Abstract
The rapid advancements in nanotechnology in the field of nanomedicine have the potential to significantly enhance therapeutic strategies for cancer treatment. There is considerable promise for enhancing the efficacy of cancer therapy through the manufacture of innovative nanocomposite materials. Metallic nanoparticles have been found to enhance the release of anticancer medications that are loaded onto them, resulting in a sustained release, hence reducing the dosage required for drug administration and preventing their buildup in healthy cells. The combination of nanotechnology with biocompatible materials offers new prospects for the development of advanced therapies that exhibit enhanced selectivity, reduced adverse effects, and improved patient outcomes. Chitosan (CS), a polysaccharide possessing distinct physicochemical properties, exhibits favorable attributes for controlled drug delivery due to its biocompatibility and biodegradability. Chitosan nanocomposites exhibit heightened stability, improved biocompatibility, and prolonged release characteristics for anticancer medicines. The incorporation of gold (Au) nanoparticles into the chitosan nanocomposite results in the manifestation of photothermal characteristics, whereas the inclusion of silver (Ag) nanoparticles boosts the antibacterial capabilities of the synthesized nanocomposite. The objective of this review is to investigate the recent progress in the utilization of Ag and Au nanoparticles, or a combination thereof, within a chitosan matrix or its modified derivatives for the purpose of anticancer drug delivery. The research findings for the potential of a chitosan nanocomposite to deliver various anticancer drugs, such as doxorubicin, 5-Fluroacil, curcumin, paclitaxel, and 6-mercaptopurine, were investigated. Moreover, various modifications carried out on the chitosan matrix phase and the nanocomposite surfaces to enhance targeting selectivity, loading efficiency, and pH sensitivity were highlighted. In addition, challenges and perspectives that could motivate further research related to the applications of chitosan nanocomposites in cancer therapy were summarized.
1. Introduction
Cancer has emerged as a significant disorder responsible for a substantial number of fatalities annually on a global scale [1]. The National Cancer Institute reported that, as of January 2022, an estimated 18.1 million cancer survivors resided in the United States, comprising approximately 5.4% of the population. Projections suggest that this number will increase by 24.4% to 22.5 million by 2032 and is anticipated to rise to 26.0 million by 2040 (Figure 1A). Figure 1B shows the distribution of cancer survivors in the U.S. by age as of January 1, 2022. The majority of survivors are 65 years and older, followed by those aged 40–64 years. The age groups of 20–39 years and 0–19 years have smaller portions of survivors. Each year, a significant number of people die from cancer, necessitating a continuous requirement and desire for the development of potent drugs to treat diverse types of malignancies [2]. Cancer is characterized by the abnormal proliferation and growth of many cells inside the body [3]. Within the human body, cellular replacement occurs in response to cellular death, damage, or aging. Cell division is a fundamental biological process that facilitates the generation of new cells within the human body. It involves the multiplication and expansion of normal, healthy cells. The disruption of this system can lead to the development and multiplication of aberrant cells, specifically malignant cells [4]. Moreover, the process of destabilizing, degrading, and invading healthy tissues in cancer disrupts the ongoing growth of cells [5].
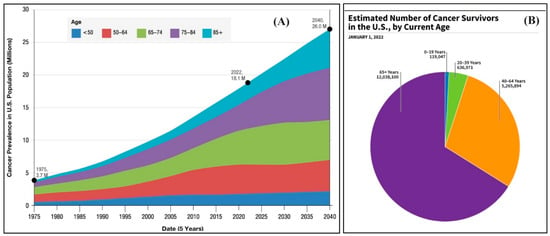
Figure 1.
(A) Cancer prevalence and projections in U.S. from 1975 to 2040; (B) estimated number of 2022 cancer survivors by age.
The development of cancer can be attributed to various factors, including virus infections, smoking, inadequate dietary habits, alcohol consumption, obesity, exposure to ionizing radiation, and hereditary predisposition [6]. The treatment must be appropriately determined for each specific situation, considering the type of cancer. The increase in cancer rates is often attributed to the environmental and climatic changes resulting from industrialization, as well as alterations in lifestyle and dietary patterns [7]. Therefore, if the symptoms indicate the early stage of the disease, the inclusion of previous diagnostic evaluations will have a minimal impact on improving cancer outcomes [8].
The delivery of anticancer drugs faces numerous challenges and difficulties, including the abundance of drugs and their corresponding physicochemical characteristics, the toxicity of administration, the potential difficulty of targeting a specific tumor location, and the complexity of treatment plans. To provide more clarification, the challenges associated with the supply of anticancer drugs can be categorized based on the administrative route [9]. The oral delivery route is a straightforward and preferable method for treating various gastrointestinal tumors, such as colorectal cancer, stomach cancer, pancreatic cancer, and anal cancer [10]. Each type of cancer may require different treatment approaches depending on its location, stage, and other factors [11]. Therefore, the drugs can be absorbed directly through the gastrointestinal tract, where many of these tumors are located, potentially leading to higher drug concentrations at the tumor site. This targeted delivery approach enhances the therapeutic effect while minimizing systemic toxicity [12]. However, the oral administration of anticancer medications presents several obstacles, including the need to improve bioavailability (solubility and/or permeability), reduce enzymatic degradation, and achieve targeted delivery to specific sites within the gastrointestinal tract [13,14]. Novel oral formulations containing non-cytotoxic targeted agents [15], lipid-based formulations [16], permeation enhancers, and gastro-retentive dosage forms [16,17] have the potential to address these challenges. The most prevalent method of delivering cancer medications is through intravenous injection, which promises a high level of absorption and minimal instability between and within patients. Several challenges arise with this delivery method, such as the unfavorable pharmacokinetic parameters of the drug, the non-specificity of some chemotherapeutic agents, leading to severe side effects (off- and on-target toxicity), and the difficulty in traversing highly impermeable barriers, such as the blood–brain barrier [9]. In addition, the subcutaneous route is frequently associated with reducing local toxicity, facilitating the injection of highly concentrated forms, providing consistent doses over an extended duration and controlling numerous release rates [9].
Nanomedicines and nanocarriers represent encouraging approaches for delivering anticancer medications with improved accuracy and effectiveness [18,19]. Nanomedicines involve the encapsulation or attachment of therapeutic substances to nanoparticles, while nanocarriers are specialized vehicles crafted to transport drugs to precise locations within the body [20,21]. In the realm of anticancer treatment delivery, both nanomedicines and nanocarriers present multiple benefits [22]. Their nanoscale size enables them to traverse biological barriers more efficiently, including tumor tissues with impaired blood vessel function. This enhanced permeability and retention (EPR) effect enables preferential accumulation of the drug at the tumor site, minimizing exposure to healthy tissues and reducing systemic toxicity [23]. Furthermore, nanocarriers have the capability to be tailored to target specific molecular markers that are excessively expressed on cancer cells, thereby refining the precision of drug delivery. Moreover, nanocarriers can protect encapsulated drugs from degradation and premature clearance in the bloodstream, prolonging circulation time and enhancing bioavailability. This feature of controlled release enables sustained delivery of drugs, maintaining therapeutic levels over an extended duration and potentially decreasing the frequency of administration [24]. Additionally, the adaptability of nanomedicines and nanocarriers permits the simultaneous delivery of multiple drugs or therapeutic agents with complementary modes of action. This coordinated approach can bolster treatment effectiveness, surmount drug resistance, and diminish the likelihood of tumor recurrence [25]. The primary novel methodologies now rely on the utilization of nanomedicines and nanocarriers and ought to be expanded for the transportation of biomolecules [26,27].
The utilization of nanotechnology in anticancer drug delivery presents several challenges, including the encapsulation of hydrophilic or hydrophobic pharmaceuticals, regulation of drug release, protecting the loaded drugs from degradation, and improving drug absorption and penetration [28]. Nevertheless, there are certain limitations for using nanotechnology, including poor drug loading, rapid or burst release before reaching the intended target, and probability of clearance through renal filtration owing to nanoscale dimensions. The utilization of polymer–prodrug systems and the integration of polymer nanocomposites can overcome these drawbacks and enable the creation of a depot beneath the skin, facilitating the controlled and gradual release of drugs with minimal adverse effects on the surrounding region [29,30].
Drug-encapsulated nanocomposites offer numerous benefits, such as enhanced pharmacokinetics and the ability to selectively administer medications to specific locations or tumors [31].
Metallic nanoparticles, including gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs), are utilized in clinical applications. This is due to their unique forms, sizes, and surface-dependent properties [32]. Gold nanoparticles (AuNPs) are highly effective in the field of cancer diagnostics. The incorporation of anticancer drugs into colloidal AuNPs has the potential to overcome drug resistance, resulting in a reduction in the required dosage and, thereby, modifying the adverse effects on healthy cells [33]. AgNPs have demonstrated favorable conductivity and chemical stability [34]. Furthermore, they have been investigated as potential carriers for delivering therapeutic substances to diseased cells. Nevertheless, AgNPs tend to aggregate and form bigger clusters that diverge from the nanoscale. This reduces the efficacy of a nano-delivery system and poses challenges to its practical implementation [35]. Hence, it is necessary to incorporate further organic or biological surface coatings to enhance the stability of AuNPs and AgNPs [36,37]. Thus, it is widely acknowledged that natural polysaccharides and natural biopolymers are advantageous stabilizers. Therefore, metal nanoparticles tend to be aggregated in biological fluids, and this can be overcome by the generated electrostatic attractive forces between polysaccharide (such as amino groups of chitosan) and metallic nanoparticle [38]. Consequently, this can provide an effective driving force for the formation and stabilization of the Au and AgNPs [39,40].
