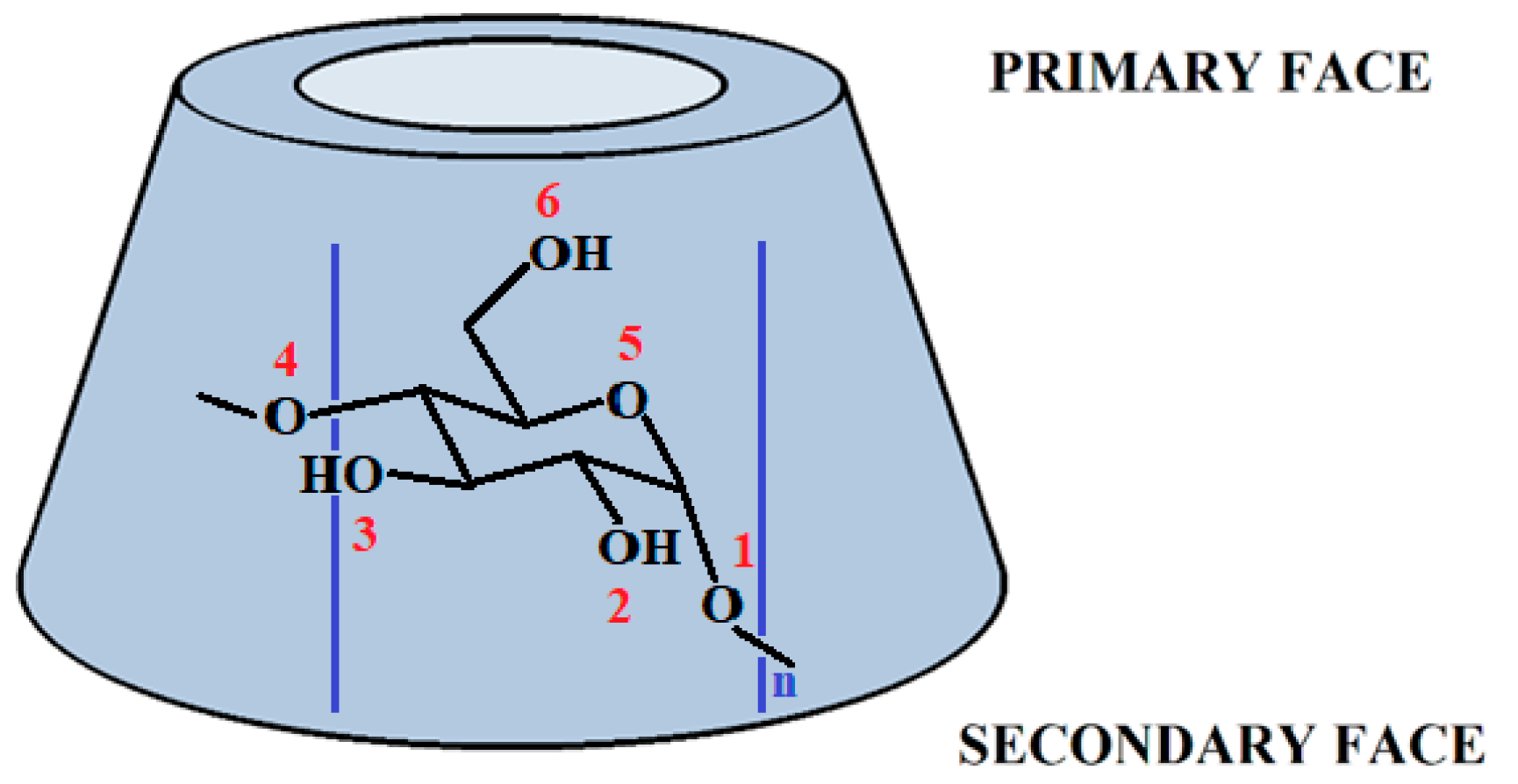
Systematic Study of Different Types of Interactions in α-, β- and γ-Cyclodextrin: Quantum Chemical Investigation
Abstract
1. Introduction
2. Results and Discussion
2.1. Benchmark Calculation for Energy Difference between the Two Conformers of CD
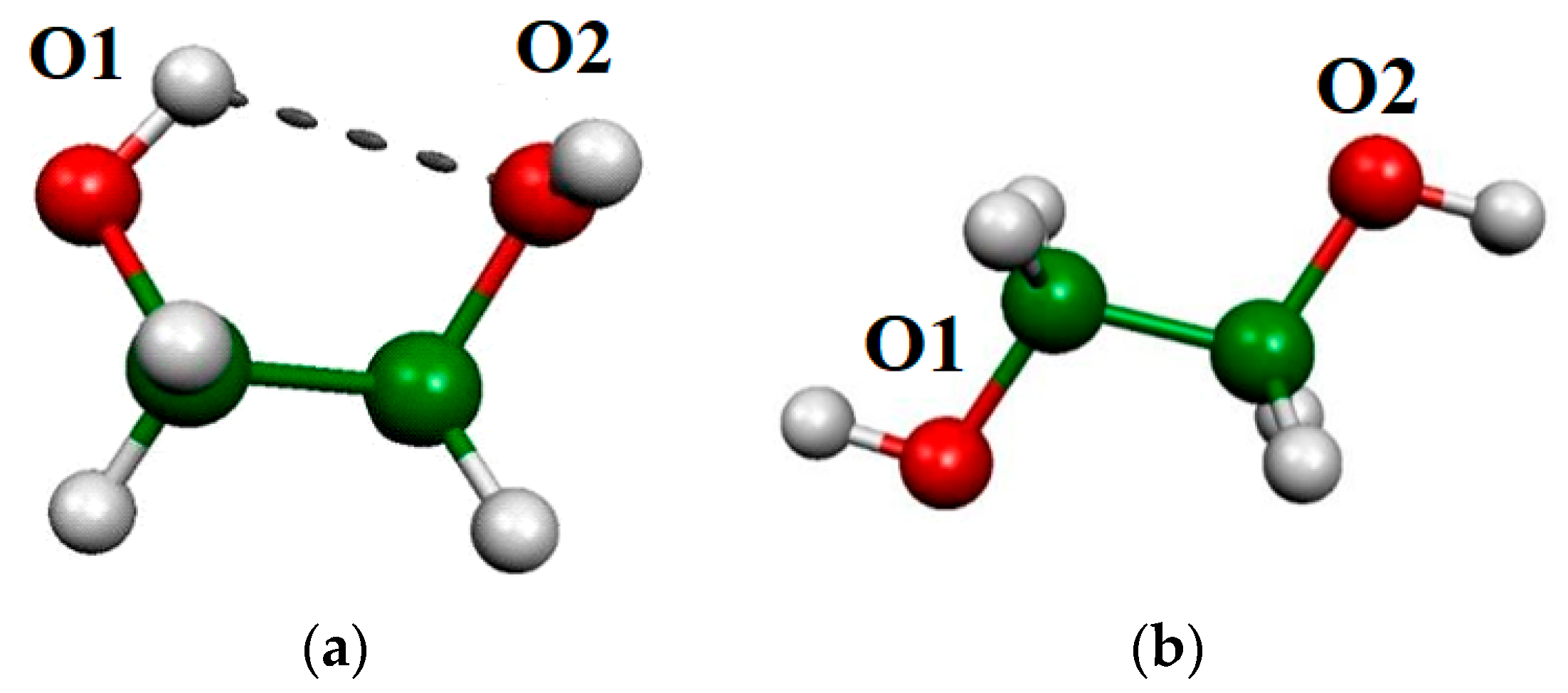
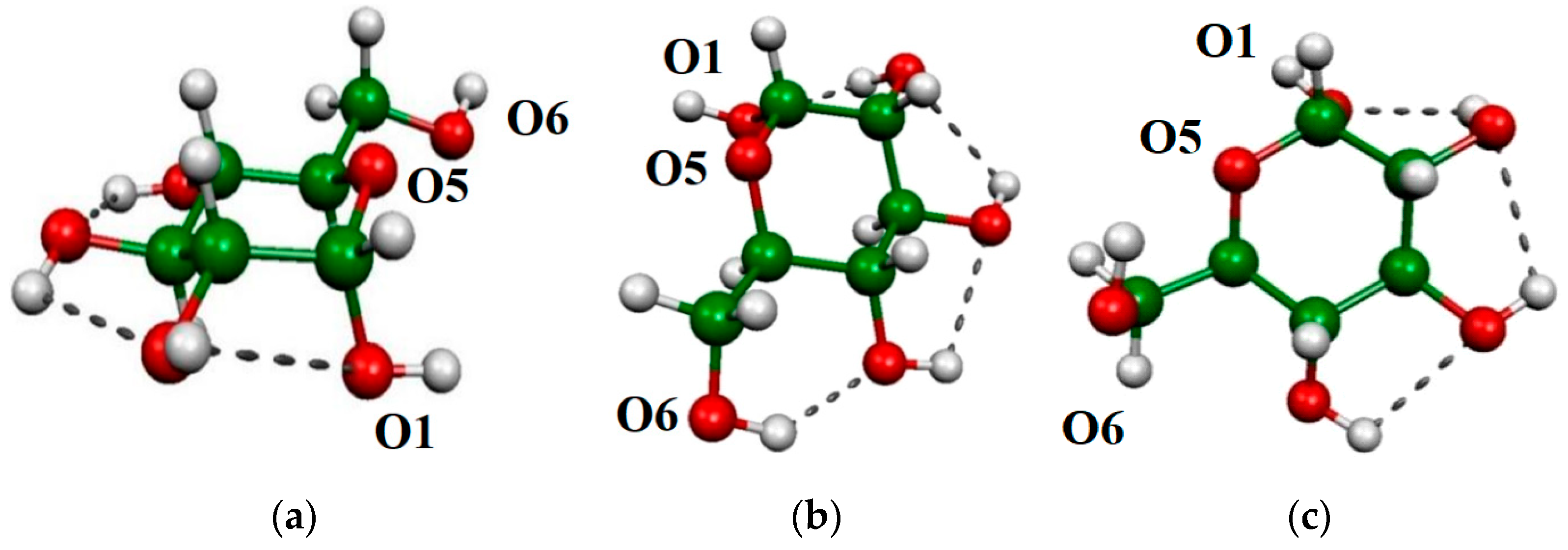
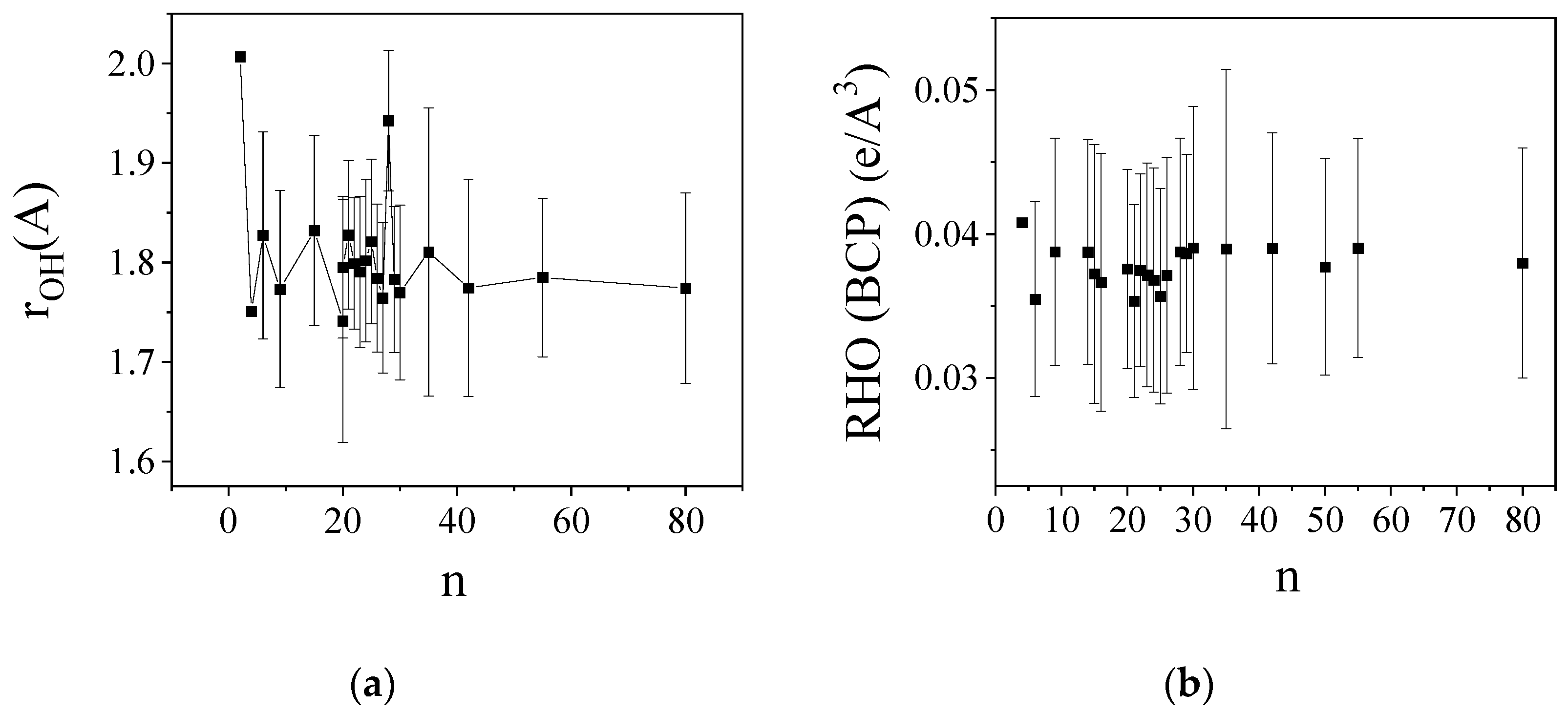
2.2. O-H Bond Lengths
2.3. OH Vibration Frequencies
2.4. Atoms in Molecules (AIM) Analyses
2.5. Natural Bond Orbital (NBO) Analyses
2.6. Bond Order and CECA
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W.; Jacob, J.L.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the Common Cyclodextrins and Their Larger Analoguess—Beyond the Doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Lipkowitz, B.K. Applications of Computational Chemistry to the Study of Cyclodextrins. Chem. Rev. 1998, 98, 1829–1874. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Jicsinszky, L.; Martina, K.; Cravotto, G. Cyclodextrins in the antiviral therapy. J. Drug Deliv. Sci. Technol. 2021, 64, 102589. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sharma, R.; Banerjee, U.C. Biotechnological Applications of Cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Medicinal applications of cyclodextrins. Med. Res. Rev. 1994, 14, 353. [Google Scholar] [CrossRef]
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- Anconi, C.P.A.; Nascimento, C.S.; Fedoce-Lopes, J.; Dos Santos, H.F.; De Almeida, W.B. Ab Initio Calculations on Low-Energy Conformers of r-Cyclodextrin. J. Phys. Chem. A 2007, 111, 12127–12135. [Google Scholar] [CrossRef] [PubMed]
- Pinjari, R.V.; Joshi, K.A.; Gejji, S.P. Molecular Electrostatic Potentials and Hydrogen Bonding in α-, β-, and γ-Cyclodextrins. J. Phys. Chem. A 2006, 110, 13073–13080. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, A.; Styrcz, A.; Korchowiec, J.; Modaressi, A.; Rogalski, M. DFT studies of cation binding by β –cyclodextrin. Theor. Chem. Acc. 2011, 130, 939–953. [Google Scholar] [CrossRef]
- Snor, W.; Liedl, E.; Weiss-Greiler, P.; Karpfen, A.; Viernstein, H.; Wolschann, P. On the structure of anhydrous β-cyclodextrin. Chem. Phys. Lett. 2007, 441, 159–162. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Defining the hydrogen bond: An account (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef]
