Anticancer Potentials of the Lignan Magnolin: A Systematic Review
Abstract
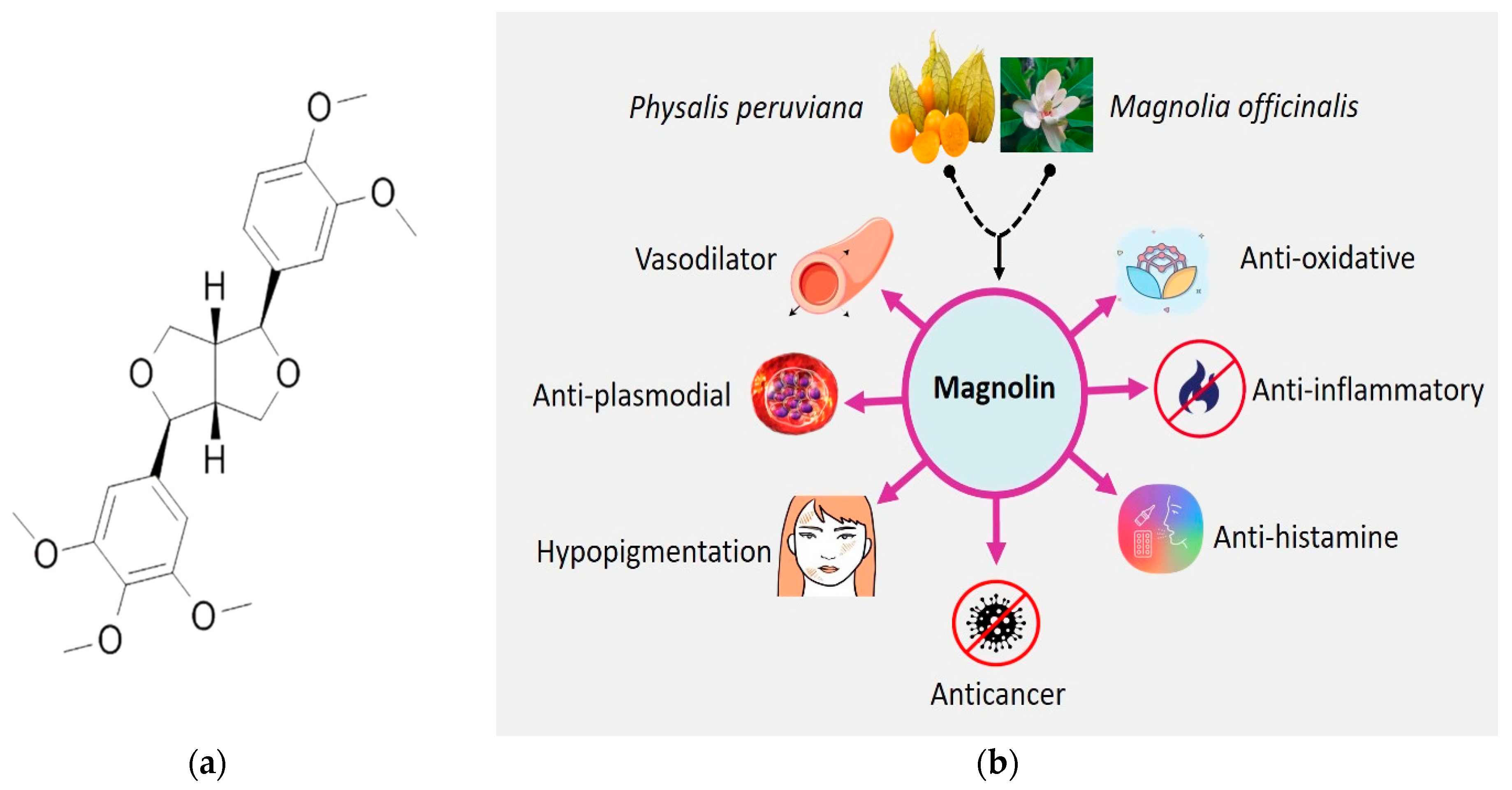
1. Introduction
2. Results and Discussion
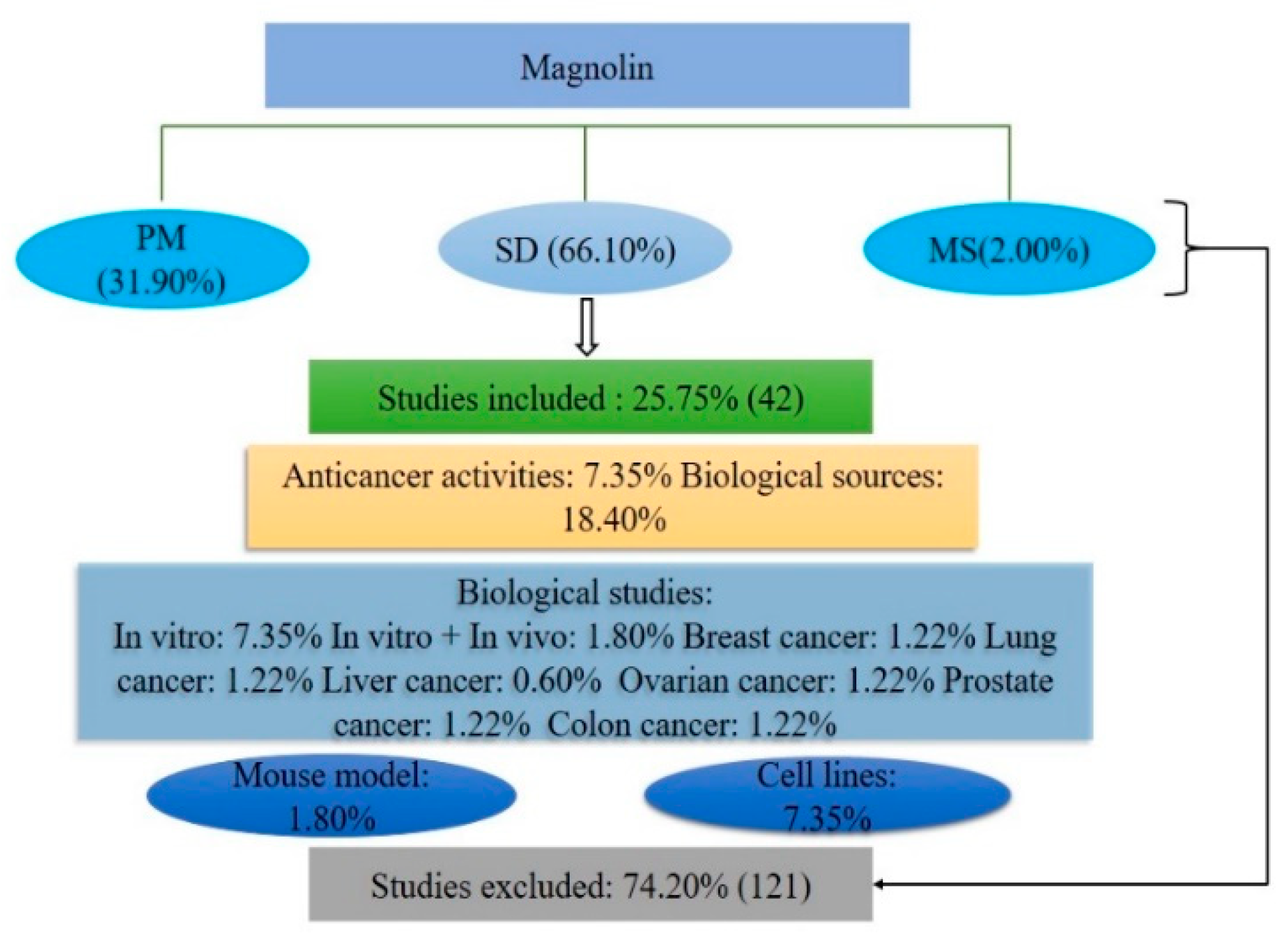
2.1. Database Reports
2.2. Botanical Sources
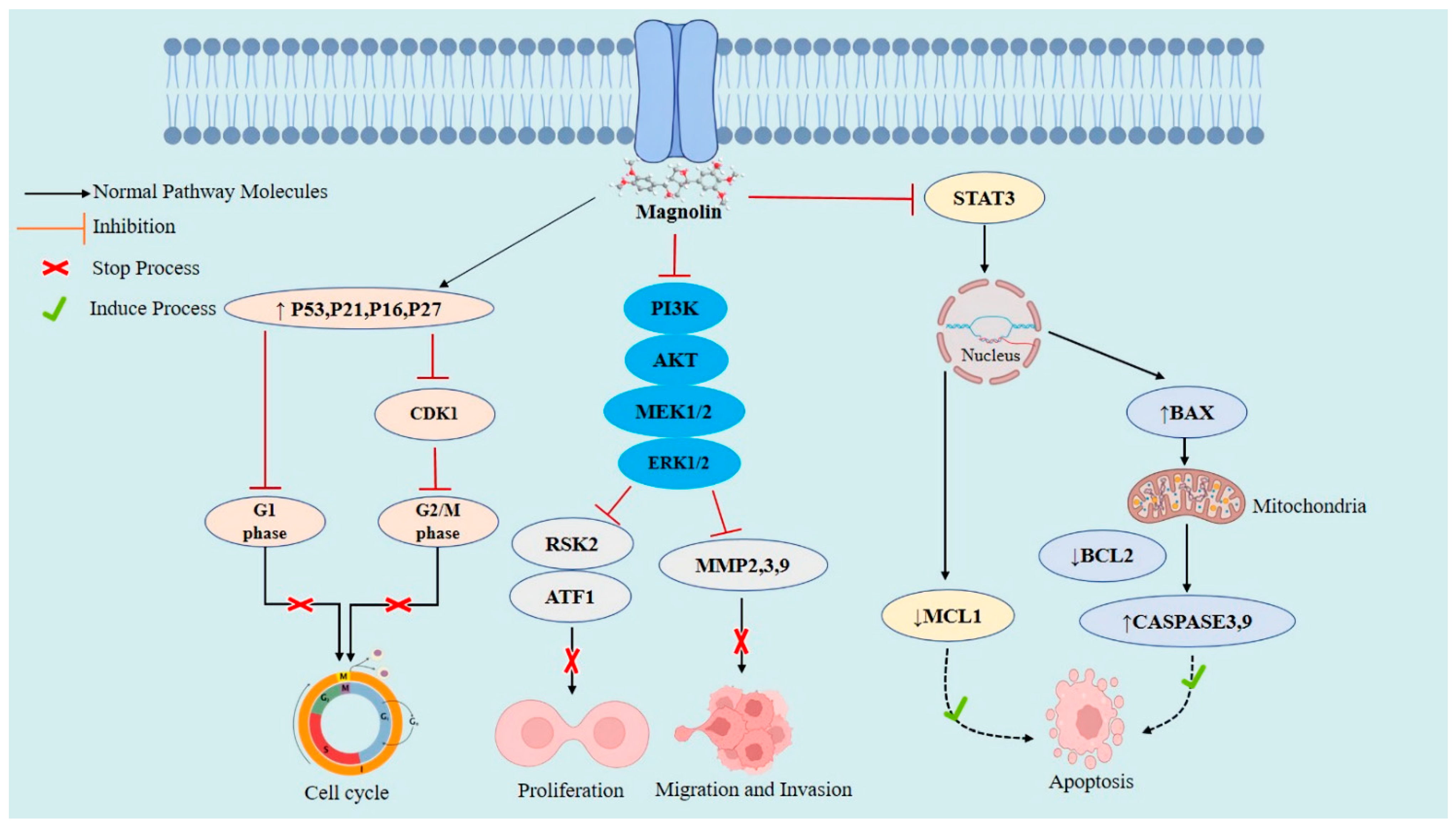
2.3. Cellular and Molecular Anticancer Mechanisms of Magnolin
2.4. Magnolin against Various Cancers
2.4.1. Breast Cancer
2.4.2. Lung Cancer
2.4.3. Liver Cancer
2.4.4. Ovarian Cancer
2.4.5. Prostate Cancer
2.4.6. Pancreatic Carcinoma
2.4.7. Colorectal Cancer
2.5. Pharmacokinetic Features
3. Methodology
3.1. Literature Searching Strategy
3.2. Inclusion Criteria
- Studies carried out in vitro, ex vivo, or in vivo with or without utilizing laboratory animals, including mice, rats, rabbits, and humans, and their derived tissues or cells.
- Studies with anticancer activities and botanical sources of magnolin.
- Studies with magnolin or its derivatives or preparations.
- Magnolin or its derivatives provide joint activity with other chemical compounds.
- Studies which do or do not imply possible mechanisms of action.
