Link between Flavor Perception and Volatile Compound Composition of Dark Chocolates Derived from Trinitario Cocoa Beans from Dominican Republic
Abstract
1. Introduction
2. Results
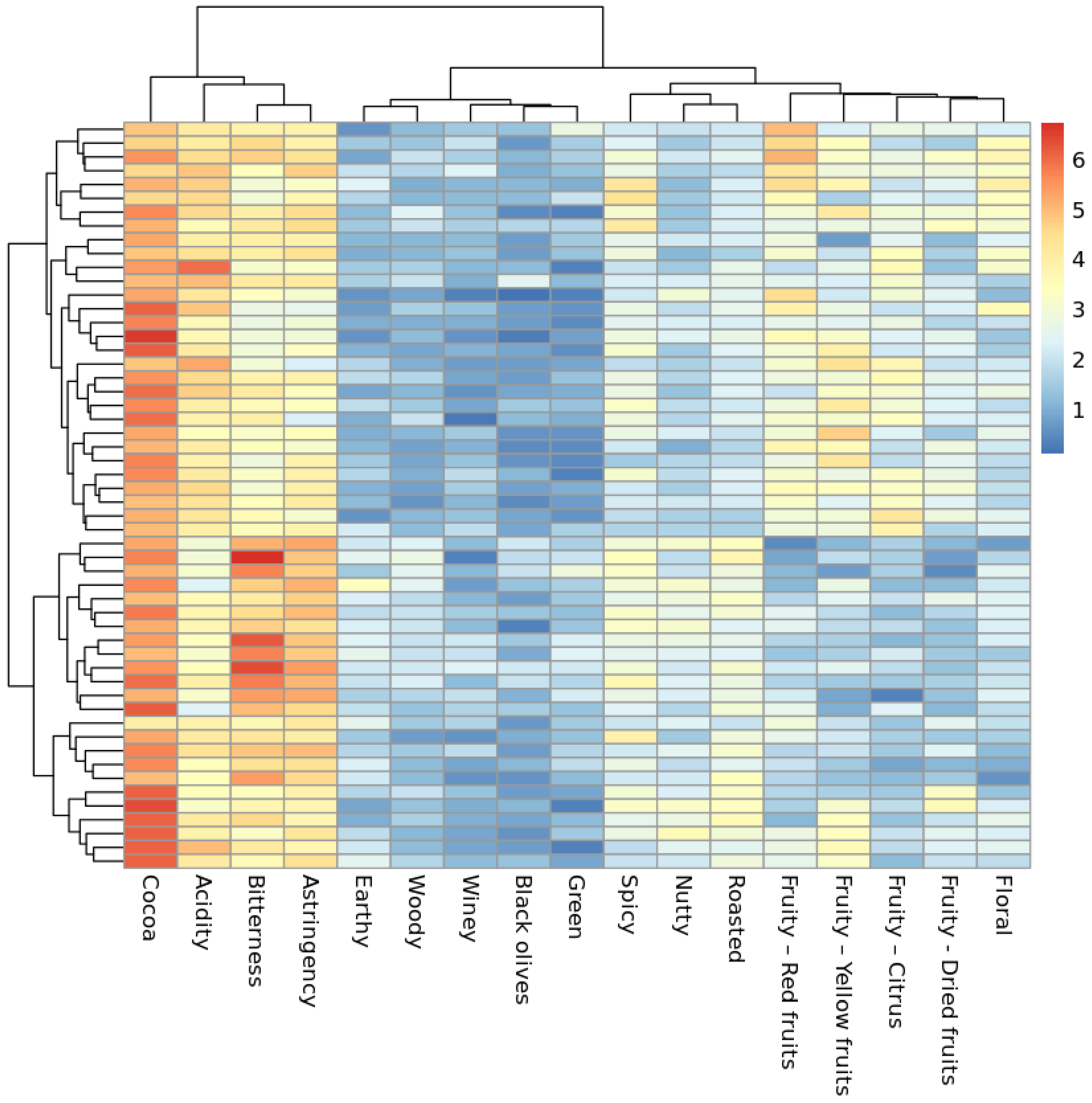
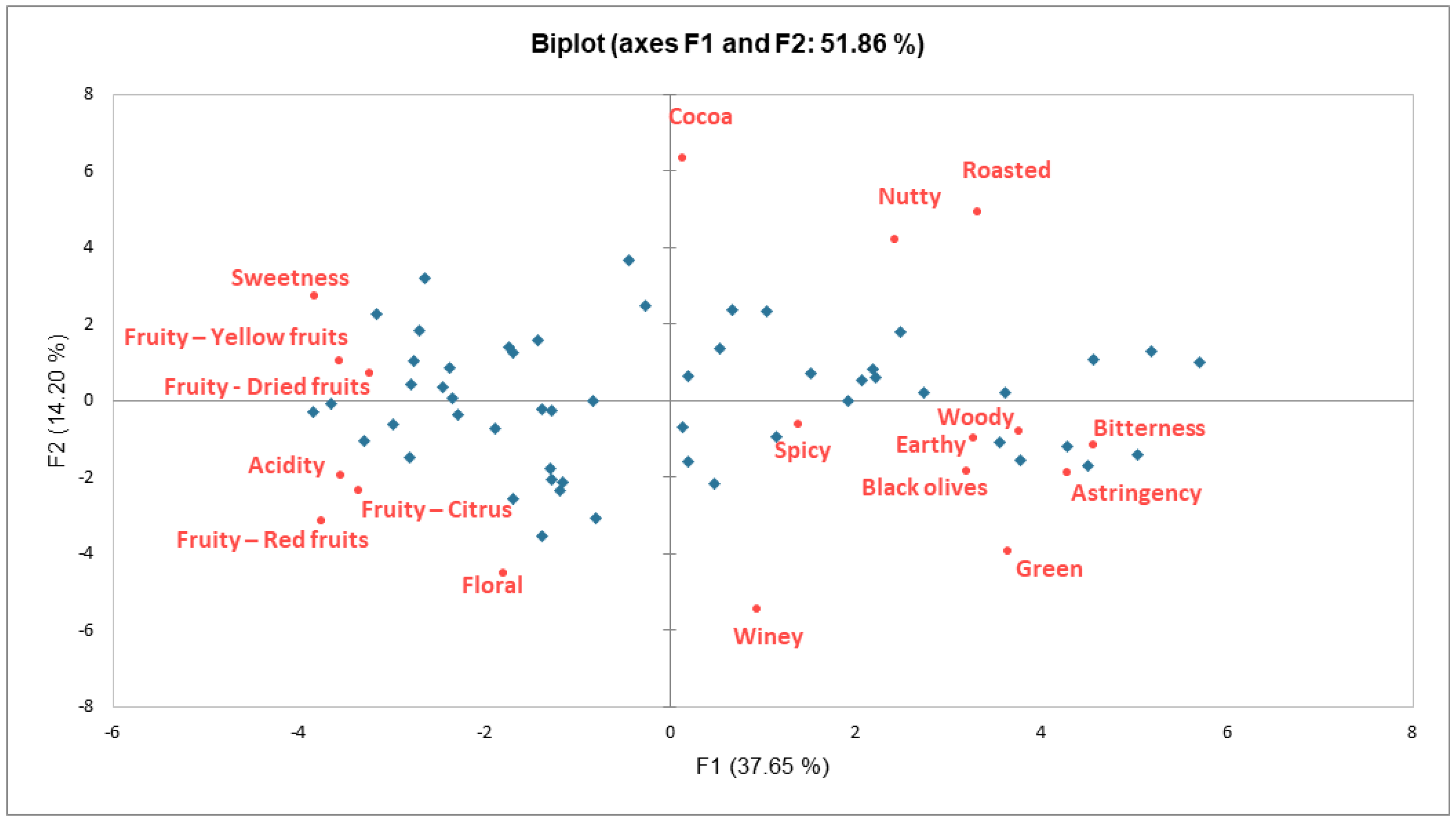
2.1. Sensory Characterization of Dark Chocolate Samples
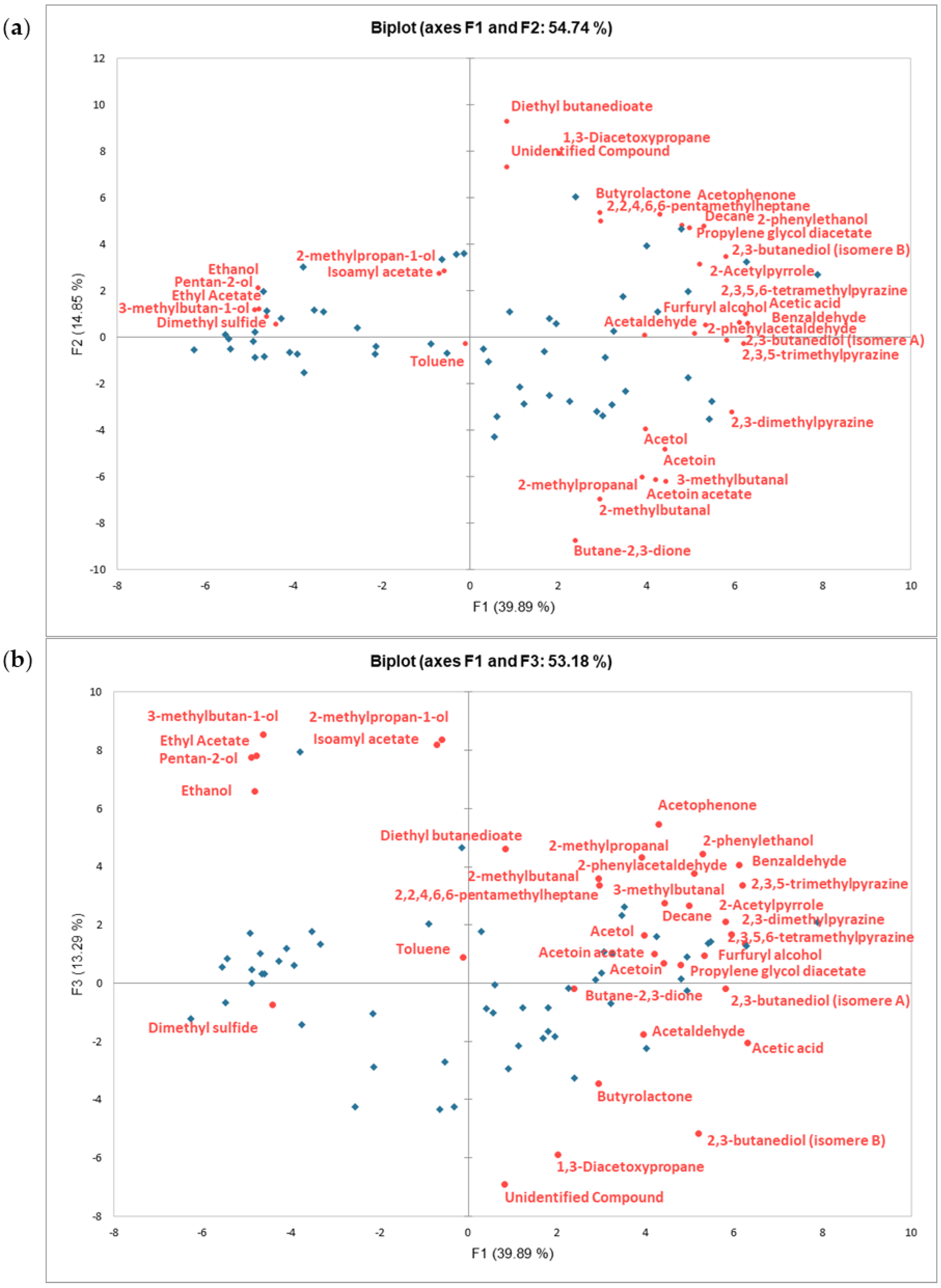
2.2. Identification and Quantification of Volatile Aroma Compounds Present in the Dark Chocolate Samples
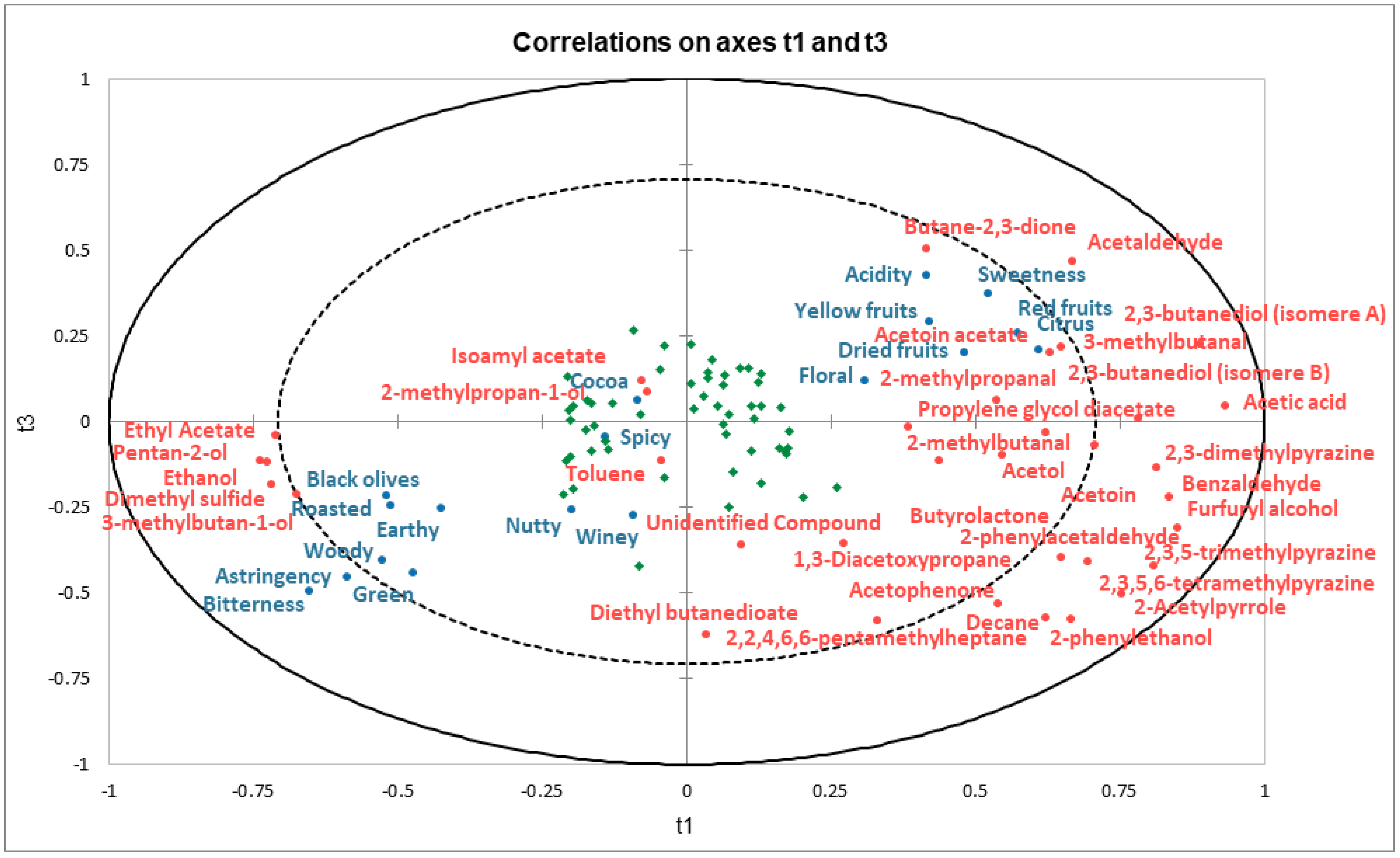
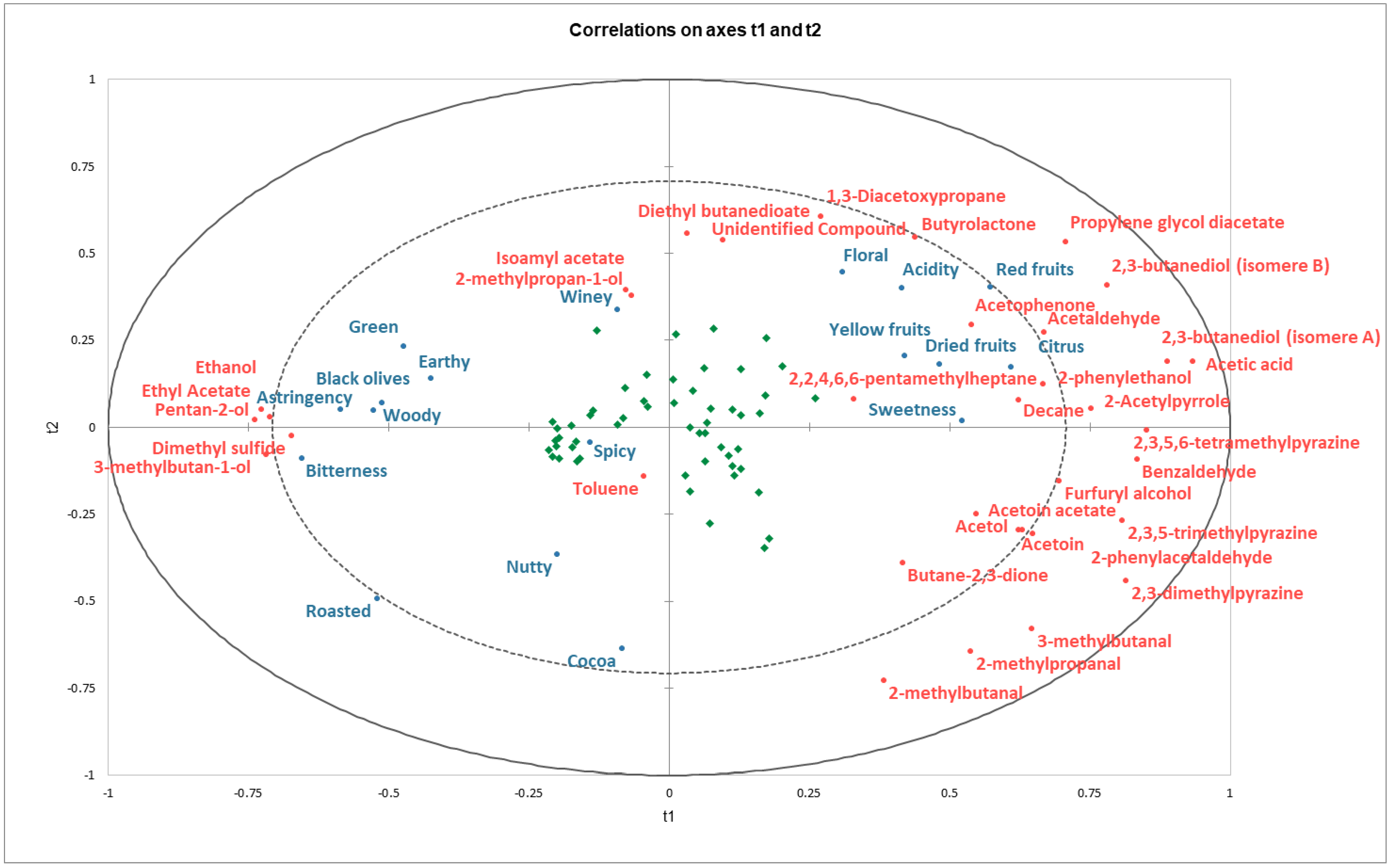
2.3. Identification of Key Aroma Compounds Based on Their Impact on the Sensory Perception of Dark Chocolate Samples
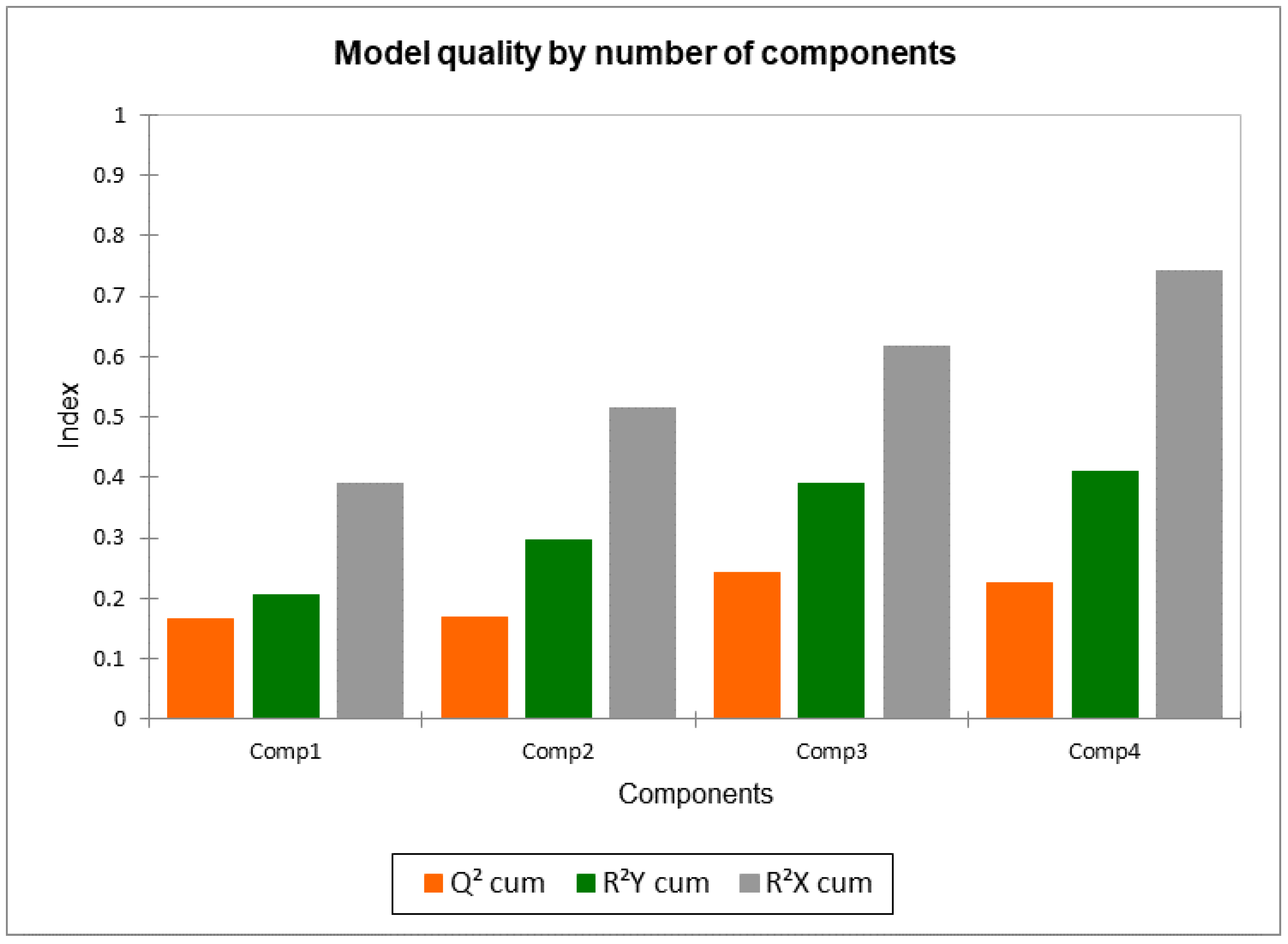
2.4. PLS Predictive Models for Individual Sensory Attributes
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Sensory Attribute | MEAN | STDEV | MAX | MIN |
|---|---|---|---|---|
| Sweetness | 4.245 | 0.535 | 5.125 | 3.125 |
| Bitterness | 4.108 | 0.991 | 6.750 | 2.750 |
| Acidity | 3.997 | 0.686 | 6.000 | 2.375 |
| Astringency | 4.038 | 0.762 | 5.375 | 2.250 |
| Cocoa | 5.447 | 0.529 | 6.563 | 4.000 |
| Yellow fruits | 2.598 | 0.983 | 4.750 | 0.750 |
| Red fruits | 2.703 | 1.033 | 5.125 | 0.500 |
| Citrus | 2.288 | 0.866 | 4.250 | 0.375 |
| Dried fruits | 2.089 | 0.710 | 3.625 | 0.500 |
| Nutty | 2.082 | 0.585 | 3.625 | 1.000 |
| Winey | 1.258 | 0.492 | 2.375 | 0.250 |
| Black olives | 1.050 | 0.506 | 2.500 | 0.125 |
| Green | 1.274 | 0.629 | 2.875 | 0.375 |
| Earthy | 1.627 | 0.625 | 3.375 | 0.625 |
| Floral | 2.232 | 0.739 | 4.000 | 0.625 |
| Woody | 1.525 | 0.525 | 2.750 | 0.625 |
| Spicy | 2.729 | 0.635 | 4.375 | 1.500 |
| Roasted | 2.533 | 0.519 | 3.750 | 1.625 |
| Volatile Compound | MEAN | STDEV | MAX | MIN |
|---|---|---|---|---|
| Acetaldehyde | 0.341 | 0.099 | 0.550 | 0.146 |
| Dimethyl sulfide | 0.092 | 0.024 | 0.145 | 0.048 |
| 2-methylpropanal | 0.319 | 0.074 | 0.489 | 0.166 |
| Ethyl acetate | 29.288 | 19.128 | 82.080 | 6.820 |
