Research Progress on Cannabinoids in Cannabis (Cannabis sativa L.) in China
Abstract
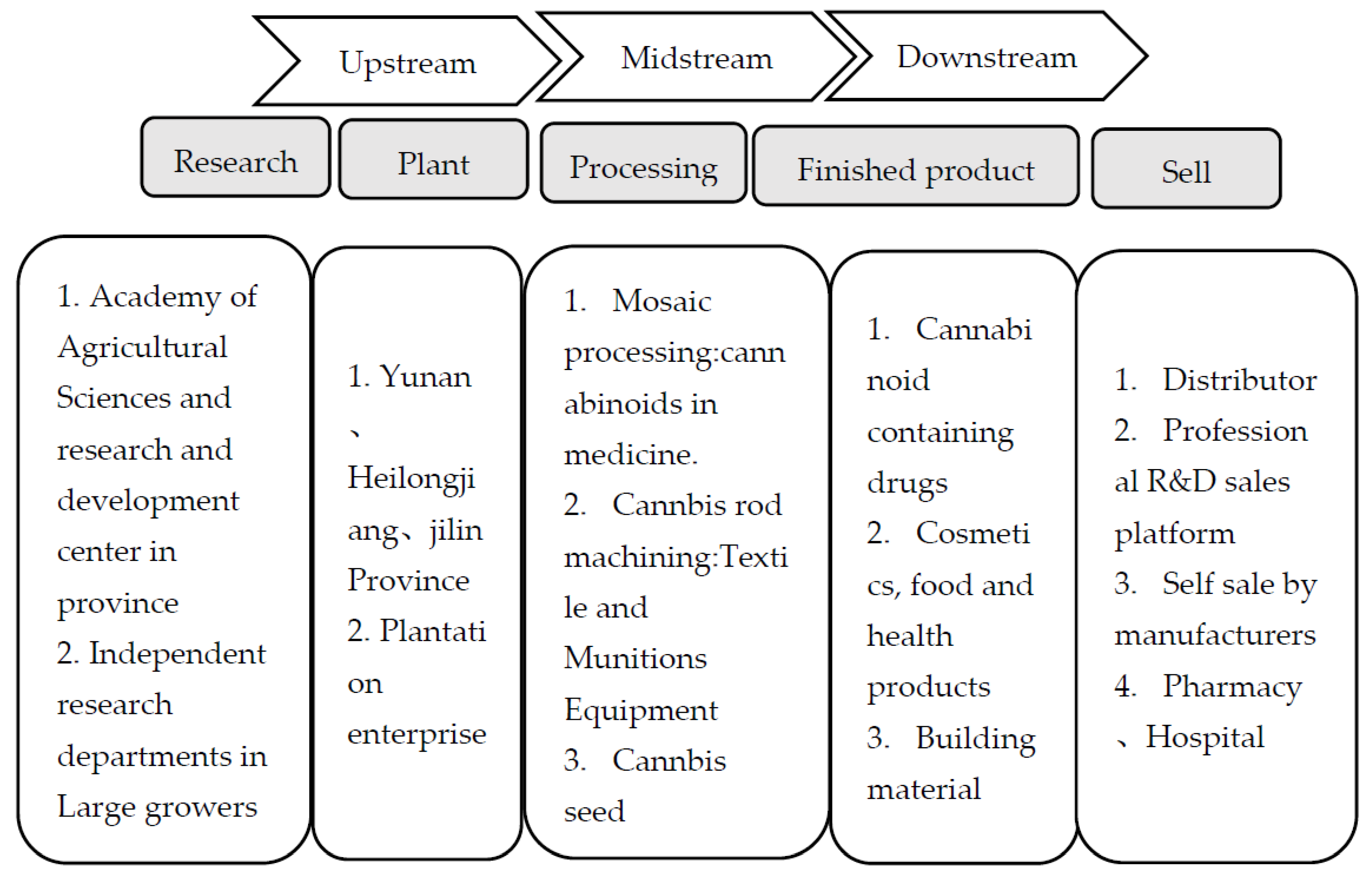
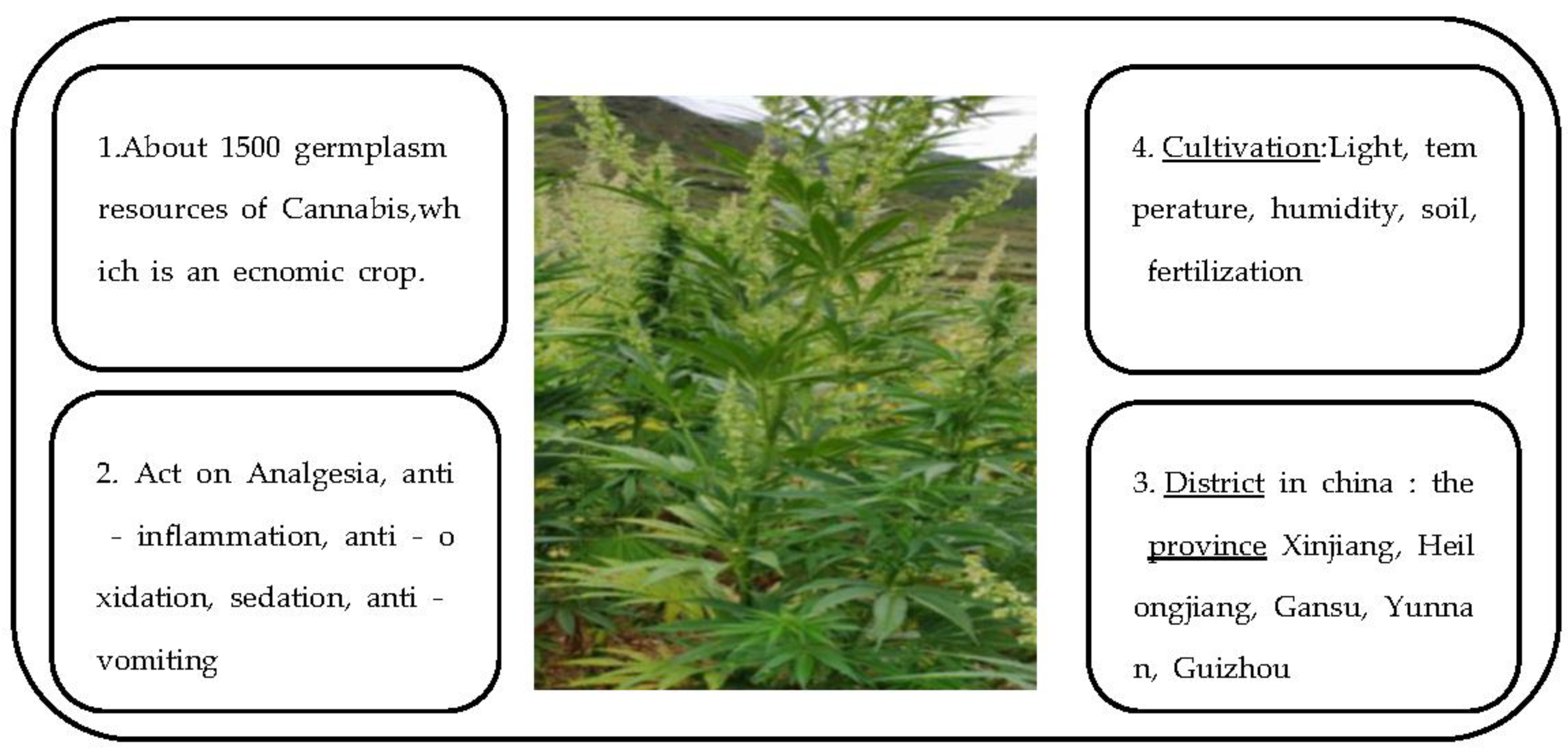
1. Introduction
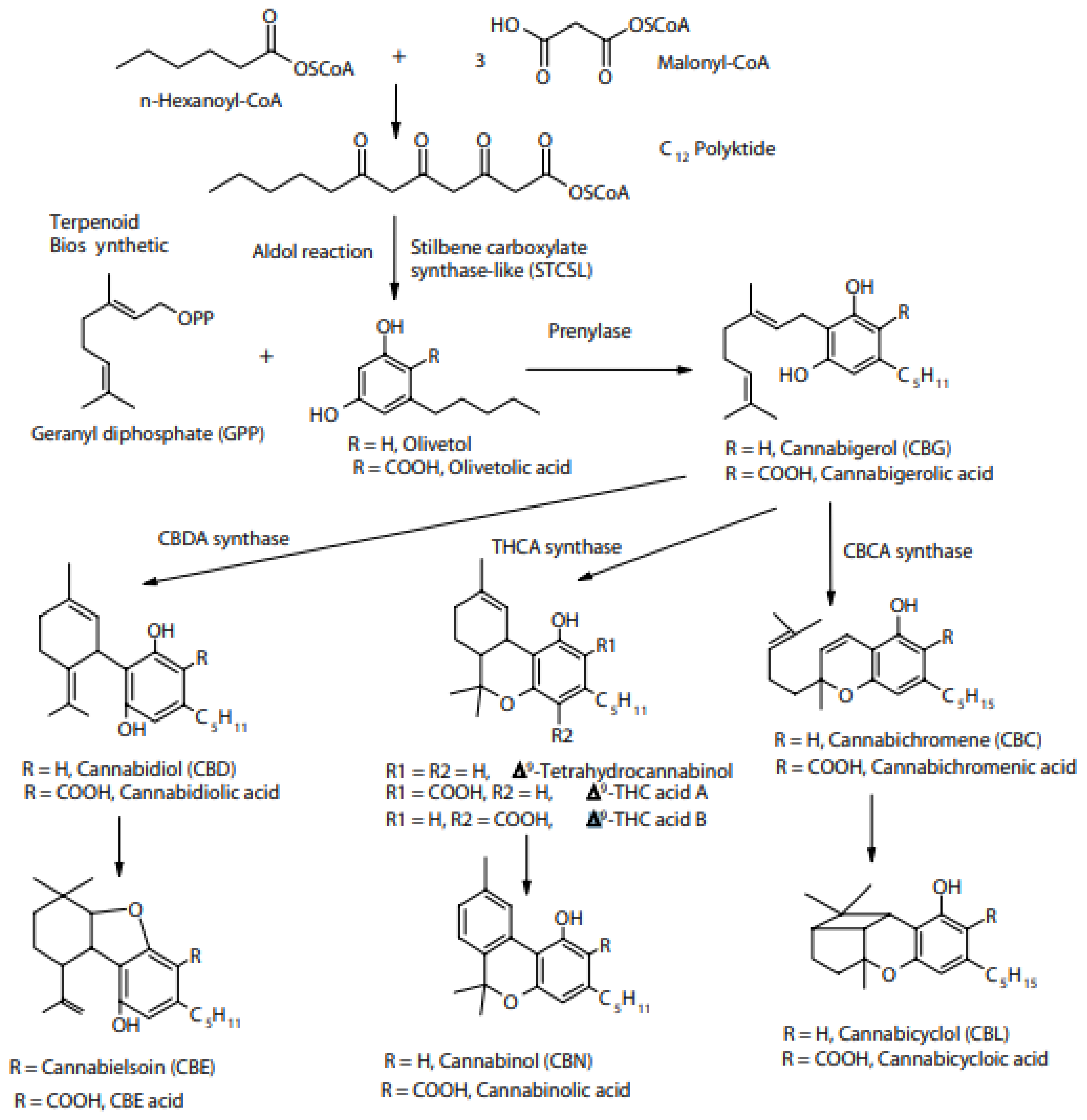
2. Main Cannabinoid Constituents
2.1. Cannabidiol (CBD)
2.2. Tetrahydrocannabinol (THC)
2.3. Cannabichromene (CBC)
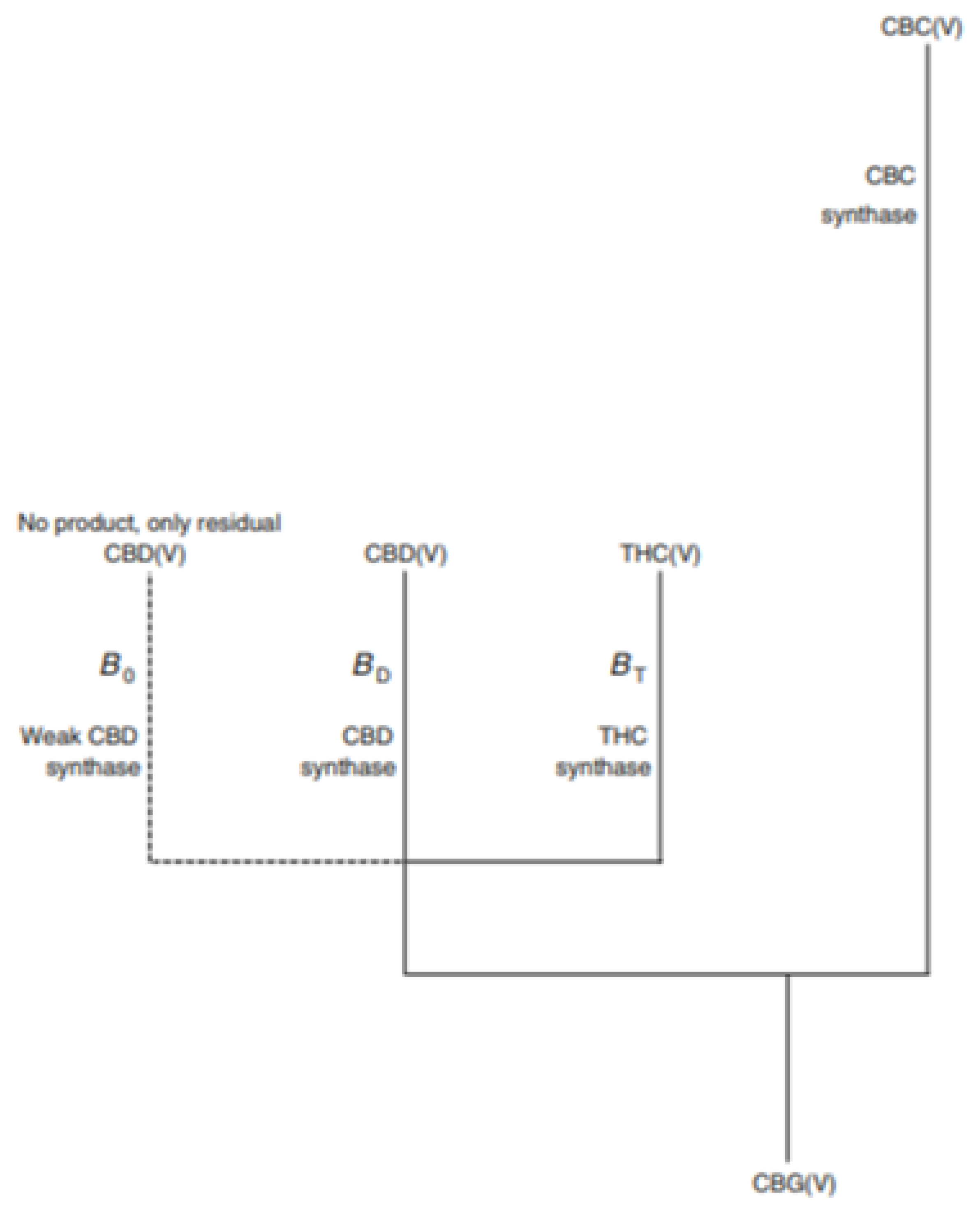
2.4. Cannabigerol (CBG)
2.5. CBN (Cannabinol)
2.6. Cannabidivarin (CBDV)
2.7. Tetrahydrocannabivarin (THCV)
2.8. Delta-8,Tetrahydrocannabinol Acid (∆8-THC)
2.9. Cannabidiolic Acid (CBDA), Tetrahydrocannabinol Acid (THCA)
3. China’s Policy on Cannabis
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandra, S.; Lata, H.; ElSohly, M.A. (Eds.) Cannabis sativa L.-Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, F.A.; Dusek, D.; Seigler, D.S.; Haney, A.W. Photosynthesis and cannabinoid content of temperate and tropical populations of Cannabis sativa. Biochem. Syst. Ecol. 1975, 3, 15–18. [Google Scholar] [CrossRef]
- Bócsa, I.; Máthé, P.; Hangyel, L. Effect of nitrogen on tetrahydrocannabinol (THC) content in hemp (Cannabis sativa L.) leaves at different positions. J. Int. Hemp. Assoc. 1997, 4, 80–81. [Google Scholar]
- Cole, M.D. The Analysis of Controlled Substances; Godin Lyttle Press: Salt Lake City, UT, USA, 2003; pp. 49–72. [Google Scholar]
- Croteau, R. Biosynthesis and catabolism of monoterpenes. Chem. Rev. 1987, 87, 929–954. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Hammond, K.M.; Micheler, M. The inheritance of chemical phenotype in Cannabis sativa L. (III): Variation in cannabichromene proportion. Euphytica 2008, 165, 293–311. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Fellermeier, M.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Biosynthesis of cannabinoids. Incorporation experiments with 13C-labeled glucoses. Eur. J. Biochem. 2001, 268, 1596–1604. [Google Scholar] [CrossRef]
- Sanchez, I.J.F.; Verpoorte, R. Polyketide Synthases in Cannabis sativa L., Chapter III: Polyketide Synthase Activities and Biosynthesis of Cannabinoids and Flavonoids in Cannabis sativa L. plants; PrintPartners Ipskamp BV: Amsterdam, The Netherlands, 2008; pp. 58–59. [Google Scholar]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef]
