Bioactive Compounds from Medicinal Plants as Potential Adjuvants in the Treatment of Mild Acne Vulgaris
Abstract
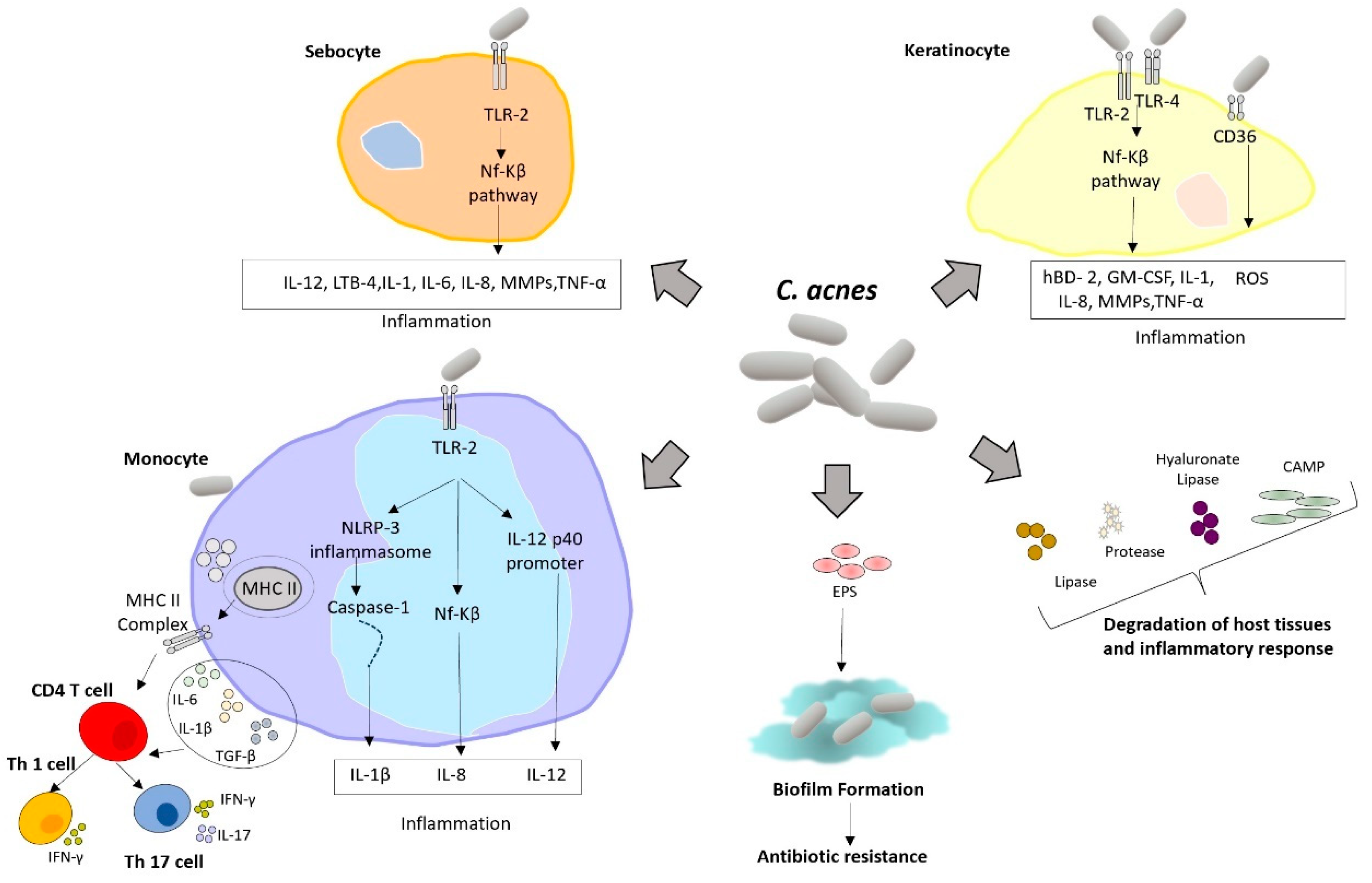
1. Introduction
2. Methodology
Search Strategy and Inclusion and Exclusion Criteria
- The search strategy used for PubMed functioned as an indication for the search strategies in other databases;
- The research included the term “acne vulgaris” combined with the terms “plants”, “extracts”, “clinical trial” using boolean operator tools AND, OR, NOT; studies did not include clinical trials;
- Overall, 89 articles were found as a result of the search;
- A total of 35 studies were considered relevant by us and therefore included in this review;
- In these 35 selected studies, the efficacy of herbal medicine in the treatment of AV was evaluated by considering in vitro and ex vivo experiments.
3. Families and Study
3.1. Lamiaceae
3.2. Anacardiaceae
3.3. Cannabaceae
3.4. Poaceae
3.5. Rosaceae
3.6. Asteraceae
3.7. Caprifoliaceae
3.8. Cistaceae
3.9. Fagaceae
3.10. Hamamelidaceae
3.11. Lauraceae
3.12. Meliaceae
3.13. Musaceae
3.14. Papaveraceae
3.15. Rubiaceae
3.16. Salicaceae
3.17. Sapindaceae
3.18. Smilacaceae
3.19. Zingiberaceae
4. Miscellaneous
5. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutaria, A.H.; Masood, S.; Saleh, H.M.; Schlessinger, J. Acne Vulgaris. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Dabash, D.; Salahat, H.; Awawdeh, S.; Hamadani, F.; Khraim, H.; Koni, A.A.; Zyoud, S.H. Prevalence of Acne and Its Impact on Quality of Life and Practices Regarding Self-Treatment among Medical Students. Sci. Rep. 2024, 14, 4351. [Google Scholar] [CrossRef]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From Pathogenesis of Acne Vulgaris to Anti-Acne Agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Proença, A.C.; Luís, Â.; Duarte, A.P. The Role of Herbal Medicine in the Treatment of Acne Vulgaris: A Systematic Review of Clinical Trials. Evid.-Based Complement. Altern. Med. 2022, 2022, 2011945. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Stein Gold, L.F.; Tan, J.K.L.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef]
- Cao, H.; Yang, G.; Wang, Y.; Liu, J.P.; Smith, C.A.; Luo, H.; Liu, Y. Complementary Therapies for Acne Vulgaris. Cochrane Database Syst. Rev. 2015, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.-T.; Tsai, T.-H.; Lien, T.-J.; Huang, W.-C.; Liu, J.-J.; Chang, H.; Chang, M.-L.; Tsai, P.-J. Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules 2018, 23, 1987. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.; Abdeltawab, N.; Shamma, R.; Abdelgayed, S.; Mohamed, S.; Farag, M.; Ramadan, M. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef]
- Abdelhamed, F.M.; Abdeltawab, N.F.; ElRakaiby, M.T.; Shamma, R.N.; Moneib, N.A. Antibacterial and Anti-Inflammatory Activities of Thymus vulgaris Essential Oil Nanoemulsion on Acne Vulgaris. Microorganisms 2022, 10, 1874. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Ferraz, C.; Coelho, S.; Pastorinho, M.R.; Sousa, A.C.; et al. Chemical Characterization and Bioactive Potential of Thymus × citriodorus (Pers.) Schreb. Preparations for Anti-Acne Applications: Antimicrobial, Anti-Biofilm, Anti-Inflammatory and Safety Profiles. J. Ethnopharmacol. 2022, 287, 114935. [Google Scholar] [CrossRef]
- Pineau, R.M.; Hanson, S.E.; Lyles, J.T.; Quave, C.L. Growth Inhibitory Activity of Callicarpa americana Leaf Extracts against Cutibacterium acnes. Front. Pharmacol. 2019, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, I.A.; Thipe, V.C.; Katti, K.V.; Mandiwana, V.; Kalombo, M.L.; Ray, S.S.; Rikhotso, R.; Janse Van Vuuren, A.; Esmear, T.; Lall, N. Targeting Acne Bacteria and Wound Healing In Vitro Using Plectranthus aliciae, Rosmarinic Acid, and Tetracycline Gold Nanoparticles. Pharmaceuticals 2022, 15, 933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Mao, Y.; Guo, M.; Yu, H.; Hao, L.; Hua, Q.; Lu, Z.; Hong, M.; An, F. Enhancement of Anti-Acne Effect of Scutellaria baicalensis Extract by Fermentation with Symbiotic Fungus Penicillium decumbens. J. Biosci. Bioeng. 2020, 130, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Poomanee, W.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Khunkitti, W.; Leelapornpisid, P. In-Vitro Investigation of Anti-Acne Properties of Mangifera indica L. Kernel Extract and Its Mechanism of Action against Propionibacterium acnes. Anaerobe 2018, 52, 64–74. [Google Scholar] [CrossRef]
