Variations in Essential Oils from the Leaves of Cinnamomum bodinieri in China
Abstract
1. Introduction
2. Results
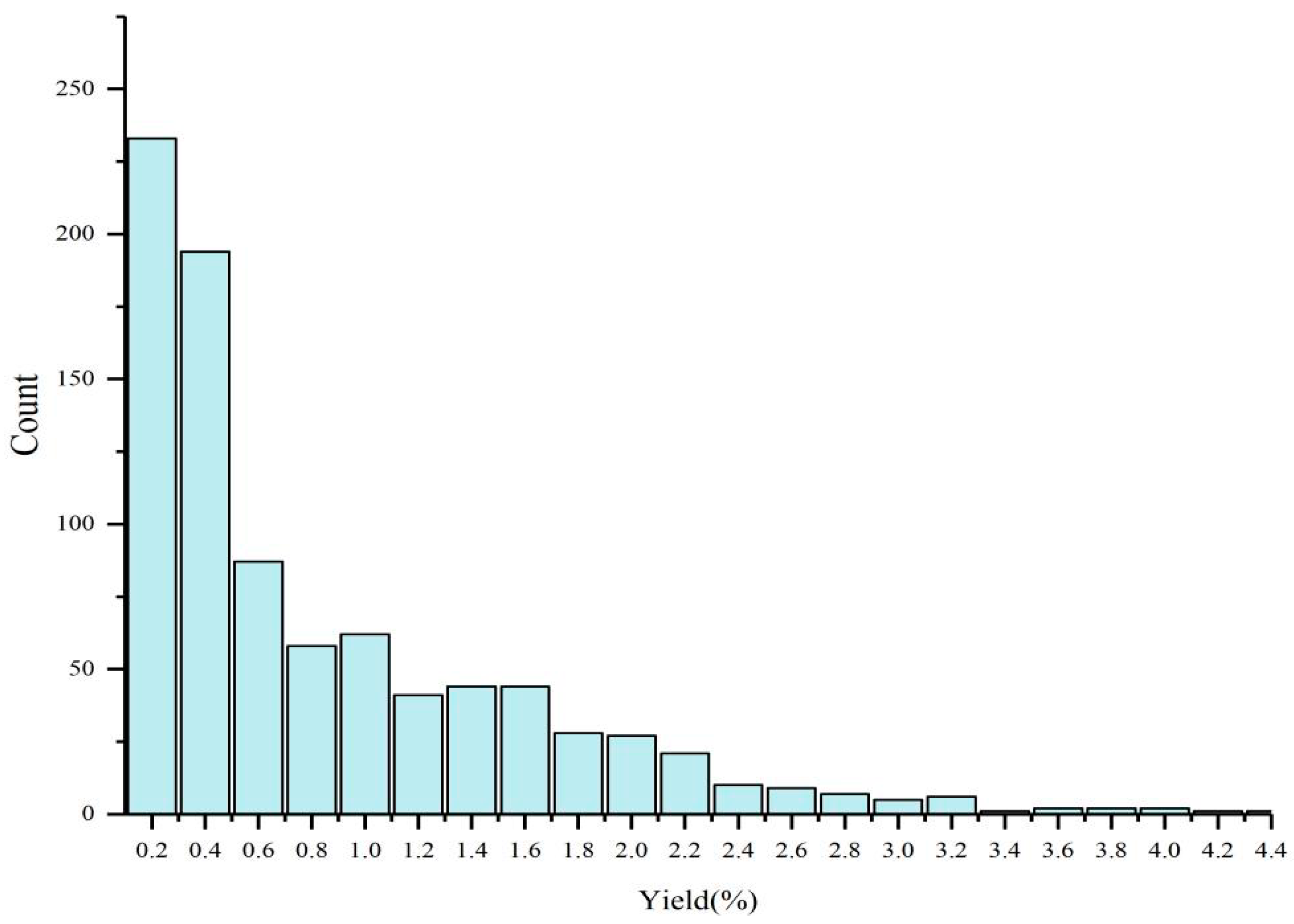
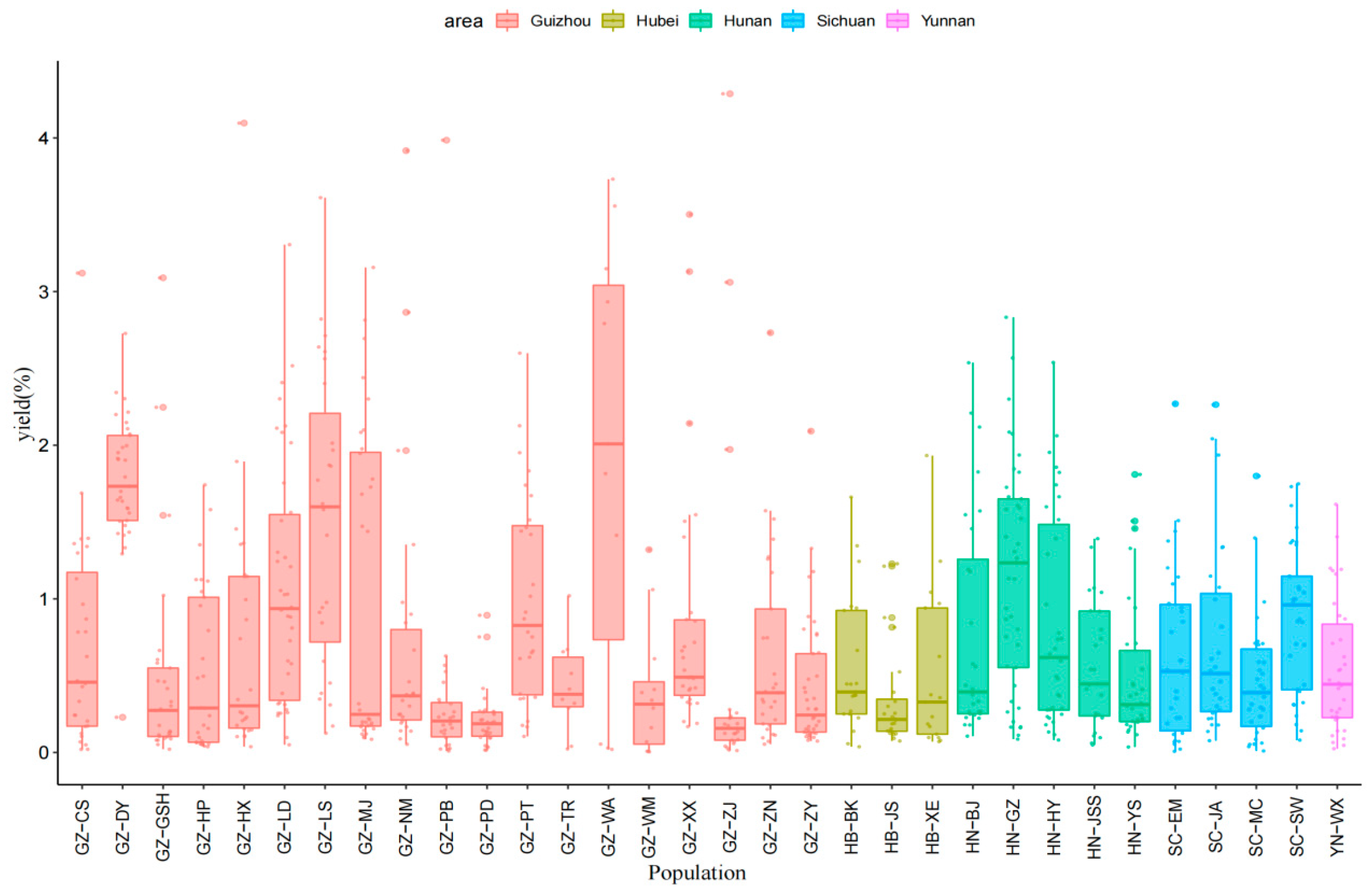
2.1. Variation in the Essential Oil Yield
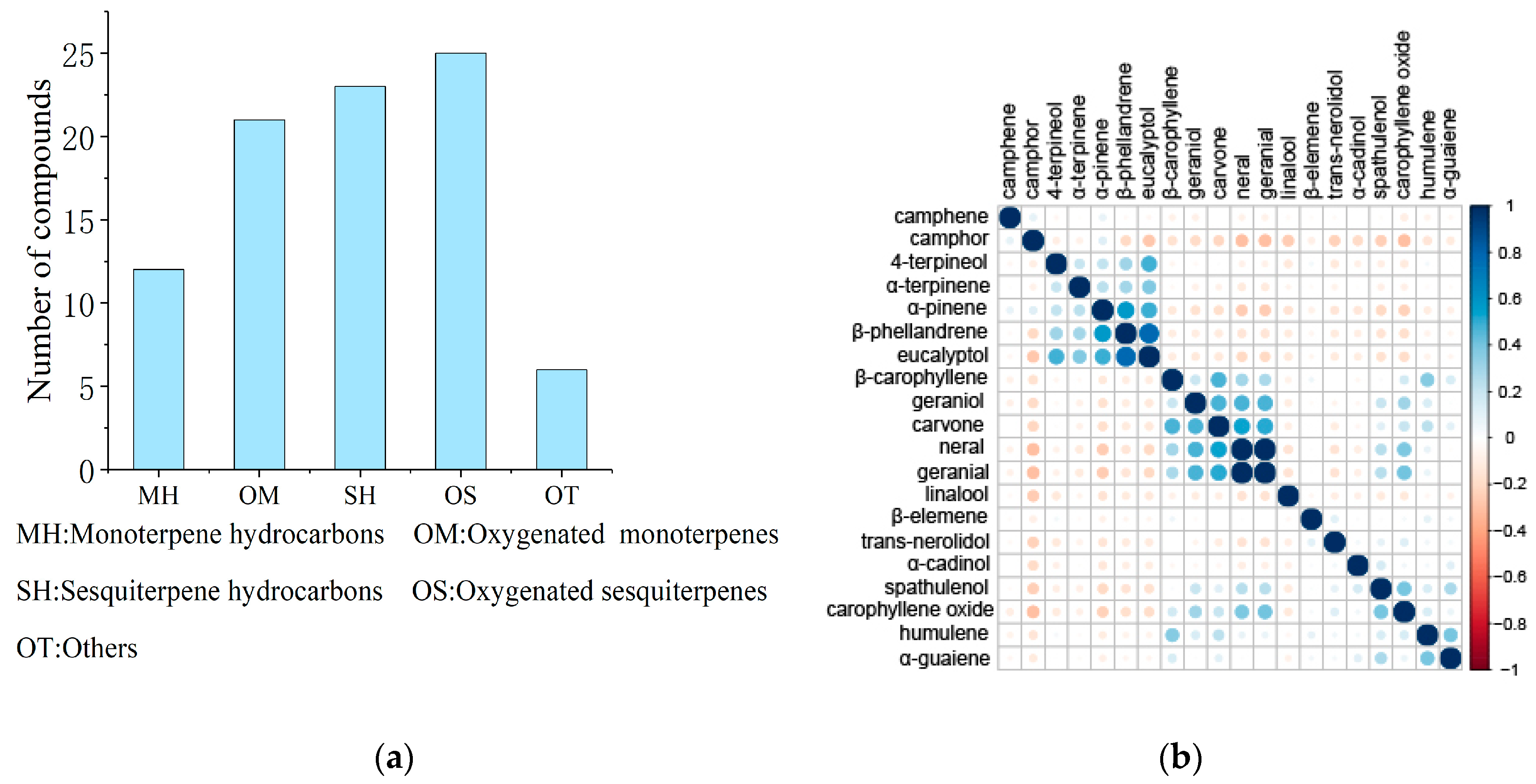
2.2. Composition and Correlations of the Main Chemical Constituents
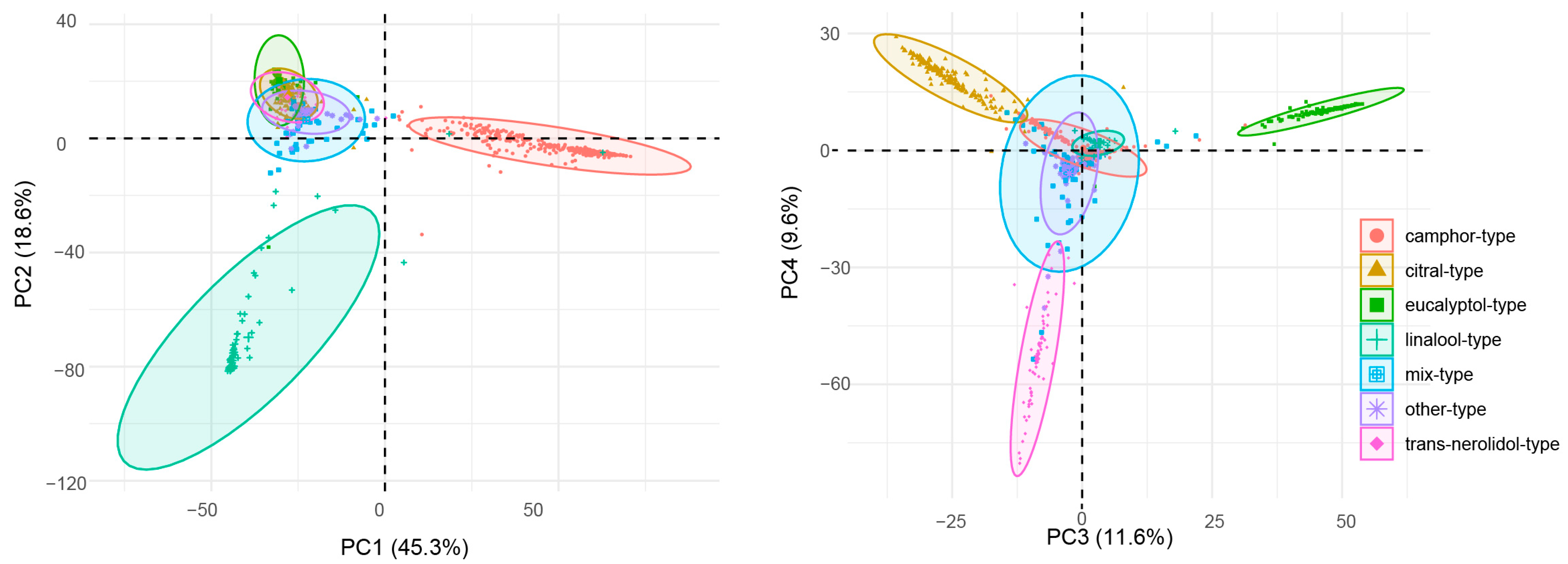
2.3. Chemotype Classification and Principal Component Analysis
2.4. Chemotypes of C. bodinieri Essential Oil
2.5. Correlation between C. bodinieri Essential Oils and Environmental Factors
3. Discussion
4. Materials and Methods
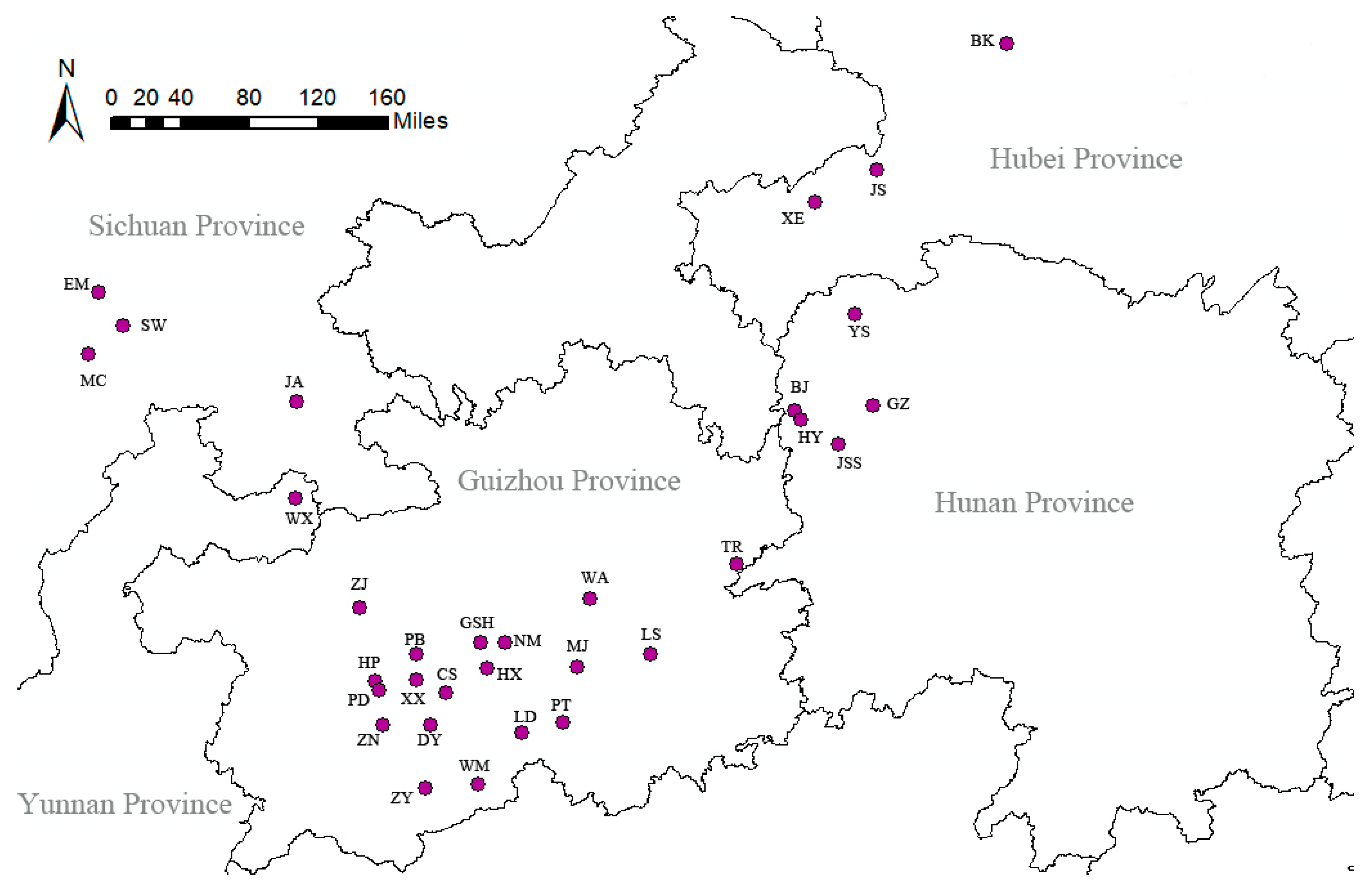
4.1. Sampling of Plant Materials
4.2. Experimental Methods
4.2.1. Distillation of Essential Oil
4.2.2. Essential Oil Chemical Composition Analysis
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, X.; Li, J.; Van der Werff, H. Cinnamomum. In Flora of China; Wu, Z., Raven, P.H., Hong, D., Eds.; Science Press: Beijing, China, 2008; pp. 166–187. [Google Scholar]
- Du, Y.; Zhou, H.; Yang, L.; Jiang, L.; Chen, D.; Qiu, D.; Yang, Y. Advances in Biosynthesis and Pharmacological Effects of Cinnamomum camphora (L.) Presl Essential Oil. Forests 2022, 13, 1020. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crops Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- Yang, Y.; Isman, M.B.; Tak, J. Insecticidal activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, D.; Park, S.; Park, H. Phytochemistry and applications of Cinnamomum camphora essential oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Guo, K. Ecophysiological adaptations to drought stress of seedlings of four plant species with different growth forms in karst habitats. Chin. J. Plant Ecol. 2011, 35, 1070–1082. [Google Scholar]
