Evaluation of Different Processes Impact on Flavor of Camellia Seed Oil Using HS-SPME-GC/MS
Abstract
1. Introduction
2. Results and Discussion
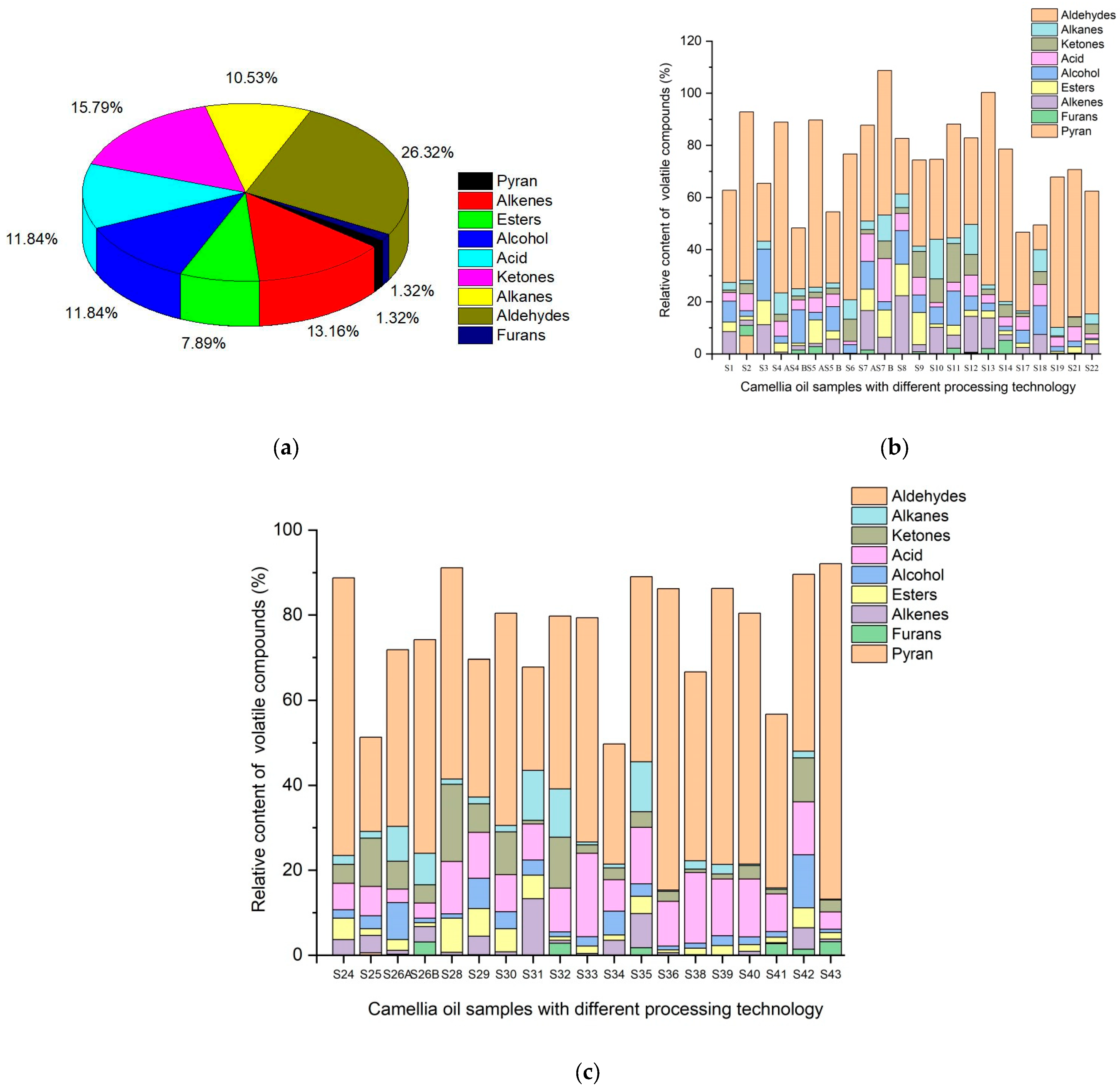
2.1. The Composition of Flavoring Substances of Camellia oleifera
2.2. Characterization of Volatile Flavor Compounds Analyzed by HS-SPME–GC/MS
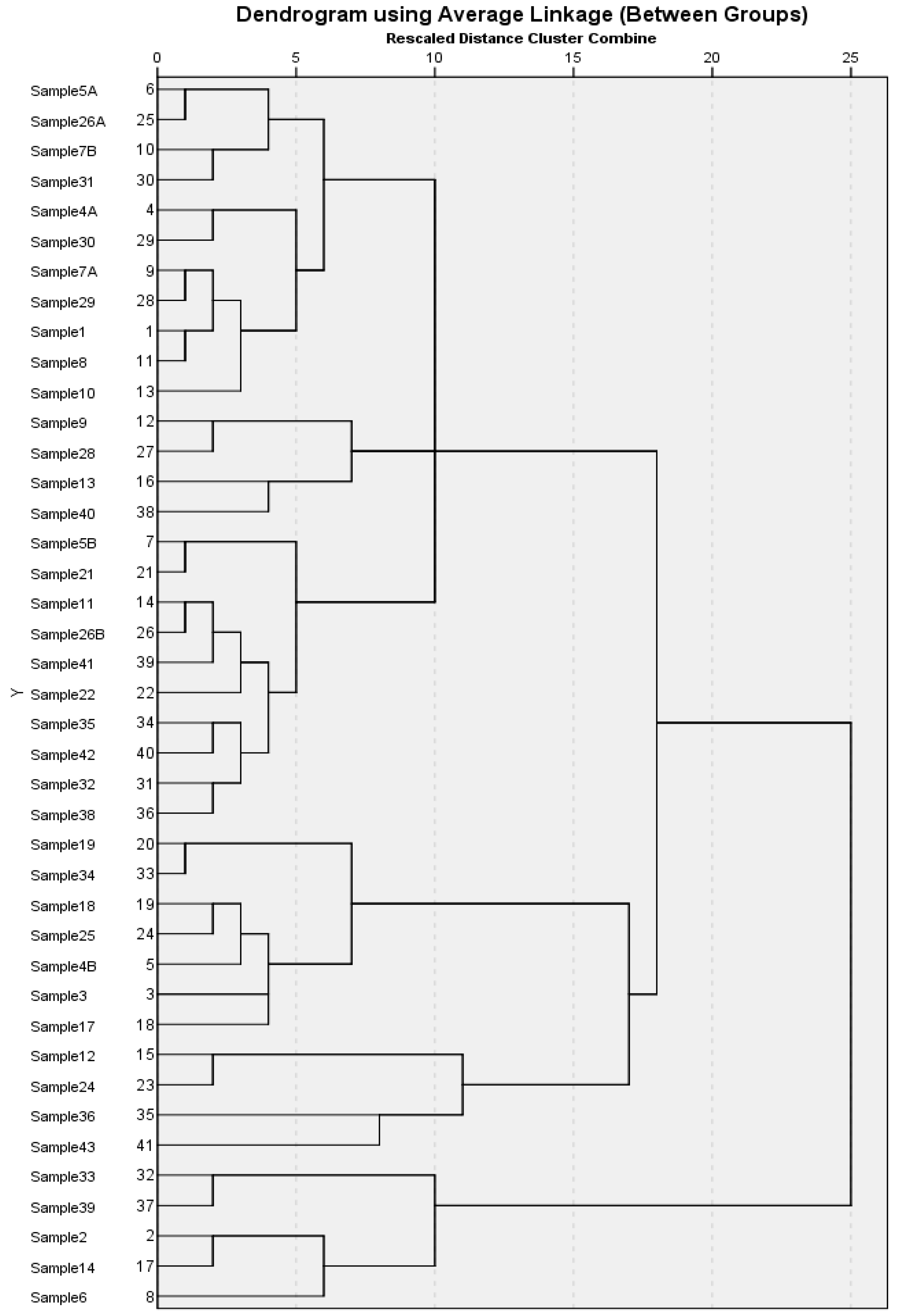
2.3. Principal Component Analysis (PCA), Hierarchical Cluster Analysis (HCA) of GC–MS Data
3. Materials and Methods
3.1. The Preparation of Oil with Different Processing Methods
3.2. Chemicals and Reagents of Flavor Collections
3.3. HS-SPME Analysis
3.4. GC–MS Analysis
3.5. Qualitative and Quantitative Detection of Volatile Compounds
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Morinaga, K. Effects of fruit load on photosynthesis and ribulose 1,5-bisphosphate carboxylase activity in satsuma mandarin (Citrus unshiu marc.) trees under long-term elevated carbon dioxide. Sci. Hortic. 2022, 71, 311–316. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.; Ryan, D.; Zhong, H. Camellia Seed Oil and Tea Oil. In Gourmet and Health Promoting Specialty Oils; Moreau, R., Kamal-Eldin, A., Eds.; AOCS Press: Urbana, IL, USA, 2009; pp. 313–343. [Google Scholar]
- Wang, T.; Wu, H.L.; Long, W.J.; Hu, Y.; Cheng, L.; Chen, A.Q.; Yu, R.Q. Rapid identification and quantification of cheaper vegetable oil adulteration in Camellia seed oil by using excitation-emission matrix fluorescence spectroscopy combined with chemometrics. Food Chem. 2019, 293, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, T.; Wang, Y.; Zhou, B.; Yan, L.; Teng, L.; Zhang, D. New method for effective identification of adulterated Camellia seed oil basing on Camellia oleifera-specific DNA. Arab J. Chem. 2018, 11, 815–826. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Lu, C.C.; Yen, G.C. Beneficial Effects of Camellia seed oil (Camellia oleifera Abel.) on Hepatoprotective and Gastroprotective Activities. J. Nutr. Sci. 2015, 61, S100–S102. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.Q.; Chen, G.C.; Ye, H.; Ma, J. Camellia as an Oilseed Crop. Hortscience 2017, 52, 488–497. [Google Scholar]
