Abstract
With the development of antimicrobial agents, researchers have developed new strategies through key regulatory systems to block the expression of virulence genes without affecting bacterial growth. This strategy can minimize the selective pressure that leads to the emergence of resistance. Quorum sensing (QS) is an intercellular communication system that plays a key role in the regulation of bacterial virulence and biofilm formation. Studies have revealed that the QS system controls 4–6% of the total number of P. aeruginosa genes, and quorum sensing inhibitors (QSIs) could be a promising target for developing new prevention and treatment strategies against P. aeruginosa infection. In this study, four series of phenyloxadiazole and phenyltetrazole sulfoxide derivatives were synthesized and evaluated for their inhibitory effects on P. aeruginosa PAO1 biofilm formation. Our results showed that 5b had biofilm inhibitory activity and reduced the production of QS-regulated virulence factors in P. aeruginosa. In addition, silico molecular docking studies have shown that 5b binds to the P. aeruginosa QS receptor protein LasR through hydrogen bond interaction. Preliminary structure–activity relationship and docking studies show that 5b has broad application prospects as an anti-biofilm compound, and further research will be carried out in the future to solve the problem of microbial resistance.
1. Introduction
As a common Gram-negative bacterium, Pseudomonas aeruginosa (P. aeruginosa or PA) is one of the main pathogens causing nosocomial infections and ventilator-associated pneumonia. It mainly affects patients that are immunocompromised, such as those with severe burns, chemotherapy, or HIV etc. [1,2,3] The ability of P. aeruginosa to form antibiotic-resistant biofilms is a significant factor for its notorious persistence in clinical settings [4]. It has been estimated that over 80% of hospital-acquired infections are biofilm-mediated, and that treatment of these biofilm-based infections costs more than USD 1 billion annually [5,6]. The biofilms’ surface-associated bacteria are microbial communities that attach to inert or living surfaces and encase them in a self-produced extracellular polymeric matrix [7]. Biofilm matrices usually contain polysaccharides, water, proteins, and extracellular DNA, but their composition varies according to bacterial species and environmental conditions [8,9,10]. The bacteria in biofilms are more tolerant to conventional antibiotics, and they are protected from environmental stress and host immune response [11]. P. aeruginosa forms biofilm on many surfaces, including cystic fibrosis-affected tissues in lungs and on medicinal implants, such as catheters, artificial hips, and contact lenses, which can cause severe infections that are difficult to treat [6,12,13]. Therefore, PA is an important model to study the growth and inhibition of bacterial biofilms.
As an effective means of evading host defenses, P. aeruginosa regulates biofilm formation and the release of virulence factors by a cell–cell communication system termed quorum sensing (QS) [14,15]. There are three major QS systems: P. aeruginosa, las, and rhl, pqs. In the las system, AHL synthase LasI encoded by the LasI gene directs the synthesis of the N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL, OdDHL) signal molecule, which binds to the LuxR type transcriptional regulator LasR and activates a series of virulence genes such as LasB elastase, LasA protease, and alkaline protease isogenic expression [15,16,17]. In the rhl system, the production of rhlI, Rhl directs the synthesis of N-butanoyl-L-homoserine lactone (C4-HSL, BHL), which forms a complex with the receptor protein RhlR. The complex activates the transcription of the rhlAB (rhamnolipid synthesis gene) and the rhlI gene itself [18,19]. In addition to the two AHL QS systems in P. aeruginosa, a third system acts via the 2-alkyl-4-quinolones autoinducer. The gene product encoded by the pqsABCD operon synthesizes 2-heptyl-4-hydroxyquinoline (HHQ), which is the precursor of PQS [20]. The pqs system controls the expression of phzA-G operons, which are closely associated with the production of pyocyanin, and regulates the production of extracellular DNA (eDNA), which is involved in the formation of stable and mature biofilms [21,22]. During the entire QS regulation process of P. aeruginosa, the LasR signal receptor is the earliest to be activated, regulating the expression of the most virulent or biofilm production-related genes, including other quorum-sensing receptors, which makes it a hot topic in this field [23].
Sulfur-containing organics are a class of compounds with a wide range of biological activities and functions that exist widely in oceans and on land. For instance, the marine-sourced natural products dithiolopyrrolone antibiotics and kottamide E have strong broad-spectrum antibacterial activity [24,25], isothiocyanates isolated from cruciferous plants can prevent cancers of the lungs and liver [26], brassilexin and methoxybrassitin were found to have antifungal activity, etc. [27,28,29] (Figure 1A). Researchers also found that garlic extracts could facilitate the rapid clearance of P. aeruginosa infection from the lungs of mice [30]. Further studies confirmed that the potent QS inhibitor in garlic is ajoene, which attenuates the main virulence factor regulated by QS [31]. Givskov et al. [32], by screening an internal compound library, found that disulfide-bonded derivatives structurally similar to ajoene showed QS inhibitory activity. Benzo heterocyclic skeleton is one of the most important structural components and biological compounds in pharmaceutical chemistry. Owing to the rich structural diversity of the bioactive benzo-heterocyclic ring, it has become an important structural component in many pharmaceutical preparations [33,34,35]. Both allicin and ajoene have sulfoxide structures, and we hypothesized that benzoheterocyclic sulfoxide might also have quorum sensing activity. In our previous work, we synthesized a series of benzoheterocyclic sulfoxide derivatives in the hope of finding new lead compounds to inhibit the quorum sensing of P. aeruginosa [36]. In addition, benzoheterocyclic sulfoxide derivatives in a planar π system in three-dimensional space structures were analyzed. We speculated that if the benzoheterocycle skeleton was stretched for phenyl heterocyclic sulfoxides, there might be multiple perspectives of three-dimensional rotation π systems. Owing to its longer skeleton structure, it may have a pocket advantage over the benzoheterocyclic structure from the perspective of protein docking (Figure 1B).
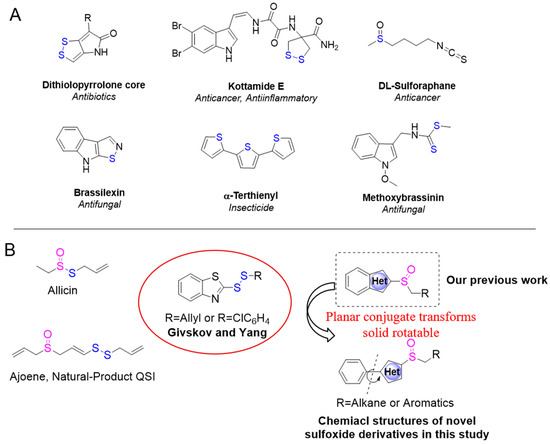
Figure 1.
(A) Sulfur compounds found in natural products and their biological activities. (B) Structures and strategies of the design of the target compounds.
Hence, in this study, four series of phenyloxadiazole and phenyltetrazole sulfoxide derivatives were designed and synthesized to optimize potent P. aeruginosa QSIs. Through the biofilm inhibitory behavior of P. aeruginosa PAO1 and the fluorescence expression analysis of reporter strains (PAO1-lasB-gfp, PAO1-rhlA-gfp, and PAO1-pqsA-gfp), 5b was found to be the most active biofilm inhibitor. We also tested 5b for QS-activated virulence factors (elastase, rhamnolipid, and pyocyanin) production. Finally, we explored the binding effects between 5b and the LasR receptor protein with molecular docking.
2. Results and Discussion
2.1. Chemistry
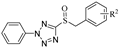
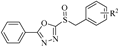
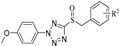
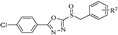
A series of phenyldioxazole and phenyltetrazole sulfoxide derivatives were synthesized. The preparation methods for the titled compounds are described in Scheme 1. The different substituted benzyl bromides 2 were added dropwise to a solution of phenyldioxazole thiol or phenyltetrazole thiol 1 and Et3N in MeCN at room temperature to obtain intermediate thioethers 3. Then, intermediates 3 were oxidized by m-chloroperoxybenzoic acid (mCPBA) in dichloromethane to obtain title compounds 4a–4o (containing phenyltetrazole), 5a–5o (containing phenyldioxazole), 6a–6o (containing 4-methoxylphenyltetrazole), and 7a–7o (containing 4-chlorophenyldioxazole) [37]. 1H NMR, 13C NMR spectra and HRMS spectra of all compounds are shown in the experimental section and Supplementary Materials.
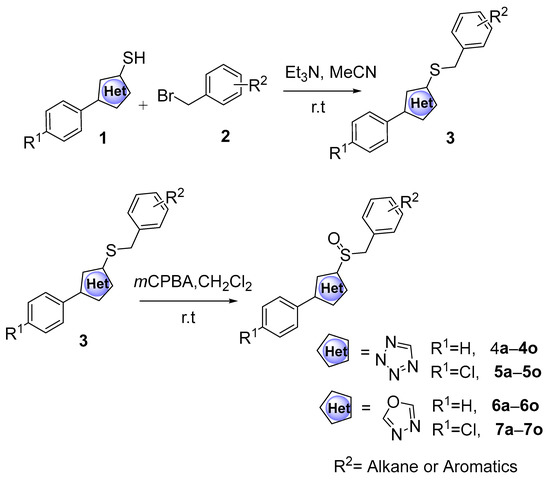
Scheme 1.
The synthesis route of the title compounds.
2.2. Evaluation of Inhibition of P. aeruginosa Biofilm and Structure–Activity Relationship (SAR) Studies
First, all target compounds were tested for their inhibitory activities against P. aeruginosa PAO1 biofilms (Table 1); 2-aminobenzimidazole was set as the positive control [38]. On the basis of previous studies, phenyldioxazole or phenyltetrazole sulfoxide derivatives were preferred for study. As shown in Table 1, in contrast to the good biofilm inhibitory activity in 5a–5o, there was little or no effect observed in the 4a–4o series compounds. We observed that the biofilm activity of most compounds in series 5 was higher than that of the positive control, which may be due to the introduction of five-membered rings by oxygen atoms. Among them, 5b (R2 was substituted by para-chloro, inhibition rate was 46.85 ± 2.76%) and 5f (R2 was substituted by 4-naphthyl; inhibition rate was 40.88 ± 0.75%) had the most significant inhibitory activity. However, the activities of 5c and 5d were weaker than those of 5b (5c, 23.26 ± 3.20%; 5d, 17.55 ± 2.85%), indicating that substitution of the para-position of the benzene ring may have better inhibitory activity. In addition, when R2 was substituted at the para-position of the benzene ring, the inhibitory activity of chlorinated derivative was higher than that of fluorinated derivative (5h, 23.17 ± 1.10%) and brominated derivative (5i, 21.94 ± 2.72%). When R2 were alkyl-branched chains, 5n and 5o showed no inhibitory activity (5n, 0.45 ± 0.19%; 5o, 4.75 ± 0.96%), indicating that the aromatic ring was a necessary active group. Based on preliminary SAR analysis, only the para-substitution on the R2 benzene ring was conducive to activity; in particular, the para-chloro group promoted the activity. It should be noted that the three aryl groups were the optimal framework for designing phenyloxazole inhibitors.

Table 1.
Biofilm formation inhibition rates of derivatives against the P. aeruginosa PAO1.
