Molecular Dynamics Study on the Aggregation Behavior of Triton X Micelles with Different PEO Chain Lengths in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Micelle Shape and Size
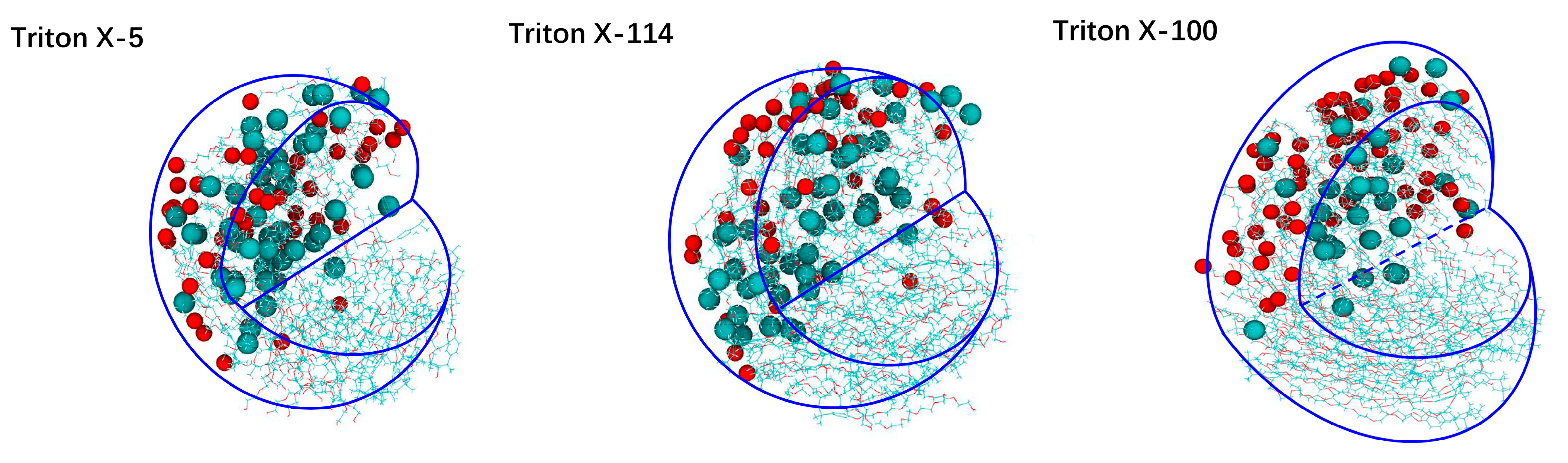
2.1.1. Micelle Shape
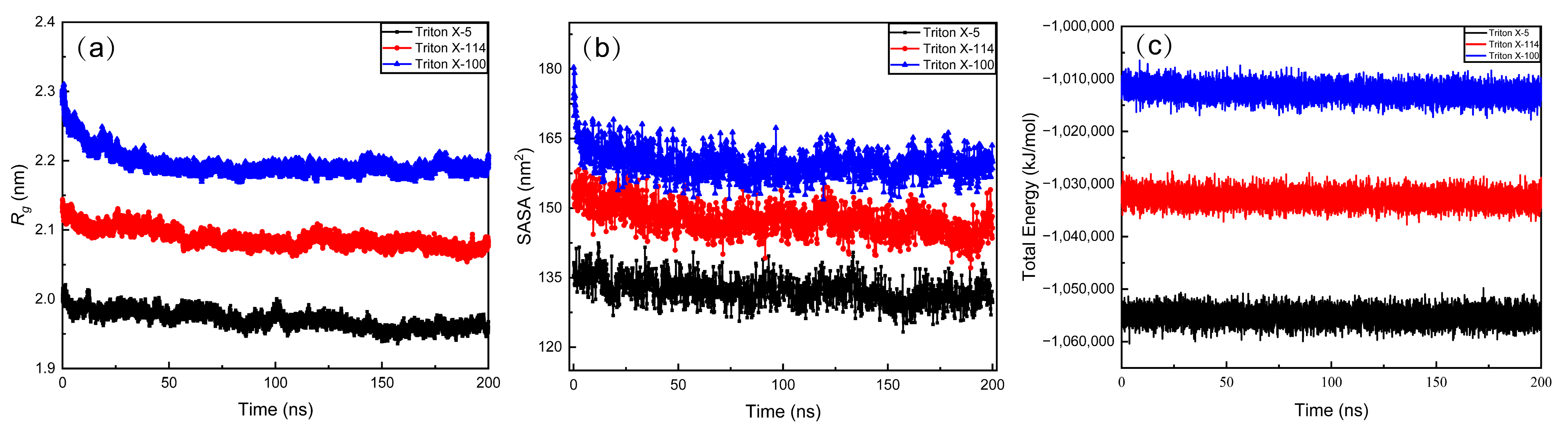
2.1.2. Micelle Size
2.1.3. Solvent Accessible Surface Area
2.2. Micelle Structure
2.3. Interactions between Micelle and Water
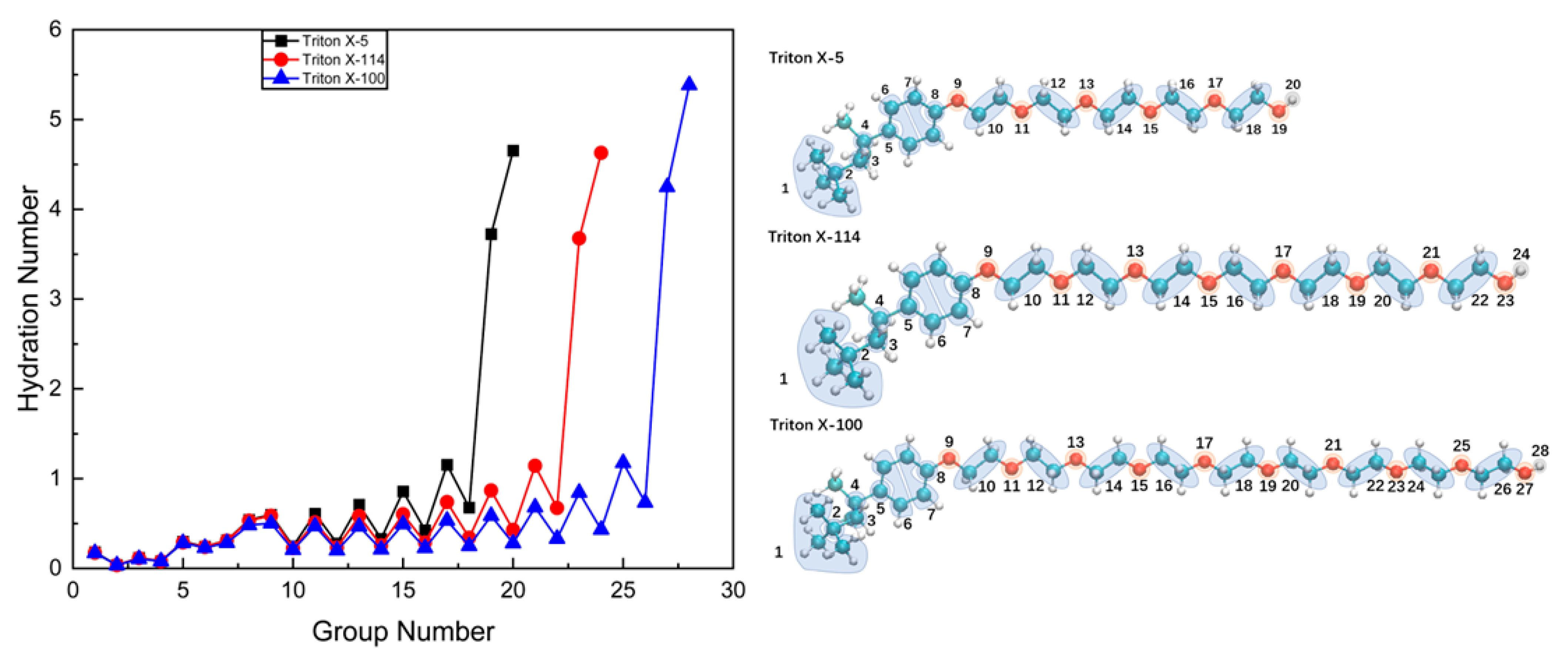
2.3.1. Hydration Numbers
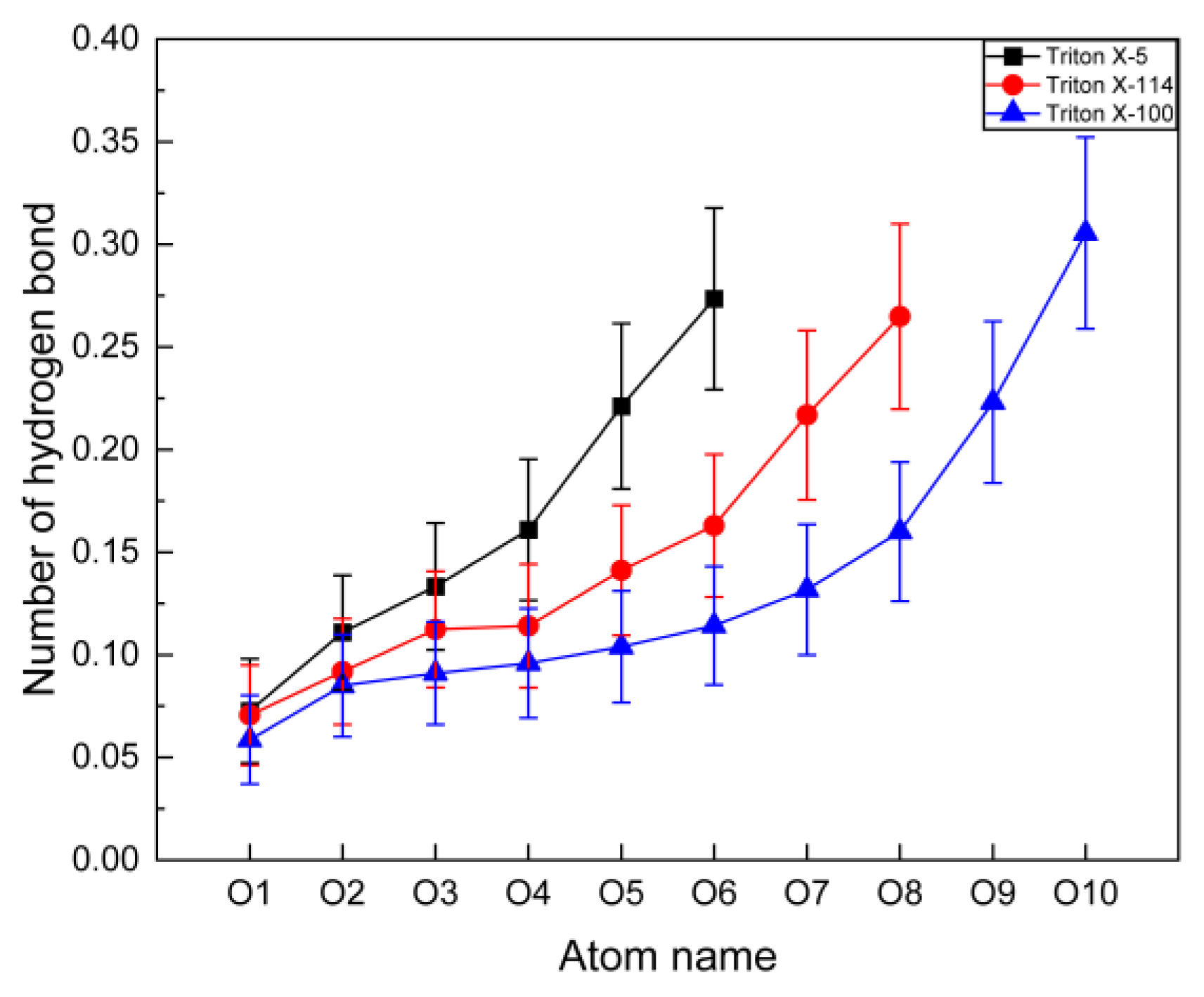
2.3.2. Number of Hydrogen Bonds
2.3.3. Time Correlation Function of the Hydrogen Bond
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barzykin, A.V.; Tachiya, M. Interpretation of passive permeability measurements on lipid-bilayer vesicles. Effect of fluctuations. BBA Biomembr. 1997, 1330, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Azum, N.; Rub, M.A.; Azim, Y.; Asiri, A.M. Micellar and spectroscopic studies of amphiphilic drug with nonionic surfactant in the presence of ionic liquids. J. Mol. Liq. 2020, 315, 113732. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mondal, R.; Paul, D.; Mukherjee, S. Investigating the micellization of the triton-X surfactants: A non-invasive fluorometric and calorimetric approach. Chem. Phys. Lett. 2016, 646, 18–24. [Google Scholar] [CrossRef]
- Abu-Ghunmi, L.; Badawi, M.; Fayyad, M. Fate of Triton X-100 Applications on Water and Soil Environments: A Review. J. Surfactants. Deterg. 2014, 17, 833–838. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Aguiar, J.; Ruiz, C.C. Growth and Hydration Of Triton X-100 Micelles In Monovalent Alkali Salts: A Light Scattering Study. J. Phys. Chem. B 2002, 106, 870–877. [Google Scholar] [CrossRef]
