σ-Aromatic MAl6S6 (M = Ni, Pd, Pt) Stars Containing Planar Hexacoordinate Transition Metals
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
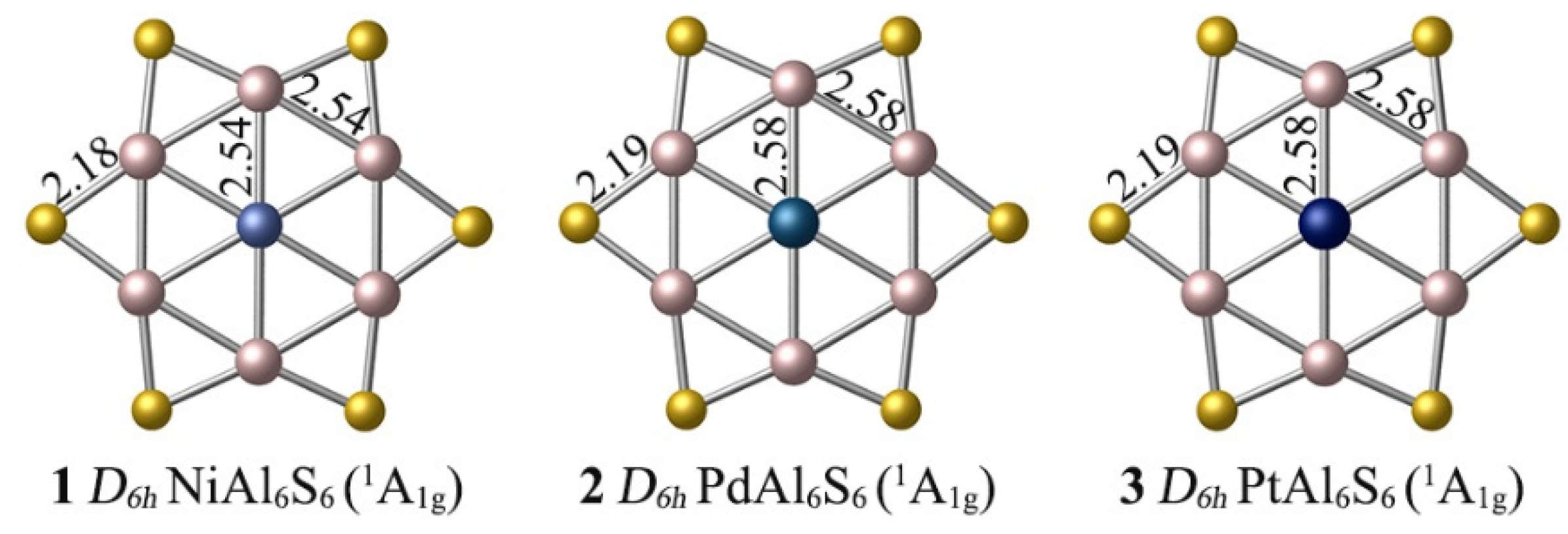
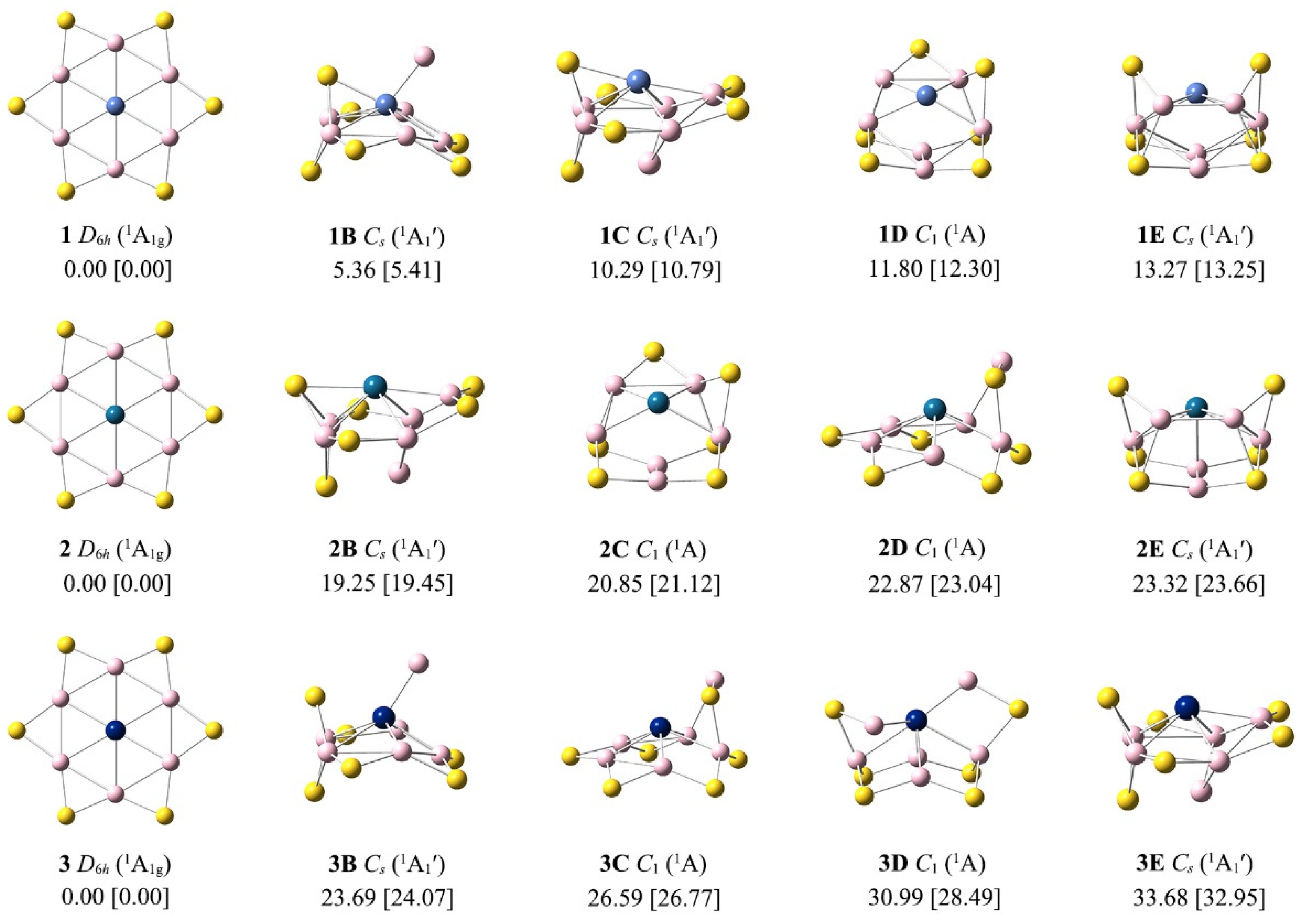
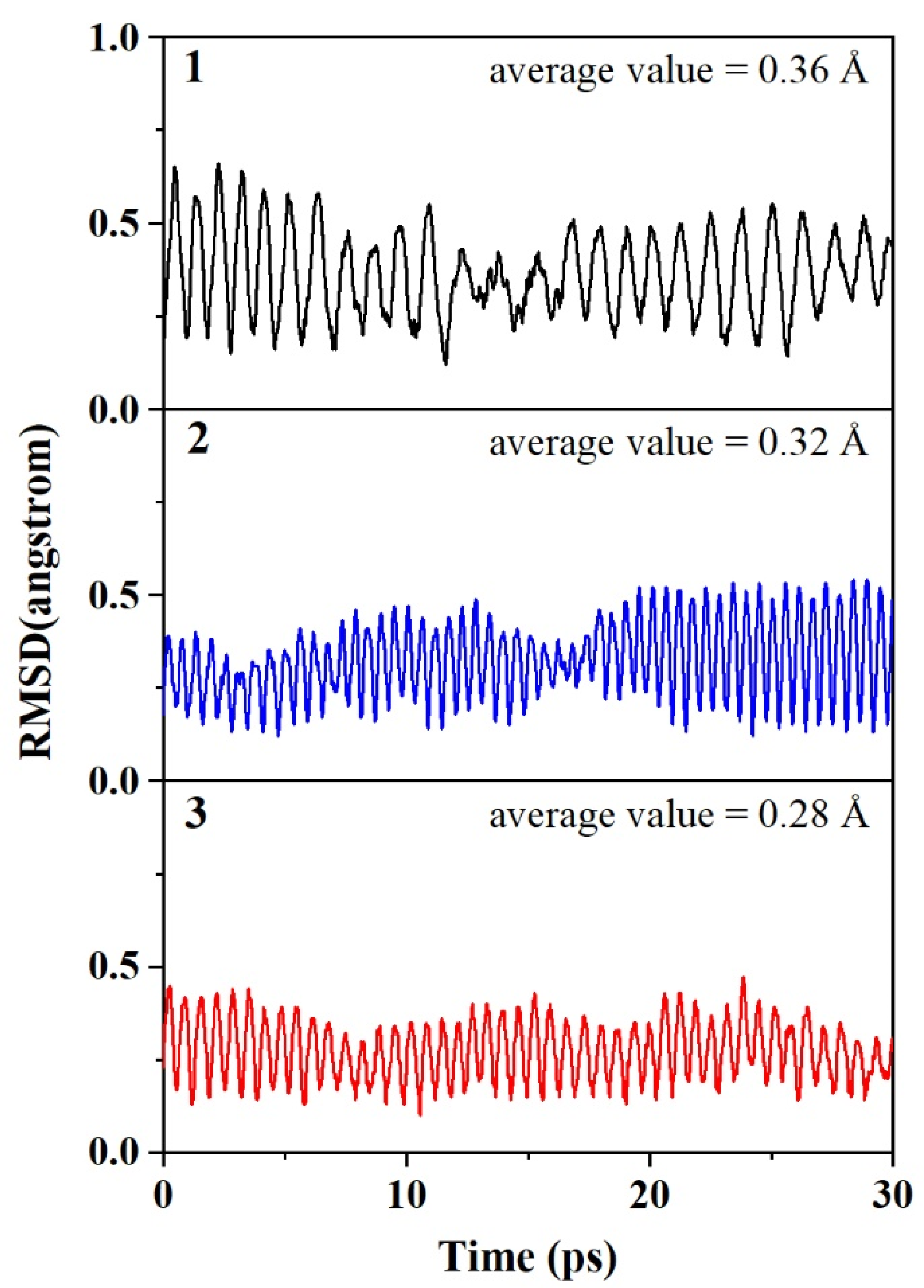
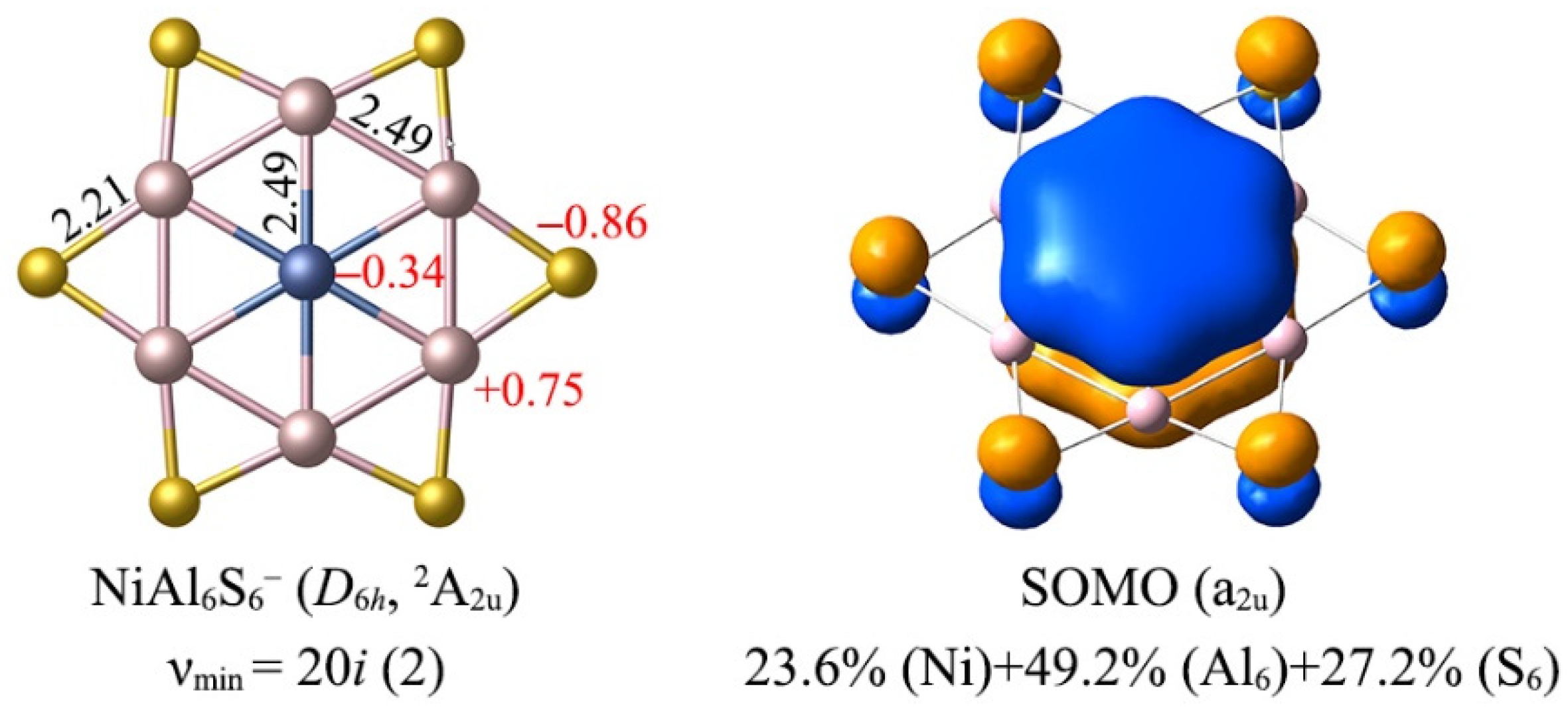
3.1. Structures and Stability
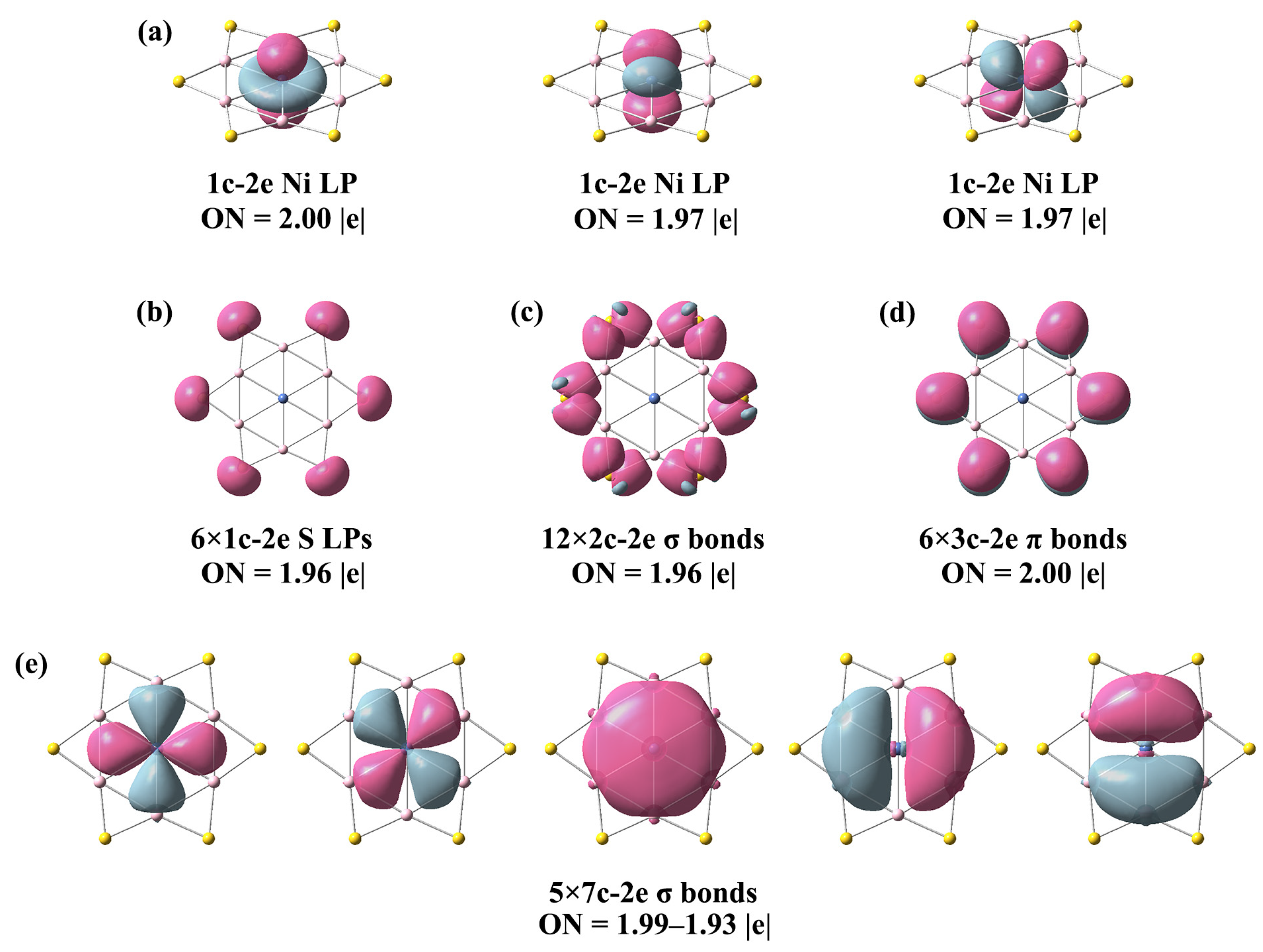
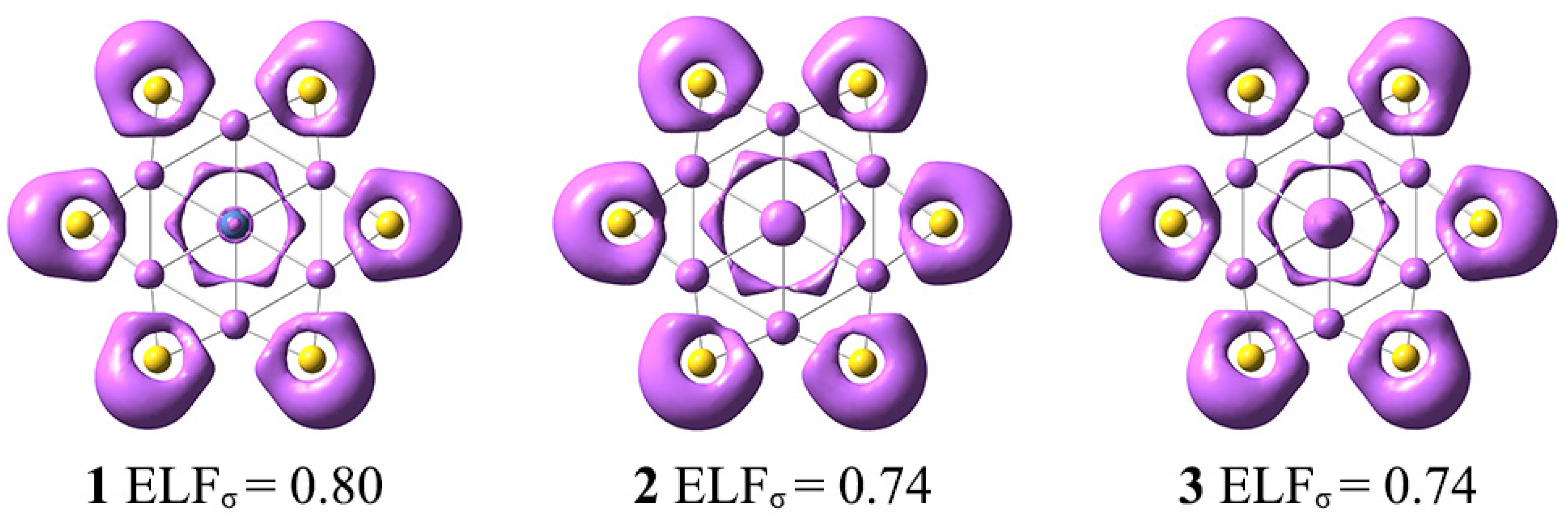
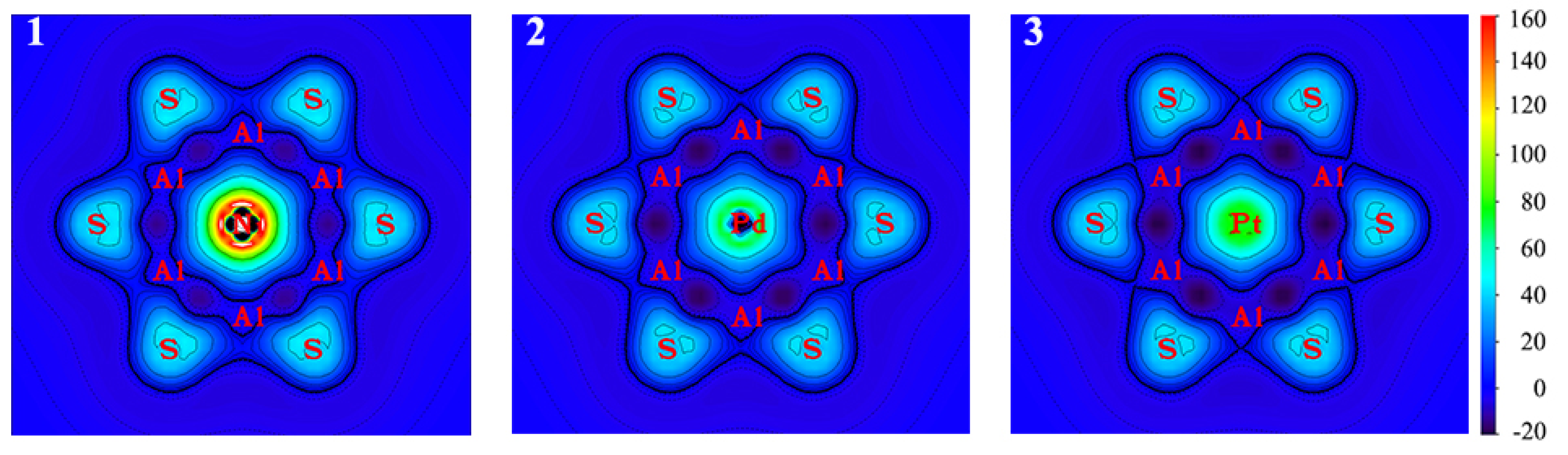
3.2. Chemical Bonding
3.3. σ-Aromaticity
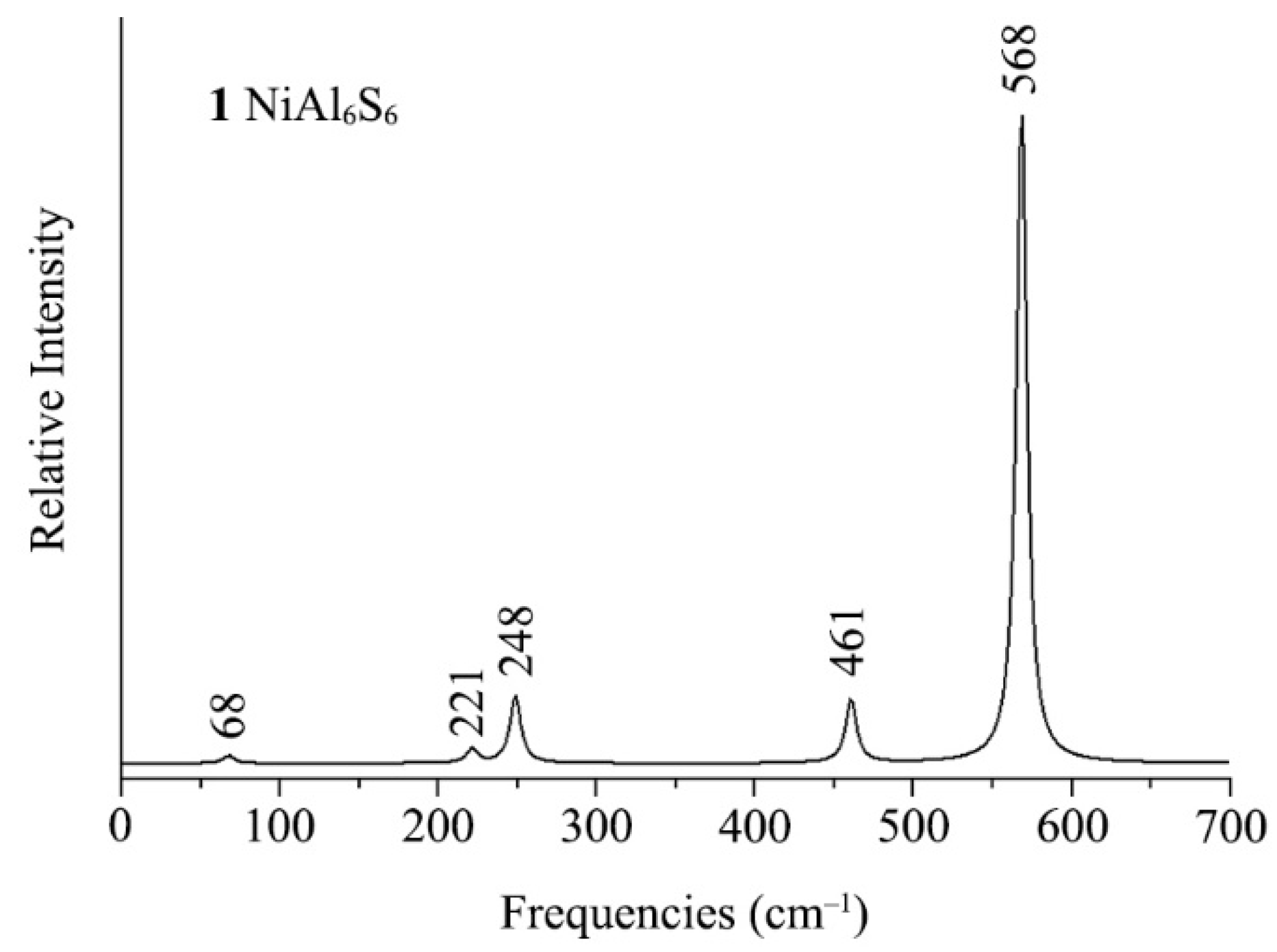
3.4. Simulated IR Spectrums
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigoreva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon film. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.D.; Abazari, R.; Yao, J.M.; Gao, J.K. Recent progress in two-dimensional metal-organic framework photocatalysts: Synthesis, photocatalytic mechanism, and applications. J. Phy. Energy 2021, 3, 032010. [Google Scholar] [CrossRef]
- Sanati, S.; Rezvani, Z.; Abazari, R.; Hou, Z.Q.; Dai, H. Hierarchical CuAl-layered double hydroxide/CoWO4 nanocomposites with enhanced efficiency in supercapacitors with long cyclic stability. New J. Chem. 2019, 43, 15240–15248. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.F.; Chen, Z.F. Planar hypercoordinate motifs in two-diemensional materials. Acc. Chem. Res. 2020, 1, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Cui, Z.H.; Wang, S.; Li, Q.; Zhao, J.J.; Chen, Z.F. A two-dimensional Be2Au monolayer with planar hexacoordinate s-block metal atoms: A superconducting global minimum Dirac material with two perfect Dirac node-loops. Chem. Sci. 2022, 13, 11099–11109. [Google Scholar] [CrossRef]
