Total Flavonoids Extracts of Apocynum L. from the Ili River Valley Region at Different Harvesting Periods and Bioactivity Analysis
Abstract
1. Introduction
2. Results and Discussion
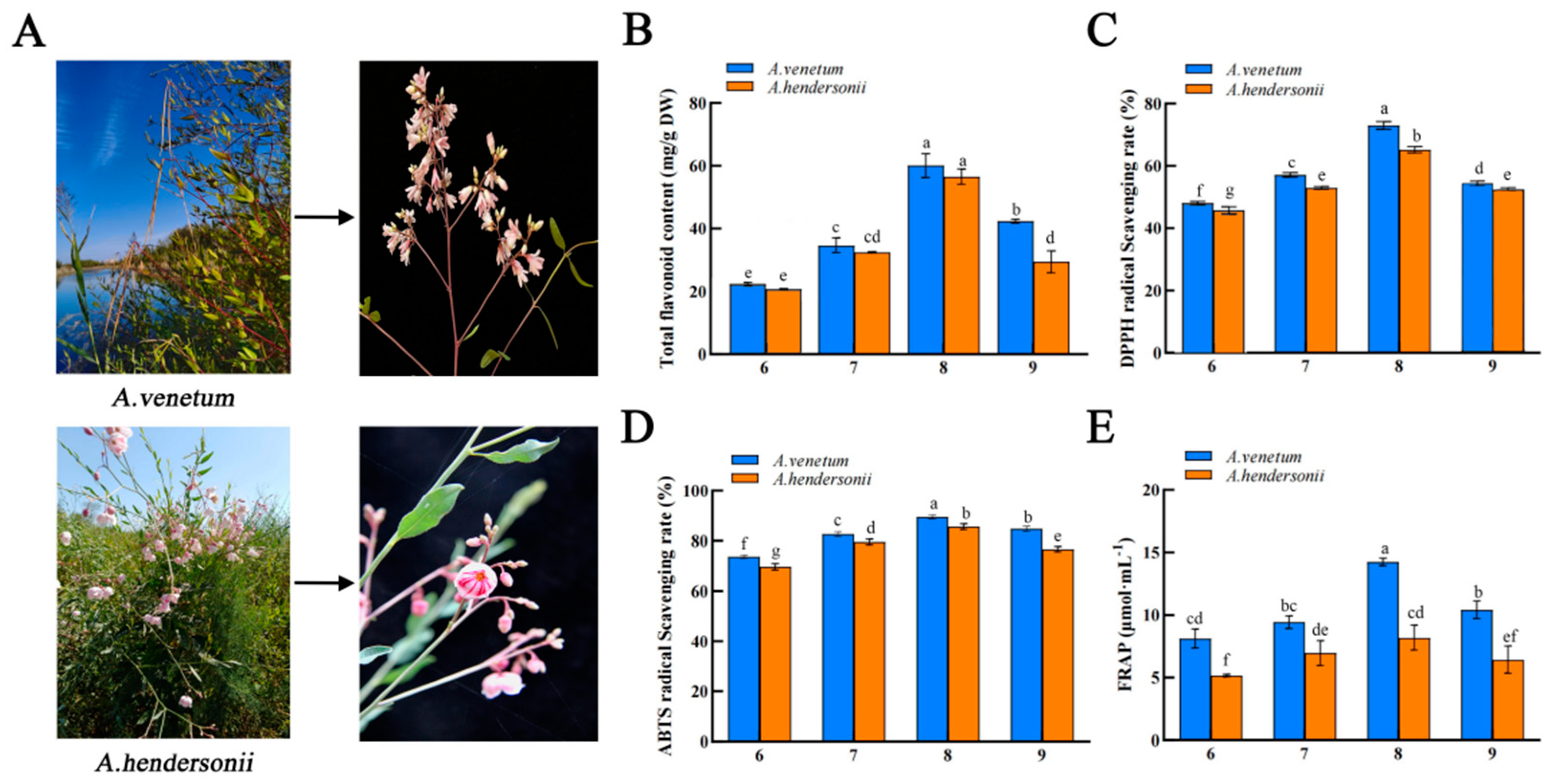
2.1. Total Flavonoid Content Determination
2.2. Antioxidant Activity Analysis of Apocynum L. at Different Harvesting Stages
2.3. Antimicrobial Activity of A. venetum and A. hendersonii Extracts
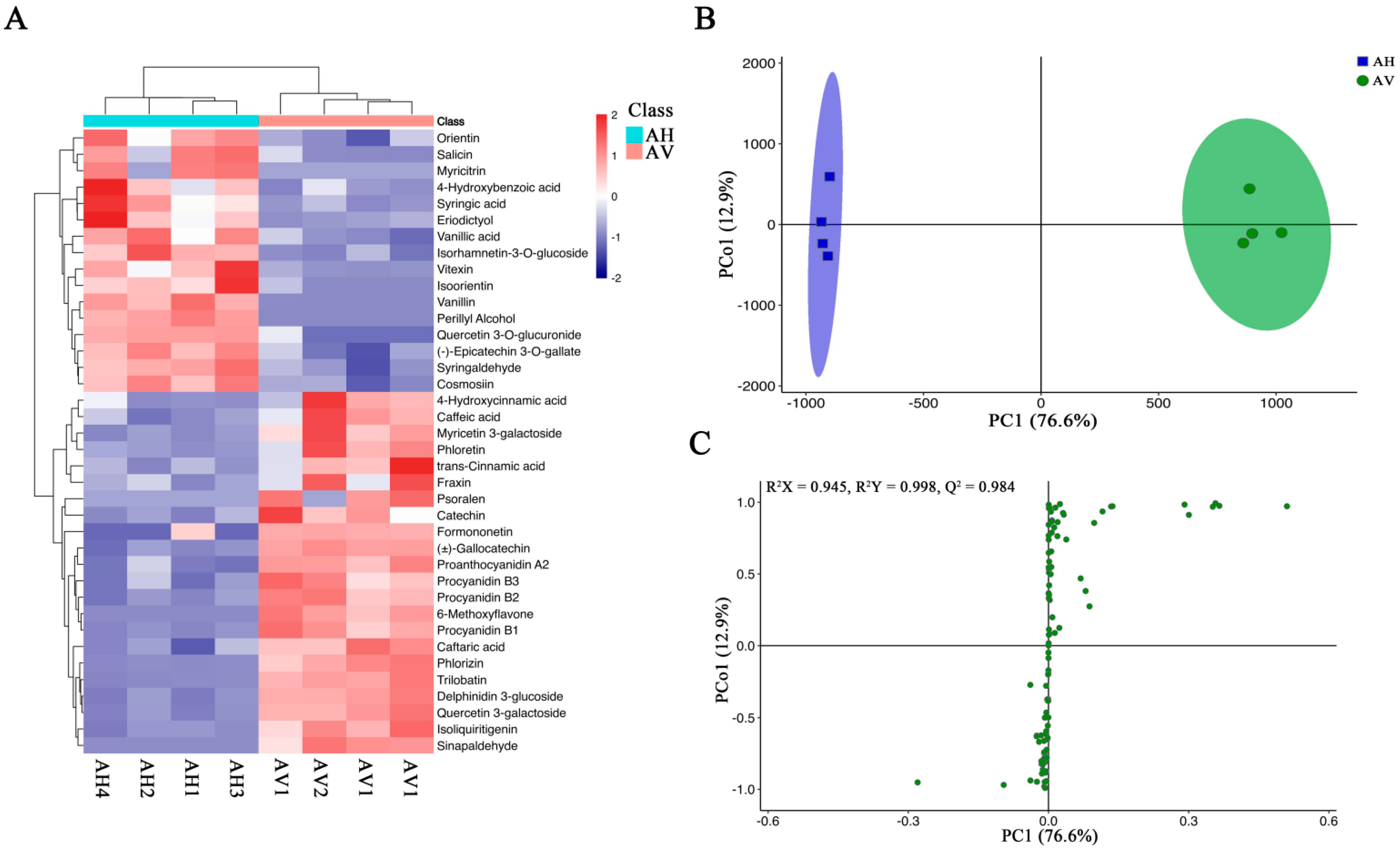
2.4. Metabolomic Profiling of the Apocynum Species
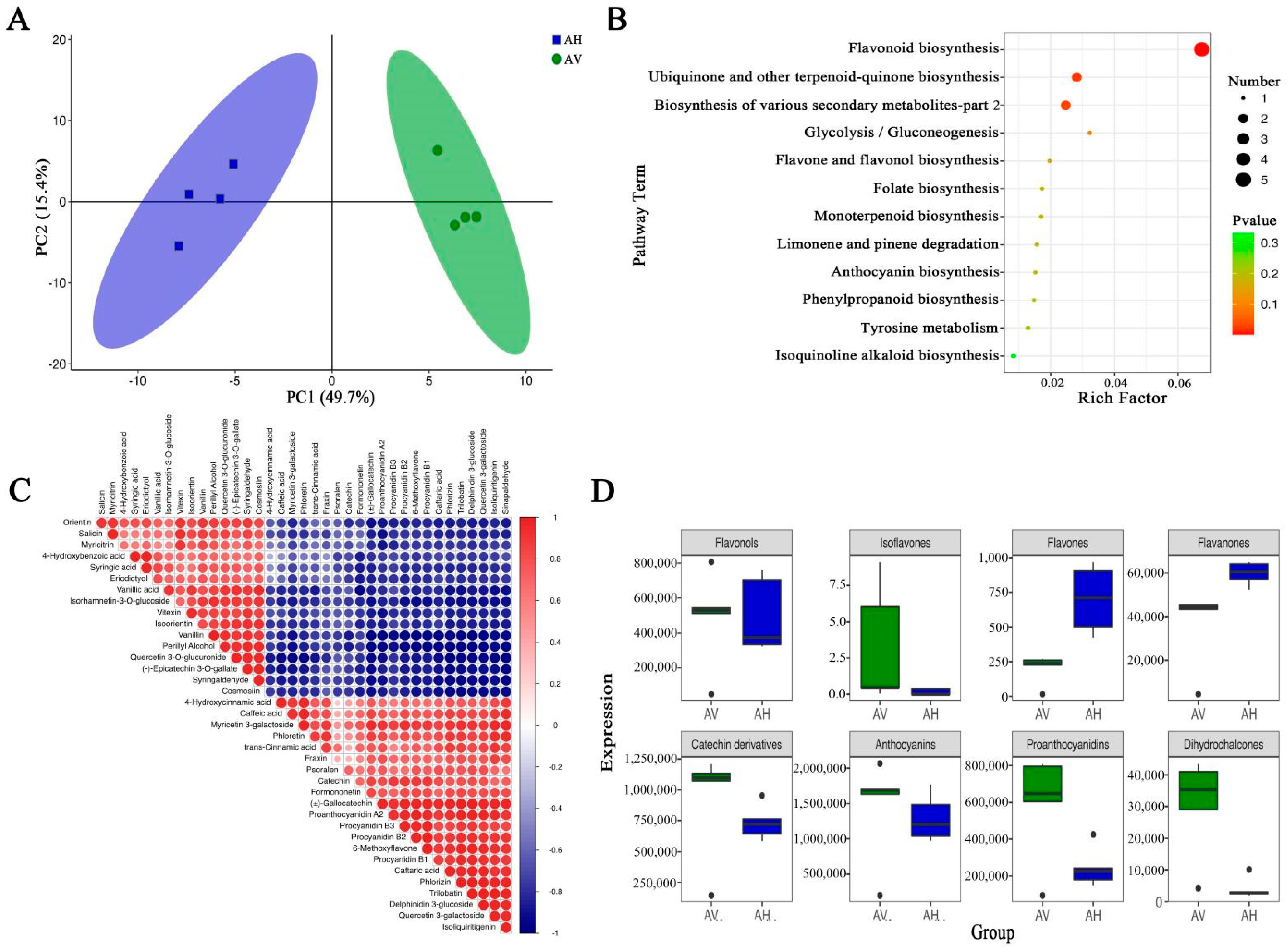
2.5. Orthogonal Projection to Latent Structures–Discriminant Analysis (OPLS-DA) and Principal Component Analysis (PCA) for A. venetum and A. hendersonii
2.6. Screening, Functional Annotation, and Enrichment Analysis of Differential Flavonoid Metabolites
2.7. Differential Flavonoid Metabolites between A. Venetum and A. Hendersonii
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Determination of Total Flavonoids
3.3. Antioxidant Assays
3.4. In Vitro Antimicrobial Assay
3.5. Qualitative and Quantitative Analysis of Flavonoid Constituents of A. venetum and A. hendersonii
3.5.1. Sample Preparation and Extraction
3.5.2. UPLC Conditions and ESI-Q TRAP-MS/MS
3.5.3. Multivariate Data Analysis
3.5.4. Kyoto Encyclopedia of Genes and Genomes (KEGG) Annotations and Metabolic Pathway Analyses of Differential Metabolites
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Les, F.; Cásedas, G.; López, V. Bioactivity of Medicinal Plants and Extracts. Biology 2021, 10, 634. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Xiao, J. 2-Polyphenols: Absorption, Bioavailability, and Metabolomics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Southen, UK, 2018; pp. 45–67. ISBN 978-0-12-813572-3. [Google Scholar]
- Cvejić, J.; Atanacković Krstonošić, M.; Mikulić, M.; Miljić, U. Polyphenols. In Nutraceutical and Functional Food Components; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–312. ISBN 978-0-323-85052-0. [Google Scholar]