- Parra, J.G.; Iza, P.; Dominguez, H.; Schott, E.; Zarate, X. Effect of Triton X-100 surfactant on the interfacial activity of ionic surfactants SDS, CTAB and SDBS at the air/water interface: A study using molecular dynamic simulations. Colloid. Surface. A 2020, 603, 125284. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, X.; Sun, P.; Yuan, B.; Jiang, B.; Zhang, X.; Liu, M. The Study of the Aggregated Pattern of TX100 Micelle by Using Solvent Paramagnetic Relaxation Enhancements. Molecules 2019, 24, 1649. [Google Scholar] [CrossRef]
- Brown, W.; Rymden, R.; Van Stam, J.; Almgren, M.; Svensk, G. Static and dynamic properties of nonionic amphiphile micelles: Triton X-100 in aqueous solution. J. Phys. Chem. 1989, 93, 2512–2519. [Google Scholar] [CrossRef]
- Li, M.; Rharbi, Y.; Huang, X.; Winnik, M.A. Small variations in the composition and properties of Triton X-100. J. Colloid Interface Sci. 2000, 230, 135–139. [Google Scholar] [CrossRef]
- Thakkar, K.; Patel, V.; Ray, D.; Pal, H.; Aswal, V.K.; Bahadur, P. Interaction of imidazolium based ionic liquids with Triton X-100 micelles: Investigating the role of the counter ion and chain length. Rsc. Adv. 2016, 6, 36314–36326. [Google Scholar] [CrossRef]
- Denkova, P.S.; Lokeren, L.V.; Verbruggen, I.; Willem, R. Self-Aggregation and Supramolecular Structure Investigations of Triton X-100 and SDP2S by NOESY and Diffusion Ordered NMR Spectroscopy. J. Phys. Chem. B 2008, 112, 10935–10941. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-Z.; Cheng, G.-Z.; Zhao, S.; Miao, X.-J.; Yu, J.-Y.; Shen, L.-F.; Du, Y.-R. Conformational Dependence of Triton X-100 on Environment Studied by 2D NOESY and 1H NMR Relaxation. Langmuir 2000, 16, 3030–3035. [Google Scholar] [CrossRef]
- Paradies, H.H. Shape and size of a nonionic surfactant micelle. Triton X-100 in aqueous solution. J. Phys. Chem. 1980, 84, 599–607. [Google Scholar] [CrossRef]
- Kumbhakar, M.; Goel, T.; Mukherjee, T.; Pal, H. Role of Micellar Size and Hydration on Solvation Dynamics: A Temperature Dependent Study in Triton-X-100 and Brij-35 Micelles. J. Phys. Chem. B 2004, 108, 19246–19254. [Google Scholar] [CrossRef]