- Li, Z.; Liu, A.; Du, Q.; Zhu, W.; Liu, H.; Naeem, A.; Guan, Y.; Chen, L.; Ming, L. Bioactive substances and therapeutic potential of camellia oil: An overview. Food Biosci. 2022, 49, 101855. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Yang, Y.; Ma, X.; Yang, C. Camellia oil (Camellia oleifera Abel.) alleviates gastric injury induced by ethanol associated with modulation of gut microbiota in mice. Oil Crop Sci. 2023, 8, 61–71. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Li, Z.; Liu, R.; Zhang, A.; Xiao, Z.; Deng, S. Simultaneous extraction of oil and tea saponin from Camellia oleifera Abel seeds under subcritical water conditions. Fuel Process. Technol. 2018, 174, 88–94. [Google Scholar] [CrossRef]
- Fang, X.; Fei, X.; Sun, H.; Jin, Y. Aqueous enzymatic extraction and demulsification of camellia seed oil (Camellia oleifera Abel.) and the oil’s physicochemical properties. Eur. J. Lipid Sci. Technol. 2016, 118, 244–251. [Google Scholar] [CrossRef]
- Muangrat, R.; Jirarattanarangsri, W. Physicochemical properties and antioxidant activity of oil extracted from assam tea seeds (Camellia sinensis var. assamica) by supercritical CO2 extraction. J. Food Process. Preserv. 2020, 44, e14364. [Google Scholar] [CrossRef]
- Jirarattanarangsri, W.; Muangrat, R. Comparison of supercritical CO2 and screw press extraction methods for producing oil from Camellia sinensis var. assamica seeds: Physicochemical properties and antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100413. [Google Scholar] [CrossRef]
- Shanying, Z.; Yong-Gui, P.; Lili, Z.; Yang, Y.; Xiaoyan, Z.; Binling, A.; Zhimin, X.; Zhanwu, S. Application of steam explosion in oil extraction of camellia seed (Camellia oleifera Abel.) and evaluation of its physicochemical properties, fatty acid, and antioxidant activities. Food Sci. Nutr. 2019, 7, 1004–1016. [Google Scholar]
