Phytochemical Analysis and Profiling of Antioxidants and Anticancer Compounds from Tephrosia purpurea (L.) subsp. apollinea Family Fabaceae
Abstract
1. Introduction
2. Results
2.1. Phytochemical Compounds
2.2. Antioxidants Capacity
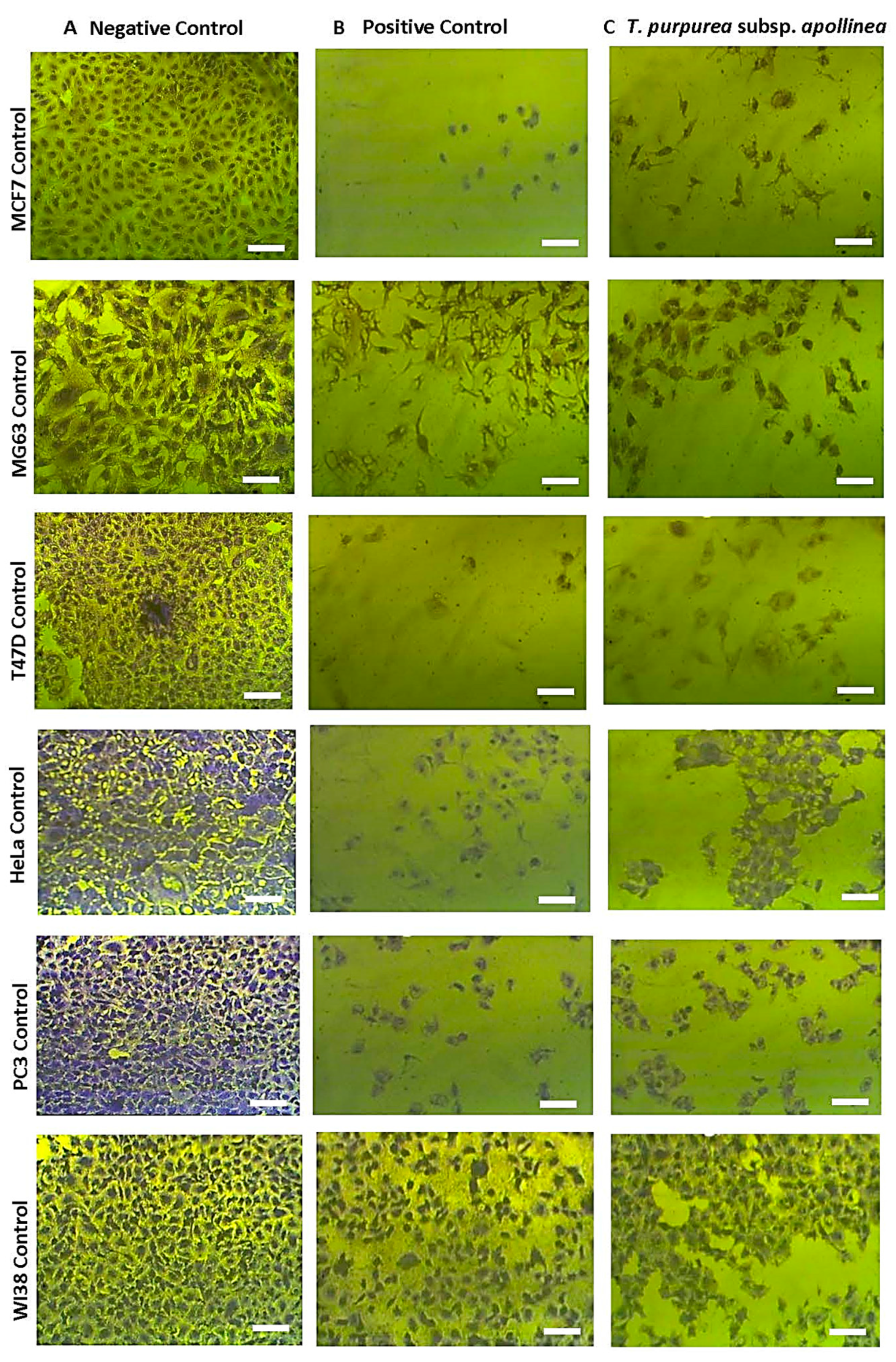
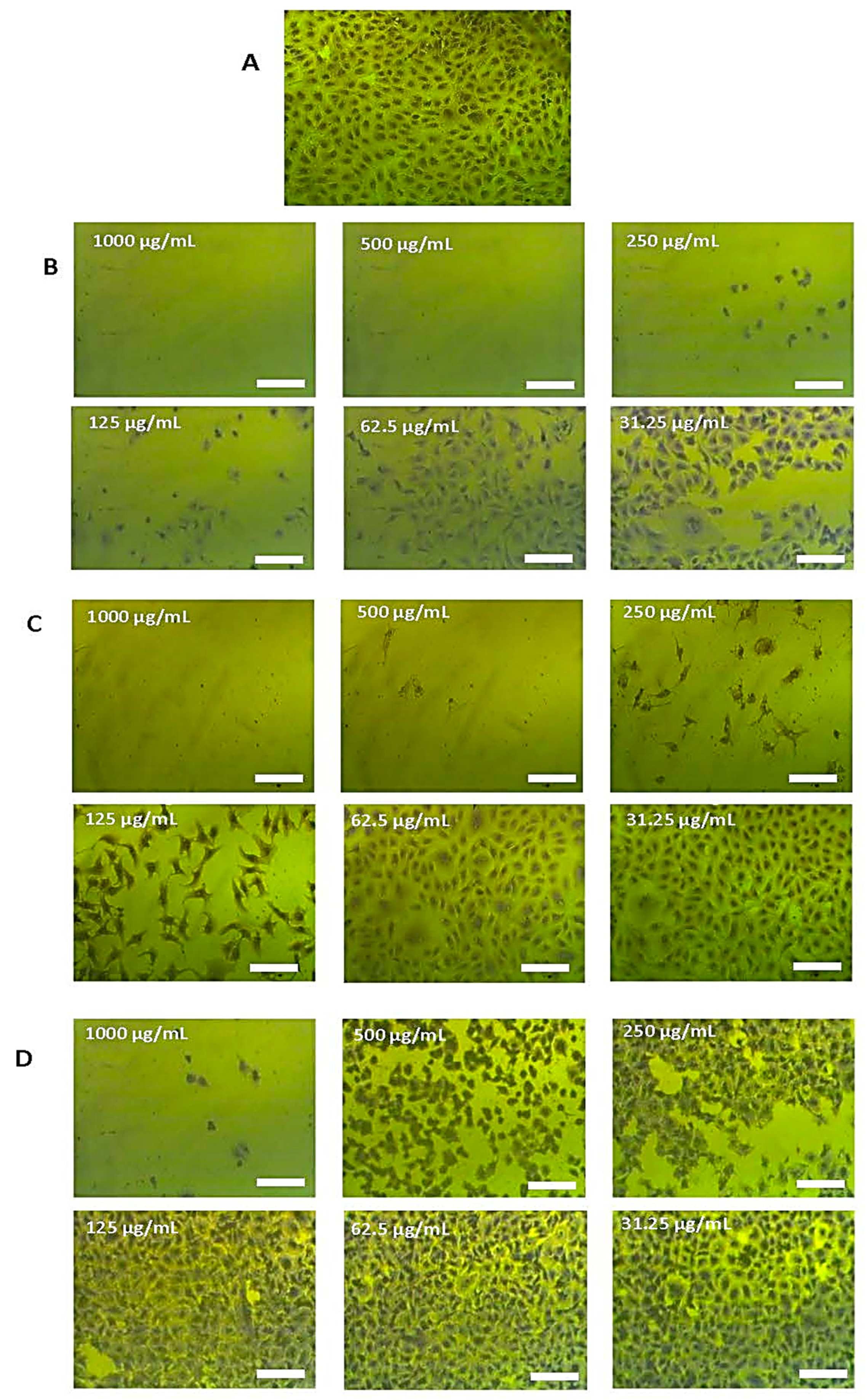
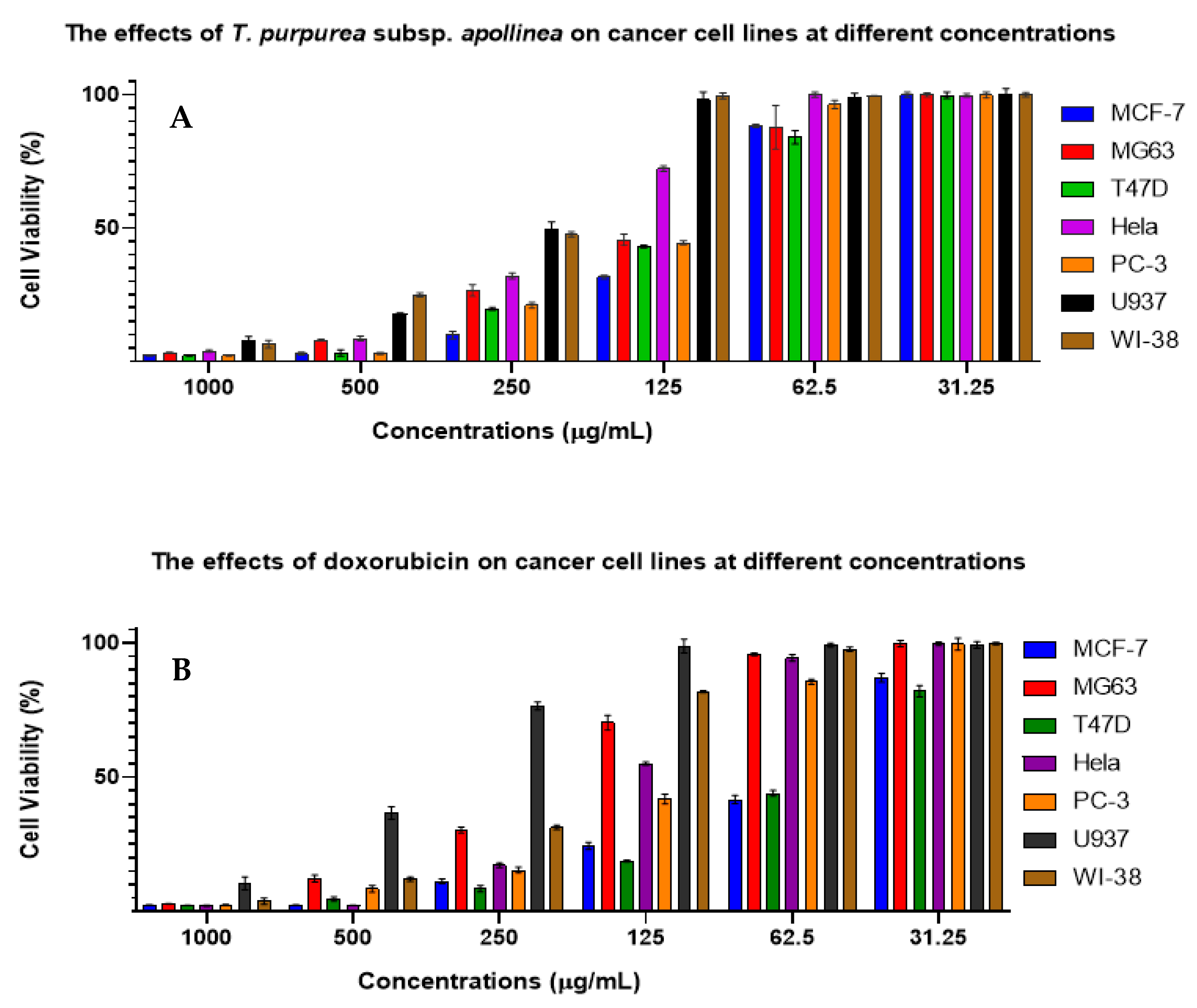
2.3. Antitumor Capacity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Methanolic Extract and GC-MS Studies
4.3. Sub-Fractionation Using n-Hexane and GC-MS Studies
4.4. Quantification of Phenolics and Flavonoids
4.5. HPLC Analysis of Phenolic Compounds
4.5.1. Standards
4.5.2. HPLC Quantitation of Phenolics
4.6. Evaluation of Antioxidant Properties
4.6.1. DPPH Antioxidant Assay
4.6.2. ABTS Antioxidant Assay
4.6.3. Measurement of IC50
4.7. Determination of Anticancer Effect
4.7.1. Viability Evaluation
4.7.2. Measurement of IC50
4.7.3. Classification of Cytotoxicity
4.7.4. Criteria for Selectivity
4.7.5. Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.O.; Youssef, A.M.; Al-Sarayreh, S.; Almuhaisen, G.H.; Alnawaiseh, N.; Al Shuneigat, J.M.; Alrawashdeh, H.M. Profiling of CYP4Z1 and CYP1B1 expression in bladder cancers. Sci. Rep. 2021, 11, 5581. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mai, Z.; Liu, C.; Yin, S.; Cai, Y.; Xia, C. Natural products in preventing tumor drug resistance and related signaling pathways. Molecules 2022, 27, 3513. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Alshammari, F.O.; Youssef, A.M.; Al-Sarayra, Y.M.; Al-Saraireh, R.A.; Al-Muhaisen, G.H.; Al-Mahdy, Y.S.; Al-Kharabsheh, A.M.; Abufraijeh, S.M.; Alrawashdeh, H.M. Cytochrome 4Z1 expression is correlated with poor prognosis in patients with cervical cancer. Curr. Oncol. 2021, 28, 3573–3584. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Alshammari, F.O.; Youssef, A.M.; Al-Sarayreh, S.; Al-Sarayra, Y.M.; Aborajooh, E.; Al-Shuneigat, J.; Alrawashdeh, H.M. Screening of glypican-6 expression in benign, primary and metastatic colon cancers. Clin. Med. Insights Oncol. 2021, 15, 11795549211036419. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, F.O.; Al-Saraireh, Y.M.; Youssef, A.M.; Al-Sarayra, Y.M.; Alrawashdeh, H.M. Glypican-1 Overexpression in Different Types of Breast Cancers. Onco Targets Ther. 2021, 14, 4309. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; Maaty, D.A.; Al-Saraireh, Y.M. Phytochemical Analysis and Profiling of Antitumor Compounds of Leaves and Stems of Calystegia silvatica (Kit.) Griseb. Molecules 2023, 28, 630. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Ayoka, T.O.; Ezema, B.O.; Eze, C.N.; Nnadi, C.O. Antioxidants for the Prevention and Treatment of Non-communicable Diseases. J. Explor. Res. Pharmacol. 2022, 7, 178–188. [Google Scholar] [CrossRef]
- Hussein, R.M.; Youssef, A.M.; Magharbeh, M.K.; Al-Dalaen, S.M.; Al-Jawabri, N.A.; Al-Nawaiseh, T.N.; Al-Jwanieh, A.; Al-Ani, F.S. Protective Effect of Portulaca oleracea Extract Against Lipopolysaccharide-Induced Neuroinflammation, Memory Decline, and Oxidative Stress in Mice: Potential Role of miR-146a and miR-let 7. J. Med. Food 2022, 25, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 1999; Volume 1. [Google Scholar]
- Samuel, V.J.; Mahesh, A.R.; Murugan, V. Phytochemical and pharmacological aspects of Tephrosia genus: A brief review. J. Appl. Pharm. Sci. 2019, 9, 117–125. [Google Scholar]