- Ruiz, C.C.; Molina-Bolívar, J.A.; Aguiar, J.; MacIsaac, G.; Moroze, S.; Palepu, R. Thermodynamic and Structural Studies of Triton X-100 Micelles in Ethylene Glycol-Water Mixed Solvents. Langmuir 2001, 17, 6831–6840. [Google Scholar] [CrossRef]
- Robson, R.J.; Dennis, E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton X-100. J. Phys. Chem. 1977, 81, 1075–1078. [Google Scholar] [CrossRef]
- Kumbhakar, M.; Nath, S.; Mukherjee, T.; Pal, H. Solvation dynamics in triton-X-100 and triton-X-165 micelles: Effect of micellar size and hydration. J. Chem. Phys. 2004, 121, 6026–6033. [Google Scholar] [CrossRef]
- Fedyaeva, O.A.; Poshelyuzhnaya, E.G. Dimensions and Orientation of Triton X-100 Micelles in Aqueous Solutions, According to Turbidimetric Data. Russ. J. Phys. Chem. A 2019, 93, 2559–2561. [Google Scholar] [CrossRef]
- Streletzky, K.; Phillies, G.D. Temperature dependence of Triton X-100 micelle size and hydration. Langmuir 1995, 11, 42–47. [Google Scholar] [CrossRef]
- Mandal, A.; Ray, S.; Biswas, A.; Moulik, S. Physicochemical studies on the characterization of Triton X 100 micelles in an aqueous environment and in the presence of additives. J. Phys. Chem. 1980, 84, 856–859. [Google Scholar] [CrossRef]
- Phillies, G.D.; Yambert, J.E. Solvent and solute effects on hydration and aggregation numbers of Triton X-100 micelles. Langmuir 1996, 12, 3431–3436. [Google Scholar] [CrossRef]
- Phillies, G.D.; Stott, J.; Ren, S. Probe diffusion in the presence of nonionic amphiphiles: Triton X 100. J. Phys. Chem. 1993, 97, 11563–11568. [Google Scholar] [CrossRef]
- Leach, A.R. Molecular Modelling: Principles and Applications; Pearson Education: London, UK, 2001. [Google Scholar]
- De Nicola, A.; Kawakatsu, T.; Rosano, C.; Celino, M.; Rocco, M.; Milano, G. Self-Assembly of Triton X-100 in Water Solutions: A Multiscale Simulation Study Linking Mesoscale to Atomistic Models. J. Chem. Theory. Comput. 2015, 11, 4959–4971. [Google Scholar] [CrossRef] [PubMed]