The chitosan (CS) biopolymer is a derivative of chitin that has undergone deacetylation, resulting in notable biological characteristics [41,42]. These characteristics include reduced toxicity, enhanced biocompatibility, stability, and effective adhesion to mucus membranes. The utilization of chitosan in drug administration facilitates the pH selectivity of nanometals, as chitosan is a cationic biopolymer that is suitable for drug delivery purposes [43]. CS molecules possess NH2 and OH functional groups that serve as chelating sites for drugs within target cells, depending on their pH sensitivity. In addition, CS demonstrates the ability to selectively target specific sites for drug delivery, resulting in an enhanced therapeutic index of the drug in comparison to alternative natural materials [44,45]. Additionally, it exhibits antibacterial characteristics and improves the use of chitosan in drug encapsulation. Nevertheless, CS exhibits some limitations, such as reduced mechanical strength, limited solubility in both neutral and alkaline pH environments, and challenges associated with pore size adjustment [46,47].
In the current review, we highlighted the role of nanotechnology in cancer treatment in addition to nanocomposites and their applications for anticancer drug delivery. Moreover, the recent advances in chitosan and its nanocomposites with bioactive metallic nanoparticles (Ag and Au) for the effectual delivery of various anticancer drugs were investigated.
2. Nanotechnology for Cancer Treatment
Nanotechnology is the scientific study of materials that exist at a scale of 10−9 m [48]. Nanotechnology is widely regarded as a means of scientific progress, as demonstrated by the significant increase in commercial products, academic publications, and patents in the medical, pharmaceutical, and cosmetic sectors. Pharmaceutical and biotechnology companies are interested in improving medications that exhibit limited efficacy due to factors, such as poor solubility, toxicity, aggregation, poor mobility, rapid degradation in living organisms, or a short half-life [49,50]. Nanoparticles (NPs) have garnered attention as carriers for anticancer medications because of their advantageous properties, such as enhanced permeability and retention effect. In addition to diminishing the negative impacts of the medication, nanoparticles (NPs) have been found to provide protection against drug degradation and enhance the drug’s bioavailability [51,52].
There are still difficulties in the fabrication of metal nanoparticles (NPs) for purposes, such as imaging, drug delivery, diagnosis, and treatment. The development of nanoparticles remains challenging due to the observed instability of several nanoformulations in biological fluids [51,53]. Biological fluids’ high ionic strengths often induce NP aggregation and losing their colloidal stability, negatively affecting their function. The high content of biomacromolecules, including lipids, sugars, nucleic acids, and proteins, also affects NPs’ stability and viability for various applications [54]. Conventional approaches of cancer treatment are associated with negative consequences, including limited solubility, insufficient bioavailability, frequent deterioration, lack of specificity, and drug resistance. In addition, the high doses of conventional medications cause adverse effects in specific anatomical places, such as the skin, hair, RBCs, bone, genitourinary system, and lymphatic system [6].
NPs possess the capability to transport pharmaceuticals directly to cancerous cells, hence enhancing their efficacy. Nevertheless, the usefulness of nanoparticles is contingent upon the growth stage and type of tumor. The utilization of nanoparticles (NPs) in the field of cancer diagnosis and treatment has seen significant advancements, enabling the identification and treatment of single cancer cells through the targeted delivery of carriers. NPs, such as carbon nanotubes (CNTs), calcium nanoparticles (CaNPs), graphene, and polymeric NPs (including chitosan), have demonstrated enhanced capabilities in cancer diagnosis and treatment owing to their significant dimensions, surface charge, and morphology. NPs undergo functionalization with various biological molecules, such as antibodies, thereby facilitating the administration of drugs and the detection of cancer cells [6,55].
2.1. Role of NPs in Cancer Treatment
Nanoparticles can enhance the concentration of medication within cancer cells and direct therapy toward solid tumors via either passive or active targeting mechanisms, as demonstrated in Figure 2 [56]. An active targeting mechanism involves the incorporation of targeting ligands or molecules onto the surface of nanocarriers, enabling selective interactions and binding to cellular receptors. The effectiveness of drug delivery depends on the overexpression or coating of nanoparticles by either cancer cells or angiogenic endothelial cells, which enhance the internalization of nanoparticles and improve their efficacy [57]. The differentiation between tumor cells and healthy cells is facilitated by the presence of distinct molecular markers within the different cell types comprising the tumor bulk. Moreover, active targeting exhibits a high degree of selectivity, remarkable versatility, and a reduced incidence of side effects.
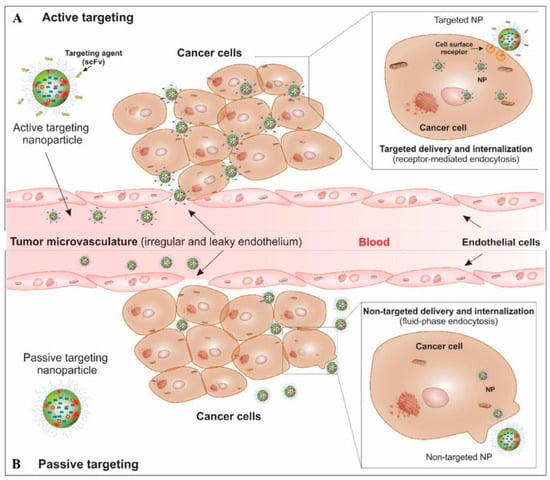
Figure 2.
A schematic diagram for the targeted therapy of solid tumors by passive and active targeting mechanisms. Reproduced with permission [56], Copyright 2024, Elsevier.
The passive targeting mechanism plays a crucial role in anticancer targeting therapy. In this mechanism, non-targeted nanoparticles can enter tumor sites through the sieve-like microvasculature of solid tumors, facilitated by permeability factors such as bradykinin and nitric oxide. This phenomenon is commonly referred to as the retention (EPR) effect [58]. Drug-loaded nanocarriers tend to accumulate within the tumor microenvironment (TME) because of their considerable size, whereas smaller molecules are liable for back diffusion and efflux. However, the EPR effect is not sufficient for an effective accumulation of molecules with low molecular weight near the tumor site [59], which are less selective, restricted in use, and cause more side effects [31].
For applications involving cancer, it is crucial to regulate the factors, such as the shape, surface charge, homogeneity, stability, and toxicity, of nanoparticles. The physicochemical properties are also influenced by the production process. Various materials can be exploited in these processes, such as metals, lipids (liposomes, solid lipid nanoparticles), and polymers (nanoparticles, micelles, and dendrimers) [60].
2.2. Nanocomposite in Cancer Therapy
Nanocomposites are often formed through the dispersion of a matrix phase with fillers, such as nanoparticles, nanolayers, and nanotubes. However, the classification of nanocomposites can vary depending on the material composition of each phase, resulting in three distinct forms. The organic–organic nanocomposite comprises organic nanoparticles distributed inside an organic polymer or lipid matrix phase. Furthermore, inorganic–inorganic nanocomposites consist of a matrix and dispersed phases made of one or more minerals. The last type of composite material is referred to as organic–inorganic nanocomposites, comprising an organic polymer matrix phase and a nanometric form of an inorganic filler phase [60]. Similarly, the utilization of nanocomposites in cancer therapy needs the process of modifying nanoparticles with biomolecules and incorporating medications into them to enhance their efficacy in tumor interaction. The hydrophilic polymer matrix leads to enhanced solubility of the nanoparticle and greater compatibility. Figure 3 depicts a proposed schematic design that provides a clear illustration of the synthesis process of the nanocomposite, which extends the potential for application in drug delivery for cancer treatment.
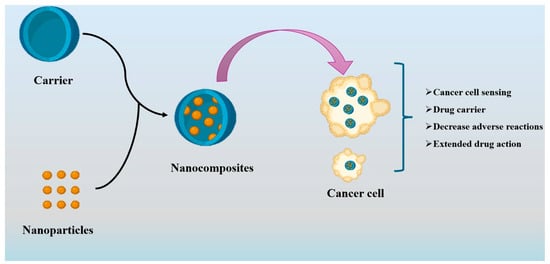
Figure 3.
Proposed diagram for the synthesis of the nanocomposite carrier in cancer treatment.
Nanocomposites in drug delivery systems are used to avoid cancer cell treatment resistance. The literature documents the synthesis and manufacture of many conjugated materials, such as graphene–gold [61], chitosan–palladium [62], gold ferrite [63], and graphene oxide/bismuth selenide [64], among others, for drug delivery [65]. Furthermore, the presence of a magnetic field gradient facilitates the vectorization of nanocomposites within the tumor, enabling the administration of medication. Some examples of possible materials include nano-graphene [66], amine-polyglycerol functional shell-modified silica-coated magnetic iron oxide [67], and hybrid protein-inorganic nanoparticles [68]. The pH level within the tumor microenvironment poses a significant challenge that nanocomposites must address. Cancer exhibits accelerated proliferation compared to healthy cells, resulting in a deficiency of oxygen and nutrients, leading to acidosis and a decrease in pH. Researchers have investigated pH-sensitive nanocomposite systems, like superparamagnetic Fe3O4 nanoparticles and magnetic nanoparticles made from chitosan, to treat cancer more effectively [69,70]. Research findings indicate that nanocomposites possess the ability to breach biological barriers and deliver drugs to specific cells within the tumor microenvironment. This consequently enhances the effectiveness of cancer therapy [71].
