Critical Review on the Different Roles of Exosomes in TNBC and Exosomal-Mediated Delivery of microRNA/siRNA/lncRNA and Drug Targeting Signalling Pathways in Triple-Negative Breast Cancer
Abstract
1. Introduction
1.1. Triple Negative Breast Cancer
1.2. Need of Delivery Vehicle—Exosomes as an Answer
2. Extracellular Vesicles
3. Exosomes in TNBC
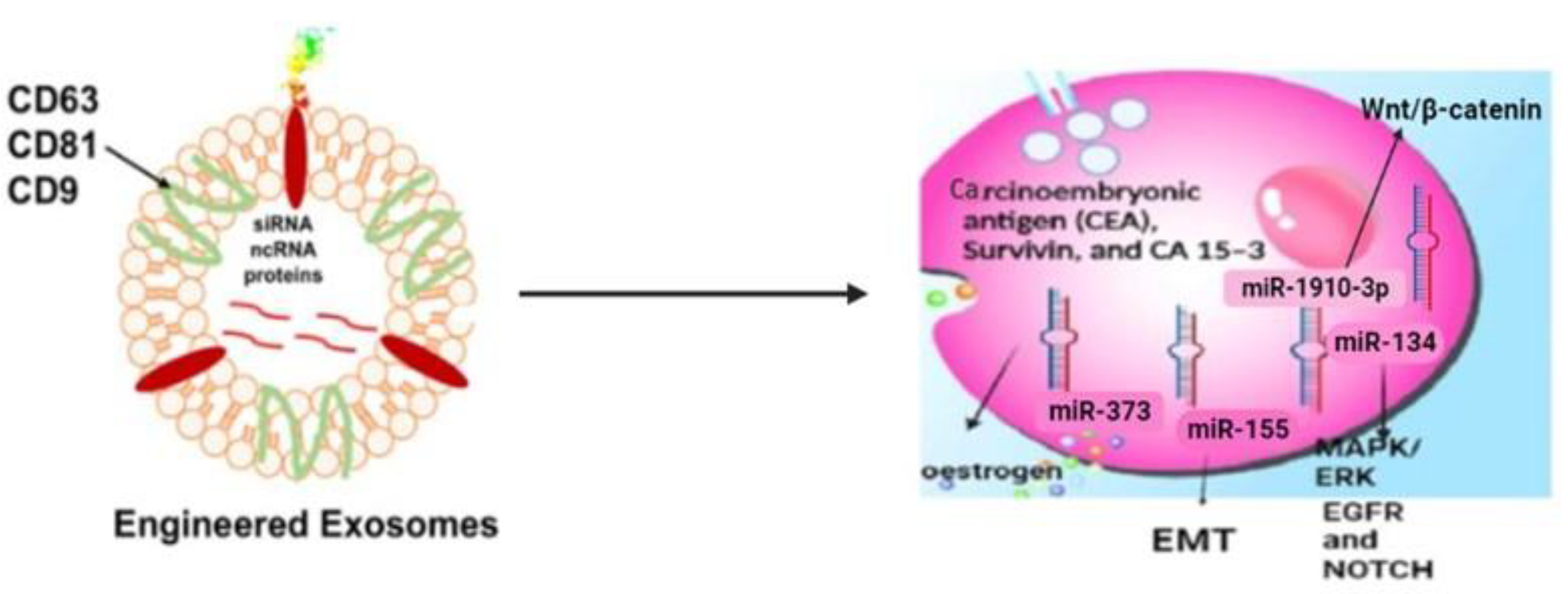
3.1. Exosomes Carry Signature Markers from Their Cells of Origin
3.2. Exosomes for Diagnosis of Triple-Negative Breast Cancer
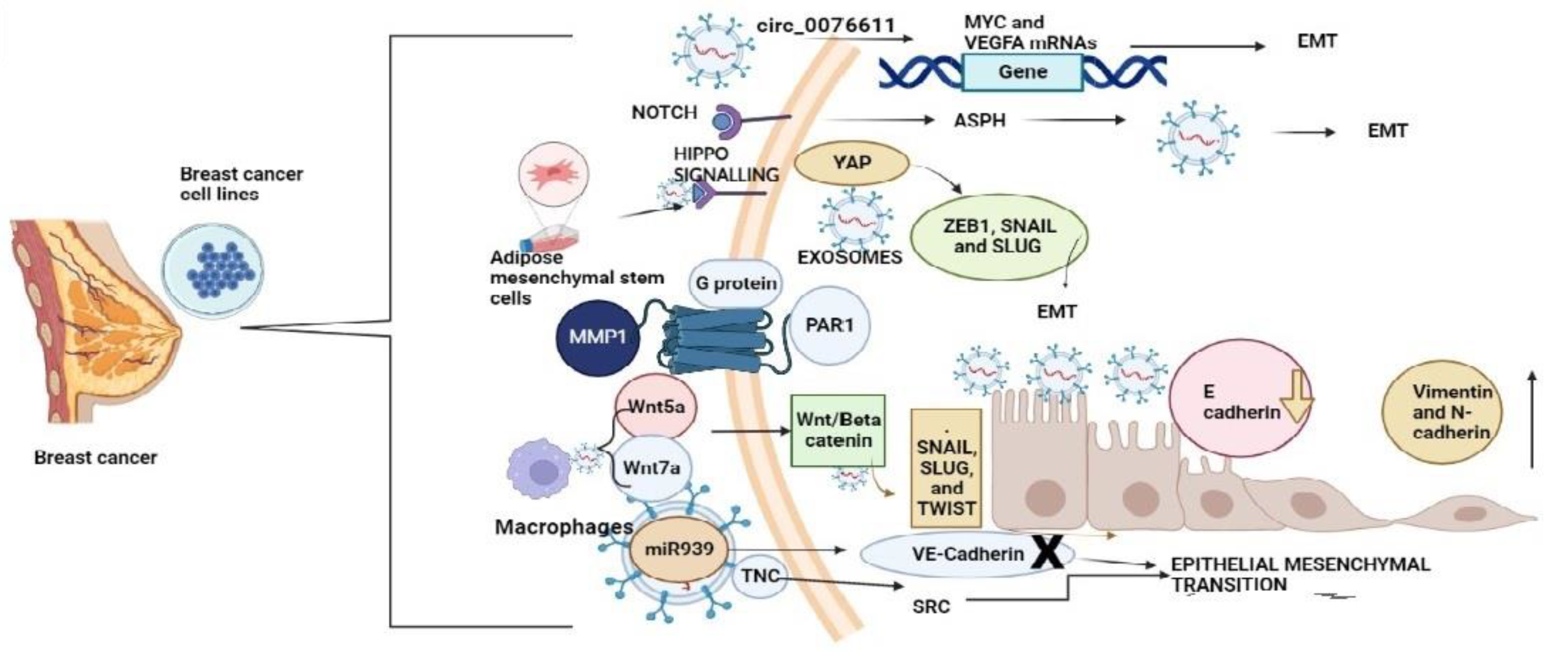
3.3. Exosomes Initiating the Epithelial-Mesenchymal Transition in Breast Cancer and TNBC Influencing the Metastasis to Other Organs
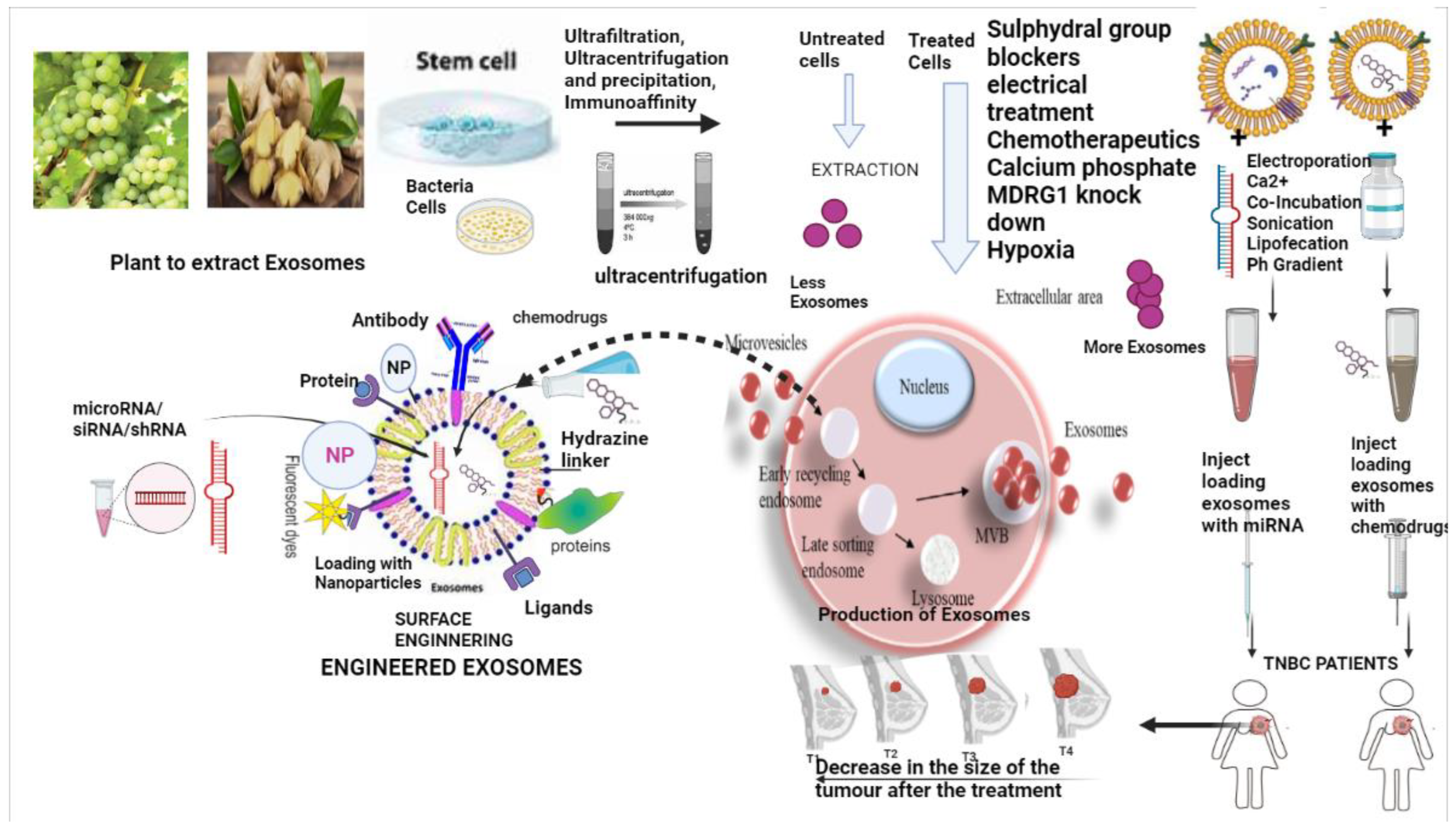
4. Exosomes—Production, Preservation, Loading, Surface Engineering, and Exosome Hybrids in Drug Delivery
5. Exosomes for the Delivery of Chemotherapeutic Drugs
6. Engineered Exosomes in Triple-Negative Breast Cancer Drug Delivery
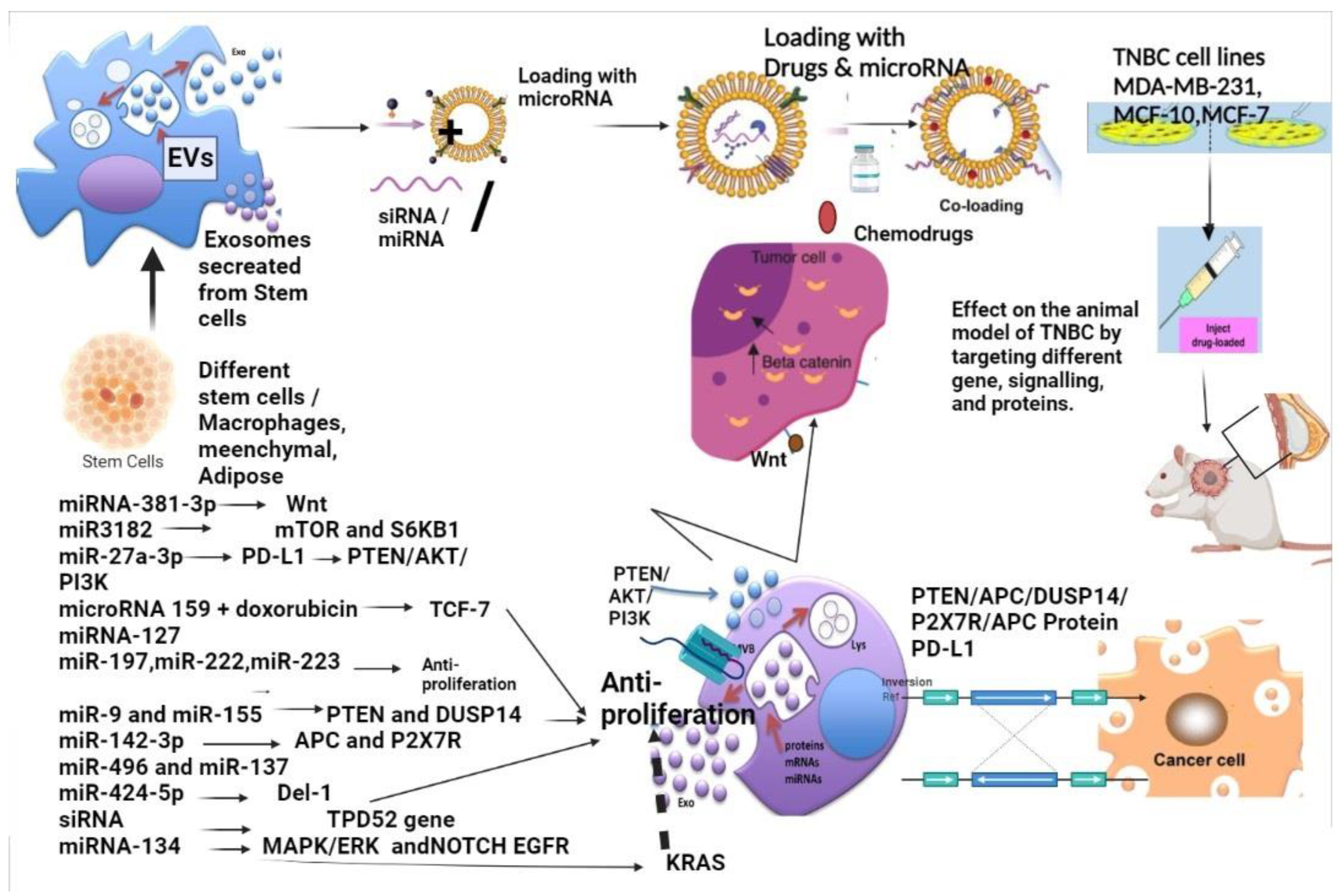
7. Exosomes as a Delivery Agent for microRNA and siRNA in TNBC Modulating Signalling
8. Exosomal-Associated Small Molecule Modulating Pathways Inhibiting Metastasis of Triple-Negative Breast Cancer to Other Organs
9. Exosomes Specifically Regulating TNBC Signallings
10. Exosomes from Plants and Bacteria as a Delivery Agent
11. Exosome-Mediated Delivery Vehicles in Pre-Clinical and Clinical Trials (Vaccines) in Triple-Negative Breast Cancer
12. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Prusty, R.K.; Begum, S.; Patil, A.; Naik, D.D.; Pimple, S.; Mishra, G. Knowledge of Symptoms and Risk Factors of Breast Cancer among Women: A Community Based Study in a Low Socio-Economic Area of Mumbai, India. BMC Womens Health 2020, 20, 106. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Al Sendi, M.; Kelly, C.M. Overview of Recent Advances in Metastatic Triple Negative Breast Cancer. World J. Clin. Oncol. 2021, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Aruga, T.; Yamashita, T.; Miyamoto, H.; Horiguchi, K.; Kitagawa, D.; Idera, N.; Goto, R.; Kuroi, K. Prolonged Survival after Diagnosis of Brain Metastasis from Breast Cancer: Contributing Factors and Treatment Implications. Jpn. J. Clin. Oncol. 2015, 45, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Adamo, B.; Cheang, M.C.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular Characterization of Basal-like and Non-basal-like Triple-negative Breast Cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Bauer, J.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. Am. Soc. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Keane, M.M.; Wink, D.A.; Callagy, G.; Glynn, S.A. Review of Triple Negative Breast Cancer and the Impact of Inducible Nitric Oxide Synthase on Tumor Biology and Patient Outcomes. Crit. Rev. Oncog. 2016, 21, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-Negative Breast Cancer: Is There a Treatment on the Horizon? Oncotarget 2016, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Nishimura, R.; Nakatsukasa, A.; Yoshida, N.; Masuda, M.; Tanabe, T.; Shien, S.; Tanaka, N.; Arima, Y.; Komoike, T.; et al. Change in Estrogen Receptor, HER2, and Ki-67 Status between Primary Breast Cancer and Ipsilateral Breast Cancer Tumor Recurrence. Eur. J. Surg. Oncol. 2015, 41, 548–552. [Google Scholar] [CrossRef]
- Nakashoji, A.; Matsui, A.; Nagayama, A.; Iwata, Y.; Sasahara, M.; Murata, Y. Clinical Predictors of Pathological Complete Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Oncol. Lett. 2017, 14, 4135–4141. [Google Scholar] [CrossRef]
- Aslam, M.S.; Naveed, S.; Ahmed, A.; Abbas, Z.; Gull, I.; Athar, M.A.; Aslam, M.S.; Naveed, S.; Ahmed, A.; Abbas, Z.; et al. Side Effects of Chemotherapy in Cancer Patients and Evaluation of Patients Opinion about Starvation Based Differential Chemotherapy. J. Cancer Ther. 2014, 5, 817–822. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of Distant Recurrence and Clinical Outcomes in Patients with Metastatic Triple-Negative Breast Cancer: High Incidence of Central Nervous System Metastases. Cancer 2008, 113, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An Approach to Chemotherapy-Associated Toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 2018, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; von der Ohe, J.; Hass, R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Du, C.; Zhang, Y.; Li, Y.; Chu, L.; Han, X.; Galons, H.; Zhang, Y.; Sun, H.; et al. Exosomes from Different Cells: Characteristics, Modifications, and Therapeutic Applications. Eur. J. Med. Chem. 2020, 207, 112784. [Google Scholar] [CrossRef]