- Murakami, W.; De Nicola, A.; Oya, Y.; Takimoto, J.-I.; Celino, M.; Kawakatsu, T.; Milano, G. Theoretical and Computational Study of the Sphere-to-Rod Transition of Triton X-100 Micellar Nanoscale Aggregates in Aqueous Solution: Implications for Membrane Protein Purification and Membrane Solubilization. ACS Appl. Nano Mater. 2021, 4, 4552–4561. [Google Scholar] [CrossRef]
- Farafonov, V.; Lebed, A.; Khimenko, N.; Mchedlov-Petrossyan, N. Molecular Dynamics Study of an Acid-Base Indicator Dye in Triton X-100 Non-Ionic Micelles. Vopr. Khimii I Khimicheskoi Tekhnologii 2020, 1, 97–103. [Google Scholar] [CrossRef]
- Pacheco-Blas, M.D.A.; Vicente, L. Molecular dynamics study of the behaviour of surfactant Triton X-100 in the extraction process of Cd2+. Chem. Phys. Lett. 2020, 739, 136920. [Google Scholar] [CrossRef]
- Ritter, E.; Yordanova, D.; Gerlach, T.; Smirnova, I.; Jakobtorweihen, S. Molecular dynamics simulations of various micelles to predict micelle water partition equilibria with COSMOmic: Influence of micelle size and structure. Fluid Phase Equilib. 2016, 422, 43–55. [Google Scholar] [CrossRef]
- Yordanova, D.; Smirnova, I.; Jakobtorweihen, S. Molecular Modeling of Triton X Micelles: Force Field Parameters, Self-Assembly, and Partition Equilibria. J. Chem. Theory Comput. 2015, 11, 2329–2340. [Google Scholar] [CrossRef]
- Rauf, M.A.; Hisaindee, S.; Graham, J.P.; Al-Zamly, A. Effect of various solvents on the absorption spectra of dithizone and DFT calculations. J. Mol. Liq. 2015, 211, 332–337. [Google Scholar] [CrossRef]
- Faramarzi, S.; Bonnett, B.; Scaggs, C.A.; Hoffmaster, A.; Grodi, D.; Harvey, E.; Mertz, B. Molecular Dynamics Simulations as a Tool for Accurate Determination of Surfactant Micelle Properties. Langmuir 2017, 33, 9934–9943. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Liu, G.; Wang, Z.; Yuan, S. A molecular dynamics study on two promising green surfactant micelles of choline dodecyl sulfate and laurate. RSC Adv. 2016, 6, 84090–84097. [Google Scholar] [CrossRef]