- Hu, J.B.; Yang, G.L. Physiochemical characteristics, fatty acid profile and tocopherol composition of the oil from Camellia oleifera Abel cultivated in Henan, China. Grasas Aceites 2018, 69, e255. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Yao, X.H.; Ren, H.D.; Wang, K.L. Determination of fatty acid composition and metallic element content of four Camellia species used for edible oil extraction in China. J. Consum. Food Saf. 2017, 12, 165–169. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.Y.; Zhang, H.; Zheng, H.; Liu, S.P.; Zhang, W.W. Study on physicochemical properties and main constituents of Camellia reticulate Lindl. seed oil. J. Yunnan Agric. Univ. 2013, 28, 102–106. [Google Scholar]
- Zhang, L.; Huang, X.; Li, P.; Na, W.; Jiang, J.; Mao, J.; Zhang, Q. Multivariate adulteration detection for sesame oil. Chemom. Intell. Lab. Syst. 2017, 161, 147–150. [Google Scholar] [CrossRef]
- Zhang, X.F.; Han, Y.Y.; Bao, G.H.; Ling, T.J.; Zhang, L.; Gao, L.P.; Xia, T. A new saponin from tea seed pomace (Camellia oleifera Abel.) and its protective effect on pc12 cells. Molecules 2012, 17, 11721–11728. [Google Scholar] [CrossRef]
- Zheng, H.; Jun, W. Detection of adulteration in camellia seed oil and sesame oil using an electronic nose. Eur. J. Lipid Sci. Technol. 2006, 108, 116–124. [Google Scholar]
- Li, S.; Zhu, X.; Zhang, J.; Li, G.; Su, D.; Shan, Y. Authentication of pure Camellia seed oil by using near infrared spectroscopy and pattern recognition techniques. J. Food Sci. 2012, 77, C374–C380. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, M.; Chen, Y.; Yan, X.; Chen, Q.; Wu, X.; Lin, J.; Xie, M. 1H NMR combined with chemometrics for the rapid detection of adulteration in Camellia seed oils. Food Chem. 2018, 242, 308–315. [Google Scholar] [CrossRef]
- Xie, J.; Liu, T.; Yu, Y.; Song, G.; Hu, Y. Rapid Detection and Quantification by GC–MS of Camellia Seed Oil Adulterated with Soybean Oil. J. Am. Oil Chem. Soc. 2013, 90, 641–646. [Google Scholar] [CrossRef]
- Yuan, J.J.; Tu, J.L.; Qin, G.F.; Li, B. Fatty acid composition and volatiles of Camellia oleifera oil by GC and SPME/GC-MS. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022068. [Google Scholar] [CrossRef]