- Kojoma, M.; Seki, H.; Yoshida, S.; Muranaka, T. DNA polymorphisms in the tetrahydrocannabinolic acid (THCA) synthase gene in “drug-type” and “fiber-type” Cannabis sativa L. Forensic Sci. Int. 2006, 159, 132–140. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef]
- Mahlberg, P.G.; Kim, E.S. Accumulation of cannabinoids in glandular trichomes of Cannabis (Cannabaceae). J. Ind. Hemp. 2004, 9, 15–36. [Google Scholar] [CrossRef]
- Marks, M.D.; Tian, L.; Wenger, J.P.; Omburo, S.N.; Soto-Fuentes, W.; He, J.; Gang, D.R.; Weiblen, G.D.; Dixon, R.A. Identification of candidate genes affecting D9 -tetrahydrocannabinol biosynthesis in Cannabis sativa. J. Exp. Bot. 2009, 60, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanuš, L.O. Cannabidiol—Recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Ross, S.A.; Slade, D.; Ahmed, S.A.; Zulfiqar, F.; ElSohly, M.A. Isolation and characterization of new cannabis constituents from a high potency variety. Planta Med. 2008, 74, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Shahidi, F. Cannabis and cannabis edibles: A review. J. Agric. Food Chem. 2021, 69, 1751–1774. [Google Scholar] [CrossRef]
- Robson, P. Human studies of cannabinoids and medicinal cannabis. In Cannabinoids Handbook of Experimental Pharmacology; Pertwee, R.G., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2005; pp. 719–756. [Google Scholar]
- Shoyama, Y.; Hirano, H.; Nishioka, I. Biosynthesis of propyl cannabinoid acid and its biosynthetic relationship with pentyl and methyl cannabinoid acids. Phytochemistry 1984, 23, 1909–1912. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Taura, F.; Tanaka, Y.; Ishikawa, Y.; Morimoto, S.; Shoyama, Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 2005, 46, 1578–1582. [Google Scholar] [CrossRef]
- Small, E.; Beckstead, H.D. Common cannabinoid phenotypes in 350 stocks of Cannabis. Lloydia 1973, 36, 144–165. [Google Scholar]