- Gulecha, V.; Sivakuma, T. Anticancer activity of Tephrosia purpurea and Ficus religiosa using MCF 7 cell lines. Asian Pac. J. Trop. Med. 2011, 4, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, T.; Gao, C.; Cao, W.; Huang, R. Natural products from the genus Tephrosia. Molecules 2014, 19, 1432–1458. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Fafal, T.; Tüzün, B.S.; KIVÇAK, B. Fatty Acid Compositions and Antioxidant Activities of Ranunculus isthmicus subsp. tenuifolius and Ranunculus rumelicus. Int. J. Nat. Life Sci. 2022, 6, 151–159. [Google Scholar] [CrossRef]
- Ibrahim, O.H.; Al-Qurashi, A.D.; Asiry, K.A.; Mousa, M.A.; Alhakamy, N.A.; Abo-Elyousr, K.A. Investigation of Potential In Vitro Anticancer and Antimicrobial Activities of Balanites aegyptiaca (L.) Delile Fruit Extract and Its Phytochemical Components. Plants 2022, 11, 2621. [Google Scholar] [CrossRef]
- Elsharkawy, E.R.; Alghanem, S.M.; Elmorsy, E. Effect of habitat variations on the chemical composition, antioxidant, and antimicrobial activities of Achillea fragrantissima (Forssk) Sch. Bip. Biotechnol. Rep. 2021, 29, e00581. [Google Scholar] [CrossRef]
- Kumar, P.; Sati, S.; Khulbe, K.; Pant, P.; Tripathi, A.N.; Sarvendra, K. Phytochemical constituents, antimicrobial and antioxidant activities of Kumaun Himalayan Hoop Pine bark extract. Nat. Prod. Res. 2022, 36, 1095–1099. [Google Scholar] [CrossRef]
- Kumar, R.S.; Anburaj, G.; Subramanian, A.; Vasantha, S.; Selvam, A.P. Preliminary phytochemical investigation, Antimicrobial activity and GC-MS analysis of leaf extract of Capparis zeylanica Linn. J. Pharm. Phytochem 2019, 8, 1399–1405. [Google Scholar]
- Belakhdar, G.; Benjouad, A.; Abdennebi, E. Determination of some bioactive chemical constituents from Thesium humile Vahl. J. Mater. Environ. Sci. 2015, 6, 2778–2783. [Google Scholar]
- Rakkimuthu, R.; Ananthi, P.; Sathishkumar, P.; Ananda kumar, A.M.; Sowmiya, D. Chemical profiling of fern Cheilosoria mysurensis (Wall. ex Hook.) Ching & Shing and its biological activity. Plant Sci. Today 2023, 10, 91–95. [Google Scholar] [CrossRef]
- Mohamed, N.T. Seperation of bioactive compounds from Haemolymph of scarab beetle Scarabaeus sacer (Coleoptera: Scarabaeidae) by GC-MS and determination of its antimicrobial activity. Int. J. Appl. Biol. 2021, 5, 98–116. [Google Scholar] [CrossRef]
- Harun, A.; Aziz, N.A.; Azenan, N.S.M.; Kamarazzaman, N.F.M.; So’ad, S.Z.M. Antimicrobial Efficacy, Antioxidant Profile and Nine Alternative Active Constituents from Petroleum Ether and Ethyl Acetate Extract of Entada spiralis. Malays. J. Anal. Sci. 2020, 24, 707–718. [Google Scholar]
- Hussein, H.J.; Hameed, I.H.; Hadi, M.Y. Using gas chromatography-mass spectrometry (GC-MS) technique for analysis of bioactive compounds of methanolic leaves extract of Lepidium sativum. Res. J. Pharm. Technol. 2017, 10, 3981–3989. [Google Scholar] [CrossRef]
- Rautela, I.; Dheer, P.; Thapliyal, P.; Joshi, T.; Sharma, N.; Sharma, M.D. GC-MS analysis of plant leaf extract of Datura stramonium in different solvent system. Eur. J. Biomed. Pharm. Sci 2018, 5, 236–245. [Google Scholar]
- Khan, N.; Ali, A.; Qadir, A.; Ali, A.; Warsi, M.H.; Tahir, A.; Ali, A. GC-MS analysis and antioxidant activity of Wrightia tinctoria R. Br. leaf extract. J. AOAC Int. 2021, 104, 1415–1419. [Google Scholar] [CrossRef]