- Banerjee, P.; Bhattacharya, I.; Chakraborty, T. Weak hydrogen bonds: Insights from vibrational spectroscopic studies. Int. Rev. Phys. Chem. 2018, 37, 83–123. [Google Scholar] [CrossRef]
- Ryu, I.S.; Liu, X.; Jina, Y.; Sun, J.; Lee, Y.J. Stoichiometric analysis of competing intermolecular hydrogen bonds using infrared spectroscopy. RSC Adv. 2018, 8, 23481–23488. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wu, R.-Z.; Yan, C.-X.; Yang, X.; Zhou, D.-G.; Zhou, P.-P. Quantitative relationships between bond lengths, stretching vibrational frequencies, bond force constants, and bond orders in the hydrogen-bonded complexes involving hydrogen halides. Struct. Chem. 2018, 29, 513–521. [Google Scholar] [CrossRef]
- Egyed, O. Spectroscopic studies on β-cyclodextrin. Vib. Spectrosc. 1990, 1, 225–227. [Google Scholar] [CrossRef]
- Egyed, O.; Weiszfeiler, V. Structure determination of copper(II)-β-cyclodextrin complex by Fourier transform infrared spectroscopy. Vib. Spectrosc. 1994, 7, 73–77. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules: An Introduction; Pearson Education: London, UK, 2000. [Google Scholar]
- Popelier, P.L.A. The Quantum Theory of Atoms in Molecules. In The Nature of the Chemical Bond Revisited; Frenking, G., Shaik, S., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2014; pp. 271–308. [Google Scholar]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Desiraju, G.; Steiner, T. The Weak Hydrogen Bond; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Mo, Y. Can QTAIM Topological Parameters Be a Measure of Hydrogen bonding strength. J. Phys. Chem. A 2012, 116, 5240–5246. [Google Scholar] [CrossRef] [PubMed]
- Silla, J.M.; Cormanich, R.A.; Rittner, R.; Freitas, M.P. Does intramolecular hydrogen bond play a key role in the stereochemistry of α- and β-d-glucose? Carbohydr. Res. 2014, 396, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.A.; Freitas, M.P. Revisiting the Case of an Intramolecular Hydrogen Bond Network Forming Four- and Five-Membered Rings in d-Glucose. ACS Omega 2018, 3, 10250–10254. [Google Scholar] [CrossRef]
- Lomas, J.S.; Joubert, L. On the importance of intramolecular hydrogen bond cooperativity in d-glucose an NMR and QTAIM approach. Magn. Reson. Chem. 2017, 55, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Csonka, G.I.; Kaminsky, J. Accurate Conformational Energy Differences of Carbohydrates: A Complete Basis Set Extrapolation. J. Chem. Theory Comput. 