- Allen, J.C.; Hamilton, R.J. Rancidity in Foods; Applied Science Publishers: London, UK, 1983; pp. 11–201. [Google Scholar]
- Granvogl, M.; Beksan, E.; Schieberle, P. New insights into the formation of aroma-active Strecker aldehydes from 3-oxazolines as transient intermediates. J. Agric. Food Chem. 2012, 60, 6312–6322. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, H.G.; Kim, C.R.; Lim, H.J.; Cho, K.M.; Choi, J.S.; Shin, D.H.; Shin, E.C. Quality evaluation on use of Camellia seed oil as an alternative method in dried seaweed preparation. Prev. Nutr. Food Sci. 2014, 19, 234–241. [Google Scholar] [CrossRef]
- Sun, B. Edible Flavoring Technique; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Zhu, G.; Liu, H.; Xie, Y.; Liao, Q.; Lin, Y.; Liu, Y.; Liu, Y.; Xiao, H.; Gao, Z.; Hu, S. Postharvest processing and storage methods for Camellia oleifera seeds. Food Rev. Inter. 2019, 36, 319–339. [Google Scholar] [CrossRef]
- Wang, X.; Song, Z.; Zhang, Y.; Ni, Y. Effect of refining process on the flavor compounds of linseed oil. Sci. Technol. Food Ind. 2016, 37, 55–59. [Google Scholar]
| Compounds | Chemical Functional Group Classification | PRO | AEO | RFO | SEO |
|---|---|---|---|---|---|
| Formic acid, octyl ester | Esters | 0.91–1.91 | 1.62–2.06 | 0.17–4.3 | 0.9 |
| Ethyl tiglate | Esters | 0.63–2.26 | 0–0.63 | 0.03–3.4 | 0 |
| Hexadecanoic acid, methyl ester | Esters | 0–0.02 | 0–0.01 | 0.02–7 | 0.04 |
| 2-Hexenoic acid, ethyl ester | Esters | 0.54–3.45 | 0–0.7 | 0.19–3.29 | 0 |
| 1-Octen-3-ol, methyl ether | Esters | 0.34–2.27 | 0–0 | 0.11–7 | 0.6 |
| Formic acid, heptyl ester | Esters | 0.51–0.6 | 0–0.41 | 0.46–4.93 | 0 |
| Esters subtotal | 0.91–10.4 | 1.62–3.81 | 1.54 | 0–12.38 | |
| Styrene | Alkenes | 0.81–5.89 | 0–4.72 | 0.54–14.9 | 0 |
| 3,5-Octadien-2-one | Alkenes | 0.13–2.14 | 0–1.19 | 0.04–7 | 0.09 |
| 4,6-Decadiene | Alkenes | 0.08–0.15 | 0.08–0.08 | 0.04–7 | 0.14 |
| 3-Octen-2-one | Alkenes | 0.3–0.31 | 0 | 0.11–2 | 0.19 |
| Bicyclo [3.1.1]hept-2-ene, 2,6-d | Alkenes | 0.04–0.06 | 0–0.08 | 0.03–7 | 0 |
