Horse Chestnut Saponins–Escins, Isoescins, Transescins, and Desacylescins
Abstract
1. Introduction
2. Results and Discussion
2.1. Saponin Extraction and Identification in the Natural Extract (NE)–Purification, Characterization, and Quantification of the Escins I, II and III in the Enriched-Extract (EE)
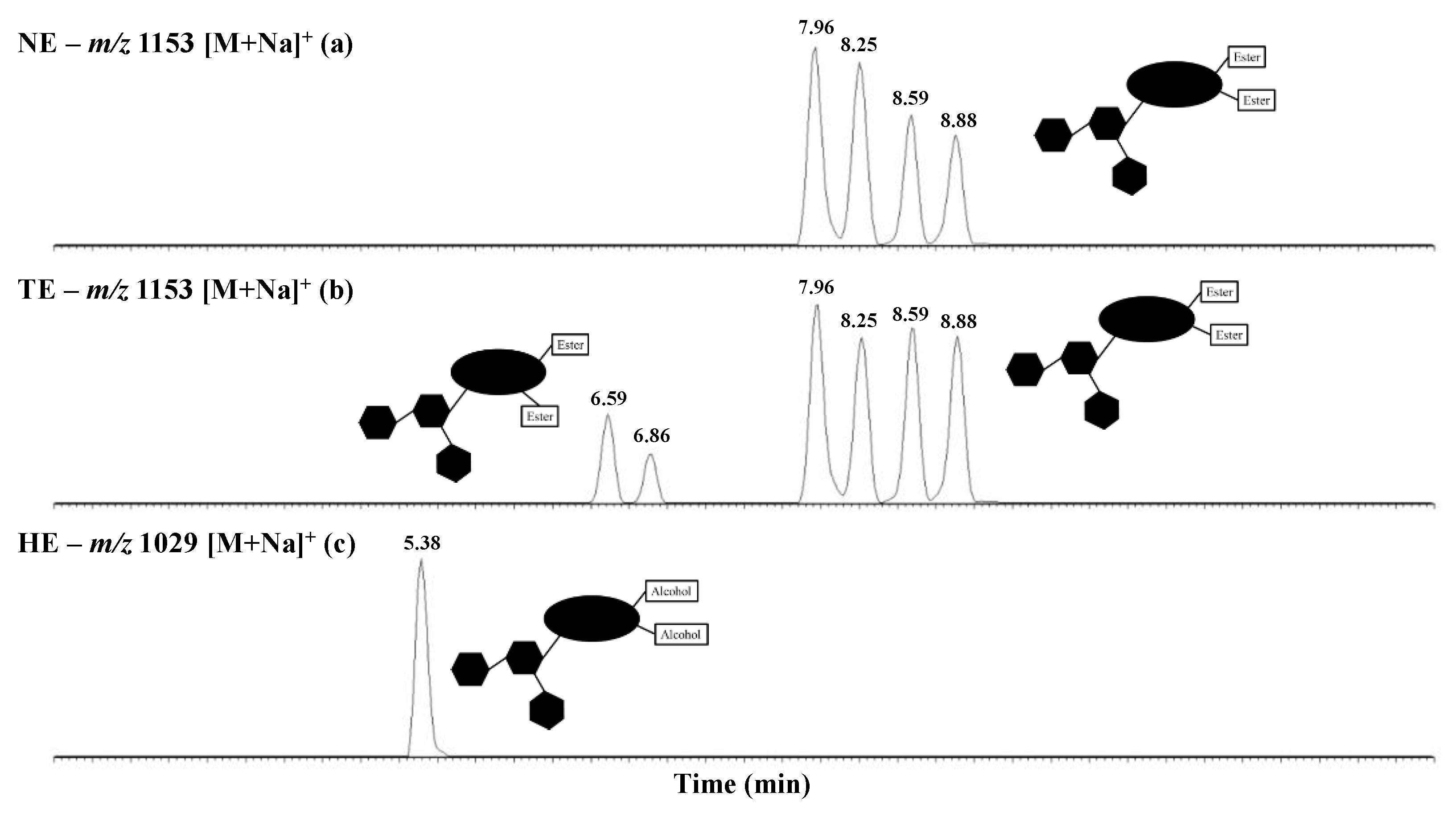
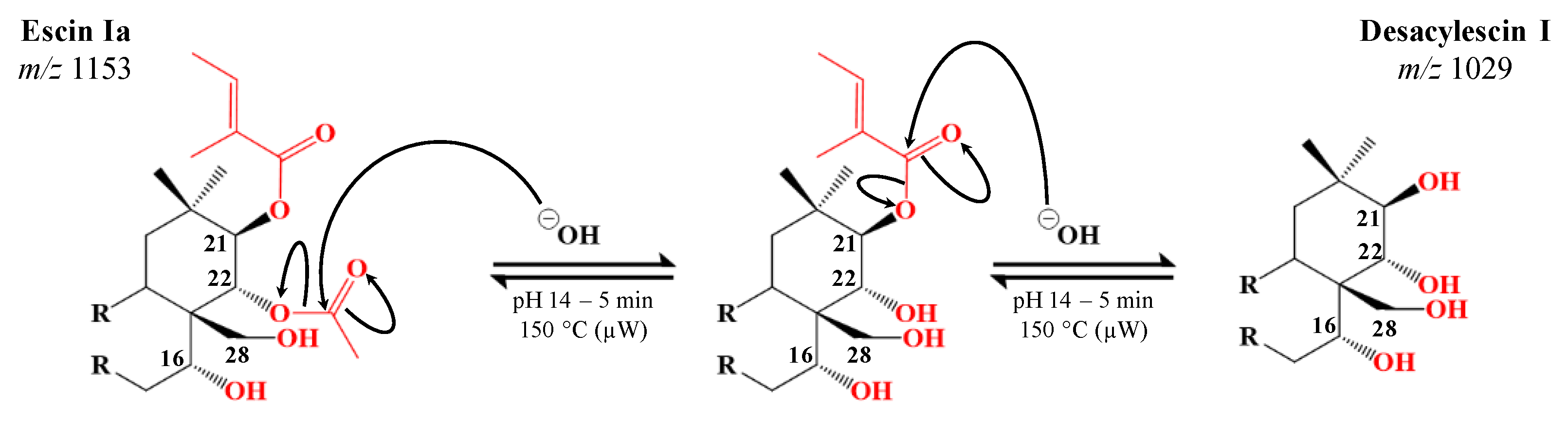
2.2. Specific Microwave-Assisted Hydrolysis of Horse Chestnut Seed Saponins
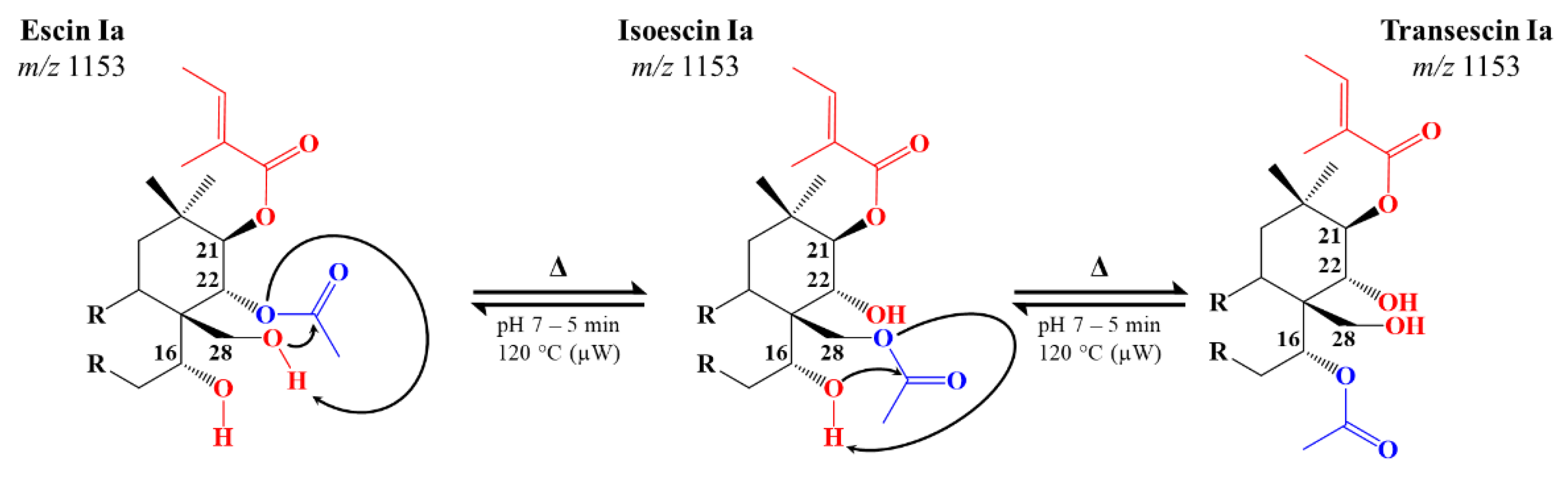
2.3. Microwave-Assisted Transesterification of Horse Chestnut Seed Saponins
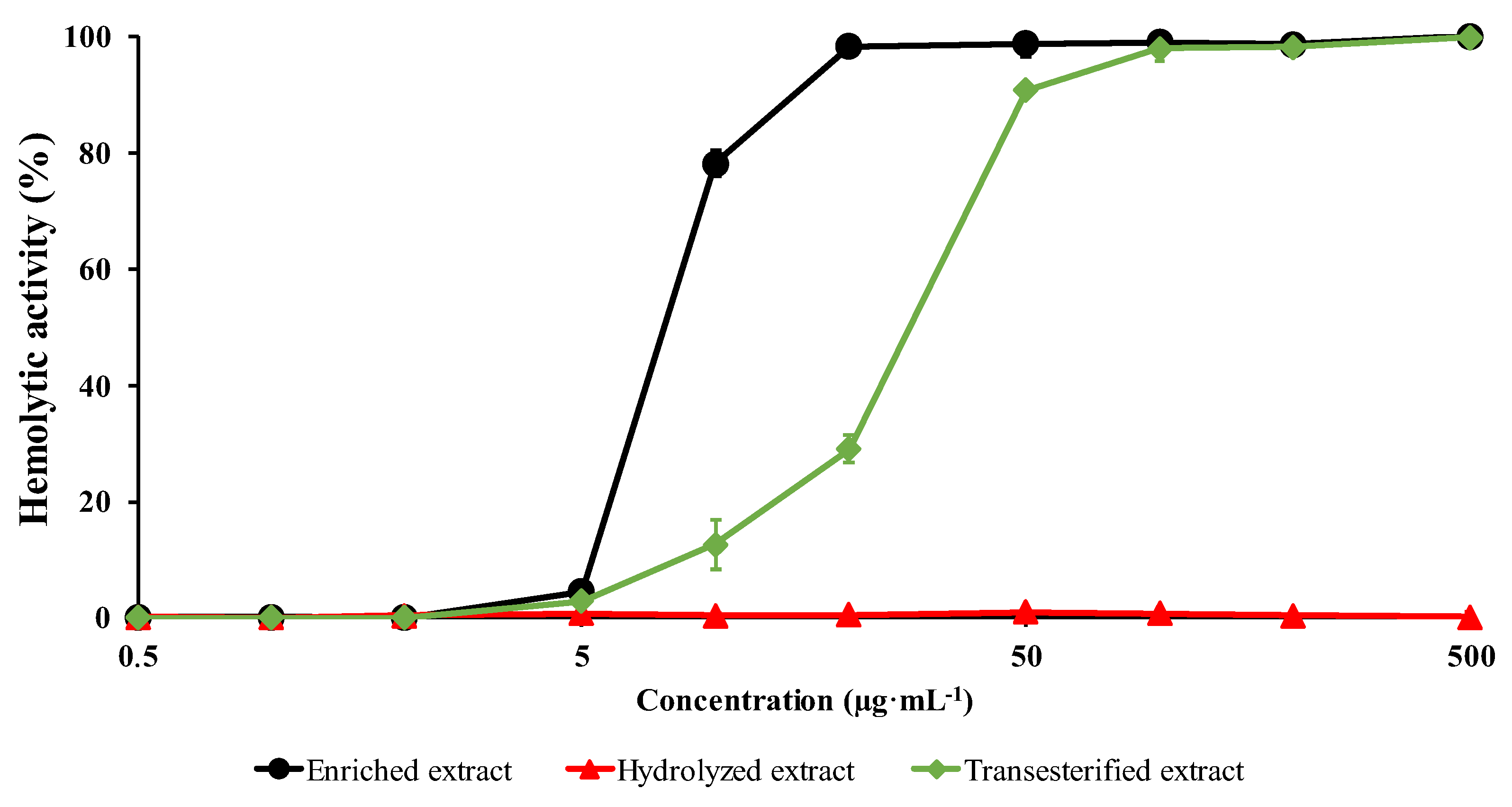
2.4. Hemolytic Activity (HA) Evaluation
3. Materials and Methods
3.1. Chemicals
3.2. Extraction and Flash Chromatography
3.3. Microwave-Assisted Hydrolysis and Transesterification
3.4. Mass Spectrometry (MS) Analyses
3.5. Hemolytic Activity (HA) Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Li, T.; Fong, C.M.V.; Chen, X.; Chen, X.J.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from chinese medicines as anticancer agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.A. Some terpenoid and steroid derivatives from echinoderms and sponges. Pure Appl. Chem. 1986, 58, 423–436. [Google Scholar] [CrossRef]
- Demeyer, M.; de Winter, J.; Caulier, G.; Eeckhaut, I.; Flammang, P.; Gerbaux, P. Molecular diversity and body distribution of saponins in the sea star Asterias rubens by mass spectrometry. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2014, 168, 1–11. [Google Scholar] [CrossRef]
- Savarino, P.; Colson, E.; Caulier, G.; Eeckhaut, I.; Flammang, P.; Gerbaux, P. Microwave-assisted desulfation of the hemolytic saponins extracted from Holothuria scabra viscera. Molecules 2022, 27, 537. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Chemistry & Pharmacology of Natural Products—Saponins; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Kassem Algfri, S.; Alshakka, M.; Tariq Munaiem, R. Study of saponins in methanol extract of the leaves of Acacia etbaica subspecies etbaica. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 803–810. [Google Scholar]
- Yang, Y.; Laval, S.; Yu, B. Chemical synthesis of saponins. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 71, pp. 137–226. [Google Scholar]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Caulier, G.; Flammang, P.; Rakotorisoa, P.; Gerbaux, P.; Demeyer, M.; Eeckhaut, I. Preservation of the bioactive saponins of Holothuria scabra through the processing of trepang. Cah. Biol. Mar. 2013, 54, 685–690. [Google Scholar]
- Tantry, M.A.; Khan, I.A. Saponins from Glycine Max Merrill (Soybean). Fitoterapia 2013, 87, 49–56. [Google Scholar] [CrossRef]
