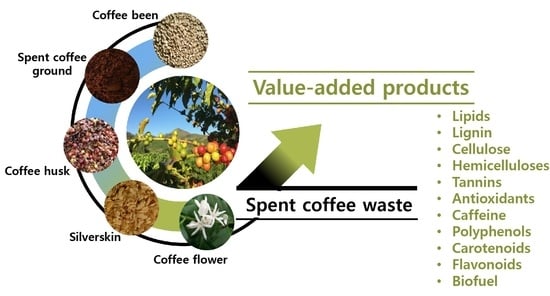
Value-Added Products from Coffee Waste: A Review
Abstract
1. Introduction
2. Components of Coffee By-Products and Their Chemical Compositions
2.1. Coffee Leaves
2.2. Coffee Flowers
2.3. Coffee Pulps and Husks
2.4. Coffee Silverskin
2.5. SCGs
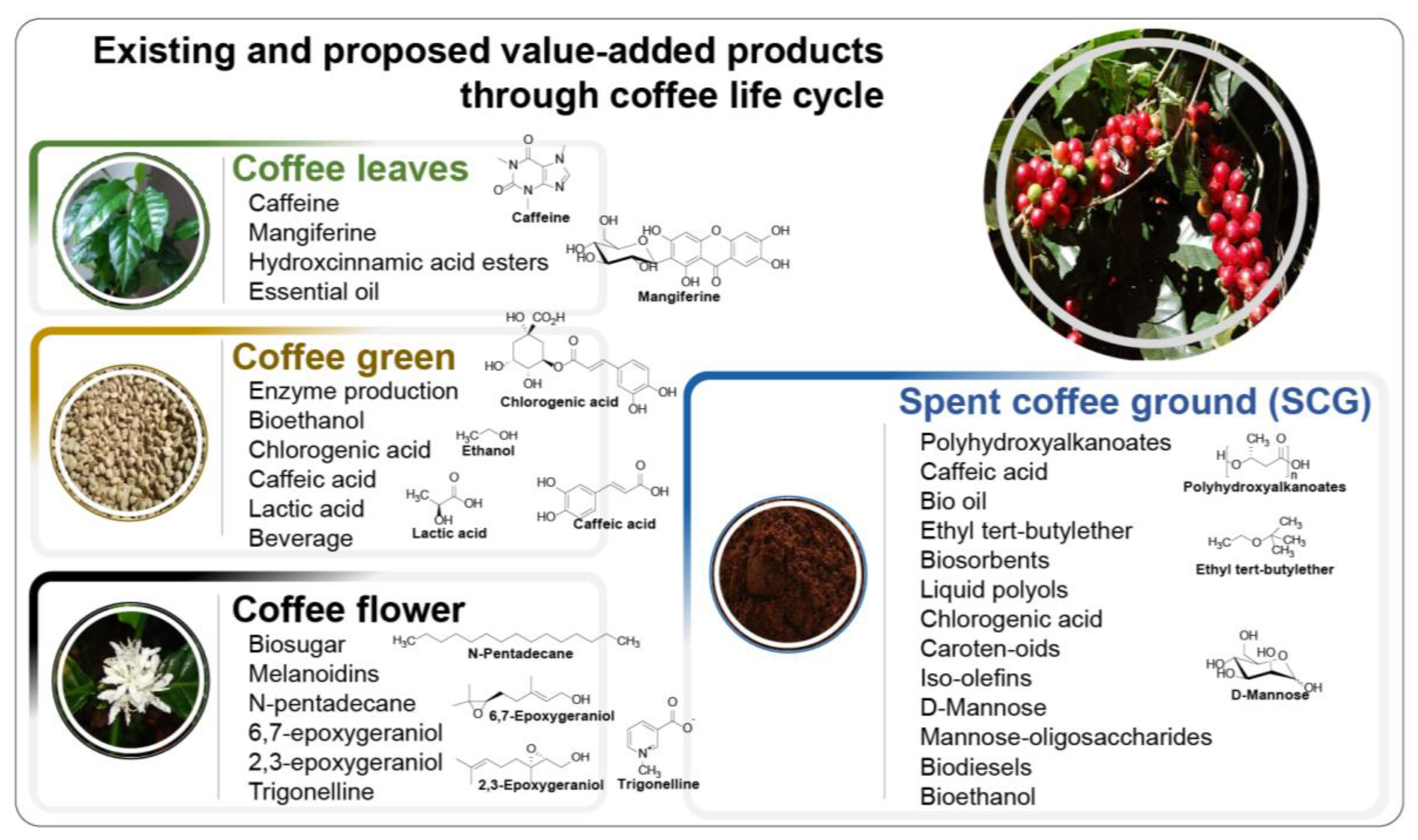
3. Value-Added Products from SCG
3.1. Biofuels
3.2. Bio-Sugars
3.3. Bio-Oils
3.4. Bioactive Compounds
3.5. Enzymes and Organic Acids
3.6. Biopolymers, Carotenoids, Biosorbents, Antioxidants, and Biocomposites
3.7. Photothermal Materials, Catalyst for Creation of Nanoparticles, and Synthetic Leather
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel approaches in the valorization of agricultural wastes and their applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Catalán, E.; Komilis, D.; Sánchez, A. Environmental impact of cellulase production from coffee husks by solid-state fermentation: A life-cycle assessment. J. Clean. Prod. 2019, 233, 954–962. [Google Scholar] [CrossRef]
- Moreira, M.D.; Melo, M.M.; Coimbra, J.M.; Reis, K.C.D.; Schwan, R.F.; Silva, C.F. Solid coffee waste as alternative to produce carotenoids with antioxidant and antimicrobial activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Peshev, D.; Mitev, D.; Peeva, L.; Peev, G. Valorization of spent coffee grounds–A new approach. Sep. Purif. Technol. 2018, 192, 271–277. [Google Scholar] [CrossRef]
- Thenepalli, T.; Ramakrishna, C.; Ahn, J.W. Environmental effect of the coffee waste and anti-microbial property of oyster shell waste treatment. J. Energy Eng. 2017, 26, 39–49. [Google Scholar] [CrossRef]
- Yun, B.Y.; Cho, H.M.; Kim, Y.U.; Lee, S.C.; Berardi, U.; Kim, S. Circular reutilization of coffee waste for sound absorbing panels: A perspective on material recycling. Environ. Res. 2020, 184, 109281. [Google Scholar] [CrossRef]
- Echeverria, M.C.; Nuti, M. Valorisation of the residues of coffee agro-industry: Perspectives and limitations. Open Waste Manag. J. 2017, 10, 13–22. [Google Scholar] [CrossRef]
- Lestari, W.; Hasballah, K.; Listiawan, M.; Sofia, S. Coffee by-products as the source of antioxidants: A systematic review. F1000Research 2022, 11, 220. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Kim, K.; Park, Y. Bioenergy Production by Establishing a System for Collecting Spent Coffee Grounds (Korean); National Assembly Research Service: Seoul, Republic of Korea, 2020. [Google Scholar]
- Blinová, L.; Sirotiak, M. Utilization of Spent Coffee Grounds for Removal of Hazardous Substances from Water: A Review. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2019, 27, 145–152. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Sholichah, E.; Apriani, R.; Desnilasari, D. By-product of arabica and robusta coffee husk as polyphenol source for antioxidant and antibacterial. J. Ind. Has. Perkeb. 2019, 14, 57–66. [Google Scholar]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rebacz-Maron, E.; Gutowska, I. Mineral composition and antioxidant potential of coffee beverages depending on the brewing method. Foods 2020, 9, 121. [Google Scholar] [CrossRef]
- Jung, S.; Gu, S.; Lee, S.-H.; Jeong, Y. Effect of roasting degree on the antioxidant properties of espresso and drip coffee eExtracted from Coffea arabica cv. Java. Appl. Sci. 2021, 11, 7025. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honorio, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Iuliano, M. Active biocatalyst for Biodiesel Production from Spent Coffee Ground. Bioresour. Technol. 2018, 266, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Wogderess, A.S. Available information on the feeding value of coffee waste and ways to improve coffee waste for animal feed. Afr. J. Biol. 2016, 3, 243–257. [Google Scholar]
- Arya, M.; Rao, L.J.M. An Impression of Coffee Carbohydrates. Crit. Rev. Food Sci. Nutr. 2007, 47, 51–67. [Google Scholar] [CrossRef]
- Rallis, C.; Codlin, S.; Bähler, J. TORC 1 signaling inhibition by rapamycin and caffeine affect lifespan, global gene expression, and cell proliferation of fission yeast. Aging Cell 2013, 12, 563–573. [Google Scholar] [CrossRef]