- Allen, D.T.; Saaka, Y.; Lawrence, M.J.; Lorenz, C.D. Atomistic description of the solubilisation of testosterone propionate in a sodium dodecyl sulfate micelle. J. Phys. Chem. B 2014, 118, 13192–13201. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, G.; Wang, Z.; Yuan, S. Molecular dynamics study on the aggregation behaviour of different positional isomers of sodium dodecyl benzenesulphonate. RSC Adv. 2016, 6, 49708–49716. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Liu, G.; Yuan, S.; Liu, C. Tetraalkylammonium interactions with dodecyl sulfate micelles: A molecular dynamics study. Phys. Chem. Chem. Phys. 2016, 18, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Eisenhaber, F.; Lijnzaad, P.; Argos, P.; Sander, C.; Scharf, M. The double cubic lattice method: Efficient approaches to numerical integration of surface area and volume and to dot surface contouring of molecular assemblies. J. Comput. Chem. 1995, 16, 273–284. [Google Scholar] [CrossRef]
- Tang, X.; Koenig, P.H.; Larson, R.G. Molecular dynamics simulations of sodium dodecyl sulfate micelles in water-the effect of the force field. J. Phys. Chem. B 2014, 118, 3864–3880. [Google Scholar] [CrossRef]
- Palazzesi, F.; Calvaresi, M.; Zerbetto, F. A molecular dynamics investigation of structure and dynamics of SDS and SDBS micelles. Soft Matter. 2011, 7, 9148–9156. [Google Scholar] [CrossRef]
- Shelley, J.; Watanabe, K.; Klein, M.L. Simulation of a sodium dodecylsulfate micelle in aqueous solution. Int. J. Quantum Chem. 1990, 38, 103–117. [Google Scholar] [CrossRef]
- Gao, J.; Ge, W.; Hu, G.; Li, J. From Homogeneous Dispersion to Micelles A Molecular Dynamics Simulation on the Compromise of the Hydrophilic and Hydrophobic Effects of Sodium Dodecyl Sulfate in Aqueous Solution. Langmuir 2005, 21, 5223–5229. [Google Scholar] [CrossRef]