- Kim, H.; Jang, H.; Cho, H.; Choi, J.; Hwang, K.Y.; Choi, Y.; Kim, S.H.; Yang, Y. Recent Advances in Exosome-Based Drug Delivery for Cancer Therapy. Cancers 2021, 13, 4435. [Google Scholar] [CrossRef]
- Kanchanapally, R.; Deshmukh, S.K.; Chavva, S.R.; Tyagi, N.; Srivastava, S.K.; Patel, G.K.; Singh, A.P.; Singh, S. Drug-Loaded Exosomal Preparations from Different Cell Types Exhibit Distinctive Loading Capability, Yield, and Antitumor Efficacies: A Comparative Analysis. Int. J. Nanomed. 2019, 14, 531–541. [Google Scholar] [CrossRef]
- Xi, X.M.; Xi, X.M.; Xia, S.J.; Lu, R. Drug Loading Techniques for Exosome-Based Drug Delivery Systems. Pharmazie 2021, 76, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Berumen Sánchez, G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular Vesicles: Mediators of Intercellular Communication in Tissue Injury and Disease. Cell Commun. Signal. 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Chen, T.; Banciu, D.D.; Cretoiu, D.; Xiao, J. Circulating Exosomes in Cardiovascular Diseases. Adv. Exp. Med. Biol. 2017, 998, 255–269. [Google Scholar] [CrossRef]
- Howitt, J.; Hill, A.F. Exosomes in the Pathology of Neurodegenerative Diseases. J. Biol. Chem. 2016, 291, 26589–26597. [Google Scholar] [CrossRef]
- Baharlooi, H.; Azimi, M.; Salehi, Z.; Izad, M. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases. Int. J. Stem Cells 2020, 13, 13–23. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.I. The Role of Exosomes in Cancer Metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Konishi, Y.; Kosaka, N.; Katsuda, T.; Kato, T.; Ochiya, T. Comparative Marker Analysis of Extracellular Vesicles in Different Human Cancer Types. J. Extracell. Vesicles 2013, 2, 20424. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Q.; Zhang, J.; Li, C.; Miao, Y.R.; Lei, Q.; Li, Q.; Guo, A.Y. EVmiRNA: A Database of MiRNA Profiling in Extracellular Vesicles. Nucleic Acids Res. 2019, 47, D89–D93. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A Compendium of RNA, Proteins, Lipids and Metabolites in Extracellular Vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in Cancer Development and Clinical Applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef]
- Zlotogorski-Hurvitz, A.; Dekel, B.Z.; Malonek, D.; Yahalom, R.; Vered, M. FTIR-Based Spectrum of Salivary Exosomes Coupled with Computational-Aided Discriminating Analysis in the Diagnosis of Oral Cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, E.L.; Lindsay, S.; Halasz, M.; Gubbins, L.C.; Weiner-Gorzel, K.; Guang, M.H.Z.; McGoldrick, A.; Collins, E.; Henry, M.; Blanco-Fernández, A.; et al. Protein and Chemotherapy Profiling of Extracellular Vesicles Harvested from Therapeutic Induced Senescent Triple Negative Breast Cancer Cells. Oncogenesis 2017, 6, e388. [Google Scholar] [CrossRef]
- Jakhar, R.; Crasta, K. Exosomes as Emerging Pro-Tumorigenic Mediators of the Senescence-Associated Secretory Phenotype. Int. J. Mol. Sci. 2019, 20, 2547. [Google Scholar] [CrossRef]
- Campisi, J.; D’Adda Di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Lianidou, E.; Pantel, K. Liquid Biopsies. Genes Chromosomes Cancer 2019, 58, 219–232. [Google Scholar] [CrossRef]
- Toth, B.; Nieuwland, R.; Liebhardt, S.; Ditsch, N.; Steinig, K.; Stieber, P.; Rank, A.; Göhring, P.; Thaler, C.J.; Friese, K.; et al. Circulating Microparticles in Breast Cancer Patients: A Comparative Analysis with Established Biomarkers. Anticancer Res. 2008, 28, 1107–1112. [Google Scholar]
- Eichelser, C.; Stückrath, I.; Müller, V.; Milde-Langosch, K.; Wikman, H.; Pantel, K.; Schwarzenbach, H. Increased Serum Levels of Circulating Exosomal MicroRNA-373 in Receptor-Negative Breast Cancer Patients. OncoTargets Ther. 2014, 5, 9650–9663. [Google Scholar] [CrossRef]
