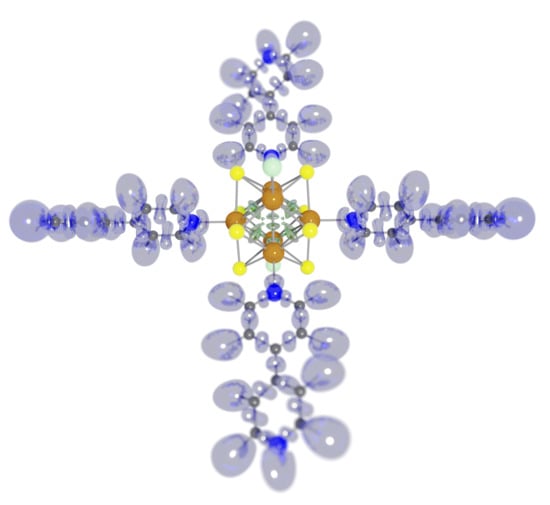
Evolution of the Electronic Structure of the trans-[Re6S8bipy4Cl2] Octahedral Rhenium Cluster during Reduction
Abstract
1. Introduction
2. Computational Details
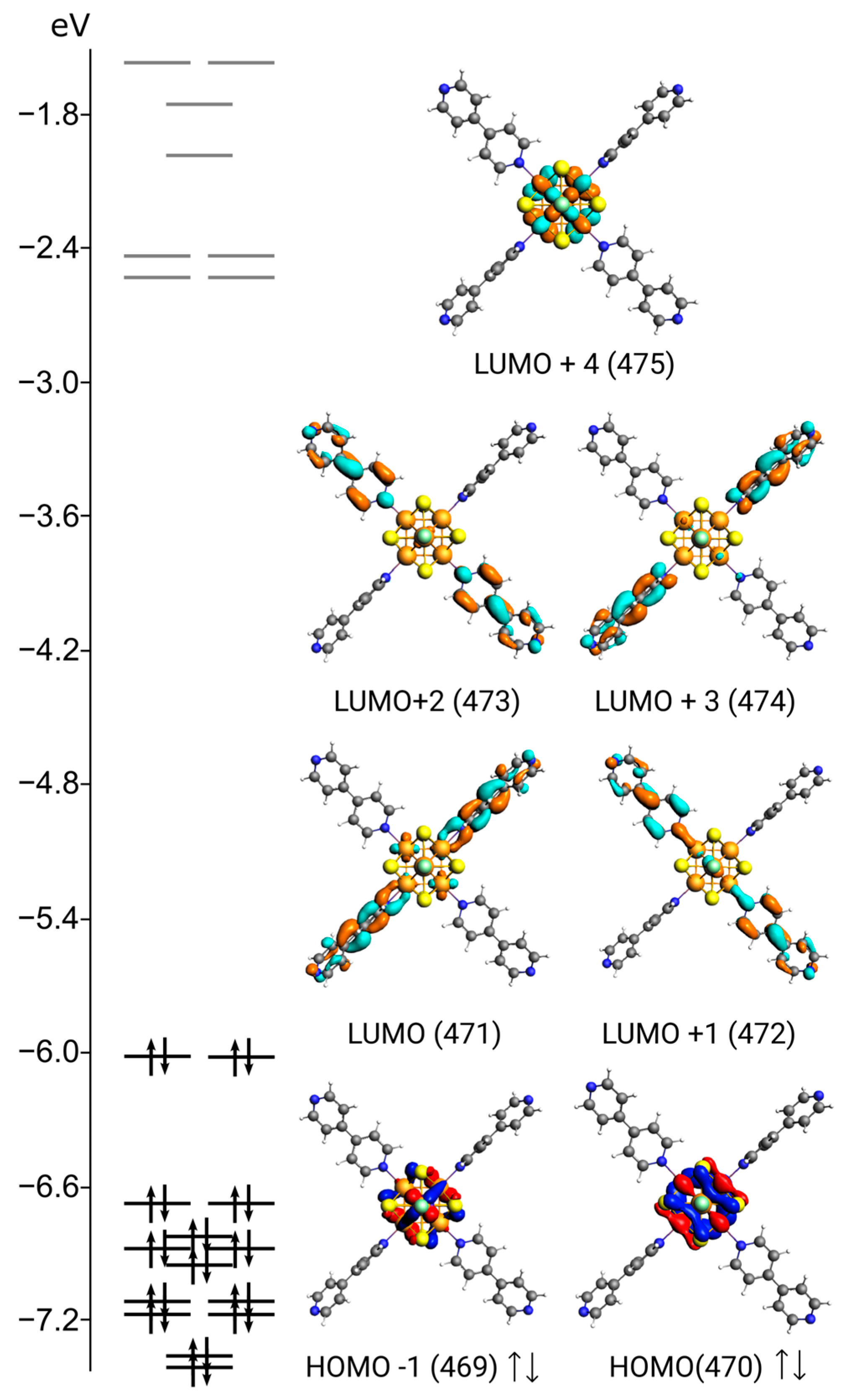
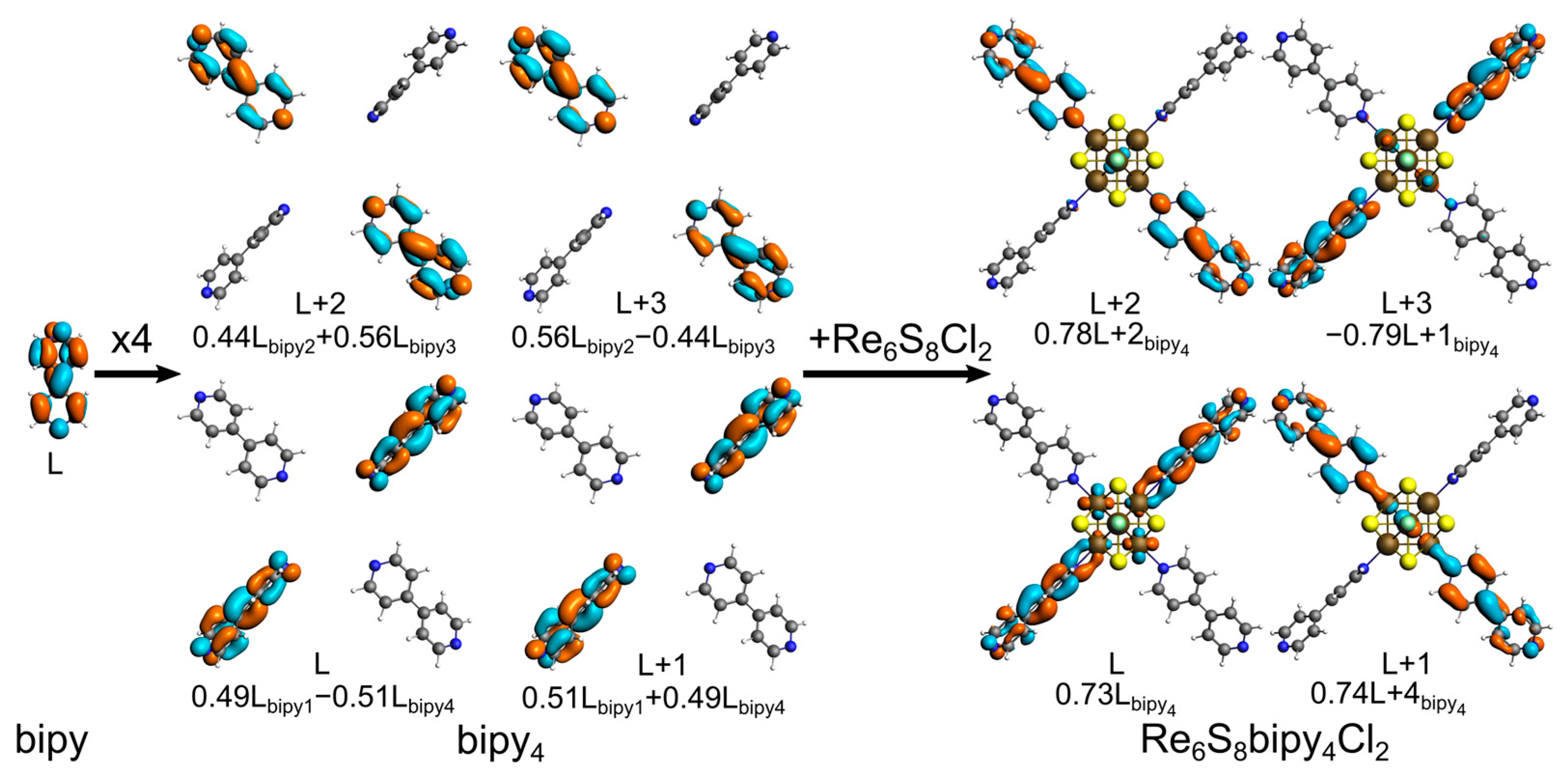
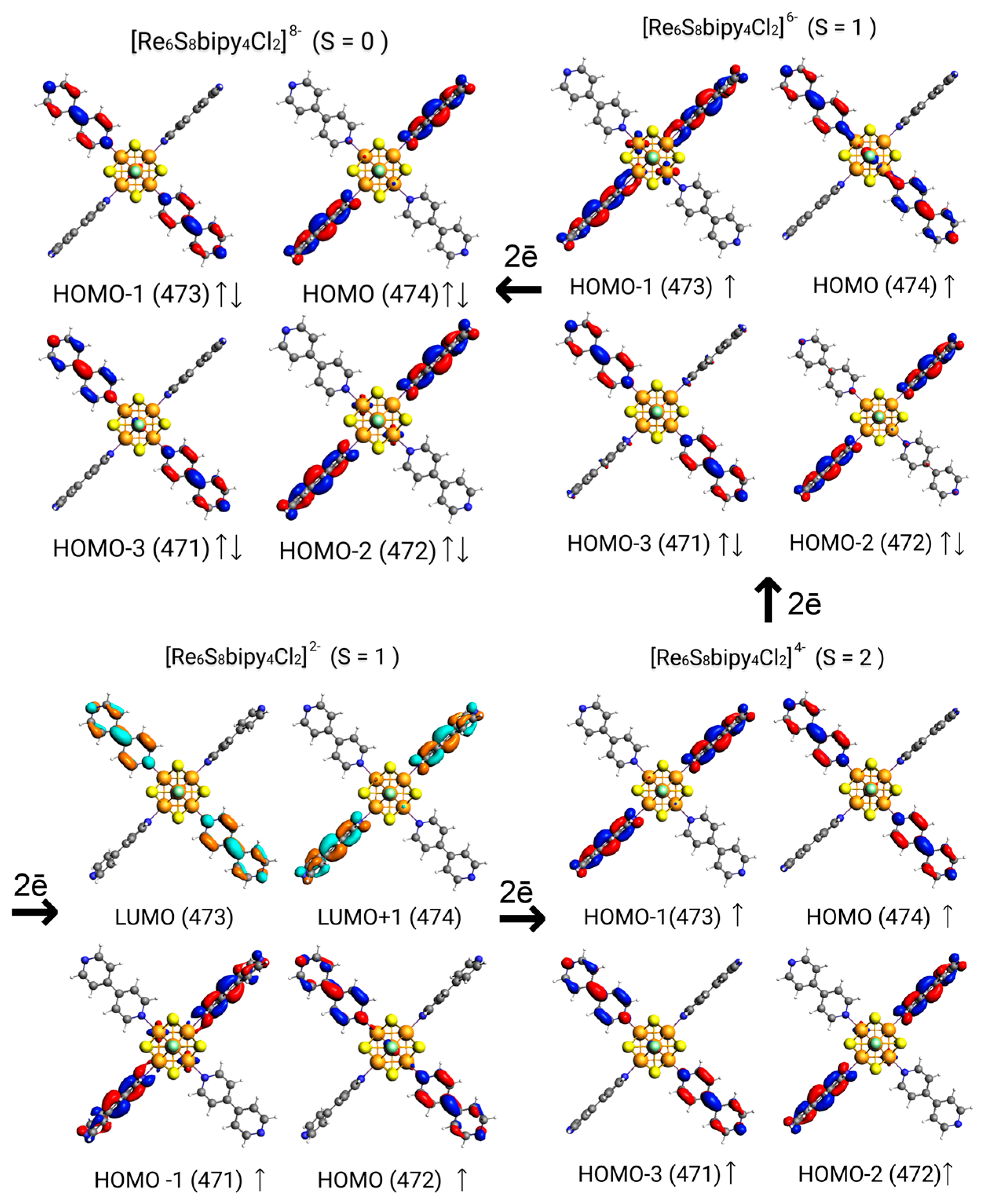
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S. Metal Complexes Containing Redox-Active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review. Catalysts 2019, 9, 1046. [Google Scholar] [CrossRef]
- Pardo, E.; Ruiz-García, R.; Cano, J.; Ottenwaelder, X.; Lescouëzec, R.; Journaux, Y.; Lloret, F.; Julve, M. Ligand design for multidimensional magnetic materials: A metallosupramolecular perspective. Dalton Trans. 2008, 21, 2780–2805. [Google Scholar] [CrossRef]
- Ma, X.; Suturina, E.A.; Rouzières, M.; Platunov, M.; Wilhelm, F.; Rogalev, A.; Clérac, R.; Dechambenoit, P. Using Redox-Active π Bridging Ligand as a Control Switch of Intramolecular Magnetic Interactions. J. Am. Chem. Soc. 2019, 141, 7721–7725. [Google Scholar] [CrossRef] [PubMed]
- Lyaskovskyy, V.; de Bruin, B. Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions. ACS Catal. 2012, 2, 270–279. [Google Scholar] [CrossRef]
- Cameron, J.M.; Holc, C.; Kibler, A.J.; Peake, C.L.; Walsh, D.A.; Newton, G.N.; Johnson, L.R. Molecular redox species for next-generation batteries. Chem. Soc. Rev. 2021, 50, 5863–5883. [Google Scholar] [CrossRef]
- Du, H.-Y.; Chen, S.-C.; Su, X.-J.; Jiao, L.; Zhang, M.-T. Redox-Active Ligand Assisted Multielectron Catalysis: A Case of CoIII Complex as Water Oxidation Catalyst. J. Am. Chem. Soc. 2018, 140, 1557–1565. [Google Scholar] [CrossRef]