- Tollenaere, M.D.; Boira, C.; Chapuis, E.; Lapierre, L.; Jarrin, C.; Robe, P.; Zanchetta, C.; Vilanova, D.; Sennelier-Portet, B.; Martinez, J.; et al. Action of Mangifera indica Leaf Extract on Acne-Prone Skin through Sebum Harmonization and Targeting C. acnes. Molecules 2022, 27, 4769. [Google Scholar] [CrossRef]
- Cefali, L.C.; Vazquez, C.; Ataide, J.A.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Foglio, M.A.; Lancellotti, M.; Mazzola, P.G. In Vitro Activity and Formulation of a Flavonoid-Containing Cashew Pulp Extract for the Topical Treatment of Acne and the Protection of Skin against Premature Aging. Nat. Prod. Res. 2021, 35, 5243–5249. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.-Y. The Ameliorative Effect of Hemp Seed Hexane Extracts on the Propionibacterium acnes-Induced Inflammation and Lipogenesis in Sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.-W.; Frank, U.; Schempp, C.M.; Wölfle, U. Hop Extract Acts as an Antioxidant with Antimicrobial Effects against Propionibacterium acnes and Staphylococcus aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef]
- Kim, C.; Park, J.; Lee, H.; Hwang, D.-Y.; Park, S.H.; Lee, H. Evaluation of the EtOAc Extract of Lemongrass (Cymbopogon citratus) as a Potential Skincare Cosmetic Material for Acne Vulgaris. J. Microbiol. Biotechnol. 2022, 32, 594–601. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Study of Biosurfactant Extract from Corn Steep Water as a Potential Ingredient in Antiacne Formulations. J. Dermatol. Treat. 2022, 33, 393–400. [Google Scholar] [CrossRef]
- Krzemińska, B.; Dybowski, M.P.; Klimek, K.; Typek, R.; Miazga-Karska, M.; Dos Santos Szewczyk, K. The Anti-Acne Potential and Chemical Composition of Two Cultivated Cotoneaster Species. Cells 2022, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, B.; Dybowski, M.P.; Klimek, K.; Typek, R.; Miazga-Karska, M.; Ginalska, G.; Dos Santos Szewczyk, K. Can Extracts from the Leaves and Fruits of the Cotoneaster Species Be Considered Promising Anti-Acne Agents? Molecules 2022, 27, 2907. [Google Scholar] [CrossRef] [PubMed]
- Miazga-Karska, M.; Michalak, K.; Ginalska, G. Anti-Acne Action of Peptides Isolated from Burdock Root—Preliminary Studies and Pilot Testing. Molecules 2020, 25, 2027. [Google Scholar] [CrossRef] [PubMed]
- Chrząszcz, M.; Miazga-Karska, M.; Klimek, K.; Granica, S.; Tchórzewska, D.; Ginalska, G.; Szewczyk, K. Extracts from Cephalaria uralensis (Murray) Roem. & Schult. and Cephalaria gigantea (Ledeb.) Bobrov as Potential Agents for Treatment of Acne Vulgaris: Chemical Characterization and In Vitro Biological Evaluation. Antioxidants 2020, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Bouabidi, M.; Salamone, F.L.; Gadhi, C.; Bouamama, H.; Speciale, A.; Ginestra, G.; Pulvirenti, L.; Siracusa, L.; Nostro, A.; Cristani, M. Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules 2023, 28, 2797. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yin, J.; Hwang, I.H.; Park, D.H.; Lee, E.K.; Kim, M.J.; Lee, M.W. Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition. Molecules 2020, 25, 2154. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Vrhovsek, U.; Masuero, D.; Fumagalli, M.; Magnavacca, A.; Pozzoli, C.; Canilli, L.; Terno, M.; Angarano, M.; et al. Anti-Inflammatory and Anti-Acne Effects of Hamamelis virginiana Bark in Human Keratinocytes. Antioxidants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Wu, H.-C.; Hwang, T.-L.; Lin, C.-H.; Cheng, Y.-H.; Wang, C.-C.; Kan, H.-L.; Kuo, Y.-H.; Chen, I.-S.; Chang, H.-S.; et al. Anti-Inflammatory and Antibacterial Activity Constituents from the Stem of Cinnamomum validinerve. Molecules 2020, 25, 3382. [Google Scholar] [CrossRef]
- Kola-Mustapha, A.T.; Raji, M.A.; Adedeji, O.; Ambrose, G.O. Network Pharmacology and Molecular Modeling to Elucidate the Potential Mechanism of Neem Oil against Acne vulgaris. Molecules 2023, 28, 2849. [Google Scholar] [CrossRef]
