocannabinoid: Inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002, 513, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Tsuchie, A.; Tokumura, A.; Muramatsu, M.; Suhara, Y.; Takayama, H.; Waku, K.; Sugiura, T. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J. Neurochem. 2003, 85, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.C.; Sauer, J.-M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Sharir, H.; Console-Bram, L.; Mundy, C.; Popoff, S.N.; Kapur, A.; Abood, M.E. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J. Neuroimmune Pharmacol. 2012, 7, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Melck, D.; Bobrov, M.Y.; Gretskaya, N.M.; Bezuglov, V.V.; De Petrocellis, L.; Di Marzo, V. N-acyl-dopamines: Novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000, 351, 817–824. [Google Scholar] [CrossRef]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef]
- Wilhelmsen, K.; Khakpour, S.; Tran, A.; Sheehan, K.; Schumacher, M.; Xu, F.; Hellman, J. The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55, 212–2 abate the inflammatory activation of human endothelial cells. J. Biol. Chem. 2014, 289, 13079–13100. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. In Reviews of Physiology Biochemistry and Pharmacology; Amara, S.G., Bamberg, E., Fleischmann, B., Gudermann, T., Hebert, S.C., Jahn, R., Lederer, W.J., Lill, R., Miyajima, A., Offermanns, S., et al., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2008; pp. 1–24. [Google Scholar] [CrossRef]
- Hussain, Z.; Uyama, T.; Tsuboi, K.; Ueda, N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2017, 1862, 1546–1561. [Google Scholar] [CrossRef]
- Deutsch, D.; Ueda, N.; Yamamoto, S. The fatty acid amide hydrolase (FAAH). Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2002, 66, 201–210. [Google Scholar] [CrossRef]
- Tsuboi, K.; Takezaki, N.; Ueda, N. The N-Acylethanolamine-Hydrolyzing Acid Amidase (NAAA). Chem. Biodivers. 2007, 4, 1914–1925. [Google Scholar] [CrossRef]
- Ueda, N.; Tsuboi, K.; Uyama, T.; Ohnishi, T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors 2011, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Archambault, A.-S.; Lavoie, J.-P.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochem. Pharmacol. 2022, 205, 115261. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Saario, S.M.; Laitinen, J.T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. 2012, 204, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Taschler, U.; Radner, F.P.W.; Heier, C.; Schreiber, R.; Schweiger, M.; Schoiswohl, G.; Preiss-Landl, K.; Jaeger, D.; Reiter, B.; Koefeler, H.C.; et al. Monoglyceride Lipase Deficiency in Mice Impairs Lipolysis and Attenuates Diet-induced Insulin Resistance*. J. Biol. Chem. 2011, 286, 17467–17477. [Google Scholar] [CrossRef] [PubMed]
- Abohalaka, R.; Karaman, Y.; Recber, T.; Onder, S.C.; Nemutlu, E.; Bozkurt, T.E. Endocannabinoid metabolism inhibition ameliorates ovalbumin-induced allergic airway inflammation and hyperreactivity in Guinea pigs. Life Sci. 2022, 306, 120808. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Duranti, A.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Cuzzocrea, S.; Di Paola, R.; et al. Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury. Int. J. Mol. Sci. 2022, 23, 2781. [Google Scholar] [CrossRef] [PubMed]
- Vujic, N.; Schlager, S.; Eichmann, T.O.; Madreiter-Sokolowski, C.T.; Goeritzer, M.; Rainer, S.; Schauer, S.; Rosenberger, A.; Woelfler, A.; Doddapattar, P.; et al. Monoglyceride lipase deficiency modulates endocannabinoid signaling and improves plaque stability in ApoE-knockout mice. Atherosclerosis 2016, 244, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Dione, N.; Lacroix, S.; Taschler, U.; Deschênes, T.; Abolghasemi, A.; Leblanc, N.; Di Marzo, V.; Silvestri, C. Mgll Knockout Mouse Resistance to Diet-Induced Dysmetabolism Is Associated with Altered Gut Microbiota. Cells 2020, 9, 2705. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Chokr, D.; Wan, J.; Hegde, P.; Mabire, M.; Siebert, M.; Ribeiro-Parenti, L.; Le Gall, M.; Lettéron, P.; Pilard, N.; et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut 2019, 68, 522. [Google Scholar] [CrossRef]
- Sara, R.N.; Floyd, F.S.; Timothy, B.W.; Adam, H.L.; Ku-Lung, H.; Steven, G.K. Monoacylglycerol Lipase Inhibition Using JZL184 Attenuates Paw Inflammation and Functional Deficits in a Mouse Model of Inflammatory Arthritis. Cannabis Cannabinoid Res. 2021, 6, 233–241. [Google Scholar] [CrossRef]
- Pertwee, R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Steffens, S.; Veillard, N.R.; Arnaud, C.; Pelli, G.; Burger, F.; Staub, C.; Zimmer, A.; Frossard, J.-L.; Mach, F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB 1 on neurons and CB 2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef]
- Horváth, B.; Magid, L.; Mukhopadhyay, P.; Bátkai, S.; Rajesh, M.; Park, O.; Tanchian, G.; Gao, R.Y.; Goodfellow, C.E.; Glass, M.; et al. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br. J. Pharmacol. 2012, 165, 2462–2478. [Google Scholar] [CrossRef]
