Abstract
The chemical investigation of branches of Cinnamomum camphora chvar. Borneol guided by mosquito larvicidal activity led to the isolation of fourteen known lignans (1–14). Their structures were elucidated unambiguously based on comprehensive spectroscopic analysis and comparison with the literature data. This is the first report of these compounds being isolated from branches of Cinnamomum camphora chvar. Borneol. Compounds 3–5 and 8–14 were isolated from this plant for the first time. All compounds isolated were subjected to anti-inflammatory, mosquito larvicidal activity and cytotoxic activity evaluation. Compounds (1–14) showed significant mosquito larvicidal activity against Culex pipiens quinquefasciatus with lethal mortality in 50% (LC50), with values ranging from 0.009 to 0.24 μg/mL. Among them, furofuran lignans(1–8) exhibited potent mosquito larvicidal activity against Cx. p. quinquefasciatus, with LC50 values of 0.009–0.021 μg/mL. From the perspective of a structure–activity relationship, compounds with a dioxolane group showed high mosquito larvicidal activity and have potential to be developed into a mosquitocide.
1. Introduction
Mosquitoes transmit various diseases such as malaria, which caused 409,000 deaths in 2019 [1]. Culex pipiens quinquefasciatus is widely distributed south of Yangtze river in China and is known as the Japanese encephalitis virus (JEV) vector in China [2]. Because of the lack of vaccines, vector control has been considered as an effective approach to reducing mosquito-borne cases [3]. However, the extensive use of limited available chemicals has caused increasing resistance. For example, Cx. p. quinquefasciatus has become more or less resistant to permethrin, deltamethrin, temephos, chlorpyrifos, malathion and dieldrin in La Réunion Island [4].
Cinnamomum camphora chvar. Bornel is a subtropical evergreen broad-leaved tree belonging to the Cinnamomum camphora of Lauraceae. This species is considered to be a special chemical type of camphor tree. The volatile oil extracted from its fresh branches and leaves is rich in D-borneol (natural borneol), which is the best plant choice for obtaining natural borneol at present. However, few studies have been conducted on the chemical components other than its non-volatile oil. Previous studies found that the crude CH2Cl2 fraction obtained from the EtOH extract of branches of Cinnamomum camphora chvar. Borneol had excellent mosquito larvicidal activity against Culex pipiens quinquefasciatus. In our further searches for mosquito larvicidal active metabolites from the crude CH2Cl2 extraction, 14 lignans were afforded. We report herein the isolation, structure elucidation and biological activity of them.
2. Results
2.1. Structure Elucidation of the Isolated Compound
The crude n-hexane, CH2Cl2, EtOAc, n-BuOH and aqueous phase fractions were obtained from the EtOH extract of branches of Cinnamomum camphora chvar. Borneol by extraction. Among the five extraction stages, the CH2Cl2 fraction displayed the most prominent mosquito larvicidal activity against Cx. p. quinquefasciatus with lethal mortality in 50% (LC50) values of 0.032 μg/mL. To further explore the mosquito larvicidal chemical components, the CH2Cl2 extract was subjected to silica gel chromatography, Sephadex LH-20, reversed phase column chromatography and Preparative HPLC. This led to the isolation of compounds 1–14 (Figure 1), including Medioresinol (1) [5], Syringaresinol (2) [6], Pinoresinol (3) [7], Kobusin (4) [8], piperitol (5) [9], sesamin (6) [10], 9(R)-hydroxy-d-sesamin (7) [11], aptosimon (8) [12], acuminatolide (9) [13], (2R, 3R)-2,3-di-(3, 4-dimethoxybenzyl)-butyrolactone (10) [14], (−)-Dihydro-3′,4′-dimethoxy-3′,4′-demethylenedloxycubebin (11) [15], balanophonin (12) [16], buddlenol D (13) [17], (7R, 7′R, 7″S,7‴S, 8S, 8′S, 8″S, 8‴S)-4″,4‴-dihydroxy-3, 3′, 3″, 3‴, 5, 5′-hexamethoxy-7, 9′; 7′, 9-diepoxy-4, 8″; 4’, 8‴-bisoxy-8 and 8′-dineolignan-7″, 7‴, 9″, 9‴-tetraol (14) [18].
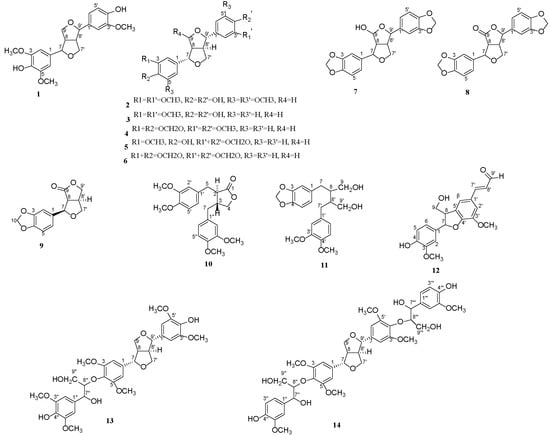
Figure 1.
Chemical structures of compounds 1–14.
Compounds 3–5 and 8–14 were first isolated from the titled plant and structures of all compounds were identified based on NMR spectroscopic methods, mass spectrometry, as well as by comparison with the literature data (See the Figures S1–S42 in Supplementary Information). Compounds 1–8 were furan lignans with the same basic mother nucleus and the relative configuration subtypes of compounds 2–6 were decided as trans-(H-7, 8, 8′, 9) by the shift difference between two protons at position 7′ and the shift difference between two protons at position 9 (ΔδH-7′ and ΔδH-9 =0.3~0.4) [19]. More specifically, the structural differences of compounds 1–3 were reflected in the substitution of hydroxyl and methoxy groups on the benzene ring. Compounds 4–5 and 6–8 differed structurally from compounds 1–3 in that they contained one or two methylenedioxy groups. Different similar compounds with different substituents may have led to different biological activities.