- Rodriguez, R.S.; Haugen, R.; Rueber, A.; Huang, C.-C. Reversible neuronal and muscular toxicity of caffeine in developing vertebrates. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 163, 47–54. [Google Scholar] [CrossRef]
- Durán-Aranguren, D.D.; Robledo, S.; Gomez-Restrepo, E.; Arboleda Valencia, J.W.; Tarazona, N.A. Scientometric overview of coffee by-products and their applications. Molecules 2021, 26, 7605. [Google Scholar] [CrossRef]
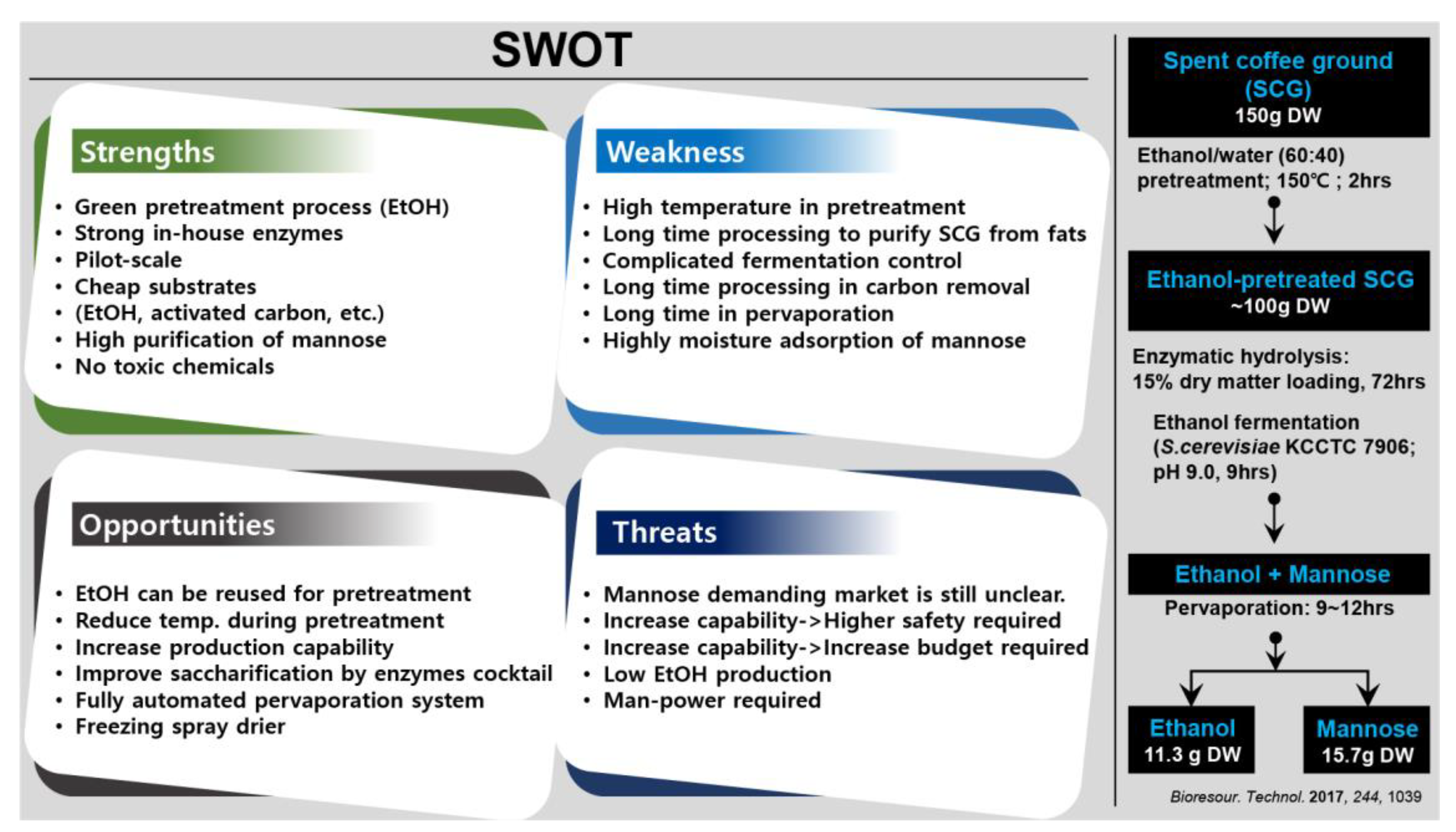
- Nguyen, Q.A.; Cho, E.J.; Trinh, L.T.P.; Jeong, J.-S.; Bae, H.-J. Development of an integrated process to produce d-mannose and bioethanol from coffee residue waste. Bioresour. Technol. 2017, 244, 1039–1048. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Cho, E.J.; Lee, D.S.; Bae, H.J. Development of an advanced integrative process to create valuable biosugars including manno-oligosaccharides and mannose from spent coffee grounds. Bioresour. Technol. 2019, 272, 209–216. [Google Scholar] [CrossRef]
- Ashihara, H.; Monteiro, A.M.; Moritz, T.; Gillies, F.M.; Crozier, A. Catabolism of caffeine and related purine alkaloids in leaves of Coffea arabica L. Planta 1996, 198, 334–339. [Google Scholar] [CrossRef]
- Mazzafera, P. Mineral nutrition and caffeine content in coffee leaves. Bragantia 1999, 58, 387–391. [Google Scholar] [CrossRef]
- Campa, C.; Mondolot, L.; Rakotondravao, A.; Bidel, L.P.R.; Gargadennec, A.; Couturon, E.; La Fisca, P.; Rakotomalala, J.-J.; Jay-Allemand, C.; Davis, A.P. A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: Biological implications and uses. Ann. Bot. 2012, 110, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, D.; Pino, J.A. Essential oil of Coffea arabica L. var. castillo leaves from colombia AU-Quijano-Célis, Clara. J. Essent. Oil Bear. Plants 2015, 18, 486–488. [Google Scholar]
- Chen, X. A review on coffee leaves: Phytochemicals, bioactivities and applications. Crit. Rev. Food Sci. Nutr. 2019, 59, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Emura, M.; Nohara, I.; Toyoda, T.; Kanisawa, T. The volatile constituents of the coffee flower (Coffea arabica L. ). Flavour Fragr. J. 1997, 12, 9–13. [Google Scholar] [CrossRef]
- Emura, M.; Toyoda, T.; Kanisawa, T. Epoxygeraniol and epoxynerol from coffee flower (Coffea arabica L.) AU-Nohara, Isao. J. Essent. Oil Res. 1997, 9, 727–729. [Google Scholar]
- Stashenko, E.E.; Martínez, J.R.; Cárdenas-Vargas, S.; Saavedra-Barrera, R.; Durán, D.C. GC–MS study of compounds isolated from Coffea arabica flowers by different extraction techniques. J. Sep. Sci. 2013, 36, 2901–2914. [Google Scholar] [CrossRef]
- Nguyen, T.M.T.; Cho, E.J.; Song, Y.; Oh, C.H.; Funada, R.; Bae, H.J. Use of coffee flower as a novel resource for the production of bioactive compounds, melanoidins, and bio-sugars. Food Chem. 2019, 299, 125120. [Google Scholar] [CrossRef]
- Wirz, K.; Schwarz, S.; Richling, E.; Walch, S.G.; Lachenmeier, D.W. Coffee flower as a promising novel food—Chemical characterization and sensory evaluation. Biol. Life Sci. Forum 2022, 18, 53. [Google Scholar]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Corro, G.; Paniagua, L.; Pal, U.; Bañuelos, F.; Rosas, M. Generation of biogas from coffee-pulp and cow-dung co-digestion: Infrared studies of postcombustion emissions. Energy Convers. Manag. 2013, 74, 471–481. [Google Scholar] [CrossRef]
- Martínez-Carrera, D.; Aguilar, A.; Martínez, W.; Bonilla, M.; Morales, P.; Sobal, M. Commercial production and marketing of edible mushrooms cultivated on coffee pulp in mexico. In Coffee Biotechnology and Quality, Proceedings of the Third International Seminar on Biotechnology in the Coffee Agro-Industry, Londrina, Brazil; Sera, T., Soccol, C.R., Pandey, A., Roussos, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 471–488. [Google Scholar]
- Velázquez-Cedeño, M.A.; Mata, G.; Savoie, J.-M. Waste-reducing cultivation of Pleurotus ostreatus and Pleurotus pulmonarius on coffee pulp: Changes in the production of some lignocellulolytic enzymes. World J. Microbiol. Biotechnol. 2002, 18, 201–207. [Google Scholar] [CrossRef]