- Buchweitz, J.P.; Karmaus, P.W.; Williams, K.J.; Harkema, J.R.; Kaminski, N.E. Targeted Deletion of Cannabinoid Receptors CB1 and CB2 Produced Enhanced Inflammatory Responses to Influenza A/PR/8/34 in the Absence and Presence of Δ9-tetrahydrocannabinol; Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Oka, S.; Yanagimoto, S.; Ikeda, S.; Gokoh, M.; Kishimoto, S.; Waku, K.; Ishima, Y.; Sugiura, T. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J. Biol. Chem. 2005, 280, 18488–18497. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, F.A.; Egan, R.W. Prostaglandins, arachidonic acid, and inflammation. Science 1980, 210, 978–984. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Dynamics of arachidonic acid mobilization by inflammatory cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 249–256. [Google Scholar] [CrossRef]
- Newton, C.A.; Chou, P.-J.; Perkins, I.; Klein, T.W. CB1 and CB2 Cannabinoid Receptors Mediate Different Aspects of Delta-9-Tetrahydrocannabinol (THC)-Induced T Helper Cell Shift Following Immune Activation by Legionella Pneumophila Infection. J. Neuroimmune Pharmacol. 2009, 4, 92–102. [Google Scholar] [CrossRef]
- Comelli, F.; Giagnoni, G.; Bettoni, I.; Colleoni, M.; Costa, B. The inhibition of monoacylglycerol lipase by URB602 showed an anti-inflammatory and anti-nociceptive effect in a murine model of acute inflammation. Br. J. Pharmacol. 2007, 152, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zheng, F.; Liu, A.; Li, Z.; Zheng, F.; Liu, Q.; Yang, L.; Chen, K.; Wang, Y.; Zhang, Z.; et al. Activation of CB2 receptor inhibits pyroptosis and subsequently ameliorates cecal ligation and puncture-induced sepsis. Int. Immunopharmacol. 2021, 99, 108038. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ribeiro, R.; Tanaka, M.; Zhang, Y. Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology 2015, 99, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Costola-de-Souza, C.; Ribeiro, A.; Ferraz-de-Paula, V.; Calefi, A.S.; Aloia, T.P.; Gimenes-Junior, J.A.; de Almeida, V.I.; Pinheiro, M.L.; Palermo-Neto, J. Monoacylglycerol lipase (MAGL) inhibition attenuates acute lung injury in mice. PLoS ONE 2013, 8, e77706. [Google Scholar] [CrossRef]
- Naidu, P.S.; Kinsey, S.G.; Guo, T.L.; Cravatt, B.F.; Lichtman, A.H. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J. Pharmacol. Exp. Ther. 2010, 334, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Wakui, J.; Ikeda, S.; Yanagimoto, S.; Kishimoto, S.; Gokoh, M.; Nasui, M.; Sugiura, T. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J. Immunol. 2006, 177, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108952. [Google Scholar] [CrossRef] [PubMed]
- Tindall, B.; Cooper, D.; Donovan, B.; Barnes, T.; Philpot, C.; Gold, J.; Penny, R. The Sydney AIDS Project: Development of acquired immunodeficiency syndrome in a group of HIV seropositive homosexual men. Aust. New Zealand J. Med. 1988, 18, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Caiaffa, W.T.; Vlahov, D.; Graham, N.; Astemborski, J.; Solomon, L.; Nelson, K.E.; Munoz, A. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus-seropositive injection drug users. Am. J. Respir. Crit. Care Med. 1994, 150, 1493–1498. [Google Scholar] [CrossRef]
- Baldwin, G.C.; Tashkin, D.P.; Buckley, D.M.; Park, A.N.; Dubinett, S.M.; Roth, M.D. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am. J. Respir. Crit. Care Med. 1997, 156, 1606–1613. [Google Scholar] [CrossRef]
- Atwood, B.K.; Wager-Miller, J.; Haskins, C.; Straiker, A.; Mackie, K. Functional Selectivity in CB2 Cannabinoid Receptor Signaling and Regulation: Implications for the Therapeutic Potential of CB2 Ligands. Mol. Pharmacol. 2012, 81, 250. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi-Carmona, M.; Barth, F.; Millan, J.; Derocq, J.-M.; Casellas, P.; Congy, C.; Oustric, D.; Sarran, M.; Bouaboula, M.; Calandra, B. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998, 284, 644–650. [Google Scholar] [PubMed]
- Li, X.; Shen, L.; Hua, T.; Liu, Z.-J. Structural and functional insights into cannabinoid receptors. Trends Pharmacol. Sci. 2020, 41, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.W.; Liddle, J.; Yu, S.; Aung, M.M.; Abood, M.E.; Wiley, J.L.; Martin, B.R. 3-(1′, 1′-Dimethylbutyl)-1-deoxy-Δ8-THC and related compounds: Synthesis of selective ligands for the CB2 receptor. Bioorganic Med. Chem. 1999, 7, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Gallant, M.; Dufresne, C.; Gareau, Y.; Guay, D.; Leblanc, Y.; Prasit, P.; Rochette, C.; Sawyer, N.; Slipetz, D.M.; Tremblay, N. New class of potent ligands for the human peripheral cannabinoid receptor. Bioorganic Med. Chem. Lett. 1996, 6, 2263–2268. [Google Scholar] [CrossRef]
- Huang, Z.-B.; Zheng, Y.-X.; Li, N.; Cai, S.-L.; Huang, Y.; Wang, J.; Hu, X.-W.; Wang, Y.; Wu, J.; Fan, X.-G. Protective effects of specific cannabinoid receptor 2 agonist GW405833 on concanavalin A-induced acute liver injury in mice. Acta Pharmacol. Sin. 2019, 40, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Parlar, A.; Arslan, S.O.; Doğan, M.F.; Çam, S.A.; Yalçin, A.; Elibol, E.; Özer, M.K.; Üçkardeş, F.; Kara, H. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 2018, 16, 4900–4908. [Google Scholar] [CrossRef]
- Schuelert, N.; Zhang, C.; Mogg, A.J.; Broad, L.M.; Hepburn, D.L.; Nisenbaum, E.S.; Johnson, M.P.; McDougall, J.J. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthr. Cartil. 2010, 18, 1536–1543. [Google Scholar] [CrossRef]
- Li, A.-L.; Carey, L.M.; Mackie, K.; Hohmann, A.G. Cannabinoid CB2 Agonist GW405833 Suppresses Inflammatory and Neuropathic Pain through a CB1 Mechanism that is Independent of CB2 Receptors in Mice. J. Pharmacol. Exp. Ther. 2017, 362, 296. [Google Scholar] [CrossRef]