2011, 7, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Marianski, M.; Supady, A.; Ingram, T.; Schneider, M.; Baldauf, C. Assessing the Accuracy of Across-the-Scale Methods for Predicting Carbohydrate Conformational Energies for the Examples of Glucose and α-Maltose. J. Chem. Theory Comput. 2016, 12, 6157–6168. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.B.; Naidoo, K.J. Stereoelectronic and Solvation Effects Determine Hydroxymethyl Conformational Preferences in Monosaccharides. J. Phys. Chem. B 2008, 112, 15450–15459. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.L.; Lozoya, M.A.; Pena, I.; Lopez, J.C.; Cabezas, C.; Mata, S.; Blanco, S. The conformational behaviour of free p-glucose. Chem. Sci. 2014, 5, 515. [Google Scholar] [CrossRef]
- Carcabal, P.; Jockusch, R.A.; Hünig, I.; Snoek, L.C.; Kroemer, R.T.; Davis, B.G.; Gamblin, D.P.; Compagnon, I.; Oomens, J.; Simons, J.P. Hydrogen Bonding and Cooperativity in Isolated and Hydrated Sugars: Mannose, Galactose, Glucose, and Lactose. J. Am. Chem. Soc. 2005, 127, 11414–11425. [Google Scholar] [CrossRef]
- Kohl, R.; Kaur, R.P. A Theoretical Study of Hydrogen-Bonded Complexes of Ethylene Glycol, Thioglycol and Dithioglycol with Water. Asian J. Chem. 2022, 34, 169–182. [Google Scholar] [CrossRef]
- Guvench, O.; MacKerell, A.D. Quantum Mechanical Analysis of 1,2-Ethanediol Conformational Energetics and Hydrogen Bonding. J. Phys. Chem. A 2006, 110, 9934–9939. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Vasudevan, S. The Conformation of Ethylene Glycol in the Liquid State: Intra- versus Intermolecular Interactions. J. Phys. Chem. B 2017, 121, 5595–5600. [Google Scholar] [CrossRef]
- Howard, D.L.; Jørgensen, P.; Kjaergaard, H.G. Weak Intramolecular Interactions in Ethylene Glycol Identified by Vapor Phase OH-Stretching Overtone Spectroscopy. J. Am. Chem. Soc. 2005, 127, 48. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R.; Contreras-García, J.; Piquemal, J.-P.; Miller, B.J.; Kjaergaard, H.G. Are Bond Critical Points Really Critical for Hydrogen Bonding? J. Chem. Theory Comput. 2013, 9, 3263–3266. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C. Valency and Bonding, A Natural Bond Orbital Donor–Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Reed, E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Szatylowicz, H.; Jezierska, A.; Sadlej-Sosnowska, N. Correlations of NBO energies of individual hydrogen bonds in nucleic acid base pairs with some QTAIM parameters. Struct. Chem. 2016, 27, 367–376. [Google Scholar] [CrossRef]
