Chitosan-Coated Solid Lipid Nanoparticles as an Efficient Avenue for Boosted Biological Activities of Aloe perryi: Antioxidant, Antibacterial, and Anticancer Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-MS of ALP Methanolic Extract
2.2. The Content of Total Phenolic and Flavonoid of ALP Extract
2.3. Characterization of ALP-SLNPs
2.3.1. Physicochemical Characterization
2.3.2. Encapsulation Efficiency and Loading Capacity
2.3.3. In Vitro Release Study
2.3.4. Morphology of ALP-SLNs-F2 and C-ALP-SLNs-F2
2.3.5. Physical Stability Study
2.4. Biological Activities of ALP-Loaded SLNs, C-SLNs, and CSNPs
2.4.1. Antioxidant Activity
2.4.2. Antibacterial Activity
2.4.3. Anticancer Activity
3. Material and Methods
3.1. Materials
3.2. Plant Collection and Extraction
3.3. Gas Chromatography and Mass Spectrometry (GC-MS) Analysis
3.4. Determination of the Total Phenolic and Flavonoid Content of AP Extract
3.5. Preparation of ALP-SLNs, C-ALP-SLNs, and ALP-CSNPs
3.6. Characterization of ALP-SLNs
3.6.1. UV–Visible Spectrophotometry
3.6.2. Measurement of Particle Size, Polydispersity Index (PDI), and Zeta Potential
3.6.3. Encapsulation Efficiency (EE) and Loading Capacity (LC)
3.6.4. Surface Morphology
3.6.5. In Vitro Release Studies
3.6.6. Antioxidant Activity
3.6.7. Antibacterial Activity
3.6.8. Cytotoxicity Activity
3.7. Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Al-Oqail, M.M.; El-Shaibany, A.; Al-Jassas, E.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Farshori, N.N. In Vitro Anti-Proliferative Activities of Aloe perryi Flowers Extract on Human Liver, Colon, Breast, Lung, Prostate and Epithelial Cancer Cell Lines. Pak. J. Pharm. Sci. 2016, 29, 723–729. [Google Scholar]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arab. J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Health benefits of chromones: Common ingredients of our daily diet. Phytochem. Rev. 2020, 19, 761–785. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Alshammari, G.M.; Omar, U.M.; Grace, M.H.; Lila, M.A.; Yahya, M.A. Hypoglycaemic, insulin releasing, and hepatoprotective effect of the aqueous extract of Aloe perryi Baker resin (Socotran Aloe) in streptozotocin-induced diabetic rats. J. Taibah Univ. Sci. 2020, 14, 1671–1685. [Google Scholar] [CrossRef]
- Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Aljatli, D.A.; Huwaizi, S.; Suliman, R.; Albadrani, G.M.; Tolayyan, A.A.; Alghanem, B. Metabolites Profiling, in Vitro, in Vivo, Computational Pharmacokinetics and Biological Predictions of Aloe perryi Resins Methanolic Extract. Plants 2021, 10, 1106. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Al-Yousef, H.M.; Alqahtani, A.S.; Tabish, R.M.; AlAjmi, M.F.; Almarfidi, O.; Amina, M.; Alshememry, A.; Syed, R. Preparation, characterization, and in vitro-in silico biological activities of Jatropha pelargoniifolia extract loaded chitosan nanoparticles. Int. J. Pharm. 2021, 606, 120867. [Google Scholar] [CrossRef]
- Lin, C.; Gao, H.; Ouyang, L. Advance Cardiac Nanomedicine by Targeting the Pathophysiological Characteristics of Heart Failure. J. Control. Release 2021, 337, 494–504. [Google Scholar] [CrossRef]
- Xing, M.; Yan, F.; Yu, S.; Shen, P. Efficacy and Cardiotoxicity of Liposomal Doxorubicin Based Chemotherapy in Advanced Breast Cancer: A Meta-Analysis of Ten Randomized Controlled Trials. PLoS ONE 2015, 10, e0133569. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Lala, S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: A review. IET Nanobiotechnol. 2021, 15, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Neme, K.; Nafady, A.; Uddin, S.; Tola, Y.B. Application of nanotechnology in agriculture, postharvest loss reduction and food processing: Food security implication and challenges. Heliyon 2021, 7, e08539. [Google Scholar] [CrossRef]
- Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive In Situ Gel-A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics 2022, 14, 2527. [Google Scholar] [CrossRef]