- Savitri, D.; Wahyuni, S.; Bukhari, A.; Djawad, K.; Hatta, M.; Riyanto, P.; Bahar, B.; Wahab, S.; Hamid, F.; Rifai, Y. Anti-Inflammatory Effects of Banana (Musa balbisiana) Peel Extract on Acne Vulgaris: In Vivo and in Silico Study. J. Taibah Univ. Med. Sci. 2023, 18, 1586–1598. [Google Scholar] [CrossRef]
- Xie, M.; Pu, Z.; Gao, L.; Yuan, R.; Dongzhi, Z.; Dikye, T.; Huang, S.; Li, B. Antibacterial Activity and Underlying Mechanism of Meconopsis Quintuplinervia Regel Extract against the Acne-Causing Bacteria Propionibacterium Acnes and Staphylococcus Aureus. Pak. J. Pharm. Sci. 2023, 36, 71–80. [Google Scholar] [PubMed]
- Seo, G.; Kim, K. Exploring the Mechanism of Action of Hedyotis diffusa Willd on Acne Using Network Analysis. Medicine 2023, 102, e33323. [Google Scholar] [CrossRef] [PubMed]
- Bassino, E.; Gasparri, F.; Munaron, L. Pleiotropic Effects of White Willow Bark and 1,2-Decanediol on Human Adult Keratinocytes. Ski. Pharmacol. Physiol. 2018, 31, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Qiu, J.; Li, L.; Xie, Y.; Yu, H.; Guo, Y.; Yao, W. Saponin Fraction from Sapindus mukorossi Gaertn as a Novel Cosmetic Additive: Extraction, Biological Evaluation, Analysis of Anti-Acne Mechanism and Toxicity Prediction. J. Ethnopharmacol. 2021, 268, 113552. [Google Scholar] [CrossRef]
- Joo, J.-H.; Han, M.-H.; Kim, J.-I.; Kim, J.-E.; Jung, K.-H.; Oh, H.S.; Chung, Y.S.; An, H.J.; Lee, J.D.; Moon, G.-S.; et al. Antimicrobial Activity of Smilax china L. Root Extracts against the Acne-Causing Bacterium, Cutibacterium acnes, and Its Active Compounds. Molecules 2022, 27, 8331. [Google Scholar] [CrossRef]
- Sitthichai, P.; Chanpirom, S.; Maneerat, T.; Charoensup, R.; Tree-Udom, T.; Pintathong, P.; Laphookhieo, S.; Sripisut, T. Kaempferia parviflora Rhizome Extract as Potential Anti-Acne Ingredient. Molecules 2022, 27, 4401. [Google Scholar] [CrossRef]
- Kılıç, S.; Okullu, S.Ö.; Kurt, Ö.; Sevinç, H.; Dündar, C.; Altınordu, F.; Türkoğlu, M. Efficacy of Two Plant Extracts against Acne Vulgaris: Initial Results of Microbiological Tests and Cell Culture Studies. J. Cosmet. Dermatol. 2019, 18, 1061–1065. [Google Scholar] [CrossRef]
- Cohen, G.; Jakus, J.; Baroud, S.; Gvirtz, R.; Rozenblat, S. Development of an Effective Acne Treatment Based on CBD and Herbal Extracts: Preliminary In Vitro, Ex Vivo, and Clinical Evaluation. Evid.-Based Complement. Altern. Med. 2023, 2023, 4474255. [Google Scholar] [CrossRef]
- Mias, C.; Chansard, N.; Maitre, M.; Galliano, M.F.; Garidou, L.; Mengeaud, V.; Bessou-Touya, S.; Duplan, H. Myrtus Communis and Celastrol Enriched Plant Cell Culture Extracts Control Together the Pivotal Role of Cutibacterium Acnes and Inflammatory Pathways in Acne. Acad. Dermatol. Venereol. 2023, 37, 12–19. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Ramos, L.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Thymus mastichina (L.) L. and Cistus ladanifer L. for Skin Application: Chemical Characterization and In Vitro Bioactivity Assessment. J. Ethnopharmacol. 2023, 302, 115830. [Google Scholar] [CrossRef]
- Sonyot, W.; Lamlertthon, S.; Luangsa-ard, J.J.; Mongkolsamrit, S.; Usuwanthim, K.; Ingkaninan, K.; Waranuch, N.; Suphrom, N. In Vitro Antibacterial and Anti-Inflammatory Effects of Novel Insect Fungus Polycephalomyces phaothaiensis Extract and Its Constituents against Propionibacterium acnes. Antibiotics 2020, 9, 274. [Google Scholar] [CrossRef]
- Dreno, B.; Martin, R.; Moyal, D.; Henley, J.B.; Khammari, A.; Seité, S. Skin Microbiome and Acne Vulgaris: Staphylococcus, a New Actor in Acne. Exp. Dermatol. 2017, 26, 798–803. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G. New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef]
- Lambrechts, I.A.; Lall, N. Traditional Usage and Biological Activity of Plectranthus madagascariensis and Its Varieties: A Review. J. Ethnopharmacol. 2021, 269, 113663. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology and Toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Schulze-Kaysers, N.; Feuereisen, M.M.; Schieber, A. Phenolic Compounds in Edible Species of the Anacardiaceae Family—A Review. RSC Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Bhat, M.G.; Nagaraja, K.V.; Rupa, T.R. Cashew Research in India. J. Hortic. Sci. 2010, 5, 1–16. [Google Scholar] [CrossRef]