2.2. Mosquito Larvicidal Activity of Lignans(1–14)
All compounds isolated were subjected to mosquito larvicidal activity evaluation. As shown in Table 1, compounds (1–14) showed significant mosquito larvicidal activity against Cx. p. quinquefasciatus, with LC50 values ranging from 0.009 to 0.24 μg/mL. No mortality was observed in the DMSO-treated group but permethrin (positive control) showed high toxicity with an LC50 value of 0.007 μg/mL. Among them, furofuran lignans (1–8) exhibited potent mosquito larvicidal activity against Cx. p. quinquefasciatus, with LC50 values of 0.009–0.021 μg/mL. In particular, kobusin (4), piperitol (5), sesamin (6) and aptosimon (8) exhibited comparable mosquito larvicidal activity against Cx. p. quinquefasciatus to that of the positive control, with LC50 values of 0.01, 0.009, 0.01 and 0.011, respectively. Other furan lignans, including Medioresinol (1), Syringaresinol (2), Pinoresinol (3), 9(R)-hydroxy-d-sesamin (7), acuminatolide (9), buddlenol D (13) and (7R, 7′R, 7″S, 7‴S, 8S, 8′S, 8″S, 8‴S)-4″,4‴-dihydroxy-3, 3’, 3″, 3‴, 5, 5′-hexamethoxy-7, 9′; 7′, 9-diepoxy-4, 8″; 4’,8‴-bisoxy-8, 8’-dineolignan-7″, 7‴, 9″, 9‴-tetraol (14) exhibited slightly weaker anti-mosquito activity compared to the above compounds, with LC50 values of 0.02, 0.021, 0.021, 0.016, 0.047, 0.039, 0.039, respectively. The mosquito larvicidal activity against Cx. p. quinquefasciatus of (2R, 3R)-2,3-di-(3, 4-dimethoxybenzyl)-butyrolactone (10), (−)-Dihydro-3′,4′-dimethoxy-3′,4′-demethylenedl-oxycubebin (11) and balanophonin (12) showed a steep decrease compared to furan lignans, with LC50 values of 0.106, 0.240, 0.185, respectively.

Table 1.
Effects of the CH2Cl2 fraction and compounds 1–14 against Culex pipiens quinquefasciatus. LC50 values (concentrations that caused mortality in 50 % of a sample population) were determined at 24 h.
2.3. Anti-Inflammatory Activity, Cytotoxic Activity and Evaluation of Lignans(1–14)
All compounds isolated were subjected to anti-inflammatory and cytotoxic activity evaluation. Unfortunately, as shown in Figure 2 and Figure 3, all compounds did not show significant NO inhibitory activity on lipopolysaccharide-induced RAW 264 cells (compared with the LPS induction group, the inhibition rate was <50% at 10 μM) or cytotoxicity against the human tumor cell HepG2 (with an inhibition rate of <50% at 50 μM).
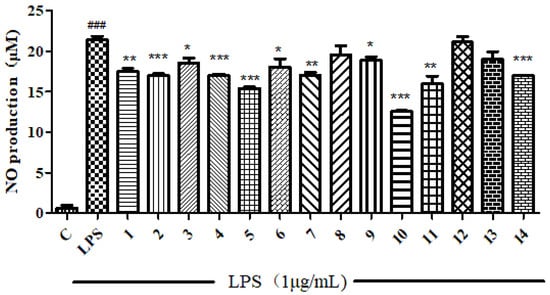
Figure 2.
The ability to inhibit LPS-induced NO production in RAW 264 cells of compounds 1–14. *** means the significant difference at p < 0.001, ** means p < 0.01, * means p < 0.05 compared compounds 1–14 with LPS. ### means p < 0.001 compared LPS with Control.
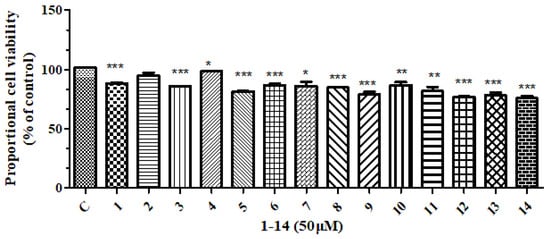
Figure 3.
The effect of compounds 1–14 on the HepG2 cell viability at 50 μM. *** means p < 0.001, ** means p < 0.01, * means p < 0.05 compared compounds 1–14 with Control.
3. Discussion
Compounds 1–9, 13 and 14 are furofuran lignans that feature with a bicyclic oxygen skeleton; these mainly showed antioxidant, insecticidal, inhibitory activity against AChE and NO production in LPS-treated BV-2 microglial cells [20]. Among them, Sesamin (7) has the effect of lowering cholesterol in clinical applications [20,21]. Two dibenzylbutane lignans (10–11) were also isolated from Virola Venosa, but their biological activity has been poorly reported [15]. Benzodihydrofuran neolignan balanophonin (12) was first isolated from the plant Balanophora japonica Makino and has obvious PGI2 induction activity [22].
Compounds 1–14 showed broad mosquito larvicidal activity against Cx. p. quinquefasciatus with LC50 values ranging from 0.009 to 0.24 μg/mL. Similarly, lignans have proven potential in mosquito control. Leptostachyol acetate was found to be lethal to Culex pipiens pallens, Aedes aegypti and Ocheratatos togoi [23]; haedoxan A exhibited high activity against Aedes aegypti larvae [24]; Phrymarolin-I, haedoxane A and haedoxane E were toxic to Cx. p. pallens [25]; and (+)-xanthoxylol-γ,γ-dimethylallylether (XDA) showed ability against Culex pipiens pallens and Aedes aegypti [26].
In a sense, as the main characteristic component of the branches of Cinamomum camphora chvar. Borneol, lignans may play a certain role in ecology such as protecting themselves from mosquitoes and pests. Among them, furofuran lignans(1–8) exhibited potent mosquito larvicidal activity against Cx. p. quinquefasciatus, with LC50 values of 0.009–0.021 μg/mL; these values are far stronger than compounds dibenzylbutane lignans (10–11) and benzodihydrofuran neolignan balanophonin (12), thus indicating the presence of a dioxolane group in compounds enhancing mosquito larvicidal activity. From the perspective of a structure–activity relationship, the mosquito larvicidal activity against Cx. p. quinquefasciatus in comparison to compounds 1–3, 4 and 6, 6–8 shows that there is no effect on the methoxy substitutions at the 3 and 3′ sites of the benzene ring. There is also no effect on whether the 3′ and 5′ methoxy groups form a ring, but the hydroxyl substitution and the formation of double bonds at position 9 have a significant effect. It is necessary to conduct further research on the resistance of lignans to mosquitoes and insects, especially furan lignan analogues with a methylenedioxy group such as structural optimization. This will determine whether it is related to configuration or whether it is related to bioecology.