3. Chitosan and Its Nanocomposites for Cancer Treatment
Chitosan (CS) is a biopolymer made up of β-(1-4)-linked glucosamine and N-acetyl-D-glucosamine units [72]. It is a linear, cationic polysaccharide produced from simple deacetylation of the chitin biopolymer, which is present in the exoskeleton of insects, marine aquatic animals, and microorganisms, like fungi, yeast, and microalgae [73]. Chitosan has drawn much attention in diverse medical and pharmaceutical fields, such as tissue engineering, wound healing, and drug delivery [74]. This is due to its excellent characteristics, including low-cost production, non-toxic, mucoadhesive, biocompatibility, biodegradability, low immunogenicity, and antimicrobial and anticancer activities [41,46]. Owing to its cationic nature, adequate stability, versatility in formulation, and ease of modification, CS exhibits considerable potential as a viable option for the formation of nanoparticles. Various methods have been employed to fabricate CS NPs, such as ionotropic gelation, spray drying, crosslinking via water-in-oil emulsion, reverse micelle formation, emulsion-droplet coalescence, nanoprecipitation, and self-assembling techniques [75,76].
The hydrophilic drug can permeate epithelial cells by adhering to negatively charged cell membranes and then opening the tight connection between them. This process extends the retention of the medication and enhances cellular uptake [77]. Hence, many chitosan derivatives, such as chitosan–thymine conjugate, carboxymethyl chitosan, sulfated chitosan, sulfated benzaldehyde chitosan, and polypyrrole–chitosan, have exhibited encouraging anticancer properties against a wide range of cancer cells [78]. Chitosan derivatives can enhance their anticancer properties by combining them with nanocomposites or other chemical agents. The bioavailability of chitosan in tumor cells was improved through the conjugation of 3-amino-2-phenyl-4(3H)-quinazolinone on a nanocomposite of PPC-silver chloride. The formulation described in reference [68] effectively captured molecules from non-cancerous cells and demonstrated a prolonged release of chitosan into cancer cells. Figure 4 shows different formulations of CS-based multifunctional targeted nanosystems for the simultaneous imaging and therapy of cancer [56].
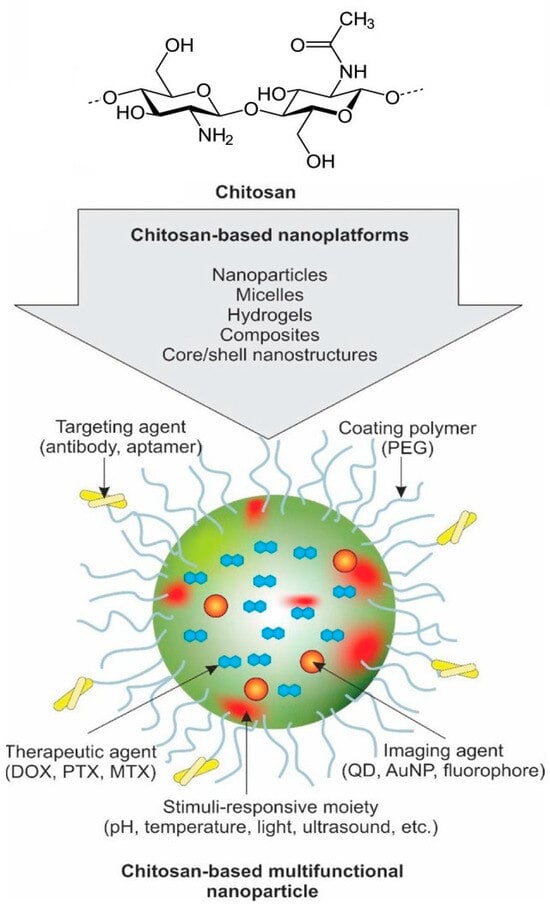
Figure 4.
A schematic representation of CS-based multifunctional targeted nanosystem for simultaneous imaging and therapy of cancer. Reproduced with permission [56], Copyright 2024, Elsevier.
3.1. Chitosan-Au Bio-Nanocomposite for Anticancer Drug Delivery
The utilization of AuNPs for drug administration is of extreme importance owing to their claimed biocompatibility and non-cytotoxicity [79,80,81]. Additionally, metal nanoparticles exhibit lower toxicity compared to other substances. They have a high surface-to-volume ratio, allowing them to encapsulate pharmaceuticals and serve as carriers for drug delivery [82]. Moreover, they exhibit exceptional infiltration into cancer tissues and, notably, lack immunogenicity within the human body. Additionally, this material exhibits distinct physical and chemical properties, including the influence of nanoscale, surface, quantum, electrical, and optical phenomena, hence extending specific advantages. In the field of photothermal therapy (PTT), gold nanoparticles (AuNPs) have been employed as a nanoscale-based photothermal agent to elevate the temperature of specific tissues through the conversion of light into heat [83]. This phenomenon can be attributed to their enhanced light absorption capabilities and increased stability under exposure to light. PTT specifically eliminates cancer cells, which are more exposed to an increase in temperature. AuNPs have certain limitations that hinder their application in the pharmaceutical industry. The toxicity of uncoated gold nanoparticles (AuNPs) is dependent upon various factors, including their composition, shape, size, coating, charge, hydrophobicity, solubility, reactivity, and the nature of biological medium qualities. Research has demonstrated that naked AuNPs tend to accumulate within lysosomes, resulting in alterations to their optical characteristics and how they are activated by radiation. This review discusses the application of chitosan-dispersed gold nanoparticle composites to load various anticancer medications, addressing the challenges associated with their encapsulation and mitigating the cytotoxic side effects. Table 1 presents the advantages of modifying matrix chitosan and dispersed gold nanoparticles to load various anticancer drugs.

Table 1.
Chitosan AuNP composite modifications and advantages for anticancer drug delivery.
3.1.1. Chitosan-Au Nanocomposite for Doxorubicin Delivery
Alle Madhusudhan et al. [84] prepared a composite of chitosan AuNPs that may load doxorubicin (DOX) according to the scheme represented in Figure 5 and exhibit anticancer properties. This composite is capable of targeting cancer cells under intracellular acidic conditions. The chitosan matrix phase was modified to carboxymethyl chitosan (CM-CS) to serve as a capping and reducing agent for the synthesis of AuNPs. This modification demonstrated the remarkable stability of AuNPs at different electrolyte concentrations and pH values. The ionization of carboxylic groups occurred at an acidic pH, resulting in increased drug release and enhanced pharmacological activity at an acidic pH that is suitable for targeting cancer cells. The structure of CM-CS is like amino acids, as it contains both amino and carboxylic groups. This unique composition enables effective drug interaction, particularly for DOX-like drugs. These drugs contain several amines and hydroxyl functional groups, which possess the ability to form hydrogen bonds with CM-chitosan-activated AuNPs. The covalent connections on the surface of AuNPs are strengthened by the hydrogen bonding and electrostatic attraction between the carboxylic groups of CM-CS and the amino groups of DOX molecules. Furthermore, cervical cancer cells exhibited a higher uptake of DOX when loaded with AuNPs compared to free DOX, suggesting a potential solution to address the issue of drug resistance in cancer. The cytotoxicity of free DOX is enhanced by CMC-AS-dispersed AuNP-loaded DOX.
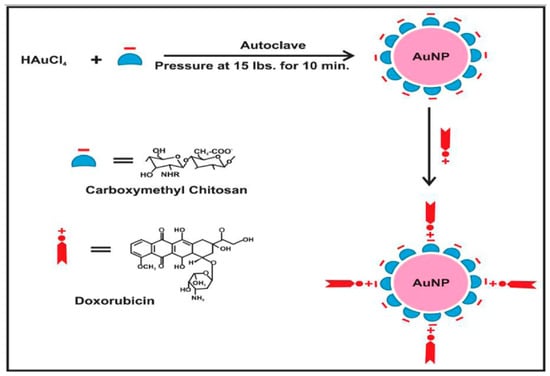
Figure 5.
Schematic diagram showing gold nanoparticles (AuNPs) stabilized in CM-chitosan and subsequent loading of cationic doxorubicin on AuNPs (the negative sign “–” refers to anionic carboxylic groups in carboxymethyl chitosan and the positive sign “+” refers to cationic NH2 groups in doxorubicin) [84].
3.1.2. Chitosan-Au Nanocomposite for 5-Fluroacil Delivery
Nivethaa et al. [85] fabricated bimetallic nanoparticles through the combination of gold nanoparticles with other metal nanoparticles. These nanoparticles were then dispersed into chitosan composites to facilitate the loading of 5-Fluroacil, an anticancer drug. Silver nanoparticles were incorporated into AuNPs, hence increasing their antibacterial efficacy. This approach aims to integrate the unique properties of Au (photothermal) and Ag nanoparticles within nanoparticle alloys comprising both metals. This merger aims to expose novel paths for research and medical treatment, owing to the distinctive properties exhibited by this alloy, which are significantly different from those of single metal components. The study involved the in situ reduction of AgNO3 and HAuCl4 in a CS solution, resulting in the formation of Ag-Au nanoparticles. A CS/Ag-Au fluorescent nanocomposite was prepared using a simple and cost-effective chemical process, with varying quantities of bimetallic nanoparticles distributed in chitosan. The manufactured composite has a distinct emission peak within the near-infrared range (700–900 nm), making it suitable for cellular imaging applications. The process of loading 5-FU drugs results in duplication of nanoparticle size, leading to a loading capacity of 97%. The incorporation of bimetallic nanoparticles resulted in an accelerated release of 5-FU, reaching 67.6% after 72 h, in contrast to the 97% release rate observed with a single metal dispersion. The cytotoxicity assessment of the nanocomposite containing 5-FU against MCF-7 cancer cells demonstrated that the bimetallic nanoparticles combined with the chitosan composite exhibited more efficacy compared to the individual Ag, Au, and CS particles.
