The Effect of the Plant Stabilisation Method on the Composition and Antioxidant Properties of Elderflower (Sambucus nigra L.) Extract
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Methods of Elderflower Stabilisation
3.3. Parameters of the Elderflower Extraction
3.4. Analysis of the Properties of the Elderflower Extracts
3.4.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.4.2. DPPH Radical Scavenging Capacity Assay
3.4.3. Total Flavonoid Content Determination
3.4.4. Total Polyphenol Content Determination
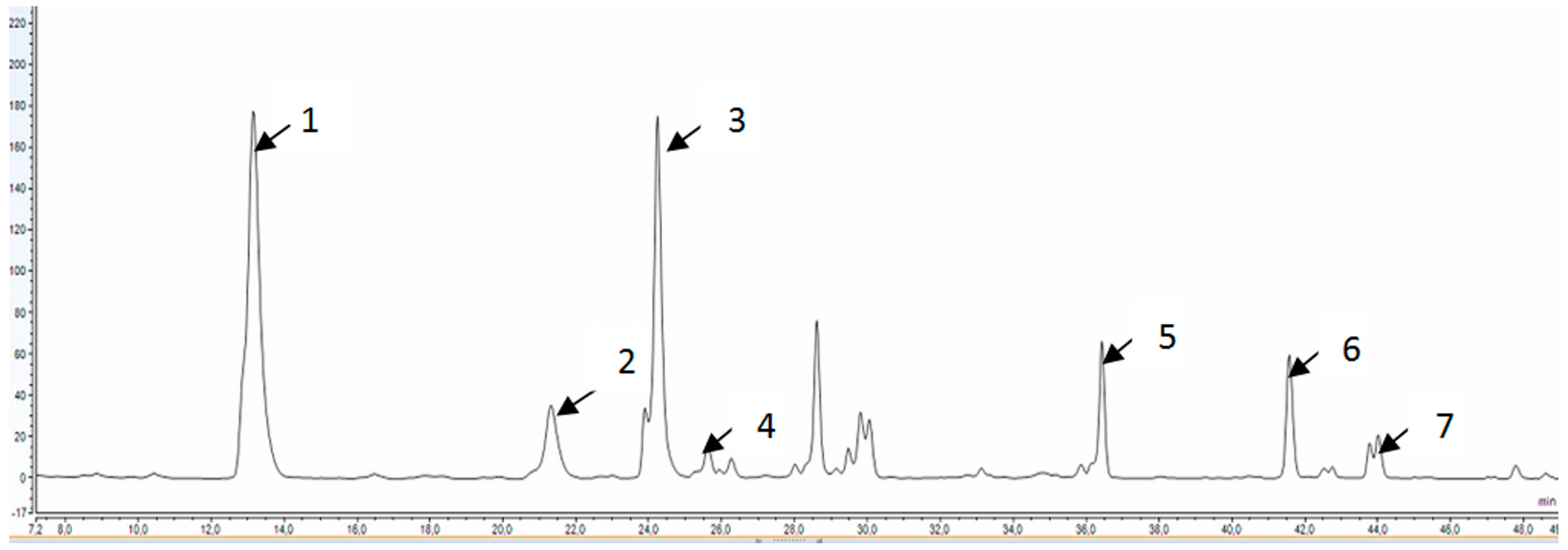
3.4.5. HPLC Analysis of the Polyphenols Profile
3.5. Dry Extract Content Determination
3.6. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Brągiel, E.; Ślusarczyk, B. Tendencje na europejskim rynku żywności ekologicznej. (Trends on the European organic food market. In Polish). Sci. J. WULS Probl. World Agric. 2017, 17, 29–38. [Google Scholar] [CrossRef]
- Senderski, M.E. (Ed.) Prawie Wszystko o Ziołach; Wydawnictwo: Warszawa, Poland, 2016. [Google Scholar]
- Ulbricht, C.; Basch, E.; Cheung, L.; Goldberg, H.; Hammerness, P.; Isaac, R.; Singh Khalsa, K.P.; Romm, A.; Rychlik, I.; Varghese, M.; et al. An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the Natural Standard Research Collaboration. J. Diet Suppl. 2014, 11, 80–120. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 144, 511–515. [Google Scholar] [CrossRef]
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. Biological flora of the British Isles. J. Ecol. 2002, 90, 895–923. Available online: https://www.jstor.org/stable/3072258 (accessed on 12 March 2022). [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Christensen, K.B.; Olsen, L.C.; Christensen, L.P.; Grevsen, K.; Færgeman, N.J.; Kristiansen, K.; Young, J.F.; Oksbjerg, N. Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 11033–11040. [Google Scholar] [CrossRef]
- Stoilova, I.; Wilker, M.; Stoyanova, A.; Krastanov, A.; Stanchev, V. Antioxidant activity of extract from elder flower (Sambucus nigra L.). Herba. Polonica. 2007, 53, 45–54. [Google Scholar]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Bartak, M.; Lange, A.; Słońska, A.; Cymerys, J. Antiviral and healing potential of Sambucus nigra extracts. Rev. Bionatura. 2020, 5, 1264–1270. [Google Scholar] [CrossRef]
- Domokos, J.; Sipos, B.; Bela, Z.; Kiss, B. Black stimulus (Sambucus nigra L.) and cosmetics. Kozmetika 2001, 50, 5–8. [Google Scholar]
- Kislichenko, V.S.; Vel’ma, V.V. Aminoacid composition of flowers, leaves and extract of Sambucus nigra flowers. Chem. Nat. Compd. 2006, 42, 125–126. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus nigra L. fruits and flowers: Chemical composition and related bioactivities. Food Rev. Int. 2022, 38, 1237–1265. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef]
- Kaack, K.; Christensen, L.; Hughes, M.; Eder, R. Relationship between sensory, quality and volatile compounds of elderberry (Sambucus nigra L.) extracts. Eur. Food Res. Technol. 2006, 223, 57–70. [Google Scholar] [CrossRef]
- Kaack, K. Aroma composition and sensory quality of fruit juices processed from cultivars of elderberry (Sambucus nigra L.). Eur. Food Res. Technol. 2008, 227, 45–56. [Google Scholar] [CrossRef]