- Balzani, V.; Juris, A.; Venturi, M.; Campagna, S.; Serroni, S. Luminescent and Redox-Active Polynuclear Transition Metal Complexes. Chem. Rev. 1996, 96, 759–834. [Google Scholar] [CrossRef]
- Crabtree, R.H. Multifunctional ligands in transition metal catalysis. New J. Chem. 2011, 35, 18–23. [Google Scholar] [CrossRef]
- Kaim, W. Manifestations of Noninnocent Ligand Behavior. Inorg. Chem. 2011, 50, 9752–9765. [Google Scholar] [CrossRef]
- Gray, T.G.; Rudzinski, C.M.; Meyer, E.E.; Holm, R.H.; Nocera, D.G. Spectroscopic and Photophysical Properties of Hexanuclear Rhenium(III) Chalcogenide Clusters. J. Am. Chem. Soc. 2003, 125, 4755–4770. [Google Scholar] [CrossRef]
- Fedorov, V.E.; Yu, V.M.; Naumov, N.G.; Sokolov, M.N.; Vladimir, P.F. Chalcogenide clusters of Group 5–7 metals. Russ. Chem. Rev. 2007, 76, 529. [Google Scholar] [CrossRef]
- Naumov, N.G.; Virovets, A.V.; Fedorov, V.E. Octahedral rhenium(III) chalcocyanide cluster anions: Synthesis, structure, and solid state design. J. Struct. Chem. 2000, 41, 499–520. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Boubekeur, K.; Uriel, S.; Batail, P. Chemistry of Hexanuclear Rhenium Chalcohalide Clusters. Chem. Rev. 2001, 101, 2037–2066. [Google Scholar] [CrossRef] [PubMed]
- Perrin, A.; Perrin, C. The molybdenum and rhenium octahedral cluster chalcohalides in solid state chemistry: From condensed to discrete cluster units. Comptes Rendus Chim. 2012, 15, 815–836. [Google Scholar] [CrossRef]
- Pinkard, A.; Champsaur, A.M.; Roy, X. Molecular Clusters: Nanoscale Building Blocks for Solid-State Materials. Acc. Chem. Res. 2018, 51, 919–929. [Google Scholar] [CrossRef]
- Yoshimura, T.; Umakoshi, K.; Sasaki, Y.; Sykes, A.G. Synthesis, Structures, and Redox Properties of Octa(μ3-sulfido)hexarhenium(III) Complexes Having Terminal Pyridine Ligands. Inorg. Chem. 1999, 38, 5557–5564. [Google Scholar] [CrossRef]