3.1.3. Chitosan-Au Nanocomposite for Curcumin Delivery
Nadia G. Kandil et al. [86] developed another photothermal chitosan–gold nanoparticle composite to load curcumin, which can be used in cancer treatments. To incorporate a recently modified curcumin derivative, metal oxide (ZnO) nanoparticles and gold (Au) nanoparticles were combined within a chitosan mixture. This modified the mechanism by which curcumin specifically targeted cancer cells. The researchers prepared a new hydrazinocurcumin derivative (HCUR) by a thermal condensation reaction of curcumin, a polyphenol that is found naturally in the rhizome with a pyridazine derivative (VII). Pyridazines are an important group of heterocycles that have gotten a lot of attention in science, especially in the pharmaceutical field, because they have a wide range of biological activities and anticancer activity.
After that, the ZnO and Au nanoparticles that were dispersed in chitosan were used to load the HCUR composite that had been made using a self-assembling mechanism. The entrapment efficiency (EE%) exhibited a range of 39.41% to 73.70%. The Au-HCUR NPs showed the highest percentage (73.70%) but less than the capacity for loading 5-Fluoracil using a bimetallic silver and gold nanoparticle composite. A prolonged drug was released, and it was noticed that it happened faster at an acidic pH than at a neutral pH. This is because the nanoparticles diffuse better in the acidic environment. The study found that the type of cell had an impact on cell viability, indicating that NP formulations were more effective against HCT-116 cells (colon cancer) than CUR. Notably, CS-HCUR NPs show the highest level of activity in terms of cell viability. Also, ZnO-HCUR nanoparticles had the strongest effect on destroying HepG-2 cells, which are a type of hepatocellular carcinoma. According to this study, nanoformulations are very suitable for medicinal use as anticancer agents.
3.1.4. Chitosan–Au Nanocomposite for Paclitaxel (PTX) Delivery
Paclitaxel (PTX) has demonstrated efficacy in the treatment of breast and ovarian cancer; however, its solubility is limited, and its therapeutic use is limited [89,90]. Manivasagan et al. prepared a nanocomposite of chitosan AuNP-loaded PTX [87] to overcome the limitations of PTX as a drug carrier, ensuring minimal side effects. These composites were intended for use as contrast agents in photoacoustic imaging (PAI) and photoacoustic tomography (PAT). The utilization of contrast agents such as nanoparticles and the application of photoacoustic imaging (PAI), a more recent technological advancement, enable the imaging of tissues and cells. A modified chitosan matrix phase was used to disperse gold nanoparticles (AuNPs) that can work with biological systems. This matrix phase consisted of chitosan oligosaccharide (COS) as a reducing and stabilizing agent. Subsequently, paclitaxel (PTX) was loaded onto the AuNPs. The study demonstrated that PTX-COS AuNPs exhibited sustained and pH-dependent drug release profiles. Additionally, these nanoparticles were recognized as a unique class of optical contrast agents for photoacoustic medical imaging. It is worth noting that no prior research has explored the utilization of PTX-COS AuNPs as innovative agents for drug delivery. The PTX-COS AuNPs have shown effective cytotoxic properties against MDA-MB-231 cells by inducing apoptosis, resulting in increased formation of reactive oxygen species (ROS) and changed levels of matrix metalloproteinases (MMPs). Additionally, these AuNPs exhibited remarkable cellular uptake in MDA-MB-231 cells. In conclusion, PTX-COS AuNPs exhibit ability as advanced anticancer agents for the treatment of breast cancer and have potential as a novel contrast agent for photoacoustic imaging (PAI). By adding new gold nanoparticles (AuNPs) to this composite material, their unique physical and chemical properties, like surface plasmon resonance (SPR), as well as their optical properties, enhance the composite’s capability to permit surface modification for biomedical applications [91]. In addition, green synthesis, a cost-effective, zero-energy, and less time-consuming approach to synthesizing AuNPs without the utilization of toxic chemicals, has been documented [92]. However, the main challenge of CS is its poor solubility in neutral pH conditions. Chitosan oligosaccharide (COS) has garnered significant interest in the field of biomedical applications due to its ability to overcome these challenges and its potential as a highly suitable option for drug administration [93].
3.1.5. Chitosan-AuNPs for 6-Mercaptopurine (6MP) Delivery
A composite of chitosan-dispersed gold nanoparticles (AuNPs) was synthesized to load 6-mercaptopurine (6MP), a therapeutic agent employed in the treatment of cancerous solid tumors [94]. Faid et al. [88] developed chitosan-encapsulated 6MP with subsequent loading on AuNPs to form 6MP-CNPs-AuNPs nanocomposites, as depicted in Figure 6. The formulated nanocomposite has the advantage of reducing the side effects of 6MP, like other chemotherapeutic agents, such as allergic reactions and hepatotoxicity [95]. The researchers proposed encapsulating 6MP into chitosan nanoparticles (CNPs) to form chemically linked 6MP-CNP complexes. These complexes additionally loaded onto AuNPs, resulting in the development of a novel nanocomposite consisting of 6MP-CNPs-AuNPs. This nanocomposite facilitates therapeutic effects for chemo-photothermal techniques. Chitosan nanoparticles (CNPs) are prepared using an ionic gelation method. To make the nanoparticles, a tri-polyphosphate (TPP) polyanionic crosslinker and positively charged chitosan strands combine electrostatically [96]. In addition, the 6MP-CNP composite was prepared using the modified ionic gelation process. This composite was then loaded to AuNPs that were synthesized using the traditional wet chemical method, with tri-sodium citrate serving as a capping and reducing agent. Chitosan nanoparticles (CNPs) improve the targeting of drugs to cells by absorbing the loaded or encapsulated drugs [97]. The composite fabricated from CNPs and gold nanoparticles demonstrates exceptional stability in zeta potential. The encapsulation efficiency of 6MP was determined to be 57%. The cytotoxicity of 6MP-CNPs and 6MP-CNPs-AuNPs on the MCF7 breast cell line was significantly increased, resulting in IC50 values of 9.3 and 8.7 m, respectively. The combined effects of nanocomposites were more effective at treating cancer than the effects of chemotherapy and photothermal therapy used alone. A decrease in the IC50 values to 5 and 4.4 m, respectively, indicated a greater reduction in cell viability following the application of a diode laser to 6MP-CNPs and 6MP-CNPs-AuNPs.
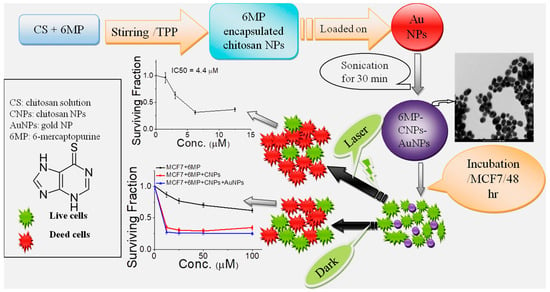
Figure 6.
Illustration of preparation of chitosan-encapsulated 6MP with subsequent loading on AuNPs to form 6MP-CNPs-AuNPs nanocomposites, as well as treatment of MCF7 with the as-prepared nanocomposites in the absence and presence of DPSS laser for chemo-photothermal therapy [88].
3.2. Chitosan-Ag Nanocomposite
Silver nanoparticles (AgNPs) are frequently used in composites for biomedical purposes to increase the surface area for drug encapsulation and improve the antibacterial efficacy [98]. When chitosan-dispersed silver nanoparticle composites are used as natural carriers for different anticancer drugs, they reduce the adverse side effects and improve the treatment profile. Ways to reach this approach through different modifications to challenge the properties of the composite are summarized in Table 2. Before that, different anticancer drug-loaded chitosan Ag nanocomposites were fabricated and explained as follows.

Table 2.
Chitosan-AgNP bio-nanocomposite modifications and advantages for anticancer drug delivery.
3.2.1. Chitosan-Ag Nanocomposite for 5-FU Delivery
Different modification processes have been performed on chitosan-dispersed AgNPs for improving the loading of 5-FU anticancer drugs despite the following challenges.
Modification of Chitosan Matrix Phase
The challenge to prepare a pH-sensitive controlled intestinal discharge of anticancer 5-FU medicine was approached by Hanna et al. [99], according to the schematic diagram in Figure 7. Silver nanoparticles (SNPs) were synthesized and dispersed within chitosan composite carriers to deliver them orally to the intestines composed of a polyelectrolyte complex (PEC)-modified chitosan matrix phase. The matrix phase of the PEC consists of chitosan that has been modified with N, N, and N trimethyl groups to enhance its solubility in water across a wide pH range (pH 2–9) and carboxymethyl Kappa-carrageenan that is derived from chloroacetic acid and Kappa-carrageenan. It is one of the carrageenan derivatives that is commonly employed in biomedical applications [105]. Silver nanoparticles were dispersed in various ratios using a one-pot green biosynthesis method. The encapsulation efficiency was further enhanced by increasing the amount of AgNPs, resulting in a peak efficiency of 92% with the maximum nanoparticle content. In addition, the incorporation of silver nanoparticles (AgNPs) leads to the prolonged release of 5-FU, achieving a 96% release rate within 24 h at a pH of 7.4. This release is attributed to distinct kinetic mechanisms at a pH of 1.2. The treated HCT116 cells exhibited significant cytotoxicity, as evidenced by an IC50 value of 31.15 µg/mL and an apoptotic cell count of 30.66%.