- Honey-Escandón, M.; Arreguín-Espinosa, R.; Solís-Marín, F.A.; Samyn, Y. Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (echinodermata, holothuroidea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 180, 16–39. [Google Scholar] [CrossRef]
- Caulier, G.; Mezali, K.; Soualili, D.L.; Decroo, C.; Demeyer, M.; Eeckhaut, I.; Gerbaux, P.; Flammang, P. Chemical characterization of saponins contained in the body wall and the cuvierian tubules of the sea cucumber Holothuria (Platyperona) sanctori (Delle Chiaje, 1823). Biochem. Syst. Ecol. 2016, 68, 119–127. [Google Scholar] [CrossRef]
- Miyazaki, S.; Ichiba, T.; Reimer, J.D.; Tanaka, J. Chemoattraction of the pearlfish Encheliophis vermicularis to the sea cucumber Holothuria leucospilota. Chemoecology 2014, 24, 121–126. [Google Scholar] [CrossRef]
- Caulier, G.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. When a repellent becomes an attractant: Harmful saponins are kairomones attracting the symbiotic harlequin crab. Sci. Rep. 2013, 3, 2639. [Google Scholar] [CrossRef]
- Moghimipour, E.; Handali, S. Saponin: Properties, methods of evaluation and applications. Ann. Res. Rev. Biol. 2015, 5, 207–220. [Google Scholar] [CrossRef]
- Costa, T.S.A.; Vieira, R.F.; Bizzo, H.R.; Silveira, D.; Gimenes, M.A. Secondary metabolites. In Chromatography and Its Applications; Dhanarasu, S., Ed.; InTech: São Paulo, Brazil, 2012. [Google Scholar]
- Chen, Z.; Duan, H.; Tong, X.; Hsu, P.; Han, L.; Morris-Natschke, S.L.; Yang, S.; Liu, W.; Lee, K.H. Cytotoxicity, Hemolytic Toxicity, and Mechanism of Action of Pulsatilla Saponin D and Its Synthetic Derivatives. J. Nat. Prod. 2018, 81, 465–474. [Google Scholar] [CrossRef]
- Mohammadizadeh, F.; Ehsanpor, M.; Afkhami, M.; Mokhlesi, A.; Khazaali, A.; Montazeri, S. Evaluation of antibacterial, antifungal and cytotoxic effects of Holothuria scabra from the north coast of the persian gulf. J. Mycol. Med. 2013, 23, 225–229. [Google Scholar] [CrossRef]
- Thaweboon, S.; Thaweboon, B. Assessment of antifungal activity of aloe vera toothpaste against Candida albicans. IOP Conf. Ser. Mater. Sci. Eng. 2020, 761, 012007. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Amoros, M.; Girre, L. Mechanism of antiviral activity of triterpenoid saponins. Phytother. Res. 1999, 13, 323–328. [Google Scholar] [CrossRef]
- Regalado, E.L.; Tasdemir, D.; Kaiser, M.; Cachet, N.; Amade, P.; Thomas, O.P. antiprotozoal steroidal saponins from the marine sponge Pandaros acanthifolium. J. Nat. Prod. 2010, 73, 1404–1410. [Google Scholar] [CrossRef]