- Yoshimura, T.; Umakoshi, K.; Sasaki, Y.; Ishizaka, S.; Kim, H.-B.; Kitamura, N. Emission and Metal- and Ligand-Centered-Redox Characteristics of the Hexarhenium(III) Clusters trans- and cis-[Re6(μ3-S)8Cl4(L)2]2-, Where L Is a Pyridine Derivative or Pyrazine. Inorg. Chem. 2000, 39, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Suo, C.; Tsuge, K.; Ishizaka, S.; Nozaki, K.; Sasaki, Y.; Kitamura, N.; Shinohara, A. Excited-State Properties of Octahedral Hexarhenium(III) Complexes with Redox-active N-heteroaromatic Ligands. Inorg. Chem. 2010, 49, 531–540. [Google Scholar] [CrossRef]
- Yoshimura, T.; Nishizawa, H.; Nagata, K.; Ito, A.; Sakuda, E.; Ishizaka, S.; Kitamura, N.; Shinohara, A. Tuning the Ground- and Excited-State Redox Potentials of Octahedral Hexanuclear Rhenium(III) Complexes by the Combination of Terminal Halide and N-Heteroaromatic Ligands. ACS Omega 2022, 7, 26965–26982. [Google Scholar] [CrossRef]
- Saito, T.; Yoshikawa, A.; Yamagata, T.; Imoto, H.; Unoura, K. Synthesis, structure and electronic properties of octakis(.mu.3-sulfido)hexakis(triethylphosphine)hexatungsten as a tungsten analog of the molecular model for superconducting Chevrel phases. Inorg. Chem. 1989, 28, 3588–3592. [Google Scholar] [CrossRef]
- Chen, Z.-N.; Yoshimura, T.; Abe, M.; Sasaki, Y.; Ishizaka, S.; Kim, H.-B.; Kitamura, N. Chelate Formation around a Hexarhenium Cluster Core by the Diphosphane Ligand Ph2P(CH2)6PPh2. Angew. Chem. Int. Ed. 2001, 40, 239–242. [Google Scholar] [CrossRef]