- Kolandaivel, P.; Nirmala, V. Study of proper and improper hydrogen bonding using Bader’s atoms in molecules (AIM) theory and NBO analysis. J. Mol. Struct. 2004, 694, 33–38. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Kuhn, L.; Krivoshchapov, N.V.; Mehaffya, P.; Medvedev, M.G. Anomeric effect, hyperconjugation and electrostatics: Lessons from complexity in a classic stereoelectronic phenomenon. Chem. Soc. Rev. 2021, 50, 10212. [Google Scholar] [CrossRef]
- Filloux, C.M. The Problem of Origins and Origins of the Problem: Influence of Language on Studies Concerning the Anomeric Effect. Angew. Chem. Int. Ed. 2015, 54, 8880–8889. [Google Scholar] [CrossRef]
- Khan, D.; Duarte, L.J.; Popelier, P.L.A. An Interacting Quantum Atoms (IQA) and Relative Energy Gradient (REG) Analysis of the Anomeric Effect. Molecules 2022, 27, 3. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, K.B.; Bailey, W.F.; Lambert, K.M.; Stempel, Z.D. The Anomeric Effect: It’s Complicated. J. Org. Chem. 2018, 83, 5242–5255. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Costas, D.; Mosquera, R.A. Complementarity of QTAIM and ELF Partitions: Deeper Understanding of the Anomeric Effect. J. Chem. Theory Comput. 2013, 9, 4816–4824. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.P. The anomeric effect on the basis of natural bond orbital analysis. Org. Biomol. Chem. 2013, 11, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I. Simple Theorems, Proofs, and Derivations in Quantum Chemistry; Springer: New York, NY, USA, 2003; Chapter 2.4. [Google Scholar]
- Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 1983, 97, 270. [Google Scholar] [CrossRef]
- Hamza, A.; Mayer, I. Physical analysis of the diatomic ‘‘chemical’’ energy components. Theor. Chem. Acc. 2003, 109, 91–98. [Google Scholar] [CrossRef]
- Mayer, I. A chemical energy component analysis. Chem. Phys. Lett. 2000, 332, 381. [Google Scholar] [CrossRef]
- Mayer, I.; Hamza, A. APOST, Version 1.0. 2000. Available online: http://occam.ttk.hu/programs (accessed on 2 May 2024).
- Vyboishchikov, S.F.; Krapp, A.; Frenking, G. Two complementary molecular energy decomposition schemes: The Mayer and Ziegler–Rauk methods in comparison. J. Chem. Phys. 2008, 129, 144111. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I. Bond Order and Energy Components Extracting Chemical Information from Molecular Wave Function; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Mayer, I. Improved chemical energy component analysis. Phys. Chem. Chem. Phys. 2012, 14, 337–344. [Google Scholar] [CrossRef]
