Qualitative and Quantitative Analysis of the Major Bioactive Components of Juniperus chinensis L. Using LC-QTOF-MS and LC-MSMS and Investigation of Antibacterial Activity against Pathogenic Bacteria
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Profiling of Juniperus chinensis L.
2.2. Quantitative Analysis of the Reference Compounds
2.3. Antibacterial Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of the Plant Extract
3.3. Qualitative Analysis Using Ultra-High-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry
3.4. Quantitative Analysis Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
3.5. Reference Compounds and Preparation of the Standard Solution
3.6. Antibacterial Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Farjon, A. Juniperus chinensis. In The IUCN Red List of Threatened Species; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2013. [Google Scholar]
- Seca, A.M.; Silva, A.M. The chemical composition of the Juniperus genus (1970–2004). Recent Prog. Med. Plants 2006, 16, 401–522. [Google Scholar]
- Asili, J.; Emami, S.A.; Rahimizadeh, M.; Fazly-Bazzaz, B.S.; Hassanzadeh, M.K. Chemical and antimicrobial studies of Juniperus excelsa subsp. excelsa and Juniperus excelsa subsp. polycarpos essential oils. J. Essent. Oil Bear. Plants 2008, 11, 292–302. [Google Scholar] [CrossRef]
- Taviano, M.F.; Marino, A.; Trovato, A.; Bellinghieri, V.; La Barbera, T.M.; Güvenç, A.; Hürkul, M.M.; De Pasquale, R.; Miceli, N. Antioxidant and antimicrobial activities of branches extracts of five Juniperus species from Turkey. Pharm. Biol. 2011, 49, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097. [Google Scholar] [CrossRef] [PubMed]
- Keskes, H.; Mnafgui, K.; Hamden, K.; Damak, M.; El Feki, A.; Allouche, N. In vitro anti-diabetic, anti-obesity and antioxidant proprieties of Juniperus phoenicea L. leaves from Tunisia. Asian Pac. J. Trop. Biomed. 2014, 4, S649–S655. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef]