4. Materials and Methods
4.1. General Experimental Procedures
NMR spectra were recorded on a Bruker AV-400 (Bruker Corporation, Switzerland) instrument with TMS as an internal standard. Optical rotation was recorded at 25 °C using a WYA-2S digital Abbe polarimeter (Shanghai Physico-optical Instrument Factory). ESI-MS spectra were recorded on a VG Auto Spec-3000 mass spectrometer (VG, Manchester, UK). High-resolution ESI-MS were recorded on an Agilent 6210 mass spectrometer employing peak matching. Preparative HPLC was performed on a Waters Prep 150 equipped with a Waters 2489 UV/visible detector and XBridge BEH C18 Column (130 Å, 5 μm, 4.6 mm × 250 mm). SephadexLH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), SiliaSphere C18 (SiliCycle, Quebec, QC, Canada) and Silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China) were used for column chromatography (CC). Thin-layer chromatography (TLC) was performed on precoated silica gel GF254 plates (Qingdao Marine Chemical Factory, Qingdao, China).
4.2. Plant Material and Mosquitoes
The branches of Cinnamomum camphora chvar. Borneol were collected from Ji An (114.62° E, 27.38° N), Jiang Xi Province, People’s Republic of China in November 2020, and authenticated by Professor Xionghui Li (Jiangxi Academy of Sciences). The voucher specimen (No. PML202102) was deposited in the herbarium of Jiangxi Academy of Sciences.
Cx. p. quinquefasciatus were reared in an insect room, maintained at 26 ± 1 °C, 65 ± 10% relative humidity (RH) in a 12 h: 12 h (light: dark cycle) with an artificial diet of yeast (50): lactose albumin (50).
4.3. Extraction and Isolation
The air-dried branches of Cinnamomum camphora chvar. Borneol (5 kg) were ground into powder and extracted with EtOH/H2O (95:5, v:v, 3 × 10 L) at room temperature. The filtrates were evaporated under reduced pressure to yield a crude gum (252 g), which was dissolved in warm water (50 °C) and extracted with petroleum ether (3 × 4 L), dichloromethane (3 × 4 L), ethyl acetate (3 × 4 L), n-BuOH (3 × 4 L) successively to obtain a CH2Cl2 extract (50 g). The CH2Cl2 extract (50 g) was subjected to vacuum liquid chromatography (VLC) on silica gel using a step gradient of CH2Cl2/MeOH (100:0, 100:1, 100:2, 100:4, 10:1, v:v) to afford 5 fractions (fraction 1- fraction 5) and compound 7 (80.5 mg, colorless crystals). Fraction 1 was eluted with a step gradient of petroleum ether/ethyl acetate (10:1, 5:1, 3:1, 1:1) to give 4 subfractions (fraction 1.1- fraction 1.4). Compound 6 (10.8 mg, tR 11.4 min) and compound 8 (4.0 mg, tR 45.6 min) were obtained from fraction 1.1 using a preparative RP-C18 HPLC (CH3CN–H2O, 38:62). Fraction 1.2 was further purified by Sephadex LH-20 CC (CH2Cl2/MeOH, 1:1) and finally by a preparative RP-C18 HPLC (CH3CN–H2O, 37:63) to yield compound 2 (7.6 mg, tR 10.4 min) and compound 9 (6.1 mg, tR 16.2 min). Fraction 1.3 was purified by Sephadex LH-20 CC (MeOH) and finally by a C18 ODS column eluted with 34% MeOH-H2O to obtain compound 1 (4.8 mg) and compound 3 (6.7 mg). Fraction 2 was eluted with a step gradient of petroleum ether/ethyl acetate (5:1, 3:1, 1:1) to give 3 subfractions (fraction 2.1- fraction 2.3). Compound 4 (99.4 mg) and compound 10 (644 mg) were isolated from fraction 2.1 and 2.3 by recrystallization, respectively. Compound 5 (28.8 mg) was acquired from fraction 3 by a silica gel CC equivalently eluted with petroleum ether/ethyl acetate (5:1). Fraction 4 was further purified by a C18 ODS column eluted with a step gradient of 30%–100% MeOH-H2O, giving 4 subfractions (fraction 4.1- fraction 4.4). Compounds 12 (18.6 mg, tR 55 min), 13 (5.1 mg, tR 34 min), 14 (2.3 mg, tR 53 min) and 11 (2.2 mg, tR 45 min) were isolated from fraction 4.3 by a preparative RP-C18 HPLC (CH3CN–H2O, 25:75).
Medioresinol (1): white powder; ESl-MS m/z, 388.0[M]+(C21H24O7); 1H NMR(400 MHz, CDCl3) δH 6.90 (1H, d, J = 2.0 Hz, H-2′), 6.88 (1H, d, J = 2.0 Hz, H-6′), 6.82 (1H, dd, J = 8.1, 1.8 Hz, H-5′), 6.59 (2H, s, H-2, 6), 5.65 (1H, s, C-4 or 4′ OH), 5.54 (1H, s, C-4 or 4′ OH), 4.74 (2H, dd, J = 11.4, 4.4 Hz, H-7, 9′), 4.36–4.17 (2H, m, H-7′a, 9a), 3.90 (9H, s, C-3, 3’, 5-OCH3), 3.84–3.77 (2H, m, H-7′b, 9b), 3.16–2.99 (2H, m, H-8, 8′); 13C-NMR (100 MHz, CDCl3) δC 147.3 (C-3, 5), 146.9 (C-3′), 145.4 (C-4′), 134.4 (C-4), 133.0 (C-1′), 132.3 (C-1), 119.1 (C-6′), 114.4 (C-5′), 108.8 (C-2′), 102.9 (C-2, 6), 86.3 (C-7), 86.0 (C-9′), 72.0 (C-9), 71.8 (C-7′), 56.5(3, 5-OCH3), 56.1 (3′-OCH3), 54.5 (C-8), 54.2 (C-8′).