3.3. Exclusion Criteria
- Studies demonstrated data duplication and titles and/or abstracts not meeting the inclusion criteria.
- Magnolin in other studies not covering the current issue.
- Papers written in languages other than English.
- Studies without full text available.
- Case reports, letters, editorials, and commentaries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes Dis. 2021, 8, 655–661. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zink, D.; Fischer, A.H.; Nickerson, J.A. Nuclear structure in cancer cells. Nat. Rev. Cancer 2004, 4, 677–687. [Google Scholar] [CrossRef]
- Farber, E.; Rubin, H. Cellular adaptation in the origin and development of cancer. Cancer Res. 1991, 51, 2751–2761. [Google Scholar] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Armstrong, B.; Doll, R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int. J. Cancer 1975, 15, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Javier, R.T.; Butel, J.S. The history of tumor virology. Cancer Res. 2008, 68, 7693–7706. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S. Cancer issue: Why cancer and inflammation? Yale J. Biol. Med. 2006, 79, 123. [Google Scholar]
- Trichopoulos, D.; Li, F.P.; Hunter, D.J. What causes cancer? Sci. Am. 1996, 275, 80–87. [Google Scholar] [CrossRef]
- Ng, R.; Sutradhar, R.; Yao, Z.; Wodchis, W.P.; Rosella, L.C. Smoking, drinking, diet and physical activity—Modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 2020, 49, 113–130. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y. Advances in mechanism and treatment strategy of cancer. Cell. Mol. Biol. 2018, 64, 1–3. [Google Scholar] [CrossRef]
- Mansouri, V.; Beheshtizadeh, N.; Gharibshahian, M.; Sabouri, L.; Varzandeh, M.; Rezaei, N. Recent advances in regenerative medicine strategies for cancer treatment. Biomed. Pharmacother. 2021, 141, 111875. [Google Scholar] [CrossRef]
- Maher, E.; Coia, L.; Duncan, G.; Lawton, P. Treatment strategies in advanced and metastatic cancer: Differences in attitude between the USA, Canada and Europe. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 239–244. [Google Scholar] [CrossRef]
- Furue, H. Chemotherapy cancer treatment during the past sixty years. Gan Kagaku Ryoho Cancer Chemother. 2003, 30, 1404–1411. [Google Scholar]
- Ruggeri, B.A.; Camp, F.; Miknyoczki, S. Animal models of disease: Pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem. Pharmacol. 2014, 87, 150–161. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 2013, 63, 570–577. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ebob, O.T.; Babiaka, S.B.; Ntie-Kang, F. Natural products as potential lead compounds for drug discovery against SARS-CoV-2. Nat. Prod. Bioprospecting 2021, 11, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Sikder, L.; Khan, M.R.; Smrity, S.Z.; Islam, M.T.; Khan, S.A. Phytochemical and pharmacological investigation of the ethanol extract of Byttneria pilosa Roxb. Clin. Phytosci. 2022, 8, 1–8. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Ražná, K.; Nôžková, J.; Vargaová, A.; Harenčár, Ľ.; Bjelková, M. Biological functions of lignans in plants. Agriculture 2021, 67, 155–165. [Google Scholar] [CrossRef]
- Magoulas, G.E.; Papaioannou, D. Bioinspired syntheses of dimeric hydroxycinnamic acids (lignans) and hybrids, using phenol oxidative coupling as key reaction, and medicinal significance thereof. Molecules 2014, 19, 19769–19835. [Google Scholar] [CrossRef]
- Kalinová, J.P.; Marešová, I.; Tříska, J.; Vrchotová, N. Distribution of lignans in Panicum miliaceum, Fagopyrum esculentum, Fagopyrum tataricum, and Amaranthus hypochondriacus. J. Food Compos. Anal. 2022, 106, 104283. [Google Scholar] [CrossRef]
- Ionkova, I. Anticancer lignans-from discovery to biotechnology. Mini Rev. Med. Chem. 2011, 11, 843–856. [Google Scholar] [CrossRef]
- Durazzo, A.; Zaccaria, M.; Polito, A.; Maiani, G.; Carcea, M. Lignan content in cereals, buckwheat and derived foods. Foods 2013, 2, 53–63. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Su, G.-Y.; Wang, K.-W.; Wang, X.-Y.; Wu, B. Bioactive lignans from Zanthoxylum planispinum with cytotoxic potential. Phytochem. Lett. 2015, 11, 120–126. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kasahara, H.; Kameoka, H. Phenolic lignans from flower buds of Magnolia fargesii. Phytochemistry 1992, 31, 3666–3668. [Google Scholar] [CrossRef]
- Pan, J.-X.; Hensens, O.D.; Zink, D.L.; Chang, M.N.; Hwang, S.-B. Lignans with platelet activating factor antagonist activity from Magnolia biondii. Phytochemistry 1987, 26, 1377–1379. [Google Scholar] [CrossRef]
- Lee, Y.D. Use of Magnolia (Magnolia grandiflora) Seeds in Medicine, and Possible Mechanisms of Action. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 727–732. [Google Scholar]
- Kim, J.Y.; Lim, H.J.; Kim, J.S.; Lee, H.J.; Kim, H.D.; Jeon, R.; Ryu, J.-H. In vitro anti-inflammatory activity of lignans isolated from Magnolia fargesii. Bioorganic Med. Chem. Lett. 2009, 19, 937–940. [Google Scholar] [CrossRef]
- Ibarra-Alvarado, C.; Rojas, A.; Mendoza, S.; Bah, M.; Gutiérrez, D.; Hernández-Sandoval, L.; Martínez, M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010, 48, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, G.; Zhou, Y.; Gui, D.; Li, J.; Xing, T.; Wang, N. Magnolin protects against contrast-induced nephropathy in rats via antioxidation and antiapoptosis. Oxidative Med. Cell. Longev. 2014, 2014, 203458. [Google Scholar] [CrossRef]