- Taura, F.; Tanaka, S.; Taguchi, C.; Fukamizu, T.; Tanaka, H.; Shoyama, Y.; Morimoto, S. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 2009, 583, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.R.; Vieira, J.E.V.; Aucélio, J.G.; Valio, I.F.M. Influence of photoperiodism on cannabinoid content of Cannabis sativa L. Bull. Narc. 1978, 30, 67–68. [Google Scholar] [PubMed]
- Yamauchi, T.; Shoyama, Y.; Aramaki, H.; Azuma, T.; Nishioka, I. Tetrahydrocannabinolic acid, a genuine substance of tetrahydrocannabinol. Chem. Pharm. Bull. 1967, 15, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure-Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Ieb, A.; Ehs, B.; Ab, A.; Mk, A.; Am, A. A comparative phytochemical profiling of essential oils isolated from three hemp (Cannabis sativa L.) cultivars grown in central-northern Morocco. Biocatal. Agric. Biotechnol. 2022, 42, 102327. [Google Scholar]
- Zheng, H.; Chen, B.; Rao, J. Nutraceutical potential of industrial hemp (Cannabis sativa L.) extracts: Physicochemical stability and bioaccessibility of cannabidiol (CBD) nanoemulsions. Food Funct. 2022, 13, 4502–4512. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Piazza, L. Ultrasound-assisted extraction of oil from hempseed (Cannabis sativa L.): Part 1. J. Sci. Food Agric. 2022, 102, 732–739. [Google Scholar] [CrossRef]
- Gildea, L.; Ayariga, J.A.; Ajayi, O.S.; Xu, J.; Villafane, R.; Samuel-Foo, M. Cannabis sativa CBD Extract Shows Promising Antibacterial Activity against Salmonella typhimurium and S. newington. Molecules 2022, 27, 2669. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, G.; Yemis, O. Modification of hemp seed protein isolate (Cannabis sativa L.) by high-intensity ultrasound treatment. Part 1: Functional properties. Food Chem. 2022, 375, 131843. [Google Scholar] [CrossRef]
- Todd, J.; Song, H.; Acker, R.V. Does pollination alter the cannabinoid composition and yield of extracts from hemp (Cannabis sativa L. cv. Finola) flowers? Ind. Crops Prod. 2022, 183, 114989. [Google Scholar] [CrossRef]
- Wise, J. FDA approves its first cannabis based medicine. BMJ 2018, 361, k2827. [Google Scholar] [CrossRef] [PubMed]