- Çakır, M.; Tekin, S.; Okan, A.; Çakan, P.; Doğanyiğit, Z. The ameliorating effect of cannabinoid type 2 receptor activation on brain, lung, liver and heart damage in cecal ligation and puncture-induced sepsis model in rats. Int. Immunopharmacol. 2020, 78, 105978. [Google Scholar] [CrossRef]
- Bai, J.; Ge, G.; Wang, Y.; Zhang, W.; Wang, Q.; Wang, W.; Guo, X.; Yu, B.; Xu, Y.; Yang, H.; et al. A selective CB2 agonist protects against the inflammatory response and joint destruction in collagen-induced arthritis mice. Biomed. Pharmacother. 2019, 116, 109025. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yu, B.; Bai, J.; Wang, X.; Guo, X.; Liu, Y.; Lin, J.; Hu, S.; Zhang, W.; Tao, Y. Cannabinoid receptor 2 agonist prevents local and systemic inflammatory bone destruction in rheumatoid arthritis. J. Bone Miner. Res. 2019, 34, 739–751. [Google Scholar] [CrossRef]
- Fukuda, S.; Kohsaka, H.; Takayasu, A.; Yokoyama, W.; Miyabe, C.; Miyabe, Y.; Harigai, M.; Miyasaka, N.; Nanki, T. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. BMC Musculoskelet. Disord. 2014, 15, 275. [Google Scholar] [CrossRef]
- Burston, J.J.; Sagar, D.R.; Shao, P.; Bai, M.; King, E.; Brailsford, L.; Turner, J.M.; Hathway, G.J.; Bennett, A.J.; Walsh, D.A. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS ONE 2013, 8, e80440. [Google Scholar] [CrossRef]
- La Porta, C.; Bura, S.A.; Aracil-Fernández, A.; Manzanares, J.; Maldonado, R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain 2013, 154, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, T.; Corsten, C.; Beier, J.P.; Kim, B.-S. The immunosuppressive effect of the endocannabinoid system on the inflammatory phenotypes of macrophages and mesenchymal stromal cells: A comparative study. Pharmacol. Rep. 2021, 73, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Khuja, I.; Yekhtin, Z.; Or, R.; Almogi-Hazan, O. Cannabinoids Reduce Inflammation but Inhibit Lymphocyte Recovery in Murine Models of Bone Marrow Transplantation. Int. J. Mol. Sci. 2019, 20, 668. [Google Scholar] [CrossRef]
- Murineddu, G.; Lazzari, P.; Ruiu, S.; Sanna, A.; Loriga, G.; Manca, I.; Falzoi, M.; Dessì, C.; Curzu, M.M.; Chelucci, G. Tricyclic Pyrazoles. 4. Synthesis and Biological Evaluation of Analogues of the Robust and Selective CB2 Cannabinoid Ligand 1-(2’, 4’-Dichlorophenyl)-6-methyl-N-piperidin-1-yl-1, 4-dihydroindeno [1, 2-c] pyrazole-3-carboxamide. J. Med. Chem. 2006, 49, 7502–7512. [Google Scholar] [CrossRef]
- Malan Jr, T.P.; Ibrahim, M.M.; Deng, H.; Liu, Q.; Mata, H.P.; Vanderah, T.; Porreca, F.; Makriyannis, A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 2001, 93, 239–245. [Google Scholar] [CrossRef]
- Porter, R.F.; Szczesniak, A.-M.; Toguri, J.T.; Gebremeskel, S.; Johnston, B.; Lehmann, C.; Fingerle, J.; Rothenhäusler, B.; Perret, C.; Rogers-Evans, M.; et al. Selective Cannabinoid 2 Receptor Agonists as Potential Therapeutic Drugs for the Treatment of Endotoxin-Induced Uveitis. Molecules 2019, 24, 3338. [Google Scholar] [CrossRef]
- Pasquini, S.; De Rosa, M.; Pedani, V.; Mugnaini, C.; Guida, F.; Luongo, L.; De Chiaro, M.; Maione, S.; Dragoni, S.; Frosini, M. Investigations on the 4-quinolone-3-carboxylic acid motif. 4. Identification of new potent and selective ligands for the cannabinoid type 2 receptor with diverse substitution patterns and antihyperalgesic effects in mice. J. Med. Chem. 2011, 54, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wolk, B.; Kendall, D.A. Effects of a CB2 subtype selective agonist ABK5-1 on cytokine production in microglia. J. Cell. Signal. 2021, 2, 85. [Google Scholar] [PubMed]
- Odan, M.; Ishizuka, N.; Hiramatsu, Y.; Inagaki, M.; Hashizume, H.; Fujii, Y.; Mitsumori, S.; Morioka, Y.; Soga, M.; Deguchi, M. Discovery of S-777469: An orally available CB2 agonist as an antipruritic agent. Bioorg. Med. Chem. Lett. 2012, 22, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Gatley, S.J.; Lan, R.; Pyatt, B.; Gifford, A.N.; Volkow, N.D.; Makriyannis, A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997, 61, PL191–PL197. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [PubMed]
- Ross, R.A.; Brockie, H.C.; Stevenson, L.A.; Murphy, V.L.; Templeton, F.; Makriyannis, A.; Pertwee, R.G. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br. J. Pharmacol. 1999, 126, 665. [Google Scholar] [CrossRef] [PubMed]
- Zarruk, J.G.; Fernandez-Lopez, D.; Garcia-Yebenes, I.; Garcia-Gutierrez, M.S.; Vivancos, J.; Nombela, F.; Torres, M.; Burguete, M.C.; Manzanares, J.; Lizasoain, I.; et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke 2012, 43, 211–219. [Google Scholar] [CrossRef]
- Gupta, B.; Hornick, M.G.; Briyal, S.; Donovan, R.; Prazad, P.; Gulati, A. Anti-apoptotic and Immunomodulatory Effect of CB2 Agonist, JWH133, in a Neonatal Rat Model of Hypoxic-Ischemic Encephalopathy. Front. Pediatr. 2020, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-K.; Guo, H.-C.; Liu, H.-Y.; Zhang, Z.-L.; Hu, M.-Y.; Zhang, Y.; Li, Q.; Guo, H.-C. Cannabinoid type 2 receptor agonist JWH133 decreases blood pressure of spontaneously hypertensive rats through relieving inflammation in the rostral ventrolateral medulla of the brain. J. Hypertens. 2020; Publish Ahead of Print. [Google Scholar] [CrossRef]
- Shapiro, L.; Wong, J.C.; Escayg, A. Reduced cannabinoid 2 receptor activity increases susceptibility to induced seizures in mice. Epilepsia 2019, 60, 2359–2369. [Google Scholar] [CrossRef]