- Frenkel, N.; Makky, A.; Sudji, I.R.; Wink, M.; Tanaka, M. Mechanistic investigation of interactions between steroidal saponin digitonin and cell membrane models. J. Phys. Chem. B 2014, 118, 14632–14639. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.; le Duff, C.S.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. Induction of highly curved structures in relation to membrane permeabilization and budding by the triterpenoid saponins, α- and δ-hederin. J. Biol. Chem. 2013, 288, 14000–14017. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.; Lins, L.; Domenech, Ò.; Quetin-Leclercq, J.; Brasseur, R.; Mingeot-Leclercq, M.P. Domain formation and permeabilization induced by the saponin α-hederin and its aglycone hederagenin in a cholesterol-containing bilayer. Langmuir 2014, 30, 4556–4569. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.; Melzig, M.F. The influence of saponins on cell membrane cholesterol. Bioorg. Med. Chem. 2013, 21, 7118–7124. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, M.; Lalun, N.; Bobichon, H.; Plé, K.; Voutquenne-Nazabadioko, L. Structure-activity relationships of some hederagenin diglycosides: Haemolysis, cytotoxicity and apoptosis induction. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1418–1427. [Google Scholar] [CrossRef]
- Savarino, P.; Contino, C.; Colson, E.; Cabrera-Barjas, G.; de Winter, J.; Gerbaux, P. Impact of the hydrolysis and methanolysis of bidesmosidic Chenopodium quinoa saponins on their hemolytic activity. Molecules 2022, 27, 3211. [Google Scholar] [CrossRef]
- Grzywacz, D.; Liberek, B.; Myszka, H. Synthesis, modification and biological activity of diosgenyl β-d-glycosaminosides: An overview. Molecules 2020, 25, 5433. [Google Scholar] [CrossRef]
- Marciani, D.J. Effects of N-acylation on the immune adjuvanticity of analogs of the quillaja saponins derivative GPI-0100. Vaccine 2022, 40, 4169–4173. [Google Scholar] [CrossRef]
- Fuentes, R.; Aguinagalde, L.; Pifferi, C.; Plata, A.; Sacristán, N.; Castellana, D.; Anguita, J.; Fernández-Tejada, A. Novel oxime-derivatized synthetic triterpene glycosides as potent saponin vaccine adjuvants. Front. Immunol. 2022, 13, 865507. [Google Scholar] [CrossRef]
- Abudayeh, Z.H.M.; al Azzam, K.M.; Naddaf, A.; Karpiuk, U.V.; Kislichenko, V.S. Determination of four major saponins in skin and endosperm of seeds of horse chestnut (Aesculus hippocastanum L.) using high performance liquid chromatography with positive confirmation by thin layer chromatography. Adv. Pharm. Bull. 2015, 5, 587–591. [Google Scholar] [CrossRef]
- Yi, H.Y.; Lee, J.Y. Poisoning due to consumption of horse chestnut seed. Clin. Exp. Emerg. Med. 2021, 8, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Ratschow, M.; Bodecker, H. Parenteral venostasin therapy. Munch. Med. Wochenschr. 1952, 94, 1368–1373. [Google Scholar] [PubMed]