Compared with the 4a–4o series, the anti-biofilm activity was not significantly improved by introducing the R1 as para-methoxy group in 6a–6o series derivatives. In the 7a–7o series, the antibiofilm activity of replacing R1 with para-chloro substitution was not as good as that of the 5a–5o series. In conclusion, when the para-chloro-substituted phenyloxazole ring and para-methoxyl-substituted phenyltetrazole were applied, the inhibitory activity against P. aeruginosa biofilm was not improved. These experimental results showed that the para-chloro substitution group was not essential for activity as R1 on the benzene ring compared to chloro as the R2 on the other side. In addition, we also tested the P. aeruginosa biofilm inhibitory activity of the corresponding intermediate thioethers of 5b and 5f (the biofilm inhibition rate of the intermediate thioether of 5b was −3.77 ± 1.16%, and 5f intermediate thioether was −5.04 ± 0.52%). These results revealed that the thioether derivatives of phenyloxazole had no PAO1 biofilm inhibitory activity, and they showed inhibitory activity against PAO1 biofilms only after being oxidized as sulfoxides. Therefore, the sulfoxide group was verified as an essential active functional group for inhibiting biofilm formation.
2.3. Effect of Sulfoxide Derivatives on QS System Reporter Strains
As previously mentioned, las, rhl, and pqs pathways are critical for the regulation of biofilm formation and the secretion of virulence factors in the QS system of P. aeruginosa. According to the regulatory process of the QS system in P. aeruginosa, Givskov et al. [18,39,40] reported the promoters in the QS pathway, including the lasB gene encoding elastase, which was shown to be under the transcriptional control of LasR; the first gene rhlA encodes the rhl operon of rhamnotransferase, and the first gene pqsA encodes the pqsABCDE of the PQS molecule. They were respectively fused with unstable green fluorescent protein (GFP) to construct three reporter strains, PAO1-lasB-gfp, PAO1-rhlA-gfp, and PAO1-pqsA-gfp, and a detection system for screening small molecule QSIs was established [41,42].
To further explore the mechanism of biofilm formation inhibition caused by the synthetic compounds, reporter strains PAO1-lasB-gfp, PAO1-rhlA-gfp, and PAO1-pqsA-gfp were introduced. Five compounds, 5a, 5b, 5f, 5j, and 5k, which showed the best anti-biofilm activities, were selected for study (Figure 2). The experimental results verified that at the concentration of 20 μM, all five compounds could inhibit the fluorescence expression of the PAO1-lasB-gfp strain; of these compounds 5b and 5f had the best inhibitory effect (Figure 2A). Although 5a, 5b, 5f, 5k, and 5j had certain inhibitory effects on the PAO1-lasB-gfp and PAO1-pqsA-gfp strains, the expression of rhlA-gfp was not particularly affected under the same experimental conditions (Figure 2B,C). The las system is upstream of the quorum sensing network [17], and biological reporter gene analysis indicated that phenyloxadiazole sulfoxide derivatives may specifically exhibited anti-biofilm activity via the las pathway.
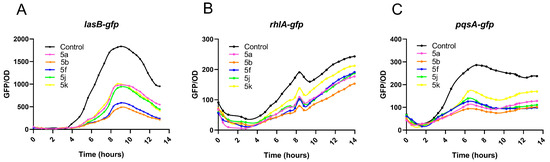
Figure 2.
The effects of 5a, 5b, 5f, 5j, and 5k on QS system reporter strains (A) PAO1-lasB-gfp, (B) PAO1-rhlA-gfp, and (C) PAO1-pqsA-gfp. The experiments were performed in triplicate.
2.4. The Effect of 5b on PAO1-lasB-gfp and P. aeruginosa PAO1 Biofilm Growth and Formation
Based on the experimental results, 5b was selected as the key research object. (Figure 3). Under the premise of no effect on the growth function of the PAO1-lasB-gfp reporter strain (Figure 3A), 5b showed dose-dependent fluorescence inhibition of the PAO1-lasB-gfp strain at different concentrations of 20 μM, 10 μM, 5 μM, 2.5 μM, and 1.25 (Figure 3B). Based on the dose-response curves obtained, the IC50 value of 5b against the PAO1-lasB-gfp strain was calculated as 3.53 ± 0.16 μM (Figure 3C). Similarly, we further verified the effect of 5b on the growth and formation of the PAO1 biofilm. The effect of 5b on the growth of P. aeruginosa PAO1 was assessed by monitoring the OD600 of the culture hourly. The results showed that the normal growth of P. aeruginosa PAO1 was not affected when the maximum concentration of 5b was 50 μM (Figure 3D). Compound 5b was incubated at concentrations of 50, 25, 12.5, 5, and 2.5 μM, and the control for 24 h and the OD values at 600 nm were evaluated before the biofilm experiments. We found that 5b could reduce the biofilm formation in a dose-dependent manner (Figure 3E). In addition, the reduction of biofilm formation by 5b was also observed by confocal laser scanning microscopy (CLSM) (Figure 3F). As shown, biofilm formed with 50 μM of 5b was shallower than the control biofilm; the height of the biofilm formed with the control group, positive group, and 5b were 35, 27, and 14 μm, respectively.
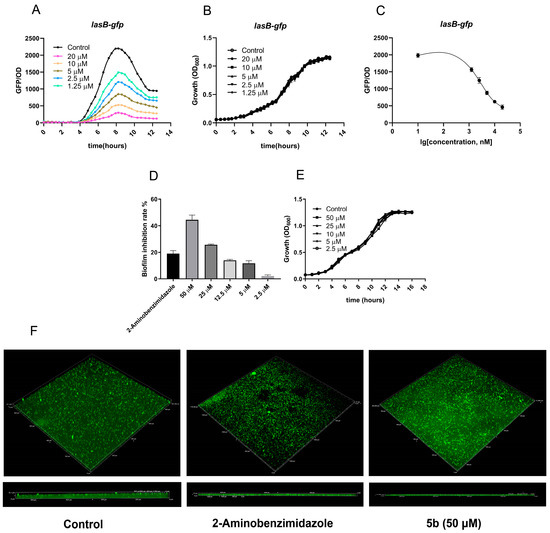
Figure 3.
Effects of 5b on PAO1-lasB-gfp and P. aeruginosa PAO1 biofilm growth and formation. (A) Growth at different concentrations of 5b (20, 10, 5, 2.5, 1.25μM). (B) Dose-dependent inhibition curves of 5b incubated with the QS monitors PAO1-lasB-gfp. (C) IC50 values calculations were based on three biological replicates and performed by nonlinear fitting, using Graphpad Prism 6 software. (San Diego, CA, USA). The IC50 values of 5b were 3.53 ± 0.16 μM for PAO1-lasB-gfp. (D) Growth at different concentrations of 5b (50, 25, 12.5, 5, 2.5 μM) for 16 h. (E) Inhibition of biofilm formation by different concentrations of 5b (50, 25, 12.5, 5, 2.5 μM) for 24 h in microtiter plate. (F) CLSM images of biofilms formed for 24 h with control and 5b (50 μM), with 2-aminobenzimidazole used as positive control (An equal amount of dimethyl sulfoxide solvent was set as the control group). Data represent the average of three independent determinations of triplicate samples.
2.5. Effect of 5b on Virulence Factors
Further, 5b was used as a template to demonstrate that it inhibits biofilm formation through the las pathway of P. aeruginosa. We measured the effect of 5b on the production of three virulence factors, elastase, pyocyanin, and rhamnolipid, in P. aeruginosa PAO1, which were regulated by las, rhl, and pqs systems, respectively. The results showed that 5b could reduce elastase production in a concentration-dependent manner at a concentration of 50 μM, 25 μM, and 12.5 μM (Figure 4A). Compound 5b inhibited pyocyanin production only at high concentrations and had little effect on rhamnolipid production. In summary, 5b suppressed the expression of the QS system lasB gene and decreased the production of the virulence factor elastase. Thus, 5b inhibited the formation of the PAO1 biofilm through the las pathway.
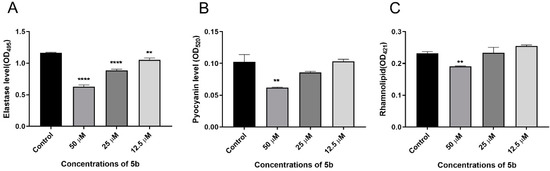
Figure 4.
Effects of 5b on the production of (A) elastase, (B) pyocyanin, and (C) rhamnolipid at different concentrations (50, 25, 12.5 μM, and control group). Error bars are means ± SDs. ** = p < 0.01 versus the control, **** = p < 0.0001 versus the control.
2.6. Molecular Docking Study
Furthermore, we explored the binding properties of 5b and QS-associated proteins by molecular docking. As the autoinducer of the las pathway in P. aeruginosa, OdDHL binds to the cytoplasmic receptor protein LasR after synthesis by the LasI protein and forms the complex LasR-OdDHL, which binds to the promoter region of the target gene. The LasR-OdDHL complex then induces gene transcription of various virulence factors and specific proteins [43]. Silico molecular docking was performed to predict the binding models of 5b and 5f to the homologous signal receptor protein LasR (Figure 5, Table 2). The lowest binding energies of the docked conformations were selected from 30 hypothetical conformations as the modes for the corresponding compounds. The docking results are shown in Figure 5, where the benzene ring in the phenyloxazole structure of compound 5f forms hydrophobic interactions with the residue Gly 126; the π bond in the naphthalene ring also developed hydrophobic interaction with the key residues Tyr 56, while the π bond in the chlorophenyl ring of 5b had strong hydrophobic interactions with Trp 88 and Phe 101. It should be noted that the para-chlorinated benzene ring also formed binding interactions with the residue Leu 110, which demonstrates the importance of the para-chlorinated characteristics in phenyloxazole sulfoxide derivatives for P. aeruginosa biofilm activity. These results are consistent with the SAR results observed above and further interpret the active mechanism of phenyloxazole derivatives from the perspective of target and protein binding.
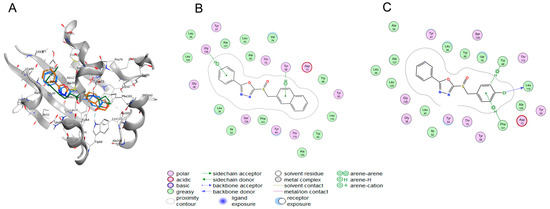
Figure 5.
Predicted binding model of compounds (OdDHL, 5b, 5f) and LasR (PDB code: 2uv0). (A) Overlay of the crystal ligand OdDHL (green) with the docked pose of 5b (blue) and 5f (brown) in the LasR LBD domain (PDB ID:2UV0). (B,C) 2D structure-diagrams of protein–ligand complexes.

Table 2.
Details of the docked complex of the LasR with OdDHL, 5b and 5f.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods for Synthetic Chemistry
All solvents and reagents were obtained from commercial sources without further purification. 1H and 13C NMR spectra were recorded on a Bruker Avance III 400 at 600 and 150 MHz or 400 and 100 MHz spectrometer. Chemical shifts were recorded as δ in units of parts per million (ppm), while tetramethylsilane (TMS) was used as an internal standard. Compounds were dissolved in CDCl3. Mass spectra were recorded on a SCIEX series X500B QTOF mass spectrometer. Thin-layer chromatography (TLC) was performed using Huanghai GF254 Silica gel plates. Column chromatography was performed using silica gel (200–300 mesh, Beijing, China) with a linear solvent gradient.