- Sun, L.; Dong, R.; Xu, X.; Yang, X.; Peng, M. Activation of cannabinoid receptor type 2 attenuates surgery-induced cognitive impairment in mice through anti-inflammatory activity. J. Neuroinflamm. 2017, 14, 138. [Google Scholar] [CrossRef]
- Zoppi, S.; Madrigal, J.L.; Caso, J.R.; Garcia-Gutierrez, M.S.; Manzanares, J.; Leza, J.C.; Garcia-Bueno, B. Regulatory role of the cannabinoid CB2 receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014, 171, 2814–2826. [Google Scholar] [CrossRef]
- Rentsch, P.; Stayte, S.; Egan, T.; Clark, I.; Vissel, B. Targeting the cannabinoid receptor CB2 in a mouse model of l-dopa induced dyskinesia. Neurobiol. Dis. 2020, 134, 104646. [Google Scholar] [CrossRef] [PubMed]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.S.; Chauhan, P.; Hu, S.; Prasad, S.; Lokensgard, J.R. Antiallodynic Effects of Cannabinoid Receptor 2 (CB(2)R) Agonists on Retrovirus Infection-Induced Neuropathic Pain. Pain Res. Manag. 2019, 2019, 1260353. [Google Scholar] [CrossRef]
- Tao, Y.; Li, L.; Jiang, B.; Feng, Z.; Yang, L.; Tang, J.; Chen, Q.; Zhang, J.; Tan, Q.; Feng, H.; et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav. Immun. 2016, 58, 118–129. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Guo, J.; Yang, L.; Tao, Y.; Li, L.; Miao, H.; Feng, H.; Chen, Z.; Zhu, G. Minocycline Attenuates Neonatal Germinal-Matrix-Hemorrhage-Induced Neuroinflammation and Brain Edema by Activating Cannabinoid Receptor 2. Mol. Neurobiol. 2015, 53, 1935–1948. [Google Scholar] [CrossRef]
- Li, L.; Yun, D.; Zhang, Y.; Tao, Y.; Tan, Q.; Qiao, F.; Luo, B.; Liu, Y.; Fan, R.; Xian, J. A cannabinoid receptor 2 agonist reduces blood–brain barrier damage via induction of MKP-1 after intracerebral hemorrhage in rats. Brain Res. 2018, 1697, 113–123. [Google Scholar] [CrossRef]
- Pan, S.D.; Grandgirard, D.; Leib, S.L. Adjuvant Cannabinoid Receptor Type 2 Agonist Modulates the Polarization of Microglia Towards a Non-Inflammatory Phenotype in Experimental Pneumococcal Meningitis. Front. Cell. Infect. Microbiol. 2020, 10, 588195. [Google Scholar] [CrossRef]
- Hall, S.; Faridi, S.; Trivedi, P.; Sultana, S.; Ray, B.; Myers, T.; Euodia, I.; Vlatten, D.; Castonguay, M.; Zhou, J.; et al. Selective CB2 Receptor Agonist, HU-308, Reduces Systemic Inflammation in Endotoxin Model of Pneumonia-Induced Acute Lung Injury. Int. J. Mol. Sci. 2022, 23, 15857. [Google Scholar] [CrossRef]
- Berger, G.; Arora, N.; Burkovskiy, I.; Xia, Y.; Chinnadurai, A.; Westhofen, R.; Hagn, G.; Cox, A.; Kelly, M.; Zhou, J. Experimental Cannabinoid 2 Receptor Activation by Phyto-Derived and Synthetic Cannabinoid Ligands in LPS-Induced Interstitial Cystitis in Mice. Molecules 2019, 24, 4239. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, J.; Kelly, M.E.; Zhou, J.; Lehmann, C. Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis. Mediat. Inflamm. 2014, 2014, 978678. [Google Scholar] [CrossRef] [PubMed]
- Tambaro, S.; Casu, M.A.; Mastinu, A.; Lazzari, P. Evaluation of selective cannabinoid CB(1) and CB(2) receptor agonists in a mouse model of lipopolysaccharide-induced interstitial cystitis. Eur. J. Pharmacol. 2014, 729, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.A.; Keenan, C.M.; Zhang, H.; Patel, K.D.; Makriyannis, A.; Sharkey, K.A. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm. Bowel Dis. 2009, 15, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Li, H.; Heller, J.; Skarica, M.; Zhang, M.; Ganea, D.; Tuma, R.F. Modulation of inflammatory responses by a cannabinoid-2-selective agonist after spinal cord injury. J. Neurotrauma 2011, 28, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Bayram, S.; Parlar, A.; Arslan, S.O. The curative effect of cannabinoid 2 receptor agonist on functional failure and disruptive inflammation caused by intestinal ischemia and reperfusion. Fundam. Clin. Pharmacol. 2020, 34, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.M.; Osman, M.; Elshabrawy, O.; Abdallah, H.M.I.; Khairallah, A. AM-1241 CB2 Receptor Agonist Attenuates Inflammation, Apoptosis and Stimulate Progenitor Cells in Bile Duct Ligated Rats. Open Access Maced J. Med. Sci. 2019, 7, 925–936. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Wang, P.; Bjorling Dale, E. Treatment with a Cannabinoid Receptor 2 Agonist Decreases Severity of Established Cystitis. J. Urol. 2014, 191, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Silva, M.; Correa, L.B.; Candéa, A.L.P.; Cavalher-Machado, S.C.; Barbosa, H.S.; Rosas, E.C.; Henriques, M.G. The cannabinoid 2 receptor agonist β-caryophyllene modulates the inflammatory reaction induced by Mycobacterium bovis BCG by inhibiting neutrophil migration. Inflamm. Res. 2016, 65, 869–879. [Google Scholar] [CrossRef]
- Leinwand, K.L.; Jones, A.A.; Huang, R.H.; Jedlicka, P.; Kao, D.J.; de Zoeten, E.F.; Ghosh, S.; Moaddel, R.; Wehkamp, J.; Ostaff, M.J.; et al. Cannabinoid Receptor-2 Ameliorates Inflammation in Murine Model of Crohn’s Disease. J. Crohn’s Colitis 2017, 11, 1369–1380. [Google Scholar] [CrossRef]
- Du, Y.; Ren, P.; Wang, Q.; Jiang, S.-K.; Zhang, M.; Li, J.-Y.; Wang, L.-L.; Guan, D.-W. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J. Inflamm. 2018, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Zhao, R.; Li, J.-Y.; Li, S.-S.; Liu, M.; Wang, M.; Zhang, M.-Z.; Dong, W.-W.; Jiang, S.-K.; Zhang, M.; et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur. J. Pharmacol. 2016, 786, 128–136. [Google Scholar] [CrossRef]