- Hoffmann, R.; Alder, R.W.; Wilcox, C.F. Planar tetracoordinate carbon. J. Am. Chem. Soc. 1970, 92, 4992–4993. [Google Scholar] [CrossRef]
- Collins, J.B.; Dill, J.D.; Jemmis, E.D.; Apeloig, Y.; Schleyer, P.V.R.; Seeger, R.; Pople, J.A. Stabilization of planar tetracoordinate carbon. J. Am. Chem. Soc. 1976, 98, 5419–5427. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Boldyrev, A.I. A new, general strategy for achieving planar tetracoordinate geometries for carbon and other second row periodic elements. J. Chem. Soc. Chem. Commun. 1991, 1536–1538. [Google Scholar] [CrossRef]
- Sorger, K.; von Ragué, P. Planar and inherently nontetrahedral tetracoordinate carbon: A status report. J. Mol. Struct. THEOCHEM 1995, 338, 317–346. [Google Scholar] [CrossRef]
- Keese, R. Carbon flatland: Planar tetracoordinate carbon and fenestranes. Chem. Rev. 2006, 106, 4787–4808. [Google Scholar] [CrossRef]
- Merino, G.; Mendez-Rojas, M.A.; Vela, A.; Heine, T. Recent advances in planar tetracoordinate carbon chemistry. J. Comput. Chem. 2007, 28, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M.; Ganz, E.; Chen, Z.F.; Wang, Z.X.; Schleyer, P.V.R. Four decades of the chemistry of planar hypercoordinate compounds. Angew. Chem. Int. Ed. 2015, 54, 9468–9501. [Google Scholar] [CrossRef] [PubMed]
- Vassilev-Galindo, V.; Pan, S.J.; Donald, K.; Merino, G. Planar pentacoordinate carbons. Nat. Rev. Chem. 2018, 2, 0114. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.S.; Boldyrev, A.I.; Simons, J. Tetracoordinated planar carbon in the Al4C− anion. A combined photoelectron spectroscopy and ab initio study. J. Am. Chem. Soc. 1999, 121, 6033–6038. [Google Scholar] [CrossRef]