- Carocho, M.; Ferreira, I.C.F.R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Iwasa, M.; Marunaka, Y. Metabolic Functions of Flavonoids: From Human Epidemiology to Molecular Mechanism. Neuropeptides 2021, 88, 102163. [Google Scholar] [CrossRef]
- Wang, X.; Ding, G.; Liu, B.; Wang, Q. Flavonoids and Antioxidant Activity of Rare and Endangered Fern: Isoetes Sinensis. PLoS ONE 2020, 15, e0232185. [Google Scholar] [CrossRef] [PubMed]
- Aibibaihan, M.-Z.; Zhang, J.; Zhu, J.; Wang, G.-P.; Li, X.-J.; Qiu, Y.-J.; He, W.; Wang, L. Comprehensive analysis of components in Chinese medicines derived from Apocynum venetum and Poacynum pictum in Xinjiang based on HPLC fingerprint and chemometrics. J. Chin. Mater. Med. 2021, 46, 3886–3892. [Google Scholar] [CrossRef]
- Abubakar, A.S.; Gao, G.; Zhu, A. Apocynum Venetum, A Bast Fiber Plant with Medicinal Significances and Potentials for Drought Tolerance and Phytoremediation Studies—A Review. J. Nat. Fibers 2021, 1–13. [Google Scholar] [CrossRef]
- Gao, G.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Liu, N.; Yu, C.; Zhu, A. Genomic Survey, Transcriptome, and Metabolome Analysis of Apocynum venetum and Apocynum hendersonii to Reveal Major Flavonoid Biosynthesis Pathways. Metabolites 2019, 9, 296. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Y.; Jiang, C.; Jiang, C.; Yang, F.; Yang, F.; Chen, Z.; Chen, Z.; Li, Z.; Li, Z. Apocynum Venetum Leaf Extract Protects against H2O2-Induced Oxidative Stress by Increasing Autophagy in PC12 Cells. Biomed. Rep. 2020, 13, 6. [Google Scholar] [CrossRef]
- Du, S.; Huang, H.; Li, X.; Zhai, L.; Zhu, Q.; Zheng, K.; Song, X.; Xu, C.; Li, C.; Li, Y.; et al. Anti-Inflammatory Properties of Uvaol on DSS-Induced Colitis and LPS-Stimulated Macrophages. Chin. Med. 2020, 15, 43. [Google Scholar] [CrossRef]