3.1.2. General Synthesis Method for Compounds 4a–7o
To a solution of heterocyclic thiol (2 mmol) and Et3N (3 mmol) dropwise in MeCN (10 mL) were added benzyl bromide (2.4 mmol). The mixture was stirred for 2–4 h at room temperature (monitored by TLC) and quenched with 6M HCl aqueous. The mixture was then extracted by EtOAc (10 mL × 3), saturated in aqueous brine (5 mL × 2), dried over Na2SO4, and concentrated to dryness. The crude residue was purified by silica gel column, followed by gradient elution with a petroleum ether/ethyl acetate mixture (50/1–30/1 ratio) to provide intermediate thioethers. Thioethers intermediate (1 mmol) and m-chloroperoxy-benzoic acid (1 mmol) were added to a solvent of CH2Cl2 (5 mL). The reaction mixture was stirred for 2–4 h at room temperature (monitored by TLC), and then washed with saturated aqueous NaHCO3 (5 mL × 2) and brine (5 mL × 2), dried over Na2SO4, and concentrated to dryness. The residue was directly loaded onto a silica gel column followed by gradient elution with petroleum ether/ethyl acetate mixture (30/1–10/1 ratio) to obtain target compounds (4a–4o, 5a–5o, 6a–6o, 7a–7o).
5-(benzylsulfinyl)-2-phenyl-2H-tetrazole (4a): white solid, yield 79%. 1H NMR (600 MHz, CDCl3) δ 7.56–7.51 (m, 1H, Ph-H), 7.46 (t, J = 7.8 Hz, 2H, Ph-H), 7.34–7.28 (m, 3H, Ph-H), 7.19–7.14 (m, 4H, Ph-H), 4.90–4.71 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 156.12(N=C-N), 132.70(Ph-C), 131.05(Ph-C), 130.62(Ph-C, 2C), 129.60(Ph-C, 2C), 129.31(Ph-C), 129.18(Ph-C, 2C), 127.32(Ph-C), 125.02(Ph-C, 2C), 60.30(CH2). ESI-HRMS: calcd. for C14H12N4OS [M + H]+, 285.0805; found, 285.0803.
5-((4-chlorobenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4b): white solid, yield 73%. 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J = 7.4 Hz, 1H, Ph-H), 7.52 (d, J = 8.1 Hz, 2H, Ph-H), 7.33–7.29 (m, 2H, Ph-H), 7.29–7.25 (m, 2H, Ph-H), 7.15–7.10 (m, 2H, Ph-H), 4.84–4.69 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 155.74(N=C-N), 135.65(Ph-C), 132.68(Ph-C), 131.90, (Ph-C, 2C) 131.14(Ph-C), 129.67(Ph-C, 2C), 129.35(Ph-C, 2C), 125.90(Ph-C), 124.91(Ph-C, 2C), 59.31(CH2). ESI-HRMS: calcd. for C14H11ClN4OS [M + H]+, 319.0415; found, 319.0419.
5-((3-chlorobenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4c): yellow oily liquid, yield 79%. 1H NMR (600 MHz, CDCl3) δ 7.59–7.55 (m, 1H, Ph-H), 7.54–7.50 (m, 2H, Ph-H), 7.35–7.32 (m, 3H, Ph-H), 7.26–7.22 (m, 2H, Ph-H), 7.08 (dt, J = 7.5, 1.3 Hz, 1H, Ph-H), 4.85–4.67 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 155.80(N=C-N), 135.02(Ph-C), 132.68(Ph-C), 131.19(Ph-C), 130.58(Ph-C), 130.39(Ph-C), 129.74(Ph-C, 2C), 129.59(Ph-C), 129.51(Ph-C), 128.77(Ph-C), 124.95(Ph-C, 2C), 59.37(CH2). ESI-HRMS: calcd. for C14H11ClN4OS [M + H]+, 319.0415; found, 319.0415.
5-((2-chlorobenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4d): white solid, yield 83%. 1H NMR (600 MHz, CDCl3) δ 7.58–7.52 (m, 3H), 7.45–7.43 (m, 2H), 7.39 (dd, J = 8.5, 1.4 Hz, 1H), 7.31–7.29 (m, 2H), 7.22–7.19 (m, 1H), 5.14–4.89 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 156.47(N=C-N), 135.21(Ph-C), 133.27(Ph-C), 132.75(Ph-C), 131.14(Ph-C), 130.91(Ph-C), 129.92(Ph-C), 129.90(Ph-C, 2C), 127.57(Ph-C), 125.96(Ph-C), 124.75(Ph-C, 2C), 58.17(CH2). ESI-HRMS: calcd. for C14H11ClN4OS [M + H]+, 319.0415; found, 319.0416.
5-((4-methylbenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4e): white solid, yield 87%. 1H NMR (600 MHz, CDCl3) δ 7.53 (t, J = 7.5 Hz, 1H, Ph-H), 7.46 (t, J = 7.9 Hz, 2H, Ph-H), 7.22–7.16 (m, 2H, Ph-H), 7.09 (d, J = 8.0 Hz, 2H, Ph-H), 7.03 (d, J = 8.0 Hz, 2H, Ph-H), 4.87–4.67 (m, 2H, CH2), 2.32 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 156.14(N=C-N), 139.33(Ph-C), 132.67(Ph-C), 130.90(Ph-C), 130.39(Ph-C, 2C), 129.77(Ph-C, 2C), 129.44(Ph-C, 2C), 124.89(Ph-C, 2C), 123.96(Ph-C), 60.01(CH2), 21.11(CH3). ESI-HRMS: calcd. for C15H14N4OS [M + H]+, 299.0961; found, 299.0964.
5-((naphthalen-2-ylmethyl) sulfinyl)-2-phenyl-2H-tetrazole (4f): white solid, yield 83%. 1H NMR (600 MHz, CDCl3) δ 7.81–7.78 (m, 1H, Ph-H), 7.75–7.71 (m, 2H, Ph-H), 7.67–7.64 (m, 1H, Ph-H), 7.53–7.48 (m, 2H, Ph-H), 7.44–7.41 (m, 1H, Ph-H), 7.29 (t, J = 8.0 Hz, 2H, Ph-H), 7.19 (dd, J = 8.4, 1.8 Hz, 1H, Ph-H), 7.06 (d, J = 7.5 Hz, 2H, Ph-H), 5.04–4.86 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 156.16(N=C-N), 133.24(Ph-C), 133.13(Ph-C), 132.54(Ph-C), 130.87(Ph-C), 130.43(Ph-C), 129.38(Ph-C, 2C), 129.01(Ph-C), 127.93(Ph-C), 127.68(Ph-C), 127.18(Ph-C), 127.08(Ph-C), 126.83(Ph-C), 124.79(Ph-C, 2C), 124.47(Ph-C), 60.66(CH2). ESI-HRMS: calcd. for C18H14N4OS [M + Na]+, 357.0781; found, 357.0764.
5-((4-nitrobenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4g): yellow solid, yield 70%. 1H NMR (600 MHz, CDCl3) δ 8.26–8.14 (m, 2H, Ph-H), 7.63–7.59 (m, 1H, Ph-H), 7.57–7.54 (m, 2H, Ph-H), 7.51–7.48 (m, 2H, Ph-H), 7.47–7.44 (m, 2H, Ph-H), 4.99–4.87 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 155.32(N=C-N), 148.41(Ph-C), 134.94(Ph-C), 132.62(Ph-C, 2C), 131.72(Ph-C), 131.34(Ph-C), 129.84(Ph-C, 2C), 124.85(Ph-C, 2C), 124.07(Ph-C, 2C), 58.72(CH2). ESI-HRMS: calcd. for C14H11N5O3S [M + H]+, 330.0655; found, 330.0653.
5-((4-fluorobenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4h): white solid, yield 76%. 1H NMR (600 MHz, CDCl3) δ 7.57–7.49 (m, 3H, Ph-H), 7.33–7.30 (m, 2H, Ph-H), 7.21–7.17 (m, 2H, Ph-H), 7.01–6.98 (m, 2H, Ph-H), 4.84–4.70 (m, 2H CH2). 13C NMR (151 MHz, CDCl3) δ 164.12(Ph-C), 162.47(Ph-C), 155.82(N=C-N), 132.72(Ph-C), 132.52(Ph-C), 132.46(Ph-C), 131.14(Ph-C), 129.68(Ph-C, 2C), 124.93(Ph-C, 2C), 116.32(Ph-C), 116.17(Ph-C), 59.15(CH2). ESI-HRMS: calcd. for C14H11FN4OS [M + Na]+, 325.0530; found, 325.0527.
5-((4-bromobenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4i): white solid, yield 88%. 1H NMR (600 MHz, CDCl3) δ 7.58–7.55 (m, 1H, Ph-H), 7.53–7.49 (m, 2H, Ph-H), 7.44–7.40 (m, 2H, Ph-H), 7.34–7.28 (m, 2H, Ph-H), 7.07–7.04 (m, 2H, Ph-H), 4.82–4.66 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 155.73(N=C-N), 132.65(Ph-C), 132.29(Ph-C, 2C), 132.14(Ph-C, 2C), 131.11(Ph-C), 129.64(Ph-C, 2C), 126.40(Ph-C), 124.89(Ph-C, 2C), 123.81(Ph-C), 59.39(CH2). ESI-HRMS: calcd. for C14H11BrN4OS [M + Na]+, 384.9729; found, 384.9728.
2-phenyl-5-((4-(trifluoromethyl) benzyl) sulfinyl)-2H-tetrazole (4j): white solid, yield 70%. 1H NMR (600 MHz, CDCl3) δ 7.58–7.55 (m, 3H, Ph-H), 7.52–7.47 (m, 2H, Ph-H), 7.34 (d, J = 8.1 Hz, 2H, Ph-H), 7.31–7.28 (m, 2H, Ph-H), 4.92–4.77 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 155.55(N=C-N), 132.62(Ph-C), 131.62(Ph-C), 131.59(Ph-C), 131.38(Ph-C, 2C), 131.20(Ph-C), 131.06(Ph-C), 129.69(Ph-C, 2C), 126.00(Ph-C), 125.97(Ph-C), 124.88(Ph-C, 2C), 59.41(CH2). ESI-HRMS: calcd. for C15H11F3N4OS [M + Na]+, 375.0498; found, 375.0492.
5-((3-methoxybenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4k): yellow solid, yield 77%. 1H NMR (600 MHz, CDCl3) δ 7.58–7.52 (m, 1H, Ph-H), 7.50–7.43 (m, 2H, Ph-H), 7.25–7.17 (m, 3H, Ph-H), 6.88–6.86 (m, 1H, Ph-H), 6.74 (dt, J = 7.5, 1.2 Hz, 1H, Ph-H), 6.68–6.61 (m, 1H, Ph-H), 4.87–4.68 (m, 2H, CH2), 3.71 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 160.05(Ph-C), 156.22(N=C-N), 132.71(Ph-C), 131.03(Ph-C), 130.19(Ph-C), 129.60(Ph-C, 2C), 128.59(Ph-C), 125.00(Ph-C, 2C), 122.71(Ph-C), 115.46(Ph-C), 115.39(Ph-C), 60.44(CH2), 55.30(OCH3). ESI-HRMS: calcd. for C15H14N4O2S [M + H]+, 315.0910; found, 315.0907.