- Blanco, M.A.; Pendás, A.M.; Francisco, E. Interacting Quantum Atoms: A Correlated Energy Decomposition Scheme Based on the Quantum Theory of Atoms in Molecules. J. Chem. Theory Comput. 2005, 1, 1096–1109. [Google Scholar] [CrossRef]
- Mayer, I. Energy partitioning schemes. Phys. Chem. Chem. Phys. 2006, 8, 4630–4646. [Google Scholar] [CrossRef] [PubMed]
- Hugas, D.; Guillaumes, L.; Duran, M.; Simon, S. Delocalization indices for non-covalent interaction: Hydrogen and DiHydrogen bond. Comput. Theor. Chem. 2012, 998, 113–119. [Google Scholar] [CrossRef]
- Silva, A.F.; Duarte, L.J.; Popelier, P.L.A. Contributions of IQA electron correlation in understanding the chemical bond and non-covalent interactions. Struct. Chem. 2020, 31, 507–519. [Google Scholar] [CrossRef]
- Mao, Y.; Loipersberger, M.; Horn, P.R.; Das, A.; Demerdash, O.; Levine, D.S.; Veccham, S.P.; Head-Gordon, T.; Head-Gordon, M. From Intermolecular Interaction Energies and Observable Shifts to Component Contributions and Back Again: A Tale of Variational Energy Decomposition Analysis. Annu. Rev. Phys. Chem. 2021, 72, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Gimferrer, M.; Danés, S.; Andrada, D.M.; Salvador, P. Merging the Energy Decomposition Analysis with the Interacting Quantum Atoms Approach. J. Chem. Theory Comput. 2023, 19, 3469–3485. [Google Scholar] [CrossRef] [PubMed]
- Bakó, I.; Mayer, I. Hierarchy of the Collective Effects in Water Clusters. J. Phys. Chem. A 2016, 120, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bakó, I.; Mayer, I.; Hamza, A.; Pusztai, L. Two- and three-body, and relaxation energy terms in water clusters: Application of the hierarchical BSSE corrected decomposition scheme. J. Mol. Liq. 2019, 285, 177. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Robertson, M.J.; Tirado-Rives, J.; Jorgensen, W.L. Improved Peptide and Protein Torsional Energetics with the OPLS-AA Force Field. J. Chem. Theory Comput. 2015, 11, 3499–3509. [Google Scholar] [CrossRef]
- Maestro; Version 12.5; Schrödinger, LLC: New York, NY, USA, 2015.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- AIMALL. TK Gristmill Software. 2019. Available online: https://aim.tkgristmill.com/ (accessed on 2 May 2024).