- Uto, T.; Tung, N.H.; Ohta, T.; Shoyama, Y. (+)-Magnolin enhances melanogenesis in melanoma cells and three-dimensional human skin equivalent; involvement of PKA and p38 MAPK signaling pathways. Planta Med. 2022, 88, 1199–1208. [Google Scholar] [CrossRef]
- Ma, P.; Che, D.; Zhao, T.; Zhang, Y.; Li, C.; An, H.; Zhang, T.; He, H. Magnolin inhibits IgE/Ag-induced allergy in vivo and in vitro. Int. Immunopharmacol. 2019, 76, 105867. [Google Scholar] [CrossRef]
- Shen, Y.; Pang, E.C.; Xue, C.C.; Zhao, Z.; Lin, J.; Li, C.G. Inhibitions of mast cell-derived histamine release by different Flos Magnoliae species in rat peritoneal mast cells. Phytomedicine 2008, 15, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zou, X.; Zhang, X.; Wang, F.; Zhu, W.; Zhang, G.; Xiao, J.; Chen, M. Magnolin inhibits prostate cancer cell growth in vitro and in vivo. Biomed. Pharmacother. 2017, 87, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Ortet, R.; Prado, S.; Regalado, E.L.; Valeriote, F.A.; Media, J.; Mendiola, J.; Thomas, O.P. Furfuran lignans and a flavone from Artemisia gorgonum Webb and their in vitro activity against Plasmodium falciparum. J. Ethnopharmacol. 2011, 138, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Lee, C.J.; An, H.J.; Yoo, S.M.; Kang, H.C.; Lee, J.Y.; Kim, K.D.; Kim, D.J.; Lee, H.S.; Cho, Y.Y. Magnolin targeting of ERK1/2 inhibits cell proliferation and colony growth by induction of cellular senescence in ovarian cancer cells. Mol. Carcinog. 2019, 58, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, S.M.; Sagare, A.P.; Lee, C.-Y.; Kao, C.-L.; Tsay, H.-S. Studies on tissue culture of Chinese medicinal plant resources in Taiwan and their sustainable utilization. Bot. Bull. Acad. Sin. 2003, 44, 79–98. [Google Scholar]
- Kaky, E.; Gilbert, F. Using species distribution models to assess the importance of Egypt’s protected areas for the conservation of medicinal plants. J. Arid Environ. 2016, 135, 140–146. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Bieski, I.G.C.; Leonti, M.; Arnason, J.T.; Ferrier, J.; Rapinski, M.; Violante, I.M.P.; Balogun, S.O.; Pereira, J.F.C.A.; Figueiredo, R.d.C.F.; Lopes, C.R.A.S. Ethnobotanical study of medicinal plants by population of valley of Juruena region, legal Amazon, Mato Grosso, Brazil. J. Ethnopharmacol. 2015, 173, 383–423. [Google Scholar] [CrossRef]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the biodiversity of plants: A revolution in the making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Chen, H.; Chen, Y.; Hsu, H. On the Ca++-Antagonistic Principles of the Flower Buds of Magnolia fargesii1. Planta Med. 1988, 54, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-Y.; Liu, Y.-Q.; Deng, Y.-H.; Wang, Y.-H.; Zhou, X.-J. Lignans from the bark of Zanthoxylum simulans. J. Asian Nat. Prod. Res. 2015, 17, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zheng, L.; Liu, F.; Xu, X.; Mao, S.; Wang, X.; Liu, J.; Lu, Y.; Zhao, W.; Yu, X. Growth factor progranulin promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR signaling pathway. Oncotarget 2016, 7, 58381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhou, T.; Fan, G.; Chai, Y.; Wu, Y. Isolation and purification of lignans from Magnolia biondii Pamp by isocratic reversed-phase two-dimensional liquid chromatography following microwave-assisted extraction. J. Sep. Sci. 2007, 30, 2370–2381. [Google Scholar] [CrossRef]
- Ma, Y.; Han, G. Biologically active lignins from Magnolia biondii Pamp. Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 1995, 20, 102–104. [Google Scholar]
- Vo, T.N.; Nguyen, P.L.; Tuong, L.T.; Pratt, L.M.; Vo, P.N.; Nguyen, K.P.P.; Nguyen, N.S. Lignans and triterpenes from the root of Pseuderanthemum carruthersii var. atropurpureum. Chem. Pharm. Bull. 2012, 60, 1125–1133. [Google Scholar] [CrossRef]
- Hong, P.T.L.; Kim, H.J.; Kim, W.K.; Nam, J.H. Flos magnoliae constituent fargesin has an anti-allergic effect via ORAI1 channel inhibition. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2021, 25, 251–258. [Google Scholar] [CrossRef]
- Arango-De la Pava, L.D.; Zamilpa, A.; Trejo-Espino, J.L.; Domínguez-Mendoza, B.E.; Jiménez-Ferrer, E.; Pérez-Martínez, L.; Trejo-Tapia, G. Synergism and subadditivity of verbascoside-lignans and-iridoids binary mixtures isolated from castilleja tenuiflora benth. on NF-κB/AP-1 inhibition activity. Molecules 2021, 26, 547. [Google Scholar] [CrossRef]
- Radulović, N.S.; Mladenović, M.Z.; Ðorđević, N.D. Chemotypification of Astrantia major L. (Apiaceae): Essential-Oil and Lignan Profiles of Fruits. Chem. Biodivers. 2012, 9, 1320–1337. [Google Scholar] [CrossRef]
- Qiao, D.; Gan, L.-S.; Mo, J.-X.; Zhou, C.-X. Chemical constituents of Acorus calamus. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2012, 37, 3430–3433. [Google Scholar]
- Chávez, D.; Acevedo, L.A.; Mata, R. Tryptamine derived amides and acetogenins from the seeds of Rollinia mucosa. J. Nat. Prod. 1999, 62, 1119–1122. [Google Scholar] [CrossRef]
- Jo, Y.-H.; Seo, G.-U.; Yuk, H.-G.; Lee, S.-C. Antioxidant and tyrosinase inhibitory activities of methanol extracts from Magnolia denudata and Magnolia denudata var. purpurascens flowers. Food Res. Int. 2012, 47, 197–200. [Google Scholar] [CrossRef]
- Seo, Y. Antioxidant activity of the chemical constituents from the flower buds of Magnolia denudata. Biotechnol. Bioprocess Eng. 2010, 15, 400–406. [Google Scholar] [CrossRef]