- Zeitlhofler, A. Wildobst fur den Haus—Garten. Cadmos Verlag GmbH: München, Germany, 2008; ISBN 13: 9783704023063. [Google Scholar]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Chaurasia, J.P.; Khan, R.; Dhand, C.; Verma, S. Role of medicinal plants of traditional use in recuperating devastating COVID-19 situation. Med. Aromat. Plants (Los Angel) 2020, 9, 2167-0412. [Google Scholar] [CrossRef]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri Asl, M.; et al. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef]
- Schön, C.; Mödinger, Y.; Krüger, F.; Dobies, C.; Pischel, I.; Bonnländer, B. A new high-quality elderberry plant extract exerts antiviral and immunomodulatory effects in vitro and ex vivo. Food Agric. Immunol. 2021, 32, 650–662. [Google Scholar] [CrossRef]
- Mocanu, M.L.; Amariei, S. Elderberries—A source of bioactive compounds with antiviral action. Plants 2022, 11, 740. [Google Scholar] [CrossRef]
- Heidbohmer, E. Heilpflanze Holunder; GmbH: Twist, Germany, 2007. [Google Scholar]
- Lee, J.; Finn, C. Antocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (Sambucus nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Zalewska-Korona, M.; Kalbarczyk, J. Antioxidant capacity, ascorbic acid and phenolics content in wild edible fruits. J. Fruit Ornam. Plant Res. 2009, 17, 115–120. [Google Scholar]
- Uhl, K.R.; Mitchell, A.E. Headspace volatile organic and phenolic compounds in elderflowers and elderflower teas of blue elderberry (Sambucus nigra ssp. cerulea). ACS Food Sci. Technol. 2022, 2, 1535–1545. [Google Scholar] [CrossRef]
- Tabaszewska, M. Method for Obtaining Lyophilized Plant Extract as the Active Component of Products, Preferably Cosmetic Products. Patent No 240152, 18 November 2021. [Google Scholar]
- Dweck, A.C. Formulating Natural Cosmetics; Allured Books: Du Page County, IL, USA, 2011. [Google Scholar]
- Barizza, E.; Guzzo, F.; Fanton, P.; Lucchini, G.; Sacchi, A.G.; Lo Schiavo, F.; Nascimbene, J. Nutritional profile and productivity of bilberry (Vaccinium myrtillus L.) in different habitats of a protected area of the Eastern Italian Alps. J. Food Sci. 2013, 78, C673. [Google Scholar] [CrossRef]
- Jaakkola, M.; Korpelainen, V.; Hoppulab, K.; Virtanena, V. Chemical composition of ripe fruits of Rubus chamaemorus L. grown in different habitats. J. Sci. Food Agric. 2012, 92, 1324–1330. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Antoniewska, A.; Smoleń, S.; Łukasiewicz, M.; Baranowski, D.; Duda, I.; Pietsch, J. Red arils of Taxus baccata L.—A new source of valuable fatty acids and nutrients. Molecules 2021, 26, 723. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Mujumdar, A. Berry drying: Mechanism, pretreatment, drying technology, nutrient preservation, and mathematical models. Food Eng. Rev. 2019, 11, 61–77. [Google Scholar] [CrossRef]