- Yoshikawa, M.; Harada, E.; Murakami, T.; Matsuda, T.; Wariishi, N.; Yamahara, Y.; Murakami, N.; Kitagawa, I. Escins-Ia, Ib, IIa, IIb, and IIIa, bioactive triterpene oligoglycosides from the seeds of Aesculus hippocastanum L.: Their inhibitory effects on ethanol absorption and hypoglycemic activity on glucose tolerance test. Chem. Pharm. Bull. 1994, 42, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Li, Y.; Murakami, T.; Ninomiya, K.; Yamahara, J.; Yoshikawa, M. Effects of Escins Ia, Ib, IIa, and IIb from horse chestnut, the seeds of Aesculus hippocastanum L., on acute inflammation in animals. Biol. Pharm. Bull. 1997, 20, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Yamahara, J.; Matsuda, H. Bioactive saponins and glycosides. III. Horse chestnut. (1): The structures, inhibitory effects on ethanol absorption, and hypoglycemic activity of Escins Ia, Ib, IIa, IIb, and IIIa from the seeds of Aesculus hippocastanum L. Biol. Pharm. Bull. 1998, 46, 1764–1769. [Google Scholar] [CrossRef]
- Patlolla, J.M.R.; Rao, C.V. Anti-inflammatory and anti-cancer properties of β-Escin, a triterpene saponin. Curr. Pharm. Rep. 2015, 1, 170–178. [Google Scholar] [CrossRef]
- Colson, E.; Decroo, C.; Cooper-Shepherd, D.; Caulier, G.; Henoumont, C.; Laurent, S.; de Winter, J.; Flammang, P.; Palmer, M.; Claereboudt, J.; et al. Discrimination of regioisomeric and stereoisomeric saponins from Aesculus hippocastanum seeds by ion mobility mass spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 2228–2237. [Google Scholar] [CrossRef]
- Wagner, J.; Hoffmann, H.; Löw, I. Die acylaglyka des kryptoäscins und a-Ascins. Hoppe Seyler’s Z. Physiol. Chem. 1970, 351, 1133–1140. [Google Scholar] [CrossRef]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharm. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef]
- Aparecida de Almeida, P.; Campos Alves, M. New HPLC method for quality control of β-Escin in Aesculus hippocastanum L. Hydroalcoholic Extract. Lat. Am. J. Pharm. 2013, 32, 1082–1087. [Google Scholar]
- Wu, X.-J.; Zhang, M.-L.; Cui, X.-Y.; Gao, F.; He, Q.; Li, X.-J.; Zhang, J.-W.; Fawcett, J.P.; Gu, J.-K. Comparative pharmacokinetics and bioavailability of Escin Ia and Isoescin Ia after administration of Escin and of Pure Escin Ia and Isoescin Ia in rat. J. Ethnopharmacol. 2012, 139, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Ma, L.Y.; Cheng, X.L.; Lin, R.C.; Jin, W.T.; Khan, I.A.; Lu, J.Q. Preparative HPLC for purification of four isomeric bioactive saponins from the seeds of Aesculus Chinensis. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 763–773. [Google Scholar] [CrossRef]