- Dawoud, M. Chitosan Coated Solid Lipid Nanoparticles as Promising Carriers for Docetaxel. J. Drug Del. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Qushawy, M.; Prabahar, K.; Abd-Alhaseeb, M.; Swidan, S.; Nasr, A. Preparation and Evaluation of Carbamazepine Solid Lipid Nanoparticle for Alleviating Seizure Activity in Pentylenetetrazole-Kindled Mice. Molecules 2019, 24, 3971. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.; Kannangara, Y.Y.; Lin, Y.; Amaratunga, G.A.J.; de Silva, K.M.N. A Method for Top down Preparation of Chitosan Nanoparticles and Nanofibers. Carbohydr. Polym. 2015, 117, 731–738. [Google Scholar] [CrossRef]
- Tanzina, H.; Khan, A.; Brown, D.; Natasha, D.; Zhibin, H.; Yonghao, N. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar]
- Mohammed, H.; Khan, R.; Singh, V.; Yusuf, M.; Akhtar, N.; Sulaiman, G.; Albukhaty, S.; Ahmed, A.H.; Abdellatif; Khan, M.; et al. Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol. Rev. 2023, 12, 20220517. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Singh, V.; Yusuf, M.; Akhtar, N.; Sulaiman, G.M.; Albukhaty, S.; Abdellatif, A.A.H.; Khan, M.; Mohammed, S.A.A.; et al. Electrospun Polycaprolactone/Chitosan Nanofibers Containing Cordia myxa Fruit Extract as Potential Biocompatible AntibacterialWound Dressings. Molecules 2023, 28, 2501. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef] [PubMed]
- Aburahma, M.H.; Badr-Eldin, S.M. Compritol 888 ATO: A multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin. Drug Deliv. 2014, 11, 1865–1883. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization, and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Tanvir, S.; Qiao, L. Surface tension of nanofluid-type fuels containing suspended nanomaterials. Nanoscale Res. Lett. 2012, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Weerapol, Y.; Manmuan, S.; Chaothanaphat, N.; Limmatvapirat, S.; Sirirak, J.; Tamdee, P.; Tubtimsri, S. New