- Abdelhamid, M.S.; Kondratenko, E.I.; Lomteva, N.A. GC-MS analysis of phytocomponents in the ethanolic extract of Nelumbo nucifera seeds from Russia. J. Appl. Pharm. Sci. 2015, 5, 115–118. [Google Scholar] [CrossRef]
- Morah, F.N.; Uduagwu, D.N. Chemical composition, antioxidant and larvicidal activity of Alchornea laxiflora (Benth) leaf extracts. Edorium J. Pharmacol. 2017, 1, 1–8. [Google Scholar]
- Dawoud, S.F.; Al-Akra, T.; Zedan, A.M. Antioxidant Activity of Some Natural Compounds in Alleviating the Hepatotoxicity Effects Induced by Emamectin Benzoate in Male Mice. J. Agric. Chem. Biotechnol. 2021, 12, 145–156. [Google Scholar] [CrossRef]
- Emam, K.K.; Abdel Fattah, M.E.; El Rayes, S.M.; Hebishy, M.A.; Dessouki, A.A. Assessment of Wheat Germ Oil Role in the Prevention of Induced Breast Cancer in Rats. ACS Omega 2022, 7, 13942–13952. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.-M.; Mohammed, Y.H.; Imad, H.H. Determination of metabolites products by Cassia angustifolia and evaluate antimicobial activity. J. Pharmacogn. Phytother. 2016, 8, 25–48. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wu, K.-J.; Siao, L.-R.; Tsai, H.-Y. Trilinolein, a Natural Triacylglycerol, Protects Cerebral Ischemia through Inhibition of Neuronal Apoptosis and Ameliorates Intimal Hyperplasia via Attenuation of Migration and Modulation of Matrix Metalloproteinase-2 and RAS/MEK/ERK Signaling Pathway in VSMCs. Int. J. Mol. Sci. 2022, 23, 12820. [Google Scholar] [CrossRef]
- Ould Bellahcen, T.; Cherki, M.; Sánchez, J.A.C.; Cherif, A.; El Amrani, A. Chemical composition and antibacterial activity of the essential oil of Spirulina platensis from Morocco. J. Essent. Oil Bear. Plants 2019, 22, 1265–1276. [Google Scholar] [CrossRef]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2, 4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Siva, S.; Cui, H.; Lin, L. Chemical composition, antibacterial activity and study of the interaction mechanisms of the main compounds present in the Alpinia galanga rhizomes essential oil. Ind. Crops Prod. 2021, 165, 113441. [Google Scholar] [CrossRef]
- Gazwi, H.S.; Shoeib, N.A.; Mahmoud, M.E.; Soltan, O.I.; Hamed, M.M.; Ragab, A.E. Phytochemical Profile of the Ethanol Extract of Malvaviscus arboreus Red Flower and Investigation of the Antioxidant, Antimicrobial, and Cytotoxic Activities. Antibiotics 2022, 11, 1652. [Google Scholar] [CrossRef]
- Subash, N.; Raju, G. GC-MS analysis and antibacterial activity of Stem of Indigofera longeracemosa Boiv. Ex Baill. Nat. Pharm. Technol. 2014, 4, 1–6. [Google Scholar]
- Rhetso, T.; Shubharani, R.; Roopa, M.; Sivaram, V. Chemical constituents, antioxidant, and antimicrobial activity of Allium chinense G. Don. Future J. Pharm. Sci. 2020, 6, 102. [Google Scholar] [CrossRef]
- Sunita, A.; Ganesh, K.; Sonam, M. Screening and evaluation of bioactive components of Cenchrus ciliaris L. by GC-MS analysis. Int. Res. J. Pharm. 2017, 8, 69–76. [Google Scholar]
- Shariare, M.H.; Noor, H.B.; Khan, J.H.; Uddin, J.; Ahamad, S.R.; Altamimi, M.A.; Alanazi, F.K.; Kazi, M. Liposomal drug delivery of Corchorus olitorius leaf extract containing phytol using design of experiment (DoE): In-vitro anticancer and in-vivo anti-inflammatory studies. Colloids Surf. B. Biointerfaces 2021, 199, 111543. [Google Scholar] [CrossRef] [PubMed]