- Fu, X.; Liu, S.; Van Velzen, R.; Stull, G.W.; Tian, Q.; Li, Y.; Folk, R.A.; Guralnick, R.P.; Kates, H.R.; Jin, J.; et al. Phylogenomic Analysis of the Hemp Family (Cannabaceae) Reveals Deep Cyto-nuclear Discordance and Provides New Insights into Generic Relationships. J. Sytematics Evol. 2023, 61, 806–826. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In Vitro Evaluation of Antibacterial, Anticollagenase, and Antioxidant Activities of Hop Components (Humulus lupulus) Addressing Acne Vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef]
- Schmalreck, A.F.; Teuber, M.; Reininger, W.; Hartl, A. Structural Features Determining the Antibiotic Potencies of Natural and Synthetic Hop Bitter Resins, Their Precursors and Derivatives. Can. J. Microbiol. 1975, 21, 205–212. [Google Scholar] [CrossRef]
- Bhatt, P.; Thaker, V. A Comparative Study on 193 Plastomes of Poaceae for Validity and Implications of Individual Barcode Genes and Concatenated Protein Coding Sequences with Selected Plastomes of Grasses from the Desert of India. Meta Gene 2021, 29, 100921. [Google Scholar] [CrossRef]
- Ramabulana, T.; Ndlovu, M.; Mosa, R.A.; Sonopo, M.S.; Selepe, M.A. Phytochemical Profiling and Isolation of Bioactive Compounds from Leucosidea sericea (Rosaceae). ACS Omega 2022, 7, 11964–11972. [Google Scholar] [CrossRef]
- Kicel, A. An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, Biological Activity, and Toxicology. Antioxidants 2020, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Darqui, F.S.; Radonic, L.M.; Beracochea, V.C.; Hopp, H.E.; López Bilbao, M. Peculiarities of the Transformation of Asteraceae Family Species: The Cases of Sunflower and Lettuce. Front. Plant Sci. 2021, 12, 767459. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-X.; Liu, H.; Moore, M.J.; Landrein, S.; Liu, B.; Zhu, Z.-X.; Wang, H.-F. Plastid Phylogenomic Insights into the Evolution of the Caprifoliaceae s.l. (Dipsacales). Mol. Phylogenetics Evol. 2020, 142, 106641. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Lee, B.-S.; Yang, J.-H.; Park, K.I.; Cho, W.-K.; Ma, J.Y. Swertiajaponin Inhibits Skin Pigmentation by Dual Mechanisms to Suppress Tyrosinase. Oncotarget 2017, 8, 95530–95541. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Lytra, K.; Rallis, S.; Tzakos, A.G.; Skaltsa, H. An Updated Review of Genus Cistus L. since 2014: Traditional Uses, Phytochemistry, and Pharmacological Properties. Phytochem. Rev. 2022, 21, 2049–2087. [Google Scholar] [CrossRef]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid.-Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef]
- Shi, S.; Chang, H.T.; Chen, Y.; Qu, L.; Wen, J. Phylogeny of the Hamamelidaceae Based on the ITS Sequences of Nuclear Ribosomal DNA. Biochem. Syst. Ecol. 1998, 26, 55–69. [Google Scholar] [CrossRef]
- Damasceno, C.S.B.; Fabri Higaki, N.T.; Dias, J.D.F.G.; Miguel, M.D.; Miguel, O.G. Chemical Composition and Biological Activities of Essential Oils in the Family Lauraceae: A Systematic Review of the Literature. Planta Med. 2019, 85, 1054–1072. [Google Scholar] [CrossRef]
- Khoo, M.D.Y.; Tiong, N.J.L.; Li, T.; Lim, W.; Ng, D.J.J.; Nyanasengeran, M.; Yeo, D.C.J.; Cai, Y. The Freshwater Decapod Crustaceans of Bukit Timah Nature Reserve, Singapore. GBS 2019, 71, 575–581. [Google Scholar] [CrossRef]
- Inta, W.; Traiperm, P.; Ruchisansakun, S.; Janssens, S.B.; Viboonjun, U.; Swangpol, S.C. Evolution and Classification of Musaceae Based on Male Floral Morphology. Plants 2023, 12, 1602. [Google Scholar] [CrossRef]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Pentea, M.; Sarac, I.; Küşümler, A.S.; Özçelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver Plants: Current Insights on Phytochemical and Nutritional Composition Along with Biotechnological Applications. Oxidative Med. Cell. Longev. 2022, 2022, 1–23. [Google Scholar] [CrossRef]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Ruhsam, M.; Moat, J.; Brummitt, N.A. A Global Assessment of Distribution, Diversity, Endemism, and Taxonomic Effort in the Rubiaceae. Ann. Mo. Bot. Gard. 2009, 96, 68–78. [Google Scholar] [CrossRef]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef]
