Bioactive Compounds and Antioxidant Composition of Nut Bars with Addition of Various Edible Insect Flours
Abstract
1. Introduction
2. Results and Discussion
2.1. Polyphenol Profile of Edible Insects and Bars
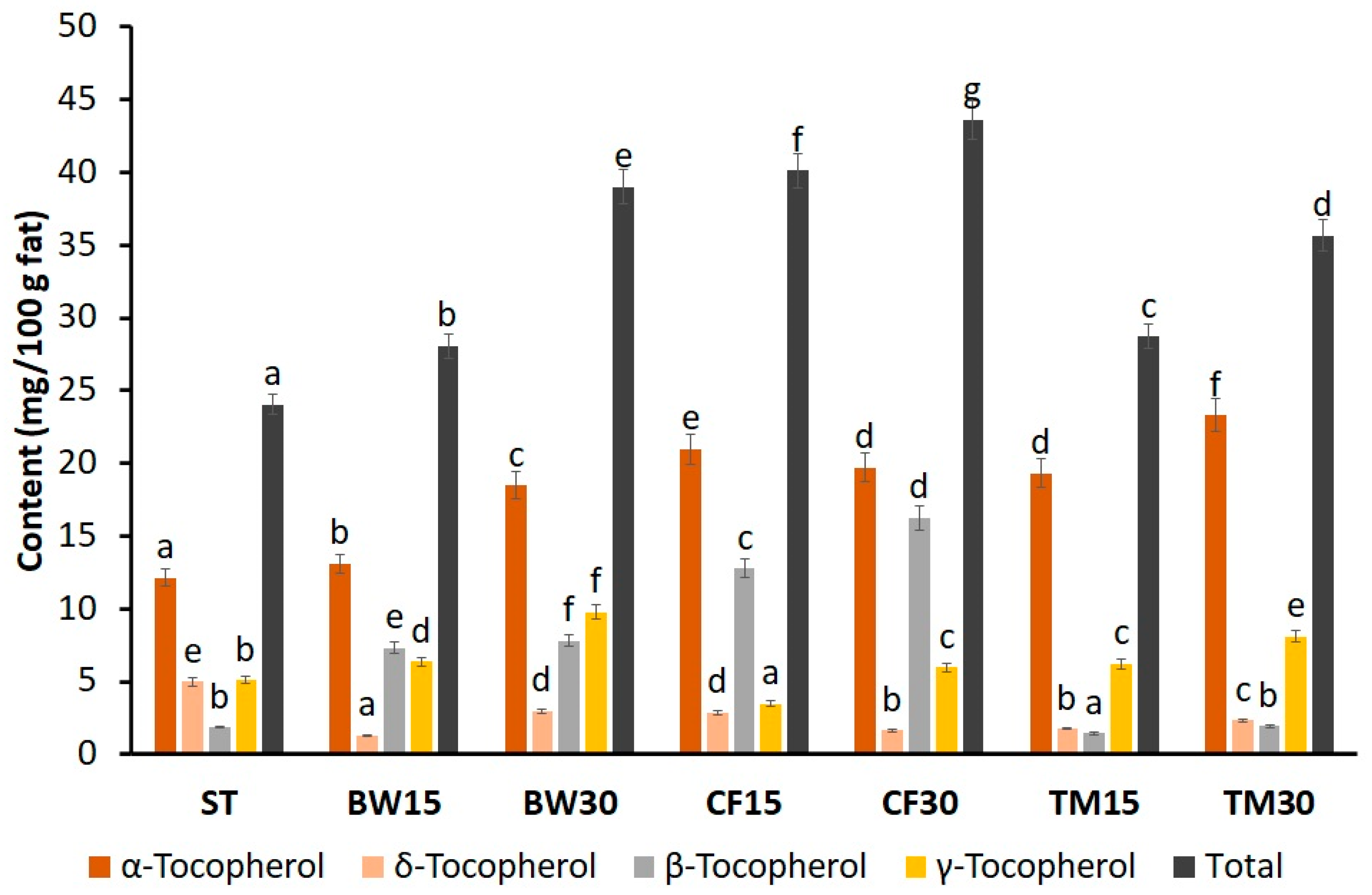
2.2. Tocopherols and Sterols in Insect Flours and Bars
2.3. Antioxidant Potential of Edible Insects and Bars
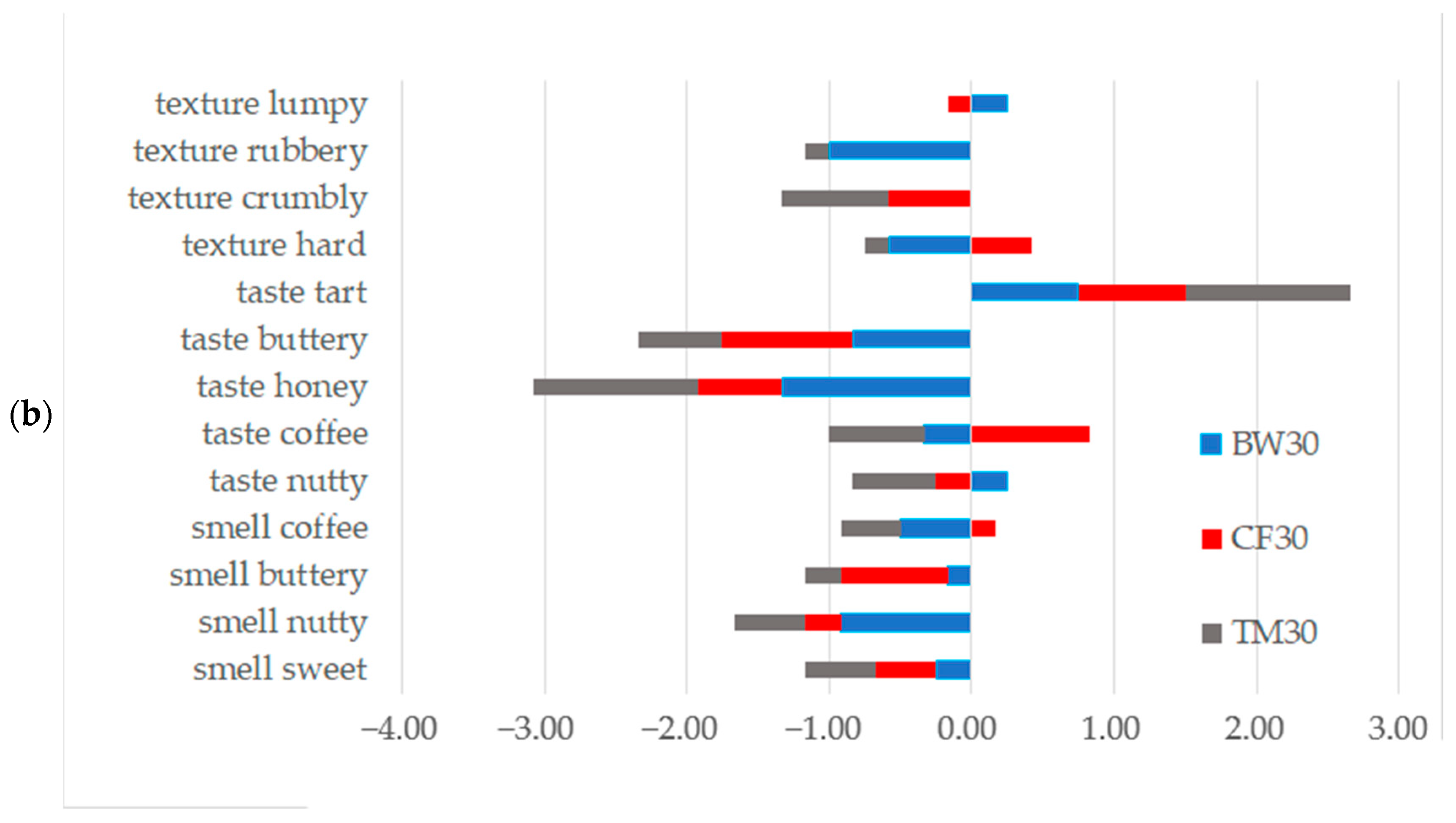
2.4. Sensory Analysis
3. Materials and Methods
3.1. Materials
Preparation of Nut Bars with Insect Powder
3.2. Methods
3.2.1. Phenolic Compounds Analysis by UHPLC-DAD-ESI-MS/MS
3.2.2. Determination of Tocopherols and Sterols
3.2.3. Analysis of Polyphenols, Flavonoids and Antioxidant Potential
- Total content of polyphenols
- Determination of flavonoids
- Free radical scavenging activity by DPPH
- Antiradical activity by ABTS
- Ferric reducing antioxidant power (FRAP)
- Determination of ferrous ion chelating activity
3.2.4. Sensory Analysis—Quantitative Descriptive Analysis
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Snacks Market Size, Growth & Trends Report. 2030. Available online: https://www.grandviewresearch.com/industry-analysis/snacks-market (accessed on 28 February 2023).
- The European Parliament; The Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (Text with EEA Relevance). Off. J. Eur. Union 2015, 327, 1–22. [Google Scholar]
- Risk Profile of Insects as Food and Feed | EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4257 (accessed on 28 February 2023).