| 2-methylbutanal | 0.467 | 0.136 | 0.838 | 0.255 |
| 3-methylbutanal | 1.454 | 0.369 | 2.437 | 0.927 |
| Ethanol | 3.032 | 1.940 | 9.798 | 0.499 |
| 2,2,4,6,6-pentamethylheptane | 20.881 | 7.421 | 41.752 | 7.318 |
| Butane-2,3-dione | 5.061 | 1.038 | 7.746 | 3.044 |
| Decane | 0.229 | 0.140 | 0.602 | 0.017 |
| Toluene | 0.794 | 0.763 | 2.900 | 0.030 |
| Isoamyl acetate | 1.325 | 0.825 | 6.224 | 0.558 |
| 2-methylpropan-1-ol | 1.304 | 0.824 | 6.300 | 0.605 |
| Pentan-2-ol | 2.081 | 1.098 | 5.184 | 0.805 |
| 3-methylbutan-1-ol | 0.745 | 0.394 | 1.872 | 0.213 |
| Acetoin | 15.747 | 3.984 | 25.335 | 8.412 |
| Acetol | 7.988 | 2.160 | 13.707 | 4.305 |
| 2,3-dimethylpyrazine | 0.687 | 0.356 | 1.797 | 0.178 |
| Acetoin acetate | 2.830 | 1.063 | 5.550 | 1.183 |
| 2,3,5-trimethylpyrazine | 2.688 | 1.229 | 5.724 | 0.857 |
| Acetic acid | 297.584 | 67.181 | 416.201 | 177.745 |
| 2,3,5,6-tetramethylpyrazine | 27.517 | 17.194 | 92.471 | 5.735 |
| Benzaldehyde | 1.767 | 0.797 | 3.913 | 0.595 |
| Propylene glycol diacetate | 1.309 | 0.950 | 4.243 | 0.192 |
| 2,3-butanediol (isomere A) | 37.814 | 10.784 | 55.346 | 17.717 |
| 2,3-butanediol (isomere B) | 12.679 | 4.402 | 21.477 | 5.786 |
| Butyrolactone | 1.456 | 0.214 | 2.112 | 1.115 |
| 2-phenylacetaldehyde | 0.568 | 0.357 | 1.909 | 0.145 |
| Acetophenone | 0.500 | 0.242 | 1.292 | 0.155 |
| 1,3-Diacetoxypropane | 4.828 | 7.718 | 34.468 | 0.098 |
| Furfuryl alcohol | 0.166 | 0.038 | 0.248 | 0.108 |
| Diethyl butanedioate | 0.156 | 0.153 | 0.714 | 0.004 |
| Unidentified Compound | 10.470 | 15.051 | 64.091 | 0.331 |
| 2-phenylethanol | 4.958 | 2.097 | 10.207 | 1.677 |
| 2-Acetylpyrrole | 0.451 | 0.209 | 1.033 | 0.165 |


References
- SQ. Global Chocolate Market to Worth 65.49 Billion. SkyQuest Technology Consulting Pvt. Ltd. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/11/17/2558106/0/en/Global-Chocolate-Market-to-Worth-65-49-Billion-Global-Cocoa-Production-is-Pegged-at-4-9-Million-Tons-Europe-Consumes-Over-628-000-Tons-of-Cocoa.html (accessed on 2 March 2023).