- Okpala, E.O.; Onocha, P.A.; Ali, M.S. Antioxidant activity of phytol dominated stem bark and leaf essential oils of Celtis zenkeri Engl. Trends Phytochem. Res. 2022, 6, 137–144. [Google Scholar]
- Abdel-Hady, H.; El-Wakil, E.A.; Abdel-Gawad, M. GC-MS analysis, antioxidant and cytotoxic activities of Mentha spicata. Eur. J. Med. Plants 2018, 26, 1–12. [Google Scholar] [CrossRef]
- Faridha Begum, I.; Mohankumar, R.; Jeevan, M.; Ramani, K. GC–MS analysis of bio-active molecules derived from Paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian J. Microbiol. 2016, 56, 426–432. [Google Scholar] [CrossRef]
- Neveen, M.K.; Emad, A.S.; Dalia, M.A.; Enas, M.A.; Ahmed, M.A.-E. Biological activities of secondary metabolites from Emericella nidulans EGCU 312. Afr. J. Microbiol. Res. 2014, 8, 2011–2021. [Google Scholar] [CrossRef]
- Kotteswari, M.; Prabhu, K.; Rao, M.R.K.; Ahamed, A.; Balaji, T.; Dinakar, S.; Sundaram, R.L. The gas chromatography-mass spectrometry study of one Ayurvedic formulation avipathi churnam. Drug Invent. Today 2020, 13, 668–671. [Google Scholar]
- Zubair, M.F.; Ajibade, S.O.; Lawal, A.Z.; Yusuf, S.A.; Babalola, J.B.; Mukadam, A.A.; Hamid, A.A. GC-MS analysis, Antioxidant and Antimicrobial Properties of Eclipta prostrata leaves. Int. J. Chem. Biochem. Sci. 2017, 11, 25–43. [Google Scholar]
- Radman, S.; Čižmek, L.; Babić, S.; Cikoš, A.-M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of less-polar fractions of Ericaria crinita and Ericaria amentacea: Developmental Toxicity and antioxidant activity. Mar. Drugs 2022, 20, 57. [Google Scholar] [CrossRef]
- Anita, A.; Selvaraj, D. In silico molecular docking study of plant-based compounds from medicinal plant Lantana camara L. against Aedes aegypti L. protein. Int. J. Mosq. Res. 2022, 9, 97–106. [Google Scholar] [CrossRef]
- El-Sayed, O.H.; Asker, M.M.; Shash, S.M.; Hamed, S.R. Isolation, structure elucidation and biological activity of Di-(2-ethylhexyl) phthalate produced by Penicillium janthinellum 62. Int. J. Chem. Tech. Res. 2015, 8, 58–66. [Google Scholar]
- Habib, M.R.; Karim, M.R. Antitumour evaluation of di-(2-ethylhexyl) phthalate (DEHP) isolated from Calotropis gigantea L. flower/Evaluacija antitumorskog djelovanja di-(2-etilheksil)-ftalata (DEHP) izoliranog iz cvjetova Calotropis gigantea L. Acta Pharm. 2012, 62, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Sirikhansaeng, P.; Tanee, T.; Sudmoon, R.; Chaveerach, A. Major phytochemical as γ-sitosterol disclosing and toxicity testing in Lagerstroemia species. Evid.-Based Complement. Altern. Med. 2017, 2017, 7209851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.-j.; Duan, B.-z.; Huang, L.-f. Gallic Acid: A Potential Anti-Cancer Agent. Chin. J. Integr. Med. 2021, 28, 661–671. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qian, C.; Kan, J.; Jin, C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocoll. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, J.K.; Kim, D.W.; Hwang, H.S.; Eum, W.S.; Park, J.; Han, K.H.; Oh, J.S.; Choi, S.Y. Antitumor activity of methyl gallate by inhibition of focal adhesion formation and Akt phosphorylation in glioma cells. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4017–4029. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Azaat, A.; Babojian, G.; Nizar, I. Phytochemical Screening, Antioxidant and Anticancer Activities of Euphorbia hyssopifolia L. against MDA-MB-231 Breast Cancer Cell Line. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 295–310. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic acid: Biological properties and biotechnological development for production processes. Afr. J. Biotechnol. 2011, 10, 4518–4523. [Google Scholar]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. A recent overview on the biological and pharmacological activities of ferulic acid. Excli J. 2019, 18, 132–138. [Google Scholar]
- Zhao, Y.; Liu, S. Bioactivity of naringin and related mechanisms. Die Pharm.-Int. J. Pharm. Sci. 2021, 76, 359–363. [Google Scholar]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M. Therapeutic potential of isoflavones with an emphasis on daidzein. Oxid. Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef] [PubMed]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharm. Rev. 2016, 10, 84. [Google Scholar]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Sahu, P.K.; Haldar, R.; Sahu, K.; Prasad, P.; Roy, A. Apigenin naturally occurring flavonoids: Occurrence and bioactivity. Pharm. Biosci. J. 2016, 4, 56–68. [Google Scholar]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Hassan, L.E.; Khadeer Ahamed, M.B.; Abdul Majid, A.S.; Iqbal, M.A.; Al Suede, F.S.R.; Haque, R.A.; Ismail, Z.; Ein, O.C.; Majid, A.M.S.A. Crystal structure elucidation and anticancer studies of (-)-pseudosemiglabrin: A flavanone isolated from the aerial parts of Tephrosia apollinea. PLoS ONE 2014, 9, e90806. [Google Scholar] [CrossRef] [PubMed]
- Azeez, K.O.; Shaker, N.M.; ElShamy, M.M.; Mogib, M.A. Phytochemical and Biological Evaluation of Tephrosia apollinea. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 195–202. [Google Scholar]
- Hassan, E.A.; Adnan Iqbal, M.; S Dahham, S.; M Tabana, Y.; B Khadeer Ahamed, M.; MS Abdul Majid, A. Colorectal, prostate and pancreas human cancers targeted bioassay-guided isolations and characterization of chemical constituents from Tephrosia apollinea. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2017, 17, 590–598. [Google Scholar]
- Cheruth, A.J.; Al Baloushi, S.A.; Karthishwaran, K.; Maqsood, S.; Kurup, S.S.; Sakkir, S. Medicinally active principles analysis of Tephrosia apollinea (Delile) DC. growing in the United Arab Emirates. BMC Res. Notes 2017, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, T.S.; Khan, A.L.; Ali, L.; Al-Mawali, N.; Mabood, F.; Hussain, J.; Adnan, M.; Al-Harrasi, A. In vitro oxidative stress regulatory potential of Citrullus colocynthis and Tephrosia apollinea. Acta Pharm. 2018, 68, 235–242. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; EL-Swaify, Z.A.S.; Maaty, D.A.; Youssef, M.M. Phytochemistry and Antiviral Properties of Two Lotus Species Growing in Egypt. Vitae 2021, 28, 348069. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; El-Swaify, Z.A.S. Anti-Tumour Effect of two Persicaria species seeds on colon and prostate cancers. Biomed. Pharmacol. J. 2018, 11, 635–644. [Google Scholar] [CrossRef]
- Youssef, A.; El-Swaify, Z.; Maaty, D.; Youssef, M. Comparative study of two Lotus species: Phytochemistry, cytotoxicity and antioxidant capacity. J. Pharm. Pharmacogn. Res. 2020, 8, 537–548. [Google Scholar]