- Chasiotis, V.; Tsakirakis, A.; Termentzi, A.; Machera, K.; Filios, A. Drying and quality characteristics of Cannabis sativa L. inflorescences under constant and time-varying convective drying temperature schemes. Therm. Sci. Eng. Prog. 2022, 28, 101076. [Google Scholar] [CrossRef]
- Welling, M.T.; Liu, L.; Shapter, T.; Raymond, C.A.; King, G.J. Characterisation of cannabinoid composition in a diverse Cannabis sativa L. germplasm collection. Euphytica 2016, 208, 463–475. [Google Scholar] [CrossRef]
- Kostanda, E.; Khatib, S. Biotic stress caused by Tetranychus urticae mites elevates the quantity of secondary metabolites, cannabinoids and terpenes, in Cannabis sativa L. Ind. Crops Prod. 2022, 176, 114331. [Google Scholar] [CrossRef]
- Roman, M.G.; Cheng, Y.; Kerrigan, S. Evaluation of tetrahydrocannabinolic acid (THCA) synthase polymorphisms for distinguishing between marijuana and hemp. J. Forensic Sci. 2022, 67, 1370–1381. [Google Scholar] [CrossRef]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Benković, E. Cannabinoid content in industrial hemp (Cannabis sativa L.) varieties grown in Slovenia. Ind. Crops Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Oomah, B.D.; Busson, M.; Godfrey, D.V. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–34. [Google Scholar] [CrossRef]
- Capasso, R.; Borrelli, F.; Aviello, G.; Romano, B.; Scalisi, C.; Capasso, F.; Izzo, A.A. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br. J. Pharmacol. 2008, 154, 1001–1008. [Google Scholar] [CrossRef]
- Kim, A.L.; Yun, Y.J.; Choi, H.W.; Hong, C.H.; Shim, H.J.; Lee, J.H.; Kim, Y.C. Profiling Cannabinoid Contents and Expression Levels of Corresponding Biosynthetic Genes in Commercial Cannabis (Cannabis sativa L.) Cultivars. Plants 2022, 11, 3088. [Google Scholar] [CrossRef]
- Gibson, L.P.; Karoly, H.C.; Ellingson, J.M.; Klawitter, J.; Sempio, C.; Squeri, J.E.; Bryan, L.A.D.; Bidwell, C.; Hutchison, K.E. Effects of cannabidiol in cannabis flower: Implications for harm reduction. Addict. Biol. 2022, 27, e13092. [Google Scholar] [CrossRef]