- Savarino, P.; Demeyer, M.; Decroo, C.; Colson, E.; Gerbaux, P. Mass spectrometry analysis of saponins. Mass Spectrom. Rev. 2021, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Shukla, U.N.; Mishra, M.L.; Sood, S. Phyto-nutritional and mineral composition of indian horse chestnut (Aesculus indica) seeds. J. Pharmacogn. Phytochem. 2018, 7, 2159–2162. [Google Scholar]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Reference Publications: Algonac, MI, USA, 1985; pp. 1–2. ISBN 0-917256-20-4. [Google Scholar]
- Shukla, U.N.; Lata Mishra, M.; Shukla, U.N. Indian horse chestnut (Aesculus indica): A wild fruit. Pop. Kheti 2017, 5, 25–27. [Google Scholar]
- Herrera, T.; Navarro del Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Acid hydrolysis of saponin-rich extracts of quinoa, lentil, fenugreek and soybean to yield sapogenin-rich extracts and other bioactive compounds. J. Sci. Food Agric. 2019, 99, 3157–3167. [Google Scholar] [CrossRef]
- Mulzer, J. Synthesis of esters, activated esters and lactones. Compr. Org. Synth. 1991, 6, 323–380. [Google Scholar]
- Tschesche, R.; Tjoa, B.T.; Wulff, G. Über Triterpene XXIII—Über struktur und chemie der priverogenine. Tet. Lett. 1968, 1, 183–188. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Prokofieva, N.G.; Likhatskaya, G.N.; Schentsova, E.B.; Agafonova, I.G.; Avilov, S.A.; Drozdova, A. Hemolytic activities of triterpene glycosides from the holothurian order dendrochirotida: Some trends in the evolution of this group of toxins. Toxicon 1996, 34, 475–483. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, X.; Liu, S. Efficient improvement of surface activity of tea saponin through gemini-like modification by straightforward esterification. Food Chem. 2015, 171, 272–279. [Google Scholar] [CrossRef]
- Vo, N.N.Q.; Fukushima, E.O.; Muranaka, T. Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. J. Nat. Med. 2017, 71, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Voutquenne, L.; Lavaud, C.; Massiot, G.; Le Men-Olivier, L. Structure-activity relationships of haemolytic saponins. Pharm. Biol. 2002, 40, 253–262. [Google Scholar] [CrossRef]
- Takechi, M.; Tanaka, Y. Structure-activity relationships of the saponin a-hederin. Phytochem. 1990, 29, 451–452. [Google Scholar] [CrossRef]
- Mackie, A.M.; Lasker, R.; Grant, P.T. Avoidance reactions of a mollusc Buccinum undatum to saponin-like surface-active substances in extracts of the starfish Asterias rubens and Marthasterias glacialis. Comp. Biochem. Physiol. 1968, 26, 415–418. [Google Scholar] [CrossRef]