- Dioufa, N.; Clark, A.M.; Ma, B.; Beckwitt, C.H.; Wells, A. Bi-Directional Exosome-Driven Intercommunication between the Hepatic Niche and Cancer Cells. Mol. Cancer 2017, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Cho, N.H. 261: Cancer-Associated Fibroblast (CAF)-Derived Exosome May Mediate Breast Cancer Progression by Reducing Exosomal MicroRNAs. Eur. J. Cancer 2014, 50, S61. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Shin, E.; Seong, K.M.; Jin, Y.W.; Youn, H.S.; Youn, B.H. The Emerging Roles of Exosomes as EMT Regulators in Cancer. Cells 2020, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Chen, X.; Meng, F.; Ogawa, K.; Li, M.; Song, R.; Zhang, S.; Zhang, Z.; Kong, X.; Xu, Q.; et al. ASPH-Notch Axis Guided Exosomal Delivery of Prometastatic Secretome Renders Breast Cancer Multi-Organ Metastasis. Mol. Cancer 2019, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, G.T.K.; Hong, W. Role of Hippo Pathway-YAP/TAZ Signaling in Angiogenesis. Front. Cell Dev. Biol. 2019, 7, 49. [Google Scholar] [CrossRef]
- Huh, H.; Kim, D.; Jeong, H.; Park, H.W. Regulation of TEAD Transcription Factors in Cancer Biology. Cells 2019, 8, 600. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Chai, J.Y.; Fang Tang, T.; Fen Wong, W.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, X.; Xu, M.; Xiao, X.; Li, X.; Li, H.; Keating, A.; Zhao, R.C. Exosomes Secreted by Mesenchymal Stromal/Stem Cell-Derived Adipocytes Promote Breast Cancer Cell Growth via Activation of Hippo Signaling Pathway. Stem Cell Res. Ther. 2019, 10, 117. [Google Scholar] [CrossRef]
- Wei, C.Y.; Zhu, M.X.; Yang, Y.W.; Zhang, P.F.; Yang, X.; Peng, R.; Gao, C.; Lu, J.C.; Wang, L.; Deng, X.Y.; et al. Downregulation of RNF128 Activates Wnt/β-Catenin Signaling to Induce Cellular EMT and Stemness via CD44 and CTTN Ubiquitination in Melanoma. J. Hematol. Oncol. 2019, 12, 21. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.N.; Anjum, K.M.; Gui, L.X.; Zhu, S.S.; Liu, J.; Chen, J.K.; Liu, Q.F.; Ye, G.D.; Wang, W.J.; et al. A Double-Negative Feedback Loop between Wnt-β-Catenin Signaling and HNF4α Regulates Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. J. Cell Sci. 2013, 126, 5692–5703. [Google Scholar] [CrossRef]
- Ekström, E.J.; Bergenfelz, C.; von Bülow, V.; Serifler, F.; Carlemalm, E.; Jönsson, G.; Andersson, T.; Leandersson, K. WNT5A Induces Release of Exosomes Containing Pro-Angiogenic and Immunosuppressive Factors from Malignant Melanoma Cells. Mol. Cancer 2014, 13, 88. [Google Scholar] [CrossRef]
- Li, C.; Yoshimura, T.; Tian, M.; Wang, Y.; Kondo, T.; Yamamoto, K.I.; Fujisawa, M.; Ohara, T.; Sakaguchi, M.; Matsukawa, A. Exosomal Wnt7a from a Low Metastatic Subclone Promotes Lung Metastasis of a Highly Metastatic Subclone in the Murine 4t1 Breast Cancer. Breast Cancer Res. 2022, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Nagaharu, K.; Zhang, X.; Yoshida, T.; Katoh, D.; Hanamura, N.; Kozuka, Y.; Ogawa, T.; Shiraishi, T.; Imanaka-Yoshida, K. Tenascin C Induces Epithelial-Mesenchymal Transition-like Change Accompanied by SRC Activation and Focal Adhesion Kinase Phosphorylation in Human Breast Cancer Cells. Am. J. Pathol. 2011, 178, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Turco, C.; Esposito, G.; Iaiza, A.; Goeman, F.; Benedetti, A.; Gallo, E.; Daralioti, T.; Perracchio, L.; Sacconi, A.; Pasanisi, P.; et al. MALAT1-Dependent Hsa_circ_0076611 Regulates Translation Rate in Triple-Negative Breast Cancer. Commun. Biol. 2022, 5, 598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tao, Z.; Chen, Y.; Lin, S.; Zhu, M.; Ji, W.; Liu, X.; Li, T.; Hu, X. Exosomal MMP-1 Transfers Metastasis Potential in Triple-Negative Breast Cancer through PAR1-Mediated EMT. Breast Cancer Res. Treat. 2022, 193, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast Cancer-Secreted MiR-939 Downregulates VE-Cadherin and Destroys the Barrier Function of Endothelial Monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Sareyeldin, R.M.; Al-Hashimi, I.; Al-Thawadi, H.A.; Al Farsi, H.; Vranic, S.; Moustafa, A.E. Al Triple Negative Breast Cancer Profile, from Gene to MicroRNA, in Relation to Ethnicity. Cancers 2019, 11, 363. [Google Scholar] [CrossRef]