- Wang, L.S.; Boldyrev, A.I.; Li, X.; Simon, J. Experimental observation of pentaatomic tetracoordinate planar carbon-containing molecules. J. Am. Chem. Soc. 2000, 122, 7681–7687. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.F.; Wang, L.S.; Geske, G.D.; Boldyrev, A.I. Pentaatomic tetracoordinate planar carbon, [CAl4]2–: A new structural unit and its salt complexes. Angew. Chem. Int. Ed. 2000, 39, 3630–3632. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.X.; Yu, S.; Ding, Y.H.; Bowen, K.H. Identifying the hydrogenated planar tetracoordinate carbon: A combined experimental and theoretical study of CAl4H and CAl4H−. J. Phys. Chem. Lett. 2017, 8, 2263–2267. [Google Scholar] [CrossRef]
- Zhang, C.J.; Dai, W.S.; Xu, H.G.; Xu, X.L.; Zheng, W.J. Structural evolution of carbon-doped aluminum clusters AlnC− (n = 6–15): Anion photoelectron spectroscopy and theoretical calculations. J. Phys. Chem. A 2022, 126, 5621–5631. [Google Scholar] [CrossRef]
- Wang, Z.X.; Schleyer, P.R. Construction principles of “Hyparenes”: Families of molecules with planar pentacoordinate carbons. Science 2001, 292, 2465–2469. [Google Scholar] [CrossRef]
- Pei, Y.; An, W.; Ito, K.; Schleyer, P.V.R.; Zeng, X.C. Planar pentacoordinate carbon in CAl5+: A global minimum. J. Am. Chem. Soc. 2008, 130, 10394–10400. [Google Scholar] [CrossRef]
- Cui, Z.H.; Vassilev-Galindo, V.; Cabellos, J.L.; Osorio, E.; Orozco, M.; Pan, S.; Ding, Y.H.; Merino, G. Planar pentacoordinate carbon atoms embedded in a metallocene framework. Chem. Commun. 2017, 53, 138–141. [Google Scholar] [CrossRef]
- Guo, J.C.; Feng, L.Y.; Barroso, J.; Merino, G.; Zhai, H.J. Planar or tetrahedral? A ternary 17-electron CBe5H4+ cluster with planar pentacoordinate carbon. Chem. Commun. 2020, 56, 8305–8308. [Google Scholar] [CrossRef] [PubMed]