- Song, F.; Zhang, M.; Su, J.; Chai, J.; Zhang, G. The comparison on cold resistance between Cinnamomum bodinieri and Cinnamomum camphora seedlings in natural decreasing process of air temperature. J. West China For. Sci. 2012, 41, 48–52. [Google Scholar]
- Xiao, Z.; Jin, Z.; Zhang, B.; Li, F.; Yu, F.; Zhang, H. Effects of IBA on rooting ability of Cinnamomum bodinieri citral type micro-shoots from transcriptomics analysis. Plant Biotechnol. Rep. 2020, 14, 467–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, X.; Wang, L.; Chen, M. Growth variability of Cinnamomum bodinieri seedlings from different geographical provenances. Southwest China J. Agric. Sci. 2014, 27, 2162–2167. [Google Scholar]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C. Chemical Composition and Antioxidant Activity of the Essential Oils of Citral-Rich Chemotype Cinnamomum camphora and Cinnamomum bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Bioch. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Ying-Jiao, L.; Li-Juan, X.; Lan, X.; Li-Min, G.; Ta-Si, L.; Nai-Hong, C. Authentication of Two Different Chemical Types of Cinnamomum Camphora Leaves by Microscopic Technique with GC-MS and GC Analysis. Pharm. Chem. 2020, 54, 154–161. [Google Scholar] [CrossRef]
- Yu, H.; Ren, X.; Liu, Y.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Extraction of Cinnamomum camphora chvar. Borneol essential oil using neutral cellulase assisted-steam distillation: Optimization of extraction, and analysis of chemical constituents. Ind. Crop. Prod. 2019, 141, 11794. [Google Scholar] [CrossRef]