- Taura, F.; Morimoto, S.; Shoyama, Y. Purification and Characterization of Cannabidiolic-acid Synthase from Cannabis sativa L. J. Biol. Chem. 1996, 271, 17411–17416. [Google Scholar] [CrossRef]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. Febs Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.E.; Xiao, L.; Zhao, Y.X.; Ferguson, D.K.; Hueber, F.; Bera, S.; Wang, Y.F.; Zhao, L.C.; Liu, C.J.; Li, C.S. A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J. Ethnopharmacol. 2006, 108, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Kojoma, M.; Iida, O.; Makino, Y. DNA fingerprinting of Cannabis sativa using inter-simple sequence repeat (ISSR) amplification. Planta Med. 2002, 68, 60–63. [Google Scholar] [CrossRef]
- Eric, Murillo-Rodríguez, Diana Cannabidiol, a constituent of Cannabis sativa, modulates sleep in rat. FEBS Lett. 2006, 580, 4337–4345. [CrossRef]
- Jett, J.; Stone, E.; Warren, G.; Cummings, K.M. Cannabis use, lung cancer, and related issues. J. Thorac. Oncol. 2018, 13, 480–487. [Google Scholar] [CrossRef]
- Izquierdo, I.; Berardi, O. Effect of cannabidiol and of other Cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacology 1973, 28, 95–102. [Google Scholar] [CrossRef]
- Hemphill, J.K.; Turner, J.C.; Mahlberg, P.G. Cannabinoid Content of Individual Plant Organs From Different Geographical Strains of Cannabis sativa L. J. Nat. Prod. 1980, 43, 112–122. [Google Scholar] [CrossRef]
- Hillestad, A.; Wold, J.K.; Engen, T. Water-soluble glycoproteins from Cannabis sativa (Thailand). Phytochemistry 1977, 16, 1953–1956. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef]
- Hillestad, A.; Wold, J.K.; Paulsen, B.S. Structural studies of water-soluble glycoproteins from Cannabis sativa L. Carbohydr. Res. 1977, 57, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.C.; Mahlberg, P.G. Effects of sample treatment on chromatographic analysis of cannabinoids in Cannabis sativa L. (Cannabaceae). J. Chromatogr. A 1984, 283, 165–171. [Google Scholar] [CrossRef]
- Vogelmann, A.F.; Turner, J.C.; Mahlberg, P.G. Cannabinoid Composition in Seedlings Compared to Adult Plants of Cannabis sativa. J. Nat. Prod. 1988, 51, 1075–1079. [Google Scholar] [CrossRef]