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as Ingredients for Bakery Goods. A Comparison Study of H. Illucens, A. Domestica and T. Molitor Flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat Bread Supplementation with Various Edible Insect Flours. Influence of Chemical Composition on Nutritional and Technological Aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Bonaccorsi, G.; Lorini, C.; Cini, E. Assessment of the Rheological Properties and Bread Characteristics Obtained by Innovative Protein Sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): Novel Food or Potential Improvers for Wheat Flour? LWT 2020, 118, 108867. [Google Scholar] [CrossRef]
- Khuenpet, K.; Pakasap, C.; Vatthanakul, S.; Kitthawee, S. Effect of Larval-Stage Mealworm Tenebrio molitor Powder on Qualities of Bread. Int. J. Agric. Technol. 2020, 16, 283–296. [Google Scholar]
- Roncolini, A.; Milanović, V.; Cardinali, F.; Osimani, A.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; et al. Protein Fortification with Mealworm (Tenebrio molitor L.) Powder: Effect on Textural, Microbiological, Nutritional and Sensory Features of Bread. PLoS ONE 2019, 14, e0211747. [Google Scholar] [CrossRef]
- Wieczorek, M.; Kowalczewski, P.; Drabińska, N.; Różańska, M.; Jeleń, H. Effect of Cricket Powder Incorporation on the Profile of Volatile Organic Compounds, Free Amino Acids and Sensory Properties of Gluten-Free Bread. Pol. J. Food Nutr. Sci. 2022, 72, 431–442. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Skotnicka, M.; Mickowska, B.; Makarewicz, M.; Sabat, R.; Wywrocka-Gurgul, A.; Mazurek, A. Effect of the Addition of Edible Insect Flour from Yellow Mealworm (Tenebrio molitor) on the Sensory Acceptance, and the Physicochemical and Textural Properties of Sponge Cake. Pol. J. Food Nutr. Sci. 2022, 72, 393–405. [Google Scholar] [CrossRef]
- Pauter, P.; Różańska, M.; Wiza, P.; Dworczak, S.; Grobelna, N.; Sarbak, P.; Kowalczewski, P.Ł. Effects of the Replacement of Wheat Flour with Cricket Powder on the Characteristics of Muffins. Acta Sci. Pol. Technol. Aliment. 2018, 17, 227–233. [Google Scholar] [CrossRef]
- Mazurek, A.; Palka, A.; Skotnicka, M.; Kowalski, S. Consumer Attitudes and Acceptability of Wheat Pancakes with the Addition of Edible Insects: Mealworm (Tenebrio molitor), Buffalo Worm (Alphitobius diaperinus), and Cricket (Acheta domesticus). Foods 2023, 12, 1. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of Insects and Pea Powder as Alternative Protein and Mineral Sources in Extruded Snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Ruszkowska, M.; Tańska, M.; Kowalczewski, P.Ł. Extruded Corn Snacks with Cricket Powder: Impact on Physical Parameters and Consumer Acceptance. Sustainability 2022, 14, 16578. [Google Scholar] [CrossRef]
- Kowalski, S.; Oracz, J.; Skotnicka, M.; Mikulec, A.; Gumul, D.; Mickowska, B.; Mazurek, A.; Sabat, R.; Wywrocka-Gurgul, A.; Żyżelewicz, D. Chemical Composition, Nutritional Value, and Acceptance of Nut Bars with the Addition of Edible Insect Powder. Molecules 2022, 27, 8472. [Google Scholar] [CrossRef] [PubMed]
- Megido, R.C.; Gierts, C.; Blecker, C.; Brostaux, Y.; Haubruge, É.; Alabi, T.; Francis, F. Consumer Acceptance of Insect-Based Alternative Meat Products in Western Countries. Food Qual. Prefer. 2016, 52, 237–243. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-Efficient Food Production to Reduce Global Warming and Ecodegradation: The Use of Edible Insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Pal, P.; Roy, S. Edible Insects: Future of Human Food—A Review. Int. Lett. Nat. Sci. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Sun, S.S.M. Application of Agricultural Biotechnology to Improve Food Nutrition and Healthcare Products. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 87–90. [Google Scholar]
- Ying, F.; Ming, C.X.; Yun, W.S.; De, Y.S.; Yong, C. Three Edible Odonata Species and Their Nutritive Value. lykxyj 2001, 14, 421–424. [Google Scholar]
- Erhirhie, E.O.; Paul, C. Edible Insects’ Bio-Actives as Anti-Oxidants: Current Status and Perspectives. J. Complement. Med. Res. 2019, 10, 89–102. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-Inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Kabir, F.; Tow, W.W.; Hamauzu, Y.; Katayama, S.; Tanaka, S.; Nakamura, S. Antioxidant and Cytoprotective Activities of Extracts Prepared from Fruit and Vegetable Wastes and By-Products. Food Chem. 2015, 167, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharm. Rev 2000, 52, 673–751. [Google Scholar] [PubMed]