- Najnin, H.; Alam, N.; Mujeeb, M.; Ahsan, H.; Siddiqui, W.A. Biochemical and toxicological analysis of Cinnamomum tamala essential oil in Wistar rats. J. Food Process. Pres. 2020, 44, 14328. [Google Scholar] [CrossRef]
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F. Study on Chemical Composition of Leaf Essential Oil and the Genes Related to the Biosynthesis of Three Important Terpenoids in Cinnamomum Porrectum, Doctorate; Chinese Academy of Forestry Sciences: Beijing, China, 2020. [Google Scholar]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Q.; Wu, H. Changes in essential oils content, antioxidant capacity and secondary metabolism in different Cinnamomum longepaniculatum varieties. Ind. Crops Prod. 2023, 192, 115996. [Google Scholar] [CrossRef]
- Wun, N.B.; Tan, F.; Xiao, W.; Wang, X. Effects of light intensity on morphologic and physiological indexes and safrol content of Cinnamomum pauciflorum seedlings. Acta Ecol. Sin. 2005, 5, 1159–1164. [Google Scholar]
- Guo, S.; Geng, Z.; Zhang, W. The chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, B.; Zhang, J.; Xiao, C.; Xiao, Z.; Zhao, J. Discovery and application prospect of citral type plant in Cinnamomum Trewand. J. Nanchang Eng. Coll. 2019, 38, 4. [Google Scholar]
- Hafsa, J.; ali Smach, M.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. Lwt-Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Juergens, U.R.; Engelen, T.; Racké, K.; Stöber, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1, 8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 2004, 17, 281–287. [Google Scholar] [CrossRef]