- Buerki, S.; Callmander, M.W.; Acevedo-Rodriguez, P.; Lowry, P.P.; Munzinger, J.; Bailey, P.; Maurin, O.; Brewer, G.E.; Epitawalage, N.; Baker, W.J.; et al. An Updated Infra-familial Classification of Sapindaceae Based on Targeted Enrichment Data. Am. J. Bot. 2021, 108, 1234–1251. [Google Scholar] [CrossRef]
- Qi, Z.; Cameron, K.M.; Li, P.; Zhao, Y.; Chen, S.; Chen, G.; Fu, C. Phylogenetics, Character Evolution, and Distribution Patterns of the Greenbriers, Smilacaceae (Liliales), a near-Cosmopolitan Family of Monocots: Phylogenetics of Smilacaceae. Bot. J. Linn. Soc. 2013, 173, 535–548. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Micale, N.; Molonia, M.S.; Citarella, A.; Cimino, F.; Saija, A.; Cristani, M.; Speciale, A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules 2021, 26, 4665. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Diversity and Traditional Utilization of the Zingiberaceae Plants in Nakhon Nayok Province, Central Thailand. Diversity 2023, 15, 904. [Google Scholar] [CrossRef]
- Alolga, R.N.; Wang, F.; Zhang, X.; Li, J.; Tran, L.-S.P.; Yin, X. Bioactive Compounds from the Zingiberaceae Family with Known Antioxidant Activities for Possible Therapeutic Uses. Antioxidants 2022, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Lopes, C.M.; Amaral, M.H. Treatment Advances for Acne Vulgaris: The Scientific Role of Cannabinoids. Cosmetics 2024, 11, 22. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic Metabolites as Therapeutic in Inflammation and Neoplasms: Molecular Pathways Explaining Their Efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef] [PubMed]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent Advances in Topical Delivery of Flavonoids: A Review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Ma, E.Z.; Khachemoune, A. Flavonoids and Their Therapeutic Applications in Skin Diseases. Arch. Dermatol. Res. 2022, 315, 321–331. [Google Scholar] [CrossRef]
- Bungau, A.F.; Radu, A.-F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Purza, A.L.; Endres, L.M. Emerging Insights into the Applicability of Essential Oils in the Management of Acne Vulgaris. Molecules 2023, 28, 6395. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Kernou, O.-N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of Rosmarinic Acid with Its Derivatives in the Treatment of Microbial Pathogens. Molecules 2023, 28, 4243. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in Membrane Fatty Acids Composition of Microbial Cells Induced by Addiction of Thymol, Carvacrol, Limonene, Cinnamaldehyde, and Eugenol in the Growing Media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhu, B.-R.; Tian, F.-L.; Hui, X.; Li, H.; Li, Y.-M.; Gao, W.-Y. Inhibitory Activity of Plant Essential Oils against E. Coli 1-Deoxy-d-Xylulose-5-Phosphate Reductoisomerase. Molecules 2019, 24, 2518. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid.-Based Complement. Altern. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular Mechanism of the Anti-Inflammatory Effects of Plant Essential Oils: A Systematic Review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef]
- Wijesekara, T.; Luo, J.; Xu, B. Critical Review on Anti-inflammation Effects of Saponins and Their Molecular Mechanisms. Phytother. Res. 2024, 38, 2007–2022. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Dréno, B.; Bettoli, V.; Bukvic Mokos, Z.; Degitz, K.; Dressler, C.; Finlay, A.Y.; Haedersdal, M.; Lambert, J.; Layton, A.; et al. European Evidence-based (S3) Guideline for the Treatment of Acne—Update 2016—Short Version. Acad Dermatol Venereol 2016, 30, 1261–1268. [Google Scholar] [CrossRef]
- Mohsin, N.; Hernandez, L.E.; Martin, M.R.; Does, A.V.; Nouri, K. Acne Treatment Review and Future Perspectives. Dermatol. Ther. 2022, 35, e15719. [Google Scholar] [CrossRef]
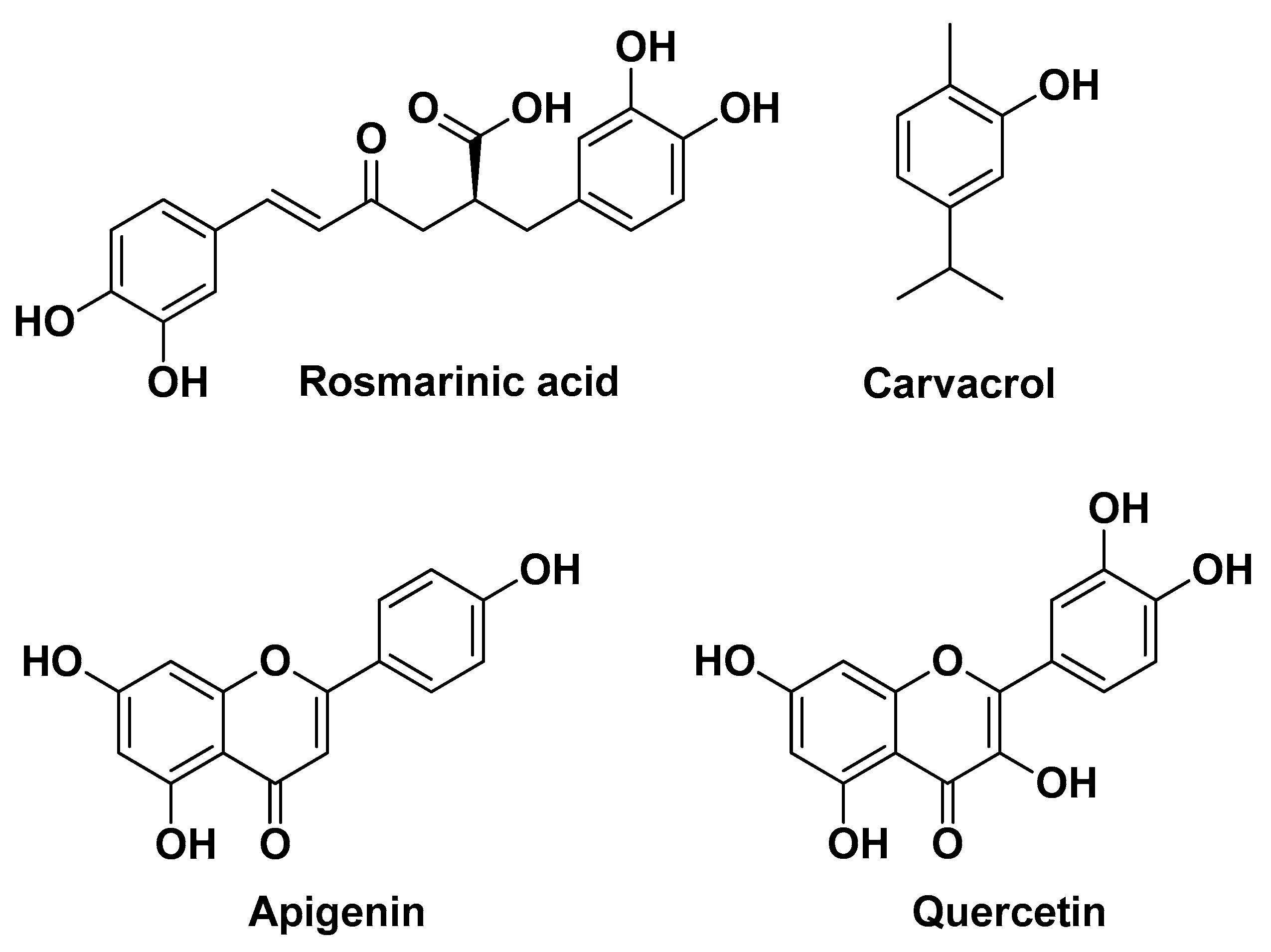
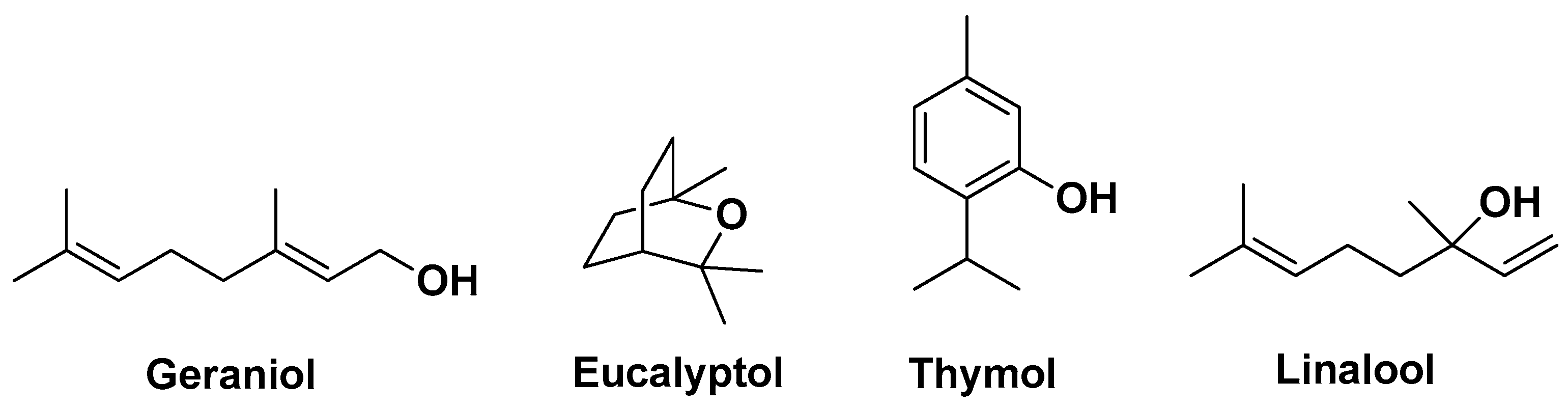
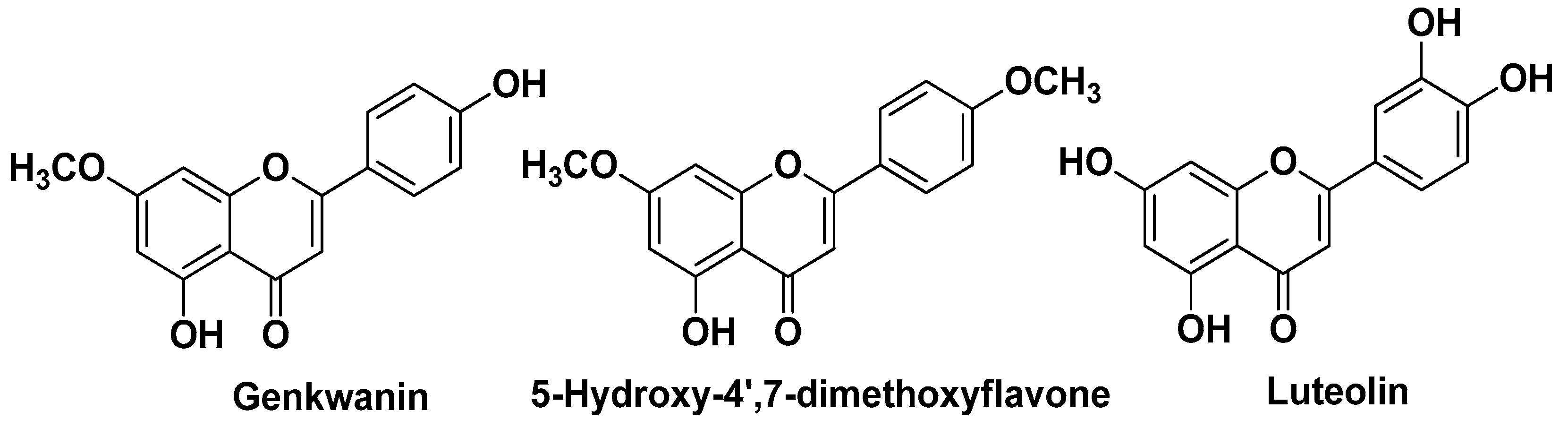
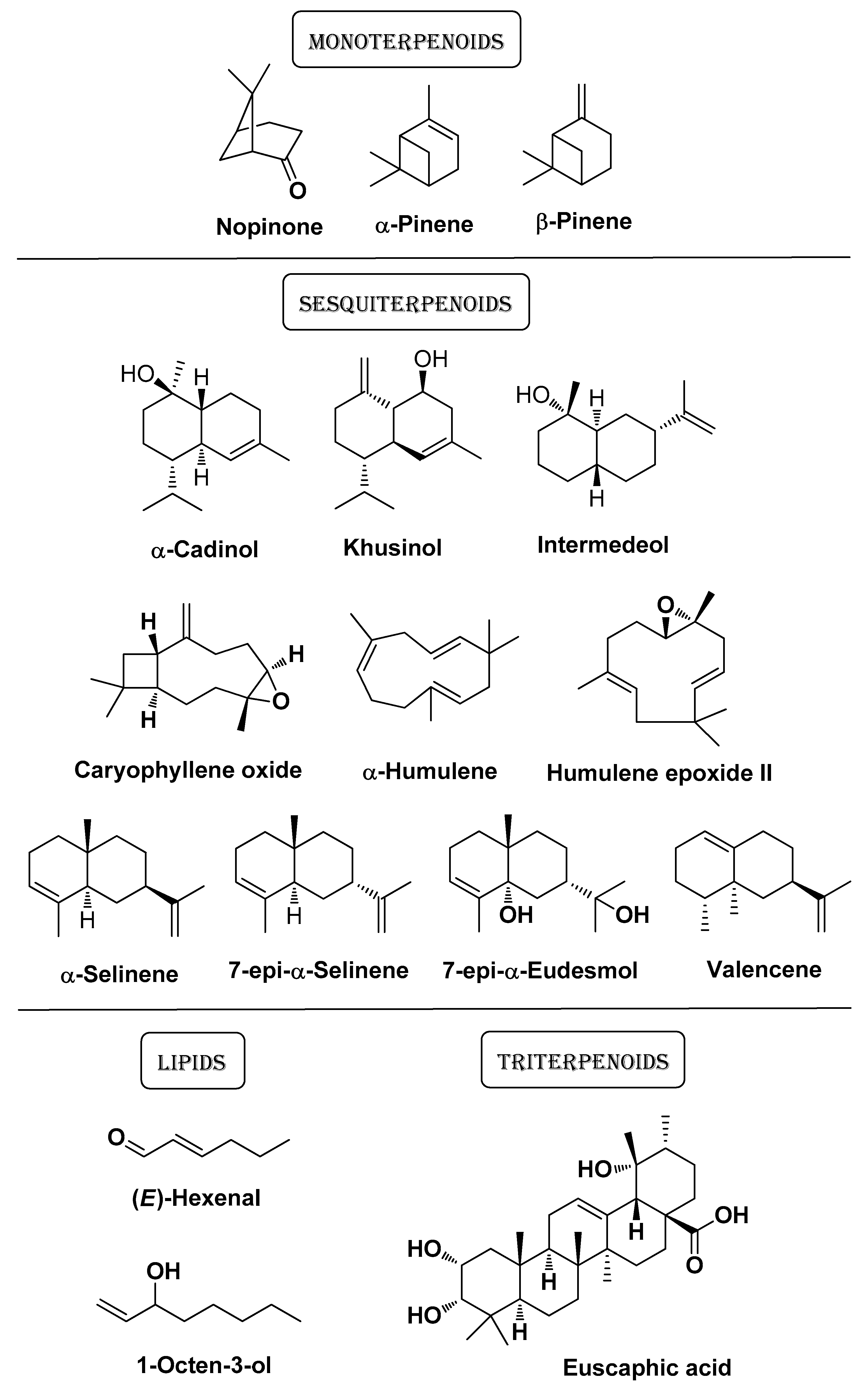
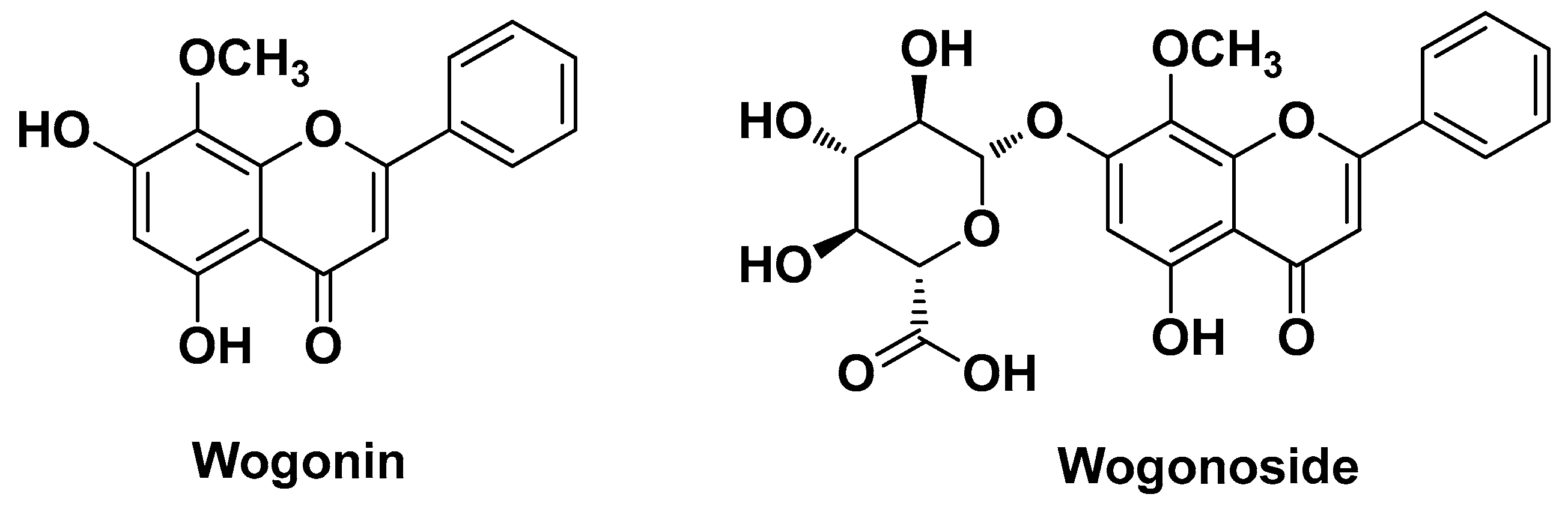
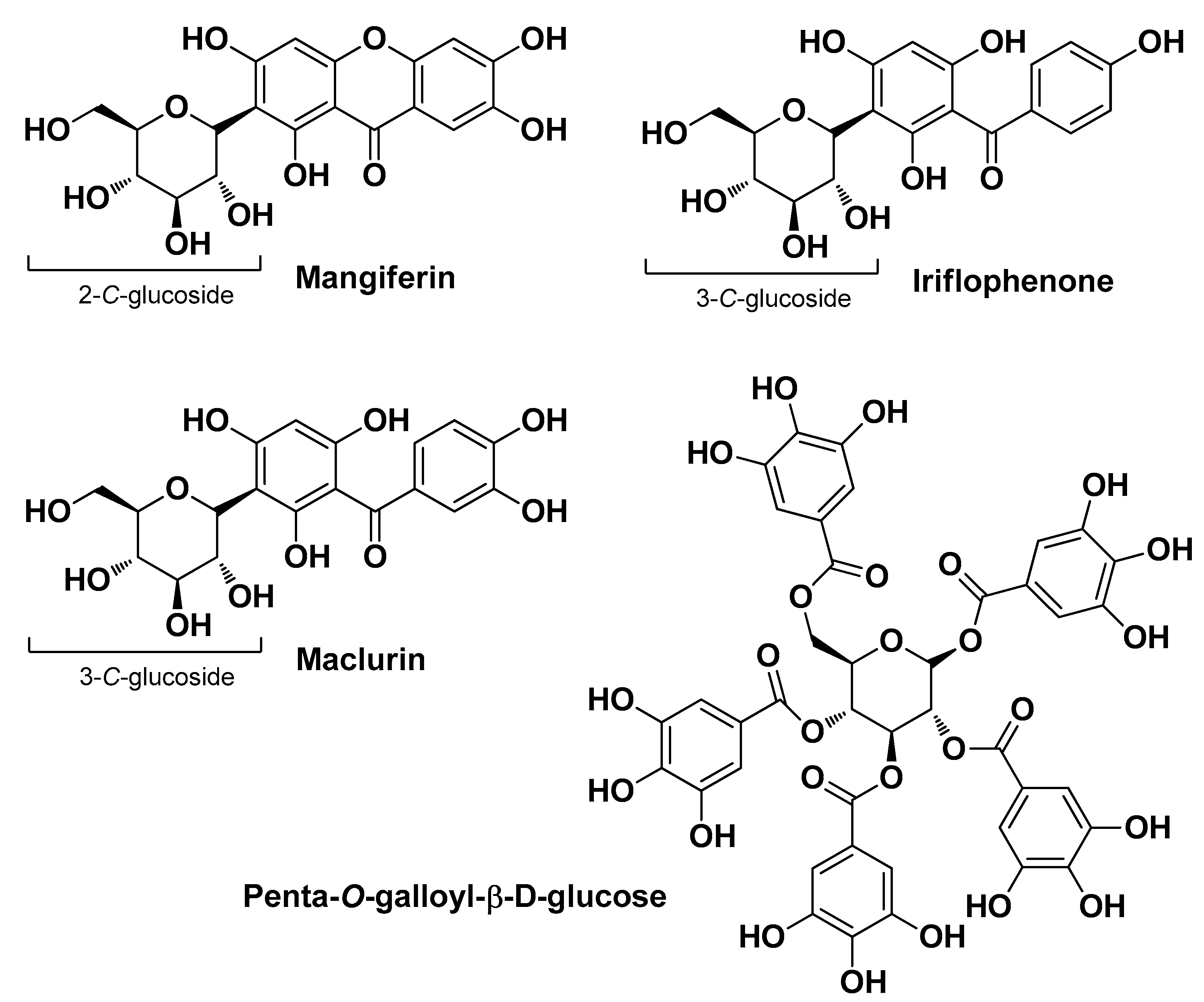