- Al-saraireh, Y.M.; Youssef, A.M.; Alshammari, F.O.; Al-Sarayreh, S.A.; Al-Shuneigat, J.M.; Alrawashdeh, H.M.; Mahgoub, S.S. Phytochemical characterization and anti-cancer properties of extract of Ephedra foeminea (Ephedraceae) aerial parts. Trop. J. Pharm. Res. 2021, 20, 1675–1681. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Youssef, A.M.; Alsarayreh, A.; Al Hujran, T.A.; Al-Sarayreh, S.; Al-Shuneigat, J.M.; Alrawashdeh, H.M. Phytochemical and anti-cancer properties of Euphorbia hierosolymitana Boiss. crude extracts. J. Pharm. Pharmacogn. Res. 2021, 9, 13–23. [Google Scholar] [CrossRef] [PubMed]


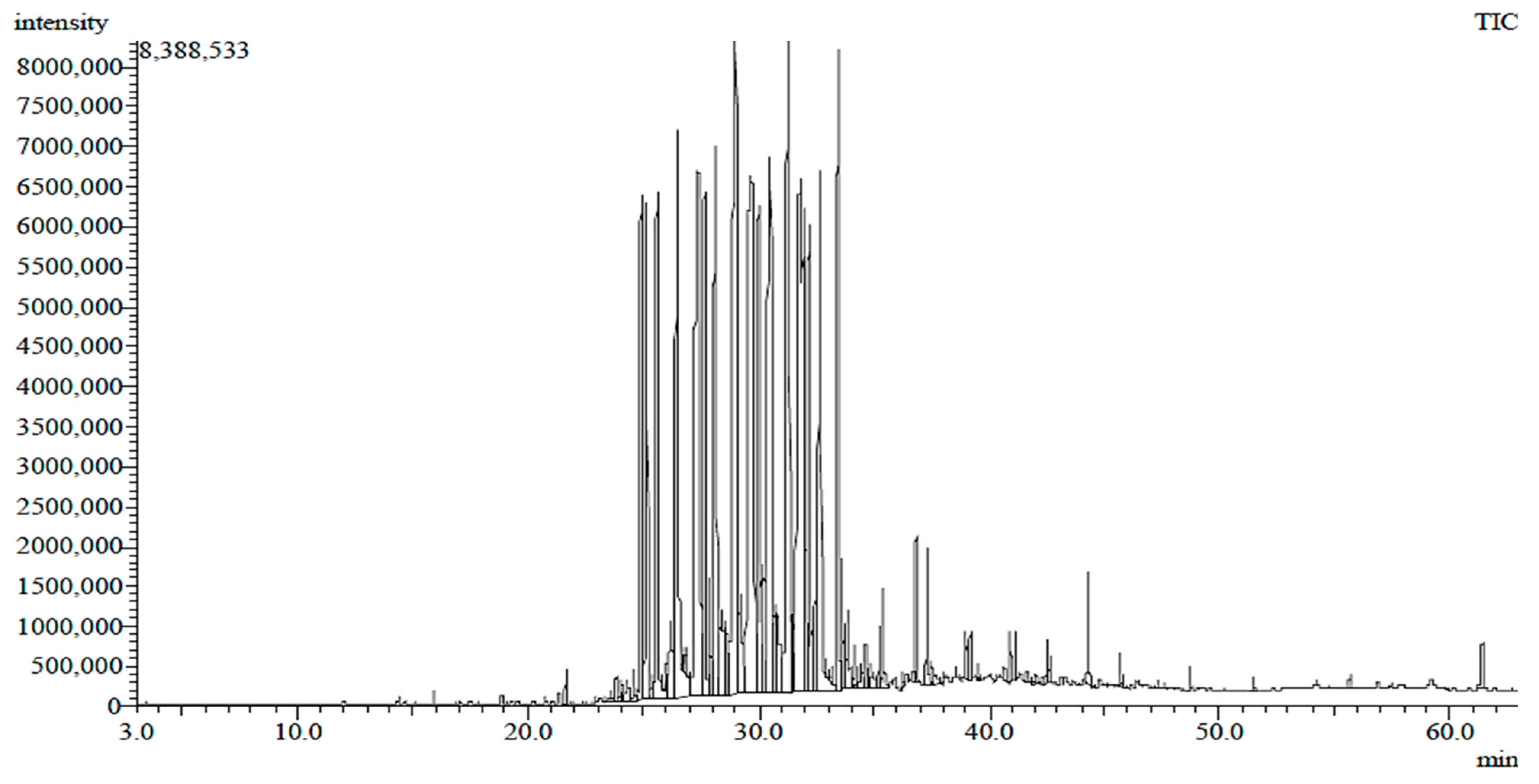

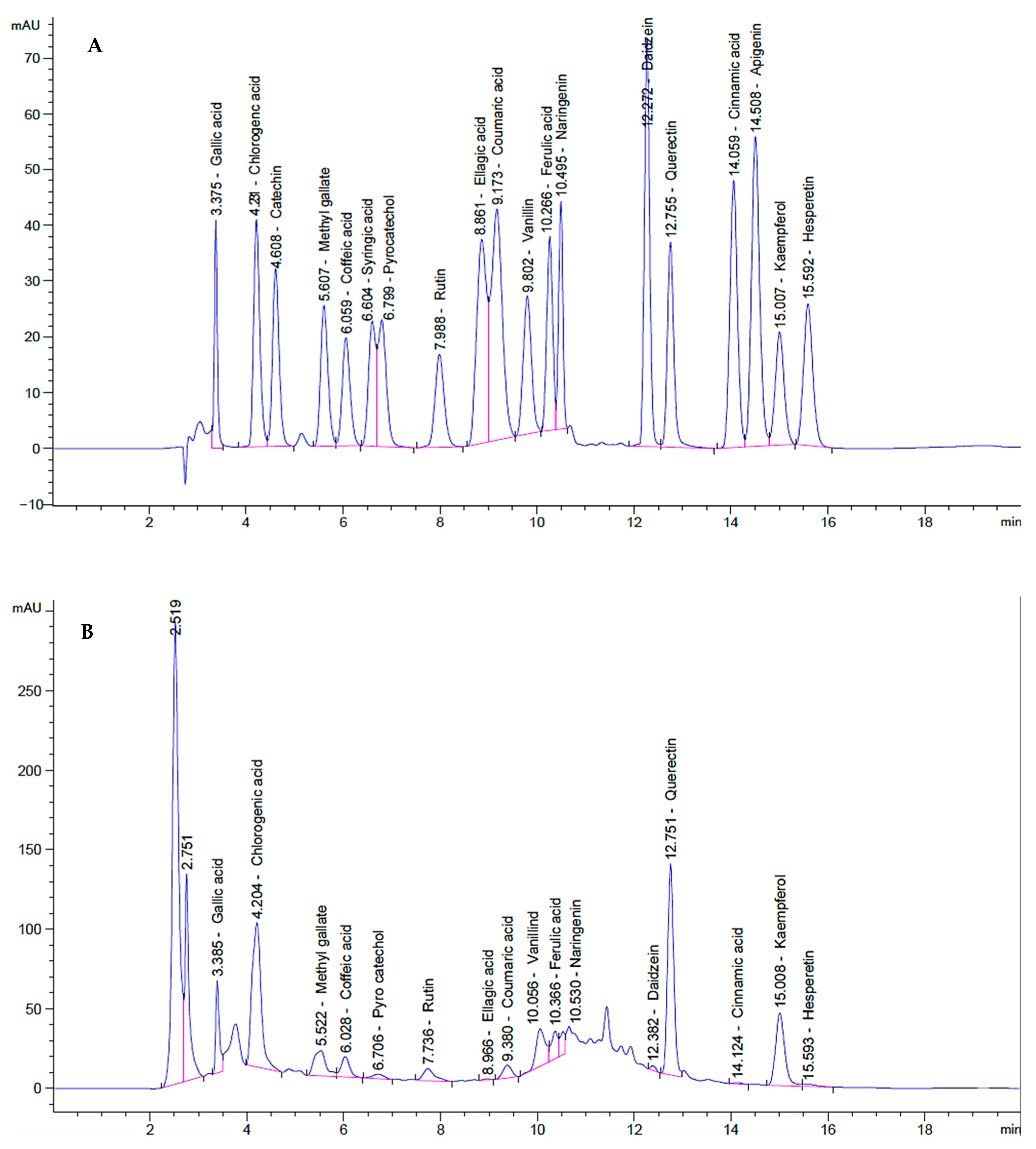
| Compounds | MW | M.F. | Category | Rt | RA% | Biological Activities | References | |
|---|---|---|---|---|---|---|---|---|
| 1 | Benzene, 1-methoxy-4-(1-propenyl)- | 148 | C10H12O | Aromatic organic compound | 9.58 | 5.88 | No data available | |
| 2 | β-Caryophyllene | 204 | C15H24 | Sesquiterpenoid | 15.04 | 2.31 | Antioxidant and Anticancer | [17] |
| 3 | Palmitic Acid methyl ester | 270 | C17H34O2 | Fatty acid methyl ester | 26.30 | 4.06 | Antioxidant and Anticancer | [18,19] |
| 4 | Pseudosolasodine diacetate | 499 | C31H49NO4 | Alkaloid Compound | 27.40 | 1.55 | Antioxidant | [20] |
| 5 | Androstan-17-one, 3-ethyl-3-hydroxy-, (5alpha) | 318 | C21H34O2 | Steroid | 28.80 | 1.20 | Antioxidant and Antibacterial | [21] |
| 6 | 9,12-octadecadienoic acid, Methyl ester | 294 | C19H34O2 | Fatty acid methyl ester | 29.43 | 3.28 | Anticancer | [22] |
| 7 | 10-Octadecenoic acid, methyl ester | 296 | C19H36O2 | Fatty acid methyl ester | 29.57 | 4.31 | Antioxidant | [23] |
| 8 | Oxiraneundecanoic acid, 3-pentyl-, Methyl ester, trans- | 312 | C19H36O3 | Fatty acid methyl esters | 30.09 | 0.99 | Antioxidant and Anticancer | [24] |
| 9 | Ethyl iso-allocholate | 436 | C26H44O5 | Steroid | 33.00 | 9.43 | Antioxidant | [25] |
| 10 | 3’,8,8’-Trimethoxy-3-piperidyl-2,2’-binaphthalene-1,1’,4,4’-tetrone | 487 | C28H25NO7 | Oxygen organic compound | 36.75 | 5.99 | Antioxidant and antimicrobial | [26] |
| 11 | Cholest-5-en-3-ol, 24-propylidene-, (3 alpha)- | 426 | C30H50O | Fatty acid | 37.45 | 0.65 | Antibacterial | [27] |
| 12 | Stigmasta-5,24(28)-dien-3-ol, (3 β,24Z)- | 412 | C29H48O | Steroid | 37.75 | 38.74 | Antioxidant | [28] |
| 13 | Olean-12-en-28-oic acid | 440 | C30H48O2 | Triterpenoids | 38.50 | 1.82 | No data available | |
| 14 | Flavone 4’-OH,5-OH,7-di-O-glucoside | 594 | C27H30O15 | Isoflavonoid | 40.36 | 2.49 | Antioxidant and Anticancer | [29,30] |
| 15 | Rhodopin | 554 | C40H58O | Carotene | 40.51 | 2.30 | Antioxidant | [31] |
| 16 | 1-heptatriacotanol | 537 | C37H76O | Alcoholic compound | 40.84 | 3.85 | Antioxidant and Anticancer | [32] |
| 17 | Glycidyl oleate | 338 | C21H38O3 | Ester | 42.51 | 3.74 | Anticancer | [33] |
| 18 | 9-Octadecenoic acid, 1,2,3-propanetriyl ester | 884 | C57H104O6 | Triglyceride | 42.93 | 3.45 | Antioxidant and immune modulators | [34,35] |
| 19 | 1,3-Dielaidin | 620 | C39H72O5 | Glycerol Derivatives | 43.70 | 1.87 | No data available |
| Compounds | MW | M.F. | Category | Rt | RA% | Biological Activities | References | |
|---|---|---|---|---|---|---|---|---|
| 1 | Tetradecane | 198 | C14H30 | Alkane | 21.64 | 0.08 | Antibacterial | [36] |