- Sablani, S.S.; Andrews, P.K.; Davies, N.M.; Walters, T.; Saez, H.; Bastarrachea, L. Effects of air and freeze drying on phytochemical content of conventional and organic berries. Drying Technol. 2011, 29, 205–216. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Wang, M.; Xu, B.N.; Du, L.J. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, a-tocopherol, b-carotene, and phenolic compounds. J. Agric. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Bhat, R.; Yeong, K.J.; Liong, M.T.; Karim, A.A.; Mansor, S.M. Influence of drying treatments on polyphenolic contents and antioxidant properties of raw and ripe papaya (Carica papaya L.). Int. J. Food Prop. 2014, 17, 283–292. [Google Scholar] [CrossRef]
- Nora, C.D.; Muller, C.D.R.; de Bona, G.S.; Rios, A.D.O.; Hertz, P.F.; de Jong Jablonski, A.; Flores, S.H. Effect of processing on the stability of bioactive compounds from red guava (Psidium cattleyanum Sabine) and guabiju (Myrcianthes pungens). J. Food Composit. Anal. 2014, 34, 18–25. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Pak Dek, M.S.; Hairuddin, M.R. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A review on the effect of drying on antioxidant potential of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Joshi, A.P.K.; Rupasinghe, H.P.V.; Khanizadeh, S. Impact of drying processes on bioactive phenolics, vitamin C and antioxidant capacity of red-fleshed apple slices. J. Food Process Preserv. 2011, 35, 453–457. [Google Scholar] [CrossRef]
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D. Bioactive properties of elderflowers (Sambucus nigra L.). World Sci. N 2017, 2, 115–119. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Kołodziej, B.; Drożdżal, K. Właściwości przeciwutleniające kwiatów i owoców bzu czarnego pozyskiwanego ze stanu naturalnego. (Antioxidant properties of black elder flowers and berries harvested from the wild). Żywność Nauka Technol. Jakość 2011, 4, 36–44. (In Polish) [Google Scholar]
- Diankov, S.; Parlapanska, K. Extraction from elderberry flowers. Examination of antioxidant capacity and total phenolic content of the extracts. J. Chem. Technol. Metall. 2013, 48, 479–482. [Google Scholar]
- Mao, L.C.; Pan, X.; Que, F.; Fang, X.H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Chen, Y.L.; Sung, J.M.; Lin, S.D. Effect of extraction methods on the active compounds and antioxidant properties of ethanolic extracts of Echinacea purpurea flower. Am. J. Plant Sci. 2015, 6, 201. [Google Scholar] [CrossRef]
- Rutkowski, L. Klucz do Oznaczania Roślin Naczyniowych Polski Niżowej; Wydawn Naukowe PWN: Warszawa, Poland, 1998. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelie, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ardestani, A.; Yazdanparast, R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007, 104, 1041–1047. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. Oxid. Antioxid. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Klimczak, I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of storage on the kontent of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compost. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
| FRAP [µmol Fe2+] | DPPH [µmol TE] | Flavonoids [mg(+)Catechin] | Total Polyphenols [mg(+)Catechin] | Dry Extract [g] | |
|---|---|---|---|---|---|
| Stabilisation method | |||||
| Fresh | 473.60 ± 14.9 b | 567.96 ± 17.0 a | 511.58 ± 23.2 b | 2072.50 ± 76.1 b | 0.257 ± 0.005 d |