- Yamatsu, A.; Yamashita, Y.; Maru, I.; Yang, J.; Tatsuzaki, J.; Kim, M. The Improvement of Sleep by Oral Intake of GABA and Apocynum venetum Leaf Extract. J. Nutr. Sci. Vitaminol. 2015, 61, 182–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ma, J.-L.; Chen, C.; Huang, P.; Ji, J.-H.; Wu, D.; Ren, L.-Q. Apocynum venetum Leaf Extract Alleviated Doxorubicin-Induced Cardiotoxicity through the AKT/Bcl-2 Signaling Pathway. Phytomedicine 2022, 94, 153815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, Z.; Chang, X.; Zhang, C.; Rong, G.; Gao, X.; Zeng, Z.; Wang, C.; Chen, Y.; Rong, Y.; et al. Protective effect of the total flavonoids from Apocynum venetum L. on carbon tetrachloride-induced hepatotoxicity in vitro and in vivo. J. Physiol. Biochem. 2018, 74, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Kwan, C.Y.; Ku, T.C.; Hsieh, W.T.; Wang, H.D.; Nishibe, S.; Dharmani, M.; Mustafa, M.R. Apocynum venetum Leaf Extract, an Antihypertensive Herb, Inhibits Rat Aortic Contraction Induced by Angiotensin II: A Nitric Oxide and Superoxide Connection. J. Ethnopharmacol. 2012, 143, 565–571. [Google Scholar] [CrossRef]
- Xie, W.; Jiang, Z.; Wang, J.; Zhang, X.; Melzig, M.F. Protective Effect of Hyperoside against Acetaminophen (APAP) Induced Liver Injury through Enhancement of APAP Clearance. Chem. Biol. Interact. 2016, 246, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; She, C.; Tian, C.; Tanveer, M.; Wang, L. Storage Period and Different Abiotic Factors Regulate Seed Germination of Two Apocynum Species—Cash Crops in Arid Saline Regions in the Northwestern China. Front. Plant Sci. 2021, 12, 671157. [Google Scholar] [CrossRef]
- Zhuang, Q.; Wu, S.; Feng, X.; Niu, Y. Analysis and Prediction of Vegetation Dynamics under the Background of Climate Change in Xinjiang, China. PeerJ 2020, 8, e8282. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Olesen, J.E.; Sun, X.; Wu, W. Inorganic Nitrogen Leaching from Organic and Conventional Rice Production on a Newly Claimed Calciustoll in Central Asia. PLoS ONE 2014, 9, e98138. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, X.; Wang, T.; Hu, J. Botany, Traditional Uses, Phytochemistry and Pharmacology of Apocynum venetum L. (Luobuma): A Review. J. Ethnopharmacol. 2012, 141, 1–8. [Google Scholar] [CrossRef]