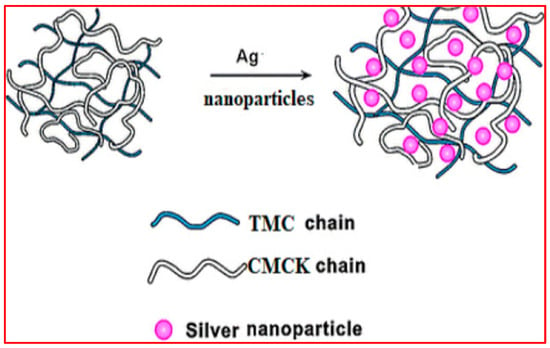
Figure 7.
Schematic diagram showing the formation of silver nanocomposites (SNC) through the incorporation of Ag nanoparticles in (TMC-CMCK) PEC. Reproduced with permission [99]. Copyright 2024, Elsevier.
Dispersed-Phase AgNPs Modification with CNT
In a study by Nivethaa et al. [100], a chitosan-incorporated sliver nanoparticle-encapsulated 5-Fu anticancer drug composite was prepared using chemical methods. The researchers observed release and cytotoxicity results that were like those reported in a previous study by Hanna et al. [99]. This study aimed to prepare systems with more prolonged release time to extend the dosage intervals. The incorporation of carbon nanotubes (CNTs) into the composite was preferred due to the numerous advantages they offer for biomedical applications. Hence, the inclusion of carbon nanotubes (CNTs) prolongs the sustained release of 5-FU to 63.1% after 72 h, compared to 88.1% for the CS-Ag nanocomposite without CNT. This is attributed to the relatively strong binding of 5-FU to multi-walled carbon nanotubes (MWCNTs). Furthermore, the IC50 value serves as an indicator of the comparative effectiveness of the CS/Ag nanocomposite encapsulated with 5-FU in destroying carcinogenic MCF-7 cells, in addition to its cytotoxic properties. This can be attributed to the characteristic capability of carbon nanotubes to facilitate targeted drug delivery to cells and tissues because of their ability to penetrate the cellular membrane and enter cellular components without inducing observable harm to the cells [100].
Dispersed-Phase AgNP Modification with GO
Another enhancement for the chitosan composite is dispersed silver nanoparticles to encapsulate 5-FU shifted towards emerging photothermal properties, along with antimicrobial and release sensitivity, which enhance cytotoxicity against tumor cells by adding two different components.
Su et al. [101] reported the incorporation of graphene oxide (GO) into the chitosan composite. Because the composite was formulated with unique physiochemical properties, it could be used in many biomedical areas, such as drug delivery, photothermal therapy, and tissue regeneration [106,107]. The unique properties of each component material were fully utilized for the first time in this kind of composite system. These include the antimicrobial properties of dispersed AgNPs that are made using green or chemical methods, the pH responsiveness of CS, and the photothermal conversion property of GO. The new multiple functions that the prepared composite system showed could make it suitable for many applications, such as antimicrobial, releasing fluorouracil drugs, and photothermal cancer treatment.
3.2.2. Chitosan-Ag Nanocomposite for Dox Delivery
Rasoulzadehzali et al. [102] prepared a bio-nanocomposite consisting of chitosan AgNPs for the controlled release of Dox. The fabrication of the bio-nanocomposite involved the dispersion of graphene oxide (GO) onto silver (Ag) nanohybrid particles. This process resulted in the formation of unique pH-sensitive bio-nanocomposite hydrogel beads, which were crosslinked using sodium, a polyanion crosslinking agent, tripolyphosphate (STPP) [108]. The dispersion of GO-Ag nanohybrid particles within the chitosan composite exhibits a notable enhancement in drug loading capacity, as well as prolonged drug release. In addition, it was observed that the release percentage of Dox was higher at pH 1.2 compared to pH 6.8. This can be attributed to the protonation of the NH2 groups of chitosan under acidic conditions, resulting in increased swelling and subsequently leading to higher release performance. The bio-nanocomposite that was prepared did not exhibit any notable toxicity on SW480 cells when DOX was not loaded. However, the burst release behavior of the loaded bio-nanocomposite led to a considerable reduction in the viability of these cells. This characteristic makes it a promising candidate for cancer therapy.
3.2.3. Chitosan-Ag Nanocomposite for Curcumin Delivery
Curcumin (CUR), an anticarcinogenic agent [109], was selected as a representative of extremely water-insoluble hydrophobic drugs that exhibit limited bioavailability, significant instability in aqueous basic environments, and a higher rate of degradation in the gastrointestinal tract (GIT). These factors pose challenges to the conventional oral administration of CUR and restrict its ability to be loaded onto carriers [110]. Therefore, there has been increased attention towards preparing novel-controlled drug delivery systems (DDS) for gastrointestinal (GIT) purposes. These systems aim to improve the effectiveness of targeting the intestines while minimizing stomach discharge and reducing the overall toxicity to the body. Therefore, El-Maadawy et al. [103] successfully prepared a composite material consisting of chitosan-dispersed AgNPs to encapsulate CUR. The composite matrix phase was modified to form a polyelectrolyte complex (PEC) that serves as a pH-sensitive carrier for the efficient and controlled delivery of CUR to cancer cells through the gastrointestinal tract (GIT). This approach is similar to the modified chitosan composite previously fabricated by Hanna et al. [99], which was designed to load 5-FU and exhibit pH sensitivity in the intestine. The chitosan matrix phase has been modified with N, N, N-trimethyl chloride formation (TMC), which exhibits water solubility through a wide pH range of 2 to 9. This modified matrix phase is also utilized for reducing silver-forming nanoparticles to be dispersed, with the inclusion of carrageenan. This incorporation of carrageenan is increasingly recognized as a biomedical benefit for the composite material. The drug-loaded carrier, AgNPs, exhibited significant antibacterial efficacy against various bacterial strains. Furthermore, the novel carrier (CUR-loaded AgNPs) exhibited a non-cytotoxic effect on human normal cells, demonstrating favorable biocompatibility. However, it also induced apoptosis in malignant cells, resulting in a significant cytotoxic effect. Therefore, this is proposed to be a safe oral formulation in the controlled transport of hydrophobic drugs. The release capacity of curcumin, while maintaining its bioactivity, was found to be greater in simulated intestinal fluid (SIF, pH 7.4) compared to gastric fluid (SGF, pH 1.2), with a sustained efficacy of up to 24 h. In addition, it was observed that the loading capacity and encapsulation efficiency of CUR increased with an increased dispersion of silver nanoparticles in an acidic solution. This may be due to the properties embedded in composite matrix phase modification based on chitosan modification and the incorporation of carrageenan. Furthermore, the adsorption of CUR on the nanoparticles facilitates an improvement in the drug loading capacity [111], thereby promoting the controlled release of the loaded drug [112].
3.2.4. Chitosan-Ag Nanocomposite for Cisplatin (CIS) Delivery
CIS, like most anticancer drugs, lacks sensitivity and specificity, leading to unpleasant side effects in patients because of administering high therapeutic doses. Hence, there is a need for novel treatment strategies to mitigate these problems. Although limited studies have been conducted on the evaluation of CIS-conjugated AgNPs functionalized with a CS biopolymer, S. Gounden et al. [104] evaluated the sensitivity and specificity of CS-functionalized AgNPs as delivery vehicles of CIS to human cancer cells. They synthesized AgNPs, functionalized with chitosan (CS), and loaded with the anticancer drug cisplatin (CIS) to be studied against Caco-2 (colorectal adenocarcinoma), MCF-7 (breast adenocarcinoma), HepG2 (hepatocellular carcinoma) and SKBR-3 (breast adenocarcinoma), and the non-cancer HEK293 (embryonic kidney) cells. The delivery system of AgNPs functionalized with chitosan exhibited desirable properties, including small size, a high positive zeta potential, and the ability to encapsulate over 80% of cisplatin. This cisplatin was released rapidly from the nano-complex at low pH, making it suitable for delivery to the tumor microenvironment. The compound demonstrated a high degree of selectivity towards breast cancer cells, resulting in more than 80% cell death at dosages below 10 μg. Additionally, it exhibited no cytotoxicity towards non-cancerous cells, even without the inclusion of a targeting moiety. The results demonstrated a potentially efficient anticancer drug delivery system with selectivity to breast cancer cells.
4. Challenges and Perspectives
The use of nanomedicine in cancer therapy sounds promising and the integration of Au and AgNPs within a chitosan matrix holds significant potential for cancer therapy advancements. Certain obstacles must be conquered in order to fully utilize their capabilities. In order to solve the difficulties that arise from the integration of medicinal and imaging elements in a nanocomposite, scientists need to develop innovative approaches to guarantee smooth operation between the different sections.