- Li, F. Resources and Development of Cinnamomum camphora Essential Oil in China; China forestry Publishing House: Beijing, China, 2020; pp. 54–56. [Google Scholar]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, Z.; Zhao, Y.X.; Gao, M.; Wang, J.Y.; Liu, K.W.; Wang, X.; Wu, L.W.; Jiao, Y.L.; Xu, Z.L.; et al. The Litsea genome and the evolution of the laurel family. Nat. Commun. 2020, 11, 1675. [Google Scholar] [CrossRef]
- Qiu, F.; Wang, X.; Zheng, Y.; Wang, H.; Liu, X.; Su, X. Full-length transcriptome sequencing and different chemotype expression profile analysis of genes related to monoterpenoid biosynthesis in cinnamomum porrectum. Int. J. Mol. Sci. 2019, 20, 6230. [Google Scholar] [CrossRef]
- Wang, X.D.; Xu, C.Y.; Zheng, Y.J.; Wu, Y.F.; Zhang, Y.T.; Zhang, T.; Xiong, Z.Y.; Yang, H.K.; Li, J.; Fu, C.; et al. Chromosome-level genome assembly and resequencing of camphor tree (Cinnamomum camphora) provides insight into phylogeny and diversification of terpenoid and triglyceride biosynthesis of Cinnamomum. Hortic. Res. Eng. 2022, 9, 1–16. [Google Scholar] [CrossRef]
- Qiu, F.; Yang, H.; Zhang, T.; Wang, X.; Wen, S.; Su, X. Chemical composition of leaf essential oil of Cinnamomum porrectum (Roxb). Kosterm. J. Essent. Oil Bear. Plants 2019, 22, 1313–1321. [Google Scholar] [CrossRef]
- Duan, B. Study on Genetic variation of leaf-oil and its main ingredients of Cinnamomum camphora(L) presl. Chin. Acad. For. 2006, 1, 60–63. [Google Scholar]
- Mahdavi, M.; Jouri, M.H.; Mahmoudi, J.; Rezazadeh, F.; Mahzooni-Kachapi, S.S. Investigating the altitude effect on the quantity and quality of the essential oil in Tanacetum polycephalum Sch-Bip. polycephalum in the Baladeh region of Nour, Iran. Chin. J. Nat. Med. 2013, 11, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Sharma, S.; Kumar, N.; Sharma, U.; Singh, B. Chemical composition of essential oil among seven populations of Zanthoxylum armatum from Himachal Pradesh: Chemotypic and seasonal variation. Nat. Prod. Commun. 2017, 12, 1643–1646. [Google Scholar] [CrossRef]