- Li, J.; Tanaka, M.; Kurasawa, K.; Ikeda, T.; Nohara, T. Studies of the chemical constituents of the flower buds of Magnolia kobus and M. salicifolia. J. Nat. Med. 2007, 61, 222–223. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, S.M.; Lee, O.K.; Jo, H.J.; Kang, H.Y.; Choi, D.H.; Paik, K.H.; Khan, M. Lignans from the bark of Magnolia kobus. Helv. Chim. Acta 2008, 91, 2361–2366. [Google Scholar] [CrossRef]
- Alegrio, L.V.; Fo, R.B.; Gottlieb, O.R.; Maia, J.G.S. Lignans and neolignans from Licaria armeniaca. Phytochemistry 1981, 20, 1963–1965. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Yoshida, M.; Gottlieb, O.R. Neolignans from the fruits of Licaria armeniaca. Phytochemistry 1986, 26, 319–321. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Mahmoud, A.A.; Ali, E.T.; Tzakou, O.; Couladis, M.; Mabry, T.J.; Gáti, T.; Tóth, G. Two highly oxygenated eudesmanes and 10 lignans from Achillea holosericea. Phytochemistry 2002, 59, 851–856. [Google Scholar] [CrossRef]
- Dutra, L.M.; Costa, E.V.; de Souza Moraes, V.R.; de Lima Nogueira, P.C.; Vendramin, M.E.; Barison, A.; do Nascimento Prata, A.P. Chemical constituents from the leaves of Annona pickelii (Annonaceae). Biochem. Syst. Ecol. 2012, 41, 115–118. [Google Scholar] [CrossRef]
- Wu, H.-B.; Liu, T.-T.; Zhang, Z.-X.; Wang, W.-S.; Zhu, W.-W.; Li, L.-F.; Li, Y.-R.; Chen, X. Leaves of Magnolia liliflora Desr. as a high-potential by-product: Lignans composition, antioxidant, anti-inflammatory, anti-phytopathogenic fungal and phytotoxic activities. Ind. Crops Prod. 2018, 125, 416–424. [Google Scholar] [CrossRef]
- Trifunović, S.; Vajs, V.; Tešević, V.; Djoković, D.; Milosavljević, S. Lignans from the plant species Achillea lingulata. J. Serb. Chem. Soc. 2003, 68, 277–280. [Google Scholar] [CrossRef]
- Öksüz, S.; Ulubelen, A.; Tuzlaci, E. Constituents of Achillea teretifolia. Fitoterapia 1990, 61, 283. [Google Scholar]
- Jain, N.; Srivastava, S.; Aggarwal, K.; Ramesh, S.; Kumar, S. Essential oil composition of Zanthoxylum alatum seeds from northern India. Flavour Fragr. J. 2001, 16, 408–410. [Google Scholar] [CrossRef]
- Nishino, C.; Mitsui, T. Lignans from Hernandia Ovigera linn. Tetrahedron Lett. 1973, 14, 335–338. [Google Scholar] [CrossRef]
- Sayed, A.M.; El-Hawary, S.S.; Abdelmohsen, U.R.; Ghareeb, M.A. Antiproliferative potential of Physalis peruviana-derived magnolin against pancreatic cancer: A comprehensive in vitro and in silico study. Food Funct. 2022, 13, 11733–11743. [Google Scholar] [CrossRef]
- Yip, H.Y.K.; Papa, A. Signaling pathways in cancer: Therapeutic targets, combinatorial treatments, and new developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef]
- Pons-Tostivint, E.; Thibault, B.; Guillermet-Guibert, J. Targeting PI3K signaling in combination cancer therapy. Trends Cancer 2017, 3, 454–469. [Google Scholar] [CrossRef]
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef]
- Callahan, R.; Egan, S.E. Notch signaling in mammary development and oncogenesis. J. Mammary Gland. Biol. Neoplasia 2004, 9, 145–163. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Olopade, O.I. MYC and breast cancer. Genes Cancer 2010, 1, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, O.; Kim, N.-H.; Quinn, L.M. MYC in brain development and cancer. Int. J. Mol. Sci. 2020, 21, 7742. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Shakya, A.; Dodson, M.; Chapman, E.; Zhang, D.D. The intricacies of NRF2 regulation in cancer. Semin. Cancer Biol. 2021, 76, 110–119. [Google Scholar] [CrossRef]
- LeBoeuf, S.E.; Wu, W.L.; Karakousi, T.R.; Karadal, B.; Jackson, S.R.; Davidson, S.M.; Wong, K.-K.; Koralov, S.B.; Sayin, V.I.; Papagiannakopoulos, T. Activation of oxidative stress response in cancer generates a druggable dependency on exogenous non-essential amino acids. Cell Metab. 2020, 31, 339–350.e4. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Fernando, J.; Mainez, J.; Sancho, P. TGF-beta signaling in cancer treatment. Curr. Pharm. Des. 2014, 20, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S. Oncogenic signaling pathways in the cancer genome atlas. Cell 2018, 173, 321–337. [Google Scholar] [CrossRef]
- Sui, X.; Jin, L.; Huang, X.; Geng, S.; He, C.; Hu, X. p53 signaling and autophagy in cancer: A revolutionary strategy could be developed for cancer treatment. Autophagy 2011, 7, 565–571. [Google Scholar] [CrossRef]
- Rader, J.; Russell, M.R.; Hart, L.S.; Nakazawa, M.S.; Belcastro, L.T.; Martinez, D.; Li, Y.; Carpenter, E.L.; Attiyeh, E.F.; Diskin, S.J. Dual CDK4/CDK6 Inhibition Induces Cell-Cycle Arrest and Senescence in NeuroblastomaCDK4/6 Inhibition in Neuroblastoma. Clin. Cancer Res. 2013, 19, 6173–6182. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Shah, U.; Shah, R.; Acharya, S.; Acharya, N. Novel anticancer agents from plant sources. Chin. J. Nat. Med. 2013, 11, 16–23. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K. Therapeutic Effectiveness of Magnolin on cancers and other Human complications. Pharmacol. Res. Mod. Chin. Med. 2022, 6, 100203. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Huang, K.; Shi, L.; Zhang, Q. Magnolin inhibits proliferation and invasion of breast cancer MDA-MB-231 cells by targeting the ERK1/2 signaling pathway. Chem. Pharm. Bull. 2020, 68, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Jun, A.Y.; Kim, H.-J.; Park, K.-K.; Son, K.H.; Lee, D.H.; Woo, M.-H.; Chung, W.-Y. Tetrahydrofurofuran-type lignans inhibit breast cancer-mediated bone destruction by blocking the vicious cycle between cancer cells, osteoblasts and osteoclasts. Investig. New Drugs 2014, 32, 1–13. [Google Scholar] [CrossRef]