- Chen, Z.-N.; Yoshimura, T.; Abe, M.; Tsuge, K.; Sasaki, Y.; Ishizaka, S.; Kim, H.-B.; Kitamura, N. Octa(μ3-selenido)hexarhenium(III) Complexes Containing Axial Monodentate Diphosphine or Diphosphine–Monoxide Ligands. Chem.–A Eur. J. 2001, 7, 4447–4455. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Brylev, K.A.; Abramov, P.A.; Gallyamov, M.R.; Novozhilov, I.N.; Kitamura, N.; Mikhaylov, M.A. Complexes of {W6I8}4+ Clusters with Carboxylates: Preparation, Electrochemistry, and Luminescence. Eur. J. Inorg. Chem. 2017, 2017, 4131–4137. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubát, P.; Langmaier, J.; Polívka, T.; Fuciman, M.; Fejfarová, K.; Lang, K. A comparative study of the redox and excited state properties of (nBu4N)2[Mo6X14] and (nBu4N)2[Mo6X8(CF3COO)6] (X = Cl, Br, or I). Dalton Trans. 2013, 42, 7224–7232. [Google Scholar] [CrossRef]
- Mikhailov, M.A.; Brylev, K.A.; Abramov, P.A.; Sakuda, E.; Akagi, S.; Ito, A.; Kitamura, N.; Sokolov, M.N. Synthetic Tuning of Redox, Spectroscopic, and Photophysical Properties of {Mo6I8}4+ Core Cluster Complexes by Terminal Carboxylate Ligands. Inorg. Chem. 2016, 55, 8437–8445. [Google Scholar] [CrossRef]
- Akagi, S.; Fujii, S.; Horiguchi, T.; Kitamura, N. pKa(L) Dependences of Structural, Electrochemical, and Photophysical Properties of Octahedral Hexamolybdenum(II) Clusters: [Mo6X8L6]2− (X = Br or I.; L = carboxylate). J. Clust. Sci. 2017, 28, 757–772. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Laricheva, Y.A.; Sokolov, M.N.; Llusar, R. Tri-and tetranuclear molybdenum and tungsten chalcogenide clusters: On the way to new materials and catalysts. Russ. Chem. Rev. 2018, 87, 670. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Laricheva, Y.A.; Abramov, P.A.; Virovets, A.V.; Vicent, C.; Sokolov, M.N.; Llusar, R. Homoleptic Molybdenum Cluster Sulfides Functionalized with NoninnocentDiimine Ligands: Synthesis, Structure, and Redox Behavior. Eur. J. Inorg. Chem. 2014, 2014, 4093–4100. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Sokolov, M.N.; Peresypkina, E.V.; Virovets, A.V.; Kozlova, S.G.; Zakharchuk, N.F.; Fedin, V.P. Crystal Structure, Electronic Structure, and Solid-State Electrochemistry of Cluster Complexes of M3Se74+ (M = Mo, W) with Noninnocent o-Phenanthroline and Se22– Ligands. Eur. J. Inorg. Chem. 2008, 2008, 3964–3969. [Google Scholar] [CrossRef]
- Ulantikov, A.A.; Gayfulin, Y.M.; Ivanov, A.A.; Sukhikh, T.S.; Ryzhikov, M.R.; Brylev, K.A.; Smolentsev, A.I.; Shestopalov, M.A.; Mironov, Y.V. Soluble Molecular Rhenium Cluster Complexes Exhibiting Multistage Terminal Ligands Reduction. Inorg. Chem. 2020, 59, 6460–6470. [Google Scholar] [CrossRef]
- Ulantikov, A.A.; Gayfulin, Y.M.; Sukhikh, T.S.; Ryadun, A.A.; Ryzhikov, M.R.; Mironov, Y.V. Synthesis, Structure, And Physicochemical Properties Of Molecular Rhenium Cluster Complexes with 4-Phenylpyridine Molecules As Terminal Ligands. J. Struct. Chem. 2021, 62, 1009–1019. [Google Scholar] [CrossRef]
- Ulantikov, A.A.; Sukhikh, T.S.; Gribov, E.N.; Maltseva, N.V.; Brylev, K.A.; Mironov, Y.V.; Gayfulin, Y.M. Thermally Controlled Synthesis of Octahedral Rhenium Clusters with 4,4′-Bipyridine and CN− Apical Ligands. Symmetry 2021, 13, 2187. [Google Scholar] [CrossRef]
- Ulantikov, A.A.; Brylev, K.A.; Sukhikh, T.S.; Mironov, Y.V.; Muravieva, V.K.; Gayfulin, Y.M. Octahedral Rhenium Cluster Complexes with 1,2-Bis(4-pyridyl)ethylene and 1,3-Bis(4-pyridyl)propane as Apical Ligands. Molecules 2022, 27, 7874. [Google Scholar] [CrossRef] [PubMed]
- ADF2017, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: https://www.scm.com (accessed on 30 March 2023).
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Swart, M. A new family of hybrid density functionals. Chem. Phys. Lett. 2013, 580, 166–171. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Lenthe, E.V.; Ehlers, A.; Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