- MacKerell, A.D. Molecular Dynamics Simulation Analysis of a Sodium Dodecyl Sulfate Micelle in Aqueous Solution: Decreased Fluidity of the Micelle Hydrocarbon Interior. J. Phys. Chem. 1995, 99, 1846–1855. [Google Scholar] [CrossRef]
- Chun, B.J.; Choi, J.I.; Jang, S.S. Molecular dynamics simulation study of sodium dodecyl sulfate micelle: Water penetration and sodium dodecyl sulfate dissociation. Colloid. Surface. A 2015, 474, 36–43. [Google Scholar] [CrossRef]
- Liu, G.; Li, R.; Wei, Y.; Gao, F.; Wang, H.; Yuan, S.; Liu, C. Molecular dynamics simulations on tetraalkylammonium interactions with dodecyl sulfate micelles at the air/water interface. J. Mol. Liq. 2016, 222, 1085–1090. [Google Scholar] [CrossRef]
- Pal, S.; Bagchi, B.; Balasubramanian, S. Hydration Layer of a Cationic Micelle, C10TAB: Structure, Rigidity, Slow Reorientation, Hydrogen Bond Lifetime, and Solvation Dynamics. J. Phys. Chem. B 2005, 109, 12879–12890. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Peng, J.; Li, Y.; Leng, X.; Liu, G.; Xia, Q.; Wei, Y. Molecular dynamics study on the aggregation behaviours of Platonic micelle in different NaCl solutions. J. Mol. Liq. 2022, 353, 118828. [Google Scholar] [CrossRef]
- Pal, A.; Pillania, A. Modulations in surface and aggregation properties of non-ionic surfactant Triton X-45 on addition of ionic liquids in aqueous media. J. Mol. Liq. 2017, 233, 243–250. [Google Scholar] [CrossRef]
- Qiao, L.; Easteal, A.J. Mass transport in Triton X series nonionic surfactant solutions: A new approach to solute-solvent interactions. Colloid Polym. Sci. 1996, 274, 974–980. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Koziara, K.B.; Stroet, M.; Malde, A.K.; Mark, A.E. Testing and validation of the Automated Topology Builder (ATB) version 2.0: Prediction of hydration free enthalpies. J. Comput. Aided Mol. Des. 2014, 28, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Lindahl, E.; Abraham, M.; Hess, B.; van der Spoel, D. GROMACS 2019.3 Source Code (2019.3). Zenodo. 2019. Available online: https://doi.org/10.5281/zenodo.3243833 (accessed on 10 March 2023).