Syringaresinol (2): white powder; ESl-MS m/z, 419.1[M + H]+(C22H27O8); 1H NMR(400 MHz, CDCl3) δH 6.57 (4H, s, H-2, 2′, 6, 6′), 5.78 (2H, s, OH), 4.72 (2H, d, J = 4.2 Hz, H-7, 9′), 4.27 (2H, dd, J = 9.0, 6.7 Hz, H-7′a, 9a), 3.87 (2H, dd, J = 9.0, 3.4 Hz, H-7′b, 9b), 3.84 (12H, s, 3, 3′, 5, 5′-OCH3), 3.09 (2H, s, H-8, 8′); 13C-NMR (100 MHz, CDCl3) δC 147.1 (C-3, 3′, 5, 5′), 134.3 (C-4, 4′), 132.0 (C-1, 1′), 102.7 (C-2, 2′, 6, 6′), 86.0 (C-7, 9′), 71.7 (C-9, 7′), 56.3(3, 3′, 5, 5′-OCH3), 54.2 (C-8, 8′).
Pinoresinol (3): Oil; HRESl-MS m/z 359.1489[M + H]+(Calcd for C20H23O6, 359.1491); 1H NMR(400 MHz, CDCl3) δH 6.81–6.90 (6H, m, H-2, 2′, 5, 5′, 6, 6′), 5.66 (2H, s, OH), 4.74 (2H, d, J = 3.7 Hz, H-7, 9′), 4.25 (2H, dd, J = 8.8, 4.4 Hz, H-7′a, 9a), 3.88 (6H, s, 3, 3′-OCH3), 3.87 (2H, dd, J = 8.8, 4.4 Hz, H-7′b, 9b), 3.10 (2H, m, H-8, 8′); 13C-NMR (100 MHz, CDCl3) δC 146.9 (C-3, 3′), 145.4 (C-4, 4′), 133.1 (C-1, 1′), 119.1 (C-6, 6′), 114.4 (C-5, 5′), 108.8 (C-2, 2′), 86.0 (C-7, 9′), 71.8 (C-7′, 9), 56.1 (3, 3′-OCH3), 54.3 (C-8, 8′).
Kobusin (4): white solid; ESl-MS m/z, 371.1[M + H]+(C21H23O6); 1H NMR(400 MHz, DMSO-d6) δH 7.06–6.76 (6H, m, H-2, 5, 6, 2′, 5′, 6′), 5.99 (2H, s, H-10), 4.66 (2H, d, J = 4.8 Hz, H-7, 9′), 4.16–4.12 (2H, m, H-9a, 7′a), 3.78 (2H, d, J = 4.0 Hz, H-9b, 7′b), 3.76 (3H, s, 4′-OCH3), 3.74 (3H, s, 3′-OCH3), 3.10–2.94 (2H, m, H-8, 8′); 13C-NMR (100 MHz, DMSO-d6) δC 148.8 (C-3′), 148.2 (C-4′), 147.4 (C-3), 146.4 (C-4), 135.5 (C-1), 133.9 (C-1′), 119.3 (C-6), 118.2 (C-6′), 111.7 (C-5), 110.0 (C-5′), 107.9 (C-2), 106.5 (C-2′), 100.8 (OCH2O), 85.0 (C-7′), 84.9 (C-7), 71.0 (C-9), 70.9 (C-9′), 55.5 (3′-OCH3), 55.5 (4′-OCH3), 53.8 (C-8), 53.6 (C-8′).
Piperitol (5): white needle crystal; ESl-MS m/z, 379.2[M + Na]+(C20H20O6Na); 1H NMR(400 MHz, DMSO-d6) δH 6.95–6.80 (4H, m, H-2, 2′, 5, 6′), 6.79–6.67 (2H, m, H-5′, 6), 5.99 (2H, s, OCH2O), 4.63 (2H, dd, J = 9.5, 4.4 Hz, H-7, 9′), 4.19–4.04 (2H, m, H-9a, 7′a), 3.77 (3H, s, 3-OCH3), 3.76–3.71 (2H, m, H-9b, 7′b), 3.09–2.93 (2H, m, H-8, 8′); 13C-NMR (100 MHz, DMSO-d6) δC 147.5 (C-3), 147.3 (C-3′), 146.4 (C-4′), 145.9 (C-4), 135.5 (C-1′), 132.2 (C-1), 119.3 (C-6′), 118.6 (C-6), 115.1 (C-5), 110.5 (C-2), 107.9 (C-5′), 106.5 (C-2′), 100.8 (OCH2O), 85.1 (C-7), 84.9 (C-9′), 71.0 (C-9), 70.8 (C-7′), 55.6 (3-OCH3), 53.8 (C-8′), 53.5 (C-8).
Sesamin (6): white needle crystal; HRESl-MS m/z 355.1163[M + H]+(Calcd for C20H19O6, 355.1165); 1H NMR(400 MHz, DMSO-d6) δH 6.90 (2H, s, H-2, 2′), 6.89–6.79 (4H, m, H-5, 5′, 6, 6′), 5.99 (4H, s, H-10, 10′), 4.64 (2H, d, J = 3.6 Hz, H-7, 9′), 4.11 (2H, dd, J = 8.6, 6.7 Hz, H-9a, 7′a), 3.76 (2H, dd, J = 9.0, 2.9 Hz, H-9b, 7′b), 3.06–2.91 (2H, m, H-8, 8′); 13C-NMR (100 MHz, DMSO-d6) δC 147.4 (C-4, 4′), 146.4 (C-3, 3′), 135.4 (C-1, 1′), 119.3 (C-6, 6′), 107.9 (C-2, 2′), 106.5 (C-5, 5′), 100.8 (C-10, 10′), 84.8 (C-7, 9′), 70.9 (C-9, 7′), 53.7 (C-8, 8′).