5-((4-methoxybenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4l): yellow oily liquid, yield 85%. 1H NMR (600 MHz, CDCl3) δ 7.54 (t, J = 7.5 Hz, 1H, Ph-H), 7.49–7.46 (m, 2H, Ph-H), 7.24–7.22 (m, 2H, Ph-H), 7.09–7.06 (m, 2H, Ph-H), 6.81–6.79 (m, 2H, Ph-H), 4.85–4.65 (m, 2H, CH2), 3.77 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 160.40(Ph-C), 156.22(N=C-N), 132.76(Ph-C), 131.87(Ph-C, 2C), 130.98(Ph-C), 129.56(Ph-C, 2C), 124.95(Ph-C, 2C), 118.86(Ph-C), 114.58(Ph-C, 2C), 59.74(CH2), 55.33(OCH3). ESI-HRMS: calcd. for C15H14N4O2S [M + Na]+, 337.0730; found, 337.0722.
5-((3, 5-dimethoxybenzyl) sulfinyl)-2-phenyl-2H-tetrazole (4m): yellow solid, yield 56%. 1H NMR (600 MHz, CDCl3) δ 7.55–7.46 (m, 4H), 7.25 (d, J = 1.4 Hz, 1H), 6.39 (s, 1H, Ph-H), 6.26 (d, J = 2.2 Hz, 2H, Ph-H), 4.82–4.64 (m, 2H, CH2), 3.69 (s, 6H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.21(Ph-C, 2C), 156.34(N=C-N), 132.71(Ph-C), 131.01(Ph-C), 129.60(Ph-C, 2C), 129.15(Ph-C, 2C), 124.97(Ph-C), 108.08(Ph-C, 2C), 101.60(Ph-C), 60.72(CH2), 55.43(OCH3, 2C). ESI-HRMS: calcd. for C16H16N4O3S [M + H]+, 345.1016; found, 345.1016.
Methyl 2-((2-phenyl-2H-tetrazol-5-yl) sulfinyl) acetate (4n): white solid, yield 55%. 1H NMR (600 MHz, CDCl3) δ 7.76–7.70 (m, 2H, Ph-H), 7.66–7.63 (m, 3H, Ph-H), 4.93–4.50 (m, 2H, CH2), 3.76 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 164.91(Ph-C), 156.30(N=C-N), 132.77(Ph-C), 131.41(Ph-C), 130.10(Ph-C, 2C), 125.00(Ph-C, 2C), 56.32(CH2), 53.31(CH3). ESI-HRMS: calcd. for C10H10N4O3S [M + Na]+, 289.0366; found, 289.0364.
Ethyl 2-((2-phenyl-2H-tetrazol-5-yl) sulfinyl) (4o): acetate yellow oily liquid, yield 74% 1H NMR (600 MHz, CDCl3) δ 7.72 (dd, J = 7.4, 2.0 Hz, 2H, Ph-H), 7.65–7.63 (m, 3H, Ph-H), 4.93–4.48 (m, 2H, CH2), 4.20 (tt, J = 7.1, 3.5 Hz, 2H, CH2), 1.26 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 164.43(Ph-C), 156.36(N=C-N), 132.78(Ph-C), 131.38(Ph-C), 130.09(Ph-C, 2C), 124.98(Ph-C, 2C), 62.78(CH2), 56.49(CH2), 13.95(CH3). ESI-HRMS: calcd. for C11H12N4O3S [M + Na]+, 303.0522; found, 303.0520.
2-(benzylsulfinyl)-5-phenyl-1,3,4-oxadiazole (5a): white solid, yield 77%. 1H NMR (600 MHz, CDCl3) δ 8.08–8.00 (m, 2H), 7.60 (t, J = 7.5 Hz, 1H, Ph-H), 7.53 (dd, J = 8.4, 7.0 Hz, 2H, Ph-H), 7.37–7.33 (m, 3H, Ph-H), 7.31 (dt, J = 5.3, 3.6 Hz, 2H, Ph-H), 4.73–4.56 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.16(C=N), 165.42(C=N), 132.82(Ph-C), 130.35(Ph-C, 2C), 129.24(Ph-C, 2C), 129.16(Ph-C, 2C), 127.84(Ph-C), 127.46(Ph-C, 2C), 122.58(Ph-C), 60.51(CH2). ESI-HRMS: calcd. for C15H12N2O2S [M + H]+, 285.0692; found, 285.0691.
2-((4-chlorobenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5b): white solid, yield 81%. 1H NMR (600 MHz, CDCl3) δ 8.05–8.03 (m, 2H, Ph-H), 7.57 (dt, J = 41.7, 7.4 Hz, 3H, Ph-H), 7.35–7.31 (m, 2H, Ph-H), 7.26 (d, J = 8.5 Hz, 2H, Ph-H), 4.72–4.50 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.31(C=N), 165.16(C=N), 135.61(Ph-C), 132.91(Ph-C), 131.74(Ph-C, 2C), 129.37(Ph-C, 2C), 129.30(Ph-C, 2C), 127.45(Ph-C, 2C), 126.37(Ph-C), 122.47(Ph-C), 59.49(CH2). ESI-HRMS: calcd. for C15H11ClN2O2S [M + Na]+, 341.0122; found, 341.0099.
2-((3-chlorobenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5c): white solid, yield 79%. 1H NMR (600 MHz, CDCl3) δ 8.07–8.04 (m, 2H, Ph-H), 7.62–7.58 (m, 1H, Ph-H), 7.53 (dd, J = 8.4, 7.0 Hz, 2H, Ph-H), 7.36–7.32 (m, 2H, Ph-H), 7.28 (t, J = 7.9 Hz, 1H, Ph-H), 7.21 (dt, J = 7.6, 1.4 Hz, 1H, Ph-H), 4.77–4.47 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.27(C=N), 165.21(C=N), 134.96(Ph-C), 132.89(Ph-C), 130.44(Ph-C), 130.33(Ph-C), 129.92(Ph-C), 129.44(Ph-C), 129.26(Ph-C, 2C), 128.59(Ph-C), 127.44(Ph-C, 2C), 122.44(Ph-C), 59.59(CH2). ESI-HRMS: calcd. for C15H11ClN2O2S [M + Na]+, 341.0122; found, 319.0102.
2-((2-chlorobenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5d): white solid, yield 72%. 1H NMR (600 MHz, CDCl3) δ 8.10–8.06 (m, 2H, Ph-H), 7.64–7.59 (m, 1H, Ph-H), 7.56–7.52 (m, 2H, Ph-H), 7.44–7.42 (m, 2H), 7.33 (td, J = 7.7, 1.7 Hz, 1H, Ph-H), 7.28 (dd, J = 7.6, 1.4 Hz, 1H, Ph-H), 5.00–4.77 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.23(C=N), 165.40(C=N), 135.03(Ph-C), 132.96(Ph-C), 132.87(Ph-C), 130.81(Ph-C), 130.02(Ph-C), 129.26(Ph-C, 2C), 127.49(Ph-C), 127.47(Ph-C,2C), 126.04(Ph-C), 122.56(Ph-C), 58.12(CH2). ESI-HRMS: calcd. for C15H11ClN2O2S [M + Na]+, 341.0122; found, 319.0100.
2-((4-methylbenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5e): white solid, yield 80%. 1H NMR (600 MHz, CDCl3) δ 8.07–8.03 (m, 2H, Ph-H), 7.62–7.58 (m, 1H, Ph-H), 7.53 (dd, J = 8.4, 6.9 Hz, 2H, Ph-H), 7.18 (d, J = 8.1 Hz, 2H, Ph-H), 7.14 (d, J = 7.9 Hz, 2H, Ph-H), 4.76–4.57 (m, 2H, CH2), 2.30 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 167.10(C=N), 165.47(C=N), 139.31(Ph-C), 132.78(Ph-C), 130.21(Ph-C, 2C), 129.88(Ph-C, 2C), 129.23(Ph-C, 2C), 127.45(Ph-C, 2C), 124.63(Ph-C), 122.63(Ph-C), 60.31(CH2), 21.18(CH3). ESI-HRMS: calcd. for C16H14N2O2S [M + H]+, 299.0849; found, 299.0849.
2-((naphthalen-2-ylmethyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5f): yellow solid, yield 67%. 1H NMR (600 MHz, CDCl3) δ 8.00–7.91 (m, 2H, Ph-H), 7.85–7.72 (m, 4H, Ph-H), 7.56 (t, J = 7.5 Hz, 1H, Ph-H), 7.52–7.44 (m, 4H, Ph-H), 7.36 (dd, J = 8.4, 1.7 Hz, 1H, Ph-H), 4.90–4.74 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.21(C=N), 165.48(C=N), 133.35(Ph-C), 133.26(Ph-C), 132.76(Ph-C), 130.28(Ph-C), 129.19(Ph-C, 2C), 129.06(Ph-C), 128.02(Ph-C), 127.73(Ph-C), 127.42(Ph-C, 2C), 127.12(Ph-C), 126.91(Ph-C), 126.71(Ph-C), 125.21(Ph-C), 122.51(Ph-C), 60.83(CH2). ESI-HRMS: calcd. for C19H14N2O2S [M + Na]+, 357.0668; found, 357.0641.
2-((4-nitrobenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5g): yellow solid, yield 63%. 1H NMR (600 MHz, CDCl3) δ 8.22 (d, J = 8.6 Hz, 2H, Ph-H), 8.05 (s, 1H, Ph-H), 7.61 (d, J = 7.4 Hz, 1H, Ph-H), 7.54 (t, J = 8.5 Hz, 5H, Ph-H), 4.80–4.70 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.55(C=N), 164.76(C=N), 148.45(Ph-C), 135.11(Ph-C), 133.09(Ph-C), 131.60(Ph-C, 2C), 129.36(Ph-C, 2C), 127.43(Ph-C, 2C), 124.10(Ph-C, 2C), 122.29(Ph-C), 59.00(CH2). ESI-HRMS: calcd. for C15H11N3O4S [M + Na]+, 352.0362; found, 352.0350.
2-((4-fluorobenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5h): white solid, yield 72%. 1H NMR (600 MHz, CDCl3) δ 8.11–8.03 (m, 2H, Ph-H), 7.67–7.50 (m, 3H, Ph-H), 7.35–7.28 (m, 2H, Ph-H), 7.05 (t, J = 8.6 Hz, 2H, Ph-H), 4.71–4.55 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.28(C=N), 165.25(C=N), 162.51(Ph-C), 132.90(Ph-C), 132.31(Ph-C), 132.25(Ph-C), 129.29(Ph-C, 2C), 127.44(Ph-C, 2C), 123.71(Ph-C), 122.50(Ph-C), 116.33(Ph-C), 116.19(Ph-C), 59.42(CH2). ESI-HRMS: calcd. for C15H11FN2O2S [M + Na]+, 325.0417; found, 325.0410.
2-((4-bromobenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5i): white solid, yield 84%. 1H NMR (600 MHz, CDCl3) δ 8.06–7.94 (m, 2H, Ph-H), 7.64–7.58 (m, 1H, Ph-H), 7.53 (dd, J = 8.4, 7.0 Hz, 2H, Ph-H), 7.50–7.45 (m, 2H, Ph-H), 7.22–7.15 (m, 2H, Ph-H), 4.74–4.43 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 167.27(C=N), 165.12(C=N), 132.89(Ph-C), 132.31(Ph-C, 2C), 131.99(Ph-C, 2C), 129.29(Ph-C, 2C), 127.43(Ph-C, 2C), 126.87(Ph-C), 123.79(Ph-C), 122.42(Ph-C), 59.51(CH2). ESI-HRMS: calcd. for C15H11BrN2O2S [M + Na]+, 384.9617; found, 384.9594.