- Tang, Y.; Wolk, B.; Britch, S.C.; Craft, R.M.; Kendall, D.A. Anti-inflammatory and antinociceptive effects of the selective cannabinoid CB2 receptor agonist ABK5. J. Pharmacol. Sci. 2021, 145, 319–326. [Google Scholar] [CrossRef]
- Haruna, T.; Soga, M.; Morioka, Y.; Imura, K.; Furue, Y.; Yamamoto, M.; Hayakawa, J.; Deguchi, M.; Arimura, A.; Yasui, K. The Inhibitory Effect of S-777469, a Cannabinoid Type 2 Receptor Agonist, on Skin Inflammation in Mice. Pharmacology 2017, 99, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Abohalaka, R.; Bozkurt, T.E.; Nemutlu, E.; Onder, S.C.; Sahin-Erdemli, I. The effect of endocannabinoid metabolism inhibition on airway inflammation in mice. Eur. Respir. J. 2019, 54, PA3878. [Google Scholar] [CrossRef]
- Giannini, L.; Nistri, S.; Mastroianni, R.; Cinci, L.; Vannacci, A.; Mariottini, C.; Passani, M.B.; Mannaioni, P.F.; Bani, D.; Masini, E. Activation of cannabinoid receptors prevents antigen-induced asthma-like reaction in guinea pigs. J. Cell. Mol. Med. 2008, 12, 2381–2394. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Y.; Liu, Y.; Wang, N.; Zhao, X.; Wen, D. CB2R agonist JWH-133 attenuates chronic inflammation by restraining M1 macrophage polarization via Nrf2/HO-1 pathway in diet-induced obese mice. Life Sci. 2020, 260, 118424. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, G.; Zhang, J.; Wei, W. Selective activation of cannabinoid receptor 2 attenuates myocardial infarction via suppressing NLRP3 inflammasome. Inflammation 2019, 42, 904–914. [Google Scholar] [CrossRef]
- Trojnar, E.; Erdelyi, K.; Matyas, C.; Zhao, S.; Paloczi, J.; Mukhopadhyay, P.; Varga, Z.V.; Hasko, G.; Pacher, P. Cannabinoid-2 receptor activation ameliorates hepatorenal syndrome. Free Radic. Biol. Med. 2019, 152, 540–550. [Google Scholar] [CrossRef]
- Ontko, C.; Kim, M.J.; Smith, T.E.; McCollum, G.W.; Yang, R.; Penn, J. Cannabinoid Receptor 2 Agonist HU-308 Demonstrates Therapeutic Potential in Inflammatory Diabetic Retinopathy Models. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3012. [Google Scholar]
- Zhao, Y.; Liu, Y.; Zhang, W.; Xue, J.; Wu, Y.Z.; Xu, W.; Liang, X.; Chen, T.; Kishimoto, C.; Yuan, Z. WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur. J. Pharmacol. 2010, 649, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Rossi, F.; Rossi, S.; D’Amico, M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: Involvement of cytokine/chemokines and PMN. J. Leukoc. Biol. 2004, 75, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-T.; Coussens, L.M. Humoral immunity, inflammation and cancer. Curr. Opin. Immunol. 2007, 19, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wolterink, R.G.J.K.; KleinJan, A.; van Nimwegen, M.; Bergen, I.; de Bruijn, M.; Levani, Y.; Hendriks, R.W. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur. J. Immunol. 2012, 42, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Christianson, C.; Irvin, C.; Gorska, M.; Alam, R. IL33 and Type 2 Innate Lymphoid Cells (ILC2) But Not Th2 Cells Are Essential For Persistence Of Chronic Experimental Asthma. J. Allergy Clin. Immunol. 2014, 133, AB170. [Google Scholar] [CrossRef]
- Mathew, A.; MacLean, J.A.; DeHaan, E.; Tager, A.M.; Green, F.H.Y.; Luster, A.D. Signal Transducer and Activator of Transcription 6 Controls Chemokine Production and T Helper Cell Type 2 Cell Trafficking in Allergic Pulmonary Inflammation. J. Exp. Med. 2001, 193, 1087–1096. [Google Scholar] [CrossRef]
- Herrick, C.A.; Bottomly, K. To respond or not to respond: T cells in allergic asthma. Nat. Rev. Immunol. 2003, 3, 405–412. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Shapiro, B.J.; Frank, I.M. Acute Effects of Smoked Marijuana and Oral Δ9-Tetrahydrocannabinol on Specific Airway Conductance in Asthmatic Subjects. Am. Rev. Respir. Dis. 1974, 109, 420–428. [Google Scholar] [CrossRef]
- Vachon, L.; FitzGerald, M.X.; Solliday, N.H.; Gould, I.A.; Gaensler, E.A. Single-Dose Effect of Marihuana Smoke. N. Engl. J. Med. 1973, 288, 985–989. [Google Scholar] [CrossRef]
- Jan, T.-R.; Farraj, A.K.; Harkema, J.R.; Kaminski, N.E. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol. Appl. Pharmacol. 2003, 188, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.A.; Klein, T.W. Cannabinoid 2 (CB2) Receptor Involvement in the Down-regulation but not Up-regulation of Serum IgE Levels in Immunized Mice. J. Neuroimmune Pharmacol. 2012, 7, 591–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zoerner, A.A.; Stichtenoth, D.O.; Engeli, S.; Batkai, S.; Winkler, C.; Schaumann, F.; Janke, J.; Holz, O.; Krug, N.; Tsikas, D.; et al. Allergen Challenge Increases Anandamide in Bronchoalveolar Fluid of Patients With Allergic Asthma. Clin. Pharmacol. Ther. 2011, 90, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, B.P.; Helou, D.G.; Shafiei-Jahani, P.; Howard, E.; Painter, J.D.; Quach, C.; Akbari, O. Cannabinoid receptor 2 engagement promotes group 2 innate lymphoid cell expansion and enhances airway hyperreactivity. J. Allergy Clin. Immunol. 2021, 149, 1628–1642.e10. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, Z.; Ferrini, M.; Roberts, K. Pulmonary NK cells prevent allergic airway sensitization: Regulation by CB2 cannabinoid receptors. J. Immunol. 2016, 196 (Suppl. S1), 192.6. [Google Scholar] [CrossRef]
- Jackson, A.R.; Nagarkatti, P.; Nagarkatti, M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS ONE 2014, 9, e93954. [Google Scholar] [CrossRef] [PubMed]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur. J. Immunol. 2016, 46, 1472–1479. [Google Scholar] [CrossRef]