9(R)-Hydroxy-d-sesamin (7): white needle crystal; ESl-MS m/z, 371.2[M + H]+(C21H23O6); 1H NMR(400 MHz, DMSO-d6) δH 7.12 (1H, s, H-2), 6.94–6.82 (5H, m, H-2′, 5, 5′, 6, 6′), 6.66 (1H, d, J = 4.8 Hz, 9-OH), 6.00 (4H, s, 2×OCH2O), 5.43 (1H, d, J = 4.6 Hz, H-9), 4.78 (2H, dd, J = 27.9, 6.6 Hz, H-7, 9′), 4.12 (1H, dd, J = 8.2, 6.4 Hz, H-7′a), 3.94 (1H, d, J = 8.3 Hz, H-7′b), 3.30–2.97(1H, m, H-8′), 2.69 (1H, m, H-8); 13C-NMR (100 MHz, DMSO-d6) δC 147.5 (C-3), 147.3 (C-3′), 146.5 (C-4), 146.3 (C-4′), 137.3 (C-1), 136.2 (C-1′), 119.4 (C-6), 119.1 (C-6′), 108.0 (C-2), 107.7 (C-2′), 106.8 (C-5), 106.2 (C-5′), 100.9 (C-9), 100.8 (OCH2O), 86.0 (C-7′), 82.6 (C-7), 71.4 (C-9′), 62.1 (C-8), 53.4 (C-8′).
Aptosimon (8): colorless oil; HRESl-MS m/z 369.0961[M + H]+(Calcd for C20H17O7, 369.0958); 1H NMR(400 MHz, DMSO-d6) δH 7.06 (1H, s, H-2), 6.96–6.82 (5H, m, H-2′, 5, 5′, 6, 6′), 6.03 (4H, d, J = 8.0 Hz, H-10, 10′), 5.44 (1H, d, J = 3.6 Hz, H-7), 5.14 (1H, d, J = 3.8 Hz, H-9′), 4.17 (1H, dd, J = 9.2, 7.4 Hz, H-7′a), 3.96 (1H, d, J = 9.4, 4.5 Hz, H-7′b), 3.78 (1H, dd, J = 9.3, 3.8 Hz, H-8), 3.31–3.24(1H, m, H-8′); 13C-NMR (100 MHz, DMSO-d6) δC 176.8 (C-9), 147.7 (C-3), 147.5 (C-3′), 147.4 (C-4), 146.8 (C-4′), 134.2 (C-1), 133.5 (C-1′), 120.1 (C-6), 119.5 (C-6′), 108.1 (C-2), 108.0 (C-2′), 106.6 (C-5), 106.4 (C-5′), 101.2 (C-10), 101.0 (C-10′), 84.3 (C-7), 82.7 (C-9′), 72.2 (C-7′), 52.3 (C-8), 48.6 (C-8′).
Acuminatolide (9): white needle crystal; HRESl-MS m/z 249.0757[M + H]+(Calcd for C13H13O5, 249.0753); 1H NMR(400 MHz, DMSO-d6) δH 6.96 (1H, s, H-2), 6.87 (2H, s, H-5, 6), 6.00 (2H, s, H-10), 4.69 (1H, d, J = 6.3 Hz, H-7), 4.48 (1H, dd, J = 9.4, 6.9 Hz, H-9′a), 4.34 (1H, dd, J = 9.5, 1.8 Hz, H-7′a), 4.18 (1H, t, J = 8.6 Hz, H-9′b), 3.95 (1H, dd, J = 9.0, 3.2 Hz, H-7′b), 3.57 (1H, td, J = 8.7, 3.2 Hz, H-8′), 3.09 (1H, dtd, J = 8.6, 6.8, 1.8 Hz, H-8); 13C-NMR (100 MHz, DMSO-d6) δC 178.5 (C-9), 147.5 (C-3), 146.8 (C-4), 134.1 (C-1), 119.5 (C-6), 108.0 (C-5), 106.5 (C-2), 101.0 (C-10), 85.3 (C-7), 70.1 (C-9′), 69.5 (C-7′), 47.6 (C-8′), 45.8 (C-8).
(2R, 3R)-2,3-Di-(3, 4-dimethoxybenzyl)-butyrolactone (10): white needle crystal; HRESl-MS m/z 409.1621[M + Na]+(Calcd for C22H26O6Na, 409.1638); 1H NMR(400 MHz, DMSO-d6) δH 6.95–6.39 (6H, m, H-2′, 5′, 6′, 2″, 5″, 6″), 4.10 (1H, d, J = 7.2 Hz, H-4a), 3.88 (1H, d, J = 7.2 Hz, H-4b), 3.71 (12H, s, 3′, 4′, 3″, 4″-OCH3), 2.82 (2H, dt, J = 20.0, 11.3 Hz, H-7), 2.70 (1H, d, J = 5.0 Hz, H-2), 2.57 –2.36 (3H, m, H-3, 5); 13C-NMR (100 MHz, DMSO-d6) δC 178.3 (C-1), 148.7 (C-3′), 148.6 (C-3″), 147.5 (C-4′), 147.4 (C-4″), 131.2 (C-1′), 130.6 (C-1″), 121.2 (C-6′), 120.4 (C-6″), 113.2 (C-2′), 112.5 (C-2″), 111.9 (C-5′), 111.8 (C-5″), 70.7 (C-4), 55.4 (3′, 4′, 3″, 4″-OCH3), 45.6 (C-2), 40.8 (C-3), 36.9 (C-7), 33.7 (C-5).
(−)-Dihydro-3′,4′-dimethoxy-3′,4′-demethylenedloxycubebin (11): White powder; [α]25D-10.2 (c 0.36, CHCl3); HRESl-MS m/z 375.1802[M + H]+(Calcd for C21H27O6, 375.1805); 1H NMR(400 MHz, DMSO-d6) δH 6.84–6.53 (6H, m, H-2, 5, 6, 2′, 5′, 6′), 5.95 (2H, s, O2CH2), 4.55 (2H, d, J = 4.1 Hz, 9, 9′-OH), 3.71 (3H, s, 3′-OCH3), 3.68 (3H, s, 4′-OCH3), 3.44 –3.36 (4H, m, H-9, 9′), 2.60–2.42 (2H, m, H-7, 7′), 1.92–1.72 (2H, m, H-8, 8′); 13C-NMR (100 MHz, DMSO-d6) δC 148.5 (C-3′), 147.0 (C-3), 146.8 (C-4′), 145.0 (C-4), 135.3 (C-1), 133.9 (C-1′), 121.7 (C-6), 120.8 (C-6′), 112.6 (C-5′), 111.7 (C-2′), 109.2 (C-5), 107.7 (C-2), 100.5 (O2CH2), 60.2 (C-9), 60.1 (C-9′), 55.5 (3′-OCH3), 55.3 (4′-OCH3), 42.7(C-8), 42.4 (C-8′), 34.0 (C-7), 33.9 (C-7′).