| 2 | 2,4-Di-tert-butylphenol | 206 | C14H22O | Phenol | 23.99 | 0.08 | Antioxidant | [37] |
| 3 | Pentadecane | 212 | C15H32 | Alkane | 24.26 | 0.20 | Antibacterial | [38] |
| 4 | Hexadecanoic acid, methyl ester | 270 | C17H34O2 | Fatty acid methyl esters | 33.62 | 0.37 | Antioxidant | [39] |
| 5 | Pentafluoropropionic acid, octadecyl ester | 416 | C21H37F5O2 | Ester | 35.24 | 0.17 | Antibacterial | [40] |
| 6 | Heneicosane | 296 | C21H44 | Aliphatic hydrocarbon | 35.42 | 0.60 | Pesticidal | [41] |
| 7 | 9,12,15-Octadecatrienoic acid, methyl ester | 292 | C19H32O2 | Fatty acid methyl ester | 36.86 | 0.67 | Anticancer | [42] |
| 8 | Phytol | 296 | C20H40O | Acyclic diterpene alcohol | 37.31 | 0.61 | Antioxidant and anticancer | [43,44] |
| 9 | Methyl stearate | 298 | C19H38O2 | Fatty acid methyl ester | 37.52 | 0.07 | Antioxidants and anticancer | [45] |
| 10 | 1-Heptacosanol | 396 | C27H56O | Long-chain fatty alcohol | 39.02 | 0.43 | Antioxidant | [46] |
| 11 | Tributyl acetylcitrate | 402 | C20H34O8 | Organic compound | 39.54 | 0.04 | Antimicrobial | [47] |
| 12 | cis-5,8,11-Eicosatrienoic acid, methyl ester | 320 | C21H36O2 | Fatty acid methyl ester | 40.631 | 0.08 | Anti-inflammatory | [48] |
| 13 | 2-Methyltetracosane | 352 | C25H52 | Alkane | 40.95 | 0.18 | Antibacterial | [49] |
| 14 | Palmitoleamide | 253 | C16H31NO | Fatty acid amide | 41.16 | 0.13 | Antioxidant | [50] |
| 15 | Acetic acid, 10,11-dihydroxy-3,7,11-trimethyl-dodeca-2,6-dienyl ester | 298 | C17H30O4 | Ester | 41.65 | 0.08 | Insecticidal | [51] |
| 16 | Bis(2-ethylhexyl) phthalate | 390 | C24H38O4 | Phthalate ester | 44.35 | 0.35 | Antioxidant and Anticancer | [52,53] |
| 17 | Gamma-Sitosterol | 414 | C29H50O | Steroid | 55.66 | 0.05 | Anticancer | [54] |
| No. | Compounds | MW | M.F. | Category | Rt | mg/100 g DWt | Biological Activities | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 170 | C7H6O5 | Phenolic acids | 3.38 | 1.24 | Antioxidant and Anticancer | [55,56] |
| 2 | Chlorogenic acid | 354 | C16H18O9 | Phenolic compound | 4.20 | 8.10 | Antioxidant and Anticancer | [57] |
| 4 | Methyl gallate | 184 | C8H8O5 | Gallate ester | 5.52 | 0.44 | Antioxidant and Anticancer | [58] |
| 5 | Coffeic acid | 180 | C9H8O4 | Polyphenol | 6.02 | 0.66 | Antioxidant and Anticancer | [59] |
| 7 | Pyrocatechol | 110 | C6H6O2 | Phenolic compounds | 6.70 | 0.36 | Antioxidant and Anticancer | [60] |
| 8 | Rutin | 610.5 | C27H30O16 | Flavonoid | 7.73 | 0.76 | Antioxidant and Anticancer | [61] |
| 9 | Ellagic acid | 302 | C14H6O8 | Tannins | 8.96 | 0.06 | Antioxidant and Anticancer | [62] |
| 10 | Coumaric acid | 164 | C9H8O3 | Phenolic compound | 9.38 | 0.15 | Antioxidant and Anticancer | [63] |
| 11 | Vanillin | 152 | C8H8O3 | Phenolic aldehyde | 10.05 | 0.45 | Antioxidant and Anticancer | [64] |
| 12 | Ferulic acid | 194 | C10H10O4 | Phenolic acid | 10.36 | 0.14 | Antioxidant and Anticancer | [65] |
| 13 | Naringenin | 580.5 | C27H32O14 | Flavanones | 10.53 | 0.12 | Antioxidant and Anticancer | [66] |
| 14 | Daidzein | 254 | C15H10O4 | Isoflavone | 12.38 | 0.07 | Antioxidant and Anticancer | [67] |
| 15 | Quercetin | 302 | C15H10O7 | Flavonoid | 12.75 | 6.76 | Antioxidant and Anticancer | [68] |
| 16 | Cinnamic acid | 148 | C9H8O2 | Monocarboxylic acid | 14.12 | 0.01 | Antioxidant and Anticancer | [69] |
| 17 | Apigenin | 270 | C15H10O5 | Flavones | 14.50 | 0.01 | Antioxidant and Anticancer | [70] |