- Exner, K.; Schleyer, P.V.R. Planar hexacoordinate Carbon: A viable possibility. Science 2000, 290, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Duan, Y.; Lu, G.; Lu, H.G.; Yang, P.; Schleyer, P.V.R.; Merino, G.; Islas, R.; Wang, Z.X. D3h CN3Be3+ and CO3Li3+: Viable planar hexacoordinate carbon prototypes. Phys. Chem. Chem. Phys. 2012, 14, 14760–14763. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Parra, L.; Diego, L.; Yanez, O.; Inostroza, D.; Barroso, J.; Vasquez-Espinal, A.; Merino, G.; Tiznado, W. Planar hexacoordinate carbons: Half covalent, half ionic. Angew. Chem. Int. Ed. 2021, 60, 8700–8704. [Google Scholar] [CrossRef] [PubMed]
- Jian, T.; Chen, X.N.; Li, S.D.; Boldyrev, A.I.; Li, J.; Wang, L.S. Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem. Soc. Rev. 2019, 48, 3550–3591. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Pan, S.; Merino, G. Structural transformations in boron clusters induced by metal doping. Chem. Soc. Rev. 2022, 51, 1098–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.J.; Alexandrova, A.N.; Birch, K.A.; Boldyrev, A.I.; Wang, L.S. Hepta- and octacoordinate boron in molecular wheels of eight- and nine-atom boron clusters: Observation and confirmation. Angew. Chem. Int. Ed. 2003, 42, 6004–6008. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Sang, R.L.; Wu, Y.Y. Structure and aromaticity of B6H5+ cation: A novel borhydride system containing planar pentacoordinated boron. J. Phys. Chem. A 2009, 113, 3382–3386. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.Q.; Cui, Z.H. σ-Aromaticity planar pentacoordinate beryllium atoms. Inorg. Chem. 2021, 60, 16053–16058. [Google Scholar] [CrossRef]