Balanophonin (12): Pale yellow powder; HRESl-MS m/z 357.1332[M + H]+(Calcd for C20H21O6, 357.1324); 1H NMR(400 MHz, DMSO-d6) δH 9.60 (1H, d, J = 7.8 Hz, H-9′), 9.07 (1H, s, 4-OH), 7.65 (1H, d, J = 15.7 Hz, H-7′), 7.32 (2H, s, H-2′, 6′), 6.92 (1H, s, H-2), 6.80 (1H, d, J = 7.8 Hz, H-8′), 6.77 (1H, s, H-5), 6.75 (1H, s, H-6), 5.56 (1H, d, J = 6.7 Hz, H-7), 5.08 (1H, t, J = 5.0 Hz, 9-OH), 3.84 (3H, s, 3′-OCH3), 3.75 (3H, s, 3-OCH3), 3.73–3.62 (2H, m, H-9), 3.53 (1H, dd, J = 12.1, 6.0 Hz, H-8); 13C-NMR (100 MHz, DMSO-d6) δC 194.0 (C-9′), 153.9 (C-7′), 150.7 (C-4′), 147.6 (C-3), 146.6 (C-4), 144.1 (C-3′), 131.7(C-1), 130.1 (C-5′), 127.7 (C-1′), 126.1 (C-8′), 118.9 (C-6′), 118.7 (C-6), 115.4 (C-5), 112.6 (C-2′), 110.5 (C-2), 88.1 (C-7), 62.7(C-9), 55.8 (3′-OCH3), 55.7 (3-OCH3), 52.4 (C-8).
Buddlenol D (13): Colorless oil; HRESl-MS m/z 667.2348[M + Na]+(Calcd for C33H40O13Na, 667.2352); 1H NMR(400 MHz, DMSO-d6) δH 8.27 (1H, s, 4′-OH), 8.13 (1H, s, 4″-OH), 6.65 (2H, s, H-2′, 6′), 6.60 (4H, s, H-2, 6, 2″, 6″), 5.17–5.09 (1H, m, H-7″), 4.81 (1H, dd, J = 7.8, 4.6 Hz, H-8″), 4.64 (2H, dd, J = 16.4, 3.7 Hz, H-7, 9′), 4.21–4.09 (3H, m, H-9, 7′a), 4.01 (1H, dd, J = 10.0, 5.8 Hz, H-7′b), 3.77 (6H, s, 3′, 5′-OCH3), 3.75 (6H, s, 3, 5-OCH3), 3.73 (6H, s, 3″, 5″-OCH3); 13C-NMR (100 MHz, DMSO-d6) δC 152.6 (C-3′, 5′), 147.9 (C-3, 5), 147.4 (C-3″, 5″), 136.8 (C-1′), 134.9 (C-4), 134.9 (C-4′), 134.3 (C-4″), 132.4 (C-1), 131.4 (C-1″), 104.3 (C-2″), 103.7 (C-2, 2′, 6, 6′), 103.3 (C-6″), 86.2 (C-8″), 85.3 (C-9′), 85.1 (C-7), 72.4 (C-7″), 71.2 (C-7′), 71.1 (C-9), 59.9(C-9″), 56.0 (3′, 5′-OCH3), 56.0 (3, 5-OCH3), 55.9(3″, 5″-OCH3), 53.7(C-8′), 53.6 (C-8).
(7R, 7′R, 7″S, 7‴S, 8S, 8′S, 8″S, 8‴S)-4″,4‴-Dihydroxy-3, 3’, 3″, 3‴, 5, 5′-hexamethoxy-7, 9’; 7′, 9-diepoxy-4, 8″; 4’, 8‴-bisoxy-8, 8’-dineolignan-7″, 7‴, 9″, 9‴-tetraol (14): White powder; HRESl-MS m/z 833.2954[M + Na]+(Calcd for C42H50O16Na, 833.2948); 1H NMR(400 MHz, DMSO-d6) δH 8.78 (2H, s, 4″, 4‴-OH), 6.92 (2H, s, H-6″, 6‴), 6.76–6.67 (4H, m, H-2″, 2‴, 3″, 3‴), 6.64 (s, H-2, 6, 2′, 6′), 5.10 (2H, d, J = 2.5 Hz, H-7″, 7‴),4.80 (2H, s, 7″, 7‴-OH), 4.67 (2H, d, J = 3.4 Hz, H-7, 9′), 4.23–4.16 (2H, m, H-8″, 8‴), 4.14– 4.07 (4H, m, H-9″, 9″), 4.03 (2H, d, J = 3.0 Hz, H-9a, 7′a), 3.85–3.79 (2H, m, H-9b, 7′b), 3.75 (18H, d, J = 8.4 Hz, 3, 3′, 3″, 5, 5′, 5″-OCH3), 3.12–2.99 (2H, m, H-8, 8′); 13C-NMR (100 MHz, DMSO-d6) δC 152.6 (C-3′, 5′), 152.6 (C-3, 5), 146.9 (C-5″, 5‴), 145.3 (C-4″, 4‴), 136.8 (C-4, 4′), 134.8 (C-1, 1′), 133.3 (C-1″, 1‴), 119.4 (C-2″, 2‴), 114.6 (C-3″, 3‴), 111.0 (C-6″, 6‴), 103.3 (C-2, 6, 2′, 6′), 86.1 (C-8″, 8‴), 85.1 (C-7, 9′), 72.1 (C-7″, 7‴), 71.3 (C-7′, 9), 59.8 (C-9″, 9‴), 56.0 (3, 3′, 5, 5′-OCH3), 55.5(5″, 5‴-OCH3), 53.6 (C-8, 8′).
4.4. Biological Assays
Larvicidal bioassays were conducted based on the WHO requirement with slight modification [27]. Serial concentrations (10, 20, 40, 60, 80 and 100 mg/L) were tested for lignans. Thirty 4th instar larvae were tested in a 150 mL glass beaker with 100 mL of sterilized water and 5 replicates were conducted. Mortality was recorded after 24 h of treatment, and no food was provided during the treatment. Dimethyl sulfoxide (DMSO) was set as the negative control and permethrin was set as the positive control. The antitumor activity of tested compounds against HepG2 was performed by the MTT method [28]. The anti-inflammatory activity was evaluated by the inflammatory model of LPS-induced RAW264.7 macrophages [29].