- MacRae, W.D.; Towers, G.N. Biological activities of lignans. Phytochemistry 1984, 23, 1207–1220. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Avila, J.G.; Garcıá, A.M.; Becerra, J.; Flores, C.; Aqueveque, P.; Bittner, M.; Hoeneisen, M.; Martinez, M.; Silva, M. Antifungal and antibacterial activities of Araucaria araucana (Mol.) K. Koch heartwood lignans. Z. Nat. C 2006, 61, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Dou, Z.; Zhou, R.; Luo, L.; Bian, L.; Chen, Y.; Tao, J.; Chen, Z. Quality evaluation of artemisia capillaris thunb. Based on qualitative analysis of the HPLC fingerprint and UFLC-Q-TOF-MS/MS combined with quantitative analysis of multicomponents. J. Anal. Methods Chem. 2021, 2021, 5546446. [Google Scholar] [CrossRef]
- Zeliou, K.; Koui, E.-M.; Papaioannou, C.; Koulakiotis, N.S.; Iatrou, G.; Tsarbopoulos, A.; Papasotiropoulos, V.; Lamari, F.N. Metabolomic fingerprinting and genetic discrimination of four Hypericum taxa from Greece. Phytochemistry 2020, 174, 112290. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ma, R.; Chen, L.; Shi, S.; Cai, P.; Zhang, S.; Xiang, H. Antioxidant profiling of vine tea (Ampelopsis grossedentata): Off-line coupling heart-cutting HSCCC with HPLC–DAD–QTOF-MS/MS. Food Chem. 2017, 225, 55–61. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.-P.; Zhou, X.; Liu, X.G.; Gao, W.; Dong, X.; Li, H.-J.; Li, P.; Yang, H. Screening of minor bioactive compounds from herbal medicines by in silico docking and the trace peak exposure methods. J. Chromatogr. A 2016, 1436, 91–99. [Google Scholar] [CrossRef]
- Avula, B.; Katragunta, K.; Wang, Y.H.; Ali, Z.; Khan, I.A. Simultaneous determination and characterization of flavonoids, sesquiterpene lactone, and other phenolics from Centaurea benedicta and dietary supplements using UHPLC-PDA-MS and LC-DAD-QToF. J. Pharm. Biomed. Anal. 2022, 216, 114806. [Google Scholar] [CrossRef]
- Wang, G.; Yao, S.; Zhang, X.-X.; Song, H. Rapid screening and structural characterization of antioxidants from the extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS method. Int. J. Anal. Chem. 2015, 2015, 849769. [Google Scholar] [CrossRef]