- Pu, Z.; Ge, M.; Li, Q. MB82– (M = Be, Mg, Ca, Sr, and Ba): Planar octacoordinated alkaline earth metal atoms enclosed by boron rings. Sci. China Chem. 2010, 53, 1737–1745. [Google Scholar] [CrossRef]
- Lin, L.; Lievens, P.; Nguyen, M.T. In search of aromatic sevenmembered rings. J. Mol. Struct. THEOCHEM 2010, 943, 23–31. [Google Scholar] [CrossRef]
- Kalita, A.J.; Sarmah, K.; Yashmin, F.; Borah, R.R.; Baruah, I.; Deka, R.P.; Guha, A.K. σ-Aromaticity in planar pentacoordinate aluminium and gallium clusters. Sci. Rep. 2022, 12, 10041. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.C.; Feng, L.Y.; Zhang, X.Y.; Zhai, H.J. Star-like CBe5Au5+ cluster: Planar pentacoordinate carbon, superalkali cation, and multifold (π and σ) aromaticity. J. Phys. Chem. A 2018, 122, 1138–1145. [Google Scholar] [CrossRef]
- Wang, M.H.; Chen, C.; Pan, S.; Cui, Z.H. Planar hexacoordinate gallium. Chem. Sci. 2021, 12, 15067–15076. [Google Scholar] [CrossRef]
- Romanescu, C.; Galeev, T.R.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. Transition-metal-centered monocyclic boron wheel clusters (M©Bn): A new class of aromatic borometallic compounds. Acc. Chem. Res. 2013, 46, 350–358. [Google Scholar] [CrossRef]
- Romanescu, C.; Galeev, T.R.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. Aromatic metal-centered monocyclic boron rings: Co©B8− and Ru©B9−. Angew. Chem. Int. Ed. 2011, 50, 9334–9337. [Google Scholar] [CrossRef]
- Galeev, T.R.; Romanescu, C.; Li, W.L.; Wang, L.S.; Boldyrev, A.I. Observation of the highest coordination number in planar species: Decacoordinated Ta©B10− and Nb©B10− anions. Angew. Chem. Int. Ed. 2012, 51, 2101–2105. [Google Scholar] [CrossRef]