- Selvam, K.; Govarthanan, M.; Kamala-Kannan, S.; Govindharaju, M.; Senthilkumar, B.; Selvankumar, T.; Sengottaiyan, A. Process optimization ofs cellulase production from alkali-treated coffee pulp and pineapple waste using Acinetobacter sp. TSK MASC RSC Adv. 2014, 4, 13045–13051. [Google Scholar] [CrossRef]
- Dias, M.; Melo, M.M.; Schwan, R.F.; Silva, C.F. A new alternative use for coffee pulp from semi-dry process to β-glucosidase production by Bacillus subtilis. Lett. Appl. Microbiol. 2015, 61, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Menezes, E.G.T.; do Carmo, J.R.; Menezes, A.G.T.; Alves, J.G.L.F.; Pimenta, C.J.; Queiroz, F. Use of Different Extracts of Coffee Pulp for the Production of Bioethanol. Appl. Biochem. Biotechnol. 2013, 169, 673–687. [Google Scholar] [CrossRef]
- Menezes, E.G.T.; do Carmo, J.R.; Alves, J.G.L.F.; Menezes, A.G.T.; Guimarães, I.C.; Queiroz, F.; Pimenta, C.J. Optimization of alkaline pretreatment of coffee pulp for production of bioethanol. Biotechnol. Prog. 2014, 30, 451–462. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Yang, J.; Bae, H.-J. Bioethanol production from individual and mixed agricultural biomass residues. Ind. Crops Prod. 2017, 95, 718–725. [Google Scholar] [CrossRef]
- Gurram, R.; Al-Shannag, M.; Knapp, S.; Das, T.; Singsaas, E.; Alkasrawi, M. Technical possibilities of bioethanol production from coffee pulp: A renewable feedstock. Clean Technol. Environ. Policy 2016, 18, 269–278. [Google Scholar] [CrossRef]
- Torres-Mancera, M.T.; Baqueiro-Peña, I.; Figueroa-Montero, A.; Rodríguez-Serrano, G.; González-Zamora, E.; Favela-Torres, E.; Saucedo-Castañeda, G. Biotransformation and improved enzymatic extraction of chlorogenic acid from coffee pulp by filamentous fungi. Biotechnol. Prog. 2013, 29, 337–345. [Google Scholar] [CrossRef]
- Torres-Mancera, M.-T.; Cordova-López, C.J.; Rodríguez-Serrano, G.; Roussos, S.; Ramírez-Coronel, M.A.; Favela-Torres, E.; Saucedo-Castañeda, G. Enzymatic extraction of hydroxycinnamic acids from coffee pulp. Food Technol. Biotechnol. 2011, 49, 369–373. [Google Scholar]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Magoni, C.; Bruni, I.; Guzzetti, L.; Dell’Agli, M.; Sangiovanni, E.; Piazza, S.; Regonesi, M.E.; Maldini, M.; Spezzano, R.; Caruso, D.; et al. Valorizing coffee pulp by-products as anti-inflammatory ingredient of food supplements acting on IL-8 release. Food Res. Int. 2018, 112, 129–135. [Google Scholar] [CrossRef]
- Pleissner, D.; Neu, A.-K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Rahman, N.K. Equilibrium, kinetics and thermodynamic of Remazol Brilliant Orange 3R dye adsorption on coffee husk-based activated carbon. Chem. Eng. J. 2011, 170, 154–161. [Google Scholar] [CrossRef]
- Bekalo, S.A.; Reinhardt, H.-W. Fibers of coffee husk and hulls for the production of particleboard. Mater. Struct. 2010, 43, 1049–1060. [Google Scholar] [CrossRef]
- Gonçalves, M.; Guerreiro, M.C.; de Oliveira, L.C.A.; de Castro, C.S. A friendly environmental material: Iron oxide dispersed over activated carbon from coffee husk for organic pollutants removal. J. Environ. Manag. 2013, 127, 206–211. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Abidha, S.; Poopathi, S. Coffee husk waste for fermentation production of mosquitocidal bacteria. J. Econ. Entomol. 2011, 104, 1816–1823. [Google Scholar]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Regazzoni, L.; Saligari, F.; Marinello, C.; Rossoni, G.; Aldini, G.; Carini, M.; Orioli, M. Coffee silver skin as a source of polyphenols: High resolution mass spectrometric profiling of components and antioxidant activity. J. Funct. Foods 2016, 20, 472–485. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Biobutanol production from coffee silverskin. Microb. Cell Factories 2018, 17, 154. [Google Scholar] [CrossRef]
- López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A biorefinery approach for the valorization of spent coffee grounds to produce antioxidant compounds and biobutanol. Biomass Bioenergy 2021, 147, 106026. [Google Scholar] [CrossRef]