- ICCO. Fine or Flavour Cocoa. 2023. Available online: https://www.icco.org/fine-or-flavor-cocoa/ (accessed on 27 February 2023).
- Reed, S. Sensory Analysis of Chocolate Liquor. Manuf. Confect. 2010, 90, 43–52. [Google Scholar]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Jolić, S.M.; Josić, D. Cocoa Processing and Impact on Composition. In Processing and Impact on Active Components in Food; Elsevier: London, UK; Waltham, MA, USA; San Diego, CA, USA, 2015; pp. 605–612. [Google Scholar]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2015, 15, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Kongor, J.E.; Hinneh, M.; Van de Walle, D.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Noor-Soffalina, S.S.; Jinap, S.; Nazamid, S.; Nazimah, S.A.H. Effect of polyphenol and pH on cocoa Maillard-related flavour precursors in a lipidic model system. Int. J. Food Sci. Technol. 2009, 44, 168–180. [Google Scholar] [CrossRef]
- Jinap, S.; Rosli, W.I.W.; Russly, A.R.; Nordin, L.M. Effect of roasting time and temperature on volatile component profiles during nib roasting of cocoa beans (Theobroma cacao). J. Sci. Food Agric. 1998, 77, 441–448. [Google Scholar]
- Rodriguez-Campos, J.; Escalona-Buendía, H.; Contreras-Ramos, S.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Volatile organic compounds and sensory profile of dark chocolates made with cocoa beans fermented with Pichia kudriavzevii and Hanseniaspora thailandica. J. Food Sci. Technol. 2021, 59, 2714–2723. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Key aroma compounds in fermented Forastero cocoa beans and changes induced by roasting. Eur. Food Res. Technol. 2019, 245, 1907–1915. [Google Scholar] [CrossRef]