- Gayfulin, Y.M.; Brylev, K.A.; Ryzhikov, M.R.; Samsonenko, D.G.; Kitamura, N.; Mironov, Y.V. Luminescent twelve-nuclear rhenium clusters. Dalton Trans. 2019, 48, 12522–12530. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Ryzhikov, M.R.; Berezin, A.S.; Kolesnikov, I.E.; Samsonenko, D.G.; Bagryanskaya, I.Y. Photoluminescence of Ag(i) complexes with a square-planar coordination geometry: The first observation. Inorg. Chem. Front. 2019, 6, 2855–2864. [Google Scholar] [CrossRef]
- Yarovoy, S.S.; Ivanova, M.; Sukhikh, T.S.; Ryzhikov, M.R.; Fedorov, V.E.; Naumov, N.G. Replenishment in the Family of Rhenium Chalcobromides; Synthesis and Structure of Molecular {Re4S4}Br8(TeBr2)4, Dimeric [{Re4S4}Br8(TeBr2)3]2, and Polymeric {Re4S4}Br8 Compounds Based on the {Re4S4}8+ Tetrahedral Cluster Core. Inorg. Chem. 2022, 61, 20472–20479. [Google Scholar] [CrossRef]
- Baranov, A.Y.; Rakhmanova, M.I.; Hei, X.; Samsonenko, D.G.; Stass, D.V.; Bagryanskaya, I.Y.; Ryzhikov, M.R.; Fedin, V.P.; Li, J.; Artemev, A.V. A new subclass of copper(i) hybrid emitters showing TADF with near-unity quantum yields and a strong solvatochromic effect. Chem. Commun. 2023, 59, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Kohout, M. DGrid; Version 4.6; SitePen: Radebeul, Germany, 2011. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Bickelhaupt, F.M.; Baerends, E.J. Kohn-Sham Density Functional Theory: Predicting and Understanding Chemistry. In Reviews in Computational Chemistry; Wiley-VCH: New York, NY, USA, 2000; pp. 1–86. [Google Scholar] [CrossRef]
- ADF 2020, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: https://www.scm.com (accessed on 30 March 2023).