- Ghasemi, P.A.; Barani, M.; Hamedi, B. Environment effect on diversity in quality and quantity of essential oil of different wild populations of Kerman thyme. Genetika 2013, 45, 441–450. [Google Scholar] [CrossRef]
- Karami, A.; Khoshbakht, T.; Esmaeili, H.; Maggi, F. Essential Oil Chemical Variability in Oliveria decumbens (Apiaceae) from Different Regions of Iran and Its Relationship with Environmental Factors. Plants 2020, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Compontnts by Gas Chromatography/Mass Spectrometry; Allured publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
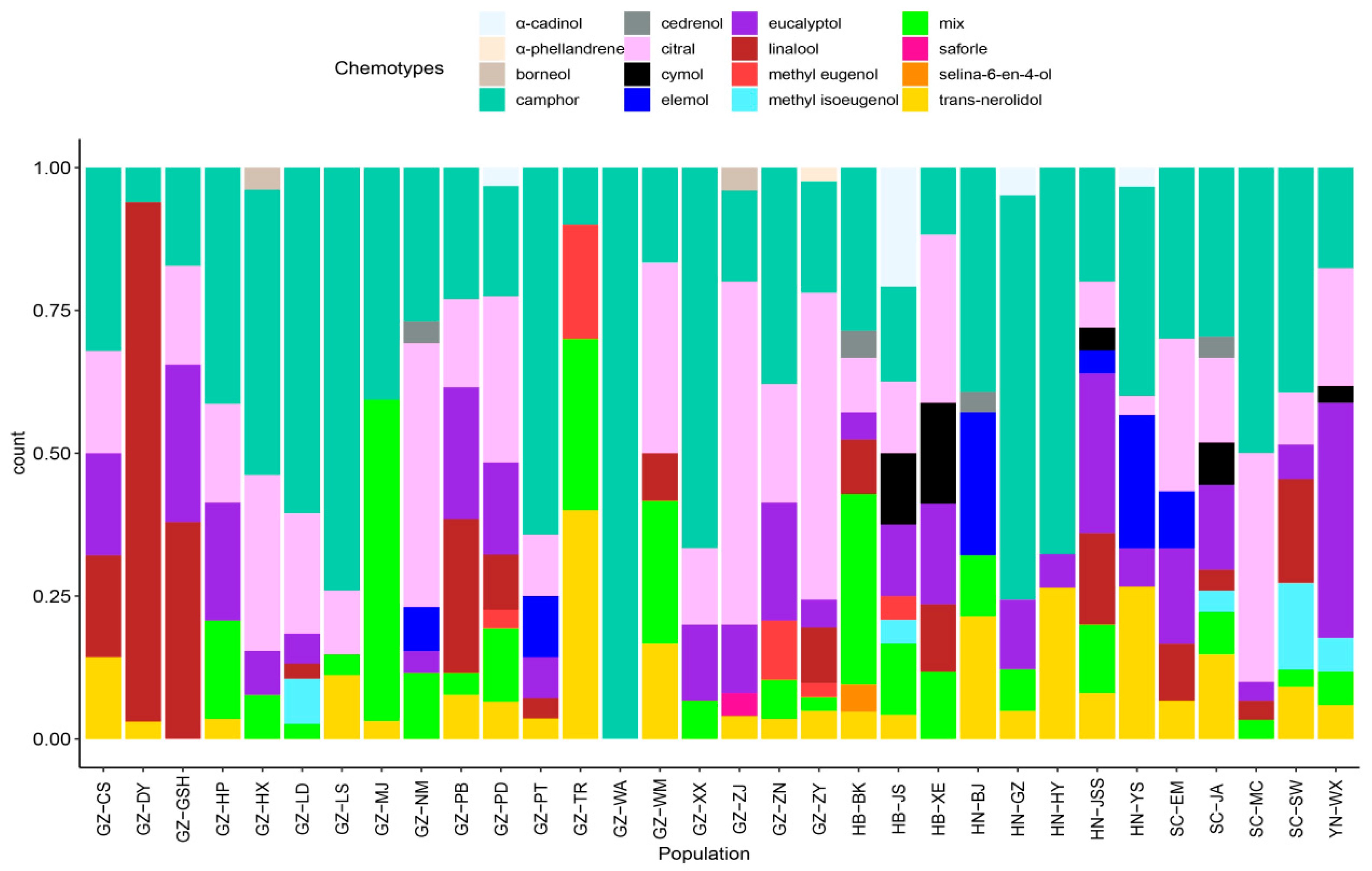
| NO | Chemotype | N | Ratio (%) | EO Mean Yield (%) | EO Min. Yield (%) | EO Max. Yield (%) | Principal Component Content (%) | |
|---|---|---|---|---|---|---|---|---|
| 1 | camphor | 333 | 37.63 | 1.18 ± 0.87 a | 0.02 | 4.28 | 30.42–98.52 | |
| 2 | citral | 160 | 18.08 | 0.26 ± 0.22 e | 0.02 | 1.51 | 31.77–82.91 | |
| 3 | eucalyptol | 101 | 11.41 | 0.73 ± 0.58 b | 0.02 | 2.86 | 41.20–67.09 | |
| 4 | linalool | 82 | 9.27 | 1.06 ± 0.74 ad | 0.02 | 2.73 | 31.39–97.14 | |
| 5 | trans-nerolidol | 65 | 7.34 | 0.31 ± 0.28 ce | 0.02 | 1.62 | 30.07–93.20 | |
| 6 | mix | 73 | 8.25 | 0.29 ± 0.32 ce | 0.02 | 1.98 | / | |
| 7 | Other | α-cadinol | 9 | 1.02 | 0.16 ± 0.07 ce | 0.07 | 0.33 | 30.01–62.79 |
| 8 | α-phellandrene | 1 | 0.11 | 0.77 | / | / | 33.32 | |
| 9 | borneol | 2 | 0.23 | 0.27 ± 0.01 bcde | 0.26 | 0.28 | 32.91–35.86 | |
| 10 | cedrenol | 4 | 0.45 | 0.27 ± 0.07 bce | 0.18 | 0.38 | 30.79–36.54 | |