- Gao, G.; Liu, N.; Yu, C.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; Liu, B.; Zhu, A. UPLC-ESI-MS/MS Based Characterization of Active Flavonoids from Apocynum spp. and Anti-Bacteria Assay. Antioxidants 2021, 10, 1901. [Google Scholar] [CrossRef]
- Abubakar, A.S.; Huang, X.; Birhanie, Z.M.; Gao, G.; Feng, X.; Yu, C.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; et al. Phytochemical Composition, Antioxidant, Antibacterial, and Enzyme Inhibitory Activities of Various Organic Extracts from Apocynum hendersonii (Hook.f.) Woodson. Plants 2022, 11, 1964. [Google Scholar] [CrossRef]
- Liang, T.; Yue, W.; Li, Q. Comparison of the Phenolic Content and Antioxidant Activities of Apocynum venetum L. (Luo-Bu-Ma) and Two of Its Alternative Species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef] [PubMed]
- Almaiman, S.A.; Albadr, N.A.; Alsulaim, S.; Alhuthayli, H.F.; Osman, M.A.; Hassan, A.B. Effects of Microwave Heat Treatment on Fungal Growth, Functional Properties, Total Phenolic Content, and Antioxidant Activity of Sorghum (Sorghum bicolor L.) Grain. Food Chem. 2021, 348, 128979. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.M.; Khatoon, S.; Rastogi, S.; Rawat, A.K.S. Determination of Flavonoids, Polyphenols and Antioxidant Activity of Tephrosia Purpurea: A Seasonal Study. J. Integr. Med. 2016, 14, 447–455. [Google Scholar] [CrossRef]
- Ouerghemmi, I.; Bettaieb Rebey, I.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Saidani Tounsi, M. Antioxidant and Antimicrobial Phenolic Compounds from Extracts of Cultivated and Wild-Grown Tunisian Ruta Chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Bahri, L.G.; Neagu, A.-N.; Gostin, I.; Mihasan, M.; Birsa, L.M.; Stefan, M. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol. 2016, 120, 630–637. [Google Scholar] [CrossRef]