- Hou, J.; Duan, Q.; Qin, J.; Shen, X.; Zhao, J.; Liang, Q.; Jiang, D.; Gao, S. Unconventional charge distribution in the planar wheel-type M©B6H6−/0/+ (M = Mn, Fe and Co): Central M with negative charges and peripheral boron ring with positive charges. Phys. Chem. Chem. Phys. 2015, 17, 9644–9650. [Google Scholar] [CrossRef]
- Guo, J.C.; Ren, G.M.; Miao, C.Q.; Tian, W.J.; Wu, Y.B.; Wang, X. CBe5Hnn−4 (n = 2–5): Hydrogen-stabilized CBe5 pentagons containing planar or quasi-planar pentacoordinate carbons. J. Phys. Chem. A 2015, 119, 13101–13106. [Google Scholar] [CrossRef]
- Zhao, X.F.; Li, J.J.; Li, H.R.; Yuan, C.X.; Tian, X.X.; Li, S.D.; Wu, Y.B.; Guo, J.C.; Wang, Z.X. Viable aromatic BenHn stars enclosing a planar hypercoordinate boron or late transition metal. Phys. Chem. Chem. Phys. 2018, 20, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Kalita, A.J.; Rohman, S.S.; Kashyap, C.; Ullaha, S.S.; Guha, A.K. Double aromaticity in a BBe6H6+ cluster with a planar hexacoordinate boron structure. Chem. Commun. 2020, 56, 12597–12599. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.H.; Wang, Y.Q.; Zhang, M.; Geng, Y.; Su, Z.M. Sulphur-bridged BAl5S5+ with 17 counting electrons: A regular planar pentacoordinate boron system. Molecules 2021, 26, 5205. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, X.F.; Jin, B.; Huo, B.; Bian, J.H.; Guan, X.L.; Yuan, C.X.; Wu, Y.B. Influence of stepwise oxidation on the structure, stability, and properties of planar pentacoordinate carbon species CAl5+. Phys. Chem. Chem. Phys. 2020, 22, 17062–17067. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Jin, B.; Huo, B.; Yuan, C.; Zhai, H.J.; Wu, Y.B. Planar pentacoordinate carbon in a sulphur-surrounded boron wheel: The global minimum of CB5S5+. Chem. Commun. 2022, 58, 2552–2555. [Google Scholar] [CrossRef]