4.5. Statistic Analysis
SPSS (version 19.0) was used to perform the statistical analyses. Standard probit analysis was conducted for the Cx. p. quinquefasciatus larvicidal bioassay and LC50 values were calculated after 24 h of exposure. Significant differences in LC50 values (p ≤ 0.05) were concluded only if there was no overlap in the confidence intervals.
5. Conclusions
Fourteen known lignans including eleven furofuran lignans (1–9, 13–14), two dibenzylbutane lignans (10–11) and a benzodihydrofuran neolignan (12) were first identified from branches of Cinnamomum camphora chvar. Borneol. Compounds 3–5 and 8–15 were isolated from this plant for the first time. Furofuran lignans (1–9, 13–14) were found to exhibit broad mosquito larvicidal activity against Culex pipiens quinquefasciatus, with LC50 values ranging from 0.009 to 0.24 μg/mL. These results suggest that it may be meaningful to conduct complementary and further studies on lignans, especially furan lignans, in mosquito repellents and plant ecology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28093769/s1, Figure S1. 1H NMR (400 MHz, CDCl3) spectrum of 1; Figure S2. 13C NMR (100 MHz, CDCl3) spectrum of 1; Figure S3. ESI-MS spectrum of 1; Figure S4. 1H NMR (400 MHz, CDCl3) spectrum of 2; Figure S5. 13C NMR (100 MHz, CDCl3) spectrum of 2; Figure S6. ESI-MS spectrum of 2; Figure S7. 1H NMR (400 MHz, CDCl3) spectrum of 3; Figure S8. 13C NMR (100 MHz, CDCl3) spectrum of 3; Figure S9. HRESI-MS spectrum of 3; Figure S10. 1H NMR (400 MHz, DMSO-d6) spectrum of 4; Figure S11. 13C NMR (100 MHz, DMSO-d6) spectrum of 4; Figure S12. ESI-MS spectrum of 4; Figure S13. 1H NMR (400 MHz, DMSO-d6) spectrum of 5; Figure S14. 13C NMR (100 MHz, DMSO-d6) spectrum of 5; Figure S15. ESI-MS spectrum of 5; Figure S16. 1H NMR (400 MHz, DMSO-d6) spectrum of 6; Figure S17. 13C NMR (100 MHz, DMSO-d6) spectrum of 6; Figure S18. HRESI-MS spectrum of 6; Figure S19. 1H NMR (400 MHz, DMSO-d6) spectrum of 7; Figure S20. 13C NMR (100 MHz, DMSO-d6) spectrum of 7; Figure S21. ESI-MS spectrum of 7; Figure S22. 1H NMR (400 MHz, DMSO-d6) spectrum of 8; Figure S23. 13C NMR (100 MHz, DMSO-d6) spectrum of 8; Figure S24. HRESI-MS spectrum of 8; Figure S25. 1H NMR (400 MHz, DMSO-d6) spectrum of 9; Figure S26. 13C NMR (100 MHz, DMSO-d6) spectrum of 9; Figure S27. HRESI-MS spectrum of 9; Figure S28. 1H NMR (400 MHz, DMSO-d6) spectrum of 10; Figure S29. 13C NMR (100 MHz, DMSO-d6) spectrum of 10; Figure S30. HRESI-MS spectrum of 10; Figure S31. 1H NMR (400 MHz, DMSO-d6) spectrum of 11; Figure S32. 13C NMR (100 MHz, DMSO-d6) spectrum of 11; Figure S33. HRESI-MS spectrum of 11; Figure S34. 1H NMR (400 MHz, DMSO-d6) spectrum of 12; Figure S35. 13C NMR (100 MHz, DMSO-d6) spectrum of 12; Figure S36. HRESI-MS spectrum of 12; Figure S37. 1H NMR (400 MHz, DMSO-d6) spectrum of 13; Figure S38. 13C NMR (100 MHz, DMSO-d6) spectrum of 13; Figure S39. HRESI-MS spectrum of 13; Figure S40. 1H NMR (400 MHz, DMSO-d6) spectrum of 14; Figure S41. 13C NMR (100 MHz, DMSO-d6) spectrum of 14; Figure S42. HRESI-MS spectrum of 14; Tables S1–S14. Comparison of 1H and 13C data between compounds 1–14 and those in literature.