- Ryzhikov, M.R.; Kozlova, S.G. Reduction of carbon and nitrogen centered trigonal prismatic tungsten clusters: Bonding patterns as viewed by ELF and AIM methods. Polyhedron 2019, 173, 114131. [Google Scholar] [CrossRef]
- Kryuchkova, N.A.; Ryzhikov, M.R.; Syrokvashin, M.M. Interatomic Interactions in Heterometallic Cubane-Type Clusters with {Mo3S4M′} (M′ = Cu, Ni, Pd) Core. J. Clust. Sci. 2021, 32, 415–421. [Google Scholar] [CrossRef]
- Michalski, M.; Berski, S. Exploring the Relationship between Reactivity and Electronic Structure in Isorhodanine Derivatives Using Computer Simulations. Molecules 2023, 28, 2360. [Google Scholar] [CrossRef]
- Kégl, T.R.; Pálinkás, N.; Kollár, L.; Kégl, T. Computational Characterization of Bidentate P-Donor Ligands: Direct Comparison to Tolman’s Electronic Parameters. Molecules 2018, 23, 3176. [Google Scholar] [CrossRef] [PubMed]


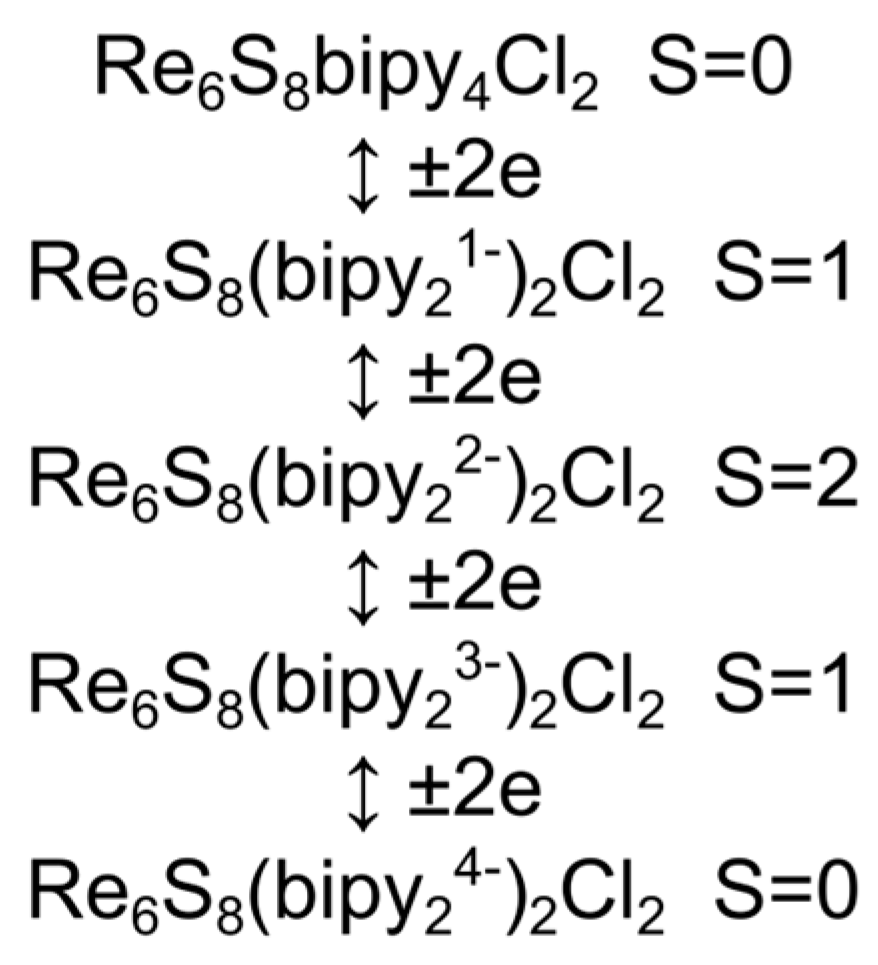

| n | 0 | 2– | 4– | 6– | 8– | ||||
|---|---|---|---|---|---|---|---|---|---|
| S | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 0 |
| <d(Re-Re)> | 2.61 | 2.61 | 2.61 | 2.62 | 2.62 | 2.61 | 2.62 | 2.62 | 2.62 |
| <d(Re-Cl)> | 2.44 | 2.46 | 2.46 | 2.48 | 2.49 | 2.48 | 2.51 | 2.51 | 2.55 |
| <d(Re-S)> | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 | 2.43 |
| <d(Re-Nin)> | 2.21 | 2.17 | 2.17 | 2.14 | 2.14 | 2.15 | 2.12 | 2.12 | 2.11 |
| ΔE(S12g/TZP) | 0.00 | −6.24 | −6.32 | −11.95 | −12.07 | −12.16 | −16.93 | −16.99 | −21.22 |
| ΔE(S12h/TZ2P//S12g/TZP) | 0.00 | −5.25 | −5.54 | −10.45 | −11.12 | −11.76 | −15.51 | −15.87 | −20.42 |
| n = 0 S = 0 | n = 2– S = 1 | n = 4– S = 2 | n = 6– S = 1 | n = 8– S = 0 | |