- Lee, C.-J.; Lee, M.-H.; Yoo, S.-M.; Choi, K.-I.; Song, J.-H.; Jang, J.-H.; Oh, S.-R.; Ryu, H.-W.; Lee, H.-S.; Surh, Y.-J. Magnolin inhibits cell migration and invasion by targeting the ERKs/RSK2 signaling pathway. BMC Cancer 2015, 15, 576. [Google Scholar] [CrossRef]
- Lee, C.-J.; Lee, H.S.; Ryu, H.W.; Lee, M.-H.; Lee, J.Y.; Li, Y.; Dong, Z.; Lee, H.-K.; Oh, S.-R.; Cho, Y.-Y. Targeting of magnolin on ERKs inhibits Ras/ERKs/RSK2-signaling-mediated neoplastic cell transformation. Carcinogenesis 2014, 35, 432–441. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Y.; Li, S.; Zhu, X.; Meng, L.; Song, C.; Yu, C.; Jiang, N.; Liu, Y. Synergistic activity of magnolin combined with B-RAF inhibitor SB590885 in hepatocellular carcinoma cells via targeting PI3K-AKT/mTOR and ERK MAPK pathway. Am. J. Transl. Res. 2019, 11, 3816. [Google Scholar]
- Yoo, S.-M.; Lee, C.-J.; Kim, S.-M.; Cho, S.-Y.; Park, J.; Cho, Y.-Y. Molecular mechanisms of magnolin resistance in ovarian cancer cells. Cancer Res. 2018, 78, 4028. [Google Scholar] [CrossRef]
- Mukhija, M.; Dhar, K.L.; Kalia, A.N. Bioactive Lignans from Zanthoxylum alatum Roxb. stem bark with cytotoxic potential. J. Ethnopharmacol. 2014, 152, 106–112. [Google Scholar] [CrossRef]
- Park, J.; Lee, G.E.; An, H.J.; Lee, C.J.; Cho, E.S.; Kang, H.C.; Lee, J.Y.; Lee, H.S.; Choi, J.S.; Kim, D.J.; et al. Kaempferol sensitizes cell proliferation inhibition in oxaliplatin-resistant colon cancer cells. Arch. Pharm. Res. 2021, 44, 1091–1108. [Google Scholar] [CrossRef]
- Yu, H.; Yin, S.; Zhou, S.; Shao, Y.; Sun, J.; Pang, X.; Han, L.; Zhang, Y.; Gao, X.; Jin, C.; et al. Magnolin promotes autophagy and cell cycle arrest via blocking LIF/Stat3/Mcl-1 axis in human colorectal cancers. Cell Death Dis. 2018, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Kim, K.M.; Jung, J. Upregulation of G Protein-Coupled Estrogen Receptor by Chrysin-Nanoparticles Inhibits Tumor Proliferation and Metastasis in Triple Negative Breast Cancer Xenograft Model. Front. Endocrinol. 2020, 11, 560605. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Verkasalo, P.K.; Banks, E. Epidemiology of breast cancer. Lancet Oncol. 2001, 2, 133–140. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Fedewa, S.A.; Goding Sauer, A.; Kramer, J.L.; Smith, R.A.; Jemal, A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J. Clin. 2016, 66, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer—Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Chan, Y.W.; Ma, H.T.; Wong, W.; Ho, C.C.; On, K.F.; Poon, R.Y. CDK1 inhibitors antagonize the immediate apoptosis triggered by spindle disruption but promote apoptosis following the subsequent rereplication and abnormal mitosis. Cell Cycle 2008, 7, 1449–1461. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.; Wang, W.; Lin, S.; Yang, L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway. Biomed. Pharmacother. 2017, 92, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liang, Y.; Zhang, T.; Wang, K.; Yang, X. ER-α36 mediates estrogen-stimulated MAPK/ERK activation and regulates migration, invasion, proliferation in cervical cancer cells. Biochem. Biophys. Res. Commun. 2017, 487, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ushijima, T.; Clark, S.J.; Tan, P. Mapping genomic and epigenomic evolution in cancer ecosystems. Science 2021, 373, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Cagle, P.T.; Allen, T.C.; Olsen, R.J. Lung cancer biomarkers: Present status and future developments. Arch. Pathol. Lab. Med. 2013, 137, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Minna, J.D.; Roth, J.A.; Gazdar, A.F. Focus on lung cancer. Cancer Cell 2002, 1, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Beasley, M.B.; Chitale, D.A.; Dacic, S.; Giaccone, G.; Jenkins, R.B.; Kwiatkowski, D.J.; Saldivar, J.-S.; Squire, J. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J. Thorac. Oncol. 2013, 8, 823–859. [Google Scholar] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [PubMed]
- Yoo, S.-M.; Cho, S.J.; Cho, Y.-Y. Molecular targeting of ERKs/RSK2 signaling axis in cancer prevention. J. Cancer Prev. 2015, 20, 165. [Google Scholar] [CrossRef]
- Chen, J.-S.; Huang, X.-H.; Wang, Q.; Huang, J.-Q.; Zhang, L.-J.; Chen, X.-L.; Lei, J.; Cheng, Z.-X. Sonic hedgehog signaling pathway induces cell migration and invasion through focal adhesion kinase/AKT signaling-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9 in liver cancer. Carcinogenesis 2013, 34, 10–19. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chen, K.-F.; Chen, P.-J. Treatment of liver cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a021535. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Bandoh, S.; Roberts, L.R. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Research 2016, 5, 879. [Google Scholar] [CrossRef]