- Silva, F.A.; Borges, F.; Guimarães, C.; Lima, J.L.; Matos, C.; Reis, S. Phenolic Acids and Derivatives: Studies on the Relationship among Structure, Radical Scavenging Activity, and Physicochemical Parameters. J. Agric. Food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Nino, M.C.; Reddivari, L.; Ferruzzi, M.G.; Liceaga, A.M. Targeted Phenolic Characterization and Antioxidant Bioactivity of Extracts from Edible Acheta domesticus. Foods 2021, 10, 2295. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Osorio, C.; Kaplan, I.; Liceaga, A.m. Insects as a Source of Phenolic Compounds and Potential Health Benefits. J. Insects Food Feed 2021, 7, 1077–1087. [Google Scholar] [CrossRef]
- Hirayama, C.; Ono, H.; Tamura, Y.; Konno, K.; Nakamura, M. Regioselective Formation of Quercetin 5-O-Glucoside from Orally Administered Quercetin in the Silkworm, Bombyx Mori. Phytochemistry 2008, 69, 1141–1149. [Google Scholar] [CrossRef]
- Lahtinen, M.; Kapari, L.; Kenttä, J. Newly Hatched Neonate Larvae Can Glycosylate: The Fate of Betula pubescens Bud Flavonoids in First Instar Epirrita autumnata. J. Chem. Ecol. 2006, 32, 537–546. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Lahtinen, M.; Lempa, K.; Kapari, L.; Haukioja, E.; Pihlaja, K. Metabolic Modifications of Birch Leaf Phenolics by an Herbivorous Insect: Detoxification of Flavonoid Aglycones via Glycosylation. Z. Für Nat. C 2004, 59, 437–444. [Google Scholar] [CrossRef]
- Cheseto, X.; Kuate, S.P.; Tchouassi, D.P.; Ndung’u, M.; Teal, P.E.A.; Torto, B. Potential of the Desert Locust Schistocerca gregaria (Orthoptera: Acrididae) as an Unconventional Source of Dietary and Therapeutic Sterols. PLoS ONE 2015, 10, e0127171. [Google Scholar] [CrossRef]
- Ochieng, B.O.; Anyango, J.O.; Nduko, J.M.; Cheseto, X.; Mudalungu, C.M.; Khamis, F.M.; Ghemoh, C.J.; Egonyu, P.J.; Subramanian, S.; Nakimbugwe, D.; et al. Dynamics in Nutrients, Sterols and Total Flavonoid Content during Processing of the Edible Long-Horned Grasshopper (Ruspolia differens Serville) for Food. Food Chem. 2022, 383, 132397. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and Sensory Quality of Edible Insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Tong, L.; Yu, X.; Liu, H. Insect Food for Astronauts: Gas Exchange in Silkworms Fed on Mulberry and Lettuce and the Nutritional Value of These Insects for Human Consumption during Deep Space Flights. Bull. Entomol. Res. 2011, 101, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Jibril, F.I.; Hilmi, A.B.M.; Manivannan, L. Isolation and Characterization of Polyphenols in Natural Honey for the Treatment of Human Diseases. Bull. Natl. Res. Cent. 2019, 43, 4. [Google Scholar] [CrossRef]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.T.N.; Thompson, L.D.; Galyean, M.L.; Brooks, J.C.; Patterson, K.Y.; Boylan, L.M. Cholesterol Content and Methods for Cholesterol Determination in Meat and Poultry. Compr. Rev. Food Sci. Food Saf. 2011, 10, 269–289. [Google Scholar] [CrossRef]
- Chizzolini, R.; Zanardi, E.; Dorigoni, V.; Ghidini, S. Calorific Value and Cholesterol Content of Normal and Low-Fat Meat and Meat Products. Trends Food Sci. Technol. 1999, 10, 119–128. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J.H. Anticancer Effects of Phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Wolska, L.; Bartoszek, A.; Namiesnik, J. Charakterystyka polifenoli: Wystepowanie, wlasciwosci, przeglad metod analitycznych. Bromatol. I Chem. Toksykol. 2005, 38, 81–92. [Google Scholar]
- Gallardo, C.; Jiménez, L.; García-Conesa, M.-T. Hydroxycinnamic Acid Composition and in Vitro Antioxidant Activity of Selected Grain Fractions. Food Chem. 2006, 3, 455–463. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, Antioxidant Activity, and Inhibitory Effect on Pancreatic Lipase of Extracts from the Edible Insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef] [PubMed]
- Musundire, R.; Zvidzai, J.; Chidewe, C. Bio-Active Compounds Composition in Edible Stinkbugs Consumed in South-Eastern Districts of Zimbabwe. Int. J. Biol. 2014, 6, 36. [Google Scholar] [CrossRef]