- Chetschik, I.; Pedan, V.; Chatelain, K.; Kneubühl, M.; Hühn, T. Characterization of the Flavor Properties of Dark Chocolates Produced by a Novel Technological Approach and Comparison with Traditionally Produced Dark Chocolates. J. Agric. Food Chem. 2019, 67, 3991–4001. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Gysel, L.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J. Time-Related Changes in Volatile Compounds during Fermentation of Bulk and Fine-Flavor Cocoa (Theobroma cacao) Beans. J. Food Qual. 2018, 2018, 1758381. [Google Scholar] [CrossRef]
- Rottiers, H.; Sosa, D.A.T.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of volatile compounds and flavor precursors during spontaneous fermentation of fine flavor Trinitario cocoa beans. Eur. Food Res. Technol. 2019, 245, 1917–1937. [Google Scholar] [CrossRef]
- Bastos, V.S.; Uekane, T.M.; Bello, N.A.; de Rezende, C.M.; Paschoalin, V.M.F.; Del Aguila, E.M. Dynamics of volatile compounds in TSH 565 cocoa clone fermentation and their role on chocolate flavor in Southeast Brazil. J. Food Sci. Technol. 2019, 56, 2874–2887. [Google Scholar] [CrossRef]
- Qin, X.W.; Lai, J.X.; Tan, L.H.; Hao, C.Y.; Li, F.P.; He, S.Z.; Song, Y.H. Characterization of volatile compounds in Criollo, Forastero, and Trinitario cocoa seeds (Theobroma cacao L.) in China. Int. J. Food Prop. 2017, 20, 2261–2275. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; De Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K.; Van Durme, J.; Messens, K.; Dewettinck, K.; Pieters, L. Non-volatile and volatile composition of West African bulk and Ecuadorian fine-flavor cocoa liquor and chocolate. Food Res. Int. 2019, 130, 108943. [Google Scholar] [CrossRef]
- Moreira, I.M.D.V.; Vilela, L.D.F.; Santos, C.; Lima, N.; Schwan, R.F. Volatile compounds and protein profiles analyses of fermented cocoa beans and chocolates from different hybrids cultivated in Brazil. Food Res. Int. 2018, 109, 196–203. [Google Scholar] [CrossRef]
- Utrilla-Vázquez, M.; Rodríguez-Campos, J.; Avendaño-Arazate, C.H.; Gschaedler, A.; Lugo-Cervantes, E. Analysis of volatile compounds of five varieties of Maya cocoa during fermentation and drying processes by Venn diagram and PCA. Food Res. Int. 2020, 129, 108834. [Google Scholar] [CrossRef]