Author Contributions
Conceptualization, Z.X., L.W., W.X. and J.C.; methodology, Z.X., L.W. and J.C.; software, R.S. and F.Y.; validation, R.S., W.X. and F.Y.; formal analysis, Z.X., C.X. and J.C.; investigation, Y.L. and X.W.; resources, Y.L. and J.F.; data curation, Z.X. and J.C.; writing—original draft preparation, Z.X. and C.X.; writing—review and editing, W.X., X.W. and L.W.; visualization, Z.X., R.S. and F.Y.; supervision, W.X. and L.W.; project administration, W.X. and L.W.; funding acquisition, W.X. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 32260250), the contract system project of Jiangxi Academy of Sciences (2021YSBG22022) and (2022YSBG22029), the Jiangxi Provincial Natural Science Foundation (20224BAB216101), Key R&D projects in Jiangxi Province (20212BBF63035) and the Doctoral Program of Jiangxi Academy of Sciences (2022YYB09) and (2020YYB07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by request through the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Zhao, T.; Lu, B. Biosystematics of Culex pipiens complex in China. Entomol. Sin. 1995, 2, 1–8. [Google Scholar] [CrossRef]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 7572, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Tantely, M.L.; Tortosa, P.; Alout, H.; Berticat, C.; Berthomieu, A.; Rutee, A.; Dehecq, J.S.; Makoundou, P.; Labbe, P.; Pasteur, N.; et al. Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from La Réunion Island. Insect Biochem. Mol. 2010, 40, 317–324. [Google Scholar] [CrossRef]
- Nakasone, Y.; Takara, K.; Wada, K.; Tanaka, J.; Yogi, S.; Nakatani, N. Antioxidative compounds isolated from Kokuto, non-centrifugal cane sugar. Biosci. Biotechnol. Biochem. 1996, 60, 1714–1716. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, J.; Ding, Z. Phenolic constituents from Manglietia crassipes. Acta Bot. Yunnanica 2000, 22, 365–367. [Google Scholar]
- Chang, C.I.; Hsu, C.M.; Li, T.S.; Huang, S.D.; Lin, C.C.; Yen, C.M.; Chou, C.H.; Cheng, H.L. Constituents of the stem of Cucurbita moschata exhibit antidiabetic activities through multiple mechanisms. J. Funct. Foods 2014, 10, 260–273. [Google Scholar] [CrossRef]
- Li, D.; Liu, M.; Zhou, X. A new dimeric lignan from Zanthoxylum simulans. China J. Chin. Mater. Med. 2015, 40, 2843–2848. [Google Scholar]
- Ina, H.; Ono, M.; Sashida, Y.; Iida, H. (+)-Piperitol from Paulownia tomentosa. Planta Med. 1987, 53, 504. [Google Scholar] [CrossRef]
- Pava, A.; Zamilpa, A.; Trejo-Espino, J.L.; Domínguez-Mendoza, B.E.; Jiménez-Ferrer, E.; Pérez-Martínez, L.; Trejo-Tapia, G. Synergism and subadditivity of verbascoside-lignans and -iridoids binary mixtures isolated from Castilleja tenuiflora Benth. on NF-κB/AP-1 inhibition activity. Molecules 2021, 26, 547. [Google Scholar] [CrossRef]
- Ye, M.; Yan, Y.; Qiao, L.; Ni, X. Studies on chemical constituents of Cuscuta chinensis. China J. Chin. Mater. Med. 2002, 27, 38–40. [Google Scholar]
- Lin, R.W.; Tsai, I.L.; Duh, C.Y.; Lee, K.H.; Chen, I.S. New lignans and cytotoxic constituents from Wikstroemia lanceolata. Planta Med. 2004, 70, 234–238. [Google Scholar] [PubMed]
- Zhang, W.; Wang, Y.; Geng, Z.; Guo, S.; Cao, J.; Zhang, Z.; Pang, X.; Chen, Z.; Du, S.; Deng, Z. Antifeedant activities of lignans from stem bark of Zanthoxylum armatum DC. against Tribolium castaneum. Molecules 2018, 23, 617. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.M.X.; Yoshida, M.; Gottlieb, O.R. Dibenzylbutyrolactone lignans from virola sebifera. Phytochemistry 1983, 22, 1516–1518. [Google Scholar] [CrossRef]
- Kato, M.J.; Yoshida, M.; Gottlieb, O.R. Flavones and lignans in flowers, fruits and seedlings of Virola Venosa. Phytochemistry 1992, 31, 283–287. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, J. Chemical constituents of Nux Prinsepiae Uniflorae. J. Shenyang Pharm. Univ. 2006, 23, 209–211. [Google Scholar]
- Cui, Y.; Mu, Q.; Hu, C. Studies on the phenylpropanoids from Caragana rosea. Nat. Prod. Res. Dev. 2003, 15, 277–283. [Google Scholar]
- Xia, Z.; Zhang, H.; Xu, T.; Chen, Y.; Zhou, G. Phenylpropanoids from Fruits of Xanthium sibiricum. Chin. Pharm. J. 2021, 56, 13–22. [Google Scholar]
- Chen, X.; Ren, Q.; Liu, Y.; Zou, Z.; Xu, K.; Tan, G. Circular dichroism in the determination of absolute configuration of lignans and neolignans. Cent. South Pharm. 2020, 18, 1–10. [Google Scholar]
- Wang, L.X.; Wang, H.L.; Huang, J.; Chu, T.Z.; Peng, C.; Zhang, H.; Chen, H.L.; Xiong, Y.A.; Tan, Y.Z. Review of lignans from 2019 to 2021: Newly reported compounds, diverse activities, structure-activity relationships and clinical applications. Phytochemistry 2022, 202, 113326. [Google Scholar] [CrossRef]
- Barre, D.E.; Mizier-Barre, K.A. Lignans’ potential in pre and post-onset type 2 diabetes management. Curr. Diabetes Rev. 2020, 16, 2–11. [Google Scholar] [CrossRef]
- Haruna, M.; Koube, T.; Ito, K.; Murata, H. Balanophonin, a new neo-lignan from balanophora japonica makino. Chem. Pharm. Bull. 1982, 30, 1525–1527. [Google Scholar] [CrossRef]
- Park, I.K.; Shin, S.C.; Kim, C.S.; Lee, H.J.; Choi, W.S.; Ahn, Y.J. Larvicidal activity of lignans identified in Phryma leptostachya Var. asiatica roots against three mosquito species. J. Agric. Food Chem. 2005, 53, 969–972. [Google Scholar] [CrossRef]
- Qie, X.; Sun, A.; Hao, H.; Lv, B.; Wu, W.; Hu, Z. A potential lignan botanical insecticide from Phryma leptostachya against Aedes aegypti: Laboratory selection, metabolic mechanism, and resistance risk assessment. J. Pest. Sci. 2021, 95, 397–408. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, Z.; Shi, B.; Wei, S.; Wu, W. Larvicidal activity of lignans from Phryma leptostachya L. against Culex pipiens pallens. Parasitol. Res. 2012, 110, 1079–1084. [Google Scholar] [CrossRef]
- Kim, S.I.; Ahn, Y.J. Larvicidal activity of lignans and alkaloid identified in Zanthoxylum piperitum bark toward insecticide-susceptible and wild Culex pipiens pallens and Aedes aegypti. Parasites Vectors 2017, 10, 221. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Xu, Z.; Xiong, B.; Xu, J. Chemical investigation of secondary metabolites produced by mangrove endophytic fungus Phyllosticta capitalensis. Nat. Prod. Res. 2019, 35, 1561–1565. [Google Scholar] [CrossRef]
- Xie, C.; Wang, S.; Cao, M.; Xiong, W.; Wu, L. (E)-9-Octadecenoic acid ethyl ester derived from lotus seedpod ameliorates inflammatory responses by regulating MAPKs and NF-κB Signalling Pathways in LPS-Induced RAW264.7 Macrophages. Evid.-Based Complement. Altern. Med. 2022, 2022, 6731360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).