- Colson, E.; Savarino, P.; Claereboudt, E.J.S.; Cabrera-Barjas, G.; Deleu, M.; Lins, L.; Eeckhaut, I.; Flammang, P.; Gerbaux, P. Enhancing the membranolytic activity of Chenopodium Quinoa saponins by fast microwave hydrolysis. Molecules 2020, 25, 1731. [Google Scholar] [CrossRef]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro, M.D.L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol. Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef]
- de Groot, C.; Müller-Goymann, C. Saponin interactions with model membrane systems—Langmuir monolayer studies, hemolysis and formation of ISCOMs. Planta Med. 2016, 82, 1496–1512. [Google Scholar] [CrossRef]
- Efimova, S.S.; Ostroumova, O.S. Is the membrane lipid matrix a key target for action of pharmacologically active plant saponins? Int. J. Mol. Sci. 2021, 22, 3167. [Google Scholar] [CrossRef]
- Claereboudt, E.J.S.; Eeckhaut, I.; Lins, L.; Deleu, M. How different sterols contribute to saponin tolerant plasma membranes in sea cucumbers. Sci. Rep. 2018, 8, 10845. [Google Scholar] [CrossRef]
- Bedini, E.; Laezza, A.; Parrilli, M.; Iadonisi, A. A review of chemical methods for the selective sulfation and desulfation of polysaccharides. Carbohydr. Polym. 2017, 174, 1224–1239. [Google Scholar] [CrossRef]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Qualitative and quantitative saponin contents in five sea cucumbers from the indian ocean. Mar. Drugs 2010, 8, 173–189. [Google Scholar] [CrossRef] [PubMed]

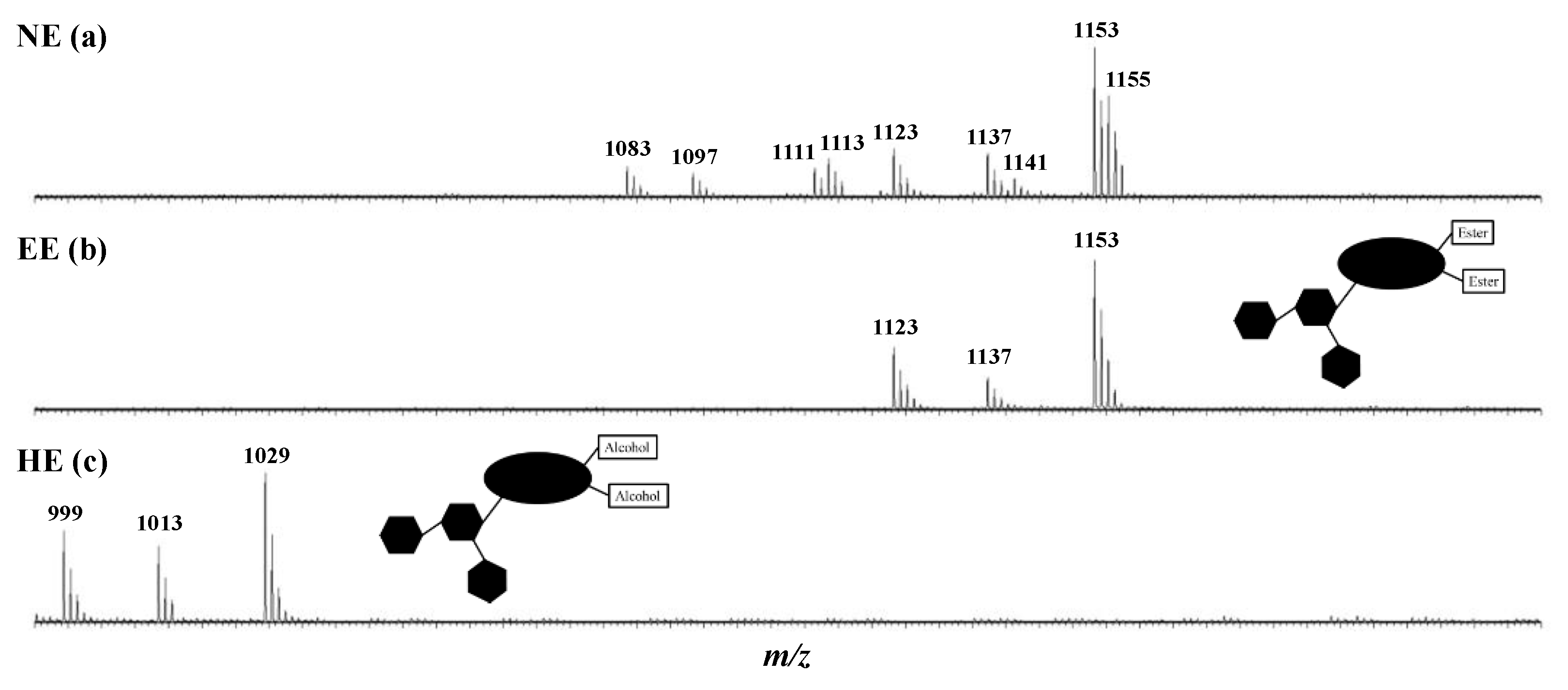
| Saponins | Elemental Compositions (M) | m/z (Δ ppm) [M + Na]+ | R1 | R2 | R3 | R4 | R5 | R6 | %-Weights in Extract (%) | Mass Fractions in Seed (mg·g−1) | Retention Time (min) | Molar Proportions (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escin Ia | C55H86O24 | 1153.5427 (1.7) | Tig | Ac | H | H | OH | Glc | 55.85 ± 0.03 | 79.46 | 7.96 | 21.72 ± 0.09 |
| Escin Ib | Ang | Ac | H | H | OH | Glc | 8.25 | 18.40 ± 0.08 | ||||
| Isoescin Ia | Tig | H | Ac | H | OH | Glc | 8.59 | 10.77 ± 0.08 | ||||
| Isoescin Ib | Ang | H | Ac | H | OH | Glc | 8.88 | 9.32 ± 0.14 | ||||
| Escin IIa | C54H84O23 | 1123.5342 (3.6) | Tig | Ac | H | H | OH | Xyl | 4.18 ± 0.11 | 5.95 | 7.98 | 1.48 ± 0.09 |
| Escin IIb | Ang | Ac | H | H | OH | Xyl | 8.28 | 1.26 ± 0.07 | ||||
| Isoescin IIa | Tig | H | Ac | H | OH | Xyl | 8.66 | 0.93 ± 0.12 | ||||
| Isoescin IIb | Ang | H | Ac | H | OH | Xyl | 8.94 | 0.99 ± 0.09 | ||||
| Escin IIIa | C55H86O23 | 1137.5453 (0.4) | Tig | Ac | H | H | H | Gal | 4.74 ± 0.08 | 6.75 | 8.45 | 1.96 ± 0.08 |
| Escin IIIb | Ang | Ac | H | H | H | Gal | 8.84 | 1.66 ± 0.06 | ||||
| Isoescin IIIa | Tig | H | Ac | H | H | Gal | 9.18 | 0.82 ± 0.03 | ||||
| Isoescin IIIb | Ang | H | Ac | H | H | Gal | 9.54 | 0.75 ± 0.04 | ||||