Approach for Preparing Solid Lipid Nanoparticles with Volatile Oil-Loaded Quercetin Using the Phase-Inversion Temperature Method. Pharmaceutics 2022, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ji, W.; Chen, S.; Bao, Y.; Tan, S.; Lu, S.; Wu, K.; Chu, Q. A novel paclitaxel-loaded poly(d,l-lactide-co-glycolide)-Tween 80 copolymer nanoparticle overcoming multidrug resistance for lung cancer treatment. Int. J. Nanomed. 2016, 11, 2119–2131. [Google Scholar]
- Anita, S.; Wahyu, U.; Ratna, Y.; Muhammad, D.; Akhmad, N. Effect of tween 80 on nanoparticle preparation of modified chitosan for targeted delivery of combination doxorubicin and curcumin analogue. IOP Conf. Ser. Mater. Sci. Eng. 2018, 311, 012024. [Google Scholar] [CrossRef]
- Amal, A.A.; Mustafa, H.A.-M.; Salim, A.; Ghassan, M.S.; Kadhim, M.I.; Elsadig, M.A. Electrospun Polycaprolactone/Chitosan Nanofibers Containing Cordia myxa Fruit Extract as Potential Biocompatible Antibacterial Wound Dressings. Molecules 2023, 28, 2501. [Google Scholar] [CrossRef]
- Miatmoko, A.; Marufah, N.A.; Nada, Q.; Rosita, N.; Erawati, T.; Susanto, J.; Purwantari, K.E.; Nurkanto, A.; Soeratri, W. The effect of surfactant type on characteristics, skin penetration and anti-aging effectiveness of transfersomes containing amniotic mesenchymal stem cells metabolite products in UV-aging induced mice. Drug Deliv. 2022, 29, 3443–3453. [Google Scholar] [CrossRef]
- Vieira, A.C.; Chaves, L.L.; Pinheiro, S.; Pinto, S.; Pinheiro, M.; Lima, S.C.; Ferreira, D.; Sarmento, B.; Reis, S. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int. J. Pharm. 2018, 536, 478–485. [Google Scholar] [CrossRef]
- Badran, M.M.; Magdy, M.M.; Ghannam, M.M.; Faiyaz, S.H. Preparation and characterization of polymeric nanoparticles surface modified with chitosan for target treatment of colorectal cancer. Int. J. Biol. Macromol. 2017, 95, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, J.H.; Liu, Y.; Wang, Z.; Zhang, Y.T.; Feng, N.P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar]