- Ran, J.; Wang, Y.; Zhang, W.; Ma, M.; Zhang, H. Research on the bioactivity of isoquercetin extracted from marestail on bladder cancer EJ cell and the mechanism of its occurrence. Artif. Cells Nanomed. Biotechnol. 2016, 44, 859–864. [Google Scholar] [CrossRef]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem.-Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- Aljubiri, S.M.; Mahmoud, K.; Mahgoub, S.A.; Almansour, A.I.; Shaker, K.H. Bioactive compounds from Euphorbia schimperiana with cytotoxic and antibacterial activities. S. Afr. J. Bot. 2021, 141, 357–366. [Google Scholar] [CrossRef]
- Ismail, A.S.; Rizal, Y.; Armenia, A.; Kasim, A. Identification of bioactive compounds in gambier (Uncaria gambir) liquid by-product in West Sumatra, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 1474–1480. [Google Scholar]
- Srinivasan, R.; Natarajan, D.; Shivakumar, M.S. Antioxidant Compound Quercetin-3-O-α-l-rhamnoside (1 → 6)-β-D-glucose (Rutin) isolated from ethyl acetate leaf extracts of Memecylon edule Roxb (Melastamataceae). Free Radic. Antioxid. 2015, 5, 35–42. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Nahar, L.; Cox, P.J.; Dinan, L.N.; Ferguson, C.A.; Finnie, D.A.; Jaspars, M.; Sarker, S.D. Biological activity of lignans from the seeds of Centaurea scabiosa. Pharm. Biol. 2003, 41, 203–206. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Chen, F.-C.; Lee, K.-H. Hinokiflavone, a Cytotoxic Principle from Rhus succedanea and the Cytotoxicity of the Related Biflavonoids1. Planta Med. 1989, 55, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Li, L.; Zhang, X.; Song, K.; Diao, X.; Sun, Y.; Zhang, L. UHPLC-Q-TOF-MS/MS method based on four-step strategy for metabolites of hinokiflavone in vivo and in vitro. J. Pharm. Biomed. Anal. 2019, 169, 19–29. [Google Scholar] [CrossRef]
- Yu, S.; Yan, H.; Zhang, L.; Shan, M.; Chen, P.; Ding, A.; Li, S.F.Y. A review on the phytochemistry, pharmacology, and pharmacokinetics of amentoflavone, a naturally-occurring biflavonoid. Molecules 2017, 22, 299. [Google Scholar] [CrossRef]
- Okigawa, M.; Hwa, C.W.; Kawano, N.; Rahman, W. Biflavones in Selaginella species. Phytochemistry 1971, 10, 3286–3287. [Google Scholar] [CrossRef]