- Tran, P.D.; Van de Walle, D.; De Clercq, N.; De Winne, A.; Kadow, D.; Lieberei, R.; Messens, K.; Tran, D.N.; Dewettinck, K.; Van Durme, J. Assessing cocoa aroma quality by multiple analytical approaches. Food Res. Int. 2015, 77, 657–669. [Google Scholar] [CrossRef]
- Escobar, S.; Santander, M.; Zuluaga, M.; Chacón, I.; Rodríguez, J.; Vaillant, F. Fine cocoa beans production: Tracking aroma precursors through a comprehensive analysis of flavor attributes formation. Food Chem. 2021, 365, 130627. [Google Scholar] [CrossRef]
- Magagna, F.; Guglielmetti, A.; Liberto, E.; Reichenbach, S.E.; Allegrucci, E.; Gobino, G.; Bicchi, C.; Cordero, C. Comprehensive Chemical Fingerprinting of High-Quality Cocoa at Early Stages of Processing: Effectiveness of Combined Untargeted and Targeted Approaches for Classification and Discrimination. J. Agric. Food Chem. 2017, 65, 6329–6341. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Res. Int. 2019, 123, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, A.; Hühn, T.; Kneubühl, M.; Chatelain, K.; Rohn, S.; Chetschik, I. Comparison of the Aroma Composition and Sensory Properties of Dark Chocolates Made with Moist Incubated and Fermented Cocoa Beans. J. Agric. Food Chem. 2022, 70, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Kadow, D.; Bohlmann, J.; Phillips, W.; Lieberei, R. Identification of main fine or flavour components in two genotypes of the cocoa tree (Theobroma cacao L.). J. Appl. Bot. Food Qual. 2013, 86, 90–98. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Identification of the Key Aroma Compounds in Cocoa Powder Based on Molecular Sensory Correlations. J. Agric. Food Chem. 2006, 54, 5521–5529. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Song, H.; Guo, J.; Wang, Y.; Yang, H.; Su, X. A comparative study of aroma-active compounds between dark and milk chocolate: Relationship to sensory perception. J. Sci. Food Agric. 2014, 95, 1362–1372. [Google Scholar] [CrossRef]