- Mirzoyan, S.; Aghekyan, H.; Vanyan, L.; Vassilian, A.; Trchounian, K. Coffee silverskin as a substrate for biobased production of biomass and hydrogen by Escherichia coli. Int. J. Energy Res. 2022, 46, 23110–23121. [Google Scholar] [CrossRef]
- Vanyan, L.; Cenian, A.; Trchounian, K. Biogas and biohydrogen production using spent coffee grounds and alcohol production waste. Energies 2022, 15, 5935. [Google Scholar] [CrossRef]
- Petrosyan, H.; Vanyan, L.; Mirzoyan, S.; Trchounian, A.; Trchounian, K. Roasted coffee wastes as a substrate for Escherichia coli to grow and produce hydrogen. FEMS Microbiol. Lett. 2020, 367, fnaa088. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef]
- Saratale, G.D.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Bhatia, S.K.; Atabani, A.; Mulone, V. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef]
- Lee, M.; Yang, M.; Choi, S.; Shin, J.; Park, C.; Cho, S.-K.; Kim, Y.M. Sequential production of lignin, fatty acid methyl esters and biogas from spent coffee grounds via an integrated physicochemical and biological process. Energies 2019, 12, 2360. [Google Scholar] [CrossRef]
- Chiyanzy, I.; Brienzo, M.; García-Aparicio, M.; Agudelo, R.; Görgens, J. Spent coffee ground mass solubilisation by steam explosion and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2015, 90, 449–458. [Google Scholar] [CrossRef]
- Mayanga-Torres, P.C.; Lachos-Perez, D.; Rezende, C.A.; Prado, J.M.; Ma, Z.; Tompsett, G.T.; Timko, M.T.; Forster-Carneiro, T. Valorization of coffee industry residues by subcritical water hydrolysis: Recovery of sugars and phenolic compounds. J. Supercrit. Fluids 2017, 120, 75–85. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Kourmentza, C.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Spent coffee grounds make much more than waste: Exploring recent advances and future exploitation strategies for the valorization of an emerging food waste stream. J. Clean. Prod. 2018, 172, 980–992. [Google Scholar] [CrossRef]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.F.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef]
- Saud, S.; Salamatullah, A.M. Relationship between the chemical composition and the biological functions of coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef]
- Eromo, A.W.; Brehanu, T.; Urga, K.; Tadessa, M.; Weledesemayat, G.T. Sensory evaluation, proximate and mineral composition of beverage prepared from matured coffee leaves growing in different areas of Ethiopia. Afr. J. Food Sci. 2020; in press. [Google Scholar] [CrossRef]
- Choi, I.S.; Wi, S.G.; Kim, S.B.; Bae, H.J. Conversion of coffee residue waste into bioethanol with using popping pretreatment. Bioresour. Technol. 2012, 125, 132–137. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.; Melo, A.C.; Martins, A.A.; Mata, T.M. Spent coffee grounds for biodiesel production and other applications. Clean Technol. Environ. Policy 2014, 16, 1423–1430. [Google Scholar] [CrossRef]
- Ding, J.; Mei, S.; Gao, L.; Wang, Q.; Ma, H.; Chen, X. Tea processing steps affect chemical compositions, enzyme activities, and antioxidant and anti-inflammatory activities of coffee leaves. Food Front. 2022, 3, 505–516. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Phuong, D.V.; Quoc, L.P.T.; Tan, P.V.; Duy, L.N.D. Production of bioethanol from Robusta coffee pulp (Coffea robusta L.) in Vietnam. Foods Raw Mater. 2019, 7, 10–17. [Google Scholar] [CrossRef]
- Braham, J.E.; Bressani, R. Coffee Pulp: Composition, Technology, and Utilization; IDRC: Ottawa, ON, Canada, 1979. [Google Scholar]
- Brienzo, M.; García-Aparicio, M.; Görgens, J. Spent coffee ground properties and application in bioenergy and bioproducts. Prod. Consum. Health Benefits 2016, 4, 67–96. [Google Scholar]
- Marín-Garza, T.; Gómez-Merino, F.C.; Aguilar-Rivera, N.; Murguía-González, J.; Trejo-Téllez, L.I.; Pastelín-Solano, M.C.; Castañeda-Castro, O. Bioactive composition of coffee leaves during an annual cycle. Rev. Fitotec. Mex. 2018, 41, 365–372. [Google Scholar]