- Stevic, I.; Müller, V.; Weber, K.; Fasching, P.A.; Karn, T.; Marmé, F.; Schem, C.; Stickeler, E.; Denkert, C.; Van Mackelenbergh, M.; et al. Specific MicroRNA Signatures in Exosomes of Triple-Negative and HER2-Positive Breast Cancer Patients Undergoing Neoadjuvant Therapy within the GeparSixto Trial. BMC Med. 2018, 16, 179. [Google Scholar] [CrossRef]
- Srivastava, A.; Amreddy, N.; Pareek, V.; Chinnappan, M.; Ahmed, R.; Mehta, M.; Razaq, M.; Munshi, A.; Ramesh, R. Progress in Extracellular Vesicle Biology and Their Application in Cancer Medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1621. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Zubair, H.; Khan, M.A.; Srivastava, S.K.; Ahmad, A.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes: Key Supporters of Tumor Metastasis. In Diagnostic and Therapeutic Applications of Exosomes in Cancer; Academic Press: Cambridge, MA, USA, 2018; pp. 261–283. [Google Scholar] [CrossRef]
- Ingato, D.; Edson, J.A.; Zakharian, M.; Kwon, Y.J. Cancer Cell-Derived, Drug-Loaded Nanovesicles Induced by Sulfhydryl-Blocking for Effective and Safe Cancer Therapy. ACS Nano 2018, 12, 9568–9577. [Google Scholar] [CrossRef] [PubMed]
- Carswell, K.S.; Papoutsakis, E.T. Culture of Human T Cells in Stirred Bioreactors for Cellular Immunotherapy Applications: Shear, Proliferation, and the IL-2 Receptor. Biotechnol. Bioeng. 2000, 68, 328–338. [Google Scholar] [CrossRef]
- Kore, R.A.; Edmondson, J.L.; Jenkins, S.V.; Jamshidi-Parsian, A.; Dings, R.P.M.; Reyna, N.S.; Griffin, R.J. Hypoxia-Derived Exosomes Induce Putative Altered Pathways in Biosynthesis and Ion Regulatory Channels in Glioblastoma Cells. Biochem. Biophys. Rep. 2018, 14, 104–113. [Google Scholar] [CrossRef]
- Fukuta, T.; Nishikawa, A.; Kogure, K. Low Level Electricity Increases the Secretion of Extracellular Vesicles from Cultured Cells. Biochem. Biophys. Rep. 2019, 21, 100713. [Google Scholar] [CrossRef]
- Frank, J.; Richter, M.; de Rossi, C.; Lehr, C.M.; Fuhrmann, K.; Fuhrmann, G. Extracellular Vesicles Protect Glucuronidase Model Enzymes during Freeze-Drying. Sci Rep. 2018, 8, 12377. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of Exosomes at Room Temperature Using Lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y.; et al. MiR-21 in the Extracellular Vesicles (EVs) of Cerebrospinal Fluid (CSF): A Platform for Glioblastoma Biomarker Development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef]
- Noguchi, K.; Hirano, M.; Hashimoto, T.; Yuba, E.; Takatani-Nakase, T.; Nakase, I. Effects of Lyophilization of Arginine-Rich Cell-Penetrating Peptide-Modified Extracellular Vesicles on Intracellular Delivery. Anticancer Res. 2019, 39, 6701–6709. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-Scale Generation of Functional MRNA-Encapsulating Exosomes via Cellular Nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef]
- Huang, L.; Wang, D.; Gu, N.; Zhang, X.E. Construction of Engineered Exosomes with High Loading Efficiency of Cellular Endogenous Proteins. Sheng Wu Gong Cheng Xue Bao 2019, 35, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; De Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-Induced SiRNA Precipitation Obscures the Efficiency of SiRNA Loading into Extracellular Vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315. [Google Scholar] [CrossRef]
- Oppel, F.; Schurmann, M.; Goon, P.; Albers, A.E.; Sudhoff, H. Specific Targeting of Oncogenes Using CRISPR Technology. Cancer Res. 2018, 78, 5506–5512. [Google Scholar] [CrossRef]
- Si, Y.; Kim, S.; Zhang, E.; Tang, Y.; Jaskula-Sztul, R.; Markert, J.M.; Chen, H.; Zhou, L.; Liu, X. Targeted Exosomes for Drug Delivery: Biomanufacturing, Surface Tagging, and Validation. Biotechnol. J. 2020, 15, 1900163. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Théry, C. Rab27a Supports Exosome-Dependent and -Independent Mechanisms That Modify the Tumor Microenvironment and Can Promote Tumor Progression. Cancer Res. 2012, 72, 4920–4930. [Google Scholar] [CrossRef]