| 11 | cymol | 10 | 1.13 | 0.28 ± 0.26 ce | 0.09 | 1.04 | 31.68–67.71 | |
| 12 | elemol | 23 | 2.60 | 0.30 ± 0.30 ce | 0.01 | 1.45 | 33.58–74.29 | |
| 13 | methyleugenol | 8 | 0.90 | 0.64 ± 0.59 bcde | 0.10 | 2.09 | 61.12–94.86 | |
| 14 | methylisoeugenol | 12 | 1.35 | 0.65 ± 0.53 bc | 0.14 | 2.30 | 49.45–88.81 | |
| 15 | safrole | 1 | 0.11 | 0.08 | / | / | 77.09 | |
| 16 | selina-6-en-4-ol | 1 | 0.11 | 0.95 | / | / | 67.15 | |
| Compounds | Latitude | Longitude | Altitude/m | Annual Average Temperature/°C | Annual Rainfall/mm | Annual Average Sunshine Duration/h |
|---|---|---|---|---|---|---|
| Essential oil yield | −0.29 | 0.250 | −1.090 ** | 0.103 ** | 0.91 * | 0.045 |
| Camphor | −0.045 | 0.086 | −0.137 * | 0.061 | −0.079 | 0.134 * |
| Eucalyptol | 0.177 | −0.149 | −0.206 * | 0.177 | 0.120 | −0.019 |
| Linalool | −0.069 | 0.076 | −0.229 * | 0.052 | 0.166 | −0.197 |
| Elemol | 0.240 | −0.471 * | 0.117 | −0.036 | −0.110 | 0.631 ** |
| Citral | −0.002 | 0.033 | −0.310 ** | 0.226 ** | 0.084 | 0.014 |
| Trans-nerolidol | −0.101 | 0.055 | 0.393 ** | 0.044 | −0.296 * | −0.007 |
| Cymol | −0.375 | 0.131 | −0.458 | 0.228 | 0.067 | 0.555 * |
| No. | Code | Sampling Location | Number of Samples | Geographical Coordinates | Altitude/m | |
|---|---|---|---|---|---|---|
| Latitude (N) | Longitude (E) | |||||
| 1 | GZ-CS | Changshun County, Guizhou Province | 28 | 26.177239 | 106.3958 | 1229–1264 |
| 2 | GZ-DY | Duyun County, Guizhou Province | 33 | 25.925159 | 107.403076 | 756–831 |
| 3 | GZ-GSH | Guanshanhu District, Guizhou Province | 29 | 26.603312 | 106.688876 | 1098–1188 |
| 4 | GZ-HX | Huaxi District, Guizhou Province | 26 | 26.385045 | 106.74266 | 987–1084 |
| 5 | GZ-LD | Luodian County, Guizhou Province | 38 | 25.392029 | 106.672442 | 368–424 |