- Muzaifa, M.; Hasni, D.; Patria, A.; Abubakar, A. Chemical composition of green and roasted coffee bean of Gayo arabica civet coffee (kopi luwak). IOP Conf. Ser. Earth Environ. Sci. 2020, 425, 012001. [Google Scholar] [CrossRef]
- dos Santos, É.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; Del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Wahyuni, N.; Rispiandi, R.; Hariyadi, T. Effect of bean maturity and roasting temperature on chemical content of robusta coffee. IOP Conf. Ser. Mater. Sci. Eng. 2020, 830, 022019. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Assabumrungrat, S.; Kiatkittipong, W.; Praserthdam, P.; Goto, S. Simulation of pervaporation membrane reactors for liquid phase synthesis of ethyl tert-butyl ether from tert-butyl alcohol and ethanol. Catal. Today 2003, 79, 249–257. [Google Scholar] [CrossRef]
- Assabumrungrat, S.; Kiatkittipong, W.; Sevitoon, N.; Praserthdam, P.; Goto, S. Kinetics of liquid phase synthesis of ethyl tert-butyl ether from tert-butyl alcohol and ethanol catalyzed by β-zeolite supported on monolith. Int. J. Chem. Kinet. 2002, 34, 292–299. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Assabumrungrat, S.; Praserthdam, P.; Goto, S. A pervaporation membrane reactor for liquid phase synthesis of ethyl tert-butyl ether from tert-butyl alcohol and ethanol. J. Chem. Eng. Jpn. 2002, 35, 547–556. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Thipsunet, P.; Goto, S.; Chaisuk, C.; Praserthdam, P.; Assabumrungrat, S. Simultaneous enhancement of ethanol supplement in gasoline and its quality improvement. Fuel Process. Technol. 2008, 89, 1365–1370. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, J.-H.; Wi, S.G.; Kim, K.H.; Bae, H.-J. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy 2013, 102, 204–210. [Google Scholar] [CrossRef]
- Wi, S.; Choi, I.; Kim, K.; Kim, H.; Bae, H.-J. Bioethanol production from rice straw by popping pretreatment. Biotechnol. Biofuels 2013, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Chung, B.Y.; Lee, Y.G.; Yang, D.J.; Bae, H.-J. Enhanced enzymatic hydrolysis of rapeseed straw by popping pretreatment for bioethanol production. Bioresour. Technol. 2011, 102, 5788–5793. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Camargos, R.R.S.; Ferraz, V.P. Coffee oil as a potential feedstock for biodiesel production. Bioresour. Technol. 2008, 99, 3244–3250. [Google Scholar] [CrossRef]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent coffee grounds as a versatile source of green energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef] [PubMed]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Oil extracted from spent coffee grounds for bio-hydrotreated diesel production. Energy Convers. Manag. 2016, 126, 1028–1036. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Ind. Crops Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- Kookos, I. Technoeconomic and environmental assessment of a process for biodiesel production from spent coffee grounds (SCGs). Resour. Conserv. Recycl. 2018, 134, 156–164. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Fulger, C.; Stahl, H.; Turek, E.; Bayha, R. Production of a Mannan Oligomer Hydrolysate; United States Patent and Trademark Office: Alexandria, VA, USA, 1985. [Google Scholar]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Hu, X.; Shi, Y.; Zhang, P.; Miao, M.; Zhang, T.; Jiang, B. D-Mannose: Properties, production, and applications: An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pan, Z.; Qian, C.; Chen, X. Isolation and purification of d-mannose from palm kernel. Carbohydr. Res. 2009, 344, 1687–1689. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Stageman, N.E.; Fortune, C.M.; Chuck, C.J. Effect of the type of bean, processing, and geographical location on the biodiesel produced from waste coffee grounds. Energy Fuels 2014, 28, 1166–1174. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, V.; Solanki, K.; Mukherjee, J.; Gupta, M.N. Combi-protein coated microcrystals of lipases for production of biodiesel from oil from spent coffee grounds. Sustain. Chem. Process. 2013, 1, 14. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, Y.G.; Song, Y.; Nguyen, D.T.; Bae, H.J. An integrated process for conversion of spent coffee grounds into value-added materials. Bioresour. Technol. 2022, 346, 126618. [Google Scholar]