- Park, N.-H.; Lee, C.-W.; Bae, J.-H.; Na, Y.J. Protective effects of amentoflavone on Lamin A-dependent UVB-induced nuclear aberration in normal human fibroblasts. Bioorganic Med. Chem. Lett. 2011, 21, 6482–6484. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Lee, J.; Jin, H.-G.; Woo, E.-R.; Lee, D.G. Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death in Candida albicans. Mycopathologia 2012, 173, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Kihara, T.; Kawano, N. Studies on bisflavones in the leaves of Podocarpus macrophylla and P. nagi. Chem. Pharm. Bull. 1969, 17, 150–154. [Google Scholar] [CrossRef]
- Bagla, V.P.; McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Antimicrobial activity, toxicity and selectivity index of two biflavonoids and a flavone isolated from Podocarpus henkelii (Podocarpaceae) leaves. BMC Complement. Altern. Med. 2014, 14, 383. [Google Scholar] [CrossRef]
- Miceli, N.; Marino, A.; Köroğlu, A.; Cacciola, F.; Dugo, P.; Mondello, L.; Taviano, M.F. Comparative study of the phenolic profile, antioxidant and antimicrobial activities of leaf extracts of five Juniperus L.(Cupressaceae) taxa growing in Turkey. Nat. Prod. Res. 2020, 34, 1636–1641. [Google Scholar] [CrossRef]
- Manel, M.; Nouzha, H.; Rim, M.; Imane, M.; Sana, A.; Yasmine, O.; Ammar, A. Antibacterial and antioxidant activity of Juniperus thurifera L. leaf extracts growing in East of Algeria. Vet. World 2018, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Ennajar, M.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Abderraba, M.; Raies, A.; Romdhane, M. Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea L. (Cupressacees). J. Food Sci. 2009, 74, M364–M371. [Google Scholar] [CrossRef] [PubMed]
- Vandenbossche, I.; Vaneechoutte, M.; Vandevenne, M.; De Baere, T.; Verschraegen, G. Susceptibility testing of fluconazole by the NCCLS broth macrodilution method, E-test, and disk diffusion for application in the routine laboratory. J. Clin. Microbiol. 2002, 40, 918–921. [Google Scholar] [CrossRef]
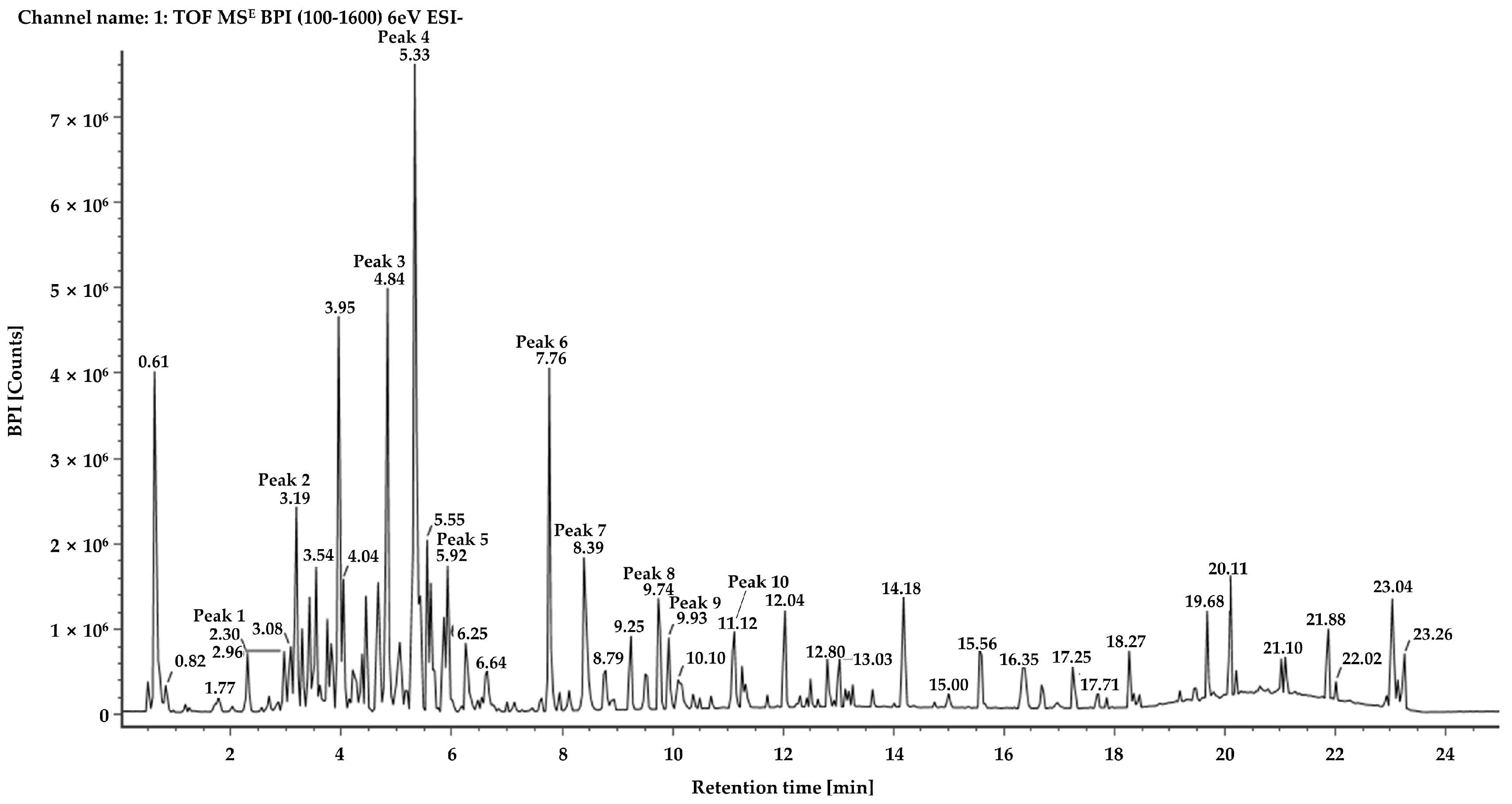