- Kamel, K.M.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S. Chitosan-Coated Cinnamon/Oregano-Loaded Solid Lipid Nanoparticles to Augment 5-Fluorouracil Cytotoxicity for Colorectal Cancer: Extract Standardization, Nanoparticle Optimization, and Cytotoxicity Evaluation. J. Agric. Food Chem. 2017, 65, 7966–7981. [Google Scholar] [CrossRef] [PubMed]
- Yahya, R.; Al-Rajhi, A.M.H.; Alzaid, S.Z.; Al Abboud, M.A.; Almuhayawi, M.S.; Al Jaouni, S.K.; Selim, S.; Ismail, K.S.; Abdelghany, T.M. Molecular Docking and Efficacy of Aloe vera Gel Based on Chitosan Nanoparticles against Helicobacter pylori and Its Antioxidant and Anti-Inflammatory Activities. Polymers 2022, 14, 2994. [Google Scholar] [CrossRef]
- Emad, A.S.; Sanaa, M.M.; Walaa, M.A.; Eman, A.H. Biological activities and antioxidant potential of different biosynthesized nanoparticles of Moringa oleifera. Sci. Rep. 2022, 12, 18400. [Google Scholar] [CrossRef]
- Ahmed, A.; Zangeneh, A.; Zangeneh, M.M. Green synthesis and chemical characterization of gold nanoparticle synthesized using Camellia sinensis leaf aqueous extract for the treatment of acute myeloid leukemia in comparison to daunorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5290. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef]
- El-Aziz, A.R.; Al-Othman, M.R.; Mahmoud, M.; Shehata, S.; Abdelazim, N. Chitosan Nanoparticles as a Carrier for Menthalongifolia Extract: Synthesis, Characterization and Antifungal Activity. Curr. Sci. 2018, 114, 2116–2122. [Google Scholar]
- Kain, D.; Kumar, S. Synthesis and characterization of chitosan nanoparticles of Achillea millefolium L. and their activities. 1000Research 2020, 9, 1297. [Google Scholar] [CrossRef]
- Khan, Z.U.; Khan, T.; Mannan, A.; Ali, A.; Ni, J. In Vitro and Ex Vivo Evaluation of Mangifera indica L. Extract-Loaded Green Nanoparticles in Topical Emulsion against Oxidative Stress and Aging. Biomedicines 2022, 10, 2266. [Google Scholar] [CrossRef]