| Z-(13,14-Epoxy)tetradec-11-en-1 | Alkenes | 0–0.09 | 0 | 0.04–0.16 | 0 |
| 1,2-Dimethyl-4-oxocyclohex-2-en | Alkenes | 0–0 | 0–0.27 | 0.06–0.29 | 0.21 |
| Naphthalene, 2-methyl- | Alkenes | 0–0 | 0–2.58 | 0.05–0.91 | 0 |
| 1H-3a,7-Methanoazulene, 2,3,4,7 | Alkenes | 0.05–0.16 | 0–0 | 0.09–7 | 0 |
| 3-Heptadecene, (Z)- | Alkenes | 0–0.15 | 0–0 | 0.04–0.18 | 0.11 |
| Dodecane | Alkanes | 0.15–0.37 | 0.57–2.56 | 0.05–7 | 0 |
| Tetradecane | Alkanes | 0.11–0.14 | 0–0.18 | 0.03–0.66 | 0 |
| Hexadecane | Alkanes | 0.06–7 | 0.06–1.34 | 0.04–7 | 0 |
| Pentadecane | Alkanes | 0–7 | 0–0 | 0.03–7 | 0 |
| 2-Bromo dodecane | Alkanes | 0.06–0.18 | 0–0 | 0.08–7 | 0.15 |
| Heptadecane | Alkanes | 0–0.18 | 0–0.06 | 0.03–7 | 0.05 |
| Ethyl 1-methylcyclopropanecarbo | Alkanes | 1.17–2.78 | 0–1.28 | 0.17–4.25 | 0 |
| Oxirane, octyl- | Alkanes | 0.05–0.08 | 0–0 | 0.08–7 | 0 |
| Alkanes subtotal | 1.64–6.29 | 3.77–5.07 | 0.6 | 0–11.78 | |
| 2(3H)-Furanone, dihydro-5-penty | Ketones | 0.17–0.3 | 0–0.19 | 0.06–2.36 | 0.31 |
| Cyclohexanone, 2-(1-methyl-2-ox | Ketones | 0.09–0.36 | 0–0.13 | 0.06–7 | 0.33 |
| 2-Sec-Butylcyclohexanone | Ketones | 0.12–0.14 | 0–7 | 0.05–0.71 | 0.23 |
| 2-Dodecanone | Ketones | 0.46–5.22 | 0.33–0.74 | 0.24–4.58 | 0.73 |
| 2(3H)-Furanone, 3-acetyldihydro | Ketones | 0.21–0.75 | 0–0.25 | 0.08–7 | 0.49 |
| 2-Nonanone | Ketones | 0.53–1.63 | 0–2.59 | 0.33–7.42 | 0.7 |
| 2-Pentadecanone | Ketones | 0–0 | 0–0 | 0.02–7 | 0 |
| 2-Decanone | Ketones | 0.29–0.35 | 0–0 | 0.11–5.43 | 0 |
| 2H-Pyran-2-one, tetrahydro-6-pr | Ketones | 0.09–0.1 | 0–7 | 0.1–2.62 | 0 |
| Ethanone, 1-(1H-pyrrol-2-yl)- | Ketones | 0.48–1.21 | 0–0 | 0.08–3.23 | 0 |
| .gamma.-Chlorobutyrophenone | Ketones | 0–0.18 | 0–0 | 0.05–0.59 | 0 |
| 2-Pentadecanone, 6,10,14-trimet | Ketones | 0–0 | 0–0.38 | 0.03–7 | 0 |
| Ketones subtotal | 1.57–6.72 | 3.7–14.9 | 2.79 | 0.79–18.16 | |
| Nonanoic acid | Acid | 0.25–4.45 | 0.63–1.63 | 0.25–11.13 | 2.19 |
| Octanoic acid | Acid | 1.05–2.68 | 0–0 | 0.58–7 | 1.19 |
| 2-Butenoic acid, 2-methyl-, 3-m | Acid | 1.02–2.03 | 0–1.51 | 0.39–4.18 | 0.41 |