- Couto, R.M.; Fernandes, J.; da Silva, M.D.R.G.; Simões, P.C. Supercritical fluid extraction of lipids from spent coffee grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Kelkar, S.; Saffron, C.M.; Chai, L.; Bovee, J.; Stuecken, T.R.; Garedew, M.; Li, Z.; Kriegel, R.M. Pyrolysis of spent coffee grounds using a screw-conveyor reactor. Fuel Process. Technol. 2015, 137, 170–178. [Google Scholar] [CrossRef]
- Kamil, M.; Ramadan, K.M.; Olabi, A.G.; Al-Ali, E.I.; Ma, X.; Awad, O.I. Economic, technical, and environmental viability of biodiesel blends derived from coffee waste. Renew. Energy 2020, 147, 1880–1894. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martinez-Avila, G.; Montanez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Madhava Naidu, M.; Srinivas, P. Production of α-amylase under solid-state fermentation utilizing coffee waste. J. Chem. Technol. Biotechnol. 2009, 84, 1246–1249. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Production and application of xylanase from Penicillium sp. utilizing coffee by-products. Food Bioprocess Technol. 2010, 5, 657–664. [Google Scholar] [CrossRef]
- Rocha, F.T.B.; Brandao-Costa, R.M.P.; Neves, A.G.D.; Cardoso, K.B.B.; Nascimento, T.P.; Albuquerque, W.W.C.; Porto, A.L.F. Purification and characterization of a protease from Aspergillus sydowii URM5774: Coffee ground residue for protease production by solid state fermentation. Acad. Bras. Cienc. 2021, 93, e20200867. [Google Scholar] [CrossRef]
- Buntic, A.V.; Pavlovic, M.D.; Antonovic, D.G.; Siler-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Utilization of spent coffee grounds for isolation and stabilization of Paenibacillus chitinolyticus CKS1 cellulase by immobilization. Heliyon 2016, 2, e00146. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Teixeira, J.A. Increase in the fructooligosaccharides yield and productivity by solid-state fermentation with Aspergillus japonicus using agro-industrial residues as support and nutrient source. Biochem. Eng. J. 2010, 53, 154–157. [Google Scholar] [CrossRef]
- Shankaranand, V.; Lonsane, B. Coffee husk: An inexpensive substrate for production of citric acid by Aspergillus niger in a solid-state fermentation system. World J. Microbiol. Biotechnol. 1994, 10, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.M.; Soccol, C.R.; de Oliveira, B.H.; Pandey, A. Gibberellic acid production by solid-state fermentation in coffee husk. Appl. Biochem. Biotechnol. 2002, 102, 179–191. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Cenian, K.; Skiadas, I.V.; Gavala, H.N. Integration of chlorogenic acid recovery and bioethanol production from spent coffee grounds. Biochem. Eng. J. 2016, 116, 54–64. [Google Scholar] [CrossRef]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P.; Ferreira, A. Spent coffee grounds as a renewable source for ecopolyols production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Safarik, I.; Horska, K.; Svobodova, B.; Safarikova, M. Magnetically modified spent coffee grounds for dyes removal. Eur. Food Res. Technol. 2011, 234, 345–350. [Google Scholar] [CrossRef]
- Dávila-Guzmán, N.E.; de Jesús Cerino-Córdova, F.; Soto-Regalado, E.; Rangel-Mendez, J.R.; Díaz-Flores, P.E.; Garza-Gonzalez, M.T.; Loredo-Medrano, J.A. Copper biosorption by spent coffee ground: Equilibrium, kinetics, and mechanism. CLEAN Soil Air Water 2013, 41, 557–564. [Google Scholar] [CrossRef]
- Utomo, H.D.; Hunter, K. Adsorption of divalent copper, zinc, cadmium and lead ions from aqueous solution by waste tea and coffee adsorbents. Environ. Technol. 2006, 27, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lamine, S.M.; Ridha, C.; Mahfoud, H.-M.; Mouad, C.; Lotfi, B.; Al-Dujaili, A.H. Chemical activation of an activated carbon prepared from coffee residue. Energy Procedia 2014, 50, 393–400. [Google Scholar] [CrossRef]