- Shen, T.; Hu, F.; Liu, Q.; Wang, H.; Li, H. Analysis of Flavonoid Metabolites in Chaenomeles Petals Using UPLC-ESI-MS/MS. Molecules 2020, 25, 3994. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Yang, H.; Wang, Y.-L.; Chen, X.-X.; Zhang, K.; Wang, Y.-L.; Sun, Y.-F.; Huang, J.; Yang, L.; Wang, J.-H. Determination of Flavonoids Compounds of Three Species and Different Harvesting Periods in Crataegi folium Based on LC-MS/MS. Molecules 2021, 26, 1602. [Google Scholar] [CrossRef]
- Das, M.C.; Samaddar, S.; Jawed, J.J.; Ghosh, C.; Acharjee, S.; Sandhu, P.; Das, A.; Daware, A.V.; De, U.C.; Majumdar, S.; et al. Vitexin Alters Staphylococcus Aureus Surface Hydrophobicity to Obstruct Biofilm Formation. Microbiol. Res. 2022, 263, 127126. [Google Scholar] [CrossRef]
- de Jesus, C.C.M.; de Araújo, M.H.; Simão, T.L.B.V.; Lasunskaia, E.B.; Barth, T.; Muzitano, M.F.; Pinto, S.C. Natural Products from Vitex polygama and Their Antimycobacterial and Anti-Inflammatory Activity. Nat. Prod. Res. 2022, 36, 1337–1341. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Sangi, S.; Arafat, H.; Ragab, E. New Phytochemical Constituent and Bioactivities of Horwoodia dicksoniae and Rumex cyprius. Pharmacogn. Mag. 2016, 12, 165. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A Review of the Most Recent Research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method: Antioxidant Activity in Brazilian Plants. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and Chemical Characterization in Hydrophilic and Lipophilic Compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Yi, Y.; Hua, H.; Sun, X.; Guan, Y.; Chen, C. Rapid Determination of Polysaccharides and Antioxidant Activity of Poria cocos Using Near-Infrared Spectroscopy Combined with Chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118623. [Google Scholar] [CrossRef]