- Whittaker, S.; Marais, R.; Zhu, A. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010, 29, 4989–5005. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, T.-Y.; Hu, B.-C.; Li, X.; Wu, Y.-T.; Sun, X.-T.; Jiang, X.-W.; Wang, S.; Qin, X.-C.; Ding, H.-W. CK-3, a novel methsulfonyl pyridine derivative, suppresses hepatocellular carcinoma proliferation and invasion by blocking the PI3K/AKT/mTOR and MAPK/ERK pathways. Front. Oncol. 2021, 11, 717626. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, Z.; Chen, Y.; Zhang, S.; Feng, L.; Meng, F.; Yu, Z. Dual blocking of PI3K and mTOR signaling by NVP-BEZ235 inhibits proliferation in cervical carcinoma cells and enhances therapeutic response. Cancer Lett. 2017, 388, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Matter, M.S.; Decaens, T.; Andersen, J.B.; Thorgeirsson, S.S. Targeting the mTOR pathway in hepatocellular carcinoma: Current state and future trends. J. Hepatol. 2014, 60, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Kunter, I.; Erdal, E.; Nart, D.; Yilmaz, F.; Karademir, S.; Sagol, O.; Atabey, N. Active form of AKT controls cell proliferation and response to apoptosis in hepatocellular carcinoma. Oncol. Rep. 2014, 31, 573–580. [Google Scholar] [CrossRef]
- Diniz, P.H.; Silva, S.D.; Vidigal, P.V.; Xavier, M.A.; Lima, C.X.; Faria, L.C.; Ferrari, T.C. Expression of MAPK and PI3K/AKT/mTOR proteins according to the chronic liver disease etiology in hepatocellular carcinoma. J. Oncol. 2020, 2020, 4609360. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Tringler, B.; Liu, W.; Corral, L.; Torkko, K.C.; Enomoto, T.; Davidson, S.; Lucia, M.S.; Heinz, D.E.; Papkoff, J.; Shroyer, K.R. B7-H4 overexpression in ovarian tumors. Gynecol. Oncol. 2006, 100, 44–52. [Google Scholar] [CrossRef]
- Gray, J.W.; Suzuki, S.; Kuo, W.-L.; Polikoff, D.; Deavers, M.; Smith-McCune, K.; Berchuck, A.; Pinkel, D.; Albertson, D.; Mills, G.B. Specific keynote: Genome copy number abnormalities in ovarian cancer. Gynecol. Oncol. 2003, 88, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Omeragić, F.; Ljuca, D. Rana detekcija raka jajnika u Federaciji Bosne i Hercegovine i uloga porodične medicine. Med. Glas. 2007, 4, 77–81. [Google Scholar]
- Trentham-Dietz, A.; Newcomb, P.A.; Nichols, H.B.; Hampton, J.M. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res. Treat. 2007, 105, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef]
- Gwinn, M.L.; Lee, N.C.; Rhodes, P.H.; Layde, P.M.; Rubin, G.L. Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. J. Clin. Epidemiol. 1990, 43, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, B.; Wei, X.; Men, K.; Zheng, F.; Zhou, Y.; Zheng, Y.; Gou, M.; Huang, M.; Guo, G. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 2012, 4, 7021–7030. [Google Scholar] [CrossRef]
- Oronsky, B.; Ray, C.M.; Spira, A.I.; Trepel, J.B.; Carter, C.A.; Cottrill, H.M. A brief review of the management of platinum-resistant–platinum-refractory ovarian cancer. Med. Oncol. 2017, 34, 1–7. [Google Scholar] [CrossRef]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent advances in tannic acid (gallotannin) anticancer activities and drug delivery systems for efficacy improvement; a comprehensive review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark Cancer 2019, 11, 1179299x19860815. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Amero, P.; Salama, S.A.; Abdelaziz, A.H.; Lopez-Berestein, G.; Rodriguez-Aguayo, C. Back to the Future: Rethinking the Great Potential of lncRNA(S) for Optimizing Chemotherapeutic Response in Ovarian Cancer. Cancers 2020, 12, 2406. [Google Scholar] [CrossRef]
- Steinmetz, R.; Wagoner, H.A.; Zeng, P.; Hammond, J.R.; Hannon, T.S.; Meyers, J.L.; Pescovitz, O.H. Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase (ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol. Endocrinol. 2004, 18, 2570–2582. [Google Scholar] [CrossRef]
- Sak, K.; Lust, H.; Kase, M.; Saar, M.; Jaal, J. Suppression of taxanes cytotoxicity by citrus flavonoid hesperetin in PPC-1 human prostate cancer cells. AntiCancer Res. 2018, 38, 6209–6215. [Google Scholar] [CrossRef] [PubMed]
- Sathiakumar, N.; Delzell, E.; Morrisey, M.A.; Falkson, C.; Yong, M.; Chia, V.; Blackburn, J.; Arora, T.; Kilgore, M.L. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011, 14, 177–183. [Google Scholar] [CrossRef]
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and therapeutic strategies for prostate cancer. Semin. Nucl. Med. 2016, 46, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Dariya, B.; Alam, A.; Nagaraju, G.P. Biology, pathophysiology, and epidemiology of pancreatic cancer. In Theranostic Approach for Pancreatic Cancer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–50. [Google Scholar]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef]
- Shuwen, H.; Yinhang, W.; Xingming, Z.; Jing, Z.; Jinxin, L.; Wei, W.; Kefeng, D. Using whole-genome sequencing (WGS) to plot colorectal cancer-related gut microbiota in a population with varied geography. Gut Pathog. 2022, 14, 50. [Google Scholar] [CrossRef]
- Bousbaa, H. Novel Anticancer Strategies. Pharmaceutics 2021, 13, 275. [Google Scholar] [CrossRef]
- Jiang, G.M.; Tan, Y.; Wang, H.; Peng, L.; Chen, H.T.; Meng, X.J.; Li, L.L.; Liu, Y.; Li, W.F.; Shan, H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.; Kimmelman, A.C.; White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016, 30, 1913–1930. [Google Scholar] [CrossRef]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Reichel, A.; Lienau, P. Pharmacokinetics in drug discovery: An exposure-centred approach to optimising and predicting drug efficacy and safety. New Approaches Drug Discov. 2016, 232, 235–260. [Google Scholar]