| Frozen | 77.40 ± 2.5 a | 243.55 ± 29.8 a | 81.40 ± 4.0 a | 619.04 ± 22.9 a | 0.189 ± 0.004 b |
| Air-flow drying | 685.59 ± 45.1 c | 1029.43 ± 221.8 b | 767.56 ± 41.2 d | 2813.32 ± 179.7 c | 0.234 ± 0.014 c |
| Freeze-drying | 777.67 ± 30.1 d | 5131.51 ± 505.9 c | 575.16 ± 25.4 c | 4070.35 ± 106.5 d | 0.185 ± 0.004 a |
| FRAP [µmol Fe2+] | DPPH [µmol TE] | Flavonoids [mg(+)Catechin] | Total Polyphenols [mg(+)Catechin] | Dry Extract [g] | |
|---|---|---|---|---|---|
| Extraction time [days] | |||||
| 0 | 283.84 ± 28.1 a | 1909.31 ± 400.7 a | 298.70 ± 17.5 a | 1598.03 ± 163.0 a | 0.141 ± 0.009 a |
| 1 | 632.77 ± 47.9 d | 3050.42 ± 541.5 b | 559.39 ± 41.74 b | 2611.04 ± 176.1 b | 0.240 ± 0.006 b |
| 2 | 586.42 ± 43.3 c | 3446.94 ± 664.4 b | 548.79 ± 39.34 b | 2752.89 ± 182.4 c | 0.244 ± 0.007 c |
| 3 | 511.21 ± 40.9 b | 3481.96 ± 672.8 b | 539.48 ± 47.0 b | 2613.27 ± 211.1 b | 0.240 ± 0.007 b |
| FRAP [µmol Fe2+] | DPPH [µmol TE] | Flavonoids [mg(+)Catechin] | Total Polyphenols [mg(+)Catechin] | Dry Extract [g] | |
|---|---|---|---|---|---|
| Extracting agent (solvent) type | |||||
| Ethanol | 479.86 ± 29.2 a | 2747.51 ± 382.9 a | 482.38 ± 28.6 a | 2347.58 ± 130.4 a | 0.210 ± 0.007 a |
| Methanol | 527.26 ± 32.7 b | 3157.79 ± 432.4 b | 488.08 ± 27.9 a | 2440.02 ± 141.2 b | 0.222 ± 0.006 b |
| FRAP [µmol Fe2+] | DPPH [µmol TE] | Flavonoids [mg(+)Catechin] | Total Polyphenols [mg(+)Catechin] | Dry Extract [g] | |
|---|---|---|---|---|---|
| Extracting agent concentration [%] | |||||
| 50 | 451.00 ± 38.4 a | 2372.29 ± 460.9 a | 463.88 ± 38.6 a | 2353.21 ± 180.6 b | 0.211 ± 0.009 a |
| 60 | 532.13 ± 44.5 c | 3274.97 ± 605.6 b | 530.02 ± 43.7 b | 2565.85 ± 192.9 d | 0.220 ± 0.009 b |
| 70 | 526.87 ± 47.0 c | 3454.42 ± 656.5 b | 485.67 ± 40.9 a | 2433.06 ± 211.1 c | 0.223 ± 0.009 b |
| 90 | 504.25 ± 45.2 b | 2721.55 ± 575.1 a | 461.83 ± 36.5 a | 2223.10 ± 183.2 a | 0.211 ± 0.009 a |
| Name of Active Component | mg in 100 mL |
|---|---|
| Caffeic acid | 10.96 ± 0.008 |
| p-coumaric acid | 1.82 ± 0.003 |
| Rutin | 38.32 ± 0.097 |
| t-cinnamic acid | 0.67 ± 0.002 |
| Kaempferol | 14.30 ± 0.020 |
| Chlorogenic acid | 5.14 ± 0.015 |
| Ferulic acid | 0.59 ± 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabaszewska, M.; Sikora, E. The Effect of the Plant Stabilisation Method on the Composition and Antioxidant Properties of Elderflower (Sambucus nigra L.) Extract. Molecules 2023, 28, 2365. https://doi.org/10.3390/molecules28052365
Tabaszewska M, Sikora E. The Effect of the Plant Stabilisation Method on the Composition and Antioxidant Properties of Elderflower (Sambucus nigra L.) Extract. Molecules. 2023; 28(5):2365. https://doi.org/10.3390/molecules28052365
Chicago/Turabian StyleTabaszewska, Małgorzata, and Elżbieta Sikora. 2023. "The Effect of the Plant Stabilisation Method on the Composition and Antioxidant Properties of Elderflower (Sambucus nigra L.) Extract" Molecules 28, no. 5: 2365. https://doi.org/10.3390/molecules28052365
APA StyleTabaszewska, M., & Sikora, E. (2023). The Effect of the Plant Stabilisation Method on the Composition and Antioxidant Properties of Elderflower (Sambucus nigra L.) Extract. Molecules, 28(5), 2365. https://doi.org/10.3390/molecules28052365