- Gobira, P.H.; Vilela, L.R.; BDC Gonçalves. Cannabidiol, a Cannabis sativa constituent, inhibits cocaine-induced seizures in mice: Possible role of the mTOR pathway and reduction in glutamate release. Neurotoxicology 2015, 50, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, M.; Yu, J.; Xu, L.; Yu, L.; Jiang, H.; Gu, Z. Tetrahydrocurcumin protects against nonalcoholic fatty liver disease by improving lipid metabolism and redox homeostasis. J. Funct. Foods 2022, 89, 104957. [Google Scholar] [CrossRef]
- Segelman, A.B.; Varma, S.D.; Wangner, H. Cannabis sativa L. (Marijuana) IX: Lens aldose reductase inhibitory activity of marijuana flavone C-glycosides. J. Pharm. Sci. 2010, 66, 1358–1359. [Google Scholar] [CrossRef]
- Schier, A.R.M.; Ribeiro, N.P.O.; Silva, A.C.O.; Hallak, J.E.C.; Crippa, J.A.S.; Nardi, A.E.; Zuardi, A.W. Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug. Braz. J. Psychiatry 2012, 34, 104–110. [Google Scholar] [CrossRef]
- Deutsch, H.M.; Green, K.; Zalkow, L.H. Isolation of ocular hypotensive agents from Cannabis sativa. J. Clin. Pharmacol. 2013, 21 (Suppl. S1), 479S–485S. [Google Scholar] [CrossRef]
- Group, L.A. New thcv standard for medical cannabis testing. Lab. Asia 2017, 24 (Suppl. S2). [Google Scholar]
- Tzimas, P.S.; Petrakis, E.A.; Halabalaki, M. Effective determination of the principal non-psychoactive cannabinoids in fiber-type Cannabis sativa L. by UPLC-PDA following a comprehensive design and optimization of extraction methodology—ScienceDirect. Anal. Chim. Acta 2021, 1150, 338200. [Google Scholar] [CrossRef]
- Alves, P.; Amaral, C.; Teixeira, N. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 2020, 157, 104822. [Google Scholar] [CrossRef] [PubMed]
- Ridout, K.K.; Young-Wolff, K.C.; Ridout, S.J. A change in blood carbamazepine levels associated with cannabis use: Implications for clinical practice. J. Clin. Psychiatry 2021, 82, 33002. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Vento, F.; Alibrando, F. Cannabis sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. A 2020, 1637, 461864. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Citti, C.; Luongo, L. Isolation of a High-Affinity Cannabinoid for the Human CB1 Receptor from a Medicinal Cannabis sativa Variety: Δ-Tetrahydrocannabutol, the Butyl Homologue of Δ-Tetrahydrocannabinol. J. Nat. Prod. 2019, 83, 88–98. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids. Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2017; Volume 69, pp. 1–36. [Google Scholar]