- Kállay, M.; Nagy, P.R.; Mester, D.; Rolik, Z.; Samu, G.; Csontos, J.; Csóka, J.; Szabó, P.B.; Gyevi-Nagy, L.; Hégely, B.; et al. The MRCC program system: Accurate quantum chemistry from water to proteins. J. Chem. Phys. 2020, 152, 074107. [Google Scholar] [CrossRef]
- Kállay, M.; Nagy, P.R.; Mester, D.; Gyevi-Nagy, L.; Csóka, J.; Szabó, P.B.; Rolik, Z.; Samu, G.; Csontos, J.; Hégely, B.; et al. MRCC, a Quantum Chemical Program Suite. Available online: www.mrcc.hu (accessed on 28 August 2023).
- Qian, P.; Song, W.; Lu, L.; Yang, Y. Ab Initio Investigation of Water Clusters (H2O)n (n = 2–34). Int. J. Quantum Chem. 2010, 110, 1923–1937. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Jia, J.; Li, W.; Cremer, D. Different Ways of Hydrogen Bonding in Water—Why Does Warm Water Freeze Faster than Cold Water? J. Chem. Theory Comput. 2017, 13, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Bakó, I.; Mayer, I. On Dipole Moments and Hydrogen Bond Identification in Water Clusters. J. Phys. Chem. A 2016, 120, 4408–4417. [Google Scholar] [CrossRef] [PubMed]
- Nowrrozi, A.; Raissi, H.; Hajiabadi, H.; Jahani, P.M. Reinvestigation of Intramolecular Hydrogen Bond in Malonaldehyde Derivatives: An Ab initio, AIM and NBO Study. Int. J. Quantum Chem. 2011, 111, 3040–3047. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, L.; Dai, Y. Combined DFT with NBO and QTAIM studies on the hydrogen bonds in (CH3OH)n (n = 2–8) clusters. Struct. Chem. 2010, 21, 565–572. [Google Scholar] [CrossRef]
- Lenz, A.; Ojamäe, L. A theoretical study of water equilibria: The cluster distribution versus temperature and pressure for (H2O)n, (n = 1–60), and ice. J. Chem. Phys. 2009, 131, 134302. [Google Scholar] [CrossRef]

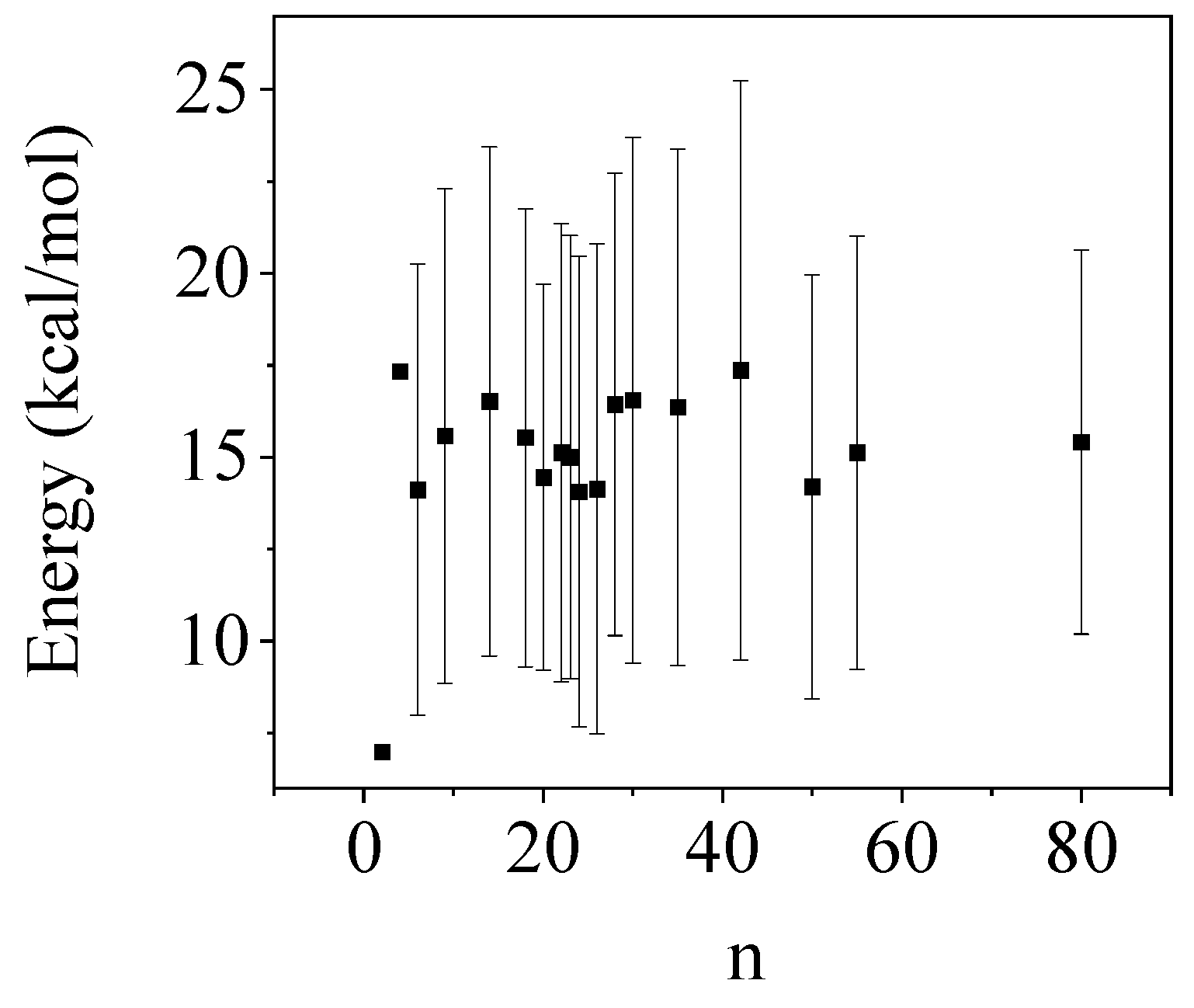

| Systems | BLYP/D3 | ωB97XD | M06-2X/D3 |
|---|---|---|---|
| α-CD | 20.5 (18.7) | 21.1 (20.8) | 17.7 (16.9) |
| β-CD | 17.6 (13.7) | 15.0 (15.6) | 8.4 (9.4) |
| γ-CD | 9.0 (3.7) | 7.0 (5.2) | 4.3 (5.3) |
| Systems | H-Bond Types | BLYP/D3 | ωB97XD | M06-2X/D3 |
|---|---|---|---|---|
| α-CD “Open” | O2H2n…O3n−1 | 1.931 | 1.889 | 1.923 |
| O3H3n…O2n | 2.374 | 2.346 | 2.349 | |
| O6H6n−1…O5n | 1.907 | 1.904 | 1.889 | |
| O6H6n−1…O6n | − | − | − | |
| α-CD “Closed” | O2H2n…O3n−1 | 2.245 | 2.175 | 2.158 |
| O3H3n…O2n | 2.465 | 2.449 | 2.432 | |
| O6H6n−1…O5n | − | − | − | |
| O6H6n−1…O6n | 1.77 | 1.775 | 1.808 | |
| β-CD “Open” | O2H2n…O3n−1 | 2.072 | 2.051 | 2.056 |
| O3H3n…O2n | 2.455 | 2.439 | 2.423 | |
| O6H6n−1…O5n | 1.991 | 2.016 | 2.051 | |