- Talha, M.; Islam, N.U.; Zahoor, M.; Sadiq, A.; Nawaz, A.; Khan, F.A.; Gulfam, N.; Alshamrani, S.A.; Nahari, M.H.; Alshahrani, M.A.; et al. Biological Evaluation, Phytochemical Screening, and Fabrication of Indigofera linifolia Leaves Extract-Loaded Nanoparticles. Molecules 2022, 27, 4707. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Anwer, M.K.; Sartaj, A.; Panda, B.P.; Ali, A.; Zafar, A.; Kumar, V.; Gilani, S.J.; Kala, C.; Taleuzzaman, M. ZnO Nanoparticles of Rubia cordifolia Extract Formulation Developed and Optimized with QbD Application, Considering Ex Vivo Skin Permeation, Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 1450. [Google Scholar] [CrossRef] [PubMed]
- CLSI M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard; CLSI document M07-A9. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Kuete, V. Potential of cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
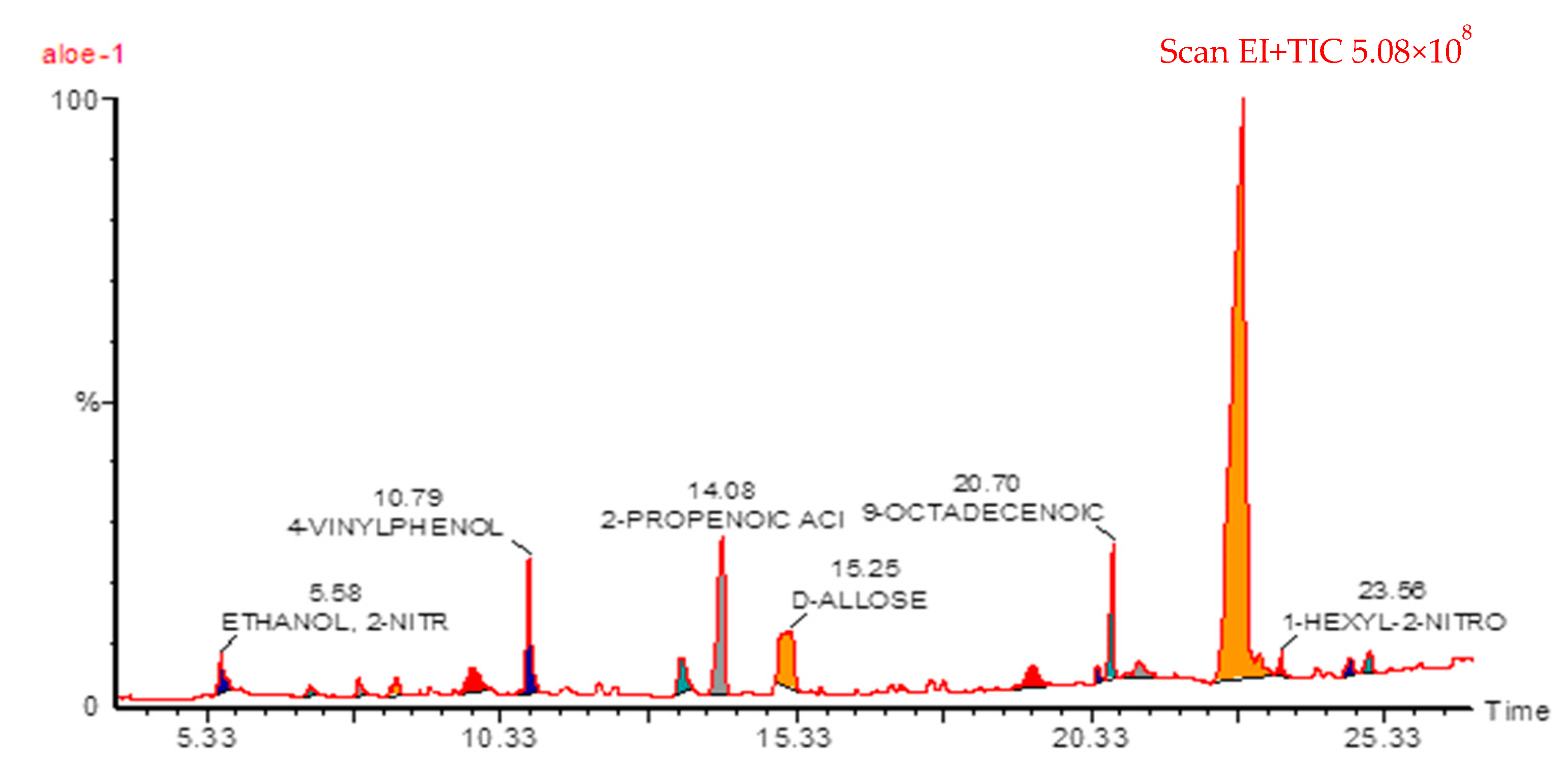


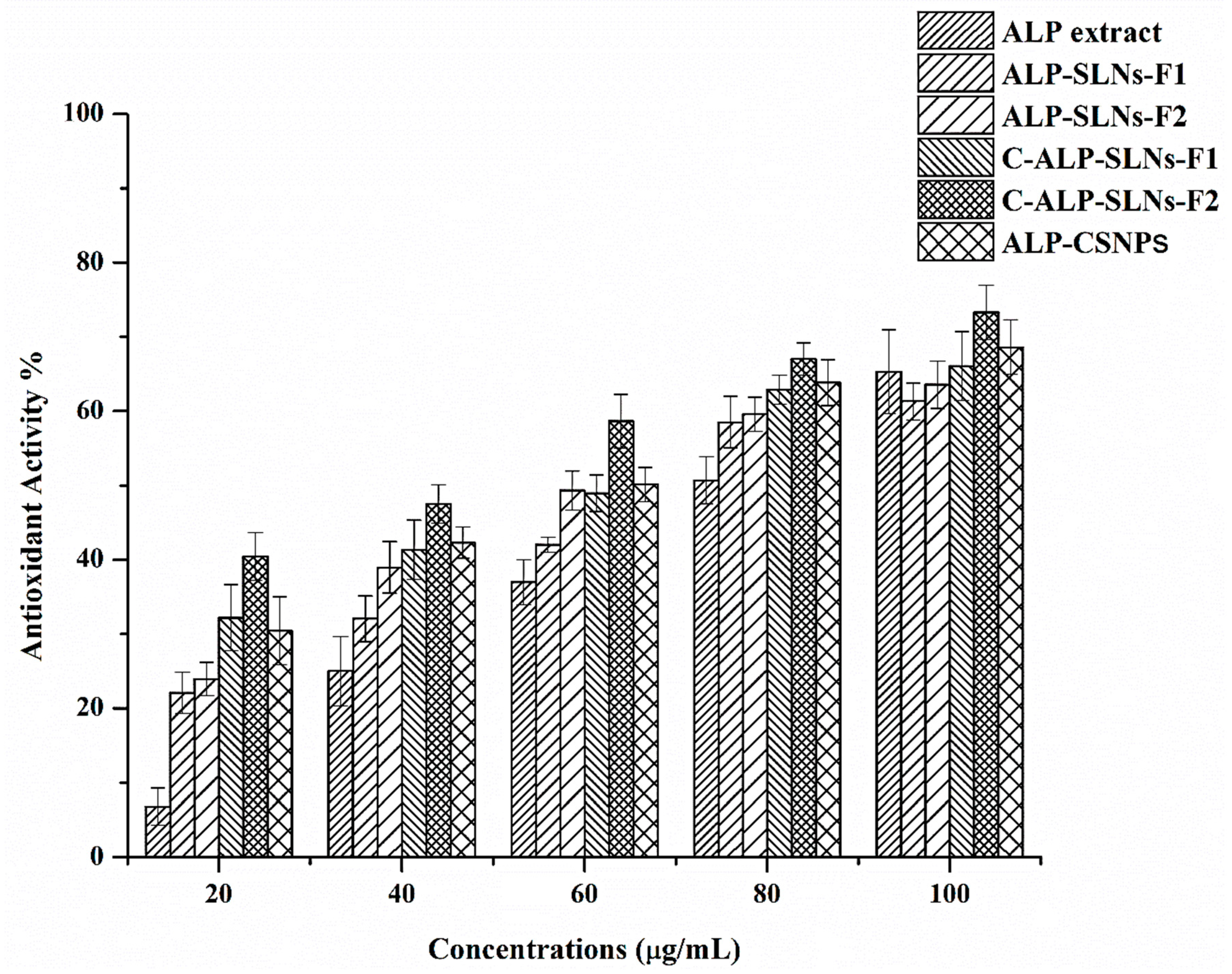
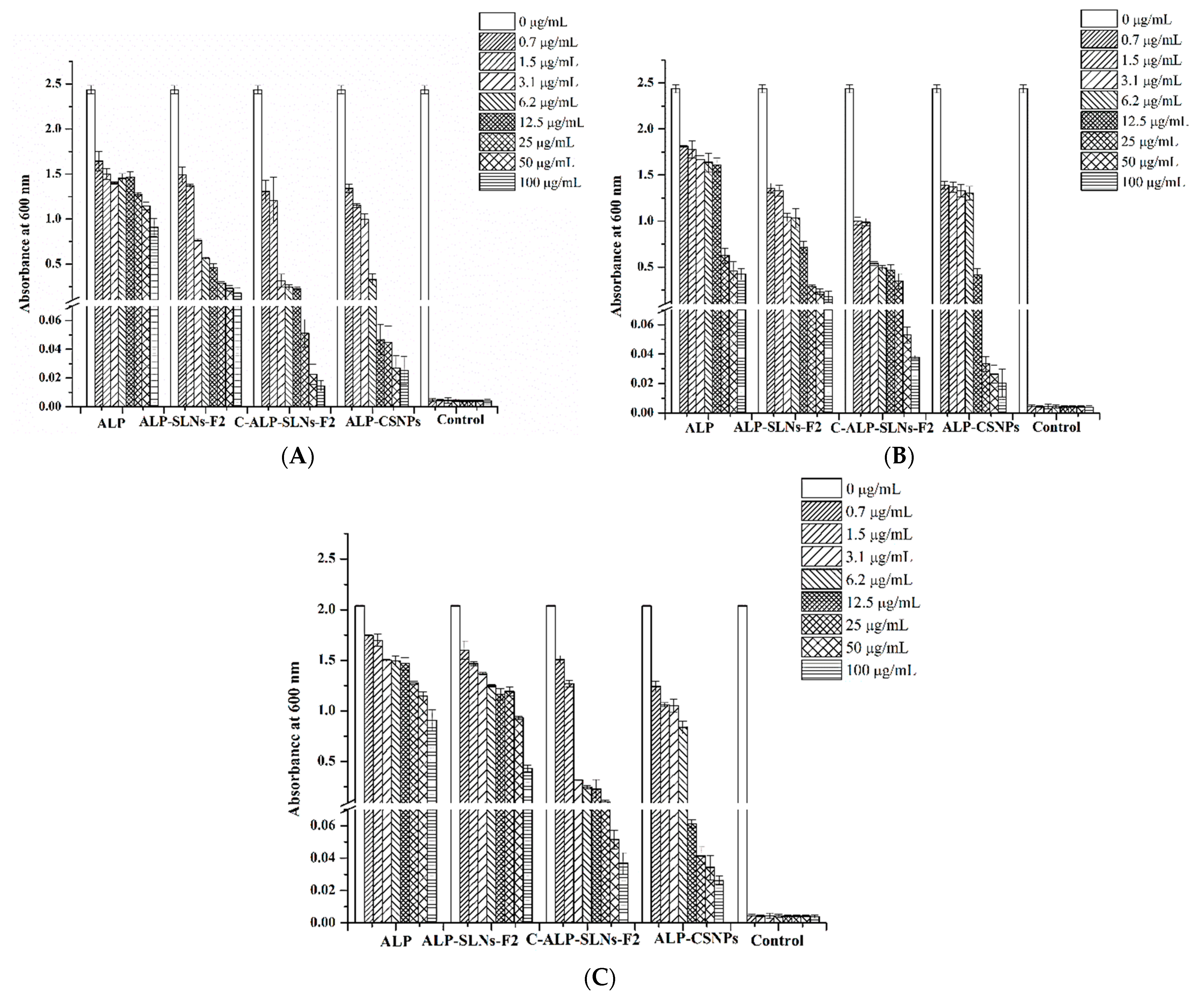

| # | Name | RT | Ares % | Area |
|---|---|---|---|---|
| 1 | 3-Amino-2-oxazolidinone | 7.07 | 1.100 | 287506 |
| 2 | N (2)-isobutyryl-2-Methylalaninamide | 7.91 | 0.960 | 250712 |
| 3 | Hydroxy dimethyl furanone | 8.54 | 1.270 | 332917 |
| 4 | 2,3-Dihydroxy-propanal | 9.86 | 6.240 | 1631520 |
| 5 | 4-Vinylphenol | 10.79 | 10.470 | 2735098 |
| 6 | 5-Methyl-1,3-benzenediol | 13.40 | 6.130 | 1602348 |
| 7 | Cinnamic acid | 14.08 | 10.030 | 2622387 |
| 8 | d-allose | 15.25 | 14.570 | 3807334 |
| 9 | 3-(4-hydroxy)-2-propenoic acid | 19.38 | 2.600 | 679033 |
| 10 | 1,19-Eicosadiene | 20.42 | 0.370 | 95965 |
| 11 | 9-Octadecenoic acid | 20.70 | 3.440 | 899762 |
| 12 | Caprinitrile | 21.15 | 0.770 | 201889 |
| 13 | 9-Hexadecenoic acid | 22.91 | 28.950 | 7566311 |
| 14 | 1-Hexyl-2-nitrocyclohexane | 23.58 | 0.440 | 114156 |
| 15 | Docos-13-enoic acid | 24.72 | 0.690 | 181277 |