- Foster, B.C.; Abramovici, H.; Harris, C.S. Cannabis and cannabinoids: Kinetics and interactions. Am. J. Med. 2019, 132, 1266–1270. [Google Scholar] [CrossRef]
- Mariana, K.; Juliane, D.; Silveira, G.F. Effects of cannabidiol, a Cannabis sativa constituent, on oral wound healing process in rats: Clinical and histological evaluation. Phytother. Res. 2018, 32, 2275–2281. [Google Scholar]
- Anna, B.S.; Marika, P.; Simone, T. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in europe and china. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Sorrentino, G. Introduction to emerging industrial applications of cannabis (Cannabis sativa L.). Rend. Lincei. Sci. Fis. E Nat. 2021, 32, 233–243. [Google Scholar] [CrossRef]
- Small, E.; Beckstead, H.D.; Chan, A. The evolution of cannabinoid phenotypes in Cannabis. Econ. Bot. 1975, 29, 219–232. [Google Scholar] [CrossRef]
- Turna, J.; Balodis, I.; Ameringen, M.V.; Busse, J.W.; MacKillop, J. Attitudes and Beliefs Toward Cannabis Before Recreational Legalization: A Cross-Sectional Study of Community Adults in Ontario. Cannabis Cannabinoid Res. 2020, 7, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Sirignano, C.; Benetti, E.; Marzaroli, V.; Collado, J.A.; de la Vega, L.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis sativa). J. Nat. Prod. 2022, 85, 1089–1097. [Google Scholar] [CrossRef]
- De Vita, S.; Finamore, C.; Chini, M.G.; Saviano, G.; De Felice, V.; De Marino, S.; Lauro, G.; Casapullo, A.; Fantasma, F.; Trombetta, F.; et al. Phytochemical Analysis of the Methanolic Extract and Essential Oil from Leaves of Industrial Hemp Futura 75 Cultivar: Isolation of a New Cannabinoid Derivative and Biological Profile Using Computational Approaches. Plants 2022, 11, 1671. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, B.; Huang, J.; Tian, K.; Shen, C. Prospects of Blockchain Technology in China’s Industrial Hemp Industry. J. Nat. Fibers 2023, 20, 2160406. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Q.; Hou, P.; Li, F.; Guo, S.; Song, W.; Zhang, H.; Liu, X.; Zhang, S.; Zhang, J.; et al. Stilbenoids and cannabinoids from the leaves of Cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct. 2018, 9, 6608–6617. [Google Scholar] [CrossRef]