- Musundire, R.; Zvidzai, C.J.; Chidewe, C.; Ngadze, R.T.; Macheka, L.; Manditsera, F.A.; Mubaiwa, J.; Masheka, A. Nutritional and Bioactive Compounds Composition of Eulepida mashona, an Edible Beetle in Zimbabwe. J. Insects Food Feed 2016, 2, 179–187. [Google Scholar] [CrossRef]
- Musundire, R.; Zvidzai, C.J.; Chidewe, C.; Samende, B.K.; Manditsera, F.A. Nutrient and Anti-Nutrient Composition of Henicus whellani (Orthoptera: Stenopelmatidae), an Edible Ground Cricket, in South-Eastern Zimbabwe. Int. J. Trop. Insect Sci. 2014, 34, 223–231. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Yang, Q. Antioxidant Activity and Phenolic Compounds of Holotrichia parallela Motschulsky Extracts. Food Chem. 2012, 134, 1885–1891. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Vita, J.A. Polyphenols and Cardiovascular Disease: Effects on Endothelial and Platelet Function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [CrossRef]
- Cybul, M.; Nowak, R. Przeglad metod stosowanych w analizie antyoksydacyjnych wyciagow roslinnych. Herba Pol. 2008, 54, 68–78. [Google Scholar]
- Oracz, J.; Zyzelewicz, D. In Vitro Antioxidant Activity and FTIR Characterization of High-Molecular Weight Melanoidin Fractions from Different Types of Cocoa Beans. Antioxidants 2019, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.; Habermeyer, M.; Kemény, M.; Weyand, U.; Niederberger, E.; Frank, O.; Hofmann, T. Maillard Reaction Products Modulating the Growth of Human Tumor Cells in Vitro. Chem. Res. Toxicol. 2003, 16, 48–55. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant Activities in Vitro of Water and Liposoluble Extracts Obtained by Different Species of Edible Insects and Invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of Enzymatic Hydrolysis on Bioactive Properties and Allergenicity of Cricket (Gryllodes sigillatus) Protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the Biological Potential of Proteins from Edible Insects through Enzymatic Hydrolysis: A Review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska, A.; Pegg, R.B. Comparison of Radical-Scavenging Activities for Selected Phenolic Acids. Pol. J. Food Nutr. Sci. 2005, 55, 165–170. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Szwajgier, D.; Pielecki, J.; Targonski, Z. Antioxidant Activities of Cinnamic and Benzoic Acid Derivatives. Acta Sci. Polonorum. Technol. Aliment. 2005, 4, 129–142. [Google Scholar]
- von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic Acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of Processing on the Antioxidant Properties of Fruit and Vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; İbanoğlu, Ş. The Effect of Extrusion Cooking Using Different Water Feed Rates on the Quality of Ready-to-Eat Snacks Made from Food by-Products. Food Chem. 2009, 114, 226–232. [Google Scholar] [CrossRef]
- Nogala-Kałucka, M.; Lampart-Szczapa, E.; Krzyżostaniak, I.; Siger, A. Natywne antyoksydacyjne biokomponenty preparatów łubinowych. Żywność Nauka Technol. Jakość 2009, 65, 70–78. [Google Scholar]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and Quantification of Free and Bound Phenolic Compounds Contained in the High-Molecular Weight Melanoidin Fractions Derived from Two Different Types of Cocoa Beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Effect of Roasting Conditions on the Fat, Tocopherol, and Phytosterol Content and Antioxidant Capacity of the Lipid Fraction from Cocoa Beans of Different Theobroma cacao L. Cultivars. Eur. J. Lipid Sci. Technol. 2014, 116, 1002–1014. [Google Scholar] [CrossRef]
- Hussain, N.; Jabeen, Z.; Li, Y.; Chen, M.; Li, Z.; Guo, W.; Shamsi, I.H.; Chen, X.; Jiang, L. Detection of Tocopherol in Oilseed Rape (Brassica napus L.) Using Gas Chromatography with Flame Ionization Detector. J. Integr. Agric. 2013, 12, 803–814. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, W.; Wei, X.; Zhang, F.; Shen, C.; Wu, B.; Zhao, Z.; Liu, H.; Deng, X. Simultaneous Determination of Tocopherols and Tocotrienols in Vegetable Oils by GC-MS. Anal. Methods 2016, 8, 7341–7346. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- El Hariri, B.; Sallé, G.; Andary, C. Involvement of Flavonoids in the Resistance of Two Poplar Cultivars to Mistletoe (Viscum album L.). Protoplasma 1991, 162, 20–26. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- ISO. ISO 13299:2016. Available online: https://www.iso.org/standard/58042.html (accessed on 9 February 2023).