- Yang, P.; Cao, X.; Cai, H.; Feng, P.; Chen, X.; Zhu, Y.; Yang, Y.; An, W.; Yang, Y.; Jie, J. The Exosomes Derived from CAR-T Cell Efficiently Target Mesothelin and Reduce Triple-Negative Breast Cancer Growth. Cell. Immunol. 2021, 360, 104262. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Z.; Xiang, M.; Chang, B.; Cui, J.; Ji, T.; Zhao, L.; Li, Q.; Deng, Y.; Xu, L.; et al. Functional Extracellular Vesicles Engineered with Lipid-Grafted Hyaluronic Acid Effectively Reverse Cancer Drug Resistance. Biomaterials 2019, 223, 119475. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ding, F.; Yang, J.; Li, J.; Gao, X.; Zhang, C.; Feng, J. Engineering Macrophage-Derived Exosomes for Targeted Chemotherapy of Triple-Negative Breast Cancer. Nanoscale 2020, 12, 10854–10862. [Google Scholar] [CrossRef]
- Quinn, Z.; Mao, W.; Xia, Y.; John, R.; Wan, Y. Conferring Receptors on Recipient Cells with Extracellular Vesicles for Targeted Drug Delivery. Bioact. Mater. 2021, 6, 749. [Google Scholar] [CrossRef]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted Exosome-Encapsulated Erastin Induced Ferroptosis in Triple Negative Breast Cancer Cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef]
- Lopatina, T.; Grange, C.; Cavallari, C.; Navarro-Tableros, V.; Lombardo, G.; Rosso, A.; Cedrino, M.; Pomatto, M.A.C.; Koni, M.; Veneziano, F.; et al. Targeting IL-3Rα on Tumor-Derived Endothelial Cells Blunts Metastatic Spread of Triple-Negative Breast Cancer via Extracellular Vesicle Reprogramming. Oncogenesis 2020, 9, 90. [Google Scholar] [CrossRef]
- Limoni, S.K.; Salimi, F.; Moghaddam, M.F. Designing PLEX-LAMP-DARPin Lentiviral Vector for Exression of HER2 Targeted DARPin on Exosome Surface. J. Maz. Univ. Med. Sci. 2017, 27, 12–23. [Google Scholar]
- Gomari, H.; Moghadam, M.F.; Soleimani, M. Targeted Cancer Therapy Using Engineered Exosome as a Natural Drug Delivery Vehicle. OncoTargets Ther. 2018, 11, 5753–5762. [Google Scholar] [CrossRef]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting Extracellular Vesicles to Injured Tissue Using Membrane Cloaking and Surface Display. J. Nanobiotechnol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Shojaei, S.; Hashemi, S.M.; Ghanbarian, H.; Sharifi, K.; Salehi, M.; Mohammadi-Yeganeh, S. Delivery of MiR-381-3p Mimic by Mesenchymal Stem Cell-Derived Exosomes Inhibits Triple Negative Breast Cancer Aggressiveness; an In Vitro Study. Stem Cell Rev. Rep. 2021, 17, 1027–1038. [Google Scholar] [CrossRef]
- Khazaei-Poul, Y.; Shojaei, S.; Koochaki, A.; Ghanbarian, H.; Mohammadi-Yeganeh, S. Evaluating the Influence of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes Loaded with MiR-3182 on Metastatic Performance of Triple Negative Breast Cancer Cells. Life Sci. 2021, 286, 120015. [Google Scholar] [CrossRef]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic Reticulum Stress-Induced Exosomal MiR-27a-3p Promotes Immune Escape in Breast Cancer via Regulating PD-L1 Expression in Macrophages. J. Cell. Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef]
- Lim, P.K.; Bliss, S.A.; Patel, S.A.; Taborga, M.; Dave, M.A.; Gregory, L.A.; Greco, S.J.; Bryan, M.; Patel, P.S.; Rameshwar, P. Gap Junction-Mediated Import of MicroRNA from Bone Marrow Stromal Cells Can Elicit Cell Cycle Quiescence in Breast Cancer Cells. Cancer Res. 2011, 71, 1550–1560. [Google Scholar] [CrossRef]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of SiRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2018, 187, 352–364. [Google Scholar] [CrossRef]
- Chen, T.; Dong, Y.; Wu, X. Plasma Exosomal MiR-335-5p Serves as a Diagnostic Indicator and Inhibits Immune Escape in Triple-Negative Breast Cancer. Mol. Ther. Nucleic Acids 2022, 38, 347–356. [Google Scholar]
- O’Brien, K.; Lowry, M.C.; Corcoran, C.; Martinez, V.G.; Daly, M.; Rani, S.; Gallagher, W.M.; Radomski, M.W.; MacLeod, R.A.; O’Driscoll, L. miR-134 inhibits epithelial to mesenchymal transition by targeting FOXM1 in non-small cell lung cancer cells. FEBS Lett. 2012, 586, 3761–3765. [Google Scholar] [CrossRef]
- O’Brien, K.; Lowry, M.C.; Corcoran, C.; Martinez, V.G.; Daly, M.; Rani, S.; Gallagher, W.M.; Radomski, M.W.; MacLeod, R.A.F.; O’Driscoll, L. MiR-134 in Extracellular Vesicles Reduces Triple-Negative Breast Cancer Aggression and Increases Drug Sensitivity. Oncotarget 2015, 6, 32774–32789. [Google Scholar] [CrossRef]