- Li, H.L. An archaeological and historical account of cannabis in China. Econ. Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Abdullaev, K. Cannabis and Other Plants With Peculiar Properties on the Silk Road. In The World of the Ancient Silk Road; Routledge: Oxfordshire, UK, 2023; pp. 148–170. [Google Scholar]
- Wen, J.; Meng, F.; Ying, T.; Belhassen, Y. A study of segmentation of cannabis-oriented tourists from China based on motivation. Curr. Issues Tour. 2020, 23, 36–51. [Google Scholar] [CrossRef]
- Brand, E.J.; Zhao, Z. Cannabis in Chinese medicine: Are some traditional indications referenced in ancient literature related to cannabinoids. Front. Pharmacol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Charlebois, S.; Somogyi, S.; Sterling, B. Cannabis-infused food and Canadian consumers’ willingness to consider “recreational” cannabis as a food ingredient. Trends Food Sci. Technol. 2018, 74, 112–118. [Google Scholar] [CrossRef]
- Toth, J.A.; Smart, L.B.; Smart, C.D.; Stack, G.M.; Carlson, C.H.; Philippe, G.; Rose, J.K. Limited effect of environmental stress on cannabinoid profiles in high-cannabidiol hemp (Cannabis sativa L.). GCB Bioenergy 2021, 13, 1666–1674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X. Research Progress on Cannabinoids in Cannabis (Cannabis sativa L.) in China. Molecules 2023, 28, 3806. https://doi.org/10.3390/molecules28093806
Sun X. Research Progress on Cannabinoids in Cannabis (Cannabis sativa L.) in China. Molecules. 2023; 28(9):3806. https://doi.org/10.3390/molecules28093806
Chicago/Turabian StyleSun, Xiangping. 2023. "Research Progress on Cannabinoids in Cannabis (Cannabis sativa L.) in China" Molecules 28, no. 9: 3806. https://doi.org/10.3390/molecules28093806
APA StyleSun, X. (2023). Research Progress on Cannabinoids in Cannabis (Cannabis sativa L.) in China. Molecules, 28(9), 3806. https://doi.org/10.3390/molecules28093806