- Newton, C.A.; Klein, T.W.; Friedman, H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect. Immun. 1994, 62, 4015–4020. [Google Scholar] [CrossRef]
- Rzeczycki, P.; Rasner, C.; Lammlin, L.; Junginger, L.; Goldman, S.; Bergman, R.; Redding, S.; Knights, A.J.; Elliott, M.; Maerz, T. Cannabinoid receptor type 2 is upregulated in synovium following joint injury and mediates anti-inflammatory effects in synovial fibroblasts and macrophages. Osteoarthr. Cartil. 2021, 29, 1720–1731. [Google Scholar] [CrossRef]
- Agudelo, M.; Newton, C.; Widen, R.; Sherwood, T.; Nong, L.; Friedman, H.; Klein, T.W. Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J. Neuroimmune Pharmacol. 2008, 3, 35–42. [Google Scholar] [CrossRef]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-κB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 2004, 173, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004, 18, 1914–1916. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Milcarek, C.A.; Xie, X.-Q. Antagonism of cannabinoid receptor 2 pathway suppresses IL-6-induced immunoglobulin IgM secretion. BMC Pharmacol. Toxicol. 2014, 15, 30. [Google Scholar] [CrossRef]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.; Tashkin, D.P.; Roth, M.D. Δ9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, P.; Cioni, C.; Mugnaini, C.; Corelli, F. Potent immunomodulatory activity of a highly selective cannabinoid CB2 agonist on immune cells from healthy subjects and patients with multiple sclerosis. J. Neuroimmunol. 2017, 303, 66–74. [Google Scholar] [CrossRef]
- Mugnaini, C.; Rabbito, A.; Brizzi, A.; Palombi, N.; Petrosino, S.; Verde, R.; Di Marzo, V.; Ligresti, A.; Corelli, F. Synthesis of novel 2-(1-adamantanylcarboxamido) thiophene derivatives. Selective cannabinoid type 2 (CB2) receptor agonists as potential agents for the treatment of skin inflammatory disease. Eur. J. Med. Chem. 2019, 161, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, T.; Price, J.; Albanese, M.; Bullman, J.; Guillard, F.; Meyer, I.; Leeson, R.; Costantin, C.; Ziviani, L.; Nocini, P.F. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin. J. Pain 2011, 27, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Werth, V.P.; Hejazi, E.; Pena, S.M.; Haber, J.; Zeidi, M.; Reddy, N.; Okawa, J.; Feng, R.; Bashir, M.M.; Gebre, K. Safety and efficacy of lenabasum, a cannabinoid receptor type 2 agonist, in patients with dermatomyositis with refractory skin disease: A randomized clinical trial. J. Investig. Dermatol. 2022, 142, 2651–2659.e1. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.F.; Flume, P.; Downey, D.G.; Dozor, A.J.; Colombo, C.; Mazurek, H.; Sapiejka, E.; Rachel, M.; Constantine, S.; Conley, B. Safety and efficacy of lenabasum in a phase 2 randomized, placebo-controlled trial in adults with cystic fibrosis. J. Cyst. Fibros. 2021, 20, 78–85. [Google Scholar] [CrossRef]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R. Safety and efficacy of lenabasum in a phase II, randomized, placebo—Controlled trial in adults with systemic sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef]
- Mackay, M.; Zurier, R.; Kamen, D.; Koumpouras, F.; Askanase, A.; Kalunian, K.; Schulz, S.; Franchin, G.; Olsen, N.; Coca, A. A Phase 2, Double-blind, Randomized, Placebo-Controlled Multicenter Study to Evaluate Efficacy, Safety, and Tolerability of JBT-101/Lenabasum in Systemic Lupus Erythematosus, an Autoimmunity Centers of Excellence Study (ALE09). In ARTHRITIS & RHEUMATOLOGY; WILEY 111 RIVER ST: Hoboken, NJ, USA, 2022; pp. 735–738. [Google Scholar]
- Spiera, R.; Khanna, D.; Kuwana, M.; Furst, D.E.; Frech, T.M.; Hummers, L.; Stevens, W.; Matucci-Cerinic, M.; Baron, M.; Distler, O. A randomised, double-blind, placebo-controlled phase 3 study of lenabasum in diffuse cutaneous systemic sclerosis: RESOLVE-1 design and rationale. Clin. Exp. Rheumatol. 2021, 39, S124–S133. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.; Kuwana, M.; Khanna, D.; Hummers, L.; Frech, T.M.; Stevens, W.; Matucci-Cerinic, M.; Kafaja, S.; Distler, O.; Jun, J.B. Efficacy and safety of lenabasum, a cannabinoid type 2 receptor agonist, in a phase 3 randomized trial in diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2023, 75, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Garcia-Caraballo, S.; Maddern, J.; Schober, G.; Lumsden, A.; Harrington, A.; Schmiel, S.; Lindstrom, B.; Adams, J.; Brierley, S.M. Olorinab (APD371), a peripherally acting, highly selective, full agonist of the cannabinoid receptor 2, reduces colitis-induced acute and chronic visceral hypersensitivity in rodents. Pain 2022, 163, e72. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Cash, B.D.; Lembo, A.; Kunkel, D.C.; English, B.A.; Lindstrom, B.; Gu, G.; Skare, S.; Gilder, K.; Turner, S. Efficacy and safety of olorinab, a full agonist of the cannabinoid receptor 2, for the treatment of abdominal pain in patients with irritable bowel syndrome: Results from a phase 2b randomized placebo-controlled trial (CAPTIVATE). Neurogastroenterol. Motil. 2023, 35, e14539. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Chappell, A.; Dethy, J.; Hoeck, H.; Arendt-Nielsen, L.; Verfaille, S.; Boulanger, B.; Jullion, A.; Johnson, M.; McNearney, T. A proof-of-concept (POC) study including experimental pain models (EPMs) to assess the effects of a CB2 agonist (LY2828360) in the treatment of patients with osteoarthritic (OA) knee pain. Clin. Pharmacol. Ther. 2013, 93, S56–S57, No. PII-11. [Google Scholar]