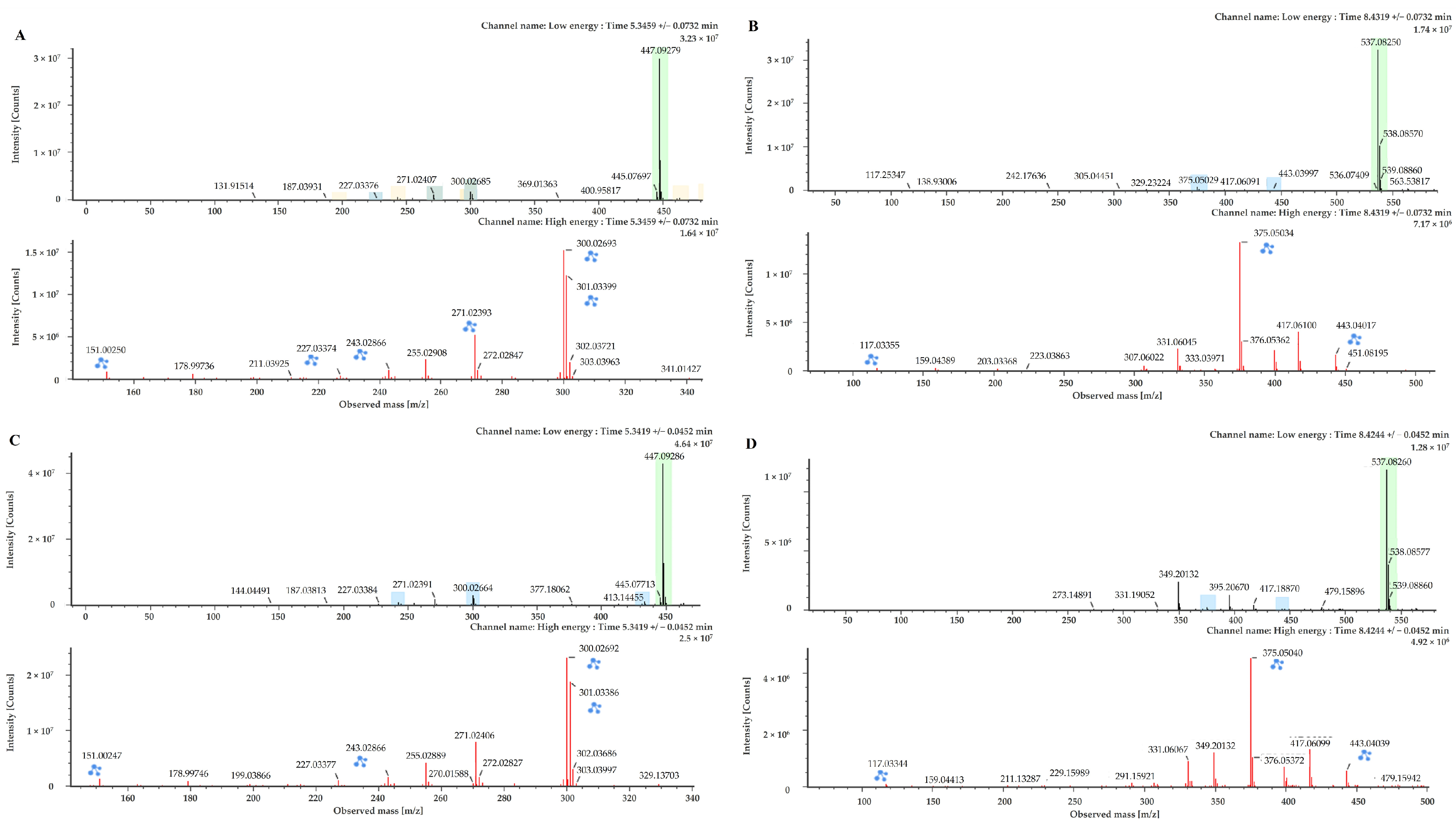
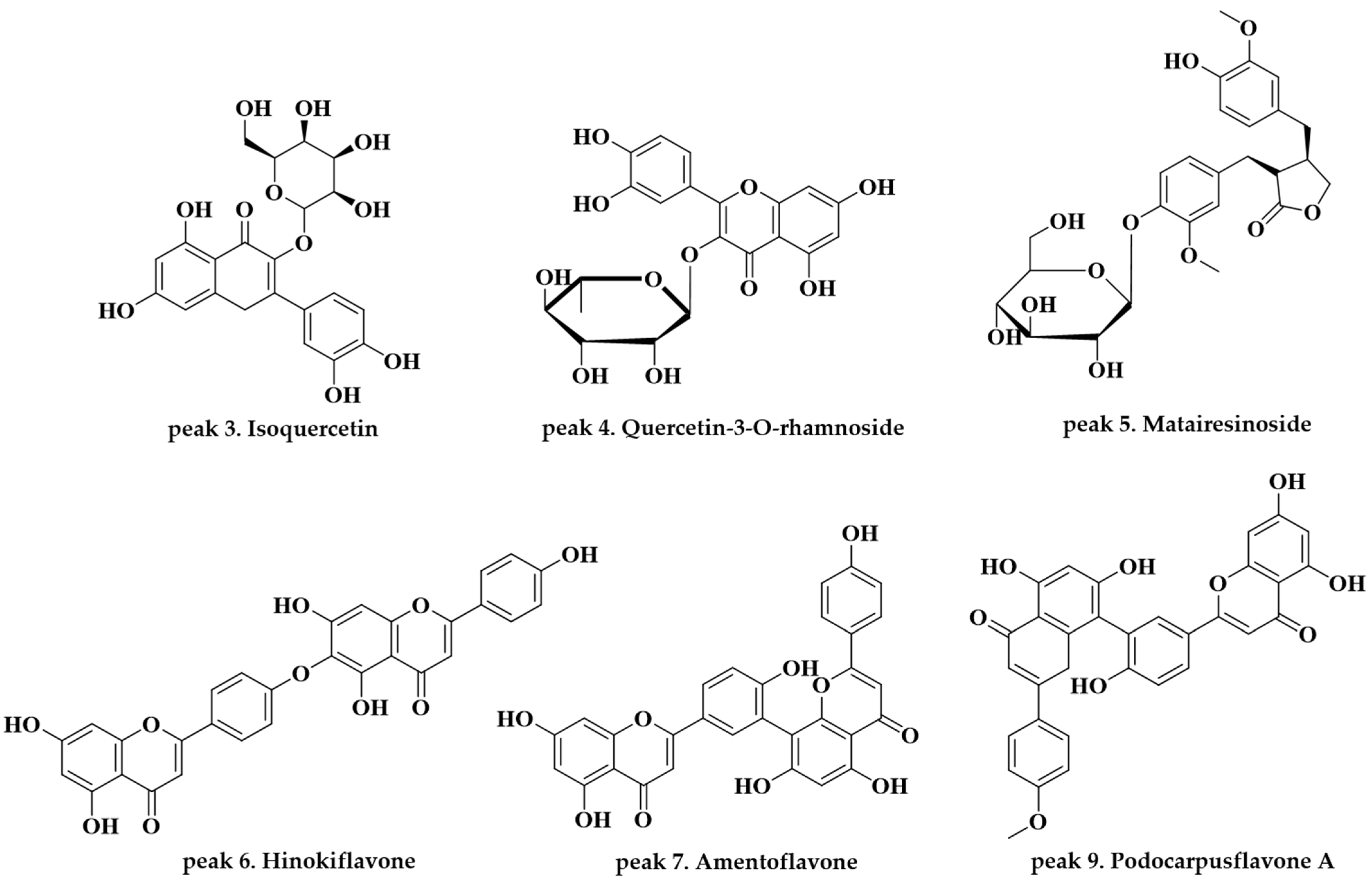
| Peak | Tentative Chemical Component | Formula | Observed RT (min) | Neutral Mass (Da) | Observed [M − H]− (m/z) | Mass Error (ppm) | Fragmentation Peaks m/z (% Base Peak) | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Unknown 1 | C21H24O11 | 2.31 | 452.1319 | 451.1242 | −0.9 | 289.0709(100); 137.0232(100) | - |
| 2 | Unknown 2 | C15H14O6 | 3.19 | 290.0790 | 289.0712 | −2 | 245.0810(78); 137.0232(100) | - |
| 3 | Isoquercetin | C21H20O12 | 4.84 | 464.0955 | 463.0878 | −0.9 | 300.0267(100) | [15,16] |
| 4 | Quercetin-3-O-α-l-rhamnoside | C21H20O11 | 5.34 | 448.1006 | 447.0929 | −1 | 300.0266(100); 243.0299(7) | Standard [16,17,18] |
| 5 | Matairesinoside | C26H32O11 | 5.94 | 520.1945 | 519.1870 | −0.3 | 357.1340(100); 342.1104(7) | [19] |
| 6 | Hinokiflavone | C30H18O10 | 7.78 | 538.0900 | 537.0826 | −0.2 | 443.0489(13); 375.0510(100); 117.0346(2) | [20] |
| 7 | Amentoflavone | C30H18O10 | 8.42 | 538.0900 | 537.0826 | −0.2 | 443.0409(14); 375.0510(100) | Standard [16,18,20] |
| 8 | Unknown 3 | C20H16O7 | 9.76 | 368.0896 | 367.0819 | −1.3 | 323.0925(85); 294.0884(100); 159.0452(97) | - |
| 9 | Podocarpusflavone A | C31H20O10 | 9.97 | 552.1056 | 551.0984 | 0 | 519.0713(2); 375.0506(100) | [20] |
| 10 | Unknown 4 | C17H26O4 | 11.12 | 294.1831 | 293.1752 | −2.1 | 249.1853(69); 193.1598(100) | - |
| Parameter | Quercetin-3-O-α-l-rhamnoside | Amentoflavone |
|---|---|---|
| Coefficients of regression equation | y = 34,990x − 205.23 | y = 3991.5x + 404.2 |
| Coefficients of determination (R2) | 0.9991 | 0.9955 |
| Linear range (ng) | 0.05–1.0 | 0.25–2.5 |
| Concentration (mg/g of dry ethanolic extract weight) | 203.78 ± 5.42 | 69.84 ± 1.94 |
| Test Pathogenic Strain | Antibacterial Activity * | ||
|---|---|---|---|
| Ethanolic Crude Extract of J. chinensis | Positive Control | ||
| Escherichia coli KCTC 2617 | ++ | ++ | ampicillin |
| Salmonella enterica serovar Enteritidis NCCP 14546 | + | +++ | ampicillin |
| Streptococcus mutans KCTC 3065 | ++ | +++ | ampicillin |
| Staphylococcus aureus NCCP 14560 | ++ | +++ | kanamycin |