| Compounds | Type of Insects | ||
|---|---|---|---|
| Buffalo Worm (A. diaperinus) | Cricket (A. domesticus) | Mealworm (T. molitor) | |
| Hydroxybenzoic acids and their derivatives (mg/100 g) | |||
| Gallic | 2.46 ± 0.06 b | 0.61 ± 0.02 a | 4.63 ± 0.12 c |
| Vanillic | 0.93 ± 0.02 b | 0.61 ± 0.02 a | 1.36 ± 0.03 c |
| Protocatechuic | 52.14 ± 0.37 b | n.d. | n.d. |
| Protocatechuic aldehyde | 19.81 ± 0.25 b | 2.89 ± 0.07 a | 23.19 ± 0.26 a |
| Syringic | 26.56 ± 0.44 c | 0.46 ± 0.01 b | n.d. |
| 2,5- Dihydroxybenzoic | 0.40 ± 0.01 b | 0.69 ± 0.02 c | 0.15 ± 0.00 a |
| Ellagic | 0.42 ± 0.01 b | 6.47 ± 0.16 c | 0.26 ± 0.01 a |
| Hydroxycinnamic acids (mg/100 g) | |||
| Caffeic | 0.10 ± 0.01 b | 0.11 ± 0.01 b | 0.06 ± 0.01 a |
| Ferulic | 0.45 ± 0.04 a | 7.23 ± 0.04 c | 0.54 ± 0.01 b |
| p-Coumaric | 0.08 ± 0.01 a | 0.05 ± 0.01 a | 0.06 ± 0.01 a |
| Chlorogenic | 0.19 ± 0.02 b | 0.20 ± 0.02 b | n.d. |
| Sinapic | 0.34 ± 0.03 b | 0.82 ± 0.08 c | 0.15 ± 0.01 a |
| Flavonols (mg/100 g) | |||
| Quercetin 3-O-galactoside | n.d. | 0.14 ± 0.01 a | n.d. |
| Quercetin 3-O-glucoside | n.d. | 1.38 ± 0.06 b | 0.40 ± 0.01 a |
| Quercetin 3-O-rutinoside (Rutin) | n.d. | 1.38 ± 0.09 b | 0.40 ± 0.02 a |
| Quercetin | n.d. | n.d. | n.d. |
| Myricetin | n.d. | n.d. | n.d. |
| Compound | Type of the Bar | ||||||
|---|---|---|---|---|---|---|---|
| ST | BW15 | BW30 | CF15 | CF30 | TM15 | TM30 | |
| Hydroxybenzoic acids and their derivatives (mg/100 g) | |||||||
| Gallic | 0.04 ± 0.00 a | 0.20 ± 0.00 d | 0.37 ± 0.01 e | 0.04 ± 0.01 a | 0.11 ± 0.00 b | 0.16 ± 0.00 c | 1.45 ± 0.04 f |
| Vanillic | 0.20 ± 0.03 b | 0.11 ± 0.02 a | 0.24 ± 0.01 b | 0.09 ± 0.00 a | 0.22 ± 0.01 b | 0.12 ± 0.00 a | 0.22 ± 0.01 b |
| Protocatechuic | 41.41 ± 1.4 b | 45.10 ± 0.36 c | 53.10 ± 0.84 d | 40.92 ± 1.03 b | 31.96 ± 0.80 a | 40.16 ± 1.01 b | 33.95 ± 0.85 a |
| Protocatechuic aldehyde | 0.48 ± 0.01 a | 1.10 ± 0.03 d | 2.20 ± 0.06 f | 0.61 ± 0.02 b | 0.66 ± 0.02 b | 0.94 ± 0.02 c | 1.32 ± 0.03 e |
| Syringic | 12.14 ± 0.30 c | 14.67 ± 0.31 e | 18.00 ± 0.19 f | 13.74 ± 0.34 d | 13.92 ± 0.35 d | 4.51 ± 0.11 b | 3.81 ± 0.10 a |
| 2,5- Dihydroxybenzoic | n.d. | 0.12 ± 0.01 a | 0.37 ± 0.00 e | 0.23 ± 0.01 c | 0.44 ± 0.01 f | 0.18 ± 0.00 b | 0.29 ± 0.01 d |
| Ellagic | 0.63 ± 0.02 d | 0.40 ± 0.01 c | 0.38 ± 0.01 bc | 1.15 ± 0.03 e | 1.59 ± 0.04 f | 0.36 ± 0.01 b | 0.31 ± 0.01 a |
| Hydroxycinnamic acids (mg/100 g) | |||||||
| Caffeic | 1.68 ± 0.07 d | 0.08 ± 0.01 a | 0.94 ± 0.09 c | 0.07 ± 0.01 a | 0.41 ± 0.04 b | 0.36 ± 0.04 b | 0.34 ± 0.03 b |