- Su, X.; Zhang, L.; Li, H.; Cheng, P.; Zhu, Y.; Liu, Z.; Zhao, Y.; Xu, H.; Li, D.; Gao, H.; et al. MicroRNA-134 Targets KRAS to Suppress Breast Cancer Cell Proliferation, Migration and Invasion. Oncol. Lett. 2017, 13, 1932–1938. [Google Scholar] [CrossRef]
- Pan, J.Y.; Zhang, F.; Sun, C.C.; Li, S.J.; Li, G.; Gong, F.Y.; Bo, T.; He, J.; Hua, R.X.; Hu, W.D.; et al. MiR-134: A Human Cancer Suppressor? Mol. Ther. Nucleic Acids 2017, 6, 140. [Google Scholar] [CrossRef]
- Du, J.; Fan, J.J.; Dong, C.; Li, H.T.; Ma, B.L. Inhibition Effect of Exosomes-Mediated Let-7a on the Development and Metastasis of Triple Negative Breast Cancer by down-Regulating the Expression of c-Myc. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5301–5314. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Sang, Y.; Song, X.; Zhang, H.; Liu, Y.; Jiang, L.; Yang, Q. MiR-770 Suppresses the Chemo-Resistance and Metastasis of Triple Negative Breast Cancer via Direct Targeting of STMN1. Cell Death Dis. 2018, 9, 14. [Google Scholar] [CrossRef]
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’Ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G.; et al. Exosome-Mediated Delivery of MiR-9 Induces Cancer-Associated Fibroblast-like Properties in Human Breast Fibroblasts. Cell Death Dis. 2016, 7, e2312. [Google Scholar] [CrossRef]
- Kia, V.; Paryan, M.; Mortazavi, Y.; Biglari, A.; Mohammadi-Yeganeh, S. Evaluation of Exosomal MiR-9 and MiR-155 Targeting PTEN and DUSP14 in Highly Metastatic Breast Cancer and Their Effect on Low Metastatic Cells. J. Cell. Biochem. 2019, 120, 5666–5676. [Google Scholar] [CrossRef]
- Naseri, Z.; Oskuee, R.K.; Jaafari, M.R.; Moghadam, M.F. Exosome-Mediated Delivery of Functionally Active MiRNA-142-3p Inhibitor Reduces Tumorigenicity of Breast Cancer In Vitro and In Vivo. Int. J. Nanomed. 2018, 13, 7727–7747. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, J.H.; Lee, J.; Park, H.Y.; Jung, J.H.; Kang, J.; Kim, E.A.; Park, N.J.Y.; Park, J.Y.; Lee, I.H.; et al. MicroRNA-496 Inhibits Triple Negative Breast Cancer Cell Proliferation by Targeting Del-1. Medicine 2021, 100, e25270. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Takeshita, F.; Yamamoto, T.; Xiao, Z.; Ochiya, T. Delivery of MiR-424-5p via Extracellular Vesicles Promotes the Apoptosis of MDA-MB-231 TNBC Cells in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 844. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Yu, T.; He, J.; Liu, J.; Chai, X.; Zhao, G.; Yin, D.; Zhang, C. Exosomes Deliver LncRNA DARS-AS1 SiRNA to Inhibit Chronic Unpredictable Mild Stress-Induced TNBC Metastasis. Cancer Lett. 2022, 543, 215781. [Google Scholar] [CrossRef]
- Xing, L.; Tang, X.; Wu, K.; Huang, X.; Yi, Y.; Huan, J. LncRNA HAND2-AS1 Suppressed the Growth of Triple Negative Breast Cancer via Reducing Secretion of MSCs Derived Exosomal MiR-106a-5p. Aging 2020, 13, 424–436. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-Mediated SiRNA Delivery to Suppress Postoperative Breast Cancer Metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Hadla, M.; Palazzolo, S.; Corona, G.; Caligiuri, I.; Canzonieri, V.; Toffoli, G.; Rizzolio, F. Exosomes Increase the Therapeutic Index of Doxorubicin in Breast and Ovarian Cancer Mouse Models. Nanomedicine 2016, 11, 2431–2441. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, B.G.; Jang, Y.; Kang, S.; Lee, J.H.; Cho, N.H. The Stromal Loss of MiR-4516 Promotes the FOSL1-Dependent Proliferation and Malignancy of Triple Negative Breast Cancer. Cancer Lett. 2020, 469, 256–265. [Google Scholar] [CrossRef]
- Singh, R.; Pochampally, R.; Watabe, K.; Lu, Z.; Mo, Y.Y. Exosome-Mediated Transfer of MiR-10b Promotes Cell Invasion in Breast Cancer. Mol. Cancer 2014, 13, 256. [Google Scholar] [CrossRef]
- Xiao, H.; Li, H.; Yu, G.; Xiao, W.; Hu, J.; Tang, K.; Zeng, J.; He, W.; Zeng, G.; Ye, Z.; et al. MicroRNA-10b Promotes Migration and Invasion through KLF4 and HOXD10 in Human Bladder Cancer. Oncol. Rep. 2014, 31, 1832–1838. [Google Scholar] [CrossRef]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Wu, X.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y.; et al. Functional Exosome-Mediated Co-Delivery of Doxorubicin and Hydrophobically Modified MicroRNA 159 for Triple-Negative Breast Cancer Therapy. J. Nanobiotechnol. 2019, 17, 93. [Google Scholar] [CrossRef]