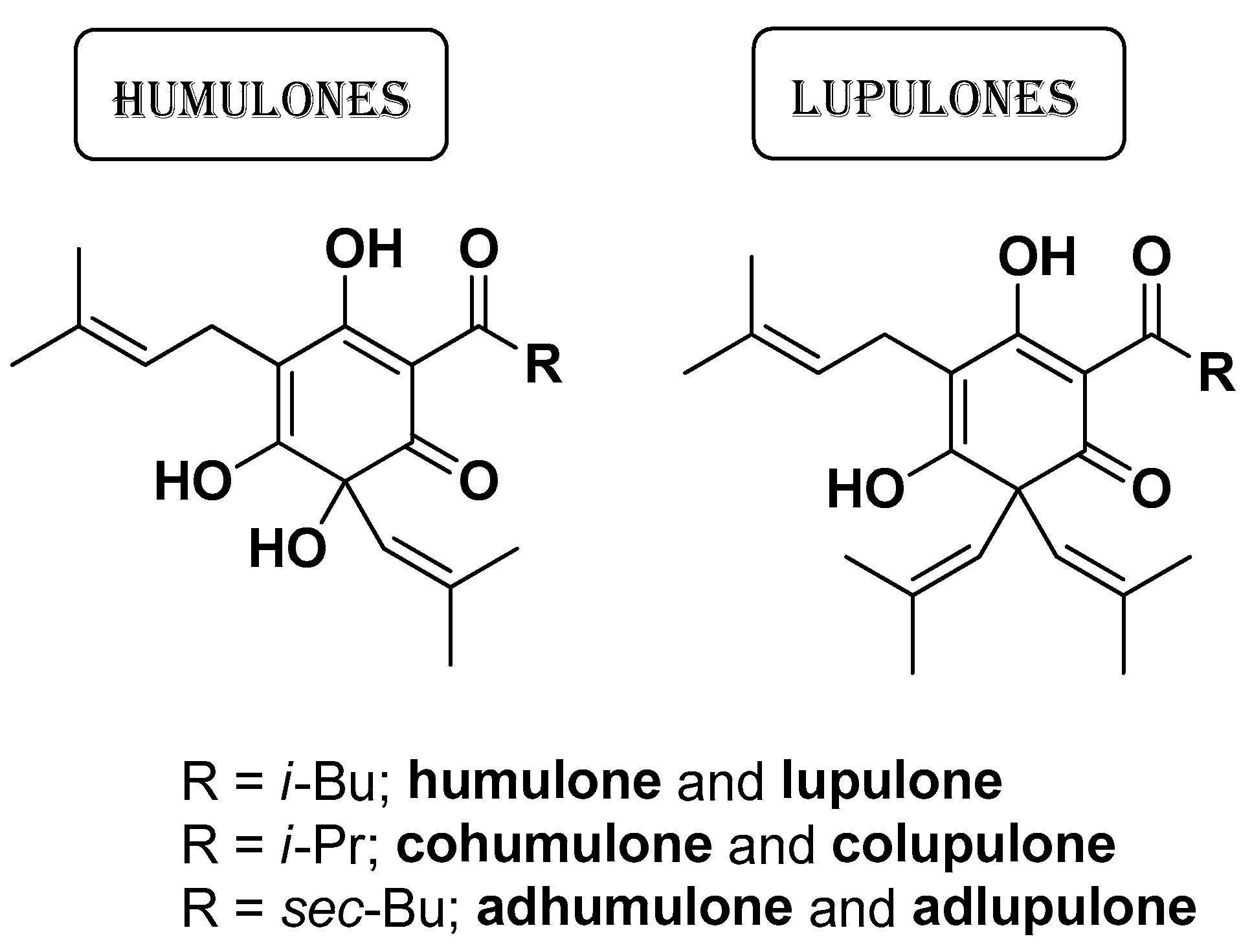
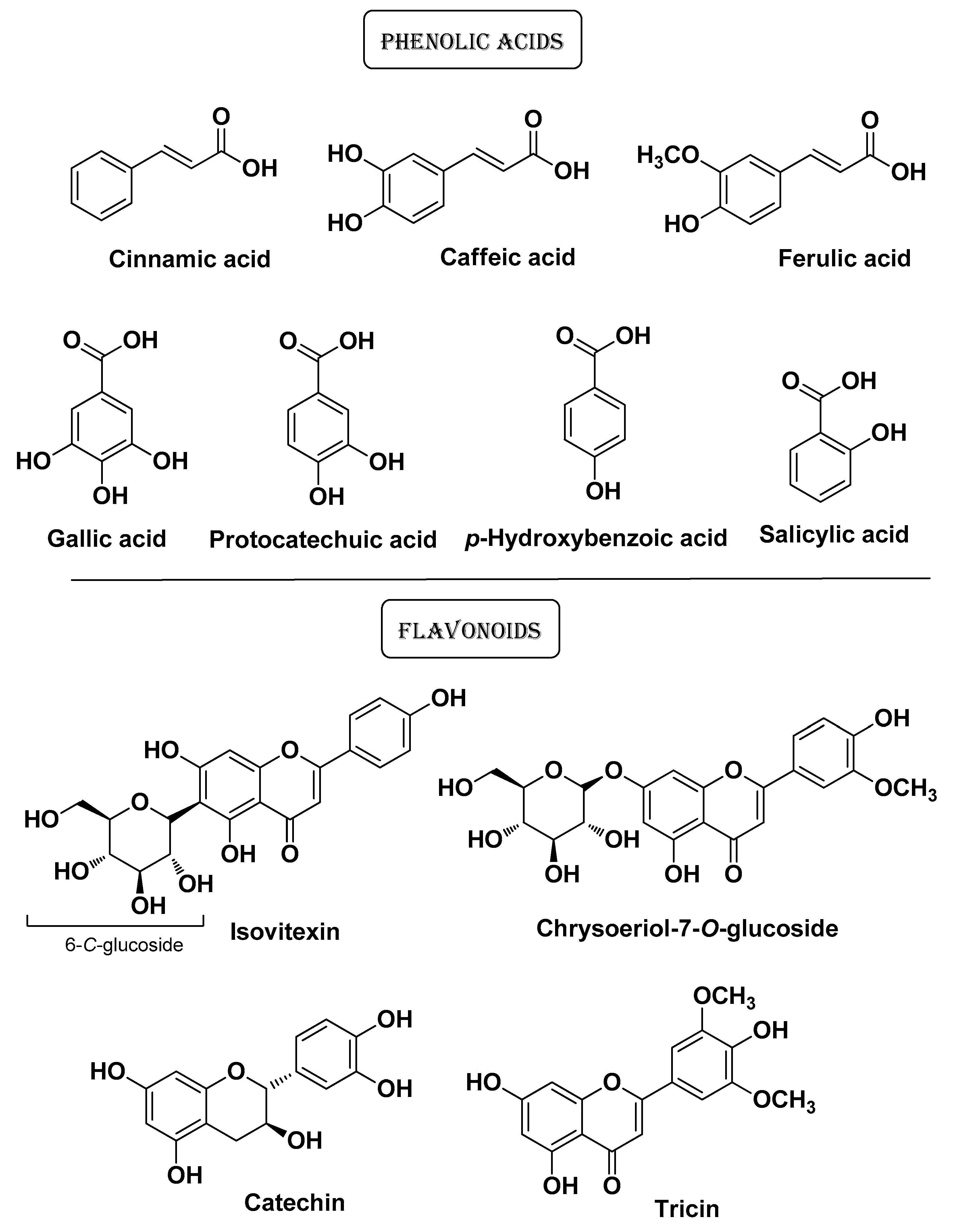
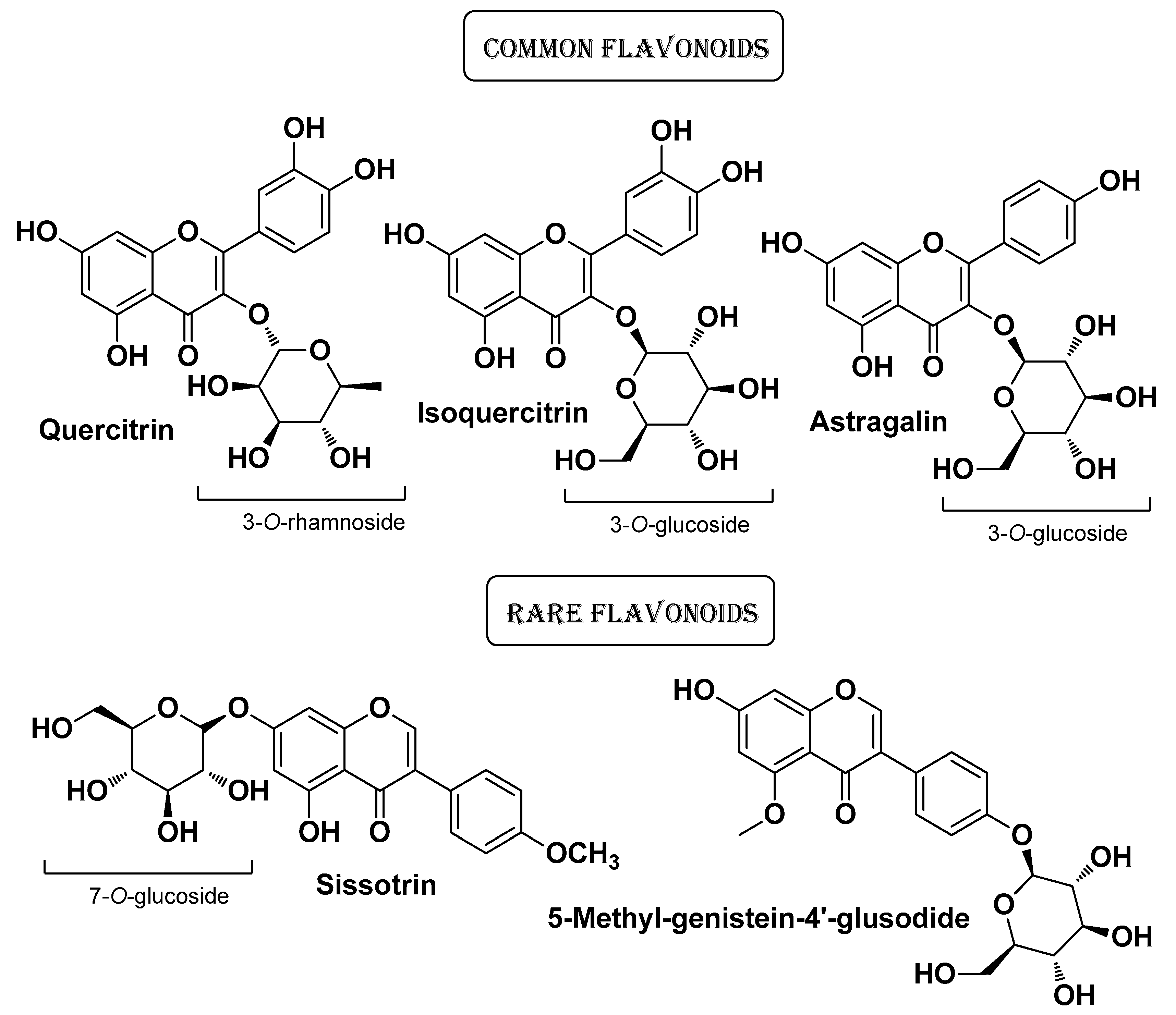
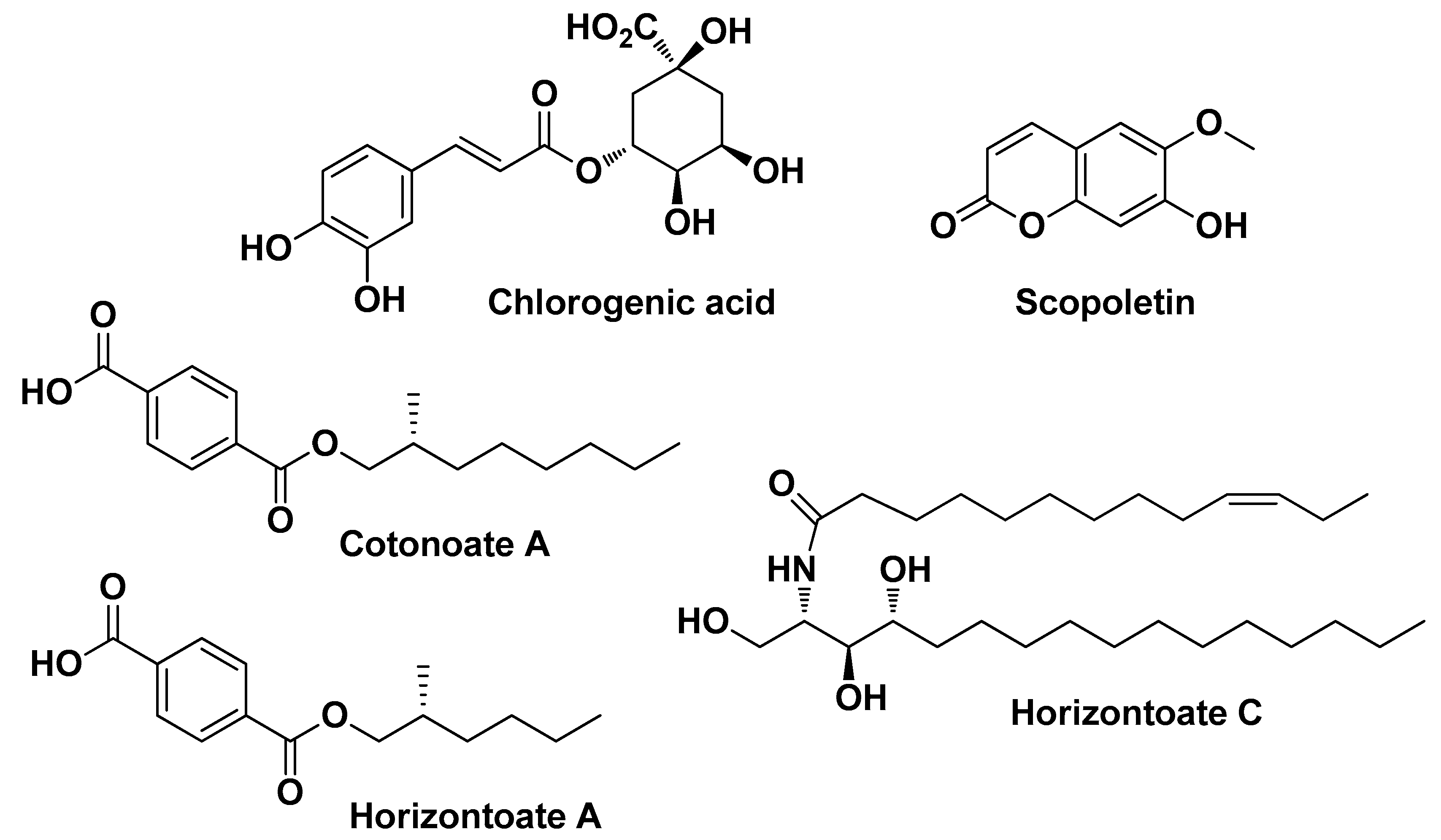
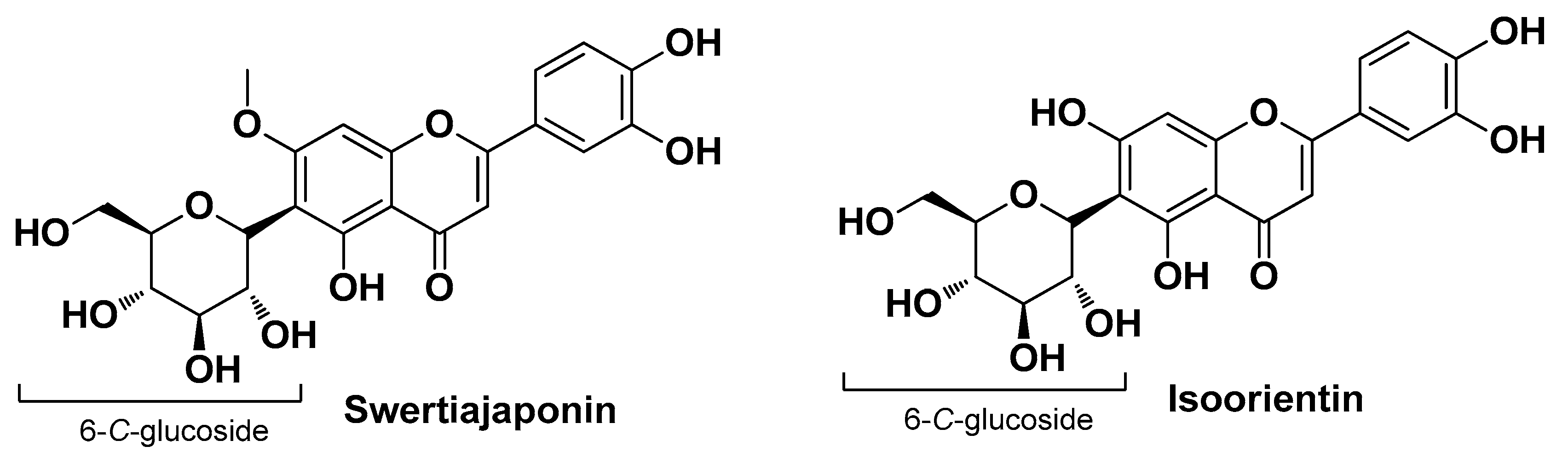


| Natural Source | Common Name | Part Used | Active Compounds | Reported Biological Activity | Ref. |
|---|---|---|---|---|---|
| Origanum vulgare L. Lamiaceae | Oregano | Leaves (extracts) | Rosmarinic acid Quercetin Luteolin Apigenin Carvacrol Thymol | Anti-inflammatory | [8] |
| Origanum vulgare L. Lamiaceae | Oregano | Essential oil | Thymol | Antimicrobial Anti-inflammatory | [9] |
| Thymus vulgaris L. Lamiaceae | Thyme | Essential oil (Nanoemulsion) | Thymol Caryophyllene Phenolic compounds Terpenoid compounds | Antimicrobial Anti-inflammatory | [10] |
| Thymus × citriodorus (Pers.) Schreb. (hybrid Thymus pulegioides L. and Thymus vulgaris L. Lamiaceae | Lemon thyme | Essential oil Hydrolate | Geraniol 1,8-Cineole Thymol Linalool | Antimicrobial Anti-biofilm Anti-inflammatory | [11] |
| Callicarpa americana L. Lamiaceae | American beautyberry | Leaves (extracts) | Six clerodane diterpenes Genkwanin 5-Hydroxy-7,4′-dimethoxyflavone Luteolin | Antimicrobial Anti-inflammatory | [12] |
| Plectranthus aliciae (Codd) van Jaarsv. & T.J. Edwards Lamiaceae | AuNP of Leaves and soft twigs (extracts) | Rosmarinic acid | Antimicrobial and Wound healing potential | [13] | |
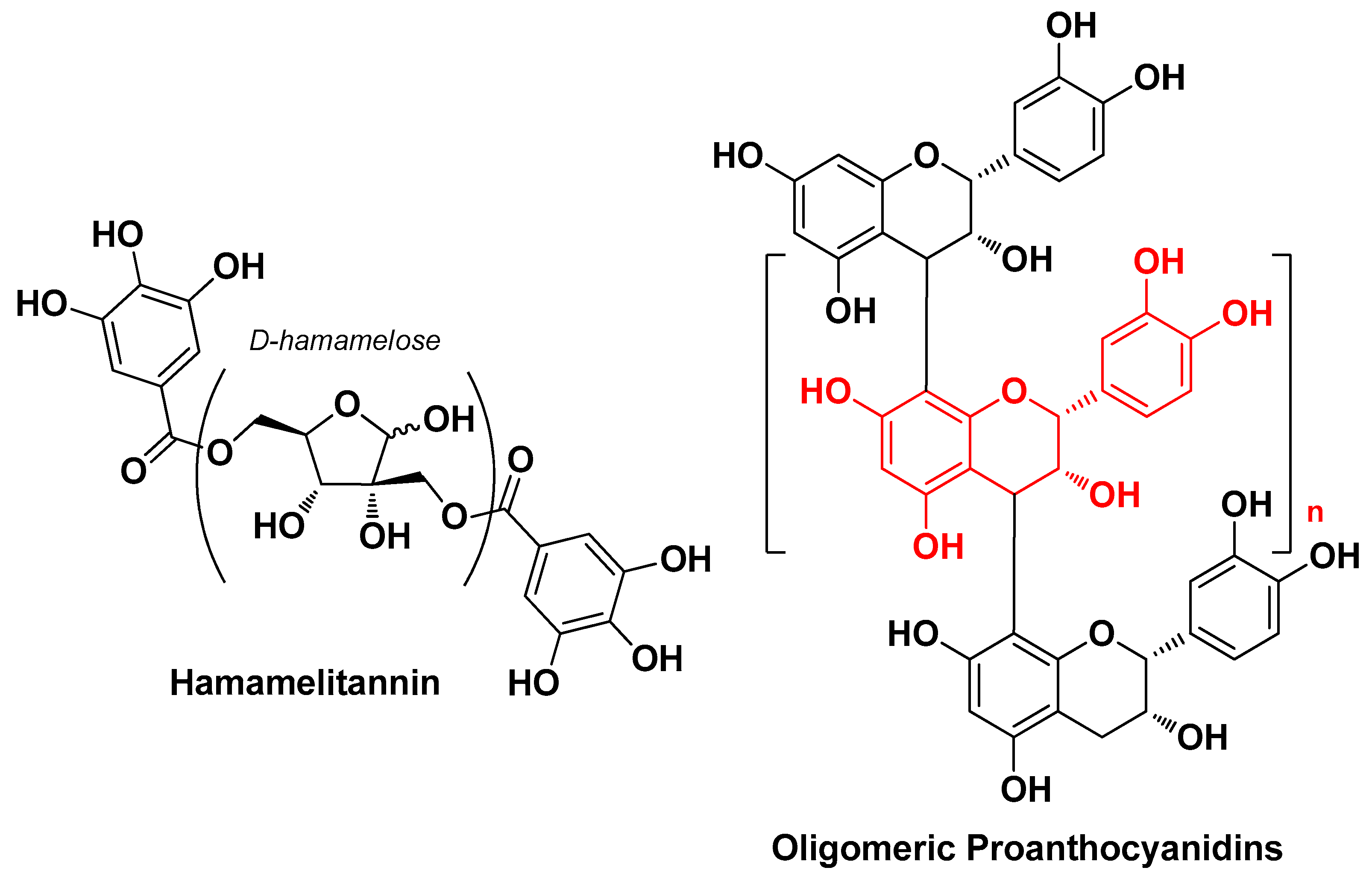
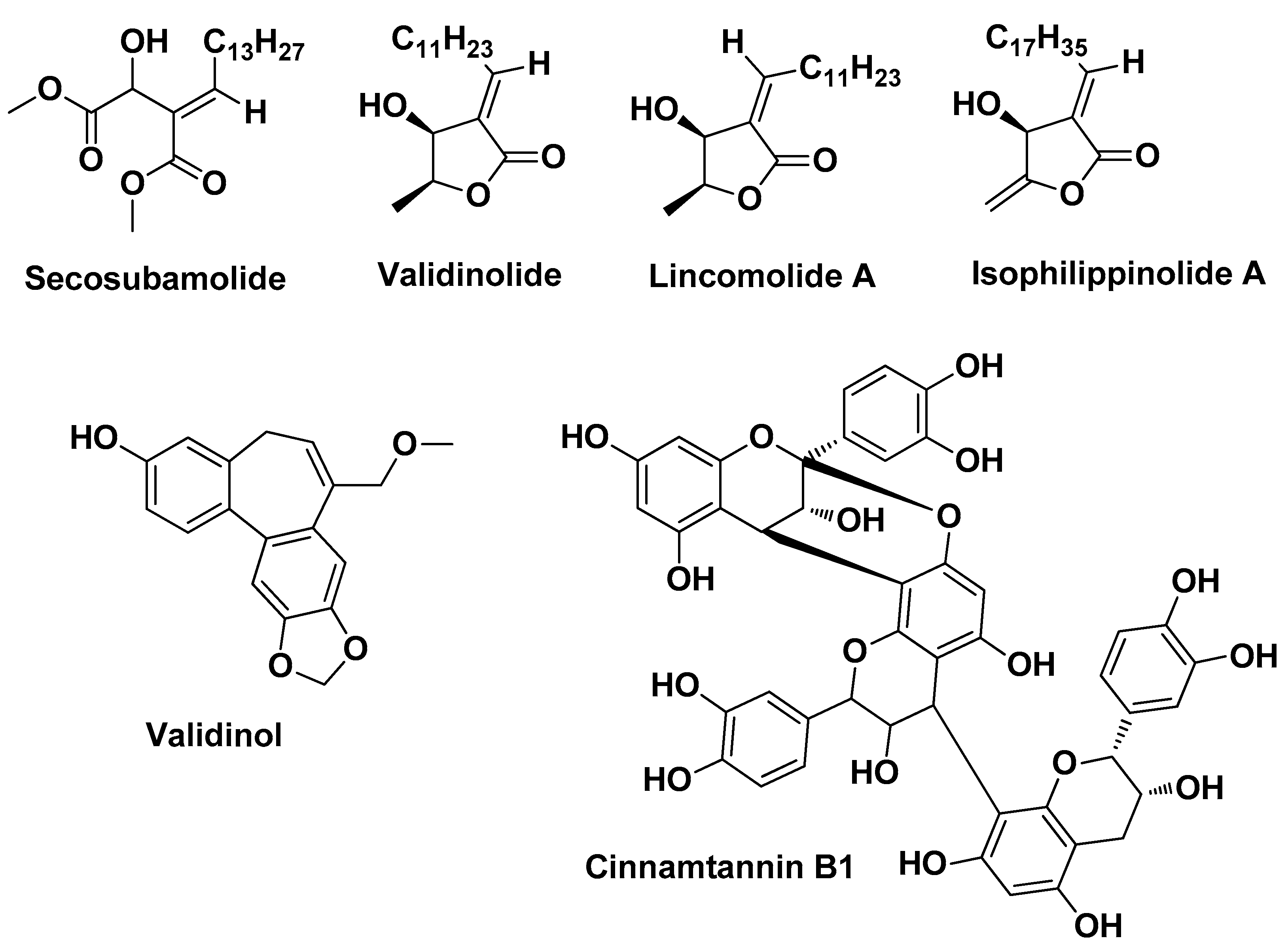
| Scutellaria baicalensis Georgi Lamiaceae | Chinese skullcap | Aereal part (extract) | Baicalin Wogonoside, Lincomolide A Secosubamolide Cinnamtannin B1 Isophilippinolide A Secosubamolide | Anti-inflammatory Antimicrobial | [14] |
| Mangifera indica L. Anacardiaceae | Mango | Raw and ripe fruits (extracts) | Gallic acid | Antioxidant Anti-inflammatory | [15] |
| Mangifera indica L. Anacardiaceae | Mango | Leaf (extract) | Mangiferin (glucosylxanthone -xanthonoid) Penta-O-galloyl-beta-D-glucose Iriflophenone-3-C-beta-glucoside Maclurin-3-C-beta-glucoside | Sebo regulation Antimicrobial | [16] |
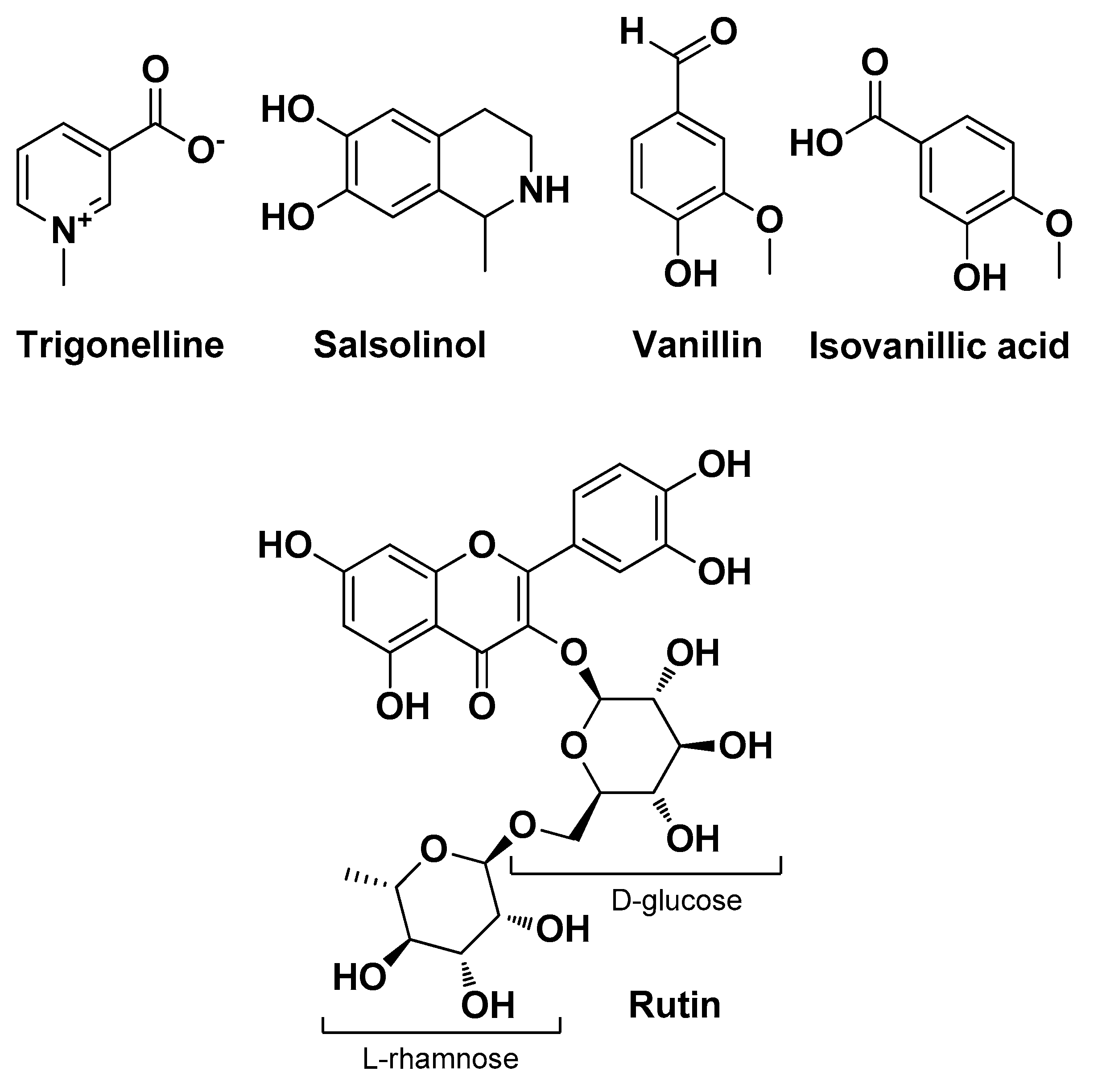
| Anacardium occidentale L. Anacardiaceae | Cashew | Peduncle pulp (extract) | Rutin | Antioxidant Antimicrobial | [17] |
| Cannabis sativa L. Cannabaceae | Hemp | Seed (extracts) | Linoleic acid Oleic acid cis-11-Eicosenoic acid Palmitic acid γ-Linolenic acid Arachidic acid, Palmitoleic acid Heneicosanoic acid | Anti-inflammation Anti-lipogenesis | [18] |
| Humulus lupulus L. Cannabaceae | Hop | Hop-CO2-extract | Humulones Lupulones | Antioxidant Anti-inflammatory Antimicrobial | [19] |
| Cymbopogon citratus Stapf Poaceae | Lemongrass | Aereal part (extracts) | Caffeic acid Salicylic acid p-Hydroxybenzoic acid, Gallic acid Ferulic acid Isovitexin Luteolin Catechin Tricin Protocatechuic acid, Chrysoriol 7-O-glucoside Catechin k | Antioxidative, Antimicrobial Anti-aging Anti-whitening | [20] |
| Zea mays L. Poaceae | Corn | Biosurfactant extract obtained from corn milling industry (named BS-CSW) | Salycilic acid | Antimicrobial | [21] |
| Cotoneaster hsingshangensis J. fryer & B. hylmö and Cotoneaster issaricus Pojatk Rosaceae | Leaves (extracts) | Isoquercitrin Rutin hyperoside Quercitrin Chlorogenic acid Gentisic acid 2-O-glucoside Scopoletin | Antioxidant Anti-cyclooxygenase Anti-lipoxygenase Anti-hyaluronidase Antimicrobial | [22] | |
| Cotoneaster nebrodensis (Guss.) K. Koch and Cotoneaster roseus Coll Rosaceae | Brickberry cotoneaster, Madagascar periwinkle | Leaves and fruits (extract) | Flavonoids (quercetin derivatives) | Anti-lipoxygenase, Anti-hyaluronidase, Anti-cyclooxygenase Antimicrobial | [23] |
| Arctium lappa L. Asteraceae | Burdock | Roots (extract) | Peptides (Br-p) isolated | Antimicrobial Antioxidant | [24] |
| Cephalaria uralensis Roem. & Schult. and Cephalaria gigantea (Ledeb.) Bobrov Caprifoliaceae | Murray and giant scabious | Aerial parts and flowers of Murray and the aerial parts of Giant scabious (extract) | 5-O-Caffeoylquinic acid Isoorinetin Swertiajaponin | Antioxidant Anti-inflammatory Antimicrobial | [25] |
| Cistus laurifolius L. and Cistus salviifolius L. Cistaceae | Aereal part (extracts) | Myricetin Quercetin Kaempferol Terflavin A Cistusin | Antioxidant Anti-Inflammatory Antimicrobial | [26] | |