| Ferulic | 0.18 ± 0.02 b | 0.05 ± 0.00 a | 0.04 ± 0.00 a | 0.28 ± 0.03 c | 0.85 ± 0.08 d | 0.04 ± 0.00 a | 0.04 ± 0.00 a |
| p-Coumaric | n.d. | 0.04 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.07 ± 0.01 a |
| Chlorogenic | 3.45 ± 0.02 e | 1.42 ± 0.06 b | 3.28 ± 0.03 e | 0.58 ± 0.06 a | 1.95 ± 0.01 d | 1.91 ± 0.02 d | 1.62 ± 0.07 c |
| Sinapic | n.d. | 0.20 ± 0.02 b | 0.17 ± 0.02 b | 0.21 ± 0.02 b | 0.18 ± 0.01 b | 0.06 ± 0.01 a | 0.15 ± 0.02 b |
| Flavonols (mg/100 g) | |||||||
| Quercetin 3-O-galactoside | 0.09 ± 0.01 a | 0.12 ± 0.01 a | 0.26 ± 0.03 b | 0.09 ± 0.01 a | 0.70 ± 0.01 c | 0.09 ± 0.01 a | 0.08 ± 0.01 a |
| Quercetin 3-O-glucoside | 0.27 ± 0.03 c | 0.26 ± 0.03 c | 0.25 ± 0.03 c | 0.11 ± 0.01 a | 0.08 ± 0.01 a | 0.29 ± 0.03 c | 0.14 ± 0.01 b |
| Quercetin 3-O-rutinoside (Rutin) | 0.71 ± 0.07 e | 0.11 ± 0.01 a | 0.46 ± 0.05 d | 0.16 ± 0.02 ab | 0.27 ± 0.01 c | 0.76 ± 0.08 e | 0.21 ± 0.02 b |
| Quercetin | n.d. | 0.03 ± 0.01 a | 0.03 ± 0.00 a | n.d. | 0.04 ± 0.00 a | 0.03 ± 0.00 a | n.d. |
| Myricetin | 0.06 ± 0.01 a | 0.08 ± 0.01 a | 0.05 ± 0.01 a | 0.06 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a | 0.05 ± 0.00 a |
| Compounds | Type of Insects | ||
|---|---|---|---|
| Buffalo Worm (A. diaperinus) | Cricket (A. domesticus) | Mealworm (T. molitor) | |
| Tocopherols (mg/100 g fat) | |||
| α-Tocopherol | 26.43 ± 1.33 b | 39.81 ± 0.33 c | 14.54 ± 0.73 a |
| δ-Tocopherol | 5.43 ± 0.27 a | 27.78 ± 0.23 c | 7.19 ± 0.36 b |
| β-Tocopherol | 7.75 ± 0.39 b | 98.15 ± 0.82 c | 5.88 ± 0.30 a |
| γ-Tocopherol | 27.91 ± 1.40 b | 75.32 ± 0.63 c | 16.59 ± 0.83 a |
| Total | 67.52 ± 6.79 b | 241.05 ± 4.02 c | 44.20 ± 4.45 a |
| Sterols (mg/100 g fat) | |||
| Cholesterol | 535.73 ± 0.77 b | 1310.41 ± 0.88 c | 452.17 ± 0.65 a |
| Campesterol | 26.38 ± 0.27 b | 55.60 ± 0.36 c | 12.32 ± 0.10 a |
| Stigmasterol | 2.80 ± 0.05 a | 45.31 ± 0.12 c | 6.92 ± 0.23 b |
| Clerosterol | 7.90 ± 0.34 a | 56.68 ± 0.67 c | 16.99 ± 0.45 b |
| β-Sitosterol | 40.39 ± 0.47 b | 60.04 ± 0.45 c | 27.67 ± 0.42 a |
| Total | 613.20 ± 4.34 b | 1528.04 ± 4.78 c | 516.07 ± 3.22 a |
| Type of Insects | ||||
|---|---|---|---|---|
| Unit of Measure | Buffalo Worm (Alphitobius diaperinus) | Cricket (Acheta domesticus) | Mealworm (Tenebrio molitor) | |
| Total Phenolic Content | (mg catechin/100 g) | 560.55 ± 0.77 b | 578.60 ± 1.54 c | 539.76 ± 2.32 a |
| (mg Gallic acid/100 g) | 283.26 ± 0.63 b | 292.37 ± 1.37 c | 273.15 ± 2.03 a | |
| DPPH | (mg Tx/g) | 15.67 ± 0.1 a | 16.27 ± 0.1 b | 15.85 ± 0.09 a |
| ABTS | (mg Tx/g) | 18.90 ± 0.14 a | 19.32 ± 0.14 c | 19.12 ± 0.07 b |