- Kim, M.; Haney, M.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering Macrophage-Derived Exosomes for Targeted Paclitaxel Delivery to Pulmonary Metastases: In Vitro and In Vivo Evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Cocco, S.; Piezzo, M.; Calabrese, A.; Cianniello, D.; Caputo, R.; di Lauro, V.; Fusco, G.; Gioia, G.D.; Licenziato, M.; de Laurentiis, M. Biomarkers in Triple-Negative Breast Cancer: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 4579. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Miao, F.; Chen, G.; Zhu, Y.; Wu, N.; Pang, L.; Chen, Z.; Chen, X. PDL1-positive Exosomes Suppress Antitumor Immunity by Inducing Tumor-specific CD8+ T Cell Exhaustion during Metastasis. Cancer Sci. 2021, 112, 3437–3454. [Google Scholar] [CrossRef]
- Shen, H.; Yang ES, H.; Conry, M.; Fiveash, J.; Contreras, C.; Bonner, J.A.; Shi, L.Z. Predictive Biomarkers for Immune Checkpoint Blockade and Opportunities for Combination Therapies. Genes Dis. 2019, 6, 232–246. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from Mesenchymal Stromal Cells Reduce Murine Colonic Inflammation via a Macrophage-Dependent Mechanism. JCI Insight 2019, 4, 131–273. [Google Scholar] [CrossRef]
- Biswas, S.; Mandal, G.; Chowdhury, S.R.; Purohit, S.; Payne, K.K.; Anadon, C.; Gupta, A.; Swanson, P.; Yu, X.; Conejo-Garcia, J.R.; et al. Exosomes Produced by Mesenchymal Stem Cells Drive Differentiation of Myeloid Cells into Immunosuppressive M2-Polarized Macrophages in Breast Cancer. J. Immunol. 2019, 203, 3447–3460. [Google Scholar] [CrossRef]
- Kim, O.Y.; Park, H.T.; Dinh, N.T.H.; Choi, S.J.; Lee, J.; Kim, J.H.; Lee, S.W.; Gho, Y.S. Bacterial Outer Membrane Vesicles Suppress Tumor by Interferon-γ-Mediated Antitumor Response. Nat. Commun. 2017, 8, 626. [Google Scholar] [CrossRef]
- Kameli, N.; Dragojlovic-kerkache, A.; Savelkoul, P.; Stassen, F.R. Plant-Derived Extracellular Vesicles: Current Findings, Challenges, and Future Applications. Membranes 2021, 11, 411. [Google Scholar] [CrossRef]
- Wang, G.; Hu, W.; Chen, H.; Shou, X.; Ye, T.; Xu, Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers 2019, 11, 1560. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T.; Somiya, M.; Yoshioka, Y.; Ochiya, T. Drug Delivery Application of Extracellular Vesicles; Insight into Production, Drug Loading, Targeting, and Pharmacokinetics. AIMS Bioeng. 2017, 4, 73–92. [Google Scholar] [CrossRef]
- Scherphof, G.L.; Dijkstra, J.; Spanjer, H.H.; Derksen, J.T.P.; Roerdink, F.H. Uptake and Intracellular Processing of Targeted and Nontargeted Liposomes by Rat Kupffer Cells In Vivo and In Vitroa. Ann. N. Y. Acad. Sci. 1985, 446, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic Cell-Derived Exosomes as Maintenance Immunotherapy after First Line Chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef]
- Tan, A.; de la Peña, H.; Seifalian, A.M. The Application of Exosomes as a Nanoscale Cancer Vaccine. Int. J. Nanomed. 2010, 5, 889–900. [Google Scholar] [CrossRef]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-Derived Exosomes Are a Source of Shared Tumor Rejection Antigens for CTL Cross-Priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in Cancer: Small Particle, Big Player. J. Hematol. Oncol. 2015, 8, 83. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell Derived Exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Dong Gao, L.J. Exosomes in Cancer Therapy: A Novel Experimental Strategy—PubMed. Am. J. Cancer Res. 2018, 8, 2165–2175. [Google Scholar]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-Term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients with Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- St-Denis-bissonnette, F.; Khoury, R.; Mediratta, K.; El-Sahli, S.; Wang, L.; Lavoie, J.R. Applications of Extracellular Vesicles in Triple-Negative Breast Cancer. Cancers 2022, 14, 451. [Google Scholar] [CrossRef]
- Mediratta, K.; El-Sahli, S.; D’costa, V.; Wang, L. Current Progresses and Challenges of Immunotherapy in Triple-Negative Breast Cancer. Cancers 2020, 12, 3529. [Google Scholar] [CrossRef]