| 18 | Kaempferol | 286 | C15H10O6 | Flavonol | 15.00 | 2.29 | Antioxidant and Anticancer | [71] |
| 19 | Hesperetin | 302 | C16H14O6 | Flavanone | 15.59 | 0.06 | Antioxidant and Anticancer | [72] |
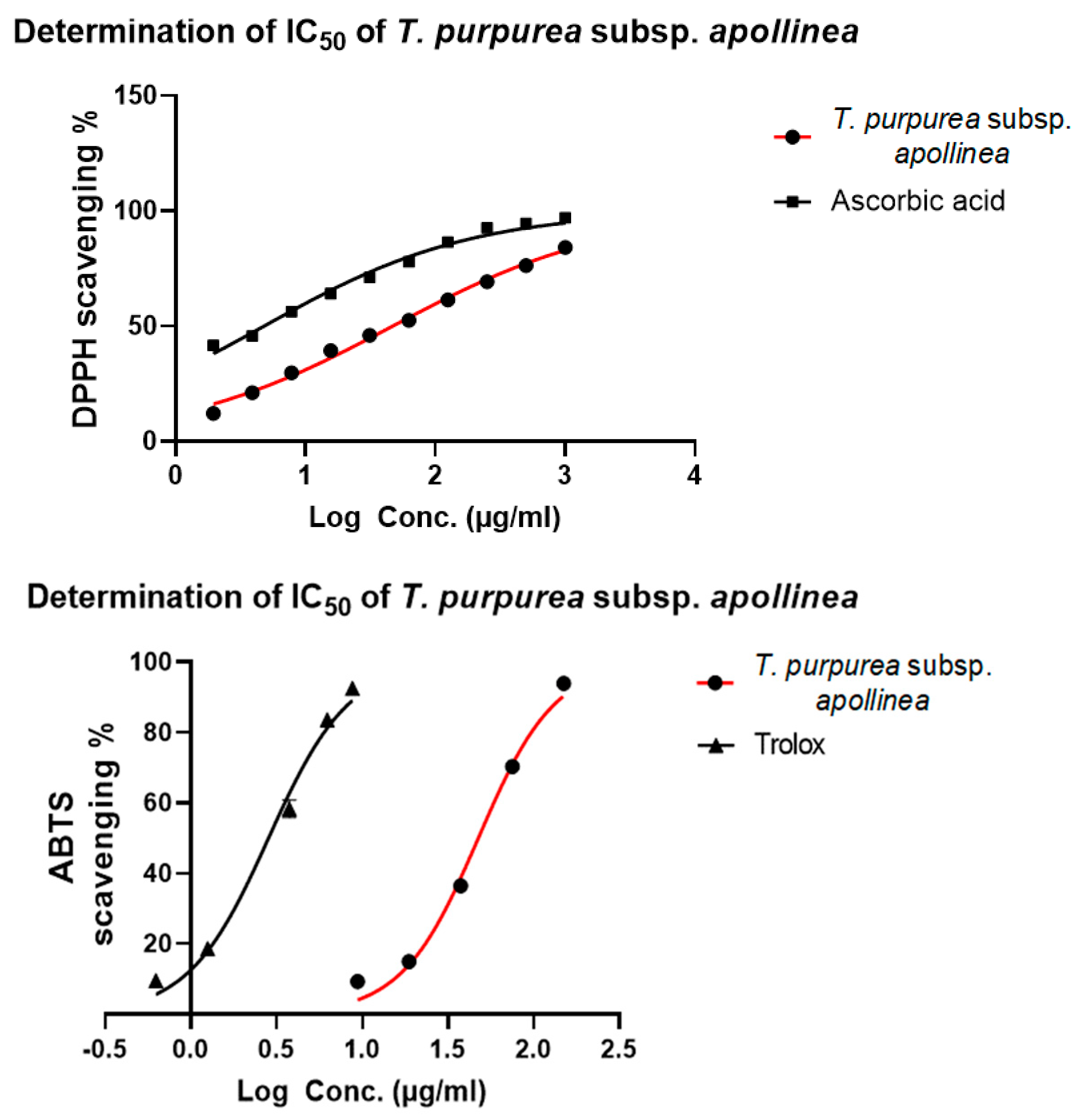
| DPPH Scavenging% | ||||
|---|---|---|---|---|
| Conc. µg/mL | T. purpurea subsp. apollinea | Ascorbic Acid | ||
| 1000 | 84.1 ± 0.3 *** | a IC50 = 46.7 ± 0.7 *** μg/mL | 97.1 ± 0.1 | a IC50 = 4.8 ± 0.1 μg/mL |
| 500 | 76.2 ± 0.1 *** | 94.5 ± 0.2 | ||
| 250 | 69.3 ± 0.3 *** | 92.7 ± 0.2 | ||
| 125 | 61.4 ± 0.1 *** | 86.4 ± 0.3 | ||
| 62.5 | 52.6 ± 0.1 *** | 77.9 ± 0.3 | ||
| 31.25 | 46.0 ± 0.2 *** | 71.2 ± 0.2 | ||
| 15.625 | 39.4 ± 0.2 *** | 64.2 ± 0.3 | ||
| 7.8125 | 29.8 ± 0.6 *** | 56.2 ± 0.2 | ||
| 3.9 | 21.2 ± 0.6 *** | 45.9 ± 0.1 | ||
| 1.95 | 12.2 ± 0.2 *** | 41.8 ± 0.5 | ||
| ABTS Scavenging% | |||||
|---|---|---|---|---|---|
| Conc. µg/mL | T. purpurea subsp. apollinea | Conc. µg/mL | Trolox | ||
| 150 | 94.1 ± 0.2 ** | a IC50 = 46.7 ± 2.6 *** μg/mL | 8.8 | 92.6 ± 0.2 | a IC50 = 2.9 ± 0.1 μg/mL |
| 75 | 72.4 ± 0.7 * | 6.2 | 83.6 ± 1.9 | ||
| 37.5 | 35.0 ± 3.7 *** | 3.8 | 49.6 ± 2.6 | ||
| 18.75 | 15.1 ± 1.3 ** | 1.3 | 18.6 ± 0.4 | ||
| 9.37 | 9.2 ± 0.1 ns | 0.6 | 9.9 ± 0.3 | ||
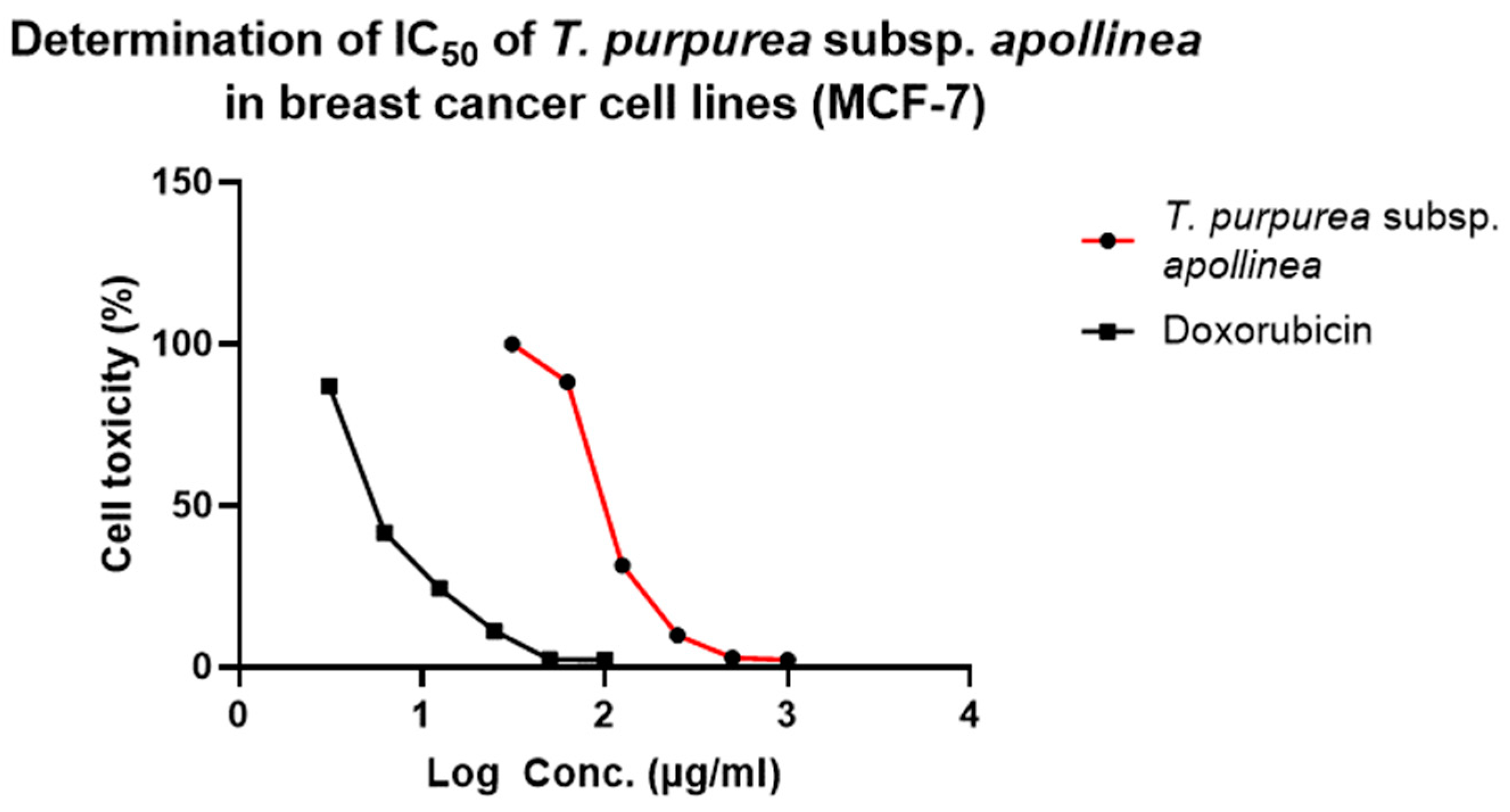
| a IC50 (µg/mL) | ||||
|---|---|---|---|---|
| Cell Lines | T. purpurea subsp. apollinea Extract | 95% Confidence Interval | R2 Value | Doxorubicin (Positive Control) |
| b MCF7 | 102.9 ± 0.5 *** | 93.8 to 113.5 | 0.9 | 37.4 ± 0.1 |
| c MG63 | 118.3 ± 2.4 *** | 102.0 to 171.0 | 0.9 | 19.0 ± 0.3 |
| d T47D | 114.7 ± 1.0 *** | 103.0 to 135.0 | 0.9 | 7.0 ± 0.1 |
| e HeLa | 196.2 ± 2.3 *** | 171.1 to 211.2 | 0.9 | 41.9 ± 0.1 |
| f PC3 | 117.6 ± 1.0 *** | 104.0 to 165.4 | 0.9 | 46.3 ± 0.2 |
| g U937 | 248.4 ± 7.5 *** | 215.2 to 311.4 | 0.9 | 41.7 ± 0.9 |
| h WI38 | 242.9 ± 1.8 *** | 207.5 to 374.8 | 0.9 | 20.1 ± 0.1 |
| a SI | ||||||
|---|---|---|---|---|---|---|
| Cell Lines | b MCF7 | c MG63 | d T47D | e HeLa | f PC3 | g U937 |
| T. purpurea subsp. apollinea Extract | 2.3 | 2.0 | 2.1 | 1.2 | 2.0 | 0.9 |
| Doxorubicin | 0.5 | 1.0 | 2.8 | 0.4 | 0.4 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, A.M.M.; Maaty, D.A.M.; Al-Saraireh, Y.M. Phytochemical Analysis and Profiling of Antioxidants and Anticancer Compounds from Tephrosia purpurea (L.) subsp. apollinea Family Fabaceae. Molecules 2023, 28, 3939. https://doi.org/10.3390/molecules28093939
Youssef AMM, Maaty DAM, Al-Saraireh YM. Phytochemical Analysis and Profiling of Antioxidants and Anticancer Compounds from Tephrosia purpurea (L.) subsp. apollinea Family Fabaceae. Molecules. 2023; 28(9):3939. https://doi.org/10.3390/molecules28093939
Chicago/Turabian StyleYoussef, Ahmed M. M., Doaa A. M. Maaty, and Yousef M. Al-Saraireh. 2023. "Phytochemical Analysis and Profiling of Antioxidants and Anticancer Compounds from Tephrosia purpurea (L.) subsp. apollinea Family Fabaceae" Molecules 28, no. 9: 3939. https://doi.org/10.3390/molecules28093939
APA StyleYoussef, A. M. M., Maaty, D. A. M., & Al-Saraireh, Y. M. (2023). Phytochemical Analysis and Profiling of Antioxidants and Anticancer Compounds from Tephrosia purpurea (L.) subsp. apollinea Family Fabaceae. Molecules, 28(9), 3939. https://doi.org/10.3390/molecules28093939