| Quercus mongolica Fisch. Fagaceae | Mongolian oak | Leaves (extract) | Pedunculagin | Anti-inflammatory 5α-Reductase inhibition | [27] |
| Hamamelis virginiana L. Hamamelidaceae | American witch hazel | Bark (extract) | Hamamelitannin Gallotannins Flavonols Proanthocyanidins | Antioxidant Anti-inflammatory Antimicrobial | [28] |
| Cinnamomum validinerve Hance Lauraceae | Cinnamomum | Stem (extract) | Validinol Validinolide Butanolide Tannins | Anti-inflammatory Antimicrobial | [29] |
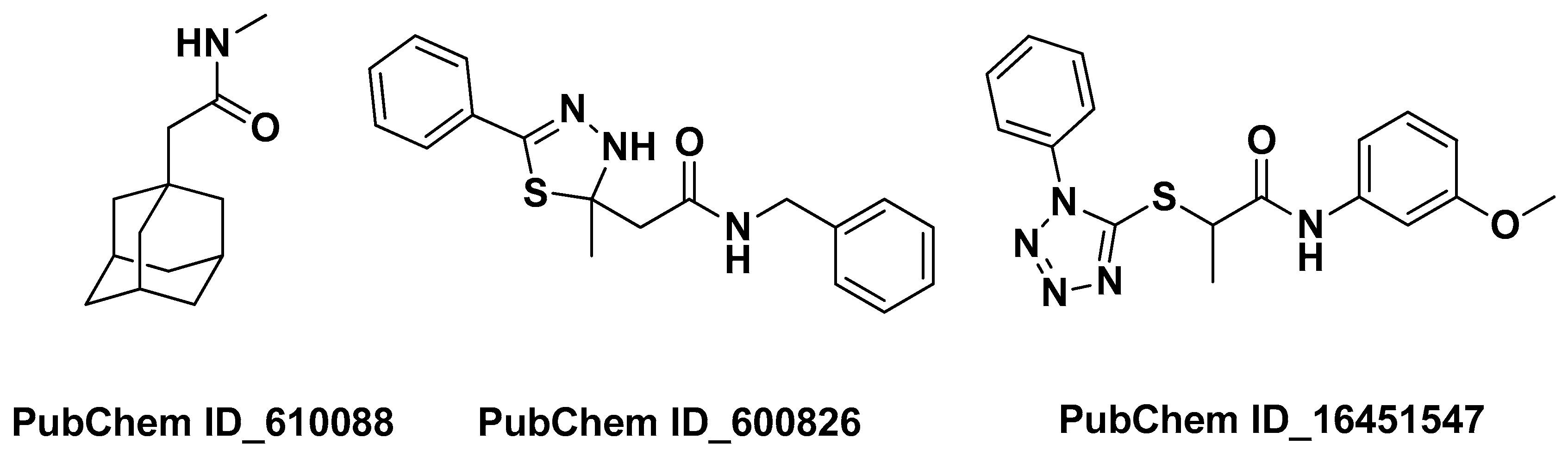
| Azadirachta indica A.Juss. Meliaceae | Neem | Leaves (oil) | 2-(1-Adamantyl)-N-methylacetamide N-benzyl-2-(2-methyl-5-phenyl-3H-1,3,4-thiadiazol-2-yl)acetamide) N-(3-methoxyphenyl)-2-(1-phenyltetrazol-5-yl)sulfanylpropanamide PubChem ID_610088, PubChem ID_600826 PubChem ID_16451547 | Anti-inflammatory | [30] |
| Musa balbisiana Colla Musaceae | Weet wild banana | Banana peels (extract) | Rutin | Anti-inflammatory Antimicrobial | [31] |
| Meconopsis quintuplinervia Regel Papaveraceae | (extract) | Alkaloids Flavonoids (quercetin and luteolin) Volatile oils | Antimicrobial | [32] | |
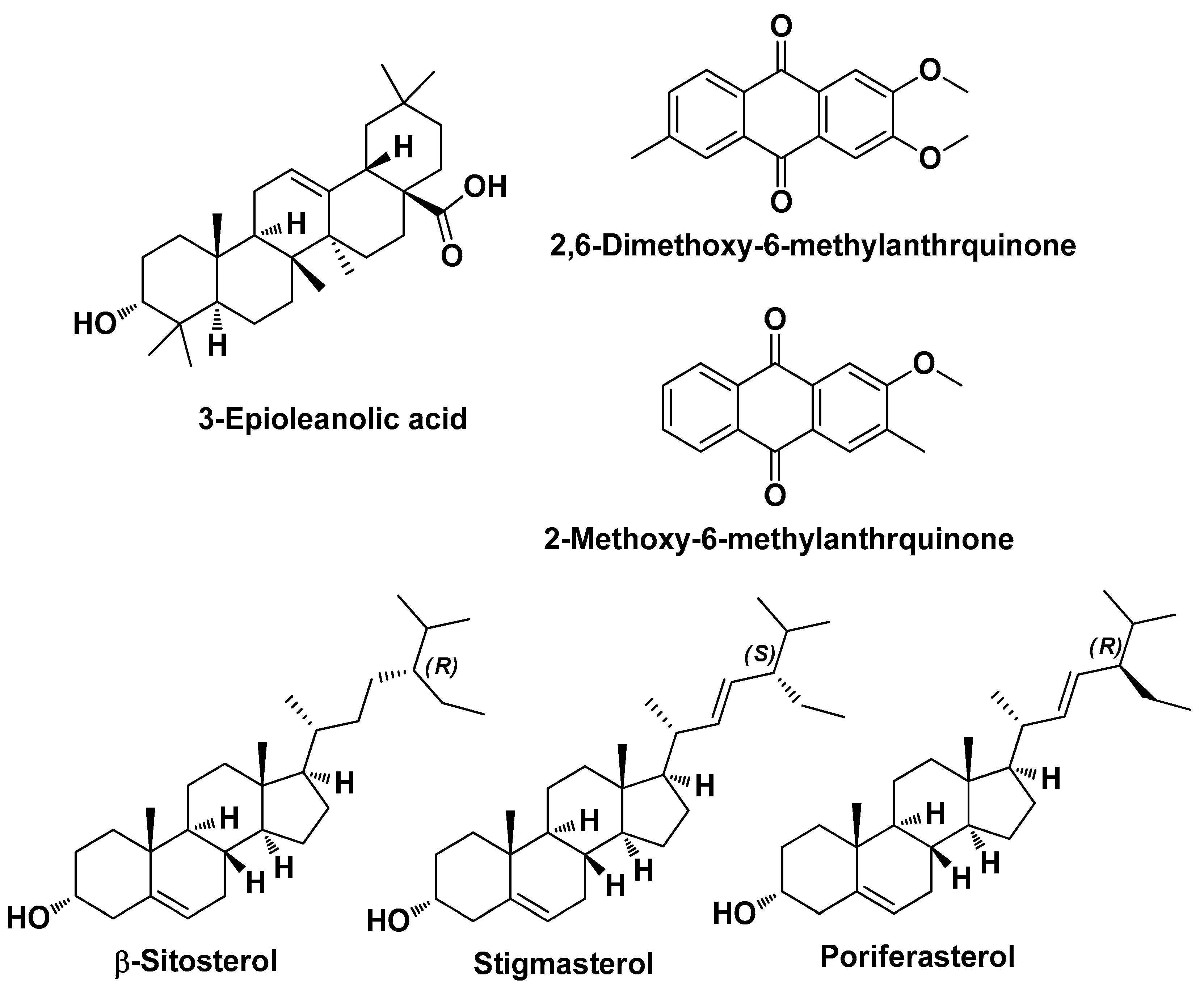
| Hedyotis diffusa Willd Rubiaceae | snake-needle grass | (extract) | 2-Methoxy-3-methyl-9,10-anthraquinone 2 3-Dimethoxy-6-methyanthraquinone Quercetin Beta-sitosterol Poriferasterol Stigmasterol 3-epioleanolic acid | Sebo reducent Anti-inflammatory | [33] |
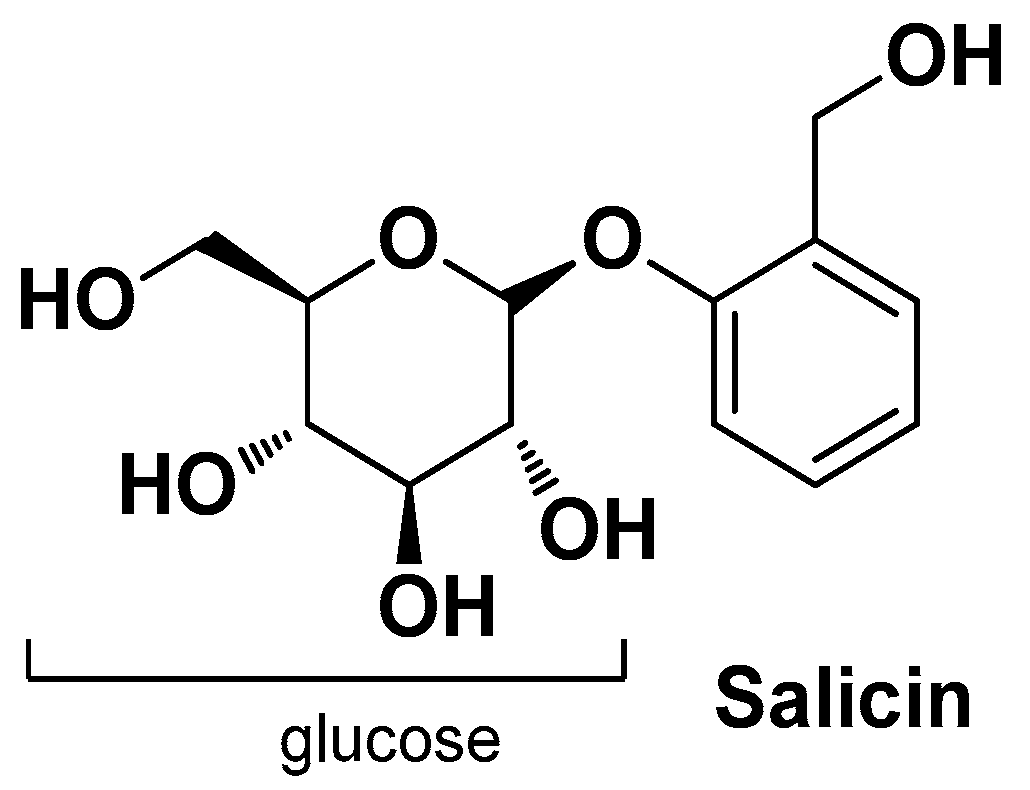
| Salix alba L. Salicaceae | White willow | Bark (extract) | Salicilin 1,2-Decanediol (beta glucoside) | Anti-inflammatory | [34] |
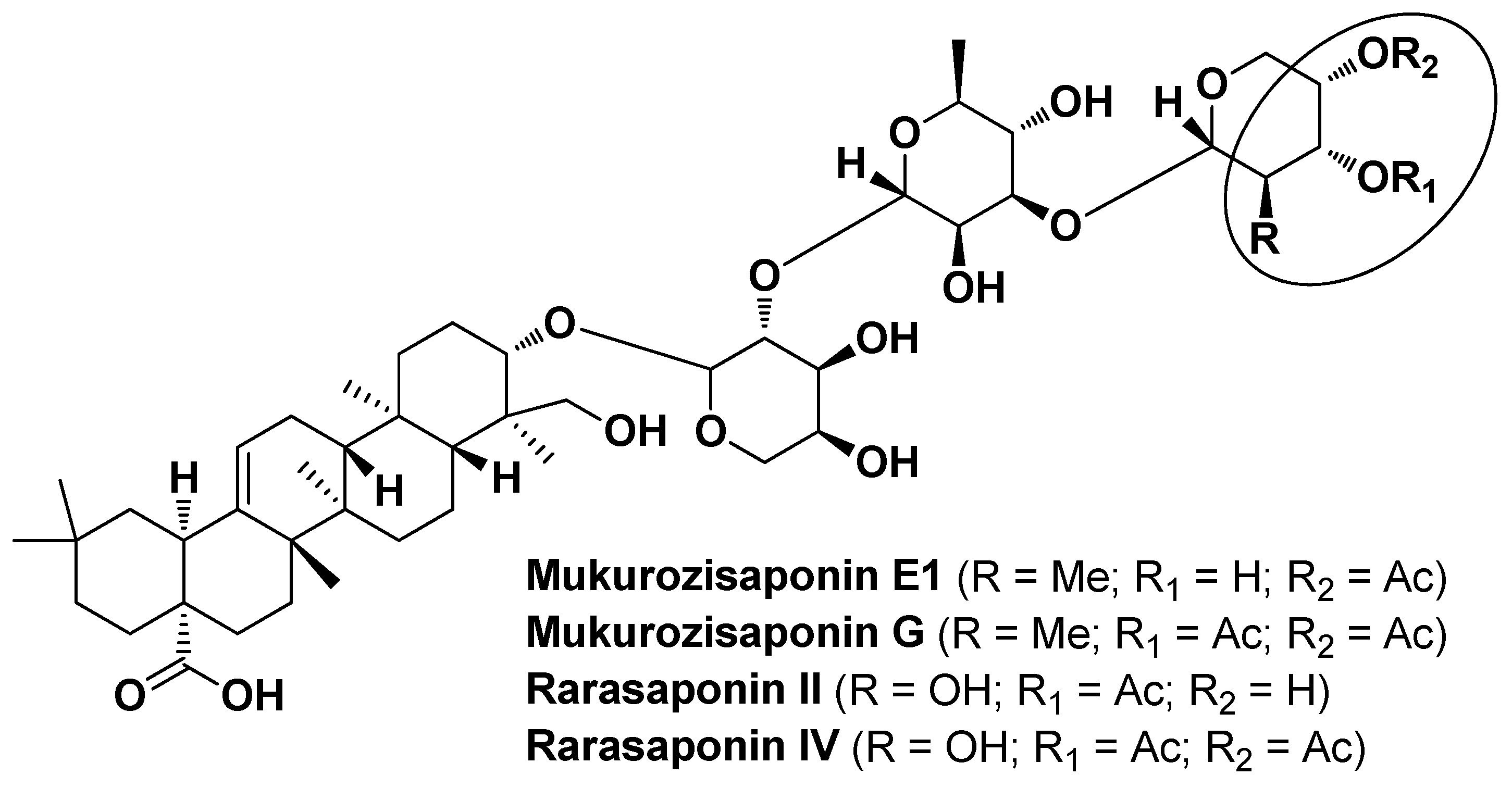
| Sapindus mukorossi Gaertn. Sapindaceae | Chinese soapberry | Peel (extract) | Saponin fraction (F4): Mukurozisaponin E1 Rarasaponin II Mukurozisaponin G Rarasaponin VI | Antimicrobial | [35] |
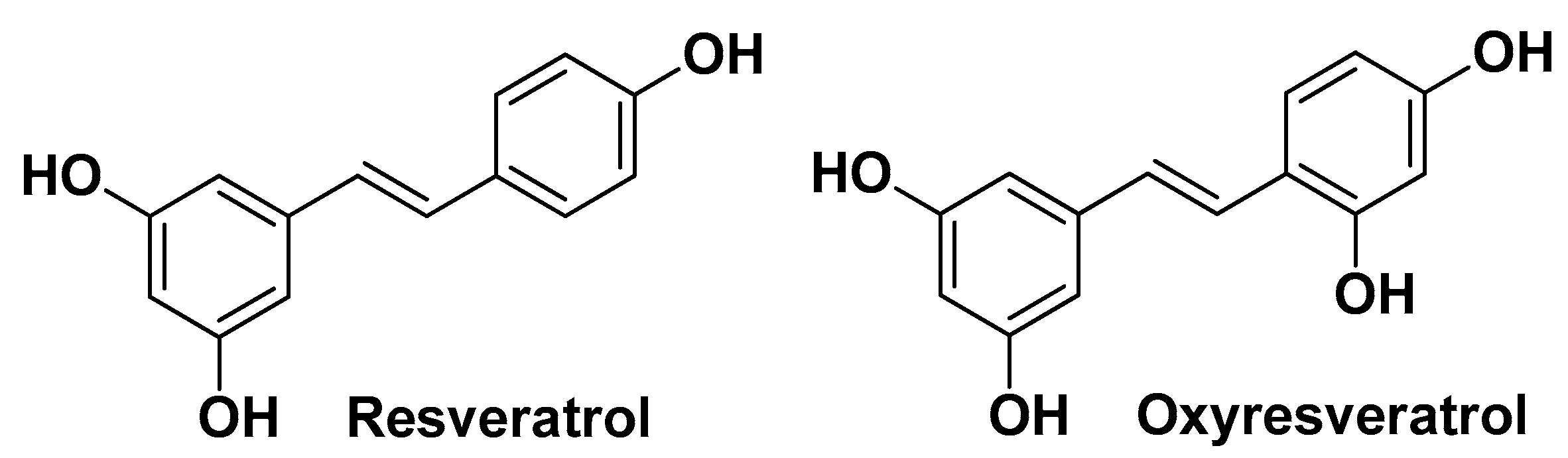
| Smilax china L. Smilacaceae | China root | Root (extract) | Quinic acid Caffeic acid Polydatin Quercetin Oxyresveratrol Catechin Resveratrol | Antimicrobial | [36] |
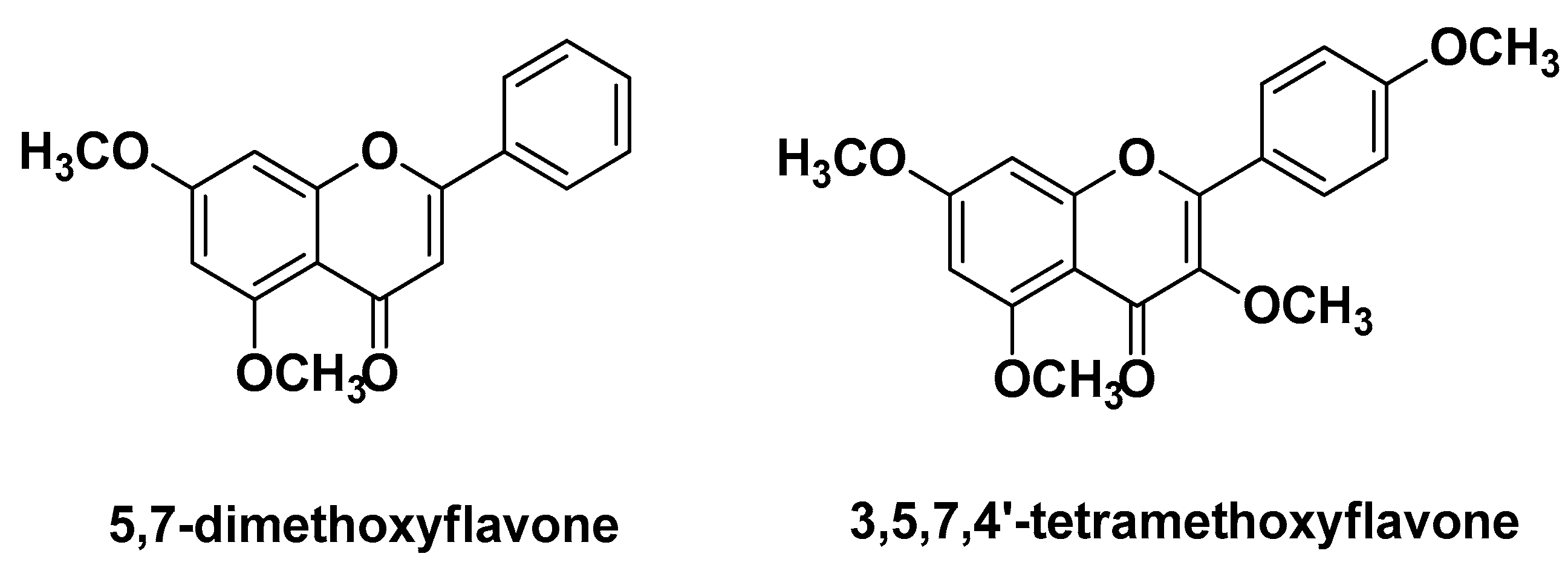
| Kaempferia parviflora Wall. Zingiberaceae | Thai ginseng | Rhizomes (extracts) | 5-hydroxy-7-methoxyflavone, 5-hydroxy-3,7-methoxyflavone 5,7-dimethoxyflavone 5-hydroxy-3,7,40-methoxyflavone | Antimicrobial Anti-inflammatory | [37] |
| Juglans regia L., Juglandaceae; Myrtus Communis L., Myrtaceae; Matricaria chamomilla L., Asteraceae; Urtica dioica L., Urticaceae; Rosa damascena Herrm., Rosaceae; Brassica oleracea var. botrytis L. Brassicaceae, and Brassica oleracea var. italica L. Brassicaceae | Walnut husk myrtle, chamomilla, stinging nettle, rose; broccoli, cauliflower | Anti-acne extract 1 (AE1): walnut husk, myrtle leaves, chamomilla flowers, stinging nettle leaves and rose flowers; Anti-acne extract 2 (AE2): broccoli and cauliflower | Main in AE1: Chlorogenic acid Caffeic acid Ferulic acid Vanillic acid catechin Juglone herbaceous (naftalenedione) Apigenin Rutin Coumarins Polyacetylenes Bisabolol Present in AE2: Alkaloids Carbohydrates Glycosides Tannins Quercetin Kaempferol | Antimicrobial Anti-inflammatory | [38] |
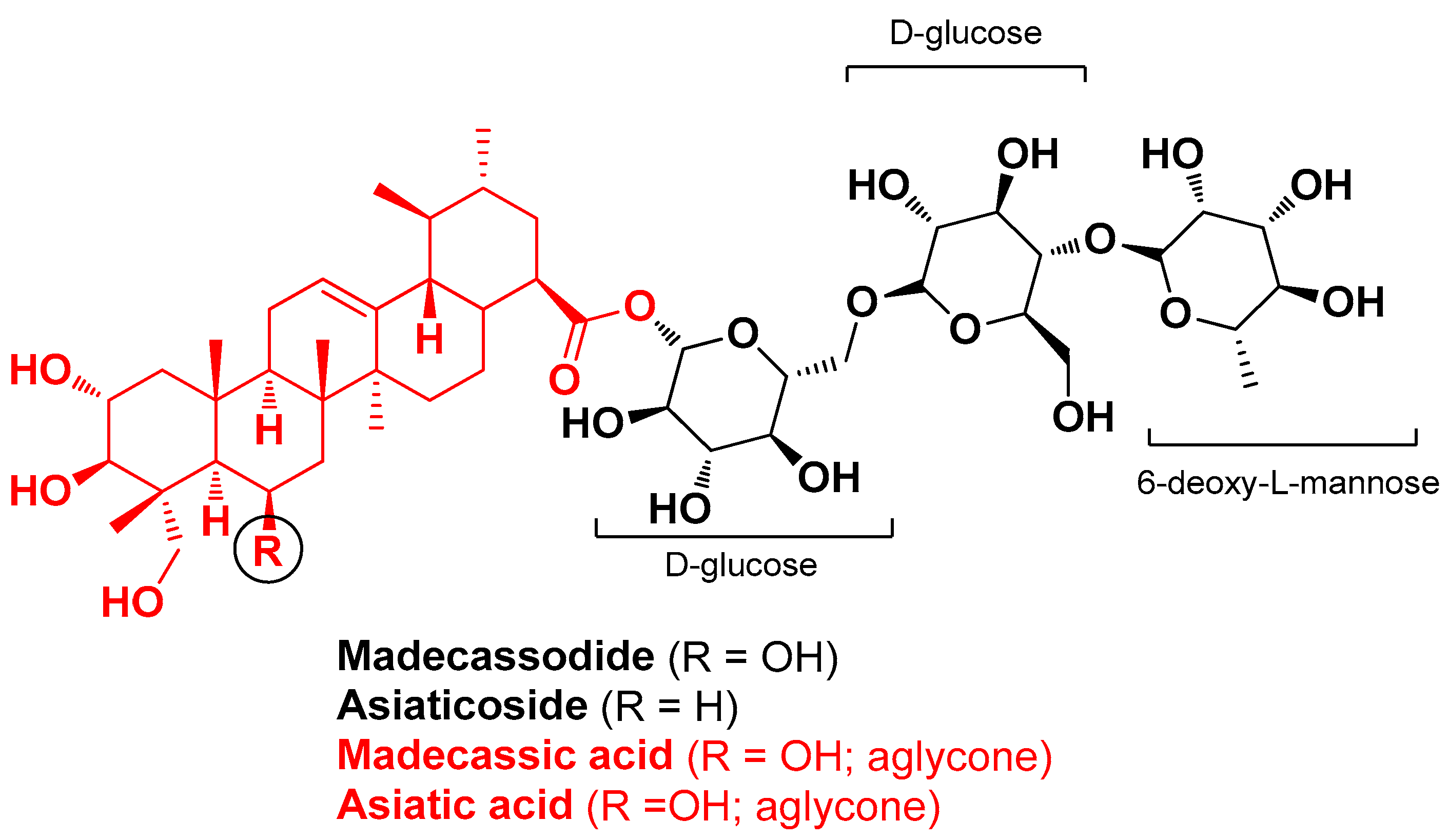
| Centella asiatica Apiaceae and Silybum marianum L. Asteraceae, Lonicera japonica flower Caprifoliaceae, Salvia miltiorrhiza Lamiaceae and Camellia sinensis L. Theaceae; Salix babylonica L. Salicaceae | Gotu kola, milk thistle, honey suckle; red sage, green tea, white willow bark | C. asiatica triterpene leaf (extract); S. marianum fruit (extract); S. miltiorrhiza root (extract); C. sinensis (extract); S. babylonica (extract) | Cannabidiol Asiaticoside Asiatic acid Madecassic acid Silymarin (as silibinin: silicristin, silibinin A and B and isosilibinin A and B); Caffeine | Anti-inflammatory Antimicrobial | [39] |
| Myrtus communis L. Myrtaceae and Tripterygium wilfordii Celastraceae | Myrtle and thunder god vine | M. communis L. (extract) (Myrtacin®) Celastrol (enriched extract) | Myrtucummulones Ursolic acid Terpenoids Alkaloids Steroids | Anti-inflammatory | [40] |