2-phenyl-5-((4-(trifluoromethyl) benzyl) sulfinyl)-1,3,4-oxadiazole (5j): white solid, yield 70%. 1H NMR (600 MHz, CDCl3) δ 8.06–8.02 (m, 2H, Ph-H), 7.66–7.61 (m, 3H, Ph-H), 7.55 (dd, J = 8.5, 7.0 Hz, 2H, Ph-H), 7.49 (d, J = 8.0 Hz, 2H, Ph-H), 4.83–4.62 (m, 2H, CH3). 13C NMR (151 MHz, CDCl3) δ 167.41(C=N), 165.01(C=N), 132.98(Ph-C), 132.02(Ph-C), 131.34(Ph-C, 2C), 130.91(Ph-C), 129.30(Ph-C, 2C), 127.44(Ph-C, 2C), 126.01(Ph-C), 124.64(Ph-C), 122.84(Ph-C), 122.38(Ph-C), 59.54(CH2). ESI-HRMS: calcd. for C16H11F3N2O2S [M + H]+, 353.0566; found, 353.0566.
2-((3-methoxybenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5k): white solid, yield 74%. 1H NMR (600 MHz, CDCl3) δ 8.16–8.05 (m, 2H, Ph-H), 7.68–7.52 (m, 3H, Ph-H), 7.29–7.27 (m, 1H, Ph-H), 6.94–6.82 (m, 3H, Ph-H), 4.75–4.53 (m, 2H, CH2), 3.77 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 167.16(C=N), 165.50(C=N), 160.03(Ph-C), 132.83(Ph-C), 130.21(Ph-C), 129.25(Ph-C, 2C), 129.18(Ph-C), 127.46(Ph-C, 2C), 122.59(Ph-C), 122.52(Ph-C), 115.60(Ph-C), 115.08(Ph-C), 60.65(CH2), 55.26(OCH3). ESI-HRMS: calcd. for C16H14N2O3S [M + H]+, 315.0798; found, 315.0782.
2-((4-methoxybenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5l): white solid, yield 57%. 1H NMR (600 MHz, CDCl3) δ 8.10–8.02 (m, 2H, Ph-H), 7.61–7.48 (m, 3H, Ph-H), 7.21 (d, J = 8.5 Hz, 2H, Ph-H), 6.89–6.81 (m, 2H, Ph-H), 4.67–4.55 (m, 2H, CH2), 3.75 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 167.12(C=N), 165.50(C=N), 160.39(Ph-C), 132.79(Ph-C), 131.63(Ph-C, 2C), 129.24(Ph-C, 2C), 127.45(Ph-C, 2C), 122.63(Ph-C), 119.49(Ph-C), 114.64(Ph-C, 2C), 60.03(CH2), 55.27(OCH3). ESI-HRMS: calcd. for C16H14N2O3S [M + Na]+, 337.0617; found, 337.0601.
2-((3,5-dimethoxybenzyl) sulfinyl)-5-phenyl-1,3,4-oxadiazole (5m): yellow solid, yield 69%. 1H NMR (600 MHz, CDCl3) δ 8.07 (d, J = 7.8 Hz, 2H, Ph-H), 7.60 (t, J = 7.4 Hz, 1H, Ph-H), 7.53 (dd, J = 8.3, 7.0 Hz, 2H, Ph-H), 6.42 (dd, J = 10.3, 2.3 Hz, 3H, Ph-H), 4.65–4.54 (m, 2H, CH2), 3.71 (d, J = 0.8 Hz, 6H, CH3). 13C NMR (151 MHz, CDCl3) δ 167.15(C=N), 165.51(C=N), 161.19(Ph-C), 132.84(Ph-C), 129.77(Ph-C, 2C), 129.25(Ph-C, 2C), 127.43(Ph-C), 125.69(Ph-C), 122.53(Ph-C), 108.11(Ph-C, 2C), 101.35(Ph-C), 60.87(CH2), 55.35(OCH3, 2C). ESI-HRMS: calcd. for C17H16N2O4S [M + H]+, 345.0904; found, 345.0894.
Methyl 2-((5-phenyl-1,3,4-oxadiazol-2-yl) sulfinyl) acetate (5n): white solid, yield 75%. 1H NMR (600 MHz, CDCl3) δ 8.18–8.11 (m, 2H, Ph-H), 7.69–7.60 (m, 1H, Ph-H), 7.58 (dd, J = 8.4, 6.9 Hz, 2H, Ph-H), 4.63–4.32 (m, 2H, CH2), 3.85 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 167.47(C=N), 165.37(C=N), 164.36(C=O), 132.99(Ph-C), 129.32(Ph-C, 2C), 127.53(Ph-C, 2C), 122.48(Ph-C), 57.09(CH2), 53.42(CH3). ESI-HRMS: calcd. For C11H10N2O4S [M + Na]+, 289.0253; found, 289.0239.
Ethyl 2-((5-phenyl-1,3,4-oxadiazol-2-yl) sulfinyl) acetate (5o): white solid, yield 61%. 1H NMR (600 MHz, CDCl3) δ 8.20–8.10 (m, 2H, Ph-H), 7.68–7.61 (m, 1H, Ph-H), 7.58 (d, J = 7.9 Hz, 2H, Ph-H), 4.58–4.39 (m, 2H, CH2), 4.30 (q, J = 7.1 Hz, 2H, CH2), 1.32–1.28 (m, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 168.03(C=N), 166.01(C=N), 164.32(C=O), 133.54(Ph-C), 129.88(Ph-C, 2C), 128.11(Ph-C, 2C), 123.08(Ph-C), 63.46(CH2), 57.92(CH2), 14.55(CH3). ESI-HRMS: calcd. for C12H12N2O4S [M + Na]+, 303.0410; found, 303.0394.
5-(benzylsulfinyl)-2-(4-methoxyphenyl)-2H-tetrazole (6a): white solid, yield 84%. 1H NMR (600 MHz, CDCl3) δ 7.38–7.29 (m, 3H, Ph-H), 7.17 (d, J = 7.5 Hz, 2H, Ph-H), 7.07 (d, J = 8.9 Hz, 2H, Ph-H), 6.93 (d, J = 8.9 Hz, 2H, Ph-H), 4.89–4.70 (m, 2H, CH2), 3.85 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.41(Ph-C), 156.08(N=C-N), 130.62(Ph-C, 2C), 129.26(Ph-C), 129.15(Ph-C, 2C), 127.41(Ph-C), 126.51(Ph-C, 2C), 125.33(Ph-C), 114.65(Ph-C, 2C), 60.17(CH2), 55.69(OCH3). ESI-HRMS: calcd. for C15H14N4O2S [M + H]+, 315.0910; found, 315.0902.
5-((4-chlorobenzyl) sulfinyl)-2-(4-methoxyphenyl)-2H-tetrazole (6b): white solid, yield 87%. 1H NMR (600 MHz, CDCl3) δ 7.27 (d, J = 7.8 Hz, 2H, Ph-H), 7.21 (dd, J = 8.7, 1.3 Hz, 2H, Ph-H), 7.16–7.11 (m, 2H, Ph-H), 6.97 (dd, J = 8.7, 1.3 Hz, 2H, Ph-H), 4.83–4.67 (m, 2H, CH2), 3.87 (d, J = 1.3 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.47(Ph-C), 155.69(N=C-N), 135.58(Ph-C), 131.91(Ph-C, 2C), 129.32(Ph-C, 2C), 126.41(Ph-C, 2C), 126.02(Ph-C), 125.31(Ph-C), 114.70(Ph-C, 2C), 59.17(CH2), 55.72(OCH3). ESI-HRMS: calcd. for C15H13ClN4O2S [M + Na]+, 371.0340; found, 371.0318.
2-(4-methoxyphenyl)-5-((4-methylbenzyl) sulfinyl)-2H-tetrazole (6e): white solid, yield 81%. 1H NMR (600 MHz, CDCl3) δ 7.09 (dd, J = 8.4, 5.8 Hz, 4H, Ph-H), 7.03 (d, J = 7.9 Hz, 2H, Ph-H), 6.95–6.91 (m, 2H, Ph-H), 4.85–4.66 (m, 2H, CH2), 3.85 (s, 3H, CH3), 2.32 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.38(Ph-C), 156.20(N=C-N), 139.34(Ph-C), 130.48(Ph-C, 2C), 129.82(Ph-C, 2C), 126.46(Ph-C, 2C), 125.39(Ph-C), 124.15(Ph-C), 114.59(Ph-C, 2C), 59.94(CH2), 55.69(OCH3), 21.18(CH3). ESI-HRMS: calcd. for C16H16N4O2S [M + Na]+, 351.0886; found, 351.0860.
2-(4-methoxyphenyl)-5-((naphthalen-2-ylmethyl) sulfinyl)-2H-tetrazole (6f): yellow solid, yield 83%. 1H NMR (600 MHz, CDCl3) δ 7.81–7.78 (m, 1H, Ph-H), 7.73 (dd, J = 8.6, 5.6 Hz, 2H, Ph-H), 7.65 (d, J = 1.7 Hz, 1H, Ph-H), 7.50 (td, J = 7.3, 1.6 Hz, 2H, Ph-H), 7.18 (dd, J = 8.5, 1.7 Hz, 1H, Ph-H), 7.01–6.89 (m, 2H, Ph-H), 6.81–6.70 (m, 2H, Ph-H), 5.02–4.84 (m, 2H, CH2), 3.78 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.21(Ph-C), 156.17(N=C-N), 133.24(Ph-C), 133.15(Ph-C), 130.44(Ph-C), 129.00(Ph-C), 127.96(Ph-C), 127.70(Ph-C), 127.22(Ph-C), 127.06(Ph-C), 126.81(Ph-C), 126.27(Ph-C, 2C), 125.21(Ph-C), 124.60(Ph-C), 114.43(Ph-C, 2C), 60.56(CH2), 55.62(OCH3). ESI-HRMS: calcd. for C19H16N4O2S [M + Na]+, 387.0886; found, 387.0866.
2-(4-methoxyphenyl)-5-((4-nitrobenzyl) sulfinyl)-2H tetrazole (6g): yellow solid, yield 85%. 1H NMR (600 MHz, CDCl3) δ 8.20–8.15 (m, 2H, Ph-H), 7.50–7.45 (m, 2H, Ph-H), 7.33 (d, J = 9.0 Hz, 2H, Ph-H), 6.99 (d, J = 9.0 Hz, 2H, Ph-H), 4.95–4.86 (m, 2H, CH2), 3.87 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.62(Ph-C), 155.28(N=C-N), 148.40(Ph-C), 135.05(Ph-C), 131.75(Ph-C, 2C), 126.37(Ph-C, 2C), 125.21(Ph-C), 124.04(Ph-C, 2C), 114.86(Ph-C, 2C), 58.63(CH2), 55.75(OCH3). ESI-HRMS: calcd. for C15H13N5O4S [M + Na]+, 382.0580; found, 382.0566.
5-((4-fluorobenzyl) sulfinyl)-2-(4-methoxyphenyl)-2H-tetrazole (6h): white solid, yield 80%. 1H NMR (600 MHz, CDCl3) δ 7.24–7.20 (m, 4H, Ph-H), 7.03–6.98 (m, 4H, Ph-H), 4.88–4.73 (m, 2H, CH2), 3.88 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 164.09(Ph-C), 162.44(Ph-C), 161.48(Ph-C), 155.76(N=C-N), 132.47, (Ph-C) 126.43(Ph-C, 2C), 125.34(Ph-C), 123.39(Ph-C), 116.28(Ph-C), 116.14(Ph-C), 114.73(Ph-C, 2C), 59.01(CH2), 55.71(OCH3). ESI-HRMS: calcd. for C15H13FN4O2S [M + Na]+, 355.0635; found, 355.0611.