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.V.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]


| TX-5 | TX-114 | TX-100 | |
|---|---|---|---|
| Imax/Imin | 1.15 ± 0.03 | 1.12 ± 0.04 | 1.26 ± 0.03 |
| e | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.12 ± 0.02 |
| Rg (nm) | 1.96 ± 0.01 | 2.07 ± 0.01 | 2.19 ± 0.01 |
| Rs (nm) | 2.53 ± 0.01 | 2.67 ± 0.01 | 2.83 ± 0.01 |
| SASA(total) (nm2) | 132.24 ± 2.77 | 147.52 ± 3.25 | 159.54 ± 2.97 |
| SASA(hydrophilic) (nm2) | 30.62 ± 2.33 | 32.72 ± 2.27 | 35.81 ± 1.96 |
| SASA(hydrophobic) (nm2) | 101.66 ± 2.19 | 114.77 ± 2.29 | 123.71 ± 2.54 |
| Molecule | TX-5 (n = 5) | TX-114 (n = 7) | TX-100 (n = 9) |
|---|---|---|---|
| Nagg | 100 | 100 | 100 |
| H2O | 30,830 | 30,264 | 29,751 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Song, X.; Li, X.; Jiang, Y.; Liu, G.; Wei, Y.; Xia, Q. Molecular Dynamics Study on the Aggregation Behavior of Triton X Micelles with Different PEO Chain Lengths in Aqueous Solution. Molecules 2023, 28, 3557. https://doi.org/10.3390/molecules28083557
Peng J, Song X, Li X, Jiang Y, Liu G, Wei Y, Xia Q. Molecular Dynamics Study on the Aggregation Behavior of Triton X Micelles with Different PEO Chain Lengths in Aqueous Solution. Molecules. 2023; 28(8):3557. https://doi.org/10.3390/molecules28083557
Chicago/Turabian StylePeng, Jin, Xiaoju Song, Xin Li, Yongkang Jiang, Guokui Liu, Yaoyao Wei, and Qiying Xia. 2023. "Molecular Dynamics Study on the Aggregation Behavior of Triton X Micelles with Different PEO Chain Lengths in Aqueous Solution" Molecules 28, no. 8: 3557. https://doi.org/10.3390/molecules28083557
APA StylePeng, J., Song, X., Li, X., Jiang, Y., Liu, G., Wei, Y., & Xia, Q. (2023). Molecular Dynamics Study on the Aggregation Behavior of Triton X Micelles with Different PEO Chain Lengths in Aqueous Solution. Molecules, 28(8), 3557. https://doi.org/10.3390/molecules28083557