- Kalliomäki, J.; Annas, P.; Huizar, K.; Clarke, C.; Zettergren, A.; Karlsten, R.; Segerdahl, M. Evaluation of the analgesic efficacy and psychoactive effects of AZD 1940, a novel peripherally acting cannabinoid agonist, in human capsaicin—Induced pain and hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2013, 40, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Elzein, E.; Yao, L. 960 TT-816, a novel small molecule immune checkpoint inhibitor targeting cannabinoid CB2 receptor, stimulates innate and adaptive immunity for cancer therapy. BMJ Spec. J. 2022, 10. [Google Scholar] [CrossRef]
- Issazadeh, S.; Mustafa, M.; Ljungdahl, Å.; Höjeberg, B.; Dagerlind, Å.; Elde, R.; Olsson, T. Interferon γ, interleukin 4 and transforming growth factor β in experimental autoimmune encephalomyelitis in Lewis rats: Dynamics of cellular mRNA expression in the central nervous system and lymphoid cells. J. Neurosci. Res. 1995, 40, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Morales, P.; Rodríguez-Cueto, C.; Fernández-Ruiz, J.; Jagerovic, N.; Franco, R. Targeting Cannabinoid CB2 Receptors in the Central Nervous System. Medicinal Chemistry Approaches with Focus on Neurodegenerative Disorders. Front. Neurosci. 2016, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’s Disease Brains. J. Neurosci. 2003, 23, 11136. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Obregon, D.; Mori, T.; Hou, H.; Sun, N.; Bai, Y.; Klein, T.; Fernandez, F.; Tan, J.; Shytle, R.D. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflamm. 2005, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, B.G.; Blázquez, C.; del Pulgar, T.G.; Guzmán, M.; de Ceballos, M.L. Prevention of Alzheimer’s Disease Pathology by Cannabinoids: Neuroprotection Mediated by Blockade of Microglial Activation. J. Neurosci. 2005, 25, 1904. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moreno, A.M.; Brera, B.; Spuch, C.; Carro, E.; García-García, L.; Delgado, M.; Pozo, M.A.; Innamorato, N.G.; Cuadrado, A.; de Ceballos, M.L. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflamm. 2012, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-M.; Astarita, G.; Yasar, S.; Vasilevko, V.; Cribbs, D.H.; Head, E.; Cotman, C.W.; Piomelli, D. An amyloid β42-dependent deficit in anandamide mobilization is associated with cognitive dysfunction in Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Ventriglia, M.; Martini, M.G.; Montesano, D.; Errante, Y.; Piscitelli, F.; Scrascia, F.; Quattrocchi, C.; Palazzo, P.; Seccia, S.; et al. Elevation of Plasma 2-arachidonoylglycerol Levels in Alzheimer’s Disease Patients as a Potential Protective Mechanism against Neurodegenerative Decline. J. Alzheimer’s Dis. 2015, 46, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Pihlaja, R.; Takkinen, J.; Eskola, O.; Vasara, J.; López-Picón, F.R.; Haaparanta-Solin, M.; Rinne, J.O. Monoacylglycerol lipase inhibitor JZL184 reduces neuroinflammatory response in APdE9 mice and in adult mouse glial cells. J. Neuroinflamm. 2015, 12, 81. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Wu, Y.; Wang, D.; Feng, G.; Tang, Y.-P.; Teng, Z.; Chen, C. Monoacylglycerol Lipase Is a Therapeutic Target for Alzheimer’s Disease. Cell Rep. 2012, 2, 1329–1339. [Google Scholar] [CrossRef]
- Aguilera-Portillo, G.; Rangel-López, E.; Villeda-Hernández, J.; Chavarría, A.; Castellanos, P.; Elmazoglu, Z.; Karasu, Ç.; Túnez, I.; Pedraza, G.; Königsberg, M. The pharmacological inhibition of fatty acid amide hydrolase prevents excitotoxic damage in the rat striatum: Possible involvement of CB1 receptors regulation. Mol. Neurobiol. 2019, 56, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Scipioni, L.; Arosio, B.; Mari, D.; Oddi, S.; Maccarrone, M. Anti-inflammatory effects of fatty acid amide hydrolase inhibition in monocytes/macrophages from alzheimer’s disease patients. Biomolecules 2021, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, P.; Liu, J.; Jiang, H.; Fan, X.; Chen, G.; Zhou, C. Elevation of arachidonoylethanolamide levels by activation of the endocannabinoid system protects against colitis and ameliorates remote organ lesions in mice. Exp. Ther. Med. 2017, 14, 5664–5670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Casarejos, M.J.; Perucho, J.; Gomez, A.; Muñoz, M.P.; Fernandez-Estevez, M.; Sagredo, O.; Fernandez Ruiz, J.; Guzman, M.; de Yebenes, J.G.; Mena, M.A. Natural Cannabinoids Improve Dopamine Neurotransmission and Tau and Amyloid Pathology in a Mouse Model of Tauopathy. J. Alzheimer’s Dis. 2013, 35, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower–Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794.e1784. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.L.; Lana, R.; Taxonera, C.; Alba, C.; Izquierdo, S.; Díaz-Rubio, M. Extraintestinal manifestations in inflammatory bowel disease: Differences between Crohn’s disease and ulcerative colitis. Med. Clin. 2005, 125, 297–300. [Google Scholar]
- Schmitz, H.; Barmeyer, C.; Fromm, M.; Runkel, N.; Foss, H.-D.; Bentzel, C.J.; Riecken, E.-O.; Schulzke, J.-D. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116, 301–309. [Google Scholar] [CrossRef]