| O6H6n−1…O6n | − | − | − | |
| β-CD “Closed” | O2H2n…O3n−1 | 2.171 | 2.108 | 2.092 |
| O3H3n…O2n | 2.465 | 2.451 | 2.434 | |
| O6H6n−1…O5n | − | − | − | |
| O6H6n−1…O6n | 1.789 | 1.787 | 1.811 | |
| γ-CD “Open” | O2H2n…O3n−1 | 2.031 | 2.014 | 2.013 |
| O3H3n…O2n | 2.432 | 2.421 | 2.406 | |
| O6H6n−1…O5n | 1.979 | 2.023 | 2.012 | |
| O6H6n−1…O6n | − | − | − | |
| γ-CD “Closed” | O2H2n…O3n−1 | 2.184 | 2.114 | 2.103 |
| O3H3n…O2n | 2.468 | 2.452 | 2.437 | |
| O6H6n−1…O5n | − | − | − | |
| O6H6n−1…O6n | 1.853 | 1.841 | 1.876 |
| Systems | Secondary Intra-Unit OH Freq. (cm−1) | Primary Inter-Unit OH Freq. (cm−1) | Secondary Inter-Unit OH Freq. (cm−1) |
|---|---|---|---|
| α-CD “Open” | 3622–3637 | 3427–3440 | 3553–3560 |
| α-CD “Closed” | 3638–3640 | 3225–3283 | 3525–3530 |
| β-CD “Open” | 3639–3640 | 3440–3460 | 3558–3561 |
| β-CD “Closed” | 3640–3641 | 3305–3346 | 3515–3517 |
| γ-CD “Open” | 3638–3639 | 3486–3489 | 3552–3556 |
| γ-CD “Closed” | 3641–3642 | 3337–3366 | 3523–3524 |
| Ethylene Glycol | O1H (cm−1) | O2H (cm−1) |
|---|---|---|
| tTt | 3700 | 3701 |
| g+Gg- | 3620 | 3658 |
| α-d-Glcp | O6H (cm−1) | O1H (cm−1) | OnH (cm−1) |
|---|---|---|---|
| G-t/cc/t | 3693 | 3688 | 3654–3660 |
| Tg+/cc/t | 3603 | 3682 | 3627–3662 |
| G-g+/cc/t | 3658 | 3683 | 3627–3668 |
| Systems | O6H6n−1…O6n Primary Inter-Unit | O6H6n−1…O5n Primary Inter-Unit | O2H2n…O3n−1 Secondary Inter-Unit |
|---|---|---|---|
| α-CD “Open” | − | 0.028 | 0.026 |
| α-CD “Closed” | 0.038 | − | 0.0132 |
| β-CD “Open” | − | 0.027 | 0.018 |
| β-CD “Closed” | 0.0356 | − | 0.0142 |
| γ-CD “Open” | − | 0.025 | 0.0205 |
| γ-CD “Closed” | 0.0307 | − | 0.015 |
| Conformers | Inter-Unit H-Bond Types | α-CD | β-CD | γ-CD |
|---|---|---|---|---|
| “Open” | (1) O6H6n−1…O5n | 6.6 | 3.19 | 3.6 |
| (2) O6H6n−1…O5n | 2.5 | 1.61 | 2.1 | |
| (1) O3H3n−1…O2n | 1.4 | 3.02 | 3.1 | |
| (2) O3H3n−1…O2n | 0.8 | 1.92 | 2.1 | |
| “Closed” | (1) O6H6n−1…O6n | 12.6 | 12.81 | 8.3 |
| (2) O6H6n−1…O6n | 3.6 | 0.0 | 3.3 | |
| (1) O3H3n−1…O2n | 1.5 | 2.1 | 1.8 | |
| (2) O3H3n−1…O2n | 0.9 | 1.14 | 1.2 |
| Type of Delocalization | α-d-Glcp Tg+/cc/t | α-CD “Open” | α-CD “Closed” | β-CD “Open” | β-CD “Closed” | γ-CD “Open” | γ-CD “Closed” |
|---|---|---|---|---|---|---|---|
| LPO1BD*C1O5 | 10.5 | 11.2 | 11.2 | 11.1 | 11.0 | 11.0 | |
| LPO5BD*C1O1 | 11.4 | 7.5 | 9.3 | 8.0 | 9.7 | 8.4 | 10.0 |
| LPO1BD*C5C4 | 4.2 | 5.0 | 4.8 | 5.1 | 4.8 | 5.3 | |
| LPO5BD*C1C2 | 3.9 | 4.7 | 4.6 | 4.7 | 4.5 | 4.6 | 4.6 |
| LPO5BD*C5H5 | 5.3 | 4.2 | 4.6 | 3.9 | 4.3 | 3.9 | 4.0 |