| Formulations | Particle Size (nm) | PDI | Zeta Potential (mV) | EE% | LC% |
|---|---|---|---|---|---|
| ALP-SLNs-F1 | 168.7 ± 3.1 | 0.18 ± 0.03 | −12.4 ± 0.6 | 67.4 ± 2.4 | 3.53 ± 0.33 |
| ALP-SLNs-F2 | 138.4 ± 9.4 | 0.17 ± 0.03 | −15.8 ± 2.4 | 82.4 ± 1.7 | 5.18 ± 0.08 |
| C-ALP-SLNs-F1 | 185.3 ± 5.5 | 0.20 ± 0.05 | 11.3 ± 1.4 | 64.7 ± 3.9 | 2.78 ± 0.14 |
| C-ALP-SLNs-F2 | 173.6 ± 11.3 | 0.21 ± 0.02 | 13.6 ± 1.1 | 80.2 ± 5.3 | 4.66 ± 0.12 |
| ALP-CSNPs | 214.8 ± 6.6 | 0.26 ± 0.05 | 27.8 ± 3.4 | 76.4 ± 7.6 | 3.01 ± 0.29 |
| Correlation Coefficient (R2) | |||||
|---|---|---|---|---|---|
| Formulations | Zero-Order | First-Order | Higuchi’s Model | Korsmeyer–Peppas Model | |
| R2 | n | ||||
| ALP-SLNs-F1 | 0.847 | 0.983 | 0.948 | 0.959 | 0.419 |
| ALP-SLNs-F2 | 0.799 | 0.985 | 0.921 | 0.943 | 0.373 |
| C-ALP-SLNs-F1 | 0.904 | 0.980 | 0.983 | 0.992 | 0.401 |
| C-ALP-SLNs-F2 | 0.905 | 0.987 | 0.982 | 0.993 | 0.360 |
| ALP-CSNPs | 0.848 | 0.984 | 0.951 | 0.996 | 0.382 |
| Formulations | SLNs Parameters | Zero Time | One Week | Four Weeks |
|---|---|---|---|---|
| ALP-SLNs-F2 | Particle size (nm) | 168.7 ± 3.1 | 170.3 ± 4.2 | 182.5 ± 3.9 |
| Zeta potential | −16.4 ± 0.6 | −16.1 ± 0.7 | −17.9 ± 0.8 | |
| EE% | 67.42 ± 2.4 | 66.23 ± 3.8 | 65.11 ± 4.1 | |
| ALP-SLNs-F2 | Particle size (nm) | 138.4 ± 9.4 | 141.5 ± 7.2 | 152.6 ± 6.1 |
| Zeta potential | −15.8 ± 2.4 | −16.3 ± 2.8 | −14.9 ± 3.3 | |
| EE% | 82.39 ± 1.7 | 84.65 ± 2.4 | 83.39 ± 2.7 | |
| C-ALP-SLNs-F1 | Particle size (nm) | 185.3 ± 5.5 | 189.1 ± 5.6 | 194.3 ± 4.7 |
| Zeta potential | 11.3 ± 1.4 | 11.8 ± 2.3 | 14.2 ± 1.9 | |
| EE% | 64.65 ± 3.9 | 62.55 ± 4.1 | 63.21 ± 3.1 | |
| C-ALP-SLNs-F2 | Particle size (nm) | 173.6 ± 11.3 | 175.1 ± 11.3 | 181.4 ± 8.3 |
| Zeta potential | 13.6 ± 1.1 | 14.2 ± 0.9 | 14.7 ± 1.8 | |
| EE% | 80.17 ± 5.3 | 78.22 ± 3.6 | 76.19 ± 5.2 | |
| ALP-CSNPs | Particle size (nm) | 214.8 ± 6.6 | 221.5 ± 4.8 | 236.4 ± 8.5 |