- Bhuia, M.S.; Islam, T.; Rokonuzzman, M.; Shamsh Prottay, A.A.; Akter, F.; Hossain, M.I.; Chowdhury, R.; Kazi, M.A.; Khalipha, A.B.R.; Coutinho, H.D.M. Modulatory effects of phytol on the antiemetic property of domperidone, possibly through the D2 receptor interaction pathway: In vivo and in silico studies. 3 Biotech 2023, 13, 116. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef]
- Bhattaram, V.A.; Graefe, U.; Kohlert, C.; Veit, M.; Derendorf, H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002, 9, 1–33. [Google Scholar] [CrossRef]
- Hassan, M.; Sallam, H.; Hassan, Z. The role of pharmacokinetics and pharmacodynamics in early drug development with reference to the cyclin-dependent kinase (Cdk) inhibitor-roscovitine. Sultan Qaboos Univ. Med. J. 2011, 11, 165. [Google Scholar] [PubMed]
- Kim, N.J.; Song, W.Y.; Yoo, S.D.; Oh, S.-R.; Lee, H.-K.; Lee, H.S. Pharmacokinetics of magnolin in rats. Arch. Pharmacal Res. 2010, 33, 933–938. [Google Scholar] [CrossRef]
- Muhamad, N.; Na-Bangchang, K. Metabolite profiling in anticancer drug development: A systematic review. Drug Des. Dev. Ther. 2020, 14, 1401–1444. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Kasahara, H.; Kameoka, H. Biotransformation of lignans: Metabolism of (+)-Yangabin in Spodoptera litura. Nat. Prod. Lett. 1996, 8, 87–88. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kasahara, H.; Kameoka, H. Biotransformation of lignans: A specific microbial oxidation of (+)-eudesmin and (+)-magnolin by Aspergillus niger. Phytochemistry 1993, 34, 1501–1507. [Google Scholar] [CrossRef]
- Kim, D.K.; Liu, K.-H.; Jeong, J.H.; Ji, H.Y.; Oh, S.-R.; Lee, H.-K.; Lee, H.S. In vitro metabolism of magnolin and characterization of cytochrome P450 enzymes responsible for its metabolism in human liver microsomes. Xenobiotica 2011, 41, 358–371. [Google Scholar] [CrossRef]
| Plant Name | Plant Part | References |
|---|---|---|
| Magnolia fargesii | Flower buds | [37,52] |
| Zanthoxylum simulans | Bark | [53] |
| M. biondii | Flower buds | [54,55,56] |
| Pseuderanthemum carruthersii | Root | [57] |
| M. officinalis | Flower buds | [58] |
| M. sargentiana and M. sprengeri | - | [42] |
| Castilleja tenuiflora | Aerial part of roots | [59] |
| Astrantia major | Fruits | [60] |
| Artemisia gorgonum Webb | Leaves and flowers | [44] |
| Acorus calamus | - | [61] |
| Rollinia mucosa | Seeds | [62] |
| M. denudata | Flowers | [63,64] |
| M. kobus | Flower buds, bark | [65,66] |
| M. salicifolia | Flower buds | [65] |
| Licaria armeniaca | Trunk wood, fruits | [67,68] |
| Achillea holosericea. | Aerial parts | [69] |
| Annona pickelii | Leaves | [70] |
| M. liliflora | Leaves | [71] |
| A. gypsicola | Aerial parts and roots | [72,73] |
| Z. alatum | Seeds | [74] |
| Hernandia ovigera | Leaves | [75] |
| Physalis peruviana | Fruits | [76] |
| Type of Cancer | Experimental Model/ Cell Line | Tested Concentrations | Efficacy, IC50 (Exposure Time) | Anticancer Effects and Mechanisms | Reference |
|---|---|---|---|---|---|
| Breast cancer | MDA-MB-231 | 20 and 50 µM | 30.34 µM (24 h) | ↓MEK1/2, ↓ERK1/2, ↓CDK1, ↓BCL2, ↓MMP 2 and 9, ↑CASPASES3 and 9 ↓proliferation, ↓invasion, ↑apoptosis | [95] |
| MDA-MB-231 | 10, 20, 40, 60, 80, and 100 µM | - | ↓mRNA expression, ↓PTHrP, ↓MMP9, ↓cathepsin K, ↑RANKL/OPG ratio ↓bone loss and bone-resorbing activity | [96] | |
| - | MCF-7 | 100 µg/mL | - | ↑cytotoxicity | [57] |
| Lung cancer | JB6CL41, NCI-H1975 and A549 | 15, 30, and 60 µM | - | ↓ERK1/2, ↓MMP2, ↓MMP9, ↓RSK2, ↓migration, ↓invasion | [97] |
| NIH3T3, A549 | 15, 30, and 60 µM | - | ↓ERK1, ↓ERK2, ↓RSK2, ↓ATF1, ↓AP1, ↓proliferation, ↓migration | [98] | |
| Hepatocellular carcinoma | BEL-7402 and SK-HEP1 | 25, 50, 75 100, and 125 μM | - | ↓MEK, ↓PI3K, ↓AKT, ↓proliferation | [99] |
| Ovarian cancer | TOV-112D | 15, 30, and 60 µM | - | ↓ERK1, ↓ERK2, ↑P16Ink4a, ↑P27Kip1, ↓G1 and G2/M phase, ↓proliferation | [45] |
| TOV-112D | - | 16 nM 68 nM | ↓ERK1, ↓ERK2, ↓ATF1, ↓RSK2 ↓proliferation | [100] | |
| Prostate cancer | PANC-1 | - | 0.51 µM | ↓MMP3, ↓proliferation, ↓migration | [76] |
| PC3 and DU145 | 50 and 100 µM | - | ↓AKT, ↓BCL2, ↑BAX, ↑CASPASE3, ↑P53, ↑P21 ↓G1, G2 phase, ↑apoptosis | [43] | |
| Pancreatic carcinoma | MIA-PaCa | - | - | ↑cytotoxicity | [101] |
| Colon cancer | HCT116 and HT29 | - | - | ↓G2/M-phases, ↓proliferation | [102] |
| Colorectal cancer | HCT116 and SW480 | 10, 20, 30, and 40 µM | - | ↓LIF, ↓STAT3, ↓MCL1 ↑ autophagy ↓cell cycle | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuia, M.S.; Wilairatana, P.; Chowdhury, R.; Rakib, A.I.; Kamli, H.; Shaikh, A.; Coutinho, H.D.M.; Islam, M.T. Anticancer Potentials of the Lignan Magnolin: A Systematic Review. Molecules 2023, 28, 3671. https://doi.org/10.3390/molecules28093671
Bhuia MS, Wilairatana P, Chowdhury R, Rakib AI, Kamli H, Shaikh A, Coutinho HDM, Islam MT. Anticancer Potentials of the Lignan Magnolin: A Systematic Review. Molecules. 2023; 28(9):3671. https://doi.org/10.3390/molecules28093671
Chicago/Turabian StyleBhuia, Md. Shimul, Polrat Wilairatana, Raihan Chowdhury, Asraful Islam Rakib, Hossam Kamli, Ahmad Shaikh, Henrique D. M. Coutinho, and Muhammad Torequl Islam. 2023. "Anticancer Potentials of the Lignan Magnolin: A Systematic Review" Molecules 28, no. 9: 3671. https://doi.org/10.3390/molecules28093671
APA StyleBhuia, M. S., Wilairatana, P., Chowdhury, R., Rakib, A. I., Kamli, H., Shaikh, A., Coutinho, H. D. M., & Islam, M. T. (2023). Anticancer Potentials of the Lignan Magnolin: A Systematic Review. Molecules, 28(9), 3671. https://doi.org/10.3390/molecules28093671