| (mM Tx/100 g) | 7.55 ± 0.08 a | 7.72 ± 0.05 c | 7.64 ± 0.03 b | |
| FRAP | (mM Fe/kg) | 69.3 ± 0.67 a | 71.15 ± 0.5 b | 70.22 ± 1.48 b |
| Ferric reduction EC50 | (mg/mL) | 51.28 ± 0.78 c | 11.76 ± 0.13 a | 18.02 ± 0.12 b |
| Unit of Measure | Type of Bar | |||||||
|---|---|---|---|---|---|---|---|---|
| ST | BW15 | BW30 | CF15 | CF30 | TM15 | TM30 | ||
| Total phenolic content | (mg catechin/100 g) | 190.19 ± 3.09 a | 221.92 ± 0.00 c | 251.46 ± 1.54 f | 241.61 ± 1.54 e | 309.45 ± 1.54 g | 213.17 ± 6.18 b | 238.33 ± 1.54 d |
| (mg gallic acid/100 g) | 96.38 ± 2.89 a | 112.07 ± 0.00 c | 127.30 ± 1.38 f | 122.22 ± 1.21 e | 156.7 ± 1.43 g | 108.02 ± 3.01 b | 120.70 ± 1.16 d | |
| DPPH | (mg Tx/g) | 6.52 ± 0.04 a | 9.94 ± 0.03 d | 11.87 ± 0.00 f | 8.87 ± 0.00 b | 10.86 ± 0.14 e | 9.32 ± 0.00 c | 9.84 ± 0.32 d |
| ABTS | (mg Tx/g) | 7.87 ± 0.02 a | 12.01 ± 0.43 d | 14.74 ± 0.23 e | 9.72 ± 0.10 b | 13.10 ± 0.32 d | 11.25 ± 0.00 c | 11.88 ± 0.42 d |
| (mM Tx/100 g) | 3.15 ± 0.01 a | 4.70 ± 0.03 d | 5.82 ± 0.02 e | 3.83 ± 0.01 b | 5.14 ± 0.02 d | 4.44 ± 0.00 c | 4.69 ± 0.04 d | |
| FRAP | (mM Fe/kg) | 28.67 ± 0.15 a | 44.4 ± 0.25 d | 51.8 ± 0.14 f | 38.2 ± 0.42 b | 48.10 ± 0.07 e | 40.7 ± 0.27 c | 43.5 ± 0.33 d |
| Ferric reduction EC50 | (mg/mL) | 416.67 ± 1.16 g | 151.58 ± 1.25 f | 46.08 ± 0.57 a | 70.63 ± 1.28 d | 49.12 ± 0.19 b | 80.56 ± 0.00 e | 59.71 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumul, D.; Oracz, J.; Kowalski, S.; Mikulec, A.; Skotnicka, M.; Karwowska, K.; Areczuk, A. Bioactive Compounds and Antioxidant Composition of Nut Bars with Addition of Various Edible Insect Flours. Molecules 2023, 28, 3556. https://doi.org/10.3390/molecules28083556
Gumul D, Oracz J, Kowalski S, Mikulec A, Skotnicka M, Karwowska K, Areczuk A. Bioactive Compounds and Antioxidant Composition of Nut Bars with Addition of Various Edible Insect Flours. Molecules. 2023; 28(8):3556. https://doi.org/10.3390/molecules28083556
Chicago/Turabian StyleGumul, Dorota, Joanna Oracz, Stanisław Kowalski, Anna Mikulec, Magdalena Skotnicka, Kaja Karwowska, and Anna Areczuk. 2023. "Bioactive Compounds and Antioxidant Composition of Nut Bars with Addition of Various Edible Insect Flours" Molecules 28, no. 8: 3556. https://doi.org/10.3390/molecules28083556
APA StyleGumul, D., Oracz, J., Kowalski, S., Mikulec, A., Skotnicka, M., Karwowska, K., & Areczuk, A. (2023). Bioactive Compounds and Antioxidant Composition of Nut Bars with Addition of Various Edible Insect Flours. Molecules, 28(8), 3556. https://doi.org/10.3390/molecules28083556