| 6 | GZ-LS | Leishan County, Guizhou Province | 27 | 26.504957 | 108.159398 | 765–872 |
| 7 | GZ-MJ | Majiang County, Guizhou Province | 32 | 26.402643 | 107.530591 | 846–886 |
| 8 | GZ-NM | Nanming District, Guizhou Province | 26 | 26.605626 | 106.901249 | 1108–1193 |
| 9 | GZ-PB | Pingba County, Guizhou Province | 26 | 26.509908 | 106.137244 | 1174–1242 |
| 10 | GZ-PD | Puding County, Guizhou Province | 31 | 26.271244 | 105.782499 | 1351–1384 |
| 11 | GZ-PT | Pingtang County, Guizhou Province | 28 | 25.835329 | 107.044541 | 660–1096 |
| 12 | GZ-TR | Tongren City, Guizhou Province | 10 | 27.287726 | 108.897560 | 812–870 |
| 13 | GZ-WA | Wengan County, Guizhou Province | 11 | 26.984027 | 107.635491 | 763–916 |
| 14 | GZ-WM | Wangmo County, Guizhou Province | 12 | 25.354239 | 106.215592 | 1060–1270 |
| 15 | GZ-XX | Xixiu District, Guizhou Province | 30 | 26.283751 | 106.133002 | 1230–1282 |
| 16 | GZ-HP | Huangping County, Guizhou Province | 29 | 26.193944 | 105.816504 | 1324–1409 |
| 17 | GZ-ZJ | Zhijin County, Guizhou Province | 25 | 26.911583 | 105.650365 | 1289–1394 |
| 18 | GZ-ZN | Zhenning County, Guizhou Province | 29 | 25.901472 | 105.851612 | 934–1093 |
| 19 | GZ-ZY | ZiYun County, Guizhou Province | 41 | 25.901472 | 106.256148 | 873–1236 |
| 20 | HB-BK | Baokang County, Hubei Province | 21 | 31.768400 | 111.234308 | 561–714 |
| 21 | HB-JS | Jianshi County, Hubei Province | 24 | 30.401166 | 109.582340 | 560–873 |
| 22 | HB-XE | Xuanen County, Hubei Province | 17 | 30.684367 | 110.112064 | 681–844 |
| 23 | HN-BJ | Baojing County, Hunan Province | 28 | 28.611383 | 109.39728 | 308–863 |
| 24 | HN-GZ | Guzhang County, Hunan Province | 41 | 28.650150 | 110.077720 | 412–767 |