Based on the present overview, the therapeutic efficacy of the CS nanocomposite is significantly influenced by its stability and the sustained drug release characteristics. The continuous resolution of hard issues, such as ensuring controlled release kinetics, minimizing premature drug leakage, and sustaining drug concentrations at the target area, is essential. The majority of studies focus on encapsulating well-known anticancer drugs such as doxorubicin, 5-Fluorouracil, curcumin, and cisplatin, often neglecting other crucial medications. Therefore, it is prudent to consider a broader spectrum of anticancer drugs, including Daunorubicin for Kaposi’s sarcoma and myeloid leukemia [113], Vinorelbine for breast cancer and solid tumors [114], Mitoxantrone (for ovarian, breast, stomach, and leukemia) [115], Methotrexate for lung cancer [116], and Disulfiram for breast cancer [117]. Additionally, the most commonly used chitosan derivatives as a matrix phase in the nanocomposite carrier are carboxymethyl-forming CS and trimethyl CS for enhanced solubility, pH control, and loading capacity, while the antimicrobial activity is dependent only on the incorporated AgNPs. So, it is recommended to develop new CS derivatives with multi-bio characteristics including self-antimicrobial activities [118].
Moreover, both metals (such as AgNPs) and metal oxides (such as ZnO NPs) are mostly involved in the modifications of dispersed AuNPs along the CS nanocomposites for improving the entrapment efficiency of the anticancer drugs. Hence, it is necessary to find alternative materials with high performance. There are several effectual carbon-based materials such as graphene oxide derivatives and carbon nanotubes in addition to quantum dots proving their biological properties, which can be used for the modification of AuNPs. These materials are expected to offer protection for the entrapped drug molecules from degradation, as well as enhance the solubility, biocompatibility, photostability, and photothermal properties [119]. It is recommended that scientists collaborate across various fields for continuous advancements in nanotechnology to effectively harness the medicinal potential of these innovative nanocomposites.
Finally, one further challenge associated with the transformation of this technology to the commercial market is the absence of regulatory guidelines and standards for ensuring the safe production and quality control of these treatments. Consequently, it is obligatory to establish legal protocols for the development and clinical application of chitosan nanocomposites.
5. Conclusions
Nanomedicine products are potentially lucrative prospects for attaining advanced targeting methods and multifunctionality. The chitosan matrix phase can be modified to enhance the composite targeting properties, making it more sensitive to pH. The nanocomposite surface has been actively functionalized to selectively target cancer cells, allowing for precise contact with cancer cell receptors. Furthermore, the surface of the nanocomposite underwent active modification, facilitating its selective interaction with cancer cell receptors, thereby permitting targeted delivery to cancer cells. The incorporation of gold and silver nanoparticles within the chitosan nanocomposite holds considerable promise for anticancer drug delivery. The present review briefly outlines the ongoing efforts in the field of nanomedicine by enabling the transformation of nanocomposites into practical applications for personalized cancer treatment.
Author Contributions
Conceptualization, A.M.O., M.A.E.-M. and A.S.E.; investigation, A.M.O., E.M.A.E.-M. and A.H.; writing—original draft preparation, M.A.E.-M., E.M.A.E.-M. and A.M.O.; writing—review and editing, A.M.O., M.S.M.-E., A.H., Z.M.Z. and A.S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This project received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie-Sklodowska-Curie grant agreement No. 945478, project No. 1381/03/02. Additionally, this work was supported by APVV-22-0568 and the FLAG-ERA grant GRAPH-OCD, by the Slovak Academy of Sciences under the grant number FLAG ERA III/2023/808/GRAPH-OCD.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Sachdeva, P.; Ghosh, S.; Ghosh, S.; Han, S.; Banerjee, J.; Bhaskar, R.; Sinha, J.K. Childhood obesity: A potential key factor in the development of glioblastoma multiforme. Life 2022, 12, 1673. [Google Scholar] [CrossRef]
- Gielecińska, A.; Kciuk, M.; Yahya, E.-B.; Ainane, T.; Mujwar, S.; Kontek, R. Apoptosis, necroptosis, and pyroptosis as alternative cell death pathways induced by chemotherapeutic agents? Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 189024. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Ben-Sahra, I. Regulation of nucleotide metabolism in cancers and immune disorders. Trends Cell Biol. 2023, 33, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, B.; Sachdeva, P.; Negi, A.; Ghosh, S.; Han, S.; Dewanjee, S.; Jha, S.K.; Bhaskar, R.; Sinha, J.K.; Paiva-Santos, A.C. Chitosan nanoparticles-based cancer drug delivery: Application and challenges. Mar. Drugs 2023, 21, 211. [Google Scholar] [CrossRef]
- Eala, M.A.B.; Robredo, J.P.G.; Dee, E.C.; Lin, V.; Lagmay, A.M.F.A. Climate crisis and cancer: Perspectives from the hardest hit. Lancet Oncol. 2022, 23, e92. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting symptoms of cancer and stage at diagnosis: Evidence from a cross-sectional, population-based study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, M.; Gaudin, A.; Nakhlé, J.; Veiman, K.-L.; Richard, J.; Chassaing, C. Challenges and opportunities in the delivery of cancer therapeutics: Update on recent progress. Ther. Deliv. 2021, 12, 55–76. [Google Scholar] [CrossRef]
- Fanotto, V.; Salani, F.; Vivaldi, C.; Scartozzi, M.; Ribero, D.; Puzzoni, M.; Montagnani, F.; Leone, F.; Vasile, E.; Bencivenga, M. Primary tumor resection for metastatic colorectal, gastric and pancreatic cancer patients: In search of scientific evidence to inform clinical practice. Cancers 2023, 15, 900. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent advances in targeted drug delivery strategy for enhancing oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Henn, J.G.; Aguirre, T.A.S.; Nugent, M.; Moura, D.J. Cancer nanomedicine: Recent developments in drug delivery systems and strategies to overcome eventual barriers to achieve a better outcome. J. Drug Deliv. Sci. Technol. 2023, 91, 105254. [Google Scholar] [CrossRef]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, A.M.; Jafari, M.; Grant, T.M.; Zhang, S.; Slater, H.C.; Wenger, E.A.; Mo, S.; Lee, Y.-A.L.; Mazdiyasni, H.; Kogan, L. Oral, ultra–long-lasting drug delivery: Application toward malaria elimination goals. Sci. Transl. Med. 2016, 8, 365ra157. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Alqosaibi, A.I. Nanocarriers for anticancer drugs: Challenges and perspectives. Saudi J. Biol. Sci. 2022, 29, 103298. [Google Scholar] [CrossRef]
- Arafat, M.; Sakkal, M.; Beiram, R.; AbuRuz, S. Nanomedicines: Emerging Platforms in Smart Chemotherapy Treatment—A Recent Review. Pharmaceuticals 2024, 17, 315. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Al Harby, N.; Batouti, M.E.; Eltaweil, A.S. Enhanced Redox Cycle of Rod-Shaped MIL-88A/SnFe2O4@ MXene Sheets for Fenton-like Degradation of Congo Red: Optimization and Mechanism. Nanomaterials 2023, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A. Smart nanocarriers as an emerging platform for cancer therapy: A review. Molecules 2021, 27, 146. [Google Scholar] [CrossRef]
- Natarajan, A.; Kirubavathy, S.J. Nanomedicine and nanocarriers for cancer treatment. In Nanotechnology for Drug Delivery and Pharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 71–110. [Google Scholar]
- Paramasivam, G.; Sanmugam, A.; Palem, V.V.; Sevanan, M.; Sairam, A.B.; Nachiappan, N.; Youn, B.; Lee, J.S.; Nallal, M.; Park, K.H. Nanomaterials for detection of biomolecules and delivering therapeutic agents in theragnosis: A review. Int. J. Biol. Macromol. 2024, 254, 127904. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Khizar, S.; Alrushaid, N.; Khan, F.A.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Nanocarriers based novel and effective drug delivery system. Int. J. Pharm. 2023, 632, 122570. [Google Scholar] [CrossRef]
- Raman, A.P.S.; Singh, P.; Jain, P. Selective treatment of tumors using nanocarriers. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 261–276. [Google Scholar]
- Choukaife, H.; Seyam, S.; Alallam, B.; Doolaanea, A.A.; Alfatama, M. Current advances in chitosan nanoparticles based oral drug delivery for colorectal cancer treatment. Int. J. Nanomed. 2022, 17, 3933–3966. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Geetanjali, S.P.; Malviya, R. Toxicity and application of nano-silver in multi-drug resistant therapy. Lett. Appl. NanoBioSci 2020, 9, 824–829. [Google Scholar]