- Baek, B.-S.; Park, J.-W.; Lee, B.-H.; Kim, H.-J. Development and application of green composites: Using coffee ground and bamboo flour. J. Polym. Environ. 2013, 21, 702–709. [Google Scholar] [CrossRef]
- Lourith, N.; Xivivadh, K.; Boonkong, P.; Kanlayavattanakul, M. Spent coffee waste: A sustainable source of cleansing agent for a high-performance makeup remover. Sustain. Chem. Pharm. 2022, 29, 100826. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Panzella, L.; Cerruti, P.; Ambrogi, V.; Agustin-Salazar, S.; D’Errico, G.; Carfagna, C.; Goya, L.; Ramos, S.; Martín, M.A.; Napolitano, A.; et al. A superior all-natural antioxidant biomaterial from spent coffee grounds for polymer stabilization, cell protection, and food lipid preservation. ACS Sustain. Chem. Eng. 2016, 4, 1169–1179. [Google Scholar] [CrossRef]
- Scully, D.S.; Jaiswal, A.K.; Abu-Ghannam, N. An investigation into spent coffee waste as a renewable source of bioactive compounds and industrially important sugars. Bioengineering 2016, 3, 33. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, K.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Del Castillo, M.D.; Gaytán-Martínez, M.; Campos-Vega, R. In vitro health promoting properties of antioxidant dietary fiber extracted from spent coffee (Coffee arabica L.) grounds. Food Chem. 2018, 261, 253–259. [Google Scholar] [CrossRef]
- Machado, E.; Mussatto, S.; Teixeira, J.; Vilanova, M.; Oliveira, J. Increasing the sustainability of the coffee agro-industry: Spent coffee grounds as a source of new beverages. Beverages 2018, 4, 105. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Wang, F.-M.; Juan, H.-Y.; Su, C.-H. Biodiesel production by direct transesterification of wet spent coffee grounds using switchable solvent as a catalyst and solvent. Bioresour. Technol. 2020, 296, 122334. [Google Scholar] [CrossRef]
- Kamgang Nzekoue, F.; Khamitova, G.; Angeloni, S.; Sempere, A.N.; Tao, J.; Maggi, F.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G. Spent coffee grounds: A potential commercial source of phytosterols. Food Chem. 2020, 325, 126836. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-E.; Mangidaan, D.; Chien, H.-W. Green sustainable photothermal materials by spent coffee grounds. J. Taiwan Inst. Chem. Eng. 2022, 137, 104259. [Google Scholar] [CrossRef]
- Chien, H.-W.; Chen, X.-E. Spent coffee grounds as potential green photothermal materials for biofilm elimination. J. Environ. Chem. Eng. 2022, 10, 107131. [Google Scholar] [CrossRef]
- Mangindaan, D.; Lin, G.-Y.; Kuo, C.-J.; Chein, H.-W. Biosynthesis of silver nanoparticles as catalyst by spent coffee ground/recycled poly(ethyleneterephtalate) composites. Food Bioprod. Process. 2020, 121, 193–201. [Google Scholar] [CrossRef]
- Atelge, M.R. Production of biodiesel and hydrogen by using a double-function heterogeneous catalyst derived from spent coffee grounds and its thermodynamic analysis. Renew. Energy 2022, 198, 1–15. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, J.; Zheng, S.; He, X.; Liu, X. Research on the preparation and application oif synthetic leather from coffee grounds for sustainable development. Sustainability 2022, 14, 13971. [Google Scholar] [CrossRef]

| Chemical Component | Green Coffee | Coffee Pulp | Coffee Husk | Silverskin | Roasted Coffee | Spent Coffee Ground | Coffee Flower | Coffee Leaf | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Carbohydrates | 60.0 | 44.0–55.0 | 57.8 | 44.0 | 58.5 | 60.3–82.0 | 51.0–63.9 | [11,77,78,79] | |
| Cellulose | 18.0–65.0 | 9.18–63.0 | 39.0–61.0 | 16.0–46.0 | 38.0–41.5 | 8.6 −47.3 | 3.1 −14.6 | 2.4 | [11,29,30,39,40,62,78,80,81,82,83,84,85,86] |
| Hemicellulose | 3.0 −15.0 | 2.0 −66.0 | 4.0 −10.0 | 4.0 −22.0 | 24.0–39.1 | 32.0–43.0 | - | - | [11,61,78,81,84,85,86,87] |
| Xylose | - | - | - | 4.7–7.6 | - | 0.3–1.1 | 2.4 | 2.7 | [29,30,39,40,62,80,83,84,88] |
| Arabinose | 20–35 | - | - | 2.0–3.5 | 0.1 | 1.7–3.6 | 0.3–3.8 | 3.5 | |
| Mannose | 10–20 | - | - | 1.8–2.6 | - | 19.1–21.6 | 0.2–1.3 | 0.6 | |
| Galactose | 55–65 | - | - | 3.8 | - | 8.2–16.4 | 2.7 | 1.0–2.3 | |
| Rhamnose | - | - | 0.1 | 0.7 | - | [39,80] | |||