| Cyclopentanecarboxylic acid, 2- | Acid | 0.15–0.66 | 0–0.21 | 0.13–0.74 | 0 |
| Heptanoic acid | Acid | 0–1.12 | 0–1.07 | 0.12–1.51 | 0 |
| Nonanoic acid, pentafluoropheny | Acid | 0.06–0.19 | 0–0 | 0.06–0.36 | 0 |
| 3-Methyl-2-butenoic acid, hepta | Acid | 0.79–4.42 | 0–0 | 0.23–4.18 | 0.23 |
| Hexanoic acid | Acid | 0–1.56 | 0–0 | 0.39–3.67 | 0 |
| 3-Cyclopentylpropionic acid, 2- | Acid | 0–7 | 0–0 | 0.03–1.27 | 0.04 |
| Acid subtotal | 3.58–16.61 | 1.7–3.35 | 4.06 | 1.28–19.67 | |
| Nonanal | Aldehydes | 6.68–14.67 | 9.29–10.37 | 4.99–19.78 | 10.66 |
| Octanal | Aldehydes | 2.54–9.93 | 4.85–10.42 | 2.09–17.17 | 8.14 |
| 2-Decenal, (E)- | Aldehydes | 1.32–2.77 | 3.49–3.49 | 0.86–9.53 | 11.94 |
| 2-Heptenal, (Z)- | Aldehydes | 0.96–1.59 | 0.96–4.02 | 0.46–7.99 | 1.21 |
| 2-Nonenal, (E)- | Aldehydes | 0.56–3.08 | 0.88–1.3 | 0.49–10.09 | 1.99 |
| Heptanal | Aldehydes | 1.03–3.03 | 2.68–3.09 | 0.24–6.37 | 1.58 |
| 2-Octenal, (E)- | Aldehydes | 0.89–2.58 | 1.21–1.26 | 0.36–4.83 | 2.32 |
| Dodecanal | Aldehydes | 0.43–1.14 | 0–0.51 | 0.23–7 | 1.01 |
| Hexanal | Aldehydes | 4.25–10.08 | 4.66–21.47 | 1.57–19.53 | 7.74 |
| 2-Undecenal | Aldehydes | 0.51–2.21 | 1.59–1.74 | 0.48–7 | 7.44 |
| 4-Oxononanal | Aldehydes | 0.12–7 | 0–0 | 0.04–7 | 0.45 |
| 2,4-Nonadienal, (E,E)- | Aldehydes | 0.34–0.65 | 0–0.17 | 0.08–1.08 | 0.38 |
| 2,4-Decadienal | Aldehydes | 0–1.51 | 0–0.7 | 0.32–3.53 | 14.4 |
| 2,4-Decadienal, (E,E)- | Aldehydes | 0.46–4.65 | 0.47–0.72 | 0.26–14.11 | 6.81 |
| Benzaldehyde | Aldehydes | 0.68–2.88 | 0–4.02 | 0.5–7.74 | 1.34 |
| Tetradecanal | Aldehydes | 0.03–0.04 | 0–0 | 0.03–7 | 0 |
| 2,4-Heptadienal, (E,E)- | Aldehydes | 0.71–8.18 | 0–0.95 | 0.21–5.62 | 1.23 |
| trans-4,5-Epoxy-(E)-2-decenal | Aldehydes | 0–0.18 | 0–0 | 0–0.3 | 0.18 |
| 2,4-Dodecadienal | Aldehydes | 0–0 | 0–0 | 0.02–7 | 0.05 |
| Benzeneacetaldehyde, .alpha.-et | Aldehydes | 0.09–0.09 | 0–0 | 0.05–0.34 | 0 |
| Aldehydes subtotal | 23.33–55.14 | 43.65–47.17 | 78.87 | 9.47–58.96 | |
| Furan, 2-pentyl- | Furans | 1.51–3.09 | 0–2.13 | 0.27–5.15 | 3.18 |
| 1-Heptanol | Alcohol | 0.42–1.23 | 0–1.7 | 0.32–2.95 | 0.48 |