- Yang, G.; Liang, K.; Zhou, Z.; Wang, X.; Huang, G. UPLC-ESI-MS/MS-Based Widely Targeted Metabolomics Analysis of Wood Metabolites in Teak (Tectona grandis). Molecules 2020, 25, 2189. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A Comparative Metabolomics Study of Flavonoids in Sweet Potato with Different Flesh Colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]

| Antioxidant Assay | Correlation Coefficient |
|---|---|
| DPPH | 0.926 ** |
| ABTS | 0.923 ** |
| FRAP | 0.734 * |
| Compounds | Class | Average (AH) | Average (AV) | VIP | FC | log2 (FC) |
|---|---|---|---|---|---|---|
| Formononetin | Isoflavones | 0.000 | 0.445 | 0.004 | 0.204 | −2.292 |
| 6-Methoxyflavone | Flavones | 0.000 | 0.629 | 0.006 | 0.000 | −19.262 |
| Vitexin | Flavones | 320.056 | 13.003 | 0.122 | 24.614 | 4.621 |
| Isoorientin | Flavones | 59.500 | 3.760 | 0.052 | 15.823 | 3.984 |
| Orientin | Flavones | 138.480 | 44.195 | 0.068 | 3.133 | 1.648 |
| Isoliquiritigenin | Flavanones | 0.413 | 1.422 | 0.007 | 0.291 | −1.783 |
| Eriodictyol | Flavanones | 191.398 | 52.812 | 0.079 | 3.624 | 1.858 |
| Myricetin 3-galactoside | Flavonols | 2479.295 | 27,784.466 | 1.156 | 0.089 | −3.486 |
| Cosmosiin | Flavonols | 1430.489 | 262.041 | 0.247 | 5.459 | 2.449 |
| Myricitrin | Flavonols | 31.270 | 0.000 | 0.037 | 9.065 | 4.898 |
| Quercetin 3-galactoside | Flavonols | 48,353.573 | 296,554.439 | 3.655 | 0.163 | −2.617 |
| Quercetin 3-O-glucuronide | Flavonols | 168,298.272 | 20,235.55 | 2.800 | 8.317 | 3.056 |
| Isorhamnetin-3-O-glucoside | Flavonols | 3351.903 | 479.293 | 0.387 | 6.993 | 2.806 |
| Delphinidin 3-glucoside | Anthocyanins | 32,306.137 | 188,173.056 | 2.911 | 0.172 | −2.542 |
| Proanthocyanidin A2 | Proanthocyanidins | 148.867 | 556.478 | 0.148 | 0.268 | −1.902 |
| Procyanidin B1 | Proanthocyanidins | 4123.930 | 38,822.697 | 1.370 | 0.106 | −3.235 |
| Procyanidin B3 | Proanthocyanidins | 431.080 | 2299.489 | 0.311 | 0.187 | −2.415 |