| Zeta potential | 27.8 ± 3.4 | 28.5 ± 4.2 | 28.3 ± 5.2 | |
| EE% | 78.83 ± 7.6 | 78.11 ± 7.6 | 79.06 ± 6.1 |
| Composition | COMP (w/w%) | LP (w/w%) | TW80 (w/w%) | CS (mg/mL) |
|---|---|---|---|---|
| ALP-SLNs-F1 | 3.0 | 2.0 | - | - |
| ALP-SLNs-F2 | 3.0 | 2.0 | 2.0 | - |
| C-ALP-SLNs-F1 | 3.0 | 2.0 | - | 1.0 |
| C-ALP-SLNs-F2 | 3.0 | 2.0 | 2.0 | 1.0 |
| ALP-CSNPs | - | - | - | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldayel, T.S.; M. Badran, M.; H. Alomrani, A.; AlFaris, N.A.; Z. Altamimi, J.; S. Alqahtani, A.; A. Nasr, F.; Ghaffar, S.; Orfali, R. Chitosan-Coated Solid Lipid Nanoparticles as an Efficient Avenue for Boosted Biological Activities of Aloe perryi: Antioxidant, Antibacterial, and Anticancer Potential. Molecules 2023, 28, 3569. https://doi.org/10.3390/molecules28083569
Aldayel TS, M. Badran M, H. Alomrani A, AlFaris NA, Z. Altamimi J, S. Alqahtani A, A. Nasr F, Ghaffar S, Orfali R. Chitosan-Coated Solid Lipid Nanoparticles as an Efficient Avenue for Boosted Biological Activities of Aloe perryi: Antioxidant, Antibacterial, and Anticancer Potential. Molecules. 2023; 28(8):3569. https://doi.org/10.3390/molecules28083569
Chicago/Turabian StyleAldayel, Tahany Saleh, Mohamed M. Badran, Abdullah H. Alomrani, Nora A. AlFaris, Jozaa Z. Altamimi, Ali S. Alqahtani, Fahd A. Nasr, Safina Ghaffar, and Raha Orfali. 2023. "Chitosan-Coated Solid Lipid Nanoparticles as an Efficient Avenue for Boosted Biological Activities of Aloe perryi: Antioxidant, Antibacterial, and Anticancer Potential" Molecules 28, no. 8: 3569. https://doi.org/10.3390/molecules28083569
APA StyleAldayel, T. S., M. Badran, M., H. Alomrani, A., AlFaris, N. A., Z. Altamimi, J., S. Alqahtani, A., A. Nasr, F., Ghaffar, S., & Orfali, R. (2023). Chitosan-Coated Solid Lipid Nanoparticles as an Efficient Avenue for Boosted Biological Activities of Aloe perryi: Antioxidant, Antibacterial, and Anticancer Potential. Molecules, 28(8), 3569. https://doi.org/10.3390/molecules28083569