- Ullrich, L.; Casty, B.; André, A.; Hühn, T.; Steinhaus, M.; Chetschik, I. Decoding the Fine Flavor Properties of Dark Chocolates. J. Agric. Food Chem. 2022, 70, 13730–13740. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Relationship of sensory and instrumental aroma measurements of dark chocolate as influenced by fermentation method, roasting and conching conditions. J. Food Sci. Technol. 2013, 50, 909–917. [Google Scholar] [CrossRef]
- Waehrens, S.S.; Zhang, S.; Hedelund, P.I.; Petersen, M.A.; Byrne, D.V. Application of the fast sensory method ‘Rate-All-That-Apply’ in chocolate Quality Control compared with DHS-GC-MS. Int. J. Food Sci. Technol. 2016, 51, 1877–1887. [Google Scholar] [CrossRef]
- Rottiers, H.; Sosa, D.A.T.; Lemarcq, V.; De Winne, A.; De Wever, J.; Everaert, H.; Jaime, J.A.B.; Dewettinck, K.; Messens, K. A multipronged flavor comparison of Ecuadorian CCN51 and Nacional cocoa cultivars. Eur. Food Res. Technol. 2019, 245, 2459–2478. [Google Scholar] [CrossRef]
- Deuscher, Z.; Andriot, I.; Sémon, E.; Repoux, M.; Preys, S.; Roger, J.M.; Boulanger, R.; Labouré, H.; Le Quéré, J.L. Volatile compounds profiling by using proton transfer reaction-time of flight-mass spectrometry (PTR-ToF-MS). The case study of dark chocolates organoleptic differences. J. Mass Spectrom. 2019, 54, 92–119. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using GC–mass spectrometry and GC–olfactometry. Food Chem. 2009, 113, 208–215. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). PubChem. Compound Summary. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 21 March 2023).
- Indraprastha Institute of Information Technology Delhi (IIIT-Delhi). FlavorDB. FlavorDB Search—A Resource for Exploring Flavor Molecules. 2017. Available online: https://cosylab.iiitd.edu.in/flavordb/search (accessed on 20 March 2023).