| Procyanidin B2 | Proanthocyanidins | 193,378.541 | 673,060.920 | 5.103 | 0.287 | −1.799 |
| Phloretin | Dihydrochalcones | 12.653 | 115.965 | 0.071 | 0.109 | −3.196 |
| Trilobatin | Dihydrochalcones | 112.871 | 1210.540 | 0.245 | 0.093 | −3.423 |
| Phlorizin | Dihydrochalcones | 2479.889 | 35,922.788 | 1.340 | 0.069 | −3.857 |
| (-)-Epicatechin 3-O-gallate | Catechin derivatives | 74.905 | 36.312 | 0.045 | 2.063 | 1.045 |
| Catechin | Catechin derivatives | 12,482.780 | 32,814.546 | 0.978 | 0.380 | −1.394 |
| (±)-Gallocatechin | Catechin derivatives | 87,069.706 | 318,346.758 | 3.573 | 0.274 | −1.870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, D.; Gao, G.; Abubakar, A.S.; Hazaisi, H.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; Wang, Y.; Chen, Y.; et al. Total Flavonoids Extracts of Apocynum L. from the Ili River Valley Region at Different Harvesting Periods and Bioactivity Analysis. Molecules 2022, 27, 7343. https://doi.org/10.3390/molecules27217343
Shao D, Gao G, Abubakar AS, Hazaisi H, Chen P, Chen J, Chen K, Wang X, Wang Y, Chen Y, et al. Total Flavonoids Extracts of Apocynum L. from the Ili River Valley Region at Different Harvesting Periods and Bioactivity Analysis. Molecules. 2022; 27(21):7343. https://doi.org/10.3390/molecules27217343
Chicago/Turabian StyleShao, Deyi, Gang Gao, Aminu Shehu Abubakar, Hanipa Hazaisi, Ping Chen, Jikang Chen, Kunmei Chen, Xiaofei Wang, Yue Wang, Yu Chen, and et al. 2022. "Total Flavonoids Extracts of Apocynum L. from the Ili River Valley Region at Different Harvesting Periods and Bioactivity Analysis" Molecules 27, no. 21: 7343. https://doi.org/10.3390/molecules27217343
APA StyleShao, D., Gao, G., Abubakar, A. S., Hazaisi, H., Chen, P., Chen, J., Chen, K., Wang, X., Wang, Y., Chen, Y., Yu, C., & Zhu, A. (2022). Total Flavonoids Extracts of Apocynum L. from the Ili River Valley Region at Different Harvesting Periods and Bioactivity Analysis. Molecules, 27(21), 7343. https://doi.org/10.3390/molecules27217343