| Lignin | 1.0–5.6 | 12.2–22 | 9.0 | 1.0–39.0 | 5.8–44.8 | 23.9–33.6 | - | - | [29,30,61,81,82,85,86,89,90] |
| Insoluble lignin | - | - | - | 21.0 | 17.6–31.9 | - | - | [29,30,69,82] | |
| Soluble lignin | - | - | - | 7.6 | 1.7–6.3 | - | - | ||
| Lipids | 8.0–18.0 | 0.3–2.5 | 0.5–6.0 | 0.3–4.0 | 11.0–17.0 | 6.0–38.6 | - | - | [11,77,84,85,89] |
| Proteins | 8.5–13.4 | 4.4–12.0 | 3.0–13.0 | 15.0–23.0 | 3.1–17.4 | 11.5–18.0 | 6.5–9.1 | 14.4–19.0 | [11,39,40,63,77,78,79,81,82,84,85,89,91,92] |
| Ash | 3.0–5.0 | 5.4–15.4 | 6.0 | 4.7–8.0 | 1.3–4.3 | 1.1–2.2 | 7.5–8.1 | 8.8–12.4 | |
| Caffeine | 0.8–4.0 | 0.8–5.7 | 0.5–2.0 | 0.0–1.4 | 1.0–2.4 | 0.02–0.4 | 0.9–1.1 | 1.6–2.5 | [11,32,39,40,77,78,79,83,84,85,86,91] |
| Tannins | - | 1.8–8.6 | 4.5–9.3 | 0.02 | - | 0.02 | - | - | [11,86] |
| Chlorogenic Acids | 3.8–10.0 | 1.0–10.7 | 2.0–12.6 | 3.0–15.8 | 0.9–8.3 | 1.8–11.5 | 1.3 | - | [11,40,77,84,86] |
| Pectins | 2.0 | 4.4–12.4 | 0.5–3.0 | 0.02 | 2.0 | 0.01 | - | - | [78,85,86] |
| Active Compounds from Spent Coffee Ground | Products | Role/Function | Application | Ref. |
|---|---|---|---|---|
| Bio-oil | Coffee oil makeup remover | Cleansing agent | Cosmetic | [139] |
| Bio-oil | Bio-polymer: poly(3-hydroxybutyrate) (PHB) | Biodegradable plastic | Packaging material | [131] |
| Hemicellulose/Cellulose | Bio-sugars (mannose, galactose, arabinose, and glucose) | Mannitol and fermentation feedstock | Chemical and food industry | [140] |
| Lipids | Ethanol, biodiesel | Biofuel/Alternative energy | Biorefinery/Transportation | [94] |
| Lignin | Hydrolyzed spent coffee grounds | Antioxidant: protect lipid oxidation | Biomedical/Industrial | [141] |
| Lignin | Bio-sugars (D-mannose, manno-oligosaccharides) | Biofuel feedstock | Value added biorefinery product | [117] |
| Lignocellulose | Bioethanol | Biofuel | Value-added biorefinery product | [50] |
| Lignocellulose | Xylanase | Xylan biodegradation | Biopulping, prebleaching of Kraft pulps, clarifying fruit juices and wine | [124] |
| Lignocellulose | glucose, galactose and mannose | Production of biofuels, amino acids and enzymes | Nutraceutical and food product | [142] |
| SCG (Ohmic heating extraction) | Dietary fiber bound with antioxidant | Antioxidant/anti-diabetic | Antidiabetic bakery product | [143] |
| Untreated SCG | Magnetically modified SCG | Biobsorbants | Xenobiotic dye removal | [134] |
| Untreated SCG | Substrate for cultivation of edible fungus | Mushrooms production | [2] | |
| Untreated/Whole SCG | Alcoholic beverages rich in ester and higher alcohol | Fermentation substrate for fermented and distilled beverages | Product diversification/Novel product development | [144] |
| Wet SCG | Biodiesel (Methanol) | Alternative energy | Biofuel production | [145] |
| SCG (Ultrasonic extraction) | Phytosterols (β-sitosterol campesterol, stigmasterol, cycloartenol) | Bioactive compound | Nutraceutical and cosmetic | [146] |
| Delignified and defatted SCG | D-mannose, manno-oligosaccharides, and bioethanol | Bio-sugar | Value-added biorefinery product | [30] |
| SCG (Ultrasonic) | Phenolic compounds (chlorogenic and protocatechuic acids) | Bioactive compounds | Biomedical and food | [123] |
| SCG (Pre-activated) | Cellulase (immobilized) | Cellulose depolymerization | Biofuel and food and pharmaceutical | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-G.; Cho, E.-J.; Maskey, S.; Nguyen, D.-T.; Bae, H.-J. Value-Added Products from Coffee Waste: A Review. Molecules 2023, 28, 3562. https://doi.org/10.3390/molecules28083562
Lee Y-G, Cho E-J, Maskey S, Nguyen D-T, Bae H-J. Value-Added Products from Coffee Waste: A Review. Molecules. 2023; 28(8):3562. https://doi.org/10.3390/molecules28083562
Chicago/Turabian StyleLee, Yoon-Gyo, Eun-Jin Cho, Shila Maskey, Dinh-Truong Nguyen, and Hyeun-Jong Bae. 2023. "Value-Added Products from Coffee Waste: A Review" Molecules 28, no. 8: 3562. https://doi.org/10.3390/molecules28083562
APA StyleLee, Y.-G., Cho, E.-J., Maskey, S., Nguyen, D.-T., & Bae, H.-J. (2023). Value-Added Products from Coffee Waste: A Review. Molecules, 28(8), 3562. https://doi.org/10.3390/molecules28083562