- Milić, M.; Leitinger, G.; Pavičić, I.; Zebić Avdičević, M.; Dobrović, S.; Goessler, W.; Vinković Vrček, I. Cellular uptake and toxicity effects of silver nanoparticles in mammalian kidney cells. J. Appl. Toxicol. 2015, 35, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mijakovic, I. Advances in gold nanoparticle technology as a tool for diagnostics and treatment of cancer. Expert Rev. Mol. Diagn. 2021, 21, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Kowalski, R.; Capasso, R.; Douglas Melo Coutinho, H.; Rose Alencar De Menezes, I. Various novel strategies for functionalization of gold and silver nanoparticles to hinder drug-resistant bacteria and cancer cells. Micro Nano Bio Asp. 2022, 1, 38–48. [Google Scholar]
- Omer, A.M.; Elgarhy, G.S.; El-Subruiti, G.M.; Abd El-Monaem, E.M.; Eltaweil, A.S. Construction of efficient Ni-FeLDH@ MWCNT@ Cellulose acetate floatable microbeads for Cr (VI) removal: Performance and mechanism. Carbohydr. Polym. 2023, 311, 120771. [Google Scholar] [CrossRef] [PubMed]
- Facchi, D.P.; da Cruz, J.A.; Bonafe, E.G.; Pereira, A.G.B.; Fajardo, A.R.; Venter, S.A.S.; Monteiro, J.P.; Muniz, E.C.; Martins, A.F. Polysaccharide-based materials associated with or coordinated to gold nanoparticles: Synthesis and medical application. Curr. Med. Chem. 2017, 24, 2701–2735. [Google Scholar] [CrossRef] [PubMed]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Krzan, M.; Ciesielski, W.; Żarska, S.; Szczepankowska, J. Polysaccharides composite materials as carbon nanoparticles carrier. Polymers 2022, 14, 948. [Google Scholar] [CrossRef]
- Moghaddam, F.D.; Zare, E.N.; Hassanpour, M.; Bertani, F.R.; Serajian, A.; Ziaei, S.F.; Paiva-Santos, A.C.; Neisiany, R.E.; Makvandi, P.; Iravani, S. Chitosan-based nanosystems for cancer diagnosis and therapy: Stimuli-responsive, immune response, and clinical studies. Carbohydr. Polym. 2024, 330, 121839. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Ayoup, M.S.; Omer, A.M.; Hammad, E.N.; Eltaweil, A.S. Sandwich-like construction of a new aminated chitosan Schiff base for efficient removal of Congo red. Appl. Water Sci. 2023, 13, 67. [Google Scholar] [CrossRef]
- AM, O.; MA, W.; TM, T.; SA, I. Novel smart pH sensitive chitosan grafted alginate hydrogel microcapsules for oral protein delivery: I. Preparation and characterization. Int. J. Pharm. Pharm. Sci. 2015, 7, 320–326. [Google Scholar]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Monaem, E.M.; Omer, A.M.; Eltaweil, A.S. Durable and low-cost Chitosan decorated Fe/MOF-5 bimetallic MOF composite film for high performance of the Congo red adsorption. J. Polym. Environ. 2023, 1–16. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Wiranowska, M. Advances in the Use of Chitosan and Chlorotoxin-Functionalized Chitosan Polymers in Drug Delivery and Detection of Glioma-A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100427. [Google Scholar] [CrossRef]
- Alibek, K.; Kakpenova, A.; Baiken, Y. Role of infectious agents in the carcinogenesis of brain and head and neck cancers. Infect. Agents Cancer 2013, 8, 7. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Cancer 2017, 8, 147–166. [Google Scholar]
- Eltaweil, A.S.; Bakr, S.S.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Magnetic hierarchical flower-like Fe3O4@ ZIF-67/CuNiMn-LDH catalyst with enhanced redox cycle for Fenton-like degradation of Congo red: Optimization and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 75332–75348. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Ataide, J.A.; Costa, J.S.R.; de Freitas Coêlho, D.; Liszbinski, R.B.; Lopes, A.M.; Oliveira-Nascimento, L.; de Jesus, M.B.; Jozala, A.F.; Ehrhardt, C. Nanotechnology as a tool to overcome macromolecules delivery issues. Colloids Surf. B Biointerfaces 2023, 222, 113043. [Google Scholar] [CrossRef]
- Jadhav, V.; Roy, A.; Kaur, K.; Rai, A.K.; Rustagi, S. Recent advances in nanomaterial-based drug delivery systems. Nano-Struct. Nano-Objects 2024, 37, 101103. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; El-Meligy, M.A.; Ghaly, Z.S.; Kenawy, M.E.; Kamoun, E.A. Novel Physically-Crosslinked Caffeine and Vitamin C-Loaded PVA/Aloe Vera Hydrogel Membranes for Topical Wound Healing: Synthesis, Characterization and In-Vivo Wound Healing Tests. J. Polym. Environ. 2023, 1–18. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.; Khan, K.; Qasim, M. Nanotechnology, a tool for diagnostics and treatment of cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef]
- Fathi, M.; Majidi, S.; Zangabad, P.S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Chitosan-based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Huang, Q.; Tang, J.-Q.; Hou, X.-Y.; Zhang, P.; Zhang, L.Z.; Jiang, G. Nanoscale drug delivery for targeted chemotherapy. Cancer Lett. 2016, 379, 24–31. [Google Scholar] [CrossRef]
- Lahooti, B.; Akwii, R.G.; Zahra, F.T.; Sajib, M.S.; Lamprou, M.; Alobaida, A.; Lionakis, M.S.; Mattheolabakis, G.; Mikelis, C.M. Targeting endothelial permeability in the EPR effect. J. Control. Release 2023, 361, 212–235. [Google Scholar] [CrossRef]
- Ghazal, H.; Waqar, A.; Yaseen, F.; Shahid, M.; Sultana, M.; Tariq, M.; Bashir, M.K.; Tahseen, H.; Raza, T.; Ahmad, F. Role of nanoparticles in enhancing chemotherapy efficacy for cancer treatment. Next Mater. 2024, 2, 100128. [Google Scholar] [CrossRef]
- Nalone, L.A.; Amaral, R.G.; de Lima Oliveira, D.M.; Andrade, L.R.; de Hollanda, L.M.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of nanocomposite materials in the delivery of anticancer drugs. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 339–352. [Google Scholar]
- Al-Ani, L.A.; AlSaadi, M.A.; Kadir, F.A.; Hashim, N.M.; Julkapli, N.M.; Yehye, W.A. Graphene–gold based nanocomposites applications in cancer diseases; Efficient detection and therapeutic tools. Eur. J. Med. Chem. 2017, 139, 349–366. [Google Scholar] [CrossRef]
- Dhanavel, S.; Nivethaa, E.; Narayanan, V.; Stephen, A. In vitro cytotoxicity study of dual drug loaded chitosan/palladium nanocomposite towards HT-29 cancer cells. Mater. Sci. Eng. C 2017, 75, 1399–1410. [Google Scholar] [CrossRef]
- Heidari, M.; Sattarahmady, N.; Azarpira, N.; Heli, H.; Mehdizadeh, A.; Zare, T. Photothermal cancer therapy by gold-ferrite nanocomposite and near-infrared laser in animal model. Lasers Med. Sci. 2016, 31, 221–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, Y.; Wu, H.; Zeng, B.; Zhang, Y.; Tian, Q.; Yang, S. Hydrophilic graphene oxide/bismuth selenide nanocomposites for CT imaging, photoacoustic imaging, and photothermal therapy. J. Mater. Chem. B 2017, 5, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Maheshwari, N.; Bag, A.; Sharma, M.C.; Prajapati, B.; Maheshwari, R. Polymer mediated light responsive therapeutics delivery system to treat cancer. Eur. Polym. J. 2024, 210, 112923. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Oh, Y.; Bharathiraja, S.; Manivasagan, P.; Rajarathinam, T.; Jang, B.; Phan, T.T.V.; Jang, H.; Oh, J. Synthesis of amine-polyglycidol functionalised Fe3O4@ SiO2 nanocomposites for magnetic hyperthermia, pH-responsive drug delivery, and bioimaging applications. RSC Adv. 2016, 6, 110444–110453. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Hemasa, A.L.; Freag, M.S. Hybrid protein-inorganic nanoparticles: From tumor-targeted drug delivery to cancer imaging. J. Control. Release 2016, 243, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Sajomsang, W.; Choi, Y.; Jang, E.; Lee, H.; Kang, B.; Kim, E.; Haam, S.; Suh, J.-S.; Chung, S.J. Chitosan-based intelligent theragnosis nanocomposites enable pH-sensitive drug release with MR-guided imaging for cancer therapy. Nanoscale Res. Lett. 2013, 8, 467. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, C.; Li, B.; Shan, M.; Wu, G. A magnetic polypeptide nanocomposite with pH and near-infrared dual responsiveness for cancer therapy. J. Polym. Res. 2017, 24, 122. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Eldin, M.M.; Hashem, A.; Tamer, T.; Omer, A.; Yossuf, M.; Sabet, M. Development of cross linked chitosan/alginate polyelectrolyte proton exchanger membranes for fuel cell applications. Int. J. Electrochem. Sci. 2017, 12, 3840–3858. [Google Scholar] [CrossRef]