|---|---|---|---|---|---|
| V(Re,Re) | 0.54 | 0.53 | 0.54 | 0.53 | 0.54 |
| V(Re3) | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| V(Re6) | 0.01 | 0.03 | - | - | - |
| V(Re5) | - | - | 0.01 | 0.01 | - |
| V(Re,Nin) | 2.59 | 2.80 | 3.05 | 3.21 | 3.45 |
| V(Nout) | 2.76 | 2.85 | 2.99 | 3.12 | 3.32 |
| V(Nin,C) | 2.42 | 2.31 | 2.18 | 2.11 | 2.01 |
| V(Re,Cl) | 1.11 | 1.14 | 1.14 | 1.16 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhikov, M.R.; Gayfulin, Y.M.; Ulantikov, A.A.; Arentov, D.O.; Kozlova, S.G.; Mironov, Y.V. Evolution of the Electronic Structure of the trans-[Re6S8bipy4Cl2] Octahedral Rhenium Cluster during Reduction. Molecules 2023, 28, 3658. https://doi.org/10.3390/molecules28093658
Ryzhikov MR, Gayfulin YM, Ulantikov AA, Arentov DO, Kozlova SG, Mironov YV. Evolution of the Electronic Structure of the trans-[Re6S8bipy4Cl2] Octahedral Rhenium Cluster during Reduction. Molecules. 2023; 28(9):3658. https://doi.org/10.3390/molecules28093658
Chicago/Turabian StyleRyzhikov, Maxim R., Yakov M. Gayfulin, Anton A. Ulantikov, Dmitry O. Arentov, Svetlana G. Kozlova, and Yuri V. Mironov. 2023. "Evolution of the Electronic Structure of the trans-[Re6S8bipy4Cl2] Octahedral Rhenium Cluster during Reduction" Molecules 28, no. 9: 3658. https://doi.org/10.3390/molecules28093658
APA StyleRyzhikov, M. R., Gayfulin, Y. M., Ulantikov, A. A., Arentov, D. O., Kozlova, S. G., & Mironov, Y. V. (2023). Evolution of the Electronic Structure of the trans-[Re6S8bipy4Cl2] Octahedral Rhenium Cluster during Reduction. Molecules, 28(9), 3658. https://doi.org/10.3390/molecules28093658