| Escin IV | C52H82O24 | 1113.5134 (3.6) | Ac | Ac | H | H | OH | Glc | 15.22 ± 0.16 | 21.66 | 6.10 | 9.16 ± 0.26 |
| Isoescin IV | Ac | H | Ac | H | OH | Glc | 6.56 | 7.84 ± 0.17 | ||||
| Escin V | C54H86O24 | 1141.5435 (2.6) | iBu | Ac | H | H | OH | Glc | 7.27 ± 0.21 | 10.34 | 7.71 | 6.81 ± 0.15 |
| Isoescin V | iBu | H | Ac | H | OH | Glc | 8.20 | 1.11 ± 0.24 | ||||
| Escin VI | C55H88O24 | 1155.5537 (2.3) | mBu | Ac | H | H | OH | Glc | 2.47 ± 0.17 | 3.52 | 8.62 | 2.18 ± 0.01 |
| Isoescin VI | mBu | H | Ac | H | OH | Glc | 9.47 | 0.48 ± 0.05 | ||||
| Escin VII | C51H80O23 | 1083.5001 (1.2) | Ac | Ac | H | H | OH | Xyl | 0.56 ± 0.10 | 0.79 | 6.12 | 0.31 ± 0.09 |
| Isoescin VII | Ac | H | Ac | H | OH | Xyl | 6.59 | 0.33 ± 0.06 | ||||
| Escin VIII | C53H84O23 | 1111.5316 (1.3) | iBu | Ac | H | H | OH | Xyl | 0.78 ± 0.13 | 1.11 | 7.42 | 0.54 ± 0.03 |
| Isoescin VIII | iBu | H | Ac | H | OH | Xyl | 7.64 | 0.33 ± 0.04 | ||||
| Escin IX | C52H82O23 | 1097.5182 (3.4) | Ac | Ac | H | H | H | Glc | 0.75 ± 0.17 | 1.07 | 6.44 | 0.46 ± 0.05 |
| Isoescin IX | Ac | H | Ac | H | H | Glc | 6.83 | 0.39 ± 0.07 |
| Elemental Compositions (M) | m/z (Δ ppm) [M + Na]+ | Isomer | Retention Time (min) | Molar Proportion (%) | %-Weights in Extract (%) | Isomer Molar Proportions (%) |
|---|---|---|---|---|---|---|
| Escin I C55H86O24 | 1153.5427 (1.7) | Escin Ia | 7.96 | 85.20 ± 0.09 | 27.21 ± 0.05 | 32.06 ± 0.04 |
| Escin Ib | 8.25 | 21.99 ± 0.09 | 25.92 ± 0.07 | |||
| Isoescin Ia | 8.59 | 19.61 ± 0.07 | 23.13 ± 0.06 | |||
| Isoescin Ib | 8.88 | 15.99 ± 0.11 | 18.89 ± 0.09 | |||
| Escin II C54H84O23 | 1123.5342 (3.6) | Escin IIa | 7.98 | 7.53 ± 0.12 | 2.75 ± 0.09 | 37.89 ± 0.08 |
| Escin IIb | 8.28 | 2.48 ± 0.06 | 34.26 ± 0.04 | |||
| Isoescin IIa | 8.66 | 1.18 ± 0.07 | 16.94 ± 0.05 | |||
| Isoescin IIb | 8.94 | 0.82 ± 0.04 | 10.91 ± 0.03 | |||
| Escin III C55H86O23 | 1137.5453 (0.4) | Escin IIIa | 8.45 | 7.26 ± 0.09 | 2.69 ± 0.06 | 38.43 ± 0.05 |
| Escin IIIb | 8.84 | 2.36 ± 0.09 | 33.92 ± 0.04 | |||
| Isoescin IIIa | 9.18 | 1.15 ± 0.05 | 17.20 ± 0.03 | |||
| Isoescin IIIb | 9.54 | 0.66 ± 0.02 | 10.45 ± 0.02 |
| Elemental Compositions (M) | m/z (Δ ppm) [M + Na]+ | R1 | R2 | R3 | R4 | R5 | R6 | Retention Time (min) | Molar Proportion (%) |
|---|---|---|---|---|---|---|---|---|---|
| Desacylescin I C48H78O22 | 1029.4876 (0.7) | H | H | H | H | OH | Glc | 5.38 | 83.71 ± 0.48 |
| Desacylescin II C47H76O21 | 999.4745 (3.2) | H | H | H | H | OH | Xyl | 5.36 | 8.15 ± 0.06 |
| Desacylescin III C48H78O21 | 1013.4953 (2.0) | H | H | H | H | H | Gal | 5.39 | 8.14 ± 0.51 |