- Tamer, T.M.; Omer, A.M.; Hassan, M.A.; Hassan, M.E.; Sabet, M.M.; Eldin, M.S.M. Development of thermo-sensitive poly N-isopropyl acrylamide grafted chitosan derivatives. J. Appl. Pharm. Sci. 2015, 5, 001–006. [Google Scholar] [CrossRef]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A review on Chitosan’s uses as biomaterial: Tissue engineering, drug delivery systems and cancer treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Liu, X.; Oyarzún, D.A.; Abdel-Azeem, A.M.; Atanasov, A.G.; Hesham, A.E.-L.; Barik, S.K.; Gupta, V.K.; Singh, B.N. Microbial Polysaccharides: An Emerging Family of Natural Biomaterials for Cancer Therapy and Diagnostics; Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 706–731. [Google Scholar]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robertson, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: In vivo pharmacokinetics and X-ray-contrast-imaging studies. Small 2007, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Huang, L.; Zhai, M.; Peng, J.; Xu, L.; Li, J.; Wei, G. Synthesis, size control and fluorescence studies of gold nanoparticles in carboxymethylated chitosan aqueous solutions. J. Colloid Interface Sci. 2007, 316, 398–404. [Google Scholar] [CrossRef] [PubMed]
- de Britto, D.; Assis, O.B. A novel method for obtaining a quaternary salt of chitosan. Carbohydr. Polym. 2007, 69, 305–310. [Google Scholar] [CrossRef]
- Cabral, R.M.; Baptista, P.V. The chemistry and biology of gold nanoparticle-mediated photothermal therapy: Promises and challenges. Nano Life 2013, 3, 1330001. [Google Scholar] [CrossRef]
- Madhusudhan, A.; Reddy, G.B.; Venkatesham, M.; Veerabhadram, G.; Kumar, D.A.; Natarajan, S.; Yang, M.-Y.; Hu, A.; Singh, S.S. Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. Int. J. Mol. Sci. 2014, 15, 8216–8234. [Google Scholar] [CrossRef]
- Nivethaa, E.; Dhanavel, S.; Narayanan, V.; Stephen, A. Chitosan stabilized Ag-Au nanoalloy for colorimetric sensing and 5-fluorouracil delivery. Int. J. Biol. Macromol. 2017, 95, 862–872. [Google Scholar]
- Kandile, N.G.; Mohamed, H.M.; Nasr, A.S. Novel hydrazinocurcumin derivative loaded chitosan, ZnO, and Au nanoparticles formulations for drug release and cell cytotoxicity. Int. J. Biol. Macromol. 2020, 158, 1216–1226. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Lim, I.G.; Oh, J. Paclitaxel-loaded chitosan oligosaccharide-stabilized gold nanoparticles as novel agents for drug delivery and photoacoustic imaging of cancer cells. Int. J. Pharm. 2016, 511, 367–379. [Google Scholar] [CrossRef]
- Faid, A.H.; Shouman, S.A.; Badr, Y.A.; Sharaky, M.; Mostafa, E.M.; Sliem, M.A. Gold nanoparticles loaded chitosan encapsulate 6-mercaptopurine as a novel nanocomposite for chemo-photothermal therapy on breast cancer. BMC Chem. 2022, 16, 94. [Google Scholar] [CrossRef]
- Debien, V.; Marta, G.N.; Agostinetto, E.; Sirico, M.; Jacobs, F.; Molinelli, C.; Moreau, M.; Paesmans, M.; De Giorgi, U.; Santoro, A. Real-world clinical outcomes of patients with stage I HER2-positive breast cancer treated with adjuvant paclitaxel and trastuzumab. Crit. Rev. Oncol./Hematol. 2023, 190, 104089. [Google Scholar] [CrossRef]
- Mustafa, G.; Hassan, D.; Ruiz-Pulido, G.; Pourmadadi, M.; Eshaghi, M.M.; Behzadmehr, R.; Tehrani, F.S.; Rahdar, A.; Medina, D.I.; Pandey, S. Nanoscale drug delivery systems for cancer therapy using paclitaxel—A review of challenges and latest progressions. J. Drug Deliv. Sci. Technol. 2023, 84, 104494. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold nanoparticles-based photothermal therapy for breast cancer. Photodiagn. Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef]
- Singh, P.; Singh, H.; Kim, Y.J.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications. Enzym. Microb. Technol. 2016, 86, 75–83. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, P.; Tian, H. Recent updates in the polysaccharides-based Nano-biocarriers for drugs delivery and its application in diseases treatment: A review. Int. J. Biol. Macromol. 2021, 182, 115–128. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ghohrodi, A.R.; Savari, Z.; Talebi, E.; Ahamdi, I.; Rahdar, A.; Pandey, S. Enhancing cancer therapy: The potential of mercaptopurine-based nanomaterials for targeted drug delivery. Next Nanotechnol. 2023, 2, 100018. [Google Scholar] [CrossRef]
- Mueller, X.M. Drug immunosuppression therapy for adult heart transplantation. Part 1: Immune response to allograft and mechanism of action of immunosuppressants. Ann. Thorac. Surg. 2004, 77, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.M.; Leong, K.W. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M.; Grecchi, S.; Chiesa, E.; Cisterna, B.; Costanzo, M.; Zancanaro, C. Internalized chitosan nanoparticles persist for long time in cultured cells. Eur. J. Histochem. EJH 2015, 59, 2492. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Hanna, D.H.; El-Mazaly, M.H.; Mohamed, R.R. Synthesis of biodegradable antimicrobial pH-sensitive silver nanocomposites reliant on chitosan and carrageenan derivatives for 5-fluorouracil drug delivery toward HCT116 cancer cells. Int. J. Biol. Macromol. 2023, 231, 123364. [Google Scholar] [CrossRef] [PubMed]
- Nivethaa, E.; Dhanavel, S.; Rebekah, A.; Narayanan, V.; Stephen, A. A comparative study of 5-Fluorouracil release from chitosan/silver and chitosan/silver/MWCNT nanocomposites and their cytotoxicity towards MCF-7. Mater. Sci. Eng. C 2016, 66, 244–250. [Google Scholar]
- Su, Z.; Sun, D.; Zhang, L.; He, M.; Jiang, Y.; Millar, B.; Douglas, P.; Mariotti, D.; Maguire, P.; Sun, D. Chitosan/silver nanoparticle/graphene oxide nanocomposites with multi-drug release, antimicrobial, and photothermal conversion functions. Materials 2021, 14, 2351. [Google Scholar] [CrossRef]
- Rasoulzadehzali, M.; Namazi, H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018, 116, 54–63. [Google Scholar] [CrossRef]
- El-Maadawy, M.W.; Mohamed, R.R.; Hanna, D.H. Preparation of carrageenan/chitosan-based (N, N, N-trimeth (yl chitosan chloride) silver nanocomposites as pH sensitive carrier for effective controlled curcumin delivery in cancer cells. OpenNano 2022, 7, 100050. [Google Scholar] [CrossRef]
- Gounden, S.; Daniels, A.; Singh, M. Chitosan-modified silver nanoparticles enhance cisplatin activity in breast cancer cells. Biointerface Res. Appl. Chem 2021, 11, 10572–10584. [Google Scholar]
- Fan, L.; Wang, L.; Gao, S.; Wu, P.; Li, M.; Xie, W.; Liu, S.; Wang, W. Synthesis, characterization and properties of carboxymethyl kappa carrageenan. Carbohydr. Polym. 2011, 86, 1167–1174. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef]
- Hani, U.; Gowda, B.J.; Siddiqua, A.; Wahab, S.; Begum, M.Y.; Sathishbabu, P.; Usmani, S.; Ahmad, M.P. Herbal approach for treatment of cancer using curcumin as an anticancer agent: A review on novel drug delivery systems. J. Mol. Liq. 2023, 390, 123037. [Google Scholar] [CrossRef]
- Kolter, M.; Wittmann, M.; Köll-Weber, M.; Süss, R. The suitability of liposomes for the delivery of hydrophobic drugs–a case study with curcumin. Eur. J. Pharm. Biopharm. 2019, 140, 20–28. [Google Scholar] [CrossRef]
- Vimala, K.; Yallapu, M.M.; Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Naidu, N.S.; Raju, K.M. Fabrication of curcumin encapsulated chitosan-PVA silver nanocomposite films for improved antimicrobial activity. J. Biomater. Nanobiotechnol. 2011, 2, 55. [Google Scholar] [CrossRef]
- Wu, H.; Su, M.; Jin, H.; Li, X.; Wang, P.; Chen, J.; Chen, J. Rutin-loaded silver nanoparticles with antithrombotic function. Front. Bioeng. Biotechnol. 2020, 8, 598977. [Google Scholar] [CrossRef]
- Fassas, A.; Anagnostopoulos, A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk. Lymphoma 2005, 46, 795–802. [Google Scholar] [CrossRef]
- Deitcher, S.; Cullis, P.; Wong, M.; Choy, G. Vinorelbine liposomes injection results in greater tumor drug exposure compared to conventional vinorelbine in tumor-bearing nude mice. Mol. Cancer Ther. 2007, 6 (Suppl. S11), C109. [Google Scholar]
- Gokhale, P.C.; Pei, J.; Zhang, C.; Ahmad, I.; Rahman, A.; Kasid, U. Improved safety, pharmacokinetics and therapeutic efficacy profiles of a novel liposomal formulation of mitoxantrone. Anticancer Res. 2001, 21, 3313–3321. [Google Scholar] [PubMed]
- Dou, J.; Mi, Y.; Daneshmand, S.; Majd, M.H. The effect of magnetic nanoparticles containing hyaluronic acid and methotrexate on the expression of genes involved in apoptosis and metastasis in A549 lung cancer cell lines. Arab. J. Chem. 2022, 15, 104307. [Google Scholar] [CrossRef]
- Solak, K.; Mavi, A.; Yılmaz, B. Disulfiram-loaded functionalized magnetic nanoparticles combined with copper and sodium nitroprusside in breast cancer cells. Mater. Sci. Eng. C 2021, 119, 111452. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, L.; Zhang, G.; Yuan, G. Preparation of chitosan derivative with polyethylene glycol side chains for porous structure without specific processing technique. Int. J. Biol. Macromol. 2006, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Jana, P.; Dev, A. Carbon quantum dots: A promising nanocarrier for bioimaging and drug delivery in cancer. Mater. Today Commun. 2022, 32, 104068. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).