| Engineered Exosomes Element in Membrane/Source | Effect | Reported Effect and Outcomes | References |
|---|---|---|---|
| pLEX-LAMP DARPin for expression of DARPin | HER2+ cells | Inhibit metastasis | [84,85] |
| Mesothelin MSLN | CAR-T cells with CARs and CD3 surface expression | Inhibit metastasis | [78] |
| Poly (lactic-co-glycolic acid) | MDA-MB-231 | Target c-Met and inhibit metastasis | [80] |
| Anti-HER2 antibody conjugated paclitaxel-loaded liposomes | MDA-MB-231 | Inhibit metastasis, boost the therapy, increase apoptosis | [81] |
| Exosome A15 derived from monocyte-derived macrophages | MDA-MB-231 | Effect on TCF-7 gene leading to improved anticancer effects | [90] |
| Cargo | Effect | Reported Effect and Outcomes | Refs. |
|---|---|---|---|
| miR-134 | Hs578Ts(i)8 | Downregulate STAT5B, HSP90, and KRAS | [93,94,95,96] |
| let-7a | MDA-MB-231 | Silence c-Myc gene | [97] |
| miRNA-770 and Doxorubicin | MDA-MB-231 | HER2+ tumour | [98] |
| CAR | MSLN + TNBC | Perforin and Granzyme B mechanism | [78] |
| Erastin | MDA-MB-231 | Ferroptosis | [82] |
| Anti-IL-3R-EV | Metastasis of TNBC to other parts, liver, lungs | Lower Vimentin, β-catenin, and TWIST1 | [83] |
| Anti-IL-3R-EVs and antago-miR-24-3p-EVs | MDA-MB-231 | Upregulate SPRY2, enhance apoptosis | [83] |
| miRNA-381-3p from adipose mesenchymal stem cells | MDA-MB-231 | Decrease Wnt signalling and factors related to EMT | [87] |
| miR3182 | MDA-MB-231 | Apoptosis in TNBC to downregulate mTOR and S6KB1 | [88] |
| miR-27a-3p | MDA-MB-231 | Modulate PD-L1 levels in macrophages causing immune evasion via the PTEN/AKT/PI3K axis | [89] |
| microRNA 159 | MDA-MB-231 | Activate protein kinase C further TCF-7 | [90] |
| miRNA-127, miR-197, miR-222, and miR-223 | MDA-MB-231 | Enhance apoptosis, metastasis | [90] |
| siRNA from HEK293T cells | HER2 Positive Breast Cancer | TPD52 gene downregulated by 70 percent | [91] |
| miR-9 and miR-155 from MDA-MB-231 | MCF-7 | PTEN and DUSP14 tumour suppressor gene | [100] |
| Anti-miR-142-3p | MDA-MB-231 | Lower miR-142-3p and miR-150 levels while increasing the level of target genes APC and P2X7R in TNBC | [101] |
| miR-496 and miR-137 from MCF10A | MDA-MB-231 | Del-1 | [102] |
| miR-424-5p | MDA-MB-231 | Suppress PD-L1 enhancing apoptosis | [103] |
| Trial (National Clinical Trial ID) | Phase | Condition | Interventions |
|---|---|---|---|
| NCT03362060 | 1 | TNBC, Metastatic TNBC | Pembrolizumab Biological: PVX-410 |
| NCT04105582 | 1 | TNBC BC | Biological: Neo-antigen pulsed DCs |
| NCT03199040 | 1 | TNBC Metastatic TNBC | Drug: Durvalumab Biological: Neoantigen DNA vaccine Device: TDS-IM system (Anchor Medical Systems) |
| NCT02316457 | I | TNBC | Biological: IVAC_W_bre1_uID Biological: IVAC_W_bre1_uID/IVAC_M_uID |
| NCT04024800 | II | TNBC | Biological: AE37 Peptide vaccine |
| NCT02826434 | 1 | BC | Biological: PVX-410 Biological: Durvalumab Drug: Hilton |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, M.; Rajeswari, V.D. Critical Review on the Different Roles of Exosomes in TNBC and Exosomal-Mediated Delivery of microRNA/siRNA/lncRNA and Drug Targeting Signalling Pathways in Triple-Negative Breast Cancer. Molecules 2023, 28, 1802. https://doi.org/10.3390/molecules28041802
Banerjee M, Rajeswari VD. Critical Review on the Different Roles of Exosomes in TNBC and Exosomal-Mediated Delivery of microRNA/siRNA/lncRNA and Drug Targeting Signalling Pathways in Triple-Negative Breast Cancer. Molecules. 2023; 28(4):1802. https://doi.org/10.3390/molecules28041802
Chicago/Turabian StyleBanerjee, Manosi, and Vijayarangan Devi Rajeswari. 2023. "Critical Review on the Different Roles of Exosomes in TNBC and Exosomal-Mediated Delivery of microRNA/siRNA/lncRNA and Drug Targeting Signalling Pathways in Triple-Negative Breast Cancer" Molecules 28, no. 4: 1802. https://doi.org/10.3390/molecules28041802
APA StyleBanerjee, M., & Rajeswari, V. D. (2023). Critical Review on the Different Roles of Exosomes in TNBC and Exosomal-Mediated Delivery of microRNA/siRNA/lncRNA and Drug Targeting Signalling Pathways in Triple-Negative Breast Cancer. Molecules, 28(4), 1802. https://doi.org/10.3390/molecules28041802