- Jin, B.; Sun, R.; Huo, B.; Yuan, C.X.; Wu, Y.B. M©B7O7+ (M = Ni, Pd, Pt): Aromatic molecular stars with a planar heptacoordinate transition metal. Chem. Commun. 2021, 57, 13716–13719. [Google Scholar] [CrossRef]
- Saunders, M. Stochastic search for isomers on a quantum mechanical surface. J. Comput. Chem. 2004, 25, 621–626. [Google Scholar] [CrossRef]
- Sergeeva, A.P.; Averkiev, B.B.; Zhai, H.J.; Boldyrev, A.I.; Wang, L.S. All-boron analogues of aromatic hydrocarbons: B17− and B18−. J. Chem. Phys. 2011, 134, 224304. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Purvis, G.D., III; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Millam, J.M.; Bakken, V.; Chen, W.; Hase, W.L.; Schlegel, H.B. Ab initio classical trajectories on the Born-Oppenheimer surface: Hessian-based integrators using fifth-order polynomial and rational function fits. J. Chem. Phys. 1999, 111, 3800–3805. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, D.Y.; Boldyrev, A.I. Developing paradigms of chemical bonding: Adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217. [Google Scholar] [CrossRef] [PubMed]
- Savin, A.; Nesper, R.; Wengert, S.; Fäsler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Dransfeld, A.; Schleyer, P.R.; Maerker, C.; Jiao, H.; van Eikema Hommes, N.J. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar]
- Kloda, S.; Kleinpeter, E. Ab initio calculation of the anisotropy effect of multiple bonds and the ring current effect of arenes application in conformational and configurational analysis. J. Chem. Soc. Perkin Trans. 2001, 2, 1893–1898. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Legault, C.Y. CYLview, 1.0b.; Universite. de Sherbrooke. 2009. Available online: http://www.cylview.org (accessed on 16 November 2022).