| Saponins | Elemental Compositions (M) | m/z (Δ ppm) [M + Na]+ | R1 | R2 | R3 | R4 | R5 | R6 | Retention Time (min) | Composition Molar Proportions (%) | %-Weights in Extract (%) | Isomer Molar Proportions (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escin Ia | C55H86O24 | 1153.5427 (1.7) | Tig | Ac | H | H | OH | Glc | 7.96 | 84.44 ± 0.18 | 19.91 ± 0.09 | 23.93 ± 0.17 |
| Escin Ib | Ang | Ac | H | H | OH | Glc | 8.25 | 15.80 ± 0.10 | 18.99 ± 0.23 | |||
| Isoescin Ia | Tig | H | Ac | H | OH | Glc | 8.59 | 16.53 ± 0.08 | 19.89 ± 0.15 | |||
| Isoescin Ib | Ang | H | Ac | H | OH | Glc | 8.88 | 14.63 ± 0.06 | 17.59 ± 0.21 | |||
| Transescin Ia | Tig | H | H | Ac | OH | Glc | 6.59 | 9.22 ± 0.03 | 11.08 ± 0.13 | |||
| Transescin Ib | Ang | H | H | Ac | OH | Glc | 6.86 | 7.10 ± 0.09 | 8.53 ± 0.21 | |||
| Escin IIa | C54H84O23 | 1123.5342 (3.6) | Tig | Ac | H | H | OH | Xyl | 7.98 | 8.72 ± 0.23 | 1.68 ± 0.08 | 21.28 ± 0.26 |
| Escin IIb | Ang | Ac | H | H | OH | Xyl | 8.28 | 1.48 ± 0.07 | 18.72 ± 0.50 | |||
| Isoescin IIa | Tig | H | Ac | H | OH | Xyl | 8.66 | 1.58 ± 0.08 | 19.98 ± 0.45 | |||
| Isoescin IIb | Ang | H | Ac | H | OH | Xyl | 8.94 | 1.57 ± 0.07 | 19.83 ± 0.68 | |||
| Transescin IIa | Tig | H | H | Ac | OH | Xyl | 6.58 | 1.01 ± 0.01 | 12.84 ± 0.24 | |||
| Transescin IIb | Ang | H | H | Ac | OH | Xyl | 6.85 | 0.69 ± 0.06 | 8.79 ± 0.27 | |||
| Escin IIa | C55H86O23 | 1137.5453 (0.4) | Tig | Ac | H | H | H | Gal | 8.45 | 6.84 ± 0.42 | 1.38 ± 0.08 | 22.53 ± 0.26 |
| Escin IIb | Ang | Ac | H | H | H | Gal | 8.84 | 1.13 ± 0.03 | 18.43 ± 0.73 | |||
| Isoescin IIa | Tig | H | Ac | H | H | Gal | 9.18 | 1.21 ± 0.07 | 19.69 ± 0.69 | |||
| Isoescin IIb | Ang | H | Ac | H | H | Gal | 9.54 | 1.10 ± 0.08 | 17.91 ± 0.20 | |||
| Transescin IIa | Tig | H | H | Ac | H | Gal | 7.00 | 0.72 ± 0.02 | 11.73 ± 0.28 | |||
| Transescin IIb | Ang | H | H | Ac | H | Gal | 7.27 | 0.60 ± 0.03 | 9.71 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savarino, P.; Colson, E.; André, J.; Gerbaux, P. Horse Chestnut Saponins–Escins, Isoescins, Transescins, and Desacylescins. Molecules 2023, 28, 2087. https://doi.org/10.3390/molecules28052087
Savarino P, Colson E, André J, Gerbaux P. Horse Chestnut Saponins–Escins, Isoescins, Transescins, and Desacylescins. Molecules. 2023; 28(5):2087. https://doi.org/10.3390/molecules28052087
Chicago/Turabian StyleSavarino, Philippe, Emmanuel Colson, Julien André, and Pascal Gerbaux. 2023. "Horse Chestnut Saponins–Escins, Isoescins, Transescins, and Desacylescins" Molecules 28, no. 5: 2087. https://doi.org/10.3390/molecules28052087
APA StyleSavarino, P., Colson, E., André, J., & Gerbaux, P. (2023). Horse Chestnut Saponins–Escins, Isoescins, Transescins, and Desacylescins. Molecules, 28(5), 2087. https://doi.org/10.3390/molecules28052087