| 1-Octen-3-ol | Alcohol | 0.33–0.45 | 0–0.75 | 0.13–3.12 | 0.2 |
| Phenylethyl Alcohol | Alcohol | 0.21–2.38 | 0–1.25 | 0.33–16.19 | 0 |
| 1-Nonanol | Alcohol | 0.09–0.35 | 0–0 | 0.06–1.38 | 0.06 |
| Benzyl alcohol | Alcohol | 0.25–0.25 | 0–0.18 | 0.11–1.67 | 0 |
| n-Nonadecanol-1 | Alcohol | 0.08–0.27 | 0–0.59 | 0.03–7 | 0.06 |
| 3-Phenylpropanol | Alcohol | 0.14–0.16 | 0–7 | 0.04–7 | 0 |
| Phenol, 2,6-dimethoxy- | Alcohol | 1.51–2.33 | 0–0.13 | 0.12–7 | 0 |
| Mequinol | Alcohol | 4.79–9.07 | 0–2.07 | 0.29–4.75 | 0 |
| Alcohol | 1.08–12.72 | 0.59–13.08 | 0.8 | 0–19.81 | |
| 1H-2-Benzopyran-1-one, 3,4-dihy | Pyran | 0.1–0.11 | 0–0 | 0.04–7 | 0 |
| Principal Components | Eigen Value | Total % of Variance | Cumulative % of Variance |
|---|---|---|---|
| 1 | 17.318 | 42.238 | 42.238 |
| 2 | 3.368 | 8.215 | 50.453 |
| 3 | 1.761 | 4.295 | 54.748 |
| 4 | 1.434 | 3.498 | 58.245 |
| 5 | 1.309 | 3.192 | 61.437 |
| 6 | 1.151 | 2.807 | 64.244 |
| 7 | 1.109 | 2.705 | 66.950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhou, X.; Li, H.; Zhou, W.; Tan, Y.; Zhang, Y.; She, J.; Lu, J.; Yu, N. Evaluation of Different Processes Impact on Flavor of Camellia Seed Oil Using HS-SPME-GC/MS. Molecules 2023, 28, 3979. https://doi.org/10.3390/molecules28103979
Li Z, Zhou X, Li H, Zhou W, Tan Y, Zhang Y, She J, Lu J, Yu N. Evaluation of Different Processes Impact on Flavor of Camellia Seed Oil Using HS-SPME-GC/MS. Molecules. 2023; 28(10):3979. https://doi.org/10.3390/molecules28103979
Chicago/Turabian StyleLi, Ziming, Xiangyu Zhou, Hongai Li, Wenhua Zhou, Yuheng Tan, Yuxin Zhang, Jiarong She, Jun Lu, and Ninghua Yu. 2023. "Evaluation of Different Processes Impact on Flavor of Camellia Seed Oil Using HS-SPME-GC/MS" Molecules 28, no. 10: 3979. https://doi.org/10.3390/molecules28103979
APA StyleLi, Z., Zhou, X., Li, H., Zhou, W., Tan, Y., Zhang, Y., She, J., Lu, J., & Yu, N. (2023). Evaluation of Different Processes Impact on Flavor of Camellia Seed Oil Using HS-SPME-GC/MS. Molecules, 28(10), 3979. https://doi.org/10.3390/molecules28103979