| LPO5BD*C5C4 | 3.4 | 4.2 | 4.3 | 4.4 | 4.2 | 4.4 | 4.1 |
| Systems | Type of Interactions | O…H (Å) | Bond Order | Electrostat. (kcal/mol) | Exchange (kcal/mol) | Overlap (kcal/mol) |
|---|---|---|---|---|---|---|
| α-CD “Open” | O6H6n−1…O5n | 1.918 | 0.096 | −23.03 | −11.48 | −14.62 |
| O2H2n…O3n−1 | 1.897 | 0.112 | −26.79 | −13.74 | −16.82 | |
| O3H3n…O2n | 2.373 | 0.057 | −16.13 | −4.77 | −7.78 | |
| α-CD “Closed” | O6H6n−1…O6n | 1.770 | 0.141 | −36.79 | −19.58 | −20.27 |
| O2H2n…O3n−1 | 2.242 | 0.078 | −19.39 | −7.28 | −12.11 | |
| O3H3n…O2n | 2.466 | 0.045 | −14.62 | −3.58 | −6.15 | |
| β-CD “Open” | O6H6n−1…O5n | 1.991 | 0.077 | −22.40 | −8.79 | −12.05 |
| O2H2n…O3n−1 | 2.078 | 0.091 | −22.40 | −9.54 | −14.37 | |
| O3H3n…O2n | 2.455 | 0.046 | −15.00 | −3.64 | −6.28 | |
| β-CD “Closed” | O6H6n−1…O6n | 1.790 | 0.129 | −35.64 | −17.57 | −19.08 |
| O2H2n…O3n−1 | 2.175 | 0.082 | −20.58 | −7.97 | −12.93 | |
| O3H3n…O2n | 2.466 | 0.044 | −14.75 | −3.51 | −6.15 | |
| γ-CD “Open” | O6H6n−1…O5n | 1.978 | 0.079 | −22.72 | −9.10 | −12.36 |
| O2H2n…O3n−1 | 2.031 | 0.093 | −23.53 | −10.17 | −14.81 | |
| O3H3n…O2n | 2.439 | 0.047 | −15.37 | −3.76 | −6.53 | |
| γ-CD “Closed” | O6H6n−1…O6n | 1.854 | 0.125 | −33.63 | −15.88 | −18.64 |
| O2H2n…O3n−1 | 2.179 | 0.079 | −20.52 | −7.72 | −12.61 | |
| O3H3n…O2n | 2.469 | 0.043 | −14.81 | −3.39 | −6.02 | |
| ethylene-glycol (g+Gg-) | 2.284 | 0.064 | −14.99 | −5.71 | −9.16 | |
| α-d-Glcp (Tg+/cc/t) | 2.547 | 0.040 | −15.69 | −3.39 | −6.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakó, I.; Jicsinszky, L.; Pothoczki, S. Systematic Study of Different Types of Interactions in α-, β- and γ-Cyclodextrin: Quantum Chemical Investigation. Molecules 2024, 29, 2205. https://doi.org/10.3390/molecules29102205
Bakó I, Jicsinszky L, Pothoczki S. Systematic Study of Different Types of Interactions in α-, β- and γ-Cyclodextrin: Quantum Chemical Investigation. Molecules. 2024; 29(10):2205. https://doi.org/10.3390/molecules29102205
Chicago/Turabian StyleBakó, Imre, László Jicsinszky, and Szilvia Pothoczki. 2024. "Systematic Study of Different Types of Interactions in α-, β- and γ-Cyclodextrin: Quantum Chemical Investigation" Molecules 29, no. 10: 2205. https://doi.org/10.3390/molecules29102205
APA StyleBakó, I., Jicsinszky, L., & Pothoczki, S. (2024). Systematic Study of Different Types of Interactions in α-, β- and γ-Cyclodextrin: Quantum Chemical Investigation. Molecules, 29(10), 2205. https://doi.org/10.3390/molecules29102205