| Acinetobacter baylyi ATCC 33305 | + | ++ | gentamycin |
| Klebsiella pneumoniae NCCP 16052 | + | ++ | ampicillin |
| Bordetella pertussis NCCP13671 | +++ | +++ | ampicillin |
| Moraxella catarrhalis ATCC 43628 | + | +++ | penicillin |
| Staphylococcus pyrogenes NCCP14783 | ++ | ++ | penicillin |
| Streptococcus pneumoniae NCCP 14774 | ++ | ++ | ampicillin |
| Component | Ion Mode | Precursor Ion m/z, (Cone Voltage, V) | Product Ions m/z, (Collision Energy, V) | |
|---|---|---|---|---|
| Quantitation | Reference | |||
| Quercetin-3-O-α-l-rhamnoside | positive | 449.18 (20) | 303.11 (9) | 84.94 (9) |
| Amentoflavone | negative | 537.48 (9) | 375.31 (33) | 443.34 (33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.J.; Song, J.-S.; Lee, B.-H.; Son, Y.K.; Kim, Y. Qualitative and Quantitative Analysis of the Major Bioactive Components of Juniperus chinensis L. Using LC-QTOF-MS and LC-MSMS and Investigation of Antibacterial Activity against Pathogenic Bacteria. Molecules 2023, 28, 3937. https://doi.org/10.3390/molecules28093937
Lim DJ, Song J-S, Lee B-H, Son YK, Kim Y. Qualitative and Quantitative Analysis of the Major Bioactive Components of Juniperus chinensis L. Using LC-QTOF-MS and LC-MSMS and Investigation of Antibacterial Activity against Pathogenic Bacteria. Molecules. 2023; 28(9):3937. https://doi.org/10.3390/molecules28093937
Chicago/Turabian StyleLim, Da Jung, Jeong-Sup Song, Byoung-Hee Lee, Youn Kyoung Son, and Yangseon Kim. 2023. "Qualitative and Quantitative Analysis of the Major Bioactive Components of Juniperus chinensis L. Using LC-QTOF-MS and LC-MSMS and Investigation of Antibacterial Activity against Pathogenic Bacteria" Molecules 28, no. 9: 3937. https://doi.org/10.3390/molecules28093937
APA StyleLim, D. J., Song, J.-S., Lee, B.-H., Son, Y. K., & Kim, Y. (2023). Qualitative and Quantitative Analysis of the Major Bioactive Components of Juniperus chinensis L. Using LC-QTOF-MS and LC-MSMS and Investigation of Antibacterial Activity against Pathogenic Bacteria. Molecules, 28(9), 3937. https://doi.org/10.3390/molecules28093937