| 25 | HN-HY | HuaYuan County, Hunan Province | 34 | 28.532613 | 109.454847 | 432–508 |
| 26 | HN-JSS | Jishou City, Hunan Province | 25 | 28.317948 | 109.783346 | 246–286 |
| 27 | HN-YS | Yongshun County, Hunan Province | 30 | 29.436850 | 109.922745 | 478–519 |
| 28 | SC-JA | Jiangan County, Sichuan Province | 27 | 28.679040 | 105.100912 | 315–402 |
| 29 | SC-EM | Emeishan City, Sichuan Province | 30 | 29.58042 | 103.44652 | 421–464 |
| 30 | SC-SW | Shawan District, Sichuan Province | 33 | 29.33298 | 103.61326 | 386–342 |
| 31 | SC-MC | Muchuan County, Sichuan Province | 30 | 29.15152 | 103.39304 | 378–482 |
| 32 | YN-WX | Weixing County, Yunnan Province | 34 | 27.842589 | 105.166708 | 1120–1148 |
| Total | 885 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Liu, X.; Liu, Q.; Qiu, F.; Yan, J.; Zhang, Y.; Zhang, T.; Li, J. Variations in Essential Oils from the Leaves of Cinnamomum bodinieri in China. Molecules 2023, 28, 3659. https://doi.org/10.3390/molecules28093659
Fu C, Liu X, Liu Q, Qiu F, Yan J, Zhang Y, Zhang T, Li J. Variations in Essential Oils from the Leaves of Cinnamomum bodinieri in China. Molecules. 2023; 28(9):3659. https://doi.org/10.3390/molecules28093659
Chicago/Turabian StyleFu, Chao, Xinliang Liu, Qian Liu, Fengying Qiu, Jindong Yan, Yueting Zhang, Ting Zhang, and Jianan Li. 2023. "Variations in Essential Oils from the Leaves of Cinnamomum bodinieri in China" Molecules 28, no. 9: 3659. https://doi.org/10.3390/molecules28093659
APA StyleFu, C., Liu, X., Liu, Q., Qiu, F., Yan, J., Zhang, Y., Zhang, T., & Li, J. (2023). Variations in Essential Oils from the Leaves of Cinnamomum bodinieri in China. Molecules, 28(9), 3659. https://doi.org/10.3390/molecules28093659