- Becke, A.D. Density Functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5659. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvettib correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–791. [Google Scholar] [CrossRef]
- Pyykkö, P. Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: A summary. J. Phys. Chem. A 2015, 119, 2326–2337. [Google Scholar] [CrossRef] [PubMed]

| Species | νmin | Gap | WBIM-Al | WBIAl-Al | WBIAl-S | qM | qAl | qS |
|---|---|---|---|---|---|---|---|---|
| 1 NiAl6S6 | 17 | 3.24 | 0.22 | 0.40 | 0.88 | −0.30 | 0.84 | −0.79 |
| 2 PdAl6S6 | 27 | 3.23 | 0.23 | 0.38 | 0.87 | −0.42 | 0.87 | −0.80 |
| 3 PtAl6S6 | 24 | 3.26 | 0.25 | 0.36 | 0.87 | −0.54 | 0.89 | −0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.-X.; Guo, J.-C. σ-Aromatic MAl6S6 (M = Ni, Pd, Pt) Stars Containing Planar Hexacoordinate Transition Metals. Molecules 2023, 28, 942. https://doi.org/10.3390/molecules28030942
Bai L-X, Guo J-C. σ-Aromatic MAl6S6 (M = Ni, Pd, Pt) Stars Containing Planar Hexacoordinate Transition Metals. Molecules. 2023; 28(3):942. https://doi.org/10.3390/molecules28030942
Chicago/Turabian StyleBai, Li-Xia, and Jin-Chang Guo. 2023. "σ-Aromatic MAl6S6 (M = Ni, Pd, Pt) Stars Containing Planar Hexacoordinate Transition Metals" Molecules 28, no. 3: 942. https://doi.org/10.3390/molecules28030942
APA StyleBai, L.-X., & Guo, J.-C. (2023). σ-Aromatic MAl6S6 (M = Ni, Pd, Pt) Stars Containing Planar Hexacoordinate Transition Metals. Molecules, 28(3), 942. https://doi.org/10.3390/molecules28030942