5-((4-bromobenzyl) sulfinyl)-2-(4-methoxyphenyl)-2H-tetrazole (6i): white solid, yield 89%. 1H NMR (600 MHz, CDCl3) δ 7.46–7.39 (m, 2H, Ph-H), 7.24–7.18 (m, 2H, Ph-H), 7.09–7.04 (m, 2H, Ph-H), 7.00–6.95 (m, 2H, Ph-H), 4.81–4.64 (m, 2H, CH2), 3.87 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.48(Ph-C), 155.66(N=C-N), 132.30(Ph-C, 2C), 132.17(Ph-C, 2C), 126.51(Ph-C, 2C), 126.42(Ph-C), 125.31(Ph-C), 123.79(Ph-C), 114.70(Ph-C, 2C), 59.29(CH2), 55.72(OCH3). ESI-HRMS: calcd. for C15H13BrN4O2S [M + Na]+, 414.9835; found 414.9834.
2-(4-methoxyphenyl)-5-((4-(trifluoromethyl) benzyl) sulfinyl) -2H-tetrazole (6g): yellow solid, yield 83%. 1H NMR (600 MHz, CDCl3) δ 7.59 (d, J = 8.0 Hz, 2H, Ph-H), 7.36 (d, J = 8.0 Hz, 2H, Ph-H), 7.24–7.19 (m, 2H, Ph-H), 7.00–6.94 (m, 2H, Ph-H), 5.01–4.67 (m, 2H, CH2), 3.87 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.51(Ph-C), 155.57(N=C-N), 131.78(Ph-C, 2C), 131.52(Ph-C), 131.31(Ph-C), 131.08(Ph-C), 126.38(Ph-C, 2C), 125.98(Ph-C), 125.25(Ph-C), 124.62(Ph-C, 2C), 114.74(Ph-C, 2C), 59.30(CH2), 55.70(OCH3). ESI-HRMS: calcd. for C16H13F3N4O2S [M + H]+, 383.0790; found, 383.0790.
5-((3-methoxybenzyl) sulfinyl)-2-(4-methoxyphenyl)-2H-tetrazole (6k): yellow oily liquid, yield 73%. 1H NMR (600 MHz, CDCl3) δ 7.22–7.18 (m, 1H, Ph-H), 7.13–7.09 (m, 2H, Ph-H), 6.96–6.92 (m, 2H, Ph-H), 6.88–6.85 (m, 1H, Ph-H), 6.76–6.72 (m, 1H, Ph-H), 6.66 (t, J = 2.0 Hz, 1H, Ph-H), 4.85–4.66 (m, 2H, CH2), 3.86 (d, J = 1.3 Hz, 3H, CH3), 3.71 (d, J = 1.2 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.41(Ph-C), 160.05(Ph-C), 156.19(N=C-N), 130.17(Ph-C), 128.69(Ph-C), 126.50(Ph-C, 2C), 125.35(Ph-C), 122.73(Ph-C), 115.48(Ph-C), 115.37(Ph-C), 114.66(Ph-C, 2C), 60.31(CH2), 55.70(OCH3), 55.30(OCH3). ESI-HRMS: calcd. for C16H16N4O3S [M + H]+, 345.1016; found, 345.1000.
5-((4-methoxybenzyl) sulfinyl)-2-(4-methoxyphenyl)-2H-tetrazole (6l): yellow solid, yield 83%. 1H NMR (600 MHz, CDCl3) δ 7.16–7.11 (m, 2H, Ph-H), 7.10–7.05 (m, 2H, Ph-H), 6.97–6.91 (m, 2H, Ph-H), 6.82–6.79 (m, 2H, Ph-H), 4.84–4.64 (m, 2H, CH2), 3.86 (s, 3H, CH3), 3.77 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.38(Ph-C), 160.39(Ph-C), 156.16(N=C-N), 131.89(Ph-C, 2C), 126.46(Ph-C, 2C), 125.43(Ph-C), 119.00(Ph-C), 114.63(Ph-C, 2C), 114.58(Ph-C, 2C), 59.62(CH2), 55.70(OCH3), 55.35(OCH3). ESI-HRMS: calcd. for C16H16N4O3S [M + Na]+, 367.0835; found 367.0825.
5-((3, 5-dimethoxybenzyl) sulfinyl)-2-(4-methoxyphenyl)-2H-tetrazole (6m): yellow solid, yield 78%. 1H NMR (600 MHz, CDCl3) δ 7.18–7.13 (m, 2H, Ph-H), 6.95 (d, J = 8.9 Hz, 2H, Ph-H), 6.42–6.25 (m, 3H, Ph-H), 4.79–4.63 (m, 2H, CH2), 3.86 (s, 3H, CH3), 3.69 (s, 6H, CH3). 13C NMR (151 MHz, CDCl3) δ 161.40(Ph-C), 161.20(Ph-C), 156.34(N=C-N), 129.27(Ph-C), 126.46(Ph-C, 2C), 125.36(Ph-C), 114.67(Ph-C, 2C), 108.12(Ph-C, 2C), 101.59(Ph-C), 60.59(CH2), 55.71(OCH3), 55.42(OCH3, 2C). ESI-HRMS: calcd. for C17H18N4O4S [M + Na]+, 397.0911; found, 397.0941.
Methyl 2-((2-(4-methoxyphenyl)-2H-tetrazol-5-yl) sulfinyl) acetate (6n): oily liquid, yield 70%. 1H NMR (600 MHz, CDCl3) δ 7.63–7.60 (m, 2H, Ph-H), 7.11–7.08 (m, 2H, Ph-H), 4.89–4.48 (m Hz, 2H, CH2), 3.91 (s, 3H, CH3), 3.76 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 164.92(Ph-C), 161.73(Ph-C), 156.29(N=C-N), 126.57(Ph-C, 2C), 125.36(Ph-C), 115.13(Ph-C, 2C), 56.27(CH2), 55.78(OCH3), 53.28(OCH3). ESI-HRMS: calcd. for C11H12N4O4S [M + Na]+, 319.0471; found 319.0467.
Ethyl 2-((2-(4-methoxyphenyl)-2H-tetrazol-5-yl) sulfinyl) acetate (6o): oily liquid, yield 77%. 1H NMR (600 MHz, CDCl3) δ 7.65–7.59 (m, 2H, Ph-H), 7.12–7.08 (m, 2H, Ph-H), 4.88–4.46 (m, 2H, CH2), 4.20 (qd, J = 7.2, 2.1 Hz, 2H, CH2), 3.91 (s, 3H, CH3), 1.26 (t, J = 7.2 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 164.44(Ph-C), 161.71(Ph-C), 156.36(N=C-N), 126.55(Ph-C, 2C), 125.39(Ph-C), 115.13(Ph-C, 2C), 62.75(CH2), 56.45(OCH3), 55.78(OCH3), 13.96(CH3). ESI-HRMS: calcd. for C12H14N4O4S [M + Na]+, 333.0628; found 333.0625.
2-(benzylsulfinyl)-5-(4-chlorophenyl)-1,3,4-oxadiazole (7a): white solid, yield 82%. 1H NMR (600 MHz, CDCl3) δ 7.99–7.96 (m, 2H, Ph-H), 7.52–7.48 (m, 2H, Ph-H), 7.35 (dd, J = 4.8, 1.9 Hz, 3H, Ph-H), 7.30 (dt, J = 4.7, 3.5 Hz, 2H, Ph-H), 4.71–4.58 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 166.35(Ph-C), 165.60(Ph-C), 139.30(Ph-C), 130.36(Ph-C, 2C), 129.70(Ph-C, 2C), 129.29(Ph-C), 129.17(Ph-C, 2C), 128.69(Ph-C, 2C), 127.80(Ph-C), 121.01(Ph-C), 60.56(CH2). ESI-HRMS: calcd. for C15H11ClN2O2S [M + Na]+, 341.0122; found, 341.0103.
2-((4-chlorobenzyl) sulfinyl)-5-(4-chlorophenyl)-1,3,4-oxadiazole (7b): white solid, yield 82%. 1H NMR (600 MHz, CDCl3) δ 7.97 (dd, J = 8.3, 6.4 Hz, 2H, Ph-H), 7.54–7.49 (m, 2H, Ph-H), 7.35–7.30 (m, 2H, Ph-H), 7.26–7.24 (m, 2H, Ph-H), 4.70–4.52 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 166.50(C=N), 165.34(C=N), 139.42(Ph-C), 135.65(Ph-C), 131.73(Ph-C, 2C), 130.92(Ph-C), 129.75(Ph-C, 2C), 129.38(Ph-C), 128.68(Ph-C, 2C), 126.30(Ph-C), 120.90(Ph-C), 59.53(CH2). ESI-HRMS: calcd. for C15H10Cl2N2O2S [M + Na]+, 374.9732; found, 374.9717.
2-(4-chlorophenyl)-5-((naphthalen-2-ylmethyl) sulfinyl)-1,3,4-oxadiazole (7f): white solid, yield 86%. 1H NMR (600 MHz, CDCl3) δ 7.88–7.83 (m, 2H, Ph-H), 7.82–7.77 (m, 4H, Ph-H), 7.51–7.46 (m, 2H, Ph-H), 7.45–7.43 (m, 2H, Ph-H), 7.35–7.33 (m, 1H, Ph-H), 4.89–4.66 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 166.35(C=N), 165.62(C=N), 139.20(Ph-C), 133.32(Ph-C), 133.21(Ph-C), 130.24(Ph-C), 129.60(Ph-C, 2C), 129.03(Ph-C), 128.61(Ph-C, 2C), 128.00(Ph-C), 127.72(Ph-C), 127.09(Ph-C), 126.94(Ph-C), 126.74(Ph-C), 125.14(Ph-C), 120.90(Ph-C), 60.88(CH2). ESI-HRMS: calcd. for C19H13ClN2O2S [M + Na]+, 391.0278; found, 391.0259.
2-(4-chlorophenyl)-5-((4-nitrobenzyl) sulfinyl) -1,3,4-oxadiazole (7g): yellow solid, yield 81%. 1H NMR (600 MHz, CDCl3) δ 8.31–8.18 (m, 2H, Ph-H), 8.02–7.99 (m, 2H, Ph-H), 7.59–7.52 (m, 4H, Ph-H), 4.88–4.65 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 166.76(C=N), 164.95(C=N), 148.47(Ph-C), 139.64(Ph-C), 135.01(Ph-C), 131.63(Ph-C, 2C), 129.82(Ph-C, 2C), 128.68(Ph-C, 2C), 124.11(Ph-C, 2C), 120.73(Ph-C), 58.98(CH2). ESI-HRMS: calcd. for C15H10ClN3O4S [M + Na]+, 385.9973; found, 385.9965.