- Bonvehí, J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur. Food Res. Technol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Machado Cuellar, L.; Ordoñez Espinosa, C.M.; Angel Sánchez, Y.K.; Guaca Cruz, L.; Suárez Salazar, J.C. Organoleptic quality assessment of Theobroma cacao L. in cocoa farms in northern Huila, Colombia. Acta Agron. 2018, 67, 46–52. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Analytical dataset of Ecuadorian cocoa shells and beans. Data Brief 2018, 22, 56–64. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Pistelli, L.; Flamini, G. Comparison of the Chemical and Sensorial Evaluation of Dark Chocolate Bars. Appl. Sci. 2021, 11, 9964. [Google Scholar] [CrossRef]
- Kouassi, A.D.D.; Koné, K.M.; Assi-Clair, B.J.; Lebrun, M.; Maraval, I.; Boulanger, R.; Fontana, A.; Guehi, T.S. Effect of spontaneous fermentation location on the fingerprint of volatile compound precursors of cocoa and the sensory perceptions of the end-chocolate. J. Food Sci. Technol. 2022, 59, 4466–4478. [Google Scholar] [CrossRef]
- Colonges, K.; Solorzano, R.G.L.; Jimenez, J.; Lahon, M.; Seguine, E.; Calderon, D.; Subia, C.; Sotomayor, I.; Fernández, F.; Lebrun, M.; et al. Variability and genetic determinants of cocoa aromas in trees native to South Ecuadorian Amazonia. Plants People Planet 2022, 4, 618–637. [Google Scholar] [CrossRef]
- Calva-Estrada, S.; Utrilla-Vázquez, M.; Vallejo-Cardona, A.; Roblero-Pérez, D.; Lugo-Cervantes, E. Thermal properties and volatile compounds profile of commercial dark-chocolates from different genotypes of cocoa beans (Theobroma cacao L.) from Latin America. Food Res. Int. 2020, 136, 109594. [Google Scholar] [CrossRef]
- Michel, S.; Baraka, L.F.; Ibañez, A.J.; Mansurova, M. Mass Spectrometry-Based Flavor Monitoring of Peruvian Chocolate Fabrication Process. Metabolites 2021, 11, 71. [Google Scholar] [CrossRef]
- Deuscher, Z.; Gourrat, K.; Repoux, M.; Boulanger, R.; Labouré, H.; Le Quéré, J.-L. Key Aroma Compounds of Dark Chocolates Differing in Organoleptic Properties: A GC-O Comparative Study. Molecules 2020, 25, 1809. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Pearl, J.; Robins, J.M. Confounding and Collapsibility in Causal Inference. Stat. Sci. 1999, 14, 29–46. [Google Scholar] [CrossRef]
- Januszewska, R. Hidden Persuaders in Cocoa and Chocolate: A Flavour Lexicon for Cocoa and Chocolate Sensory Professionals; Elsevier Science: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bonvehi, J.S.; Coll, F.V. Evaluation of bitterness and astringency of polyphenolic compounds in cocoa powder. Food Chem. 1997, 60, 365–370. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Coll, F.V. Parameters affecting the quality of processed cocoa powder: Acidity fraction. Z. Lebensm.-Forsch. A 1997, 204, 287–292. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef]
- Osborne, J.; de Orduña, R.M.; Pilone, G.; Liu, S.-Q. Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef]
- Garcia, L.; Perrin, C.; Nolleau, V.; Godet, T.; Farines, V.; Garcia, F.; Caillé, S.; Saucier, C. Impact of Acetaldehyde Addition on the Sensory Perception of Syrah Red Wines. Foods 2022, 11, 1693. [Google Scholar] [CrossRef]
- Fayeulle, N.; Vallverdu-Queralt, A.; Meudec, E.; Hue, C.; Boulanger, R.; Cheynier, V.; Sommerer, N. Characterization of new flavan-3-ol derivatives in fermented cocoa beans. Food Chem. 2018, 259, 207–212. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Zhang, S.; Marchand, S.; De Revel, G.; Barbe, J.-C. Olfactory impact of dimethyl sulfide on red wine fruity esters aroma expression in model solution. OENO One 2014, 48, 75. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Assi-Clair, B.J.; Koné, M.K.; Kouamé, K.; Lahon, M.C.; Berthiot, L.; Durand, N.; Lebrun, M.; Julien-Ortiz, A.; Maraval, I.; Boulanger, R.; et al. Effect of aroma potential of Saccharomyces cerevisiae fermentation on the volatile profile of raw cocoa and sensory attributes of chocolate produced thereof. Eur. Food Res. Technol. 2019, 245, 1459–1471. [Google Scholar] [CrossRef]
| Compound Group | Compound Name | Odor/Flavor Attributes |
|---|---|---|
| Aldehydes | 2-methylbutanal | Chocolate [35] |
| 2-methylpropanal | Chocolate [35] | |
| 2-phenylacetaldehyde | Berry, geranium, honey [36] | |
| 3-methylbutanal | Chocolate [35] | |
| Acetaldehyde | Tart (acidic), pungent fruity [36] | |
| Benzaldehyde | Nutty, almond [35] | |
| Esters | 1,3-diacetoxypropane | Acetic, fruit [37] |
| Diethyl butanedioate | Cotton, fabric, floral, fruit [36] | |
| Ethyl acetate | Pineapple [14] | |
| Isoamyl acetate | Fruity, banana [36] | |
| Propylene glycol diacetate | Fruit [36] | |
| Alcohols and Phenols | 3-methylbutan-1-ol | Pungent, repulsive taste [36] |
| 2,3-butanediol (isomere A) | Sweet [36] | |
| 2,3-butanediol (isomere B) | Sweet [36] | |
| 2-methylpropan-1-ol | Sweet, whiskey [36] | |
| 2-phenylethanol | Rose, lilac, flowery, caramel [28] | |
| Acetoin | Buttery [36] | |
| Ethanol | - | |
| Furfuryl alcohol | Bitter [36] | |
| Pentan-2-ol | Fuel oil, green [36] | |
| Ketones | Acetoin acetate | Fruit [36] |
| Acetol | Pungent, sweet, caramel-like [36] | |
| Acetophenone | Must, flower, almond, sweet [38] | |
| Butane-2,3-dione | Buttery [36] | |
| Pyrazines | 2,3,5,6-tetramethylpyrazine | Milk-coffee, roasted, chocolate [35] |
| 2,3,5-trimethylpyrazine | Cocoa, roasted, cooked [35] | |
| 2,3-dimethylpyrazine | Cooked, nutty [35] | |
| Other | 2,2,4,6,6-pentamethylheptane | Unspecified |
| 2-acetylpyrrole | Bread, cocoa, hazelnut, licorice, walnut [36] | |
| Acetic acid | Sour, astringent, vinegar [35] | |