| Thymus mastichina L. Lamiaceae and Cistus ladanifer L. Cistaceae | White thyme and gum rockrose | Essential oil Hydrolated | CL EO: α-pinene and camphene TM EO: 1,8-cineole, p-cymene In both EO: sesquiterpene hydrocarbons and oxygen-containing sesquiterpenes | Antioxidant Anti-inflammatory Wound healing Antimicrobial | [41] |
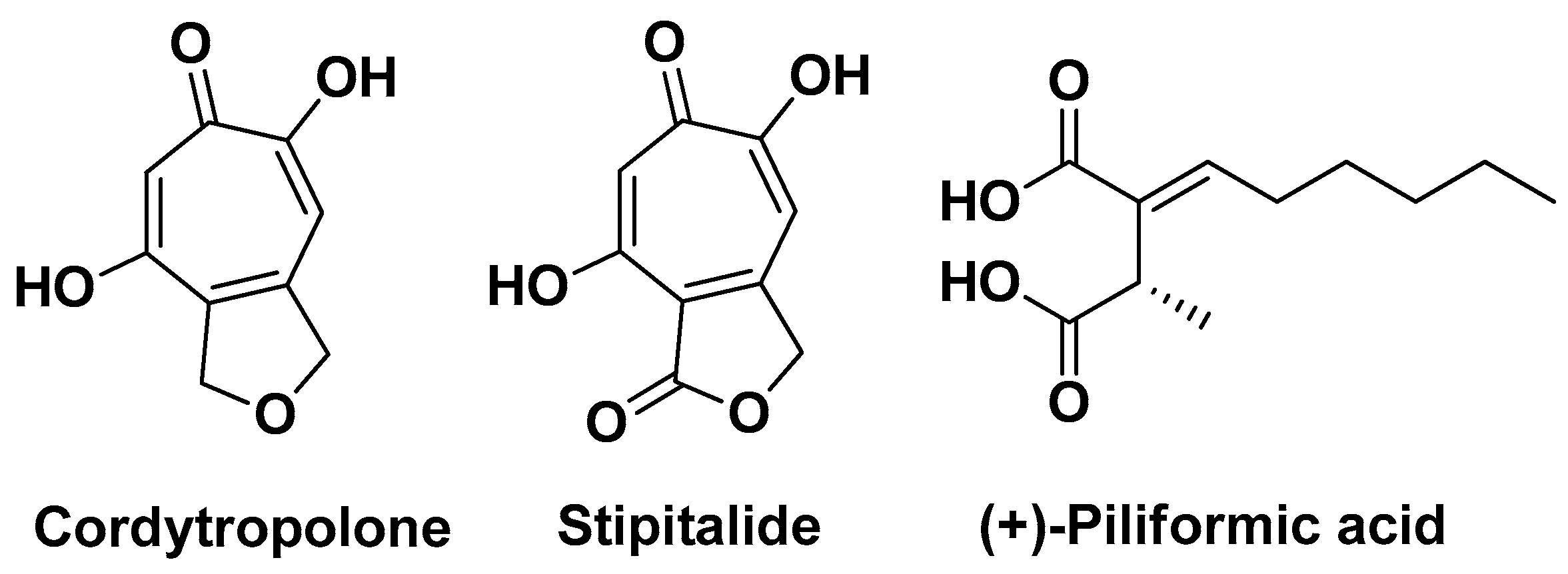
| Polycephalomyces phaothaiensis | Fungi | extracts | Cordytropolone Stipitalide (+)-piliformic acid | Anti-inflammatory, Antimicrobial | [42] |
| Mechanisms | Compounds | Refs. | |
|---|---|---|---|
| Antimicrobial | Disintegration of bacterial outer membrane or phospholipid bilayer Increase of membrane fluidity with leakage of potassium ions and protons Complexes with cholesterol membrane with increase in permeability and leakage of cytoplasmic contents Inhibition of peptidoglycan synthesis | Apigenin Catechin Ferulic acid Caffeic acid Chrysoriol 7-O-glucoside Gallic acid Kaempferol Isovitexin Luteolin Naringenin p-Hydroxybenzoic acid Protocatechuic acid Rhamnetin Rosmarinic acid Quercetin Salicylic acid Tricin β-Caryophyllene p-Cymene Carvacrol Linalool Menthol Thymol Humulones Lupulones Saponins | [19,20,21,80,81,82,83] |
| Inhibition of nucleic acid synthesis or cell envelope synthesis or fatty acid synthase or ATP synthase | Apigenin Baicalein Catechin Kaempferol Luteolin Myricetin Naringenin Quercetin | [80] | |
| Inhibition of bacterial virulence | Quercetin glycoside Kaempferol | [80] | |
| Inhibition of efflux pumps | Catechin Genestein Quercetin | [80] | |
| Alteration of fatty acid composition | Carvacrol Thymol | [84] | |
| Interference with glucose uptake | Humulones Lupulones | [19,85] | |
| Interference with oxidative phosphorylation or oxygen uptake | Carvacrol Linalool | [81] | |
| Anti-inflammatory | Inhibition/modulation/suppression of NLRP3 inflammasome | Apigenin, Catechin Quercetin Resveratrol Rutin | [86] |
| Modulation/stimulation of AhR/Nrf2 pathway | Catechin Luteolin | [86] | |
| Inhibition of the expression of inflammatory factors via the MAPK and NF-kb signaling pathways | Baicalin Cinnamtannin B1 Isophilippinolide A Lincomolide A Secosubamolide Wogonoside | [14] | |
| Suppression of the NF-κB p65 translocation and block of the phosphorylation of IKK and IκB | Linalool | [87] | |
| Reduction of mRNA or protein expression of pro-inflammatory cytokines (IL-1 β, IL-6, and TNF-α) Up-regulation of mRNA and protein expression of anti-inflammatory cytokines (IL-10) | Geraniol 1,8-Cineole Linalool Thymol Salicilin 1,2, decanediol Salicylic acid Chlorogenic acid Caffeic acid Ferulic acid Vanillic acid Pedunculagin Cannabidiol Asiaticoside Asiatic acid Madecassic acid Silymarin Caffeine Saponin Cordytropolone Stipidalide | [21,27,34,38,39,42,88,89] | |
| Inhibition of COX and LOX activity | Geraniol 1,8-Cineole Linalool Thymol Caffeoylmalic acid 5-O-caffeoylquinic acid Chlorogenic acid Gentisic acid 2-O-glucoside Isoorinetin Isoquercitrin Quercitrin Hyperoside Rutin Swertiajaponin Scopoletin Linoleic acid Oleic acid cis-11-eicosenoic acid Palmitic acid Arachidic acid Palmitoleic acid Heneicosanoic acid Saponin Cordytropolone Stipidalide | [18,22,25,42,88,89] | |
| Inhibition of inducible nitric oxide synthase (iNOS) and tyrosinase expression | 1,8-Cineole Geraniol Linalool Thymol | [88] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristani, M.; Micale, N. Bioactive Compounds from Medicinal Plants as Potential Adjuvants in the Treatment of Mild Acne Vulgaris. Molecules 2024, 29, 2394. https://doi.org/10.3390/molecules29102394
Cristani M, Micale N. Bioactive Compounds from Medicinal Plants as Potential Adjuvants in the Treatment of Mild Acne Vulgaris. Molecules. 2024; 29(10):2394. https://doi.org/10.3390/molecules29102394
Chicago/Turabian StyleCristani, Mariateresa, and Nicola Micale. 2024. "Bioactive Compounds from Medicinal Plants as Potential Adjuvants in the Treatment of Mild Acne Vulgaris" Molecules 29, no. 10: 2394. https://doi.org/10.3390/molecules29102394
APA StyleCristani, M., & Micale, N. (2024). Bioactive Compounds from Medicinal Plants as Potential Adjuvants in the Treatment of Mild Acne Vulgaris. Molecules, 29(10), 2394. https://doi.org/10.3390/molecules29102394