2-(4-chlorophenyl)-5-((4-fluorobenzyl) sulfinyl)-1,3,4-oxadiazole (7h): white solid, yield 87%. 1H NMR (600 MHz, CDCl3) δ 8.01–7.97 (m, 2H, Ph-H), 7.54–7.50 (m, 2H, Ph-H), 7.33–7.28 (m, 2H, Ph-H), 7.08–7.01 (m, 2H, Ph-H), 4.77–4.41 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 166.45(C=N), 165.42(C=N), 164.15(Ph-C), 162.50(Ph-C), 139.39(Ph-C), 132.25(Ph-C), 129.73(Ph-C, 2C), 128.66(Ph-C, 2C), 123.65(Ph-C), 120.91(Ph-C), 116.33(Ph-C), 116.18(Ph-C), 59.42(CH2). ESI-HRMS: calcd. for C15H10ClFN2O2S [M + Na]+, 359.0028; found, 359.0009.
2-((4-bromobenzyl) sulfinyl)-5-(4-chlorophenyl)-1,3,4-oxadiazole (7i): white solid, yield 79%. 1H NMR (600 MHz, CDCl3) δ 8.00–7.94 (m, 2H, Ph-H), 7.54–7.51 (m, 2H, Ph-H), 7.50–7.47 (m, 2H, Ph-H), 7.20–7.17 (m, 2H, Ph-H), 4.68–4.52 (m, 2H, CH2). 13C NMR (151 MHz, CDCl3) δ 166.51(C=N), 165.31(C=N), 139.42(Ph-C), 132.34(Ph-C, 2C), 131.99(Ph-C, 2C), 129.76(Ph-C, 2C), 128.69(Ph-C, 2C), 126.81(Ph-C), 123.86(Ph-C), 120.88(Ph-C), 59.61(CH2). ESI- HRMS: calcd. for C15H10BrClN2O2S [M + Na]+, 418.9227; found 418.9229.
2-(4-chlorophenyl)-5-((4-methoxybenzyl) sulfinyl)-1,3,4-oxadiazole (7l): white solid, yield 68%. 1H NMR (600 MHz, CDCl3) δ 8.00–7.93 (m, 2H, Ph-H), 7.55–7.49 (m, 2H, Ph-H), 7.18–7.12 (m, 4H, Ph-H), 4.68–4.50 (m, 2H, CH2), 2.31 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 166.29(C=N), 165.66(C=N), 139.34(Ph-C), 139.27(Ph-C), 130.21(Ph-C, 2C), 129.87(Ph-C, 2C), 129.67(Ph-C, 2C), 128.68(Ph-C, 2C), 124.57(Ph-C), 121.06(Ph-C), 60.35(CH2), 21.19(CH3). ESI-HRMS: calcd. for C16H13ClN2O3S [M + Na]+, 371.0228; found, 371.0238.
2-(4-chlorophenyl)-5-((3,5-dimethoxybenzyl) sulfinyl)-1,3,4-oxadiazole (7m): yellow solid, yield 71%. 1H NMR (600 MHz, CDCl3) δ 8.03–7.97 (m, 2H, Ph-H), 7.55–7.49 (m, 2H, Ph-H), 6.42 (s, 3H, Ph-H), 4.63–4.54 (m, 2H, CH2), 3.72 (s, 6H, CH3). 13C NMR (151 MHz, CDCl3) δ 166.35(C=N), 165.76(C=N), 161.23(Ph-C), 139.33(Ph-C), 129.75(Ph-C), 129.72(Ph-C, 2C), 128.69(Ph-C, 2C), 126.96(Ph-C), 121.02(Ph-C), 108.13(Ph-C, 2C), 101.35(Ph-C), 60.99(CH2), 55.39(OCH3, 2C). ESI-HRMS: calcd. for C17H15ClN2O4S [M + Na]+, 401.0333; found, 401.0324.
Methyl 2-((5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl) sulfinyl) acetate (7n): white solid, yield 88%. 1H NMR (600 MHz, CDCl3) δ 8.15–8.03 (m, 2H, Ph-H), 7.59–7.51 (m, 2H, Ph-H), 4.71–4.32 (m, 2H, CH2), 3.86 (s, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 166.76(C=N), 165.54(C=N), 164.25(C=O), 139.55(Ph-C), 129.80(Ph-C, 2C), 128.82(Ph-C, 2C), 120.94(Ph-C), 57.14(CH2), 53.48(OCH3). ESI-HRMS: calcd. for C11H9ClN2O4S [M + Na]+, 322.9864; found, 322.9850.
Ethyl 2-((5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl) sulfinyl) acetate (7o): yellow solid, yield 81%. 1H NMR (600 MHz, CDCl3) δ 8.09–8.06 (m, 2H, Ph-H), 7.56–7.53 (m, 2H, Ph-H), 4.55–4.38 (m, 2H, CH2), 4.28 (q, J = 7.1 Hz, 2H, CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3) δ 166.68(C=N), 165.64(C=N), 163.73(C=O), 139.52(Ph-C), 129.79(Ph-C, 2C), 128.80(Ph-C, 2C), 120.97(Ph-C), 62.95(CH2), 57.38(OCH2), 14.00(CH3). ESI-HRMS: calcd. for C12H11ClN2O4S [M + Na]+, 337.0020; found, 337.0016.
3.2. Biological Investigations
3.2.1. Biofilm Formation Assay
The biofilm was quantitatively analyzed by the crystal violet method [44]. The log phase of P. aeruginosa PAO1 was diluted 1000-fold with fresh Luria broth (LB) medium, then the diluted bacterial solution and the compound solution were added to a 96-well plate, making the final concentration of the compounds 50 μM. The control group was added to 0.5% DMSO per well. The 96-well plates were incubated at 37 °C for 24 h, then OD of the suspended cells was measured at 600 nm using a microplate reader (Molecular Devices, Spectra Max M5, Sunnyvale, CA, USA). The bacterial culture was removed and washed three times with PBS and dried; 0.1% crystal violet solution was then added to each well for 15 min staining. After the excess crystal violet solution was removed and washed three times with PBS, the pigment was dissolved in 95% ethanol. Finally, the absorbance value was measured at 595 nm by a microplate reader. The formula for calculating the biofilm inhibition rate is: OD595control − OD595/OD595control × 100%.
3.2.2. P. aeruginosa QS Inhibition Assays [32,45]
Test compounds were dissolved in 100% DMSO to a concentration of 10 mM and mixed with ABTGC medium, and they were then added to 96-well microtiter plates (Corning, Corning, NY, USA,) in the first well, giving a final concentration of 40 μM in a volume of 100 μL. An overnight culture of the PAO1-lasB-gfp strain, which had grown in LB medium at 37 °C with 200 rpm, was diluted in ABTGC medium to an optical density at 600 nm (OD600) of 0.2. Then 100 μL bacterial suspension was added to the wells of the microtiter plate to reach a final inhibitor concentration of 20 μM. DMSO control with 0.2% final concentration was used. The microtiter plate was incubated in a Molecular Devices SpectraMax microplate reader at 37 °C, with GFP fluorescence signals (excitation 485 nm, emission 535 nm) and cell density (OD600) measured every 20 min for at least 12 h. The Inhibition assay of all test compounds and controls were determined in triplicate. P. aeruginosa Rhl and Pqs inhibition assays were performed using a similar method to that of the LasB inhibition assay.
3.2.3. CLSM Images
P. aeruginosa PAO1 was cultured overnight and diluted 100-fold, then the test compounds and bacterial suspension were added to the plate, followed by incubation at 37 °C for 24 h. Floating bacteria were poured out and washed with water three times, then fixed with 4% paraformaldehyde for 15 min and stained with 0.01% acridine orange for 15 min in the dark; excess dye was then washed with PBS. The established model was observed by confocal laser scanning microscope (Nikon-Eclipse-Ti) under green fluorescence light (excitation wavelength: 488 nm, emission wavelength: 515 nm). The signal was received by the FITC channel, where the objective lens was ×10, and scanned layer by layer along the Z-axis from outside to inside.
3.2.4. Quantification Analysis of Elastase
The elastase quantification assay was performed as previously described [46]. An overnight culture of P. aeruginosa PAO1 was diluted to a density of OD600 = 0.01 in LB medium and inoculated with compounds in a 20 mL conical flask, then incubated at 37 °C with shaking at 200 rpm for 24 h. The cultures were centrifuged at 10,000 rpm at 4 °C for 10 min, and the supernatant was collected and filtered with a 0.22 mm-pore size filter. A supernatant fraction of 100 µL was added to 900 µL of Elastin-Congo Red reaction buffer (2 mg/mL ECR, 0.1 mM Tris-HCl), which was shaken at 37 °C for 18 h. The reaction was placed on ice and 100 μL of 0.12 M EDTA was added to terminate the reaction, before being centrifuged at 4 °C, 12,000 r/min for 10 min; the supernatant was measured at 495 nm.
3.2.5. Determination of Pyocyanin Production
The pyocyanin quantification assay is based on the absorbance of pyocyanin at 520 nm in acidic solution [47]. P. aeruginosa PAO1 was cultured overnight and diluted to OD600 = 0.01 in LB medium, and then inoculated with compounds in a 20 mL conical flask before being incubated at 37 °C, with shaking at 200 rpm for 24 h. The bacterial culture was centrifuged at 10,000 rpm for 10 min; the supernatant was collected and extracted with chloroform, then to the chloroform layer was added 0.2 M HCl for extraction (after the hydrochloric acid mixed reaction turned pink). The absorbance of the HCl layer was measured at 520 nm by microplate reader.
3.2.6. Detection of Rhamnolipid Production
Rhamnolipid production was directly quantified using the orcinol assay according to the original protocol by Koch et al. [48]. P. aeruginosa PAO1 overnight culture was diluted 100-fold in LB medium and inoculated with compounds in a 20 mL conical flask. The cultures were incubated for 24 h at 37 °C, under shaking condition (200 rpm). Supernatants were collected after the mixture was centrifuged at 10,000 rpm for 10 min and extracted twice by diethyl ether. The ether fraction was evaporated to dry and then resuspended in deionized water and supplemented with orcinal solution (0.19% (w/v) orcinol, 50% H2SO4). The mixture was incubated in a water bath at 80 °C for 30 min, and then cooled at room temperature for 15 min; the OD value at 421 nm was measured.
3.3. Molecular Docking
Compounds and OdDHL were drawn with ChemBioDraw Ultra (ver. 13.0) software (Cambridge, MA, USA) and minimized with Molecular Operate Environment software (Innovation Center of Pesticide Research, Department of Applied Chemistry, College of Science, China Agricultural University, Beijing, China). The receptor protein lasR in PDB format was downloaded from the RCSB Protein Data Bank (http://www.pdb.org (accessed on 20 July 2022)). A LasR X-ray crystal structure with 1.80 A° resolution (PDB ID: 2UV0) was used for the docking study [49]. The process of deleting water, adding hydrogen, adding Gasteiger charges, and so on, was prepared by Autodock Tools software (San Diego, CA, USA). The OdDHL binding pocket was selected as the docking site, and the docking environment was set in the solvent. Docking was performed after the setting method (placement: triangular matcher, refinement: rigid receptor), score (placement: London dG, refinement: GBVI/WSA dG), and posture (placement: 30, refinement: 5). The optimal docking posture was selected to analyze the interaction between LasR and the target compounds.