| Butyrolactone | Sweet, caramel-like [36] | |
| Decane | Gasoline-like [36] | |
| Dimethyl sulfide | Unpleasant, cabbage-like [36] | |
| Toluene | Fuel-like [36] | |
| Unidentified compound | Unspecified |
| Training Dataset Metrics | Validated Model Performance for Prediction | |||||||
|---|---|---|---|---|---|---|---|---|
| Attribute Model | Number of Components | ROC | Sensitivity | Specificity | Accuracy | 95% CI | Sensitivity | Specificity |
| Bitterness | 4 | 0.8508 | 0.8117 | 0.7300 | 0.7963 | (0.6647, 0.8937) | 0.7714 | 0.8421 |
| Astringency | 3 | 0.9263 | 0.8083 | 0.8608 | 0.7963 | (0.6647, 0.8937) | 0.8214 | 0.7692 |
| Citrus | 1 | 0.8146 | 0.6600 | 0.7283 | 0.7222 | (0.5836, 0.8354) | 0.7692 | 0.6786 |
| Acidity | 3 | 0.7621 | 0.6817 | 0.7008 | 0.7037 | (0.5639, 0.8202) | 0.7500 | 0.6667 |
| Red fruits | 3 | 0.8579 | 0.7058 | 0.7867 | 0.7037 | (0.5639, 0.8202) | 0.7407 | 0.6667 |
| Dried fruits | 2 | 0.7846 | 0.6283 | 0.8250 | 0.6852 | (0.5445, 0.8048) | 0.7273 | 0.6562 |
| Green | 2 | 0.8192 | 0.8225 | 0.6792 | 0.6852 | (0.5445, 0.8048) | 0.7143 | 0.6316 |
| Black olives | 1 | 0.6538 | 0.7342 | 0.5933 | 0.6667 | (0.5253, 0.7891) | 0.6562 | 0.6818 |
| Woody | 4 | 0.8038 | 0.7550 | 0.7500 | 0.6667 | (0.5253, 0.7891) | 0.6071 | 0.7308 |
| Sweetness | 3 | 0.7767 | 0.7042 | 0.7550 | 0.6667 | (0.5253, 0.7891) | 0.6667 | 0.6667 |
| Attribute Model | Variable | VIP | Corr. Coeff. | Attribute Model | Variable | VIP | Corr. Coeff. |
|---|---|---|---|---|---|---|---|
| Bitterness | Acetaldehyde | 100.00 | −0.782 | Dried fruits | Ethyl Acetate | 100.00 | −0.554 |
| Dimethyl sulfide | 64.98 | 0.594 | 3-methylbutan-1-ol | 96.36 | −0.566 | ||
| 2,3-butanediol (isomere A) | 49.53 | −0.694 | Ethanol | 91.13 | −0.439 | ||
| 3-methylbutan-1-ol | 41.43 | 0.598 | Acetaldehyde | 89.82 | 0.505 | ||
| Acetic acid | 37.21 | −0.636 | Pentan-2-ol | 87.1 | −0.553 | ||
| Astringency | Acetaldehyde | 100.00 | −0.749 | Green | Acetaldehyde | 100.00 | −0.571 |
| 3-methylbutanal | 79.16 | −0.632 | Diethyl butanedioate | 86.36 | 0.344 | ||
| 2,3-butanediol (isomere A) | 75.10 | −0.622 | Dimethyl sulfide | 82.36 | 0.529 | ||
| 2-methylpropanal | 72.22 | −0.487 | 3-methylbutanal | 77.51 | −0.589 | ||
| Dimethyl sulfide | 59.42 | 0.531 | Butane-2,3-dione | 60.84 | −0.411 | ||
| Citrus | 2,3-butanediol (isomere B) | 100.00 | 0.596 | Black olives | Dimethyl sulfide | 100.00 | 0.530 |
| Acetic acid | 96.23 | 0.596 | 2,3-butanediol (isomere A) | 92.87 | −0.591 | ||
| 3-methylbutan-1-ol | 91.39 | −0.564 | Acetic acid | 88.45 | −0.486 | ||
| Ethyl Acetate | 88.51 | −0.503 | Pentan-2-ol | 75.66 | 0.310 | ||
| Pentan-2-ol | 87.34 | −0.532 | 2,3-butanediol (isomere B) | 71.44 | −0.340 | ||
| Acidity | Acetaldehyde | 100.00 | 0.603 | Woody | 3-acetyloxypropyl acetate | 100.00 | 0.151 |
| Butyrolactone | 73.30 | 0.451 | Unknown compound | 98.14 | 0.248 | ||
| Isoamyl acetate | 65.34 | 0.179 | Acetaldehyde | 91.19 | −0.561 | ||
| 2,3-butanediol (isomere A) | 64.38 | 0.557 | 2,3-butanediol (isomere A) | 84.28 | −0.626 | ||
| 2,3-butanediol (isomere B) | 64.24 | 0.567 | Butyrolactone | 78.3 | −0.257 | ||
| Red fruits | Acetaldehyde | 100.00 | 0.628 | Sweetness | Acetaldehyde | 100.00 | 0.605 |
| Propylene glycol diacetate | 83.08 | 0.665 | Acetol | 89.51 | −0.042 | ||
| 2,3-butanediol (isomere A) | 57.04 | 0.607 | Dimethyl sulfide | 73.49 | −0.557 | ||
| Dimethyl sulfide | 51.26 | −0.506 | 2,3-butanediol (isomere A) | 67.46 | 0.582 | ||
| 2,3-butanediol (isomere B) | 47.27 | 0.534 | 3-methylbutanal | 59.84 | 0.512 |
| Looped Tested Model Performance | |||||||
|---|---|---|---|---|---|---|---|
| Attribute Model | Accuracy | 95% CI | Sensitivity | Specificity | Variable | VIP | Corr. Coeff. |
| Bitterness | 0.7963 | (0.6647, 0.8937) | 0.8919 | 0.5882 | Acetaldehyde | 100.00 | −0.782 |
| 2,3-butanediol (isomere A) | 67.29 | −0.694 | |||||
| 3-methylbutan-1-ol | 65.25 | 0.598 | |||||
| 2,3-butanediol (isomere B) | 62.41 | −0.548 | |||||
| Dimethyl sulfide | 57.28 | 0.594 | |||||
| Astringency | 0.8148 | (0.6857, 0.9075) | 0.8974 | 0.6000 | 2,3-butanediol (isomere A) | 100.00 | −0.622 |
| Acetaldehyde | 98.52 | −0.749 | |||||
| Acetic acid | 97.44 | −0.519 | |||||
| 2,3-butanediol (isomere B) | 88.46 | −0.378 | |||||
| 3-methylbutan-1-ol | 86.76 | 0.397 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán Penella, S.; Boulanger, R.; Maraval, I.; Kopp, G.; Corno, M.; Fontez, B.; Fontana, A. Link between Flavor Perception and Volatile Compound Composition of Dark Chocolates Derived from Trinitario Cocoa Beans from Dominican Republic. Molecules 2023, 28, 3805. https://doi.org/10.3390/molecules28093805
Guzmán Penella S, Boulanger R, Maraval I, Kopp G, Corno M, Fontez B, Fontana A. Link between Flavor Perception and Volatile Compound Composition of Dark Chocolates Derived from Trinitario Cocoa Beans from Dominican Republic. Molecules. 2023; 28(9):3805. https://doi.org/10.3390/molecules28093805
Chicago/Turabian StyleGuzmán Penella, Santiago, Renaud Boulanger, Isabelle Maraval, Gabi Kopp, Marcello Corno, Bénédicte Fontez, and Angélique Fontana. 2023. "Link between Flavor Perception and Volatile Compound Composition of Dark Chocolates Derived from Trinitario Cocoa Beans from Dominican Republic" Molecules 28, no. 9: 3805. https://doi.org/10.3390/molecules28093805
APA StyleGuzmán Penella, S., Boulanger, R., Maraval, I., Kopp, G., Corno, M., Fontez, B., & Fontana, A. (2023). Link between Flavor Perception and Volatile Compound Composition of Dark Chocolates Derived from Trinitario Cocoa Beans from Dominican Republic. Molecules, 28(9), 3805. https://doi.org/10.3390/molecules28093805