- Sartor, R.B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn’s disease. Gastroenterol. Clin. N. Am. 1995, 24, 475–507. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Xavier, R.; Podolsky, D. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Storr, M.; Devlin, S.; Kaplan, G.G.; Panaccione, R.; Andrews, C.N. Cannabis Use Provides Symptom Relief in Patients with Inflammatory Bowel Disease but Is Associated with Worse Disease Prognosis in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Mbachi, C.; Attar, B.; Wang, Y.; Paintsil, I.; Mba, B.; Fugar, S.; Agrawal, R.; Simons-Linares, R.C.; Jaiswal, P.; Trick, W.; et al. Association Between Cannabis Use and Complications Related to Crohn’s Disease: A Retrospective Cohort Study. Dig. Dis. Sci. 2019, 64, 2939–2944. [Google Scholar] [CrossRef]
- Tartakover Matalon, S.; Ringel, Y.; Konikoff, F.; Drucker, L.; Pery, S.; Naftali, T. Cannabinoid receptor 2 agonist promotes parameters implicated in mucosal healing in patients with inflammatory bowel disease. United Eur. Gastroenterol. J. 2020, 8, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Baik, D.; Schey, R. The role of cannabinoids in regulation of nausea and vomiting, and visceral pain. Curr. Gastroenterol. Rep. 2015, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Marquéz, L.; Suárez, J.; Iglesias, M.; Bermudez-Silva, F.J.; de Fonseca, F.R.; Andreu, M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS ONE 2009, 4, e6893. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Bellini, G.; Miele, E.; Martinelli, M.; Cenni, S.; Tortora, C.; Tolone, C.; Miraglia del Giudice, E.; Rossi, F. Cannabinoid Receptor 2 Functional Variant Contributes to the Risk for Pediatric Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2018, 52, e37–e43. [Google Scholar] [CrossRef] [PubMed]
- D’argenio, G.; Valenti, M.; Scaglione, G.; Cosenza, V.; Sorrentini, I.; Di Marzo, V. Up—Regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006, 20, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 9, 2358. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef]
- Gentili, M.; Ronchetti, S.; Ricci, E.; Di Paola, R.; Gugliandolo, E.; Cuzzocrea, S.; Bereshchenko, O.; Migliorati, G.; Riccardi, C. Selective CB2 inverse agonist JTE907 drives T cell differentiation towards a Treg cell phenotype and ameliorates inflammation in a mouse model of inflammatory bowel disease. Pharmacol. Res. 2019, 141, 21–31. [Google Scholar] [CrossRef]
- Shamran, H.; Singh, N.P.; Zumbrun, E.E.; Murphy, A.; Taub, D.D.; Mishra, M.K.; Price, R.L.; Chatterjee, S.; Nagarkatti, M.; Nagarkatti, P.S.; et al. Fatty acid amide hydrolase (FAAH) blockade ameliorates experimental colitis by altering microRNA expression and suppressing inflammation. Brain Behav. Immun. 2017, 59, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Sałaga, M.; Mokrowiecka, A.; Zakrzewski, P.K.; Cygankiewicz, A.; Leishman, E.; Sobczak, M.; Zatorski, H.; Małecka-Panas, E.; Kordek, R.; Storr, M.; et al. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH). J. Crohn’s Colitis 2014, 8, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.A.; Keenan, C.M.; Emmerdinger, D.; Zhang, H.; Yüce, B.; Sibaev, A.; Massa, F.; Buckley, N.E.; Lutz, B.; Göke, B. Targeting endocannabinoid degradation protects against experimental colitis in mice: Involvement of CB 1 and CB 2 receptors. J. Mol. Med. 2008, 86, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011, 25, 2711–2721. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2017, 27, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Adejumo, A.C.; Adegbala, O.M.; Adejumo, K.L.; Bukong, T.N. Reduced incidence and better liver disease outcomes among chronic HCV infected patients who consume cannabis. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9430953. [Google Scholar] [CrossRef] [PubMed]
- Achebe, I.; Mbachi, C.; Wang, Y.; Ezekiel, C.; Paintsil, I.; Attar, B. S1148 Effect of Cannabis Use on Progression of Non-Alcoholic Fatty Liver Disease in Obese Patients: A Propensity-Matched Retrospective Cohort Study. Off. J. Am. Coll. Gastroenterol.|ACG 2020, 115, S574–S575. [Google Scholar] [CrossRef]
- Auguet, T.; Berlanga, A.; Guiu-Jurado, E.; Terra, X.; Martinez, S.; Aguilar, C.; Filiu, E.; Alibalic, A.; Sabench, F.; Hernández, M. Endocannabinoid receptors gene expression in morbidly obese women with nonalcoholic fatty liver disease. BioMed Res. Int. 2014, 2014, 502542. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Sanchez, N.; Zamora-Valdes, D.; Pichardo-Bahena, R.; Barredo-Prieto, B.; Ponciano-Rodriguez, G.; Bermejo-Martínez, L.; Chavez-Tapia, N.C.; Baptista-González, H.A.; Uribe, M. Endocannabinoid receptor CB2 in nonalcoholic fatty liver disease. Liver Int. 2007, 27, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer cells in the pathogenesis of liver disease. World J. Gastroenterol. WJG 2006, 12, 7413. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Metlakunta, A.; Dedousis, N.; Zhang, P.; Sipula, I.; Dube, J.J.; Scott, D.K.; O’Doherty, R.M. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010, 59, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Benkdane, M.; Teixeira-Clerc, F.; Bonnafous, S.; Louvet, A.; Lafdil, F.; Pecker, F.; Tran, A.; Gual, P.; Mallat, A. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014, 59, 130–142. [Google Scholar] [CrossRef]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Muñoz-Luque, J.; Ros, J.; Fernández-Varo, G.; Tugues, S.; Morales-Ruiz, M.; Alvarez, C.E.; Friedman, S.L.; Arroyo, V.; Jiménez, W. Regression of Fibrosis after Chronic Stimulation of Cannabinoid CB2 Receptor in Cirrhotic Rats. J. Pharmacol. Exp. Ther. 2008, 324, 475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).