4. Conclusions
The establishment of biofilms can protect P. aeruginosa and help to elude eradication by human immunity and antibacterial drugs. Clinically, once the biofilm is formed on abiotic or tissue surfaces, it is generally considered a pathogenic characteristic of chronic infection. The difficulty in treating P. aeruginosa infections with antibiotics is that almost all patients with cystic fibrosis eventually contract an incurable resistant strain [3,22]. QSIs that reduce the virulence of pathogens without killing pathogenic bacteria are considered feasible targets for the development of antimicrobial agents against P. aeruginosa. They can also alleviate the pressure of drug resistance to a certain extent. In this study, phenyloxadiazole sulfoxide derivative 5b could inhibit the biofilm formation of P. aeruginosa but had no growth inhibitory effect. Similarly, in phenotypic experiments with virulence factors, the decreased production of extracellular virulence factor elastase was observed. Mechanism research results confirm that 5b can effectively inhibit the las system in a dose-dependent manner, and the IC50 value of inhibitory concentration against the PAO1-lasB-gfp strain was 3.53 ± 0.16 μM. Molecular docking analysis showed that 5b and LasR receptor proteins inhibited the production of virulence factors and Pseudomonas aeruginosa biofilms by forming hydrogen bonds and hydrophobic interactions. In conclusion, sulfoxide derivatives have been proposed as a new QSIs model, which provides a new strategy for the development of new antimicrobial agents and attenuating the pathogenicity of P. aeruginosa.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28093879/s1, Figures S1–S53: 1H NMR and 13C NMR spectra of title target compounds 4a–7o; Figures S54–S105: HRMS spectrum of the compounds 4a–7o; Figures S106–S113: IR (KBr) spectrum of the compound 5a, 5b, 5c, 5f, 5g, 5i, 5j, 5k.
Author Contributions
Writing—original draft, S.M. and X.Y.; writing—review and editing, Y.L. and A.D.; molecular docking, Z.Y.; supervision, H.W. and X.Y.; funding acquisition, X.Y. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC (42276137, 22101255, and 82204202), Key Research and Development Program of Zhejiang (2021C03084), High-Level Talent Special Support Plan of Zhejiang Province (2019R52009), Zhejiang University of Technology (2020414801729), and platform support from Zhejiang International Scientific & Technological Cooperation Base of Development and Utilization of Nature Products. In particular, we thank Liang Yang, Southern University of Science and Technology, for providing reporter strains.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rada, B.; Leto, T.L. Pyocyanin effects on respiratory epithelium: Relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013, 21, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Anju, C.P.; Biswas, L.; Kumar, V.A.; Mohan, C.G.; Biswas, R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int. J. Med. Microbiol. 2016, 306, 48–58. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, J.; Huang, Z.X.; Yu, J.H.; Xu, X.F.; Sun, P.H.; Lin, J.; Chen, W.M. Design, synthesis and biological evaluation of 2-substituted 3-hydroxy-6-methyl-4H-pyran-4-one derivatives as Pseudomonas aeruginosa biofilm inhibitors. Eur. J. Med. Chem. 2018, 158, 753–766. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbone, D.; Deng, D.; Cascioferro, S.M.; Diana, P.; Giovannetti, E. Biofilm formation as valuable target to fight against severe chronic infections. Curr. Med. Chem. 2022, 29, 4307–4310. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Cascioferro, S.; Parrino, B.; Carbone, D.; Pecoraro, C.; Schillaci, D.; Cusimano, M.G.; Cirrincione, G.; Diana, P. Thiazole analogues of the marine alkaloid nortopsentin as inhibitors of bacterial biofilm formation. Molecules 2021, 26, 81. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Yang, L.; Barken, K.B.; Skindersoe, M.E.; Christensen, A.B.; Givskov, M.; Tolker-Nielsen, T. Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 2007, 153, 1318–1328. [Google Scholar] [CrossRef]
- de Kievit, T.R. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2009, 11, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Shim, S.H.; Lee, J. Antibiofilm activities of norharmane and its derivatives against Escherichia coli O157:H7 and other bacteria. Phytomedicine 2017, 36, 254–261. [Google Scholar] [CrossRef]
- Straub, H.; Zuber, F.; Eberl, L.; Maniura-Weber, K.; Ren, Q. In situ investigation of Pseudomonas aeruginosa biofilm development: Interplay between flow, growth medium, and mechanical properties of substrate. ACS Appl. Mater. Interfaces 2023, 15, 2781–2791. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.Ø.; Burmølle, M.; Hentzer, M.; Haagensen, J.A.J.; Hougen, H.P.; Calum, H.; Madsen, K.G.; Moser, C.; Molin, S.; et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 2005, 151, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Givskov, M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1300–1307. [Google Scholar] [CrossRef]
- Liu, J.; Hou, J.S.; Li, Y.B.; Miao, Z.Y.; Sun, P.H.; Lin, J.; Chen, W.M. Novel 2-substituted 3-Hydroxy-1,6-dimethylpyridin-4(1H) ones as dual-acting biofilm inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2020, 63, 10921–10945. [Google Scholar] [CrossRef]
- Soukarieh, F.; Williams, P.; Stocks, M.J.; Cámara, M. Pseudomonas aeruginosa quorum sensing systems as drug discovery targets: Current position and future perspectives. J. Med. Chem. 2018, 61, 10385–10402. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef]
- Li, Q.; Mao, S.; Wang, H.; Ye, X. The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors. Mar. Drugs 2022, 20, 488. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Bjarnsholt, T.; Jensen, P.Ø.; Givskov, M.; Høiby, N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: Current and emerging inhibitors. Future Microbiol. 2013, 8, 901–921. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, J. Quorum sensing in Pseudomonas aeruginosa and its relationship to biofilm development. ACS Symp. Ser. 2019, 1323, 1–16. [Google Scholar]
- Das, T.; Manefield, M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e46718. [Google Scholar] [CrossRef]
- Zou, Y.; Nair, S.K. Molecular basis for the recognition of structurally distinct autoinducer mimics by the Pseudomonas aeruginosa LasR quorum-sensing signaling receptor. Chem. Biol. 2009, 16, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wever, W.J.; Walshc, C.T.; Bowers, A.A. Dithiolopyrrolones: Biosynthesis, synthesis, and activity of a unique class of disulfide-containing antibiotics. Nat. Prod. Rep. 2014, 31, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Appleton, D.R.; Copp, B.R.; Kottamide, E. The first example of a natural product bearing the amino acid 4-amino-1,2-dithiolane-4-carboxylic acid (Adt). Tetrahedron Lett. 2003, 44, 8963–8965. [Google Scholar] [CrossRef]
- Stoner, G.D.; Morse, M.A. Isothiocyanates and plant polyphenols as inhibitors of lung and esophageal cancer. Cancer Lett. 1997, 114, 113–119. [Google Scholar] [CrossRef]
- Soledade, M.; Pedras, C.; Suchy, M. Design, synthesis, and antifungal activity of inhibitors of brassilexin detoxification in the plant pathogenic fungus Leptosphaeria maculans. Bioorg. Med. Chem. 2006, 14, 714–723. [Google Scholar]
- Gross, D.; Porzel, A.; Schmidt, J. Phytoalexine mit indolstruktur aus Kohlrabi (Brassica oleracea var. gongylodes)+/Indole phytoalexins from the Kohlrabi (Brassica oleracea var. gongylodes)+. Z. Naturforsch. 1994, 49, 281–285. [Google Scholar]
- Champagne, D.E.; Arnason, J.T.; Philogène, B.J.R.; Campbell, G.; McLachlan, D.G. Photosensitization and feeding deterrence of Euxoa messoria (Lepidoptera: Noctuiidae) by α-terthienyl, a naturally occurring thiophene from the Asteraceae. Cell. Mol. Life Sci. 1983, 40, 577–578. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.Ø.; Rasmussen, T.B.; Christophersen, L.; Calum, H.; Hentzer, M.; Hougen, H.P.; Rygaard, J.; Moser, C.; Eberl, L.; et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiol.-Sgm 2005, 151, 3873–3880. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef]
- Fong, J.; Yuan, M.; Jakobsen, T.H.; Mortensen, K.T.; Delos Santos, M.M.S.; Chua, S.L.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Disulfide bond-containing ajoene analogues as novel quorum sensing inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017, 60, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Rastegarnia, S.; Pordel, M.; Allameh, S. Synthesis, characterization, antibacterial studies and quantum-chemical investigation of the new fluorescent Cr(III) complexes. Arab. J. Chem. 2020, 13, 3903–3909. [Google Scholar] [CrossRef]
- Ramezani, S.; Pordel, M.; Davoodnia, A. Synthesis, spectral, DFT calculations and antibacterial studies of Fe(III) complexes of new fluorescent Schiff bases derived from imidazo[4′,5′:3,4]benzo[1,2-c]isoxazole. Appl. Organomet. Chem. 2018, 32, e4178. [Google Scholar] [CrossRef]
- Rahimizadeh, M.; Pordel, M.; Bakavoli, M.; Bakhtiarpoor, Z.; Orafaie, A. Synthesis of imidazo[4,5-a] acridones and imidazo[4,5-a] acridines as potential antibacterial agents. Monatsh. Chem. 2009, 140, 633–638. [Google Scholar] [CrossRef]
- Mao, S.; Li, Q.; Yang, Z.; Li, Y.; Ye, X.; Wang, H. Design, synthesis, and biological evaluation of benzoheterocyclic sulfoxide derivatives as quorum sensing inhibitors in Pseudomonas aeruginosa. J. Enzym. Inhib. Med. Chem. 2023, 38, 2175820. [Google Scholar] [CrossRef]
- Ye, X.; Moeljadi, A.M.P.; Chin, K.F.; Hirao, H.; Zong, L.; Tan, C.H. Enantioselective sulfoxidation catalyzed by a bisguanidinium diphosphatobisperoxotungstate ion pair. Angew. Chem. Int. Ed. 2016, 55, 7101–7105. [Google Scholar] [CrossRef]
- Frei, R.; Breitbach, A.S.; Blackwell, H.E. 2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa Biofilms. Angew. Chem. Int. Ed. 2012, 51, 5226. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; van Gennip, M.; Jakobsen, T.H.; Christensen, L.D.; Jensen, P.Ø.; Givskov, M. In vitro screens for quorum sensing inhibitors and in vivo confirmation of their effect. Nat. Protoc. 2010, 5, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Köte, M.; Nielsen, J.; Eberl, L.; Givskov, M. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 2005, 187, 1799–1814. [Google Scholar] [CrossRef]
- Williams, P.; Camara, M.; Hardman, A.; Swift, S.; Milton, D.; Hope, V.J.; Winzer, K.; Middleton, B.; Pritchard, D.I.; Bycroft, B.W. Quorum sensing and the population-dependent control of virulence. Philos. Trans. R. Soc. B 2000, 355, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.A.; McKnight, S.L.; Kuznetsova, M.S.; Pesci, E.C.; Manoil, C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 2002, 184, 6472–6480. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.Y.; Chua, S.L.; Chen, Y.; Rice, S.A.; Kjelleberg, S.; Nielsen, T.E.; Yang, L.; Givskova, M. Identification of five structurally unrelated quorum-sensing inhibitors of Pseudomonas aeruginosa from a natural-derivative database. Antimicrob. Agents Chemother. 2013, 57, 5629–5641. [Google Scholar] [CrossRef]
- Hamood, A.N.; Griswold, J.; Colmer, J. Characterization of elastase-deficient clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 1996, 64, 3154–3160. [Google Scholar] [CrossRef] [PubMed]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.K.; Käppeli, O.; Fiechter, A.; Reiser, J. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol. 1991, 173, 4212–4219. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).